
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 30 January 2023
Sec. T Cell Biology
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1098847
This article is part of the Research TopicCancer cell therapy using non-MHC-restricted T cellsView all 5 articles
 Anna Maria Corsale1,2,3†
Anna Maria Corsale1,2,3† Marta Di Simone1,2,3†
Marta Di Simone1,2,3† Elena Lo Presti4
Elena Lo Presti4 Francesco Dieli1,3
Francesco Dieli1,3 Serena Meraviglia1,3*
Serena Meraviglia1,3*In recent years, research has focused on colorectal cancer to implement modern treatment approaches to improve patient survival. In this new era, γδ T cells constitute a new and promising candidate to treat many types of cancer because of their potent killing activity and their ability to recognize tumor antigens independently of HLA molecules. Here, we focus on the roles that γδ T cells play in antitumor immunity, especially in colorectal cancer. Furthermore, we provide an overview of small-scale clinical trials in patients with colorectal cancer employing either in vivo activation or adoptive transfer of ex vivo expanded γδ T cells and suggest possible combinatorial approaches to treat colon cancer.
Colorectal cancer (CRC) is the third most frequent cancer, with about 1.8 million new cases worldwide in 2018, and the second leading cause of cancer-related deaths worldwide (1). At the onset of CRC, there is a combination of various intrinsic and extrinsic factors, such as genetic factors, gut dysbiosis, dysregulation of the immune system, and environmental factors (2). Although family history is involved in 10%–30% of cases in the pathogenesis of CRC, the majority are sporadic, and the most commonly associated mutations accumulate stepwise, following the so-called adenoma-carcinoma sequence (3). Although the 5-year survival rates for localized tumors are over 90% and 71% for regional tumors, 86% of patients with distant metastases die within 5 years (4). This indicates that, despite the adoption of several screening protocols and currently available standard treatments (surgical resection, radiotherapy, and chemotherapy), there is an urgent need to develop more effective treatments to reduce mortality and tumor relapse.
In this context, immunotherapy appears to be of considerable importance thanks to the unprecedented success obtained in clinical practice. Indeed, in recent years, various studies have been developed on CRC immunotherapy, showing very promising results, especially regarding the application of immune checkpoint inhibitors (ICIs). Pembrolizumab and nivolumab have been authorized for the treatment of advanced and metastatic CRC patients with microsatellite instability-high/DNA mismatch repair deficiency (MSI-H/dMMR) in a chemotherapy-refractory scenario (5). However, ICIs are unsatisfactory in treating microsatellite stable/DNA mismatch repair proficient (MSS/pMMR) CRC because of the low tumor mutation burden and the lack of immune cell infiltration, which are regarded as immune resistance mechanisms (6).. Furthermore, along with chemotherapy, targeted therapies such as monoclonal antibodies targeting epidermal growth factor receptor (EGFR) (e.g., cetuximab) and vascular endothelial growth factor (VEGF) (e.g., bevacizumab) are the gold standard treatments for advanced or metastatic colorectal cancer. At present, all of these treatment options are hampered by the possibility of primary or acquired resistance (7). Therefore, new approaches must be developed to enable more CRC patients to benefit from immunotherapy.
The presence of immune cells in the tumor microenvironment (TME) is known to be a strong predictor of clinical outcome (8). The number of tumor-infiltrating immune cells is referred to as the immunoscore, which is used in conjunction with other characteristics to categorize colorectal cancer. To demonstrate this, Pagès et al. observed a significant link between a 5-year survival rate and a high immunoscore in patients with microsatellite instability or microsatellite-stable CRC; this benefit was not identified when immuscore was low (9). Among the immune cells that make up TME, a noteworthy population is γδ T cells, an innate-like subset of T lymphocytes (10). As well as being located in peripheral blood, they are located in the intestinal epithelium, representing the major population of intraepithelial lymphocytes (IELs). Here, they carry out powerful anticancer and anti-inflammatory actions and are involved in maintaining homeostasis and regulating the microbiome (11, 12). γδ T cells are a valid therapeutic option in the field of anticancer immunotherapy, ensuring efficacy and safety in side effects thanks to their unique recognition capabilities, their remarkable immunosurveillance properties, and their easy handling in vitro (13).
This review aims to highlight the role of γδ T cells in colorectal cancer and to summarize the different approaches that allow their use as a therapeutic tool in the treatment of colorectal cancer.
γδ T cells are a subset of CD3+ lymphocytes that, differently than αβ T cells, express a T-cell receptor (TCR) composed of γ and δ chains. They bridge innate and adaptive immunity, generating quick immune responses with macrophages and neutrophils to several pathogens and helping adaptive immune cells such as T cells and B cells to exert their effector functions. Moreover, thanks to the expression of TCR and typical natural killer (NK) receptors, such as NKG2D and CD94, they can kill target cells and activate other immune cells (14). The vast majority of γδ T cells (~70%) are CD4−CD8−, ~30% are CD8+CD4−, and only <1% are CD4+CD8− (15). Based on differences in TCR δ-chain usage, human γδ T cells are classified into three cellular subsets with specific tropisms: Vδ1+, Vδ2+, and Vδ1−Vδ2− (16). Vδ1+ T cells are predominantly expressed in mucosal and epithelial tissues and represent a large portion of human intestinal IELs (17). They show cytotoxic activities thanks to their ability to recognize stress antigens expressed by transformed cells, such as MHC class I-related molecules A and B (MICA/MICB) and UL16-binding proteins via the NKG2D receptor (18) (19). Also, they recognize self-glycolipids presented by CD1c and CD1d (20) (21) and are expanded during Cytomegalovirus (CMV) infection (22). The Vδ1 chain can be paired with several Vγ chains (Vγ2, Vγ3, Vγ4, Vγ5, Vγ8, and Vγ10) (23), but in the gut, it is paired with the Vγ4 chain (12).
The most representative γδ T-cell subset expresses the Vδ2 chain typically associated with Vγ9 chain. These cells are generally referred to as Vγ9Vδ2 T cells (24), and they account for 1%–10% of circulating T cells and 50%–95% of the total γδ T-cell compartment (25). The distinctive feature of Vγ9Vδ2 T cells is to recognize and respond to phosphorylated metabolites called phosphoantigens (P-Ags) (14). These are intermediates of the endogenous mevalonate (MVA) pathway (e.g., isopentenyl pyrophosphate (IPP)), upregulated during tumor transformation, or the exogenous 1-deoxy-d-xylulose-5-phosphate (MEP) pathway (e.g., (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP)), upregulated during infections (26, 27). As a rule, P-Ags recognition is not MHC-restricted but requires butyrophilin (BTN)2A1 and BTN3A1, two type-I glycoproteins of the B7 family. The BTN2A1–BTN3A1 complex contacts with the γδ TCR in two sites: BTN2A1 binds to the Vγ9 domain, whereas BTN3A1 may bind to the Vδ2 and γ-chain regions on the opposite side of the TCR (28). Once activated, Vγ9Vδ2 T cells kill infected, stressed, and tumor cells using the same mechanisms as NK and CD8+ T cells. Moreover, after activation, they interact with B cells, promoting CD4+ and CD8+ T-cell priming and inducing maturation of dendritic cells (DC) and survival of monocytes and neutrophils (14, 29, 30). These properties let Vγ9Vδ2 T cells orchestrate an innate-like response against pathogens and tumor cells. Recently, Davey et al. have described a population of Vγ9−Vδ2+ T cells that do not respond to P-Ags and have adaptive-like functions (31).
The Vδ1−Vδ2− compartment consists of distinct minor subsets, such as Vδ3+ and Vδ5+ T cells. Vδ3+ T cells amount to 0.2% of T lymphocytes in the blood and are also located in the liver and gut. Currently, its paired γ chain is unknown. Due to a lack of specific antibodies for their identification, generally, they are characterized as Vδ1−Vδ2−cells (32).Vδ3+ T cells recognize CD1d molecules expressed on target cells, have cytotoxic activity, and release Th1-, Th2-, and Th17-like proinflammatory cytokines (32). A recent study by Petrasca et al. described a role for this subset in the maturation of B cells and the production of IgM antibodies, without promoting class-switching (33).
Vδ5+ γδ T cells are the rarest subset; in particular, Willcox et al. described a clone of these Vδ5+Vγ4+ T cells that recognize the endothelial protein C receptor expressed by stressed human cells (34).
γδ T cells are one of the components of the tumor immune milieu and are well-known for influencing the antitumor immune response to a broad range of tumors (35). Indeed, γδ T cells are recruited into various types of malignancies, including breast cancer (36), melanoma (37), and lung cancer (38). In particular, with regard to colon cancer, we have previously shown that γδ T lymphocytes, of which Vδ1 was the most represented subset, amounted to 4.5% of the total leukocyte population in CRC (39). In addition, Corvaisier et al. have described a Vγ9Vδ2 TCR clone isolated from colon cancer ascites (40). Recently, a TCR γδ+CD103+PD-1+ subset was exclusively found by high-dimensional single-cell mass cytometry in MMR-deficient CRC, accounting for 8.4% of CD45+ cells (41, 42).
Despite some studies that have identified the presence of γδ T cells among tumor-infiltrating lymphocytes (TILs) as a positive prognostic factor, it is not yet fully known whether they can promote or hamper tumor growth (43). Particularly, Gentles et al., analyzing the gene expression profile of 18,000 tumor fragments from 39 types of malignancies on the prognostic role of the individual infiltrating cell populations, showed that the neutrophils and plasma cells present in tumor infiltrate have a significant but opposite role in different tumors, while the presence of γδ T cells was found to be the most favorable prognostic factor in analyzed tumors, both hematological and solid (44), including CRC. However, Tosolini et al., applying the CIBERSORT algorithm, showed that in Gentle’s study, the presence of Vγ9Vδ2 was wrongly identified as CD4+CD8+ T cells or NK and vice versa (45). Therefore, using an improved deconvoluted CIBERSORT matrix to investigate the abundance of TCR Vγ9Vδ2, they found that CRC patients with a high TCR Vγ9Vδ2 infiltration had a better survival rate than those with a low infiltration (45). Similarly, we have shown by transcriptomic analysis of a cohort of 557 CRC patients that 5-year disease-free survival was significantly higher in CRC patients with higher γδ TILs (39). In addition, the decrease of NKp46+/Vδ1 IELs in tumor-free specimens from CRC patients correlated with a higher risk of metastasis (12).
In contrast to these findings, a previous study highlighted the role of IL-17A-producing γδ TILs in the promotion of tumor progression in patients with CRC (46), but these results were not confirmed in subsequent studies (39).
This twofold role can be linked to the plasticity of γδ T cells, which includes the capacity to differentiate toward different functional cell subsets based on the microenvironment, which can direct their polarization. This implies that the positive or negative correlation with the tumor prognosis can be attributed to the specific γδ T cell subsets recruited or resident at the tumor site (10).
Notwithstanding that γδ T cells exert antitumor effects, it is not surprising that they also have protumor activity due to their plasticity. Indeed, after recruitment to the tumor site, they can acquire a phenotype that mirrors Tfh, Treg, Th1, Th2, and Th17, influenced by a plethora of cytokines present in the tumor microenvironment (47). Particularly, the polarized γδ T17 and γδ Treg cells, which produce IL-17A or TGF-β, respectively, have been reported to support cancer progression and be associated with poor survival in the gallbladder (48), ovarian (49), and colorectal cancer (39, 46).
In a study by Wu et al., γδ T cells were the major cellular source of IL-17A in human CRC. Activated inflammatory DC polarized Vγ9Vδ2 T cells into IL-17A-producing γδ T cells. Together with the secretion of IL-8, TNF-α, and granulocyte-macrophage colony-stimulating factor (GM-CSF), they induced an immunosuppressive microenvironment in human CRC, thus promoting tumor progression. The tumor infiltration of IL-17A-producing γδ T cells was positively associated with advanced tumor stages of human CRC and with other clinicopathological features, including tumor size, lymphatic and vascular invasion, and lymph node metastasis (46). However, in a subsequent study (39) with two very large cohorts of CRC patients, we failed to confirm the results of Wu and colleagues. In ovarian cancer, γδ T cells and their Vδ1 subset produced IL-17A, impaired antitumor responses, and enhanced immunosuppressive activities, as documented by their ability to inhibit the proliferation of naive CD4+ T cells upon co-culture with ovarian cancer tissue supernatants (49). Rong et al. described an imbalance in Vδ1 and Vδ2 subset distribution that may contribute to the growth of rectal cancer. Unlike Vδ2 T cells, Vδ1+ TILs were significantly increased, when compared with para-carcinoma tissues, and had strong inhibitory effects (50). Moreover, a CD39+ γδ Treg subset was identified in the human CRC, showing a superior immunosuppressive activity than CD4+ or CD8+ Tregs via the adenosine pathway. CD39+ γδ Treg cells also established an immunosuppressive milieu due to the chemo-attraction of myeloid-derived suppressor cells (MDSCs) in an IL-17A and GM-CSF-dependent manner. In addition, their infiltration was correlated with unfavorable clinicopathological features in CRC (51). Recently, in the microenvironment of human breast cancer, a regulatory subset of CD73+ γδ T cells was identified, which exerted immunosuppressive functions through the production of IL-10, IL-8, and adenosine (52).
Consequently, this evidence, although still very preliminary, suggests that γδ T cells may establish an immunosuppressive microenvironment to inhibit antitumor immune responses, evade immune surveillance, and promote the progression of cancer.
γδ T cells, particularly Vδ1 and Vδ2 subsets, have a significant antitumor activity that is exerted by direct and indirect mechanisms and through the involvement of several pathways. Cancer cells are recognized not only via TCR-dependent antigen recognition but also through NK receptors and or antibody-dependent cell-mediated cytotoxicity (ADCC) (Figures 1, 2).
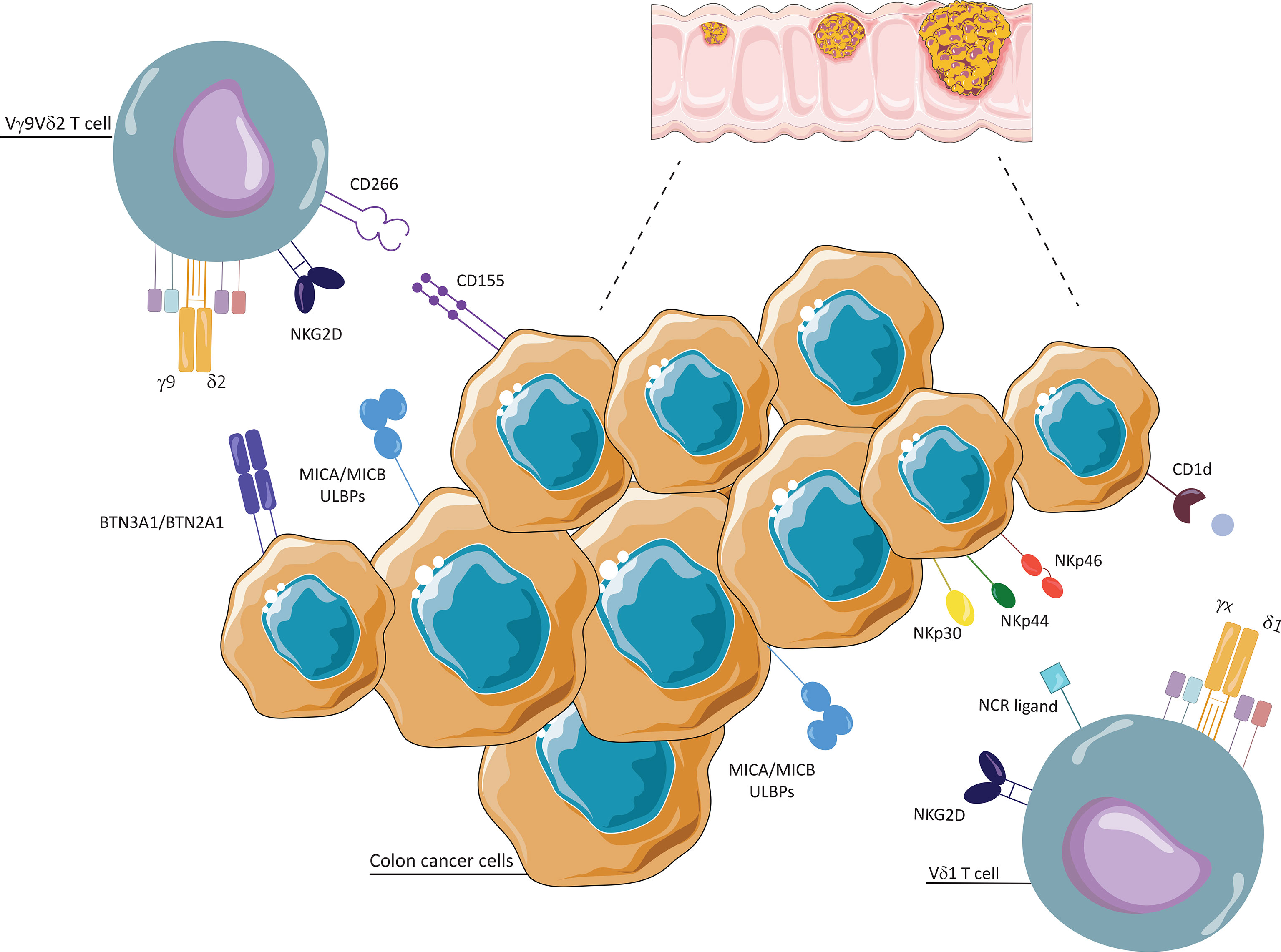
Figure 1 Interaction between γδ T cells and colon cancer cells. Vδ1 and Vδ2 T cells conduct cytotoxic activities thanks to their ability to recognize stress antigens expressed by transformed cells, such as MHC class I-related molecules A and B (MICA/MICB) and UL16-binding proteins (ULBPs), via NKG2D receptor. Vδ1 T cells recognize self-glycolipids presented by CD1c and CD1d, and tumor cells through natural cytotoxicity receptors (NKp30, NKp44, NKp46). Vδ2 T cells recognize and respond to phosphoantigens (P-Ags), through unrestricted MHC manner, with two types I glycoproteins of the B7 family, butyrophilin (BTN)2A1 and BTN3A1. They interact with tumor cells through CD226 (DNAM-1)/CD155 (Nectin-2).
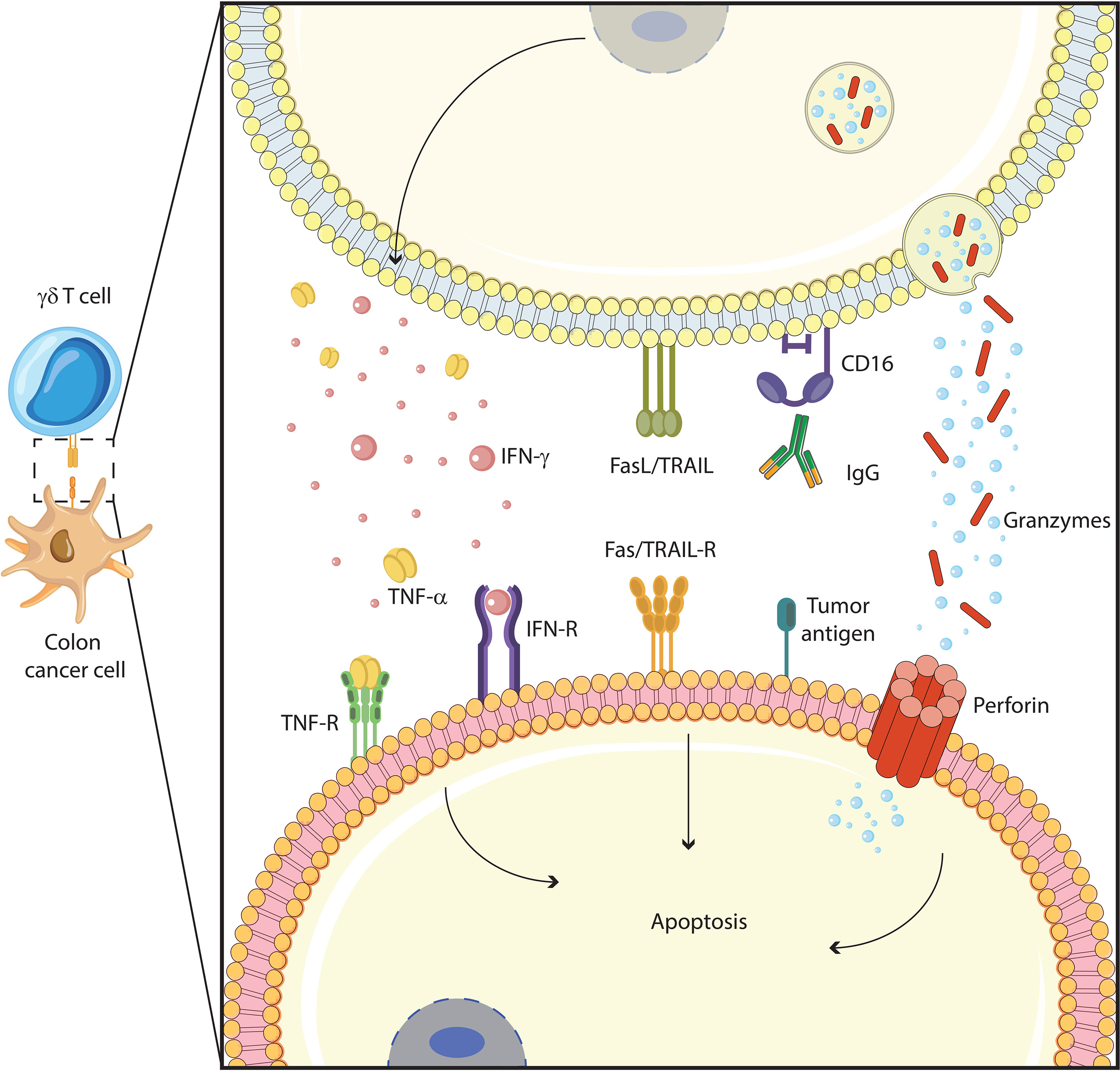
Figure 2 γδ T cells function against colon cancer cells. γδ T cells exert their cytotoxic functions through several mechanisms: induction of apoptotic cell death with Fas/TRAIL pathway, antibody-dependent cellular cytotoxicity (ADCC) with IgG/CD16 interaction, cytolytic granule (granzyme, perforin) polarization and degranulation, and proinflammatory cytokines (TNF-α, IFN-γ) production.
Vγ9Vδ2 T cells generally recognize tumor target cells either through the TCR or through stimulatory NK receptors, such as NKG2D and DNAX accessory molecule 1 (DNAM-1), that recognize their specific ligands overexpressed on tumor cells. In addition to NKG2D, Vδ1+ T cells used natural cytotoxicity receptors (NKp30, NKp44, NKp46) for tumor cell recognition, although their expression is linked to specific conditions, such as the presence of IL-15 or IL-2 (12, 53). A recent study shows the strong antitumor activity of NKp46+ γδ IELs against human colon adenocarcinoma cell lines (12).
Similar to NK cells, human γδ T cells, once activated, upregulate on their surface type-III Fcγ receptor (CD16) and promote ADCC on opsonized tumor cells. Relevant to cancer immunotherapy, CD16-expressing γδ T cells have been shown to improve the efficacy of therapeutic antibodies such as rituximab and trastuzumab for the treatment of lymphoma, chronic lymphocytic leukemia, and human epidermal growth factor receptor 2 (HER2)-positive breast cancer cells (54, 55). Recent studies have shown that bispecific antibodies binding TCR-HER2 and CD19-specific triple bodies increase γδ T cells cytotoxicity, improving ADCC (56) (57),. Finally, Vγ9Vδ2 T lymphocytes with high CD16 expression exert ADCC in the 3D cultures of colon cancer cells, inducing a decrease in size and cell number in spheroids (58). Whatever the mechanism of target cell recognition, killing by γδ T cells involves the perforin/granzyme pathways (59, 60) and Fas/FasL, TRAIL/TRAIL-R, and TNF/TNF-RII interactions (61–63), depending on the nature of the target cells.
Instead, indirect antitumor mechanisms occur via interaction between γδ T cells and other immune cells, such as B cells, DC, αβ T cells, and NK cells. Acting similarly to Tfh cells, γδ T cells can secrete cytokines that promote B cells to secrete specific antibodies (29). Furthermore, they take part in the maturation of DC, thus inducing helper and cytotoxic functions of CD4+ and CD8+ T cells (64, 65). Likewise, they improve NK cell-mediated cytotoxicity through CD137/CD137L engagement and upregulating NKG2D (66).
In the last few years, immunotherapy has had great prominence in cancer treatment. It is based on enhancing the immune system to kill tumor cells and block or limit their growth. Most immunotherapeutic approaches make use of αβ T cells, but efficacy is limited due to the characteristics of this subset, namely, their activation and functional activities rely on MHC restriction, costimulatory signal, and specific tumor-associated antigen (TAA) recognition (67).
γδ T cells provide themselves with a valid alternative to overcome these limits thanks to their unique features (68). Indeed, they recognize conserved antigens widely expressed on stressed and tumor cells without the need for MHC restriction and costimulatory molecules. Both of these aspects allow γδ T cells to avoid immune escape due to the downregulation of MHC molecules and loss of antigen expression by tumor cells. In addition, they perform effector activity relevant to antitumor immune responses, such as cytotoxicity and cytokine (IFN-γ and TNF-α) production. Finally, their inbred tissue tropism, particularly for Vδ1+ T cells, makes them more suitable than αβ T cells for performing rapid antitumor responses. These features, together with their easy activation and expansion, make γδ T cells attractive candidates for tumor immunotherapy (69).
Most γδ T-cell-based immunotherapy clinical trials have used the Vγ9Vδ2 subset (Figure 3). According to the modality of their expansion and activation, two types of Vγ9Vδ2 T-cell immunotherapy have been considered: in vivo activation and adoptive transfer of ex vivo expanded Vγ9Vδ2 T cells (17). The ex vivo approach requires the isolation of γδ T cells from peripheral blood mononuclear cells (PBMCs), their expansion in vitro, and then their adoptive transfer into recipient patients. Specific stimulants for Vγ9Vδ2 T cells include natural (IPP and HMBPP) or synthetic (bromohydrin pyrophosphate (BrHPP)) P-Ags. Furthermore, different drugs can be used to enhance the accumulation of endogenous P-Ags, among which are nitrogenous bisphosphonates (N-BPs), a class of drugs generally used in the treatment of osteoporosis and bone disorders. They act by inhibiting the farnesyl pyrophosphate synthase (FPPS) enzyme of the mevalonate pathway, thus upregulating isoprenoid biosynthesis within cells (70). Zoledronic acid (zoledronate, Zometa (ZA)) is the most common in vivo and in vitro using N-BP and it also shows antitumor effects through induction of apoptosis and autophagy in cancer cell lines (71).
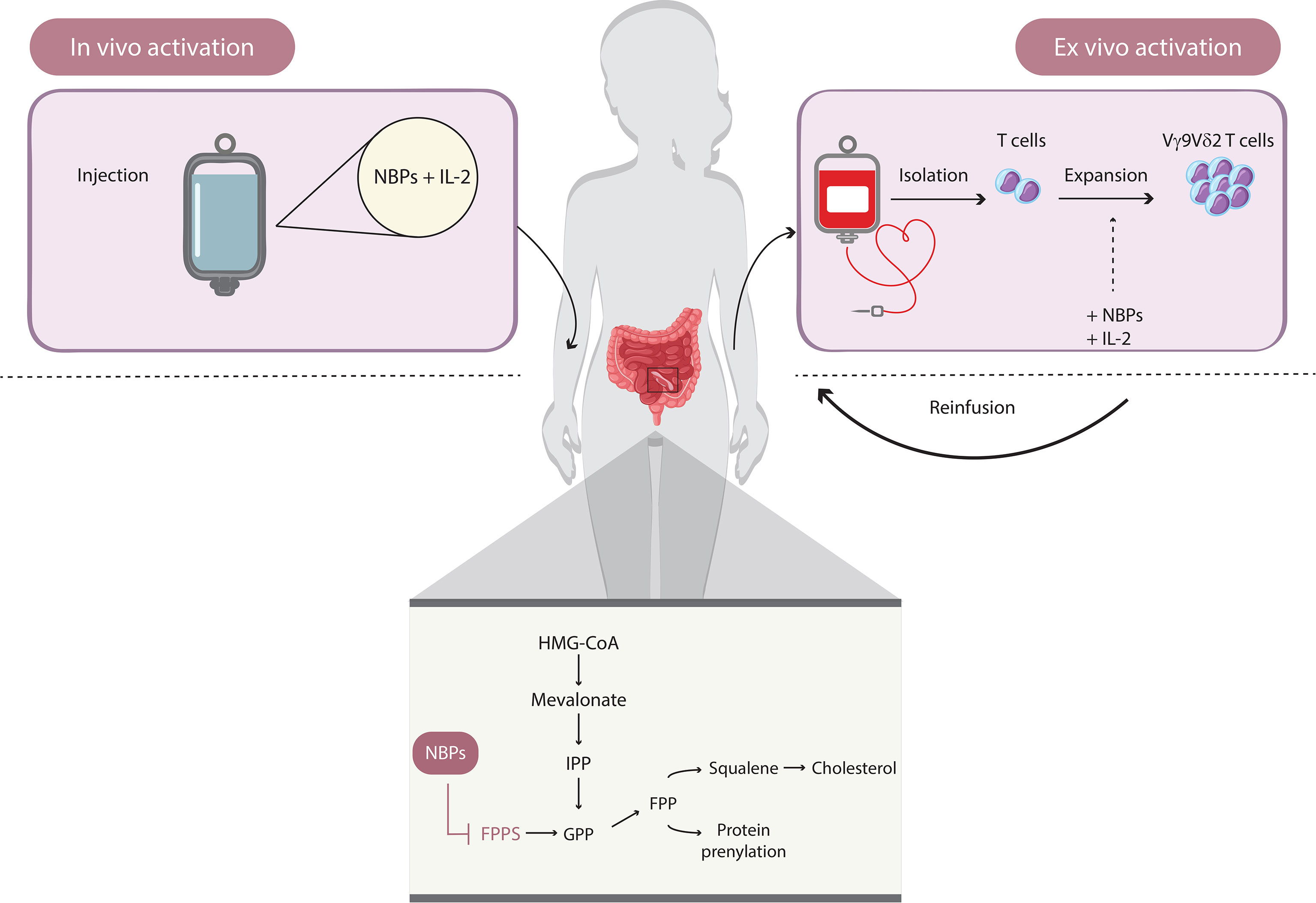
Figure 3 How to use γδ T cells in immunotherapy. Immunotherapeutic approaches based on Vγ9Vδ2 T cells involve two types of mechanisms: in vivo activation and adoptive transfer of ex vivo-expanded Vγ9Vδ2 T cells. The in vivo activation requires the administration of amino bisphosphonates (e.g., zoledronate), which induce the accumulation of endogenous phosphoantigens (P-Ags; such as IPP) by inhibiting the farnesyl pyrophosphate synthase of the mevalonate pathway and upregulating isoprenoid biosynthesis within cells. The ex vivo approach is based on the isolation of γδ T cells from peripheral blood mononuclear cells and the activation of Vγ9Vδ2 T cell, induced directly by natural (e.g., IPP and HMBPP) or synthetic (e.g., BrHPP) P-Ags plus IL-2 and the adoptive transfer.
Another alternative approach is the in vivo activation of Vγ9Vδ2 T cells by the administration of P-Ags or N-BPs in combination with IL-2, which is necessary for Vγ9Vδ2 expansion (72). This strategy increases with success the number of circulating Vγ9Vδ2, but it does not ensure their recruitment to the target tissue, where they can exert antitumor functions.
However, it has been shown both in humans and in nonhuman primate models that, upon repeated injections of P-Ags, Vγ9Vδ2 do not respond to P-Ags, probably because of activation-induced energy or exhaustion of their functions, promoting terminal differentiation (73).
In light of these limits, Vδ1+ T cells apply for a possible alternative to or support to Vγ9Vδ2-based immunotherapy. Indeed, two studies conducted on primary malignant melanoma and colorectal cancer pinpointed Vδ1+ T cells as the major γδ T-cell subset among TILs (37) (39),. Indeed, as well as showing a higher cytotoxic capacity compared to Vδ2, Vδ1+ T cells have the advantage of tissue residency, so the adoptive transfer of Vδ1 may be efficient in reaching the tumor site. Once activated by a broad variety of ligands, Vδ1+ T cells are also able to circulate and persist for a long time in the body, promoting long-term therapeutic effects. This is probably due to the fact that they are resistant to activation-induced cell death (74). Nevertheless, their clinical application is difficult due to the lack of a well-established expansion protocol. Recently, one of the protocols developed that has given promising results is established by Almeida et al., in which they expanded Vδ1+ from the peripheral blood of healthy donors and patients with chronic lymphocytic leukemia (CLL). These cells differentiate into a highly cytotoxic subset and inhibit tumor growth and metastasis in xenograft models of CLL in vivo (53). Based on this expansion procedure, GammaDelta Therapeutics has developed an allogeneic, nonengineered Vδ1 T-cell therapy (GDX012) for the treatment of patients with acute myeloid leukemia with minimal residual disease (NCT05001451) (75).
The newest γδ T-cell-based therapeutic strategies include the use of bispecific γδ T-cell engagers and γδ CAR-T cells (76). Vγ9-TCR-specific engagers binding to either CD123 (77) or HER2 (56) mediate cytotoxicity against tumor cells in acute myeloid leukemia and pancreatic adenocarcinomas, respectively. Moreover, they have the potential to enhance the efficacy of adoptively transferred γδ T cells (78). Lava Therapeutics is currently conducting two open-label phase I/IIa studies to assess the efficacy and safety of LAVA-051 (79), a humanized bispecific single-domain antibody that directly engages CD1d and the Vδ2 chain of Vγ9Vδ2 T cells in patients with relapsed/refractory chronic lymphocytic leukemia, multiple myeloma, and acute myeloid leukemia (NCT04887259) and LAVA-1207, which activates Vγ9Vδ2 T cells upon crosslinking to prostate-specific membrane antigen (PSMA) in patients with metastatic castration-resistant prostate cancer (NCT05369000).
In addition to Vγ9Vδ2 T cells, recently γδ CAR-T-cell-based therapy is also based on genetically modified Vδ1 T cells (80, 81). Adicet Bio is currently conducting a phase I clinical trial on anti-CD20 allogeneic γδ CAR-T cells for patients with B-cell malignancies (NCT04735471) (81). Compared to αβ CAR-T, the autologous or allogenic Vδ1/Vδ2 T-cell-expressing CAR ensures a reduced risk of a cytokine-related syndrome and a lower risk of off-target toxicity, as well as a long lifespan and preserved ability to act as antigen-presenting cells (82).
A growing body of evidence has allowed us to understand the role of γδ T cells in the tumor milieu and pave the way for their potential use in new anti-CRC immunotherapy approaches. CRC immunotherapy based on γδ T cells has only produced a small number of clinical results so far, although it has proven to be safe in terms of tolerability and side effects.
In a clinical trial with six CRC patients who had undergone lung metastasectomy, Izumi and colleagues used an injection of autologous Vγ9Vδ2 T cells, expanded with 5 μM of ZA and 1,000 IU/ml of IL-2, for 8 weeks. During treatment, the number of Vγ9Vδ2 T cells increased and remained stable long after the last infusions in terms of percentage and absolute number. Moreover, Vγ9Vδ2 T cells expressed more IFN-γ and CD107a ex vivo than other Vγ9− CD3+ T cells (83). In a phase I trial, Bennouna et al. tested in vivo the pharmacodynamic profile of γδ-agonist, BrHPP (IPH1101), in 28 patients with solid tumors, among which there were three CRC patients. They first evaluated the maximum-tolerated dose of BrHPP administered alone during the first cycle. Because the expansion and activation of Vγ9Vδ2 T lymphocytes required IL-2, they combined BrHPP with a low dose of IL-2. This approach proved to be safe, generally well-tolerated, and induced a strong γδ T lymphocyte expansion in these patients (84). Noguchi et al. performed a clinical trial enrolling 25 patients with advanced solid tumors, one of them with colon cancer. Once stimulated and expanded ex vivo with ZA and IL-2, γδ T cells were reinfused into patients. This approach did not cause severe toxicity, but rather restored the number of effector γδ T cells (85). Another phase I clinical study, including three patients with CRC, tested the adoptive transfer of ex vivo expanded autologous Vγ9Vδ2 T cells to evaluate whether the approach was doable and safe. No dose-dependent toxicity was found, but for some patients, the therapy was ineffective (86).
It is known that colon cancer is generated by a small population of cancer stem cells, which are relevant players in tumor persistence and drug resistance. Colon cancer stem cells are resistant to Vγ9Vδ2 T-cell cytotoxicity, but when sensitized with ZA (or even chemotherapeutic agents), they are efficiently killed by Vγ9Vδ2 T cells (60, 87). However, due to the biodistribution of ZA, including its bone tropism, its use in vivo can often be ineffective in properly reaching and stimulating γδ T lymphocytes in tissues. In this regard, recent work has shown that it is possible to encapsulate ZA in spherical polymeric nanoparticles (SPNs), which, thanks to the enhanced permeability and retention effect, can easily reach the tumor site. ZA-SPNs are engulfed by CRC cells, and Vδ2+ T cells interact with ZA-SPN-treated CRC cells, promoting their killing. In addition, Vδ2+ T cells also kill ZA-SPN-treated CRC tumor spheroids and autologous tumor organoids (88).
Ang et al. expanded Vγ9Vδ2 T cells with ZA/IL2 and constructed NKG2D ligand-specific chimeric antigen receptors (NKG2DL) using mRNA electroporation. Upon interaction with NKG2DL+ cancer cells, the CAR-γδ T cells enhanced their cytotoxic activity against a variety of cultured solid tumor cell lines, including those resistant to ZA treatment. They also evaluated the in vivo tumor-killing effect of the NKG2Dz RNA CAR-modified Vγ9Vδ2 T cells in a colorectal cancer mouse model using repeated intraperitoneal injections of aggressive human colorectal cancer cell lines. NKG2Dz RNA CAR-modified Vγ9Vδ2 T cells significantly slowed tumor progression, resulting in a median survival time of 57 days (89). Gadeta’s second cell therapy product, GDT201, which combines HLA-independent tumor recognition capabilities of γδ TCRs with proliferative capacity and robust tumor killing of αβ T cells, demonstrated long-lasting cytotoxicity effect on CRC cell lines, patient-derived tumor cells, and in a xenograft tumor model, suggesting the potential to address well-known clinical hurdles such as lack of tumor restricted antigens and safety (90).
As the functions of γδ T lymphocytes are often switched off by the microenvironment, a further useful approach would be to combine their expansion with the use of inhibitors of their negative regulators, thus restoring their ability to mediate protective immunity against cancer cells. Lu et al. have shown a high expression of B7-H3, an important member of the B7 superfamily, on CRC-infiltrating Vδ2+ T cells. B7-H3 appears to be an important negative regulator of their Vδ2+ T-cell antitumor functions, specifically via the downregulation of IFN-γ and perforin/granzyme B expression. Indeed, by inhibiting B7-H3 with a specific blocking antibody (MIH35) or B7-H3 siRNA, they witness the reactivation of effector functions, a decrease of apoptosis rates, and an upregulation of the activation markers CD25 and CD69. These treatments promoted the cytotoxic activity of Vδ2+ T cells on colon cancer cell lines. Hereupon, this suggests that it would be useful to combine in vivo activation of Vδ2+ T cells with the inhibition or blockade of B7-H3 as another immunotherapeutic approach for colon cancer (91).
Tim-3 is another inhibitory receptor that can influence the activity of γδ T lymphocytes. Its signaling significantly hindered the killing activity of Vγ9Vδ2 T cells against colon cancer cells and reduced the expression of perforin and granzyme B cells in an ERK1/2-dependent manner (92); because γδ T cells express high levels of Tim-3 in peripheral blood and colon cancer tissue, Tim-3 blockade by neutralizing antibody restores their cytotoxicity towards colon cancer cells. Consistent with this strategy, the inhibition of glycogen synthase kinase-3β with TWS119 inhibitor, boost proliferation, differentiation, and cytolytic activity of γδ T cells against colon cancer cells, activating mammalian target rapamycin (mTOR) pathway and upregulating the antiapoptotic protein Bcl-2 as well as the expression of perforin and granzyme B in vitro and in vivo (59).
Exploiting γδ T cells as a therapeutic tool can also be beneficial to overcome the failure of immunotherapies caused by the mutational state of the tumor. In this regard, de Bruin et al. generated and applied in vitro and in vivo a bispecific nanobody-based construct that, on the one hand, inhibited the signaling of EGFR and, on the other, induced target-dependent activation of Vγ9Vδ2 T cells. When co-cultured with two different EGFR+ human colon cancer cell lines, each with KRAS and BRAF mutations, and in the presence of the bispecific construct, Vγ9Vδ2 T cells produced IFN-γ and TNF-α and induced lysis of both cancer cell lines, irrespective of their mutational state. The same effects were found in vivo, combining the administration of Vγ9Vδ2 T cells with the bispecific construct (93).
Although most immunotherapy protocols are based on Vγ9Vδ2 T cells, promising results have been achieved using Vδ1+ T cells. After their isolation from PBMC and expansion with PHA and IL-7, Vδ1+ T cells were performed in vitro with strong cytotoxic and cytolytic effects against adherent and sphere-forming human colon cancer cells in a contact-dependent manner. Ex vivo expanded Vδ1+ T cells were able to block tumor growth in a CRC xenograft model (94). Furthermore, in another murine model of metastatic colon cancer obtained from orthotropic implantation of human HT29 cells, the administration of CMV-induced Vδ1+ T cells suppressed the progression of the primary tumor and the onset of metastasis (95).
CRC is a very complex and multifactorial tumor, characterized by a high mutation burden. Due to these features, standard treatment regimens have been proven to be ineffective in many patients. Here comes immunotherapy into play, especially targeting the checkpoint blockade pathway to enhance antitumor responses, although so far in CRC it does not seem to be always applicable compared to other solid tumors that respond well. Indeed, in MSI-H/dMMR CRC, this therapeutic approach is useful, whereas the majority of MSS/pMMR CRC patients do not benefit from immunotherapy (2). Hence, new immunotherapeutic approaches are needed, taking advantage of the interactions between tumors and the immune system. In recent years, the role of γδ T lymphocytes has emerged as an excellent therapeutic platform in hematological and solid tumors, including CRC. This is made possible by their hallmarks, which make them easily available, manipulable, and safe. Furthermore, to improve their immunogenicity and infiltration at the tumor sites in CRC, a combination of different strategies has been recently suggested, although their clinical relevance has yet to be evaluated. Little is known about the nature of the antigens and contacts that activate Vδ1 T cells, and a deeper understanding of their biology is required to maximize their therapeutic potential. Despite the beneficial aspects of γδ-based immunotherapy, clinical trials for CRC are still in their early stages. Therefore, future colon cancer immunotherapy could benefit from new engineering technologies, such as CAR-γδ T cells (96) and γδ T-cell-based bispecific constructs (97), to improve their antitumor efficacy.
AC, MDS, and SM designed the entire review. AC and MDS wrote the paper. ELP, FD, and SM reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
This study was financially supported by the Ministry of Education and Research (PRIN 2017-2017M8YMR8_001 to FD and PRIN 2017-20178ALPCM_004 to SM).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Lichtenstern CR, Ngu RK, Shalapour S, Karin M. Immunotherapy, inflammation and colorectal cancer. Cells. (2020) 9(3):618. doi: 10.3390/cells9030618
3. Yamagishi H, Kuroda H, Imai Y, Hiraishi H. Molecular pathogenesis of sporadic colorectal cancers. Chin J Cancer. (2016) 35:4–. doi: 10.1186/s40880-015-0066-y
4. Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal cancer statistics, 2017. CA: Cancer J Clin (2017) 67(3):177–93. doi: 10.3322/caac.21395
5. Ganesh K, Stadler ZK, Cercek A, Mendelsohn RB, Shia J, Segal NH, et al. Immunotherapy in colorectal cancer: Rationale, challenges and potential. Nat Rev Gastroenterol hepatology. (2019) 16(6):361–75. doi: 10.1038/s41575-019-0126-x
6. Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Sci (New York NY). (2006) 313(5795):1960–4. doi: 10.1126/science.1129139
7. Xie Y-H, Chen Y-X, Fang J-Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduction Targeted Ther (2020) 5(1):22. doi: 10.1038/s41392-020-0116-z
8. Wu T, Dai Y. Tumor microenvironment and therapeutic response. Cancer letters. (2017) 387:61–8. doi: 10.1016/j.canlet.2016.01.043
9. Pagès F, Mlecnik B, Marliot F, Bindea G, Ou FS, Bifulco C, et al. International validation of the consensus immunoscore for the classification of colon cancer: A prognostic and accuracy study. Lancet (London England). (2018) 391(10135):2128–39. doi: 10.1016/S0140-6736(18)30789-X
10. Lo Presti E, Pizzolato G, Corsale AM, Caccamo N, Sireci G, Dieli F, et al. γδ T cells and tumor microenvironment: From immunosurveillance to tumor evasion. Front Immunol (2018) 9:1395. doi: 10.3389/fimmu.2018.01395
11. Nielsen MM, Witherden DA, Havran WL. γδ T cells in homeostasis and host defence of epithelial barrier tissues. Nat Rev Immunol (2017) 17(12):733–45. doi: 10.1038/nri.2017.101
12. Mikulak J, Oriolo F, Bruni E, Roberto A, Colombo FS, Villa A, et al. NKp46-expressing human gut-resident intraepithelial Vδ1 T cell subpopulation exhibits high antitumor activity against colorectal cancer. JCI Insight (2019) 4(24):e125884. doi: 10.1172/jci.insight.125884
13. Buccheri S, Guggino G, Caccamo N, Li Donni P, Dieli F. Efficacy and safety of γδT cell-based tumor immunotherapy: A meta-analysis. J Biol regulators homeostatic agents. (2014) 28(1):81–90.
14. Vantourout P, Hayday A. Six-of-the-best: Unique contributions of γδ T cells to immunology. Nat Rev Immunol (2013) 13(2):88–100. doi: 10.1038/nri3384
15. Garcillán B, Marin AVM, Jiménez-Reinoso A, Briones AC, Muñoz-Ruiz M, García-León MJ, et al. Gd T lymphocytes in the diagnosis of human T cell receptor immunodeficiencies. Front Immunol (2015) 6:20. doi: 10.3389/fimmu.2015.00020
16. Fichtner AS, Ravens S, Prinz I. Human γδ TCR repertoires in health and disease. Cells. (2020) 9(4):800.
17. Yazdanifar M, Barbarito G, Bertaina A, Airoldi I. γδ T cells: The ideal tool for cancer immunotherapy. Cells. (2020) 9(5):1305. doi: 10.3390/cells9051305
18. Xu B, Pizarro JC, Holmes MA, McBeth C, Groh V, Spies T, et al. Crystal structure of a γδ T-cell receptor specific for the human MHC class I homolog MICA. PNAS (2011) 201015433:2414–9. doi: 10.1073/pnas.1015433108
19. Catellani S, Poggi A, Bruzzone A, Dadati P, Ravetti J, Gobbi M, et al. Expansion of Vδ1 T lymphocytes producing IL-4 in low-grade non-Hodgkin lymphomas expressing UL-16-binding proteins. Blood. (2007) 109:2078–85. doi: 10.1182/blood-2006-06-028985
20. Uldrich AP, Le Nours J, Pellicci DG, Gherardin NA, McPherson KG, Lim RT, et al. CD1d-lipid antigen recognition by the γδ TCR. Nat Immunol (2013) 14(11):1137–45. doi: 10.1038/ni.2713
21. Luoma AM, Castro CD, Mayassi T, Bembinster LA, Bai L, Picard D, et al. Crystal structure of Vδ1 T cell receptor in complex with CD1d-sulfatide shows MHC-like recognition of a self-lipid by human γδ T cells. Immunity. (2013) 39(6):1032–42. doi: 10.1016/j.immuni.2013.11.001
22. Khairallah C, Déchanet-Merville J, Capone M. γδ T cell-mediated immunity to cytomegalovirus infection. Front Immunol (2017) 8:105–. doi: 10.3389/fimmu.2017.00105
23. Sant S, Jenkins MR, Dash P, Watson KA, Wang Z, Pizzolla A, et al. Human γδ T-cell receptor repertoire is shaped by influenza viruses, age and tissue compartmentalisation. Clin Transl Immunol (2019) 8(9):e1079–e. doi: 10.1002/cti2.1079
24. Hayday AC. [gamma][delta] cells: A right time and a right place for a conserved third way of protection. Annu Rev Immunol (2000) 18:975–1026. doi: 10.1146/annurev.immunol.18.1.975
25. Gogoi D, Chiplunkar SV. Targeting gamma delta T cells for cancer immunotherapy: Bench to bedside. Indian J Med Res (2013) 138(5):755–61.
26. Gu S, Nawrocka W, Adams EJ. Sensing of pyrophosphate metabolites by Vγ9Vδ2 T cells. Front Immunol (2014) 5:688. doi: 10.3389/fimmu.2014.00688
27. De Rosa SC, Andrus JP, Perfetto SP, Mantovani JJ, Herzenberg LA, Herzenberg LA, et al. Ontogeny of γδ T cells in humans. J Immunol (2004) 172(3):1637–45. doi: 10.4049/jimmunol.172.3.1637
28. Rigau M, Ostrouska S, Fulford TS, Johnson DN, Woods K, Ruan Z, et al. Butyrophilin 2A1 is essential for phosphoantigen reactivity by γδ T cells. Sci (New York NY) (2020) 367(6478):eaay5516. doi: 10.1126/science.aay5516
29. Caccamo N, Battistini L, Bonneville M, Poccia F, Fournié JJ, Meraviglia S, et al. CXCR5 identifies a subset of Vgamma9Vdelta2 T cells which secrete IL-4 and IL-10 and help b cells for antibody production. J Immunol (2006) 177(8):5290–5. doi: 10.4049/jimmunol.177.8.5290
30. Caccamo N, Sireci G, Meraviglia S, Dieli F, Ivanyi J, Salerno A. γδ T cells condition dendritic cells in vivo for priming pulmonary CD8 T cell responses against mycobacterium tuberculosis. Eur J Immunol (2006) 36(10):2681–90. doi: 10.1002/eji.200636220
31. Davey MS, Willcox CR, Hunter S, Kasatskaya SA, Remmerswaal EBM, Salim M, et al. The human Vδ2(+) T-cell compartment comprises distinct innate-like Vγ9(+) and adaptive Vγ9(-) subsets. Nat Commun (2018) 9(1):1760. doi: 10.1038/s41467-018-04076-0
32. Mangan BA, Dunne MR, O’Reilly VP, Dunne PJ, Exley MA, O’Shea D, et al. Cutting edge: CD1d restriction and Th1/Th2/Th17 cytokine secretion by human Vδ3 T cells. J Immunol (2013) 191(1):30–4. doi: 10.4049/jimmunol.1300121
33. Petrasca A, Melo AM, Breen EP, Doherty DG. Human Vδ3(+) γδ T cells induce maturation and IgM secretion by b cells. Immunol letters. (2018) 196:126–34. doi: 10.1016/j.imlet.2018.02.002
34. Willcox CR, Pitard V, Netzer S, Couzi L, Salim M, Silberzahn T, et al. Cytomegalovirus and tumor stress surveillance by binding of a human γδ T cell antigen receptor to endothelial protein c receptor. Nat Immunol (2012) 13(9):872–9. doi: 10.1038/ni.2394
35. Silva-Santos B, Serre K, Norell H. γδ T cells in cancer. Nat Rev Immunol (2015) 15(11):683–91. doi: 10.1038/nri3904
36. Hidalgo JV, Bronsert P, Orlowska-Volk M, Díaz LB, Stickeler E, Werner M, et al. Histological analysis of γδ T lymphocytes infiltrating human triple-negative breast carcinomas. Front Immunol (2014) 5:[632 p.]. [Internet]. doi: 10.3389/fimmu.2014.00632
37. Cordova A, Toia F, La Mendola C, Orlando V, Meraviglia S, Rinaldi G, et al. Characterization of human γδ T lymphocytes infiltrating primary malignant melanomas. PloS One (2012) 7(11):e49878. doi: 10.1371/journal.pone.0049878
38. Kakimi K, Matsushita H, Murakawa T, Nakajima J. γδ T cell therapy for the treatment of non-small cell lung cancer. Transl Lung Cancer Res (2014) 3(1):23–33. doi: 10.3978/j.issn.2218-6751.2013.11.01
39. Meraviglia S, Lo Presti E, Tosolini M, La Mendola C, Orlando V, Todaro M, et al. Distinctive features of tumor-infiltrating γδ T lymphocytes in human colorectal cancer. Oncoimmunology. (2017) 6(10):e1347742. doi: 10.1080/2162402X.2017.1347742
40. Corvaisier M, Moreau-Aubry A, Diez E, Bennouna J, Mosnier JF, Scotet E, et al. V Gamma 9V delta 2 T cell response to colon carcinoma cells. J Immunol (2005) 175(8):5481–8. doi: 10.4049/jimmunol.175.8.5481
41. de Vries NL, van Unen V, Ijsselsteijn ME, Abdelaal T, van der Breggen R, Farina Sarasqueta A, et al. High-dimensional cytometric analysis of colorectal cancer reveals novel mediators of antitumour immunity. Gut. (2020) 69(4):691. doi: 10.1136/gutjnl-2019-318672
42. Lo Presti E, Corsale AM, Di Simone M, Dieli F, Meraviglia S. Characterisation of γδ T cells infiltrating colorectal cancer. Gut. (2021) 70(5):1001–3. doi: 10.1136/gutjnl-2020-322101
43. Zhao Y, Niu C, Cui J. Gamma-delta (γδ) T cells: Friend or foe in cancer development? J Trans Med (2018) 16(1):3. doi: 10.1186/s12967-017-1378-2
44. Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med (2015) 21(8):938–45. doi: 10.1038/nm.3909
45. Tosolini M, Pont F, Poupot M, Vergez F, Nicolau-Travers ML, Vermijlen D, et al. Assessment of tumor-infiltrating TCRVγ9Vδ2 γδ lymphocyte abundance by deconvolution of human cancers microarrays. Oncoimmunology. (2017) 6(3):e1284723. doi: 10.1080/2162402X.2017.1284723
46. Wu P, Wu D, Ni C, Ye J, Chen W, Hu G, et al. γδT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity. (2014) 40(5):785–800. doi: 10.1016/j.immuni.2014.03.013
47. Xiang Z, Tu W. Dual face of Vγ9Vδ2-T cells in tumor immunology: Anti- versus pro-tumoral activities. Front Immunol (2017) 8:1041. doi: 10.3389/fimmu.2017.01041
48. Patil RS, Shah SU, Shrikhande SV, Goel M, Dikshit RP, Chiplunkar SV. IL17 producing γδT cells induce angiogenesis and are associated with poor survival in gallbladder cancer patients. Int J Cancer. (2016) 139(4):869–81. doi: 10.1002/ijc.30134
49. Chen X, Shang W, Xu R, Wu M, Zhang X, Huang P, et al. Distribution and functions of γδ T cells infiltrated in the ovarian cancer microenvironment. J Trans Med (2019) 17(1):144. doi: 10.1186/s12967-019-1897-0
50. Rong L, Li K, Li R, Liu H-M, Sun R, Liu X-Y. Analysis of tumor-infiltrating gamma delta T cells in rectal cancer. World J Gastroenterol (2016) 22(13):3573–80. doi: 10.3748/wjg.v22.i13.3573
51. Hu G, Wu P, Cheng P, Zhang Z, Wang Z, Yu X, et al. Tumor-infiltrating CD39(+)γδTregs are novel immunosuppressive T cells in human colorectal cancer. Oncoimmunology. (2017) 6(2):e1277305. doi: 10.1080/2162402X.2016.1277305
52. Chabab G, Barjon C, Abdellaoui N, Salvador-Prince L, Dejou C, Michaud HA, et al. Identification of a regulatory Vδ1 gamma delta T cell subpopulation expressing CD73 in human breast cancer. J leukocyte Biol (2020) 107(6):1057–67. doi: 10.1002/JLB.3MA0420-278RR
53. Almeida AR, Correia DV, Fernandes-Platzgummer A, da Silva CL, da Silva MG, Anjos DR, et al. Delta one T cells for immunotherapy of chronic lymphocytic leukemia: Clinical-grade Expansion/Differentiation and preclinical proof of concept. Clin Cancer Res an Off J Am Assoc Cancer Res (2016) 22(23):5795–804. doi: 10.1158/1078-0432.CCR-16-0597
54. Tokuyama H, Hagi T, Mattarollo SR, Morley J, Wang Q, Fai-So H, et al. Vγ9Vδ2 T cell cytotoxicity against tumor cells is enhanced by monoclonal antibody drugs–rituximab and trastuzumab. Int J Cancer. (2008) 122(11):2526–34. doi: 10.1002/ijc.23365
55. Capietto A-H, Martinet L, Fournié J-J. Stimulated γδ T cells increase the In vivo efficacy of trastuzumab in HER-2+ breast cancer. J Immunol (2011) 187(2):1031–8. doi: 10.4049/jimmunol.1100681
56. Oberg HH, Peipp M, Kellner C, Sebens S, Krause S, Petrick D, et al. Novel bispecific antibodies increase γδ T-cell cytotoxicity against pancreatic cancer cells. Cancer Res (2014) 74(5):1349–60. doi: 10.1158/0008-5472.CAN-13-0675
57. Schiller CB, Braciak TA, Fenn NC, Seidel UJE, Roskopf CC, Wildenhain S, et al. CD19-specific triplebody SPM-1 engages NK and γδ T cells for rapid and efficient lysis of malignant b-lymphoid cells. Oncotarget. (2016) 7(50):83392–408. doi: 10.18632/oncotarget.13110
58. Varesano S, Zocchi MR, Poggi A. Zoledronate triggers Vδ2 T cells to destroy and kill spheroids of colon carcinoma: Quantitative image analysis of three-dimensional cultures. Front Immunol (2018) 9:998–. doi: 10.3389/fimmu.2018.00998
59. Chen YQ, Zheng L, Aldarouish M, Zhou ZH, Pan N, Liu JQ, et al. Wnt pathway activator TWS119 enhances the proliferation and cytolytic activity of human γδT cells against colon cancer. Exp Cell Res (2018) 362(1):63–71. doi: 10.1016/j.yexcr.2017.11.003
60. Todaro M, D'Asaro M, Caccamo N, Iovino F, Francipane MG, Meraviglia S, et al. Efficient killing of human colon cancer stem cells by gammadelta T lymphocytes. J Immunol (2009) 182(11):7287–96. doi: 10.4049/jimmunol.0804288
61. Dalton JE, Howell G, Pearson J, Scott P, Carding SR. Fas-fas ligand interactions are essential for the binding to and killing of activated macrophages by gamma delta T cells. J Immunol (2004) 173(6):3660–7. doi: 10.4049/jimmunol.173.6.3660
62. Todaro M, D'Asaro M, Caccamo N, Iovino F, Francipane MG, Meraviglia S, et al. Efficient killing of human colon cancer stem cells by γδ T lymphocytes. J Immunol (2009) 182(11):7287–96. doi: 10.4049/jimmunol.0804288
63. Ramstead AG, Jutila MA. Complex role of γδ T-cell-derived cytokines and growth factors in cancer. J Interferon Cytokine Res Off J Int Soc Interferon Cytokine Res (2012) 32(12):563–9. doi: 10.1089/jir.2012.0073
64. Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T cells. Sci (New York NY). (2005) 309(5732):264–8. doi: 10.1126/science.1110267
65. Landmeier S, Altvater B, Pscherer S, Juergens H, Varnholt L, Hansmeier A, et al. Activated human gammadelta T cells as stimulators of specific CD8+ T-cell responses to subdominant Epstein Barr virus epitopes: Potential for immunotherapy of cancer. J Immunother. (2009) 32(3):310–21. doi: 10.1097/CJI.0b013e31819b7c30
66. Maniar A, Zhang X, Lin W, Gastman BR, Pauza CD, Strome SE, et al. Human gammadelta T lymphocytes induce robust NK cell-mediated antitumor cytotoxicity through CD137 engagement. Blood. (2010) 116(10):1726–33. doi: 10.1182/blood-2009-07-234211
67. Liu Y, Zhang C. The role of human γδ T cells in anti-tumor immunity and their potential for cancer immunotherapy. Cells. (2020) 9(5):111719. doi: 10.3390/cells9051206
68. Lee D, Rosenthal CJ, Penn NE, Dunn ZS, Zhou Y, Yang L. Human γδ T cell subsets and their clinical applications for cancer immunotherapy. Cancers (Basel) [Internet]. (2022) 14(12):3005. doi: 10.3390/cancers14123005
69. Lo Presti E, Pizzolato G, Gulotta E, Cocorullo G, Gulotta G, Dieli F, et al. Current advances in γδ T cell-based tumor immunotherapy. Front Immunol (2017) 8:1401–. doi: 10.3389/fimmu.2017.01401
70. Rodriguez JB, Falcone BN, Szajnman SH. Approaches for designing new potent inhibitors of farnesyl pyrophosphate synthase. Expert Opin Drug Discovery. (2016) 11(3):307–20. doi: 10.1517/17460441.2016.1143814
71. Lin JF, Lin YC, Lin YH, Tsai TF, Chou KY, Chen HE, et al. Zoledronic acid induces autophagic cell death in human prostate cancer cells. J urology. (2011) 185(4):1490–6. doi: 10.1016/j.juro.2010.11.045
72. Dieli F, Vermijlen D, Fulfaro F, Caccamo N, Meraviglia S, Cicero G, et al. Targeting human {gamma}delta} T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res (2007) 67(15):7450–7. doi: 10.1158/0008-5472.CAN-07-0199
73. Fournié JJ, Sicard H, Poupot M, Bezombes C, Blanc A, Romagné F, et al. What lessons can be learned from γδ T cell-based cancer immunotherapy trials? Cell Mol Immunol (2013) 10(1):35–41. doi: 10.1038/cmi.2012.39
74. Siegers GM, Lamb LS Jr. Cytotoxic and regulatory properties of circulating Vδ1+ γδ T cells: a new player on the cell therapy field? Mol Ther J Am Soc Gene Ther (2014) 22(8):1416–22. doi: 10.1038/mt.2014.104
75. Knowles LJ, Malik M, Nussebaumer O, Brown A, van Wetering S, Koslowski M. Abstract CT525: GDX012U-001 a phase 1, open-label, dose escalation, and dose expansion study to assess the safety, tolerability, and preliminary antileukemic activity of GDX012 in patients with MRD positive AML. Cancer Res (2022) 82(12_Supplement):CT525–CT. doi: 10.1158/1538-7445.AM2022-CT525
76. Corsale AM, Di Simone M, Dieli F, Meraviglia S. Where could gammadelta T cells take us in the treatment of cancer? Expert Opin Biol Ther (2022) 23(1):1–5. doi: 10.1080/14712598.2022.2147424
77. Ganesan R, Chennupati V, Ramachandran B, Hansen MR, Singh S, Grewal IS. Selective recruitment of γδ T cells by a bispecific antibody for the treatment of acute myeloid leukemia. Leukemia. (2021) 35(8):2274–84. doi: 10.1038/s41375-021-01122-7
78. Yang R, He Q, Zhou H, Gong C, Wang X, Song X, et al. Vγ2 x PD-L1, a bispecific antibody targeting both the Vγ2 TCR and PD-L1, improves the anti-tumor response of Vγ2Vδ2 T cell. Front. Immunol. Vol. 13. (2022).
79. Broijl A, NWCJvd D, Bosch F, Mateos M-V, Rodríguez-Otero P, Tucci A, et al. Phase I dose escalation of LAVA-051, a novel bispecific gamma-delta T-cell engager (Gammabody), in relapsed/refractory hematological malignancies. Journal of Clinical Oncology Vol. 40. (2022). pp. 2577–.
80. Makkouk A, Yang XC, Barca T, Lucas A, Turkoz M, Wong JTS, et al. Off-the-shelf Vδ1 gamma delta T cells engineered with glypican-3 (GPC-3)-specific chimeric antigen receptor (CAR) and soluble IL-15 display robust antitumor efficacy against hepatocellular carcinoma. J Immunother Cancer (2021) 9(12):e003441. doi: 10.1186/s12967-019-1897-0
81. Neelapu SS, Stevens DA, Hamadani M, Frank MJ, Holmes H, Jacobovits A, et al. A phase 1 study of ADI-001: Anti-CD20 CAR-engineered allogeneic gamma Delta1 (γδ) T cells in adults with b-cell malignancies. Blood (2022) 140(Supplement 1):4617–9. doi: 10.1182/blood-2022-157400
82. Ganapathy T, Radhakrishnan R, Sakshi S, Martin S. CAR γδ T cells for cancer immunotherapy. is the field more yellow than green? Cancer Immunology Immunotherapy (2022). doi: 10.1007/s00262-022-03260-y
83. Izumi T, Kondo M, Takahashi T, Fujieda N, Kondo A, Tamura N, et al. Ex vivo characterization of γδ T-cell repertoire in patients after adoptive transfer of Vγ9Vδ2 T cells expressing the interleukin-2 receptor β-chain and the common γ-chain. Cytotherapy. (2013) 15(4):481–91. doi: 10.1016/j.jcyt.2012.12.004
84. Bennouna J, Levy V, Sicard H, Senellart H, Audrain M, Hiret S, et al. Phase I study of bromohydrin pyrophosphate (BrHPP, IPH 1101), a Vgamma9Vdelta2 T lymphocyte agonist in patients with solid tumors. Cancer immunology immunotherapy CII. (2010) 59(10):1521–30. doi: 10.1007/s00262-010-0879-0
85. Noguchi A, Kaneko T, Kamigaki T, Fujimoto K, Ozawa M, Saito M, et al. Zoledronate-activated Vγ9γδ T cell-based immunotherapy is feasible and restores the impairment of γδ T cells in patients with solid tumors. Cytotherapy. (2011) 13(1):92–7. doi: 10.3109/14653249.2010.515581
86. Nicol AJ, Tokuyama H, Mattarollo SR, Hagi T, Suzuki K, Yokokawa K, et al. Clinical evaluation of autologous gamma delta T cell-based immunotherapy for metastatic solid tumours. Br J Cancer. (2011) 105(6):778–86. doi: 10.1038/bjc.2011.293
87. Todaro M, Orlando V, Cicero G, Caccamo N, Meraviglia S, Stassi G, et al. Chemotherapy sensitizes colon cancer initiating cells to Vγ9Vδ2 T cell-mediated cytotoxicity. PloS One (2013) 8(6):e65145. doi: 10.3389/fimmu.2018.00800
88. Di Mascolo D, Varesano S, Benelli R, Mollica H, Salis A, Zocchi MR, et al. Nanoformulated zoledronic acid boosts the Vδ2 T cell immunotherapeutic potential in colorectal cancer. Cancers (Basel). (2019) 12(1):104. doi: 10.3390/cancers12010104
89. Ang WX, Ng YY, Xiao L, Chen C, Li Z, Chi Z, et al. Electroporation of NKG2D RNA CAR improves V9V2T cell responses against human solid tumor xenografts. Mol Ther - Oncolytics. (2020) 17:421–30. doi: 10.1016/j.omto.2020.04.013
90. Gadeta. . Available at: https://www.gadeta.nl/.
91. Lu H, Shi T, Wang M, Li X, Gu Y, Zhang X, et al. B7-H3 inhibits the IFN-γ-dependent cytotoxicity of Vγ9Vδ2 T cells against colon cancer cells. Oncoimmunology. (2020) 9(1):1748991. doi: 10.1080/2162402X.2020.1748991
92. Li X, Lu H, Gu Y, Zhang X, Zhang G, Shi T, et al. Tim-3 suppresses the killing effect of Vγ9Vδ2 T cells on colon cancer cells by reducing perforin and granzyme b expression. Exp Cell Res (2020) 386(1):111719. doi: 10.1016/j.yexcr.2019.111719
93. de Bruin RCG, Veluchamy JP, Lougheed SM, Schneiders FL, Lopez-Lastra S, Lameris R, et al. A bispecific nanobody approach to leverage the potent and widely applicable tumor cytolytic capacity of Vγ9Vδ2-T cells. Oncoimmunology. (2017) 7(1):e1375641–e. doi: 10.1080/2162402X.2017.1375641
94. Wu D, Wu P, Wu X, Ye J, Wang Z, Zhao S, et al. Ex vivo expanded human circulating Vδ1 γδT cells exhibit favorable therapeutic potential for colon cancer. Oncoimmunology. (2015) 4(3):e992749. doi: 10.4161/2162402X.2014.992749
95. Devaud C, Rousseau B, Netzer S, Pitard V, Paroissin C, Khairallah C, et al. Anti-metastatic potential of human Vδ1(+) γδ T cells in an orthotopic mouse xenograft model of colon carcinoma. Cancer immunology immunotherapy CII. (2013) 62(7):1199–210. doi: 10.1007/s00262-013-1402-1
96. Sebestyen Z, Prinz I, Déchanet-Merville J, Silva-Santos B, Kuball J. Translating gammadelta (γδ) T cells and their receptors into cancer cell therapies. Nat Rev Drug Discovery. (2020) 19(3):169–84. doi: 10.1038/s41573-019-0038-z
Keywords: gamma delta T cells, immunotherapy, colon rectal cancer, MHC- unrestricted activation, tumor
Citation: Corsale AM, Di Simone M, Lo Presti E, Dieli F and Meraviglia S (2023) γδ T cells and their clinical application in colon cancer. Front. Immunol. 14:1098847. doi: 10.3389/fimmu.2023.1098847
Received: 15 November 2022; Accepted: 11 January 2023;
Published: 30 January 2023.
Edited by:
Paolo Dellabona, San Raffaele Scientific Institute (IRCCS), ItalyReviewed by:
Lauren J. Howson, The University of Melbourne, AustraliaCopyright © 2023 Corsale, Di Simone, Lo Presti, Dieli and Meraviglia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Serena Meraviglia, c2VyZW5hLm1lcmF2aWdsaWFAdW5pcGEuaXQ=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.