- 1Department of Oncological Surgery, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 2College of Life Sciences, Key Laboratory for Cell and Gene Engineering of Zhejiang Province, Zhejiang University, Hangzhou, China
- 3Laboratory for Marine Biology and Biotechnology, Qingdao National Laboratory for Marine Science and Technology, Qingdao, China
Macrophages originating from the yolk sac or bone marrow play essential roles in tissue homeostasis and disease. Bone marrow-derived monocytes differentiate into Ly6Chi and Ly6Clo macrophages according to the differential expression of the surface marker protein Ly6C. Ly6Chi and Ly6Clo cells possess diverse functions and transcriptional profiles and can accelerate the disease process or support tissue repair and reconstruction. In this review, we discuss the basic biology of Ly6Chi and Ly6Clo macrophages, including their origin, differentiation, and phenotypic switching, and the diverse functions of Ly6Chi and Ly6Clo macrophages in homeostasis and disease, including in injury, chronic inflammation, wound repair, autoimmune disease, and cancer. Furthermore, we clarify the differences between Ly6Chi and Ly6Clo macrophages and their connections with traditional M1 and M2 macrophages. We also summarize the limitations and perspectives for Ly6Chi and Ly6Clo macrophages. Overall, continued efforts to understand these cells may provide therapeutic approaches for disease treatment.
Introduction
Macrophages contribute to homeostasis and disease through their extensive tissue distribution, functional diversity and plasticity. Tissue-resident macrophages (TRMs) arise from two sources: embryonic precursors and circulating monocytes (1–3). Embryonic macrophages contribute to self-maintenance, tissue remodeling and genotoxic stress resistance (4, 5), whereas bone marrow monocyte-derived macrophages act as short-lived effector cells contributing to various physiological activities, such as atherosclerosis and fibrosis (6). Conventionally, macrophages with different functions are described as M1 and M2 macrophage subsets (7). The M1 macrophages are known as classically activated macrophages, which contribute to primary host defense against pathogens (8). The M2 macrophages are known as alternatively activated macrophages, which heal tissue injury or damage caused by M1 macrophages and are involved in stimulating antibody production in adaptive humoral immunity (9). In addition, an increasing number of tumor-associated macrophages (TAMs) have been identified, which execute diverse functions such as suppression of antitumor immunity (10). The concept of M1 and M2 macrophage subsets is mainly derived from the in vitro polarization inducing assays, hence the M1 and M2 classifications are more suitable to describe the activation state of macrophages in vitro.
During the recent decade, the new classification of Ly6Chi and Ly6Clo macrophages has been widely applied to investigate monocyte-derived macrophages and to depict the precise state of macrophages in an intricate internal microenvironment (11). This classification system represents two different macrophage populations that are distinct in phenotype, function and even origin (12–14). Ly6C is a glycoprotein that is expressed on macrophage/dendritic cell precursors during mid-stage development. Differential Ly6C expression can identify functionally distinct macrophage populations in the steady state or disease (15, 16). In mice, the circulating monocytes derived from bone marrow are composed of at least Ly6Chi and Ly6Clo subsets. The CX3CR1midCCR2+Ly6Chi and CX3CR1hiCCR2-Ly6Clo phenotypes are functional equivalent with CD14hiCD16lo and CD14loCD16hi phenotypes in humans (17, 18). Recently, emerging studies have shown that the continuum of macrophage phenotypes, not the two circumscribed profiles originally proposed, plays important roles in various diseases, including kidney injury (19), liver fibrosis (13), rheumatoid arthritis (20), and breast cancer (21). Here, we review the new classification and perspectives in monocyte-derived macrophage research, including the origin, heterogeneity, conversion, and function of Ly6Chi and Ly6Clo macrophages.
Basic Biology of LY6CHI and LY6CLO Macrophages
Origin, Development, and Functional Heterogeneity
For half a century or more, the prevailing doctrine for tissue macrophages has been that these cells originate from circulating monocytes (22). Recently, it has become obvious that most tissue macrophages originate during embryonic development (23). TRM populations are mainly contributed by yolk sac (YS)-derived macrophages, erythro-myeloid progenitors (EMPs), and fetal hematopoietic stem cells (HSCs) (24, 25). In mice, yolk sac- or fetal liver-derived macrophages are located in different adult tissues, including the brain, epidermis and kidneys, and contribute to tissue homeostasis independent of bone marrow-derived monocytic precursors (5, 24, 25). Embryonic- derived and monocytes-derived subsets contribute to macrophage in adult tissues, including the gut and dermis (26–29). In addition, in the heart and pancreas, the macrophage population is a mixed population of yolk sac-derived macrophages, fetal liver-derived monocytes and bone marrow-derived monocytes (5).
Although tissues are populated with fetal macrophages, monocyte-derived macrophages might replace TRMs to a greater or lesser extent. Monocyte-derived macrophages are classified as CD11BhiF4/80hiLy6Chi macrophages (namely, Ly6Chi macrophages) and CD11Bhi F4/80hiLy6Clo macrophages (namely, Ly6Clo macrophages) based on the expression of Ly6C, a cell-surface glycoprotein (14, 19). Ly6Chi macrophages develop from recruited classical CCR2+CX3XR1loLy6Chi monocytes (analogous to human CD14+CD16- monocytes) during inflammation and are then converted into Ly6Clo macrophages. With the development of techniques such as single-cell sequencing and mass cytometry, new dimensions of the richness and heterogeneity of macrophages have been mapped. According to the latest single-cell analysis studies, four subpopulations of Ly6Chi inflammatory macrophages have been found to be present in kidney injury through relatively meticulous research (3). Here, we focus on the two most unlike subsets, Ly6Chi and Ly6Clo macrophages, to reveal the phenotypic and functional differences between them.
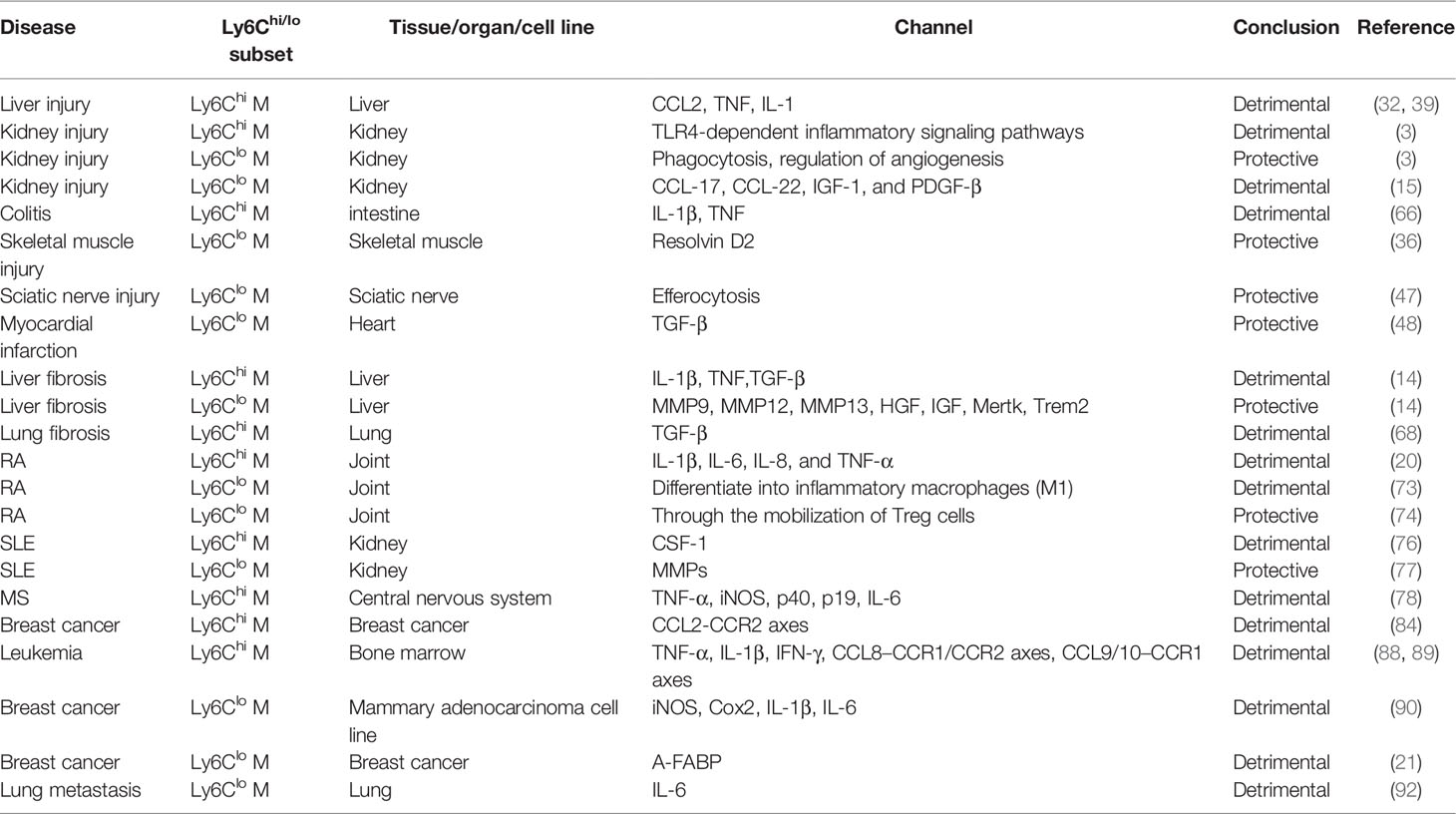
The Ly6Chi and Ly6Clo subsets exhibit functional heterogeneity, which is indicated by the high diversity in cell-surface marker, cytokine release and transcriptional profiles (14, 30–32). Indeed, Ly6Chi macrophages derived from circulating Ly6Chi monocytes are more enriched in the acute inflammatory response and show a proinflammatory ability (3). They exert proinflammatory and profibrotic functions mediated through various inflammatory and secreted factors, including tumor necrosis factor (TNF), interleukin (IL)-1β, and transforming growth factor (TGF)-β (33, 34). In contrast, Ly6Clo macrophages attract wide attention for their protective roles in wound healing, anti-inflammatory processes, and antifibrotic processes (14, 35, 36). Ly6Clo macrophages play diverse roles in maintaining the stability of the endothelium, regulating vasculogenesis, and transporting ions (3). They supposedly derived from Ly6Clo monocytes which are known to patrol endothelial cell of the blood vasculature (37). The detailed functions of the two subsets are discussed below (Table 1).
Conversion of Ly6Chi Macrophages Into Ly6Clo Macrophages
Conversion of Ly6Chi macrophages into Ly6Clo macrophages through phenotypic switching is an important source of tissue macrophages (13, 14, 33), but the precise regulatory mechanism underlying this process is still unclear. However, studies have revealed the signals driving Ly6Chi/Ly6Clo monocyte conversion and its molecular bases (55, 56). For example, it was proposed that the conversion of Ly6Chi monocytes into Ly6Clo monocytes might be a functional transition caused by loss of microenvironmental signals that sustain the expression of genes specific to Ly6Chi cells rather than a true developmental, terminal differentiation program (57, 58). The transcription factors Nr4a1 and Cebpβ were reported to be master regulators that promote the conversion of Ly6Chi monocytes into Ly6Clo monocytes (56, 59, 60). Both Nr4a1−/− mice and Cebpβ−/− mice lack Ly6Clo monocytes (59, 61). In early studies, macrophage colony-stimulating factor 1 (CSF-1) was shown to promote the maturation of macrophages from bone marrow-derived macrophage precursors accompanied by a rapid decrease in Ly6C expression, which indicated the significance of CSF-1 in phenotypic switching (62, 63). Apart from this, IL-4 and IL-10 were found to have the ability to promote liver-derived Ly6Chi macrophage conversion into Ly6Clo macrophages, and a synergistic effect was observed between these two cytokines (13). Resolving D2, a specialized proresolving lipid mediator, significantly improves muscle regeneration by promoting Ly6Chi/Ly6Clo macrophage conversion (36). Phagocytosis by Ly6Chi macrophages fosters Ly6Chi/Ly6Clo macrophage conversion in the fibrotic liver through liposomal stimulation (14). In addition, the CX3CR1-CX3CL1 signaling axis indirectly regulates the phenotypic switch between Ly6Chi/lo macrophages (38, 40, 43, 64). Since Ly6Clo macrophages are beneficial for hepatic fibrosis resolution, the number of Ly6Chi macrophages was significantly increased in CX3CR1-/- mice, followed by chronic inflammation and increased hepatic fibrosis (40). Moreover, neutrophils are involved in the Ly6Chi/Ly6Clo macrophage switch by expressing reactive oxygen species (ROS) to orchestrate liver repair (41) (Figure 1). Although the master regulators involved in Ly6Chi/Ly6Clo macrophage conversion are not fully understood, many investigations are contributing to the answer.

Figure 1 The distinct functions of Ly6Chi and Ly6Clo macrophages in injury, chronic inflammation and wound repair. During the initial stage of inflammation, Ly6Chi monocytes are recruited to the injury site via cytokines, including CC-chemokine ligand 1 (CCL1), CC-chemokine ligand 2 (CCL2), and fractalkine (CX3CL1), wherein they develop into Ly6Chi macrophages. These cells express proinflammatory mediators, such as nitric oxide (NO), tumor necrosis factor (TNF) and interleukin-1β (IL-1β), which exacerbate tissue injury. These Ly6Chi cells interact with kidney-resident macrophages through the S100a8/a9-Tlr4 axis, initiating and amplifying the inflammatory response. During fibrosis, Ly6Chi cells promote this process through the effects of transforming growth factor-β (TGFβ) on quiescent hepatic stellate cell activation, platelet-derived growth factor (PDGF) on myofibroblast proliferation and TNF and IL-1β on activated hepatic stellate cells. Ly6Clo macrophages accumulate via the recruitment of Ly6Clo monocytes or phenotype switching from Ly6Chi macrophages. Proresolution macrophages inhibit the inflammatory response and T-cell function through anti-inflammatory factors and CD52-HMGB1 binding. They also engulf apoptotic T cells, which play anti-inflammatory roles. During the phagocytic process, Ly6Clo cells produce matrix metalloproteinases (MMPs), such as MMP9, MMP12, and MMP13, accelerating extracellular matrix (ECM) degradation and inhibiting fibrosis. During tissue repair and reconstruction, Ly6Clo cells secrete hepatocyte growth factor (HGF) and insulin-like growth factor (IGF) to promote wound healing and tissue regeneration. Regarding the mechanism underlying phenotypic switching, various factors and processes, including cytokines (CSF-1, IL-4, and IL-10), the CX3CR1-CX3CL1 axis, neutrophil-released ROS, phagocytosis, and Resolving D2, have been explored.
Ly6CHI and Ly6CLO Macrophages in Homeostasis and Pathology
Ly6Chi and Ly6Clo Macrophages in Homeostasis
In the steady state, tissue-resident macrophages, such as microglia, Langerhans cells, and Kupffer cells, exhibit a F4/80hiLy6Clo phenotype. These tissue-resident macrophages play fundamental homeostatic roles in the clearance of apoptotic cells and participate in tissue immune surveillance (42). They maintain themselves locally and independently of circulating precursors. For instance, the most important cardiac macrophages in the steady state are F4/80hiLy6CloMHCIIhi and F4/80hiLy6CloMHCIIlo subsets. These subsets exist independently of bone marrow-derived monocytes and are renewed through in situ proliferation. They perform more antigen sampling and efferocytosis than infiltrating Ly6Chi macrophages (27). In contrast, Ly6Chi macrophages are rarely involved in tissue homeostasis. Classical Ly6Chi monocytes do not enter tissues on a large scale, and they intend to switch to a Ly6Clo phenotype with time of residency. Ly6Chi monocytes remain in an undifferentiated state instead of becoming committed macrophages or DCs, which is different from the differentiation of Ly6Chi monocytes into macrophages or DCs during inflammation (23). However, when homeostasis is disrupted, bone marrow-derived Ly6Chi monocytes are recruited to the site of inflammation.
Ly6Chi/Ly6Clo Macrophages in Injury, Chronic Inflammation and Wound Repair
The proinflammatory and profibrotic roles of Ly6Chi macrophages has been reported in various diseases, among which liver injury and fibrosis are typical models used to investigate the function of the Ly6Chi subset. During acute liver injury and chronic liver diseases such as liver fibrosis, CCR2+Ly6Chi monocytes are recruited to the liver in a manner dependent on the CCL2/CCR2 or CCL1/CCR8 chemokine-receptor interaction (39, 61). In the liver, these cells develop into infiltrating Ly6Chi macrophages and exhibit a proinflammatory phenotype. Ly6Chi macrophages express inflammatory genes, including inducible nitric oxide synthase (iNOS) and TNF, which aggravate the inflammatory response (65). In other inflammatory diseases, such as acute lung injury (19) colitis (66) and skin wound healing (28, 44, 67), the Ly6Chi subset is the source of IL-1β and TNF, and Ly6Chi macrophage-targeted therapies are useful for decreasing inflammation. Functionally, Ly6Chi macrophages intensify the scarring occurring during liver fibrosis by promoting hepatic stellate cell (HSC) survival via IL-1β and TNF-induced NF-κB activation and TGF-β/PDGF-mediated HSC transdifferentiation and proliferation (45, 46). Inhibiting infiltrating Ly6Chi monocytes in CCR2-/- mice was shown to relieve liver fibrosis (39). Similarly, the profibrotic function of Ly6Chi macrophages in lung and kidney fibrosis has been revealed (19, 43, 68). Mechanistically, Ly6Chi macrophages with high S100a8 and S100a9 expression were found to have a strong interaction with kidney-resident macrophages through the S100a8/a9-Tlr4 axis, thereby initiating and amplifying the inflammatory response during kidney injury (3) (Figure 1).
In contrast to Ly6Chi macrophages, Ly6Clo macrophages play important roles in inhibiting inflammation, promoting wound healing, improving regeneration and decreasing fiber deposition during tissue injury and fibrosis (Figure 1). Taking liver fibrosis as an example, the restorative Ly6Clo subset upregulates phagocytosis-related genes (Fcrls, Cd5l, Mertk, Trem2, and Axl), matrix degradation-related genes (Mmp9, Mmp12, and Mmp13), and growth factors (Hgf, Igf1 and Mif), which facilitate fiber degradation, fibrosis resolution, and tissue protection (14). In sciatic nerve injury, inflammation-resolving Ly6Clo macrophages derived from Ly6Chi cells promote an anti-inflammatory milieu by efferocytosis (47). In skeletal muscle injury, the proresolving lipid mediator resolvin D2 increases Ly6Clo macrophages and improves muscle regeneration (36). Ly6Clo macrophages express genes closely related to the mitotic cell cycle and cell division and are involved in various biological processes, including defense reactions and responses to cytokine stimuli and viruses, after resolvin D2 treatment (36). In myocardial infarction, Ly6Clo macrophages play crucial roles in postinfarct healing and optimal scar formation by secreting immunoregulatory factors, such as TGF-β (48). However, a destructive role for Ly6Clo macrophages has also been reported. For example, bone marrow-derived Ly6Clo macrophages worsen renal fibrosis by secreting various cytokines that promote the transdifferentiation of fibroblasts into myofibroblasts (69). This viewpoint is quite different from those in previous reports, and the debate on this needs to be resolved.
Ly6Chi and Ly6Clo Macrophages in Autoimmune Disease
The detrimental functions of Ly6Chi macrophages in autoimmune disease has been revealed in various reports. However, Ly6Clo macrophages show harmful or beneficial functions in diverse pathological conditions and different autoimmune disease types (Table 1). Rheumatoid arthritis (RA) is a complex autoimmune disease influenced by both genetic and environmental factors (70). Macrophages and monocytes have been reported to play important roles in the pathophysiology of RA (71). Ly6Chi macrophages have been reported to aggravate the progression of RA. Decreases in Ly6Chi macrophage numbers and chemokines are favorable markers for clinical improvement with treatment (49). Infliximab was used to improve RA in human TNF transgenic (hTNF-Tg) mice, functioning mainly by inducing apoptosis in Ly6Chi macrophages and inhibiting the recruitment of Ly6Chi monocytes (49). Ly6Clo macrophages are believed to have diverse functions in RA. Serum transfer-induced arthritis (STIA) mice are good model for RA studies (72, 73). Researchers revealed that Ly6Clo monocytes were recruited to arthritic joints and developed into Ly6CloMHC-II+ and Ly6CloMHCII-macrophages; among these cells, Ly6CloMHCII-macrophages drove the development of joint pathology (73). However, in contrast, Ly6Clo monocytes developed into Ly6Clo macrophages, which resembled anti-inflammatory M2 macrophages and contributed to reducing joint inflammation through the mobilization of regulatory T (Treg) cells (74).
Systemic lupus erythematosus (SLE) is a heterogeneous systemic rheumatic disease with profound effects on multiple organs (75). In a mouse model of lupus nephritis (MRL-Faslpr mice), CSF-1 shifted circulating Ly6Chi monocytes toward inflammatory Ly6Chi macrophages that induce apoptosis in tubular epithelial cells, damaging the kidneys (76). A similar study showed that the Ly6Chi subset increased notably and secreted proinflammatory cytokines and chemokines during SLE (77). However, Ly6Clo macrophages possess distinct functions in SLE. At nephritis onset, Ly6Clo macrophages upregulate the cell-surface marker CD11b, acquire cathepsin and matrix metalloproteinase activity, and protect cells from death. However, these changes reverse after the induction of remission (77). Multiple sclerosis (MS) is a chronic autoimmune disease mediated by a complex interaction between autoreactive lymphocytes and myeloid cells in the central nervous system (CNS) and is the most common inflammatory neurological disease in young adults (78). Experimental autoimmune encephalomyelitis (EAE), characterized by immune cell infiltration of the CNS, is an ideal model for investigating MS (79). Various studies in EVE models have revealed that CCR2+Ly6Chi monocytes are required for the initiation and progression of EVE (50, 51, 79). Ly6Chi macrophages derived from Ly6Chi monocytes are essential for the maintenance of chronic inflammation and the progression of EVE. Acetylcholine-producing natural killer (NK) cells were shown to be cytotoxic to Ly6Chi cells in EVE, acting by inhibiting the production of proinflammatory cytokines and thereby attenuating CNS inflammation (80). Autoimmune (noninfectious) uveitis is a group of intraocular inflammatory diseases that target the neuroretina, and this disease can affect the CNS (81). In mice, experimental autoimmune uveitis (EAU) is a model of organ autoimmunity in the eye. By using this model, HSC-derived Ly6Chi and Ly6Clo macrophages with relatively high MHC-II expression were found to be associated with EAU through their antigen-presenting and CD4+ T cell-activating activities (52).
Ly6Chi and Ly6Clo Macrophages in Cancer
Ly6Chi Macrophages in Cancer
Ly6Chi macrophages extensively enhance tumor initiation and malignant progression. They build an inflammatory microenvironment to promote tumor growth, invasion and metastasis. The roles of CCL2/CCR2 signaling and Ly6Chi monocyte recruitment have been implicated as poor prognostic factors in multiple malignancies (53, 54, 82, 83). CCL2/CCR2 signaling was reported to foster metastasis and prolong the survival of tumor-bearing mice, and CCL2 expression and macrophage infiltration are correlated with a poor prognosis and metastatic disease in human breast cancer (84). An anti-CCL2 antibody was found to inhibit the infiltration of Ly6Chi monocytes and tumor metastasis (84). However, CCR2-independent pathway also influenced recruitment under noninflammatory conditions (85). CSF-1 signaling has been reported to determine monocyte recruitment and differentiation in the tumor microenvironment. CSF1R signaling blockade impairs the extravasation of tumor-infiltrating Ly6Chi monocytes (86). In addition, Ly6Chi macrophages are closely connected with immune resistance to ablative radiotherapy in pancreatic ductal adenocarcinoma, as depletion of this subset delays tumor growth after radiotherapy (87). In leukemic mice, an increase in monocyte-derived Ly6Chi leukemia-associated macrophages (LAMs) was detected in extramedullary tissue. Ly6Chi LAMs differ from TAMs in their gene expression profile and activation phenotype. They actively express TNF-α and IL-1β, which contribute to sterile inflammation. Ly6Chi LAMs have high migratory and phagocytotic potentials and promote the extramedullary distribution of leukemia cells (88, 89).
Ly6Clo Macrophages in Cancer
The Ly6Clo macrophages also demonstrate detrimental roles during tumor progression. They promote angiogenesis, exert immunosuppressive effect, and are associated with poor prognosis. The monocyte pool in tumors almost exclusively consists of Ly6ChiCX3CR1lo monocytes, which renew TAM subsets (90). These inflammatory monocytes undergo rapid differentiation into TAMs and, in doing so, lose Ly6C expression (87). TAMs are distinguished as normoxic M1-like Ly6CloMHC-IIhi TAMs and hypoxic M2-like Ly6CloMHC-IIlo TAMs (90, 91). Among TAMs, the Ly6CloMHC-IIlo subset was found to be the main population involved in tumor growth, invasion and metastasis in the mammary adenocarcinoma TS/A model. Although the Ly6CloMHC-IIlo subset exhibits weak antigen presentation, they promote angiogenesis and suppress T-cell proliferation (90). The differentiation of Ly6Chi monocytes into the Ly6CloMHC-IIlo subset is facilitated by CSF1R signaling (86). In breast cancer, the expression of adipocyte/macrophage fatty acid binding protein (A-FABP) in TAMs, especially the Ly6CloMHC-IIlo subset, was shown to facilitate tumor progression. A-FABP expression in the Ly6CloMHC-IIlo subset promoted protumor IL-6/STAT3 signaling through regulation of the NF-κB/miR-29b pathway (21). During lung metastasis, tumor cell-released microparticles (T-MPs) foster the recruitment of inflammatory monocytes, and these cells mature into Ly6Clo macrophages. Ly6Clo cells not only produce IL-6 but also trigger fibrin deposition, facilitating the growth and survival of tumor-repopulating cells, thus setting the stage for lung metastasis (92). As described above, Ly6Clo TAMs are associated with a poor prognosis and tumor progression in multiple cancers. However, a proinflammatory Ly6CloMHC-II+ macrophage subset was confirmed to promote responsiveness to PD-L1 blockade instead of resistance; thus, this subset may have a host-protective role in immune checkpoint blockade therapies (93).
Correlation Between Ly6CHI/LO Macrophages and the M1/M2 Paradigm
Macrophages with distinct functions are traditionally classified as M1 macrophages (classically activated macrophages) and M2 macrophages (alternatively activated macrophages). Cells with the M1 phenotype participate in host defense against pathogens and antitumor immunity. However, those with the M2 phenotype possess anti-inflammatory function and facilitate wound healing and tumor progression (11). Strictly, M1 and M2 macrophages represent only the states polarized by IFN-γ/LPS and IL-4/IL-13 in vitro, respectively (94). This taxonomic lineage clearly defines the two extreme types of the macrophage spectrum, which is especially beneficial for in vitro research. However, in a complex microenvironment in vivo, such as that in CCL4-induced liver fibrosis (14) or chronic alcoholic liver injury (95), macrophages can exist along a continuous spectrum, and the simple M1/M2 paradigm cannot describe the state of macrophages. Therefore, researchers have begun to focus on the Ly6Chi/lo phenotype outside the M1/M2 classification and depict various roles in homeostasis and pathology according to this classification system. Ly6Chi/lo and M1/M2 macrophages have overlap in the gene expression profile. Ly6Chi macrophages express some signature M1 markers, including TNF, iNOS and IFN-γ, and M2 markers, including Chi3l3, TGF-β and IL-10. Ly6Clo macrophages upregulate traditional M1 genes, such as CD16 and CD32, and express some M2-specific markers, including CD206 and CD301 (14, 96). Therefore, when the activation state of macrophages in vivo is described, these cells can be defined more accurately through the combination of macrophage origin, surface markers and factors inducing the macrophage activation state.
Current Research Gaps and Future Perspectives
In summary, macrophages are a key innate immune cell subset that plays various roles in multiple biological processes. The conventional M1/M2 paradigm is widely applied to describe the state of macrophages. Owing to the limitations of the M1/M2 paradigm, the Ly6Chi/lo classification is increasingly used to describe cells involved in various diseases because this precise depiction is based on cell origin, stimuli, and identification markers (94). Here, we summarize the indispensable functions of Ly6Chi and Ly6Clo macrophages in homeostasis and pathology. Recent study highlighted the importance of tissue niches (blood vessels and nerves) to the two subsets (97). After blood monocytes recruitment and differentiation, the two distinct subsets preferentially reside within different, but conserved, subtissular niches located adjacent to either nerve fibers (Ly6CloMHCIIhi) or blood vessels (Ly6ChiMHCIIlo), which demonstrate conserved niche-dependent functional programming (97). In fact, whether these Ly6Chi and Ly6Clo macrophage subsets interact with their respective surroundings in function and metabolism needs to be further explored. New genetic tools promote the disclosure of macrophage heterogeneity. Recently, Kim et al. established a binary transgenic split Cre system that allows differential targeting and translatome analysis of CNS border-associated macrophages (98). Genetics-based RiboTag translatome profiling can be a valuable and complementary addition to single cell transcriptomics and can be widely applied in the future.
Author Contributions
Y-hL and J-zS searched the literature and wrote the manuscript. D-cL prepared the figures. L-xX, YZ, and GP carefully checked the manuscript and helped to improve paragraphs. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from Stem Cell and Translational Research, the National Key Research and Development Program of China (2016YFA0101001, 2018YFD0900503, 2018YFD0900505), the National Natural Science Foundation of China (32173003, 31630083).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, De Prijck S, et al. Alveolar Macrophages Develop From Fetal Monocytes That Differentiate Into Long-Lived Cells in the First Week of Life via GM-CSF. J Exp Med (2013) 210:1977–92. doi: 10.1084/jem.20131199
2. Garbi N, Lambrecht BN. Location, Function, and Ontogeny of Pulmonary Macrophages During the Steady State. Pflugers Arch (2017) 469:561–72. doi: 10.1007/s00424-017-1965-3
3. Yao W, Chen Y, Li Z, Ji J, You A, Jin S, et al. Single Cell RNA Sequencing Identifies a Unique Inflammatory Macrophage Subset as a Druggable Target for Alleviating Acute Kidney Injury. Adv Sci (Weinh) (2022) 9(12):e2103675. doi: 10.1002/advs.202103675
4. Price JG, Idoyaga J, Salmon H, Hogstad B, Bigarella CL, Ghaffari S, et al. CDKN1A Regulates Langerhans Cell Survival and Promotes Treg Cell Generation Upon Exposure to Ionizing Irradiation. Nat Immunol (2015) 16:1060–8. doi: 10.1038/ni.3270
5. Ginhoux F, Guilliams M. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity (2016) 44:439–49. doi: 10.1016/j.immuni.2016.02.024
6. Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, et al. Macrophage Plasticity, Polarization, and Function in Health and Disease. J Cell Physiol (2018) 233:6425–40. doi: 10.1002/jcp.26429
7. Sica A, Mantovani A. Macrophage Plasticity and Polarization: In Vivo Veritas. J Clin Invest (2012) 122:787–95. doi: 10.1172/JCI59643
8. Gordon S, Martinez FO. Alternative Activation of Macrophages: Mechanism and Functions. Immunity (2010) 32:593–604. doi: 10.1016/j.immuni.2010.05.007
9. Biswas SK, Mantovani A. Macrophage Plasticity and Interaction With Lymphocyte Subsets: Cancer as a Paradigm. Nat Immunol (2010) 11:889–96. doi: 10.1038/ni.1937
10. Anderson NR, Minutolo NG, Gill S, Klichinsky M. Macrophage-Based Approaches for Cancer Immunotherapy. Cancer Res (2021) 81:1201–8. doi: 10.1158/0008-5472.CAN-20-2990
11. Murray PJ, Wynn TA. Protective and Pathogenic Functions of Macrophage Subsets. Nat Rev Immunol (2011) 11:723–37. doi: 10.1038/nri3073
12. Liu L, Wang P, Wang YS, Zhang YN, Li C, Yang ZY, et al. MiR-130a-3p Alleviates Liver Fibrosis by Suppressing HSCs Activation and Skewing Macrophage to Ly6C(lo) Phenotype. Front Immunol (2021) 12:696069. doi: 10.3389/fimmu.2021.696069
13. Li YH, Shen S, Shao T, Jin MT, Fan DD, Lin AF, et al. Mesenchymal Stem Cells Attenuate Liver Fibrosis by Targeting Ly6C(hi/lo) Macrophages Through Activating the Cytokine-Paracrine and Apoptotic Pathways. Cell Death Discovery (2021) 7:239. doi: 10.1038/s41420-021-00584-z
14. Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, Ali A, et al. Differential Ly-6C Expression Identifies the Recruited Macrophage Phenotype, Which Orchestrates the Regression of Murine Liver Fibrosis. Proc Natl Acad Sci U.S.A. (2012) 109:E3186–3195. doi: 10.1073/pnas.1119964109
15. Lin SL, Castano AP, Nowlin BT, Lupher ML Jr., Duffield JS. Bone Marrow Ly6Chigh Monocytes are Selectively Recruited to Injured Kidney and Differentiate Into Functionally Distinct Populations. J Immunol (2009) 183:6733–43. doi: 10.4049/jimmunol.0901473
16. Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, et al. The Healing Myocardium Sequentially Mobilizes Two Monocyte Subsets With Divergent and Complementary Functions. J Exp Med (2007) 204:3037–47. doi: 10.1084/jem.20070885
17. Ingersoll MA, Spanbroek R, Lottaz C, Gautier EL, Frankenberger M, Hoffman R, et al. Comparison of Gene Expression Profiles Between Human and Mouse Monocyte Subsets. Blood (2010) 115:e10–19. doi: 10.1182/blood-2009-07-235028
18. Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, et al. Human CD14dim Monocytes Patrol and Sense Nucleic Acids and Viruses via TLR7 and TLR8 Receptors. Immunity (2010) 33:375–86. doi: 10.1016/j.immuni.2010.08.012
19. Wen Y, Yan HR, Wang B, Liu BC. Macrophage Heterogeneity in Kidney Injury and Fibrosis. Front Immunol (2021) 12:681748. doi: 10.3389/fimmu.2021.681748
20. Liu W, Zhang Y, Zhu W, Ma C, Ruan J, Long H, et al. Sinomenine Inhibits the Progression of Rheumatoid Arthritis by Regulating the Secretion of Inflammatory Cytokines and Monocyte/Macrophage Subsets. Front Immunol (2018) 9:2228. doi: 10.3389/fimmu.2018.02228
21. Hao J, Yan F, Zhang Y, Triplett A, Zhang Y, Schultz DA, et al. Expression of Adipocyte/Macrophage Fatty Acid-Binding Protein in Tumor-Associated Macrophages Promotes Breast Cancer Progression. Cancer Res (2018) 78:2343–55. doi: 10.1158/0008-5472.CAN-17-2465
22. Locati M, Curtale G, Mantovani A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu Rev Pathol (2020) 15:123–47. doi: 10.1146/annurev-pathmechdis-012418-012718
23. Epelman S, Lavine KJ, Randolph GJ. Origin and Functions of Tissue Macrophages. Immunity (2014) 41:21–35. doi: 10.1016/j.immuni.2014.06.013
24. Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, et al. Tissue-Resident Macrophages Originate From Yolk-Sac-Derived Erythro-Myeloid Progenitors. Nature (2015) 518:547–51. doi: 10.1038/nature13989
25. Hoeffel G, Chen J, Lavin Y, Low D, Almeida FF, See P, et al. C-Myb(+) Erythro-Myeloid Progenitor-Derived Fetal Monocytes Give Rise to Adult Tissue-Resident Macrophages. Immunity (2015) 42:665–78. doi: 10.1016/j.immuni.2015.03.011
26. Tamoutounour S, Guilliams M, Montanana Sanchis F, Liu H, Terhorst D, Malosse C, et al. Origins and Functional Specialization of Macrophages and of Conventional and Monocyte-Derived Dendritic Cells in Mouse Skin. Immunity (2013) 39:925–38. doi: 10.1016/j.immuni.2013.10.004
27. Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, et al. Embryonic and Adult-Derived Resident Cardiac Macrophages are Maintained Through Distinct Mechanisms at Steady State and During Inflammation. Immunity (2014) 40:91–104. doi: 10.1016/j.immuni.2013.11.019
28. Hoeffel G, Debroas G, Roger A, Rossignol R, Gouilly J, Laprie C, et al. Sensory Neuron-Derived TAFA4 Promotes Macrophage Tissue Repair Functions. Nature (2021) 594:94–9. doi: 10.1038/s41586-021-03563-7
29. De Schepper S, Verheijden S, Aguilera-Lizarraga J, Viola MF, Boesmans W, Stakenborg N, et al. Self-Maintaining Gut Macrophages Are Essential for Intestinal Homeostasis. Cell (2018) 175:400–415.e413. doi: 10.1016/j.cell.2018.07.048
30. McDonald B, Kubes P. Innate Immune Cell Trafficking and Function During Sterile Inflammation of the Liver. Gastroenterology (2016) 151:1087–95. doi: 10.1053/j.gastro.2016.09.048
31. Conway BR, O'Sullivan ED, Cairns C, O'Sullivan J, Simpson DJ, Salzano A, et al. Kidney Single-Cell Atlas Reveals Myeloid Heterogeneity in Progression and Regression of Kidney Disease. J Am Soc Nephrol (2020) 31:2833–54. doi: 10.1681/ASN.2020060806
32. Mossanen JC, Krenkel O, Ergen C, Govaere O, Liepelt A, Puengel T, et al. Chemokine (C-C Motif) Receptor 2-Positive Monocytes Aggravate the Early Phase of Acetaminophen-Induced Acute Liver Injury. Hepatology (2016) 64:1667–82. doi: 10.1002/hep.28682
33. Nishizawa N, Ito Y, Eshima K, Ohkubo H, Kojo K, Inoue T, et al. Inhibition of Microsomal Prostaglandin E Synthase-1 Facilitates Liver Repair After Hepatic Injury in Mice. J Hepatol (2018) 69:110–20. doi: 10.1016/j.jhep.2018.02.009
34. Krenkel O, Tacke F. Liver Macrophages in Tissue Homeostasis and Disease. Nat Rev Immunol (2017) 17:306–21. doi: 10.1038/nri.2017.11
35. Kimball A, Schaller M, Joshi A, Davis FM, denDekker A, Boniakowski A, et al. Ly6C(Hi) Blood Monocyte/Macrophage Drive Chronic Inflammation and Impair Wound Healing in Diabetes Mellitus. Arterioscler Thromb Vasc Biol (2018) 38:1102–14. doi: 10.1161/ATVBAHA.118.310703
36. Giannakis N, Sansbury BE, Patsalos A, Hays TT, Riley CO, Han X, et al. Dynamic Changes to Lipid Mediators Support Transitions Among Macrophage Subtypes During Muscle Regeneration. Nat Immunol (2019) 20:626–36. doi: 10.1038/s41590-019-0356-7
37. Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, et al. Monitoring of Blood Vessels and Tissues by a Population of Monocytes With Patrolling Behavior. Science (2007) 317:666–70. doi: 10.1126/science.1142883
38. Chousterman BG, Boissonnas A, Poupel L, Baudesson de Chanville C, Adam J, Tabibzadeh N, et al. Ly6Chigh Monocytes Protect Against Kidney Damage During Sepsis via a CX3CR1-Dependent Adhesion Mechanism. J Am Soc Nephrol (2016) 27:792–803. doi: 10.1681/ASN.2015010009
39. Karlmark KR, Weiskirchen R, Zimmermann HW, Gassler N, Ginhoux F, Weber C, et al. Hepatic Recruitment of the Inflammatory Gr1+ Monocyte Subset Upon Liver Injury Promotes Hepatic Fibrosis. Hepatology (2009) 50:261–74. doi: 10.1002/hep.22950
40. Karlmark KR, Zimmermann HW, Roderburg C, Gassler N, Wasmuth HE, Luedde T, et al. The Fractalkine Receptor CX(3)CR1 Protects Against Liver Fibrosis by Controlling Differentiation and Survival of Infiltrating Hepatic Monocytes. Hepatology (2010) 52:1769–82. doi: 10.1002/hep.23894
41. Yang W, Tao Y, Wu Y, Zhao X, Ye W, Zhao D, et al. Neutrophils Promote the Development of Reparative Macrophages Mediated by ROS to Orchestrate Liver Repair. Nat Commun (2019) 10:1076. doi: 10.1038/s41467-019-09046-8
42. Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-Resident Macrophages. Nat Immunol (2013) 14:986–95. doi: 10.1038/ni.2705
43. Peng X, Zhang J, Xiao Z, Dong Y, Du J. CX3CL1-CX3CR1 Interaction Increases the Population of Ly6C(-)CX3CR1(hi) Macrophages Contributing to Unilateral Ureteral Obstruction-Induced Fibrosis. J Immunol (2015) 195:2797–805. doi: 10.4049/jimmunol.1403209
44. Eming SA, Werner S, Bugnon P, Wickenhauser C, Siewe L, Utermohlen O, et al. Accelerated Wound Closure in Mice Deficient for Interleukin-10. Am J Pathol (2007) 170:188–202. doi: 10.2353/ajpath.2007.060370
45. Tacke F, Zimmermann HW. Macrophage Heterogeneity in Liver Injury and Fibrosis. J Hepatol (2014) 60:1090–6. doi: 10.1016/j.jhep.2013.12.025
46. Pradere JP, Kluwe J, De Minicis S, Jiao JJ, Gwak GY, Dapito DH, et al. Hepatic Macrophages But Not Dendritic Cells Contribute to Liver Fibrosis by Promoting the Survival of Activated Hepatic Stellate Cells in Mice. Hepatology (2013) 58:1461–73. doi: 10.1002/hep.26429
47. Kalinski AL, Yoon C, Huffman LD, Duncker PC, Kohen R, Passino R, et al. Analysis of the Immune Response to Sciatic Nerve Injury Identifies Efferocytosis as a Key Mechanism of Nerve Debridement. Elife (2020) 9:e60223. doi: 10.7554/eLife.60223
48. Jia D, Jiang H, Weng X, Wu J, Bai P, Yang W, et al. Interleukin-35 Promotes Macrophage Survival and Improves Wound Healing After Myocardial Infarction in Mice. Circ Res (2019) 124:1323–36. doi: 10.1161/CIRCRESAHA.118.314569
49. Huang QQ, Birkett R, Doyle R, Shi B, Roberts EL, Mao Q, et al. The Role of Macrophages in the Response to TNF Inhibition in Experimental Arthritis. J Immunol (2018) 200:130–8. doi: 10.4049/jimmunol.1700229
50. Shaked I, Hanna RN, Shaked H, Chodaczek G, Nowyhed HN, Tweet G, et al. Transcription Factor Nr4a1 Couples Sympathetic and Inflammatory Cues in CNS-Recruited Macrophages to Limit Neuroinflammation. Nat Immunol (2015) 16:1228–34. doi: 10.1038/ni.3321
51. King IL, Dickendesher TL, Segal BM. Circulating Ly-6C+ Myeloid Precursors Migrate to the CNS and Play a Pathogenic Role During Autoimmune Demyelinating Disease. Blood (2009) 113:3190–7. doi: 10.1182/blood-2008-07-168575
52. Lipski DA, Dewispelaere R, Foucart V, Caspers LE, Defrance M, Bruyns C, et al. MHC Class II Expression and Potential Antigen-Presenting Cells in the Retina During Experimental Autoimmune Uveitis. J Neuroinflamm (2017) 14:136. doi: 10.1186/s12974-017-0915-5
53. Tanaka K, Kurebayashi J, Sohda M, Nomura T, Prabhakar U, Yan L, et al. The Expression of Monocyte Chemotactic Protein-1 in Papillary Thyroid Carcinoma is Correlated With Lymph Node Metastasis and Tumor Recurrence. Thyroid (2009) 19:21–5. doi: 10.1089/thy.2008.0237
54. Yang H, Zhang Q, Xu M, Wang L, Chen X, Feng Y, et al. CCL2-CCR2 Axis Recruits Tumor Associated Macrophages to Induce Immune Evasion Through PD-1 Signaling in Esophageal Carcinogenesis. Mol Cancer (2020) 19:41. doi: 10.1186/s12943-020-01165-x
55. Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, et al. Fate Mapping Reveals Origins and Dynamics of Monocytes and Tissue Macrophages Under Homeostasis. Immunity (2013) 38:79–91. doi: 10.1016/j.immuni.2012.12.001
56. Thomas GD, Hanna RN, Vasudevan NT, Hamers AA, Romanoski CE, McArdle S, et al. Deleting an Nr4a1 Super-Enhancer Subdomain Ablates Ly6C(low) Monocytes While Preserving Macrophage Gene Function. Immunity (2016) 45:975–87. doi: 10.1016/j.immuni.2016.10.011
57. Polletti S, Natoli G. Understanding Spontaneous Conversion: The Case of the Ly6C(-) Monocyte. Immunity (2017) 46:764–6. doi: 10.1016/j.immuni.2017.04.010
58. Murray PJ. Macrophage Polarization. Annu Rev Physiol (2017) 79:541–66. doi: 10.1146/annurev-physiol-022516-034339
59. Mildner A, Schonheit J, Giladi A, David E, Lara-Astiaso D, Lorenzo-Vivas E, et al. Genomic Characterization of Murine Monocytes Reveals C/EBPbeta Transcription Factor Dependence of Ly6C(-) Cells. Immunity (2017) 46:849–862.e847. doi: 10.1016/j.immuni.2017.04.018
60. Hanna RN, Carlin LM, Hubbeling HG, Nackiewicz D, Green AM, Punt JA, et al. The Transcription Factor NR4A1 (Nur77) Controls Bone Marrow Differentiation and the Survival of Ly6C- Monocytes. Nat Immunol (2011) 12:778–85. doi: 10.1038/ni.2063
61. Hanna RN, Shaked I, Hubbeling HG, Punt JA, Wu R, Herrley E, et al. NR4A1 (Nur77) Deletion Polarizes Macrophages Toward an Inflammatory Phenotype and Increases Atherosclerosis. Circ Res (2012) 110:416–27. doi: 10.1161/CIRCRESAHA.111.253377
62. Leenen PJ, de Bruijn MF, Voerman JS, Campbell PA, van Ewijk W. Markers of Mouse Macrophage Development Detected by Monoclonal Antibodies. J Immunol Methods (1994) 174:5–19. doi: 10.1016/0022-1759(94)90005-1
63. McCormack JM, Leenen PJ, Walker WS. Macrophage Progenitors From Mouse Bone Marrow and Spleen Differ in Their Expression of the Ly-6C Differentiation Antigen. J Immunol (1993) 151:6389–98.
64. Donnelly DJ, Longbrake EE, Shawler TM, Kigerl KA, Lai W, Tovar CA, et al. Deficient CX3CR1 Signaling Promotes Recovery After Mouse Spinal Cord Injury by Limiting the Recruitment and Activation of Ly6Clo/iNOS+ Macrophages. J Neurosci (2011) 31:9910–22. doi: 10.1523/JNEUROSCI.2114-11.2011
65. Zigmond E, Samia-Grinberg S, Pasmanik-Chor M, Brazowski E, Shibolet O, Halpern Z, et al. Infiltrating Monocyte-Derived Macrophages and Resident Kupffer Cells Display Different Ontogeny and Functions in Acute Liver Injury. J Immunol (2014) 193:344–53. doi: 10.4049/jimmunol.1400574
66. Jones GR, Bain CC, Fenton TM, Kelly A, Brown SL, Ivens AC, et al. Dynamics of Colon Monocyte and Macrophage Activation During Colitis. Front Immunol (2018) 9:2764. doi: 10.3389/fimmu.2018.02764
67. Knipper JA, Willenborg S, Brinckmann J, Bloch W, Maass T, Wagener R, et al. Interleukin-4 Receptor Alpha Signaling in Myeloid Cells Controls Collagen Fibril Assembly in Skin Repair. Immunity (2015) 43:803–16. doi: 10.1016/j.immuni.2015.09.005
68. Gibbons MA, MacKinnon AC, Ramachandran P, Dhaliwal K, Duffin R, Phythian-Adams AT, et al. Ly6Chi Monocytes Direct Alternatively Activated Profibrotic Macrophage Regulation of Lung Fibrosis. Am J Respir Crit Care Med (2011) 184:569–81. doi: 10.1164/rccm.201010-1719OC
69. Yang Q, Wang Y, Pei G, Deng X, Jiang H, Wu J, et al. Bone Marrow-Derived Ly6C(-) Macrophages Promote Ischemia-Induced Chronic Kidney Disease. Cell Death Dis (2019) 10:291. doi: 10.1038/s41419-019-1531-3
70. Scott DL, Wolfe F, Huizinga TW. Rheumatoid Arthritis. Lancet (2010) 376:1094–108. doi: 10.1016/S0140-6736(10)60826-4
71. Chaudhari K, Rizvi S, Syed BA. Rheumatoid Arthritis: Current and Future Trends. Nat Rev Drug Discovery (2016) 15:305–6. doi: 10.1038/nrd.2016.21
72. Archer AM, Saber R, Rose S, Shaffer A, Misharin AV, Tsai F, et al. ApoE Deficiency Exacerbates the Development and Sustainment of a Semi-Chronic K/BxN Serum Transfer-Induced Arthritis Model. J Transl Med (2016) 14:170. doi: 10.1186/s12967-016-0912-y
73. Misharin AV, Cuda CM, Saber R, Turner JD, Gierut AK, Haines GK 3rd, et al. Nonclassical Ly6C(-) Monocytes Drive the Development of Inflammatory Arthritis in Mice. Cell Rep (2014) 9:591–604. doi: 10.1016/j.celrep.2014.09.032
74. Brunet A, LeBel M, Egarnes B, Paquet-Bouchard C, Lessard AJ, Brown JP, et al. NR4A1-Dependent Ly6C(low) Monocytes Contribute to Reducing Joint Inflammation in Arthritic Mice Through Treg Cells. Eur J Immunol (2016) 46:2789–800. doi: 10.1002/eji.201646406
75. Durcan L, O'Dwyer T, Petri M. Management Strategies and Future Directions for Systemic Lupus Erythematosus in Adults. Lancet (2019) 393:2332–43. doi: 10.1016/S0140-6736(19)30237-5
76. Menke J, Rabacal WA, Byrne KT, Iwata Y, Schwartz MM, Stanley ER, et al. Circulating CSF-1 Promotes Monocyte and Macrophage Phenotypes That Enhance Lupus Nephritis. J Am Soc Nephrol (2009) 20:2581–92. doi: 10.1681/ASN.2009050499
77. Bethunaickan R, Berthier CC, Ramanujam M, Sahu R, Zhang W, Sun Y, et al. A Unique Hybrid Renal Mononuclear Phagocyte Activation Phenotype in Murine Systemic Lupus Erythematosus Nephritis. J Immunol (2011) 186:4994–5003. doi: 10.4049/jimmunol.1003010
78. Collaborators, G. B. D. M. S. Global, Regional, and National Burden of Multiple Sclerosis 1990-2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol (2019) 18:269–85. doi: 10.1016/S1474-4422(18)30443-5
79. Croxford AL, Lanzinger M, Hartmann FJ, Schreiner B, Mair F, Pelczar P, et al. The Cytokine GM-CSF Drives the Inflammatory Signature of CCR2+ Monocytes and Licenses Autoimmunity. Immunity (2015) 43:502–14. doi: 10.1016/j.immuni.2015.08.010
80. Jiang W, Li D, Han R, Zhang C, Jin WN, Wood K, et al. Acetylcholine-Producing NK Cells Attenuate CNS Inflammation via Modulation of Infiltrating Monocytes/Macrophages. Proc Natl Acad Sci U.S.A. (2017) 114:E6202–11. doi: 10.1073/pnas.1705491114
81. Horai R, Caspi RR. Microbiome and Autoimmune Uveitis. Front Immunol (2019) 10:232. doi: 10.3389/fimmu.2019.00232
82. Soria G, Ben-Baruch A. The Inflammatory Chemokines CCL2 and CCL5 in Breast Cancer. Cancer Lett (2008) 267:271–85. doi: 10.1016/j.canlet.2008.03.018
83. Negus RP, Stamp GW, Relf MG, Burke F, Malik ST, Bernasconi S, et al. The Detection and Localization of Monocyte Chemoattractant Protein-1 (MCP-1) in Human Ovarian Cancer. J Clin Invest (1995) 95:2391–6. doi: 10.1172/JCI117933
84. Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 Recruits Inflammatory Monocytes to Facilitate Breast-Tumour Metastasis. Nature (2011) 475:222–5. doi: 10.1038/nature10138
85. Tagliani E, Shi C, Nancy P, Tay CS, Pamer EG, Erlebacher A. Coordinate Regulation of Tissue Macrophage and Dendritic Cell Population Dynamics by CSF-1. J Exp Med (2011) 208:1901–16. doi: 10.1084/jem.20110866
86. Van Overmeire E, Stijlemans B, Heymann F, Keirsse J, Morias Y, Elkrim Y, et al. M-CSF and GM-CSF Receptor Signaling Differentially Regulate Monocyte Maturation and Macrophage Polarization in the Tumor Microenvironment. Cancer Res (2016) 76:35–42. doi: 10.1158/0008-5472.CAN-15-0869
87. Kalbasi A, Komar C, Tooker GM, Liu M, Lee JW, Gladney WL, et al. Tumor-Derived CCL2 Mediates Resistance to Radiotherapy in Pancreatic Ductal Adenocarcinoma. Clin Cancer Res (2017) 23:137–48. doi: 10.1158/1078-0432.CCR-16-0870
88. Yang F, Feng W, Wang H, Wang L, Liu X, Wang R, et al. Monocyte-Derived Leukemia-Associated Macrophages Facilitate Extramedullary Distribution of T-Cell Acute Lymphoblastic Leukemia Cells. Cancer Res (2020) 80:3677–91. doi: 10.1158/0008-5472.CAN-20-0034
89. Chen SY, Yang X, Feng WL, Liao JF, Wang LN, Feng L, et al. Organ-Specific Microenvironment Modifies Diverse Functional and Phenotypic Characteristics of Leukemia-Associated Macrophages in Mouse T Cell Acute Lymphoblastic Leukemia. J Immunol (2015) 194:2919–29. doi: 10.4049/jimmunol.1400451
90. Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, et al. Different Tumor Microenvironments Contain Functionally Distinct Subsets of Macrophages Derived From Ly6C(high) Monocytes. Cancer Res (2010) 70:5728–39. doi: 10.1158/0008-5472.CAN-09-4672
91. Laoui D, Van Overmeire E, Di Conza G, Aldeni C, Keirsse J, Morias Y, et al. Tumor Hypoxia Does Not Drive Differentiation of Tumor-Associated Macrophages But Rather Fine-Tunes the M2-Like Macrophage Population. Cancer Res (2014) 74:24–30. doi: 10.1158/0008-5472.CAN-13-1196
92. Zhang H, Yu Y, Zhou L, Ma J, Tang K, Xu P, et al. Circulating Tumor Microparticles Promote Lung Metastasis by Reprogramming Inflammatory and Mechanical Niches via a Macrophage-Dependent Pathway. Cancer Immunol Res (2018) 6:1046–56. doi: 10.1158/2326-6066.CIR-17-0574
93. Qu Y, Wen J, Thomas G, Yang W, Prior W, He W, et al. Baseline Frequency of Inflammatory Cxcl9-Expressing Tumor-Associated Macrophages Predicts Response to Avelumab Treatment. Cell Rep (2020) 32:108115. doi: 10.1016/j.celrep.2020.108115
94. Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity (2014) 41:14–20. doi: 10.1016/j.immuni.2014.06.008
95. Wang M, You Q, Lor K, Chen F, Gao B, Ju C. Chronic Alcohol Ingestion Modulates Hepatic Macrophage Populations and Functions in Mice. J Leukoc Biol (2014) 96:657–65. doi: 10.1189/jlb.6A0114-004RR
96. Fujiu K, Shibata M, Nakayama Y, Ogata F, Matsumoto S, Noshita K, et al. A Heart-Brain-Kidney Network Controls Adaptation to Cardiac Stress Through Tissue Macrophage Activation. Nat Med (2017) 23:611–22. doi: 10.1038/nm.4326
97. Chakarov S, Lim HY, Tan L, Lim SY, See P, Lum J, et al. Two Distinct Interstitial Macrophage Populations Coexist Across Tissues in Specific Subtissular Niches. Science (2019) 363(6432):eaau0964. doi: 10.1126/science.aau0964
Keywords: Ly6Chi and Ly6Clo macrophages, differentiation, inflammation, autoimmune disease, cancer
Citation: Li Y-h, Zhang Y, Pan G, Xiang L-x, Luo D-c and Shao J-z (2022) Occurrences and Functions of Ly6Chi and Ly6Clo Macrophages in Health and Disease. Front. Immunol. 13:901672. doi: 10.3389/fimmu.2022.901672
Received: 22 March 2022; Accepted: 03 May 2022;
Published: 30 May 2022.
Edited by:
Joao P. B. Viola, National Cancer Institute (INCA), BrazilReviewed by:
Guillaume Hoeffel, INSERM U1104 Centre d’immunologie de Marseille-Luminy (CIML), FranceEmanuele Azzoni, University of Milano Bicocca, Italy
Copyright © 2022 Li, Zhang, Pan, Xiang, Luo and Shao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian-zhong Shao, c2hhb2p6QHpqdS5lZHUuY24=; Ding-cun Luo, bGRjNjVAMTYzLmNvbQ==; Li-xin Xiang, eGlhbmdseEB6anUuZWR1LmNu
 Yuan-hui Li
Yuan-hui Li Yu Zhang1
Yu Zhang1 Li-xin Xiang
Li-xin Xiang Jian-zhong Shao
Jian-zhong Shao