- 1Department of Biochemistry and Molecular Biology, Centre of Postgraduate Medical Education, Warsaw, Poland
- 2Department of Gastroenterology, Hepatology and Clinical Oncology Centre of Postgraduate Medical Education, Warsaw, Poland
- 3Clinic of Polish Gastroenterology Foundation, Warsaw, Poland
Background and Aims: Metalloproteinases (MMPs) are involved in many distinct processes in the liver. Matrix metalloproteinase-3 (MMP-3) plays an important role in connective tissue remodeling, degradation of collagen (types II, III, IV, IX, and X), proteoglycans, fibronectin, laminin, and elastin. In addition, MMP-3 can also activate other MMPs such as MMP-1, MMP-7, and MMP-9. Primary biliary cholangitis (PBC) is a cholestatic, autoimmune liver disease, characterized by the progressive destruction of intrahepatic bile ducts, leading to cholestasis, fibrosis, cirrhosis, and liver failure. Fibrosis is the result of an imbalance between production and degradation of the extracellular matrix surrounding hepatocytes. Our aim in the present study was to determine whether the measurement of serum MMP-3 is clinically useful for assessing ongoing liver fibrosis in patients with PBC.
Methods: The MMP-3 concentration was determined in 182 PBC patients and 80 non-PBC controls using a commercially available ELISA kit.
Results: Higher concentrations of MMP-3 were found in 61% of PBC patients. PBC subjects had greater MMP-3 levels than controls: 68.9 ± 62.6 vs 21.3 ± 7.4 ng/mL, p < 0.001 for healthy subjects; 68.9 ± 62.6 vs 22.7 ± 7.6 ng/mL, p = 0.022 for autoimmune hepatitis controls; and 68.9 ± 62.6 vs 37.2 ± 17.4 ng/mL, p = 0.002 for primary sclerosing cholangitis controls. The serum MMP-3 concentration was significantly elevated in patients with higher bilirubin concentration (107.6 ± 85.8 vs 61.6 ± 46.1 ng/mL, p < 0.001) and was correlated with the level of antimitochondrial antibodies specific for PBC. The concentration of MMP-3 in sera of PBC patients was also found to correlate with the state of liver fibrosis (OR = 4.3; p < 0.01).
Conclusions: Our study demonstrated significantly higher MMP-3 levels in PBC patients than in healthy and pathological controls. Increased MMP-3 concentrations were positively correlated with various clinical and immunological parameters, and advanced liver fibrosis. The level of MMP-3 was associated with hepatic dysfunction and could play a role in the pathophysiology of hepatic fibrosis in PBC.
Introduction
Primary biliary cholangitis (PBC) is an autoimmune chronic progressive cholestatic liver disease. PBC is characterized by infiltration of lymphocytes and plasma cells into the bile duct and, as a result of inflammation and destruction, leads to fibrosis and cirrhosis of the liver (1–7). The most characteristic immunological features of the disease are antimitochondrial antibodies (AMAs) (8–10). The M2 fraction of AMAs, directed against the 2-oxoacid-dehydrogenase complex of the inner mitochondrial membrane, is detected in up to 95% of PBC patients. In addition, approximately 50% of PBC patients have different types of antinuclear antibodies (ANAs) (11–18). Several nuclear structures are considered as targets for the antinuclear antibodies ANA) at the PBC. The detection of two antinuclear immunofluorescence patterns (“Rim-like/membranous” and “Multiple nuclear dots”) display very high diagnostic accuracy for PBC diagnosis as previously demonstrated (19). Antibodies giving these patterns recognize the gp210 nuclear pore membrane protein and the sp100 nuclear body protein, respectively. ANA against nuclear envelope proteins such as anti-gp210 antibodies are not common but highly specific for PBC. PBC-specific MND pattern produces antibodies directed against the structural components of the nuclear protein of promyelocytic leukemia body - NB PML. Among the numerous substructures of the nucleus of the NB PML the proteins PML and Sp100 appear to be the most important.
Matrix metalloproteinases (MMPs) are a 28-membered family of zinc-dependent endopeptidases. They are the main enzymes that degrade extracellular matrix proteins and play an important role in the process of tissue remodeling and repair under physiological and pathological conditions (20–22). They are produced by most normal cells, including mast cells, osteoblasts, odontoblasts, dendritic cells, microglial cells, smooth myocytes, keratinocytes and endothelial cells. These enzymes are also secreted by inflammatory cells, macrophages and T lymphocytes. The MMP family consists of four major subtypes: collagenases, gelatinases, stromelysins and membrane-type metalloproteinases (23).
MMPs are involved in many distinct physiological processes in the liver. They are also critical in the pathogenicity and progression of liver diseases, including fibrosis cirrhosis and cancer, however the mechanisms underlying this phenomenon are largely unknown (24, 25). The pathobiology of liver disease is associated with dysregulated remodeling of the extracellular matrix (ECM) and its excessive production. As the catalytic activity of MMPs is specific to collagen-like peptides, this may result in neoplastic metastases and fibrosis (26–28). MMPs have been also described as biomarkers in various liver diseases (29–33). Some research groups have proposed to recognize circulating serum MMPs as specified reliable biomarkers of active fibrosis (34–40).
It has been reported that the altered expression of MMP-3 is linked with liver inflammation and fibrosis (41). MMP-3 is a stromelysin that is expressed in a variety of cell types, including hepatocellular carcinoma cells and hepatic stellate cells (42–44). MMP-3 is also associated with the excretion of protein ectodomains from the cell surface and degradation of extracellular matrix substrates, including collagens (type III, IV and V) and non-collagen ECM components such as laminins, fibronectin, osteopontin and proteoglycans (45). MMP-3 is involved in the release of chemotactic cytokines that initiate macrophage and leukocyte infiltration, and activate the tumor necrosis factor. MMP-3 can also stimulate other MMPs such as MMP-1, MMP-7, and MMP-9 (46, 47).
The polymorphism of MMP-3 (genetic variants) is associated with poor prognosis in hepatocellular carcinoma and primary sclerosing cholangitis (PSC) (47–50).
The aim of the study was to determine whether the measurement of serum MMP-3 is clinically useful for assessing ongoing liver fibrosis in patients with PBC and assess whether an increased MMP-3 concentration may be associated with biochemical parameters, primarily with the level of bilirubin, and presence of specific antibodies.
Materials and Methods
Patients
Serum samples were collected from 182 patients (176 women, 6 men; median age: 50; range: 27–75 years), diagnosed at the Centre of Postgraduate Medical Education (Warsaw, Poland). The diagnosis of PBC was established using generally accepted criteria, according to the practical guidelines of the European Association For The Study of Liver Diseases (EASL) for PBC (51, 52). A biopsy was performed in all subjects. In AMA -negative PBC patients diagnosis was supported by antinuclear antibody positivity or by liver biopsy. We excluded patients with serum levels positive for the hepatitis B surface antigen (HBsAg), anti-hepatitis A (IgM) and hepatitis C virus, patients with alcoholism and AIH (autoimmune hepatitis)/PBC overlap syndrome. In most patients, the diagnosis was made within one year after the onset of symptoms. The main measurements were time of death from liver failure or time to liver transplantation. The pathologic control group consisted of: 40 patients (16 females, 24 males; median age: 48; age range: 20-65 years) with PSC; 10 patients with AIH (9 females, 1 male; median age: 48; age range: 22-68 years). Serum samples from 30 healthy adult blood donors (22 females, 8 males; median age: 33; age range: 19-53 years) were collected at the Warsaw Blood Bank.
The study protocol was conducted in accordance with the ethical guidelines of the Declaration of Helsinki and was approved by the Ethical Committee of the Centre of Postgraduate Medical Education, Warsaw (approval number 71/PB/2019).
Detection of MMP-3
The MMP-3 concentration was assessed using a commercially available ELISA kit AESKULISA® MMP-3 (AESKU, Wendelsheim, Germany), according to the manufacturer’s instructions. Intra-assay and inter-assay performances were 3.4% and 4.6%, respectively. MMP-3 concentrations > 30 ng/mL were considered positive.
Detection of AMA M2 Autoantibodies
AMAs type M2 were determined using the commercially available kit QUANTA Lite® M2 EP- MIT3 ELISA (Inova Diagnostics, San Diego, CA, USA), according to the manufacturer’s instructions.
Statistical Analysis
Prevalence rates were compared between groups using the chi-square test and Fisher’s exact test. Continuous data were summarized as mean ± standard deviation (SD), and categorical data were summarized as frequencies. Continuous variables were evaluated using the Mann-Whitney test and were expressed as median ± interquartile range (IQR). P < 0.05 was considered statistically significant. All statistical analyses were performed using the Statistica 8.0 software (Stat-Soft, Cracow, Poland) and MedCal for Windows, version 7.4.1.0 (MedCal Software, Mariakerke, Belgium).
Results
Clinical, Histological and Laboratory Features of PBC Patients and Control Groups
The clinical, histological and laboratory features of PBC patients are presented in Table 1. The mean age at PBC diagnosis was 50 years and 176 patients out of 182 were female. Over 50% of patients had elevated total bilirubin levels. In over 70% of the tested samples increased activity of AP, g-GT, AST and ALT was found, and 172 (86%) patients were positive for AMA M2. Liver biopsies were obtained in all patients: stages I or II were observed in 119 subjects, while stages III or IV were found in 58 cases.
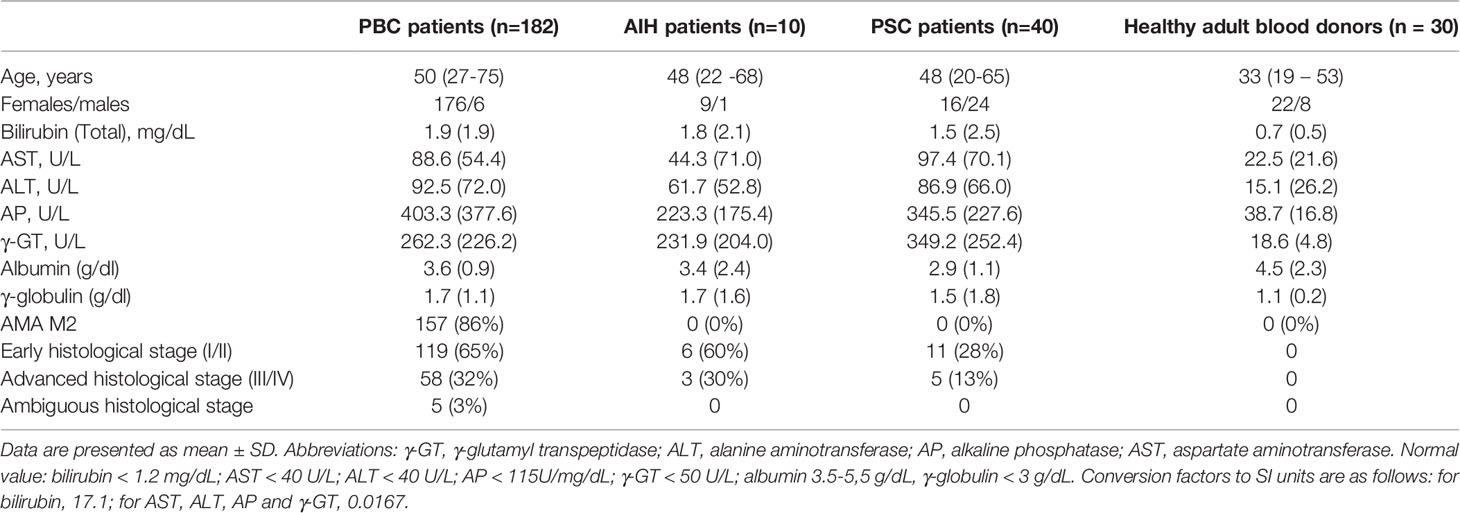
Table 1 The demographic, biochemical, immunological and histological features of PBC patients and control groups.
Occurrence and Diagnostic Value of MMP-3
In the tested PBC patients, a higher concentration of MMP-3 was found in 112 out of 182 samples (61%). In the control group, among PSC and AIH patients, an increased concentration of MMP-3 was found in 22 out of 40 (55%) and 1 out of 10 (10%) patients, respectively. We did not observe an elevated concentration of MMP-3 in any of the healthy control sera. The concentration of MMP-3 in sera of PBC patients and control groups is presented in Figure 1.
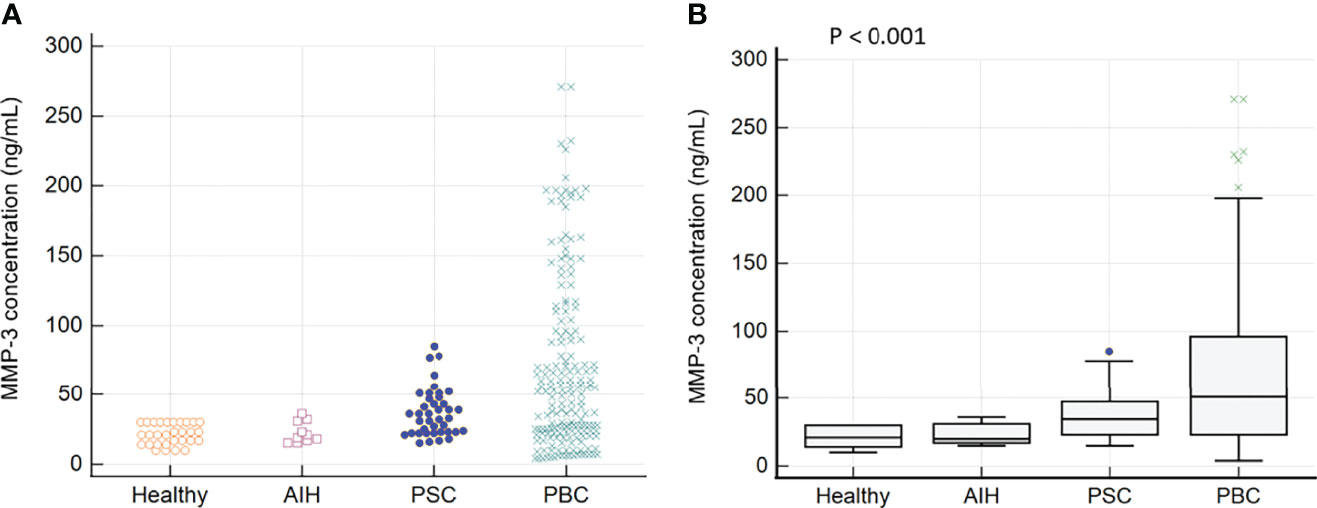
Figure 1 The MMP-3 concentration in sera of patients with PBC and control groups: (A) Distribution of MMP-3 in PBC patients and the control groups (B) The mean concentration of MMP-3 in each of the tested groups.
There was a significant difference between the mean concentration of MMP-3 in the group of PBC patients and the healthy control group: 68.9 ± 62.6 ng/mL vs 21.3 ± 7.4 ng/mL, p < 0.0001. PBC subjects also had greater MMP-3 levels than AIH controls (68.9 ± 62.6 vs 22.7 ± 7.6 ng/mL, p = 0.022), PSC controls (68.9 ± 62.6 vs 37.2 ± 17.4 ng/mL, p = 0.002). In accordance to the manufacturer’s instructions, MMP-3 concentrations > 30 ng/mL were considered positive and for this cut-off value, the specificity and sensitivity were 67.5% and 61%, respectively (Figure 2).
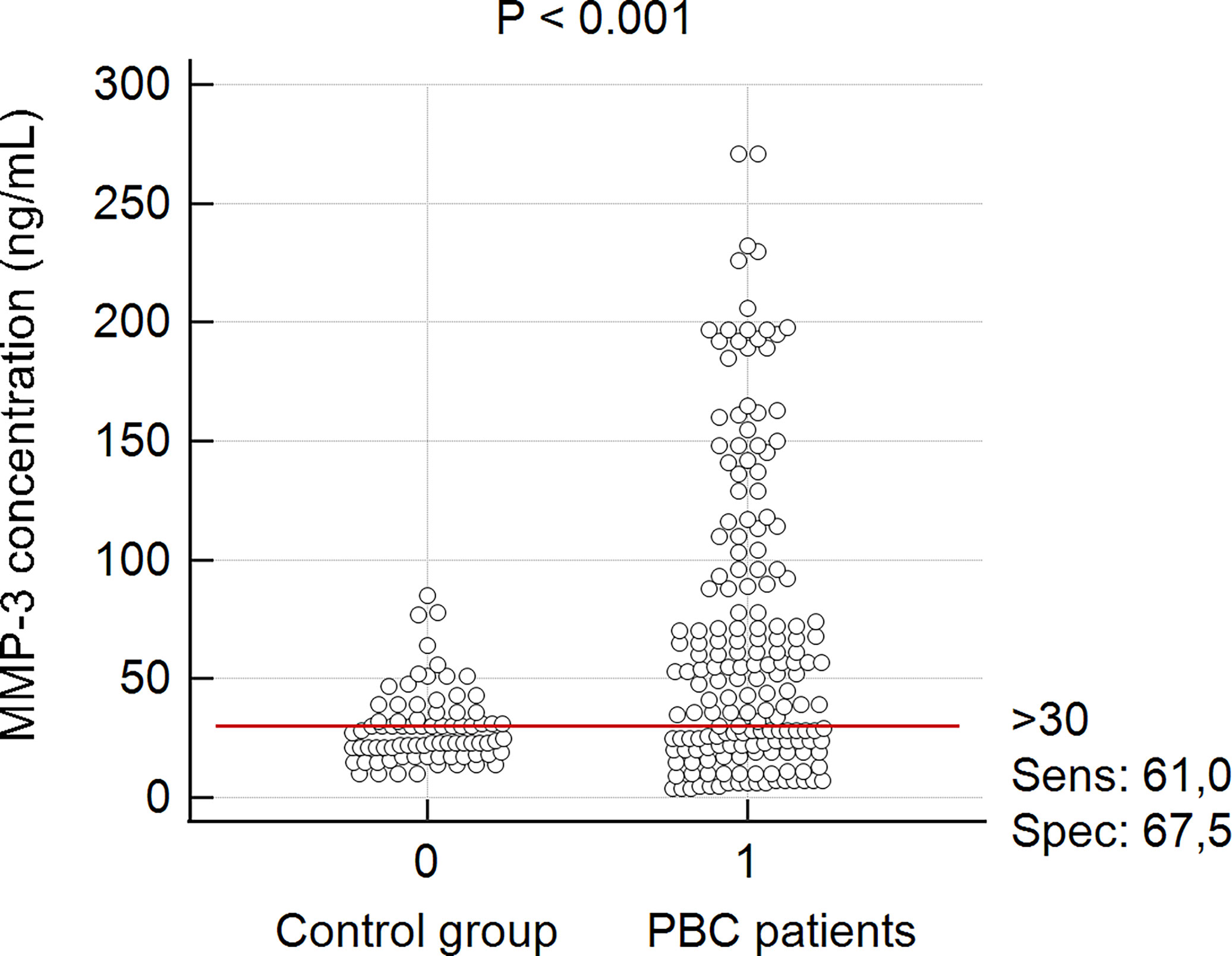
Figure 2 MMP-3 concentrations in accordance to the cut off value from manufacturer’s instructions. Control group – sera from patients with PSC, AIH and healthy donors.
Receiver operating characteristic curve analysis for serological detection of MMP-3 in PBC patients is presented in Figure 3.
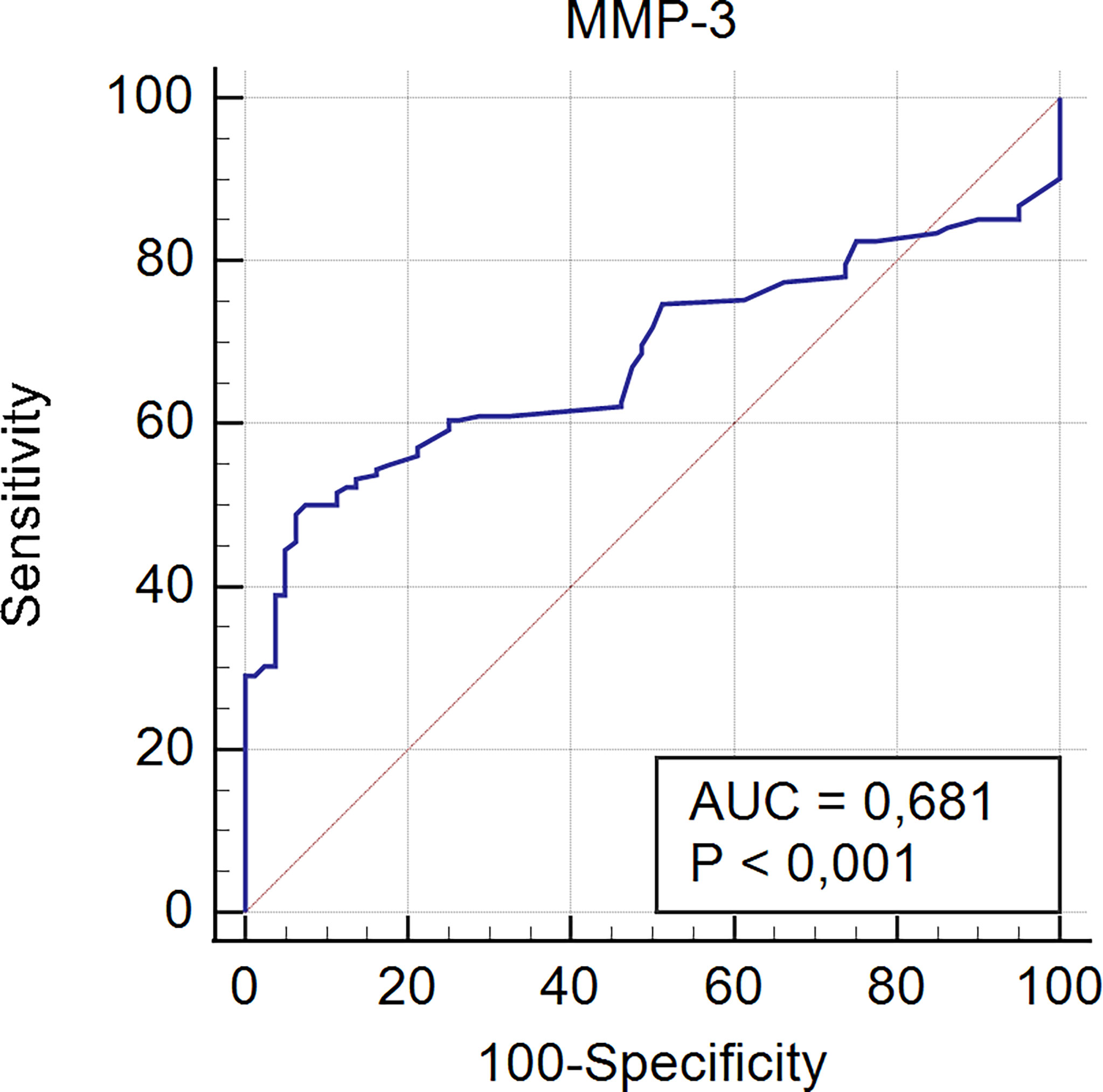
Figure 3 Receiver operating characteristic curve analysis for serological detection of MMP-3 in PBC patients.
MMP-3 Concentration and PBC-Specific Antibodies
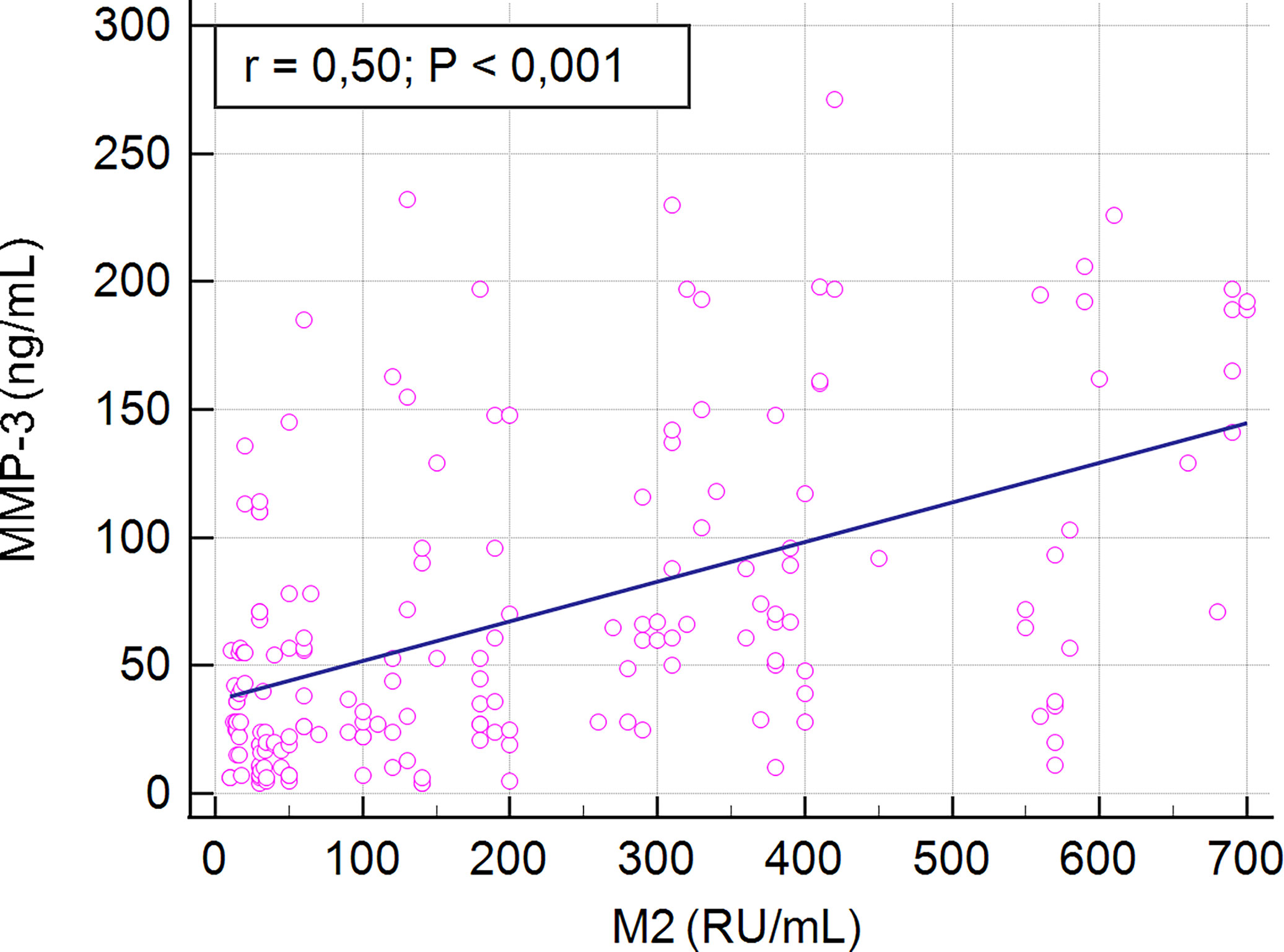
We found a positive correlation between serum MMP-3 concentration and AMA M2 levels in PBC patients (r = 0.5, p < 0.001) (Figure 4).
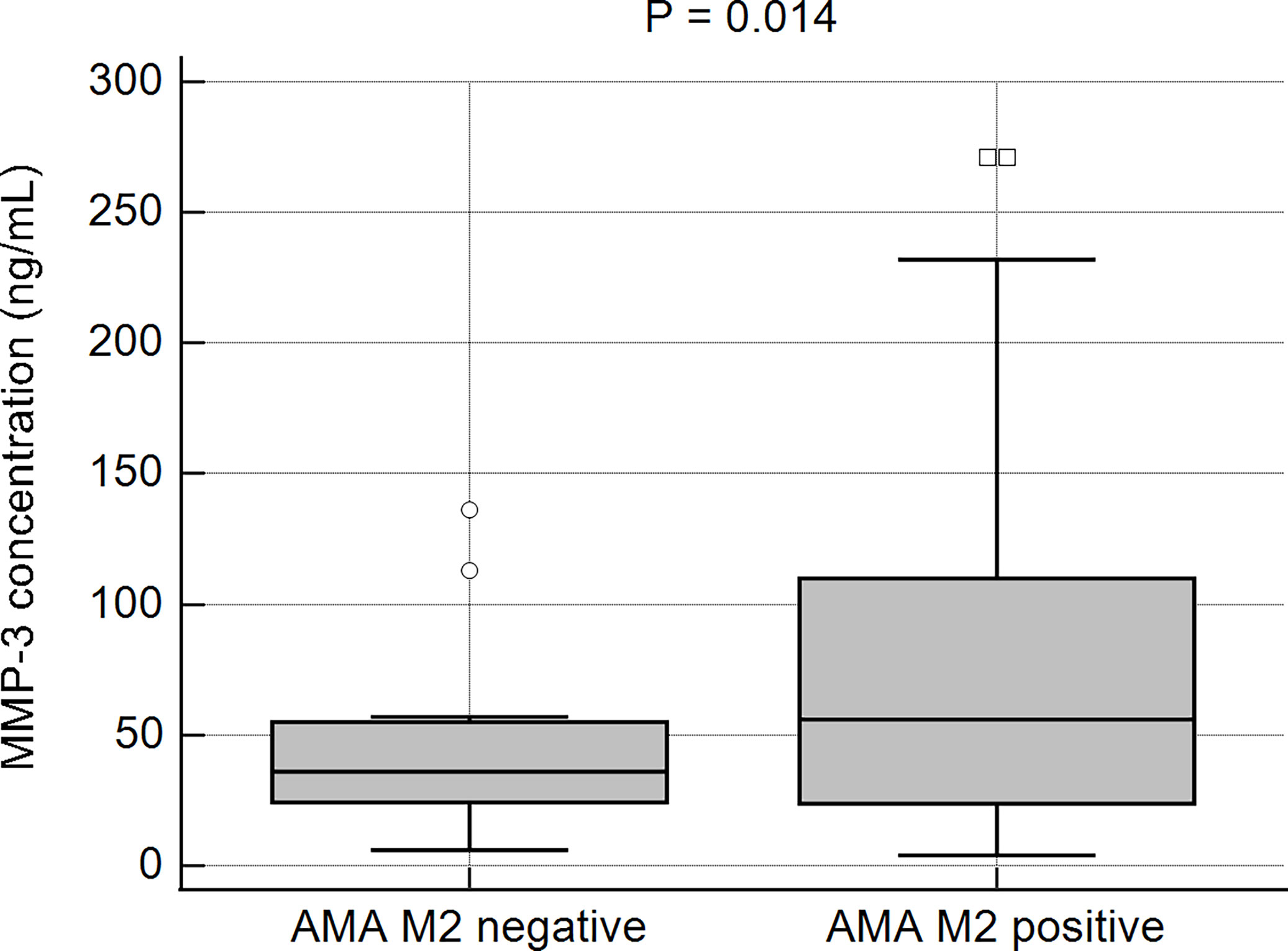
The prevalence of patients with higher MMP-3 concentrations in the studied AMA type M2- positive and -negative populations was further evaluated. Higher MMP-3 concentrations were observed more frequently in AMA M2-positive (64%; 100/157) than in AMA M2-negative patients (48%; 12/25). This difference was not found to be statistically significant (p > 0.05). However, the mean concentration of MMP-3 in the AMA M2-positive patients was significantly higher than in the negative group: 73 ± 65.0 ng/mL vs 40 ± 30.1 ng/mL, p = 0.014 (Figure 5).
Biochemical Features of PBC Patients According to the Level of MMP-3
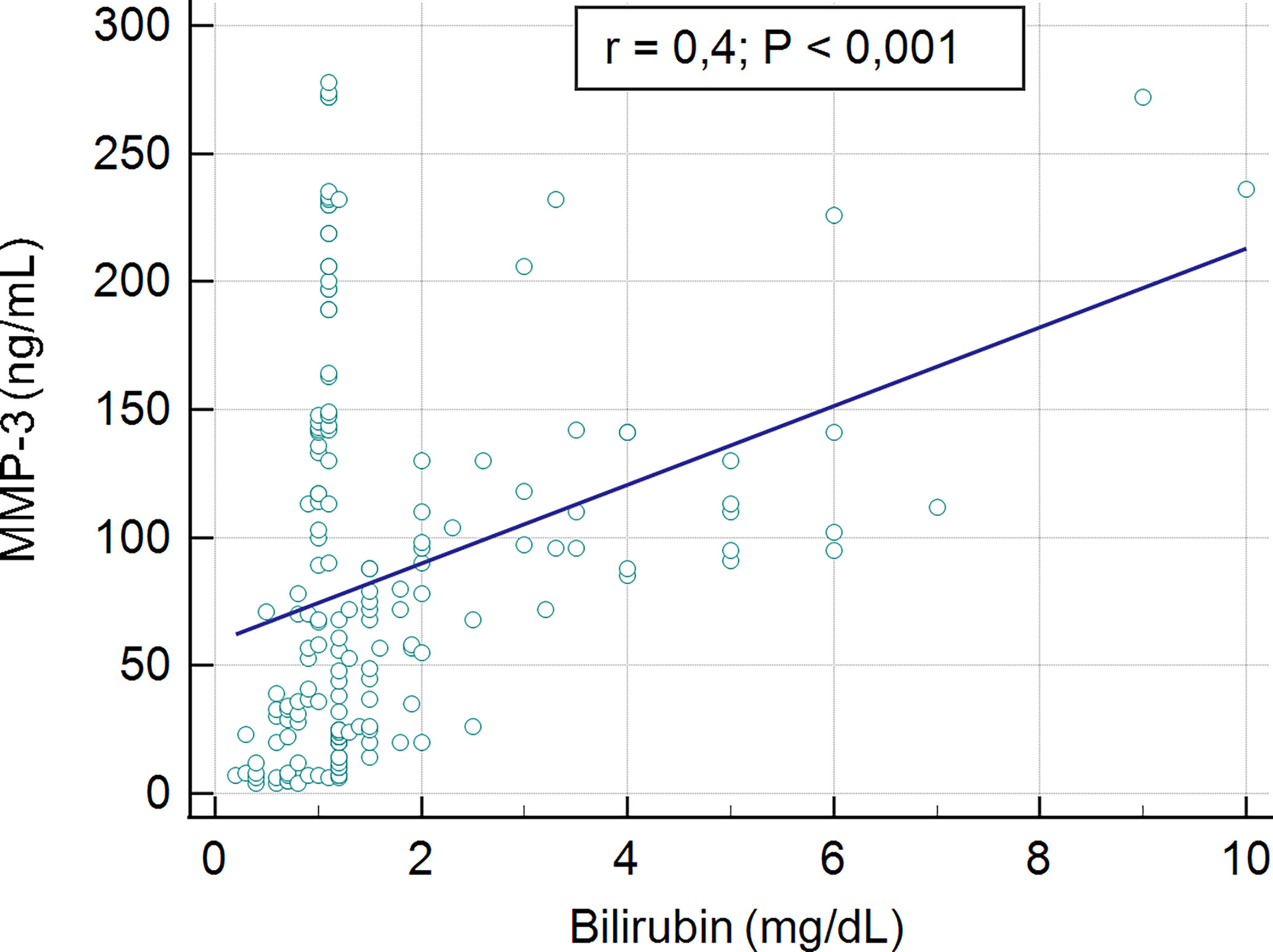
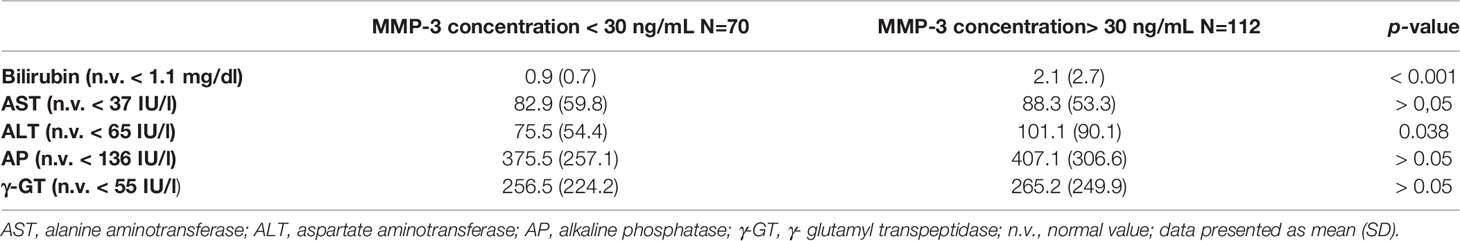
No association was found between the serum concentration of MMP-3 and biochemical markers such as AST, AP and γ-GT (r = 0.04, p > 0.05, r = 0.08, p > 0.05, r = 0.07, p > 0.05; respectively). A low, statistically significant correlation was shown between the serum concentration of MMP-3 and ALT level, r = 0,2, p = 0.04. The significant moderate correlation was presented for serum bilirubin level and MMP-3 concentration, r = 04, p < 0.001. The association between serum MMP-3 and bilirubin present Figure 6.
A comparison of groups of PBC patients who were positive and negative for MMP-3 showed that the symptoms of the disease began at the same age in each group of patients. The results of laboratory tests performed at the time of diagnosis were comparable in patients with normal and higher concentrations of MMP-3, with the exception of bilirubin and alanine aminotransferase (ALT) levels. Data from biochemical analyses performed at the time of diagnosis of 182 PBC patients, according to their MMP-3 status, are presented in Table 2.
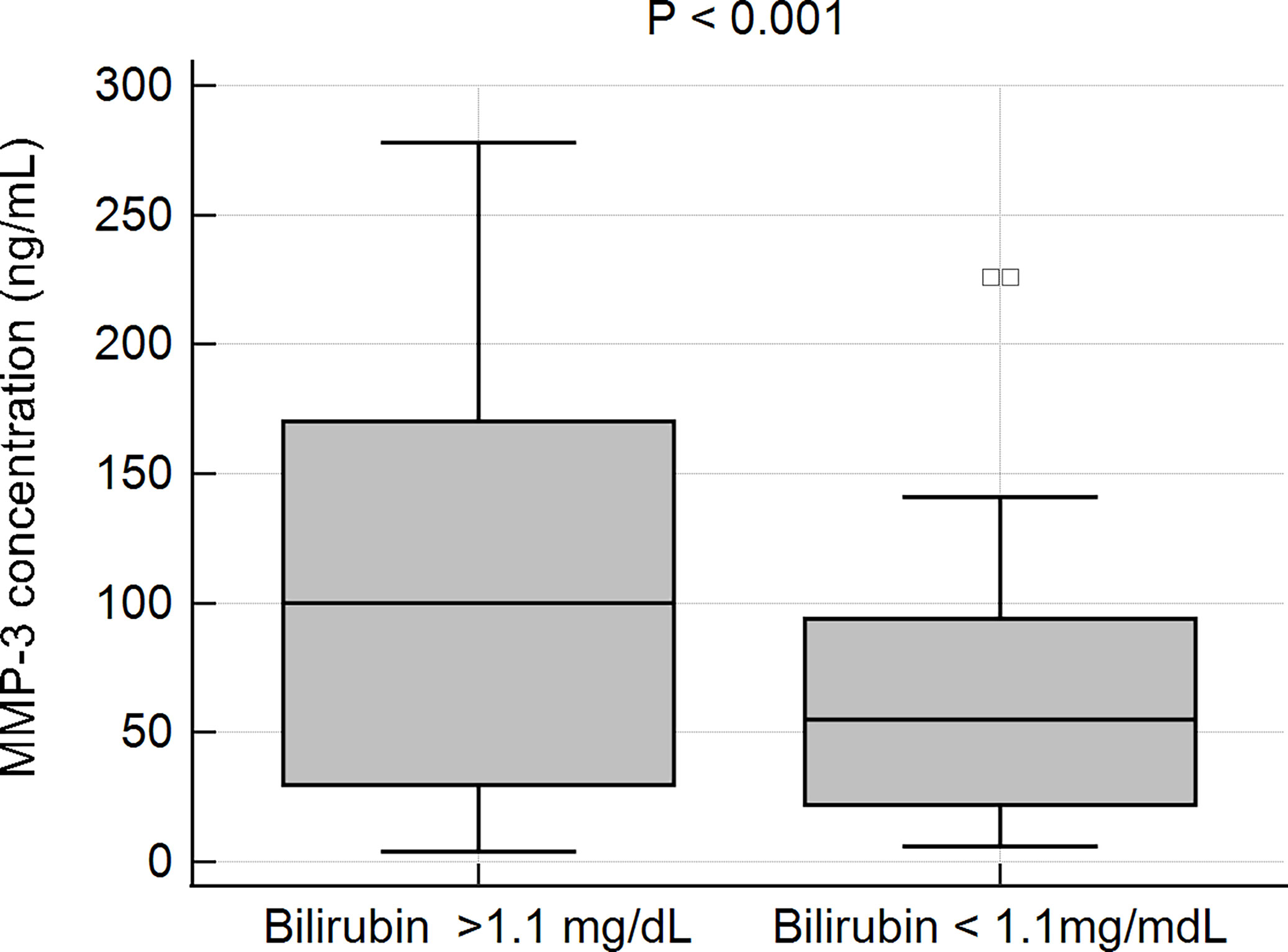
In patients with elevated levels of bilirubin, the mean concentration of MMP-3 was observed to be 107.6 6 ± 85.8 ng/mL, while in patients with a normal bilirubin level the MMP-3 concentration was 61.6 ± 46.1 ng/mL. This difference was statistically very significant, p < 0.001 (Figure 7).
Analysis of the Correlation Between Histological Parameters of PBC Patients and the Concentration of MMP-3
The serum MMP-3 concentrations were significantly correlated with the degree of liver fibrosis (r = 0.5, p<0.0001).
Patients with slight changes in liver tissue and patients with advanced fibrosis, according to Ludwig’s classification, were compared. More subjects with a higher concentration of MMP-3 were found in the group of patients with advanced fibrosis. The calculated OR (95% CI) for the histological score was 4.3 (2.0-9.2), p < 0.01. Among 119 PBC patients with early histological stages (I/II) of the disease, 63 (53%) had higher levels of MMP-3, while in the group of patients with advanced histological stages (III/IV), 48 out of 58 (83%) subjects were positive for MMP-3. This difference was statistically significant (p < 0.01).
The concentration of MMP-3 was also significantly higher in the group of patients with advanced fibrosis 103.1 ± 73.9 ng/mL vs 69.9 ± 68.7 ng/mL, p < 0.001 (Figure 8).
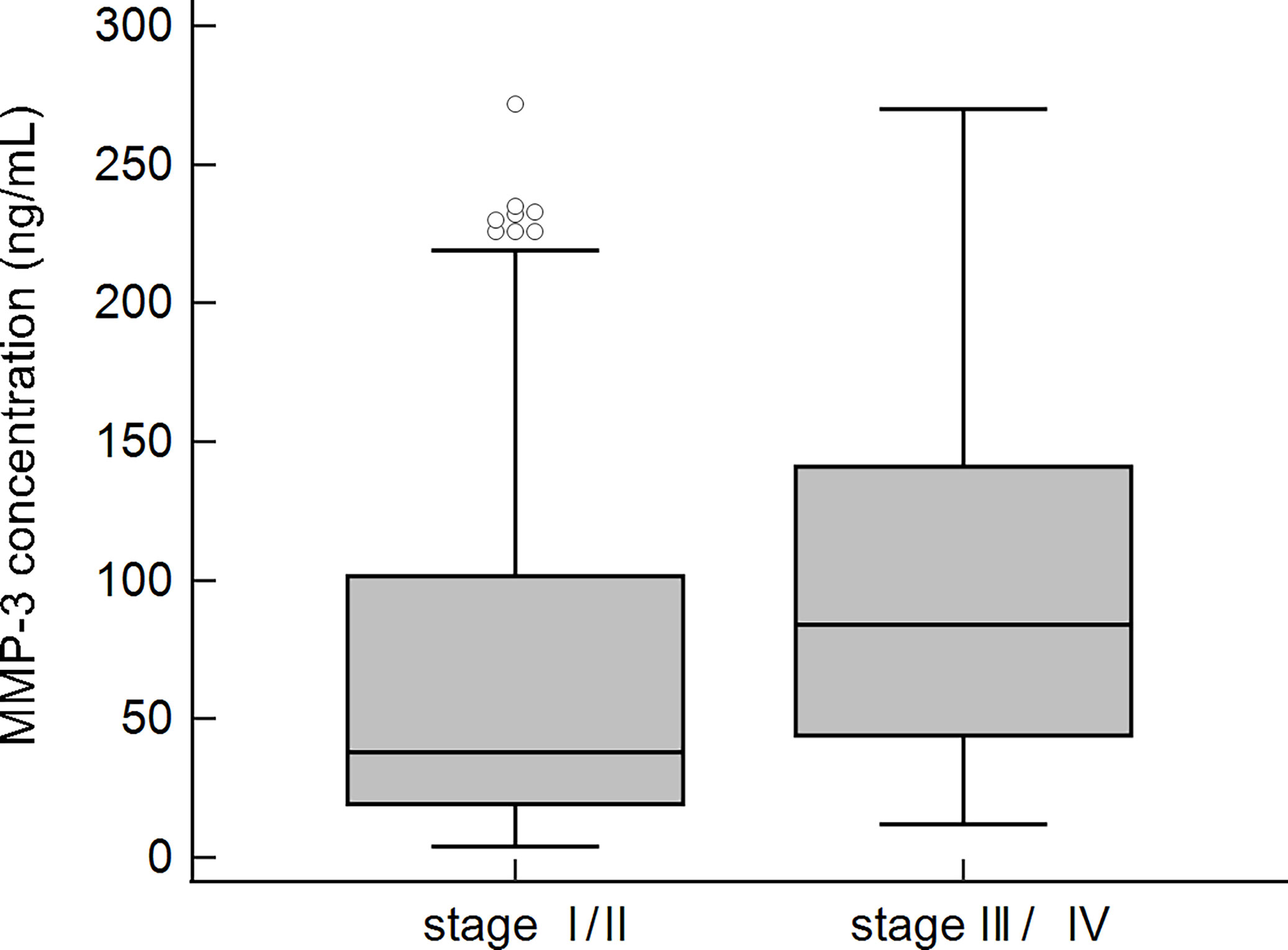
Figure 8 MMP-3 concentration in sera of PBC patients and the stage of fibrosis according to Ludwig’s classification.
Discussion
MMP-3 is associated with the occurrence and development of various diseases (53). Elevated serum MMP-3 levels have previously been reported in connective tissue disease: systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) (54–57) and also in myasthenia gravis (58). However, little is known about the role of MMP-3 in others autoimmune disorders. Therefore, in the presented study we aimed to determine MMP-3 concentrations in sera obtained from PBC patients.
Analyses of biochemical parameters and specific autoantibodies are standard procedures in the detection of PBC. Moreover, determination of markers of inflammation or fibrosis can be supportive for the diagnosis. We decided to study MMPs as they are involved in many distinct processes in the liver and play a critical role in the development of liver fibrosis. There is relatively little data in literature on the relationship between PBC and MMP-3. It has already been shown that MMP-3 is involved in liver inflammation, degradation of normal ECM, and release of cytokines that initiate macrophage and leukocyte infiltration. Our main aim was to assess whether MMP-3 is involved in liver progression and may be considered as a marker of fibrosis. We found significantly higher concentrations of MMP-3 in PBC patients in comparison to control subjects (healthy, AIH and PSC patients). PBC and PSC are both fibroinflammatory cholangiopathies, so it is interesting that PBC has higher levels of MMP-3 than PSC. The increase in MMP-3 in the liver and thus in the serum of patients may be related to the increased transforming growth factor (TGF) -β1 in the fibrotic liver. Expression of TGF-β1 is increased in patients with chronic liver disease. MMP-3 expression may also be associated with an increased amount of type IV collagen. Initial damage to cholangiocytes in autoimmune liver diseases is related to the innate immune response. Cytokines and metalloproteinases may be involved in the process of fibrosis both at PBC and at PSC. However, the mechanism of the fibrosis process involving metalloproteinases is not fully understood. Perhaps higher MMP-3 level in PBC is related to the location of the disease process. PBC targets the small bile ducts, PSC targets the large bile ducts. Despite sharing common symptoms (such as itching and fatigue) and having comparable acronyms, PSC and PBC are distinct entities and exhibit important differences, which include the site of tissue damage within the liver, associations with inflammatory bowel disease (IBD, which includes ulcerative colitis and Crohn’s disease) response to treatment, risks of disease progression and cancer. However, the two diseases are not the same, despite sharing certain similar characteristics and symptoms. Each condition creates different needs among patients, and each requires different treatments and monitoring. Some PSC patients suffer from concomitant inflammatory bowel diseases, but also from infectious diseases of the biliary tract. Individuals with PSC can occasionally develop abdominal pain and fever, which may suggest infection of the bile ducts called bacterial cholangitis. Although the latter can be treated with antibiotics, no currently known treatment has been shown to slow the progression or cure PSC. Perhaps treatment with antibiotics affects the level of MMP-3. The use of tetracycline-like antibiotics, which inhibit MMPs, were previously approved for treating infection (59). The reason for the increased activity of MMP-3 and the relationship of MMP-3 to liver fibrosis is not entirely clear. Higher MMP-3 activity in pathological conditions may be associated with liver fibrosis through the cell-matrix interaction mechanism. Changing the matrix composition results in the activation of cells in the liver, leading to proliferation and fibrogenesis. These processes need not be identical for the PBC and the PSC.
Very high MMP-3 concentrations have previously been reported in SLE and RA patients (above 250 ng/mL and 180 ng/mL, respectively), which are higher than in our PBC patients. As shown by Honsawek et al., who studied diseases of the bile ducts, serum MMP-3 levels in biliary atresia (BA) patients were markedly higher than those in healthy controls. Untreated BA patients were more likely to develop severe liver fibrosis, biliary cirrhosis, and eventually die before they were 2 years old (60). Although the diseases affect different age groups, it can be considered that the pathophysiological mechanism may be similar. Honsawek et al. revealed that BA patients with high ALT had increased concentrations of serum MMP-3, as compared to those with normal ALT. In our study, a correlation between the presence of higher concentrations of MMP-3, and higher levels of bilirubin and ALT was also found. It has been known that high serum ALT is a specific indicator for liver injury. This is important as it has been suggested that bilirubin levels predict liver transplantation or death in PBC patients and are used in selection of treatment strategies (61, 62). The group of PBC patients with an elevated level of bilirubin presented a significantly higher concentration of MMP-3. No association was found between the serum concentration of MMP-3 and biochemical markers such as AST, AP.
We also evaluated the concentration of MMP-3 in the group of PBC patients with AMA type M2 antibodies, because of their high sensitivity and specificity for diagnosis of PBC. We found that the increased concentration of MMP-3 in PBC was determined more often in the AMA M2 positive group. We found a positive correlation between serum MMP-3 concentration and AMA M2 levels in PBC patients
Finally, we studied MMP-3 concentrations in patients with both very slight changes in liver tissue and with advanced fibrosis, I/II stages and III/IV stages, respectively, according to Ludwig’s classification. In the groups with advanced fibrosis we found more patients with increased concentrations of MMP-3. The calculated OR was above 4, when compared to patients with slight changes in liver tissue. The concentration of MMP-3 was also significantly enhanced in the group of patients with advanced fibrosis.
Previous studies have presented correlations between increased MMP-3 and production of endostatin (by collagen XVIII) (63), which is connected with lung epithelial cell apoptosis (64) and thus promotes fibrosis. Perhaps a similar scenario may occur in the case of liver fibrosis, as elevated levels of MMP-3 were found in the sera of these patients. Our results may suggest that a high serum concentration of MMP-3 can be related to the degree of liver fibrosis.
We propose that the concentration of MMP-3 in sera, in connection with the level of bilirubin and presence of specific antibodies could be used as a non-invasive biomarker to determine the possible severity of liver disease.
We can try to reflect if MMP-3 could be a therapeutic target? The process of liver fibrosis may be reversible and the removal of damaging factors may be an effective treatment option, for example remodeling the ECM, affecting the activity of MMPs. A therapeutic target in the case of fibrosis could be the regulation of ECM-degrading enzymes. Some studies show that interfering with the activity or expression of MMPs reduces fibrosis. Giannandrea and Parks present fibrosis treatment outcomes for various MMPs (25). In turn, Hemman and colleagues note that MMP expression is elevated in both the early and advanced stages of fibrosis, prior to scar tissue accumulation, and that levels decline after treatment. The authors discuss MMPs for the treatment of fibrosis (65). Our study shows that the concentration of MMP-3 also increases in the early stage of fibrosis. Perhaps it is the inhibition of MMP-3 that could play an important role in the treatment that slows down the fibromyalysis process. The relationship between fibrosis and MMPs like MMP-1, MMP-3, MMP-7, MMP-9, MMP-13 and MMP-19, in macrophages and neutrophils was increased for example in BALF. These infiltrating macrophages and neutrophils are associated with the inflammatory response and are essential in pulmonary fibrosis (66, 67). Perhaps we could observe similar processes concerning the liver. Hong-Meng Chuang presents inhibitors of MMPs, such as PD166793 hydrate S-2- (4’-bromobiphenyl-4-sulfonylamino) -3-methylbutyric acid), effective in animal models of pulmonary, liver and myocardial fibrosis (68). However, most of them are drugs used in animal models and must be tested in preclinical studies before they can be used in patients.
In conclusion, we found a significantly higher MMP-3 concentration in PBC patients than in healthy controls. A positive correlation between higher MMP-3 levels, and presence of specific AMA type M2 autoantibodies and increased bilirubin concentration suggests that MMP-3 can be associated with a rapidly evolving disease of the liver, including hepatic fibrosis in PBC patients.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethical Committee of the Centre of Postgraduate Medical Education, Warsaw (approval number 71/PB/2019). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
AB obtained the funding, was responsible for conception, design, and coordination of the study, performed the serum assays, performed the statistical analysis, elaborating the table and figure, analysed and interpreted the data, drafted the manuscript and submitted the manuscript. AH evaluated patients, collected the clinical samples and data, analysed and interpreted the data. All authors have given final approval of the version to be published.
Funding
The study was supported by grant: 501-1-025-01-21 from the Centre of Postgraduate Medical Education, Warsaw, Poland.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Carey EJ, Ali AH, Lindor KD. Primary Biliary Cirrhosis. Lancet (2015) 386:1565–75. doi: 10.1016/S0140-6736(15)00154-3
2. Hirschfield GM, Chazouillères O, Cortez-Pint H, Macedo G, de Lédinghen V, Adekunle F, et al. A Consensus Integrated Care Pathway for Patients With Primary Biliary Cholangitis: A Guideline-Based Approach to Clinical Care of Patients. Expert Rev Gastroenterol Hepatol (2021) 15(8):929–39. doi: 10.1080/17474124.2021.1945919
3. Chew M, Bowlus C. Primary Biliary Cholangitis: Diagnosis and Treatment. Liver Res (2018) 2(2):81–6. doi: 10.1016/j.livres.2018.03.004
4. Beretta-Piccoli T, Mieli-Vergani BG, Vergani D, Vierling JM, Adams D, Alpini G, et al. The Challenges of Primary Biliary Cholangitis: What Is New and What Needs To Be Done. J Autoimmun (2019) 105:102328. doi: 10.1016/j.jaut.2019.102328
5. Tsuneyama K, Baba H, Morimoto Y, Tsunematsu T, Ogawa H. Primary Biliary Cholangitis: Its Pathological Characteristics and Immunopathological Mechanisms. J Med Investig (2017) 64(1.2):7–13. doi: 10.2152/jmi.64.7
6. Cichoz-Lach H, Grywalska E, Michalak A. A Deviations in Peripheral Blood Cell Populations Are Associated With the Stage of Primary Biliary Cholangitis and Presence of Itching. Arch Immunol Ther Ex (2017) 66:443–52. doi: 10.1007/s00005-018-0515-9
7. You H, Ma X, Efe C, Wang G, Jeong SH, Abe K, et al. APASL Clinical Practice Guidance: The Diagnosis and Management of Patients With Primary Biliary Cholangitis. Hepatol Int (2022) 16:1–23. doi: 10.1007/s12072-021-10276-6
8. Lindor KD, Bowlus C, Boyer J, Levy C, Mayo M. Primary Biliary Cholangitis: Practice Guidance From the American Association for the Study of Liver Diseases. Hepatology (2019) 69(1):394–419. doi: 10.1002/hep.30145
9. Kouroumalis E, Samonakis D, Voumvouraki A. Biomarkers for Primary Biliary Holangitis: Current Perspectives. Hepat Med: Evidence Res (2018) 10:43–53. doi: 10.2147/HMER.S135337
10. Cancado ELR, Harriz M. The Importance of Autoantibody Detection in Primary Biliary Cirrhosis. Front Immunol (2015) 6:309. doi: 10.3389/fimmu.2015.00309
11. Wang C, Zheng X, Jiang P, Tang R, Gong Y, Dai Y, et al. Genome-Wide Association Studies of Specific Antinuclear Autoantibody Subphenotypes in Primary Biliary Cholangitis. Hepatology (2019) 70(1):294–307. doi: 10.1002/hep.30604
12. Bauer A, Habior A, Wieszczy P, Gaweł D. Analysis of Autoantibodies Against Promyelocytic Leukemia Nuclear Body Com-Ponents and Biochemical Parameters in Sera of Patients With Primary Biliary Cholangitis. Diagnostics (2021) 11(4):1–15. doi: 10.3390/diagnostics11040587
13. Ozaslan E, Efe C, Ozaslan NG. The Diagnosis of Antimitochondrial Antibody-Negative Primary Biliary Cholangitis. Clin Res Hepatol Gastroenterol (2016) 40(5):553–61. doi: 10.1016/j.clinre.2016.06.001
14. Granito A, Yang WH, Muratori L. PML Nuclear Body Component Sp140 is a Novel Autoantigen I Primary Biliary Cirrhosis. Am J Gastroenterol (2010) 105(1):125–31. doi: 10.1038/ajg.2009.596
15. Granito A, Muratori P, Quarneti C, Pappas G, Cicola R, Muratori L. Antinuclear Antibodies as Ancillary Markers in Primary Biliary Cirrhosis. Expert Rev Mol Diagn (2012) 12(1):65–74. doi: 10.1586/erm.11.82
16. Granito A, Muratori L, Tovoli F, Muratori P. Autoantibodies to Speckled Protein Family in Primary Biliary Cholangitis. Allergy Asthma Clin Immunol (2021) 17(1):35. doi: 10.1186/s13223-021-00539-0
17. Zhang Q, Liu Z, Wu S, Duan W, Chen S, Ou X, et al. Meta-Analysis of Antinuclear Antibodies in Thediagnosis of Antimitochondrial Antibody-Negative Primary Biliary Cholangitis. Hindawi Gastroent Res Pract (2019) 10:8959103.
18. Bauer A, Habior A. Detection of Autoantibodies Against Nucleoporin P62 in Sera of Patients With Primary Biliary Cholangitis. Ann Lab Med (2019) 39:291–8. doi: 10.3343/alm.2019.39.3.291
19. Granito A, Muratori P, Muratori L. Antinuclear Antibodies Giving the ‘Multiple Nuclear Dots’ or the ‘Rim-Like/Membranous’ Patterns: Diagnostic Accuracy for Primary Biliary Cirrhosis. Aliment Pharmacol Ther (2006) 24(11-12):1575–83. doi: 10.1111/j.1365-2036.2006.03172.x
20. Bassiouni W, Ali MAM, Schulz R. Multifunctional Intracellular Matrix Metalloproteinases: Implications in Disease. FEBS J (2021) 288(24):7162–82. doi: 10.1111/febs.15701
21. Laronha H, Caldeira J. Structure and Function of Human Matrix. Cells (2020) 9(5):1076. doi: 10.3390/cells9051076
22. He J, Qin M, Chen Y, Hu Z, Xie F, Ye L, et al. Epigenetic Regulation of Matrix Metalloproteinases in Inflammatory Diseases: A Narrative Review. Cell Biosci (2020) 10:86. doi: 10.1186/s13578-020-00451-x
23. Cui N, Hu M, Khalil RA. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog Mol Biol Transl Sci (2017) 147:1–73. doi: 10.1016/bs.pmbts.2017.02.005
24. Roeb E. Matrix Metalloproteinases and Liver Fibrosis (Translational Aspects). Matrix Biol (2020) 68–69:463–73. doi: 10.3390/cells9051212
25. Fingleton B. MMPs as Therapeutic Targets—Still a Viable Option? Semin Cell Dev Biol (2008) 19:61–8. doi: 10.1016/j.semcdb.2007.06.006
26. Giannandrea M, Parks WC. Diverse Functions of Matrix Metalloproteinases During Fibrosis. Dis Model Mech (2014) 7(2):193–203. doi: 10.1242/dmm.012062
27. Attallah A, El-Far M, Malak CAA, Omran MM, Farid K, Hussien MA, et al. Fibro-Check: A Combination of Direct and Indirect Markers for Liver Fibrosis Staging in Chronic Hepatitis C Patients. Ann Hepatol (2015) 14:225–33. doi: 10.1016/S1665-2681(19)30785-9
28. Lichtinghagen R, Bahr MJ, Wehmeier M, Michels D, Haberkorn CI. Expression and Coordinated Regulation of Matrix Metalloproteinases in Chronic Hepatitis C and Hepatitis C Virus-Induced Liver Cirrhosis Clin. Sci (2003) 105:373–82. doi: 10.1042/CS20030098
29. Sharma P, Arora A. Clinical Presentation of Alcoholic Liver Disease and non-Alcoholic Fatty Liver Disease: Spectrum and Diagnosis. Transl Gastroenterol Hepatol (2020) 5:19. doi: 10.21037/tgh.2019.10.02
30. Prystupa A, Boguszewska-Czubara A, Bojarska-Junak A, Toruń-Jurkowska A, Roliński J, Załuska W. Activity of MMP-2, MMP-8 and MMP-9 in Serum as a Marker of Progression of Alcoholic Liver Disease in People From Lublin Region, Eastern Poland. Ann Agric Environ Med (2015) 22:325–8. doi: 10.5604/12321966.1152088
31. Lam S, Singh R, Dillman JR, Trout AT, Serai SD, Sharma D, et al. Serum Matrix Metalloproteinase 7 Is a Diagnostic Biomarker of Biliary Injury and Fibrosis in Pediatric Autoimmune Liver Disease. Hepatol Commun (2020) 4(11):1680–93. doi: 10.1002/hep4.1589
32. Naim A, Pan Q, Baig MS. Matrix Metalloproteinases (MMPs) in Liver Diseases. J Clin Exp Hepatol (2017) 7(4):367–72. doi: 10.1016/j.jceh.2017.09.004
33. Roeb E. Matrix Metalloproteinases and Liver Fibrosis (Translational Aspects). Matrix Biol (2018) 68-69:463–73. doi: 10.1016/j.matbio.2017.12.012
34. Ando W, Yokomori H, Tsutsui N, Yamanouchi E, Suzuki Y, Oda M, et al. Serum Matrix Metalloproteinase-1 Level Represents Disease Activity as Opposed to Fibrosis in Patients With Histologically Proven Nonalcoholic Steatohepatitis. Clin Mol Hepatol (2018) 24:61–76. doi: 10.3350/cmh.2017.0030
35. Irvine KM, Okano S, Patel PJ, Horsfall LU, Williams S, Russell A, et al. Serum Matrix Metalloproteinase 7 (MMP7) Is a Biomarker of Fibrosis in Patients With Non-Alcoholic Fatty Liver Disease. Sci Rep (2021) 11:2858. doi: 10.1038/s41598-021-82315-z
36. Veidal SS, Karsdal MA, Vassiliadis E, Nawrocki A, Martin Larsen R, Nguyen QHT, et al. MMP Mediated Degradation of Type VI Collagen Is Highly Associated With Liver Fibrosis—Identification and Validation of a Novel Biochemical Marker Assay. PloS One (2011) 6:e24753. doi: 10.1371/journal.pone.0024753
37. Monvoisin A, Bisson C, Si-Tayeb K, Balabaud C, Desmoulière A, Rosenbaum J. Involvement of Matrix Metalloproteinase Type-3 in Hepatocyte Growth Factor-Induced Invasion of Human Hepatocellular Carcinoma Cells. Int J Cancer (2002) 97:157–62. doi: 10.1002/ijc.1595
38. Martinez-Castillo M, Hernandez-Barragan A, Flores-Vasconcelos I, Rosique-Oramas D, Perez-Hernandez JL, et al. Production and Activity of Matrix Metalloproteinases During Liver Fibrosis Progression of Chronic Hepatitis C Patients. World J Hepatol (2021) 13(2):218–32. doi: 10.4254/wjh.v13.i2.218
39. Geervliet E, Bansal R. Matrix Metalloproteinases as Potential Biomarkers and Therapeutic Targets in Liver Diseases. Cells (2020) 9(5):1212. doi: 10.3390/cells9051212
40. Mak KM, Sehgal P, Harris CK. Type VI Collagen: Its Biology and Value as a Biomarker of Hepatic Fibrosis. Austin Biomark Diagn (2014) 1(2):9. doi: 10.1007/978-94-007-7675-3_6
41. Terada T, Okada Y, Nakanuma Y. Expression of Matrix Proteinases During Human Intrahepatic Bile Duct Development. A Possible Role in Biliary Cell Migration. Am J Pathol (1995) 147:1207–13.
42. Si-Tayeb K, Monvoisin A, Mazzocco C, Lepreux S, Decossas M, Cubel G, et al. Matrix Metalloproteinase 3 Is Present in the Cell Nucleus and Is Involved in Apoptosis. Am J Pathol (2006) 169:1390–401. doi: 10.2353/ajpath.2006.060005
43. Benyon RC, Arthur MJ. Extracellular Matrix Degradation and the Role of Hepatic Stellate Cells. Semin Liver Dis (2001) 21:373–84. doi: 10.1055/s-2001-17552
44. Arthur MJ. Fibrogenesis II Metalloproteinases and Their Inhibitors in Liver Fibrosis. Am J Physiol Gastrointest Liver Physiol (2000) 279:G245–9. doi: 10.1152/ajpgi.2000.279.2.G245
45. Nagase H, Visse R, Murphy G. Structure and Function of Matrix Metalloproteinases and TIMPs. Cardiovasc Re (2006) 69(3):562–73. doi: 10.1016/j.cardiores.2005.12.002
46. Cursio R, Mari B, Louis K, Rostagno P, Saint-Paul MC, Giudicelli J, et al. Rat Liver Injury Following Normothermic Ischemia Is Prevented by a Phosphinic Matrix Metalloproteinase Inhibitor. FASEB J (2002) 16:93–5. doi: 10.1096/fj.01-0279fje
47. Okamoto K, Mimura K, Murawaki Y, Yuasa I. Association of Functional Gene Polymorphisms of Matrix Metalloproteinase (MMP)-1, MMP-3 and MMP-9 With the Progression of Chronic Liver Disease. J Gastroenterol Hepatol (2005) 20:1102–8. doi: 10.1111/j.1440-1746.2005.03860.x
48. Lichtinghagen R, Breitenstein K, Arndt B, Kühbacher T, Böker KH. Comparison of Matrix Metalloproteinase Expression in Normal and Cirrhotic Human Liver. Virchows Arch (1998) 432:153–8. doi: 10.1007/s004280050149
49. Okamoto K, Mandai M, Mimura K, Murawaki Y, Yuasa I. The Association of MMP-1, -3 and -9 Genotypes With the Prognosis of HCV-Related Hepatocellular Carcinoma Patients. Res Commun Mol Pathol Pharmacol (2005) 117-118:77–89.
50. Okamoto K, Ishida C, Ikebuchi Y, Mandai M, Mimura K, Murawaki Y, et al. The Genotypes of IL-1 Beta and MMP-3 Are Associated With the Prognosis of HCV-Related Hepatocellular Carcinoma. Intern Med (2010) 49:887–95. doi: 10.2169/internalmedicine.49.3268
51. European Association for the Study of the Liver. EASL Clinical Practice Guidelines; Management of Cholestatic Liver Diseases. J Hepatol (2009) 51:237–67. doi: 10.1016/j.jhep.2009.04.009
52. European Association for the Study of the LiverEASL Clinical Practice Guidelines: The Diagnosis and Management of Patients With Primary Biliary Cholangitis. J Hepatol (2017) 67:145–72. doi: 10.1016/j.jhep.2017.03.022
53. Wan J, Zhang G, Li X, Qiu X, Ouyang J, Dai J, et al. Matrix Metalloproteinase 3: A Promoting and Destabilizing Factor in the Pathogenesis of Disease and Cell Differentiationuly. Front Physiol (2021) 12:66397812. doi: 10.3389/fphys.2021.66397812
54. Zucker S, Lysik RM, Zarrabi MH, Greenwald RA, Gruber B, Tickle S, et al. Elevated Plasma Stromelysin Levels in Arthritis. J Rheumatol (1994) 21(12):2329–33.
55. Kotajima L, Aotsuka S, Fujimani M, Okawa-Takatsuji M, Kinoshita M, Sumiya M, et al. Increased Levels of Matrix Metalloproteinase-3 in Sera From Patients With Active Lupus Nephritis. Clin Exp Rheumatol (1998) 16(4):409–15.
56. Lernera A, Neidhöfer S, Reuter S, Matthias T. MMP3 is a Reliable Marker for Disease Activity, Radiological Monitoring, Disease Outcome Predictability, and Therapeutic Response in Rheumatoid Arthritis. Best Pract Res Clin Rheumatol (2018) 32(4):550–62. doi: 10.1016/j.berh.2019.01.006
57. Tuncer T, Kaya A, Gulkesen A, Kal GA, Kaman D, Akgol G. Matrix Metalloproteinase-3 Levels in Relation to Disease Activity and Radiological Progression in Rheumatoid Arthritis. Adv Clin Exp Med (2019) 28(5):665–70. doi: 10.17219/acem/94065
58. Luckman SP, Gilhus NE, Romi F. Clinical Study Matrix Metalloproteinase-3 in Myasthenia Gravis Compared to Other Neurological Disorders and Healthy Controls. Autoimmune Dis (2011) 2011:151258. doi: 10.4061/2011/151258
59. Bhattacharyya P, Nag S, Bardhan S, Acharya D, Paul R, Dey R, et al. The Role of Long-Term Doxycycline in Patients of Idiopathic Pulmonary Fibrosis: The Results of an Open Prospective Trial. Lung India (2009) 26:81–5. doi: 10.4103/0970-2113.53231
60. Honsawek S, Praianantathavorn K, Chongsrisawat V, Vejchapipat P, Theamboonlers A, Poovorawan Y. High Serum Matrix Metalloproteinase-3 and Liver Stiffness in Postoperative Biliary Atresia. Pediatr Surg Int (2011) 27:681–7. doi: 10.1007/s00383-010-2816-x
61. Lammers WJ, van Buuren HR, Hirschfield GM, Janssen HLA, Invernizzi P, Mason AL, et al. Levels of Alkaline Phosphatase and Bilirubin Are Surrogate End Points of Outcomes of Patients With Primary Biliary Cirrhosis: An International Follow-Up Study. Gastroenterology (2014) 147(6):1338. doi: 10.1053/j.gastro.2014.08.029
62. Perez CFM, Harms MH, Lindor KD, van Buuren HR, Hirschfield GM, Corpechot C, et al. Goals of Treatment for Improved Survival in Primary Biliary Cholangitis: Treatment Target Should Be Bilirubin Within the Normal Range and Normalization of Alkaline Phosphatase. Am J Gastroenterol (2020) 115(7):1066–74. doi: 10.14309/ajg.0000000000000557
63. Heljasvaara R, Nyberg P, Luostarinen J, Parikka M, Heikkilä P, Rehn M, et al. Generation of Biologically Active Endostatin Fragments From Human Collagen XVIII by Distinct Matrix Metalloproteases. Exp Cell Res (2005) 307:292–304. doi: 10.1016/j.yexcr.2005.03.021
64. Richter AG, McKeown S, Rathinam S, Harper L, Rajesh P, McAuley DF. Soluble Endostatin Is a Novel Inhibitor of Epithelial Repair in Idiopathic Pulmonary Fibrosis. Thorax (2009) 64:156–61. doi: 10.1136/thx.2008.102814
65. Hemmann S, Graf J, Roderfeld M, Roeb E. Expression of MMP and TIMP in Liver Fibrosis - A Systematic Review With Special Emphasis on Anti-Fibrotic Strategies. J Hepatol (2007) 46:955–75. doi: 10.1016/j.jhep.2007.02.003
66. Yamashita CM, Dolgonos L, Zemans RL, Young SK, Robertson J, Briones N, et al. Matrix Metalloproteinase 3 Is a Mediator of Pulmonary Fibrosis. Am J Pathol.; (2011) 179:1733–45. doi: 10.1016/j.ajpath.2011.06.041
67. Willems S, Verleden SE, Vanaudenaerde BM, Wynants M. Multiplex Protein Profiling of Bronchoalveolar Lavage in Idiopathic Pulmonary Fibrosis and Hypersensitivity Pneumonitis. Ann Thorac Med (2013) 8:38–45. doi: 10.4103/1817-1737.105718
Keywords: primary biliary cholangitis, liver fibrosis, autoantibodies, metalloproteinases, MMP-3
Citation: Bauer A and Habior A (2022) Concentration of Serum Matrix Metalloproteinase-3 in Patients With Primary Biliary Cholangitis. Front. Immunol. 13:885229. doi: 10.3389/fimmu.2022.885229
Received: 27 February 2022; Accepted: 29 March 2022;
Published: 22 April 2022.
Edited by:
Alessandro Granito, University of Bologna, ItalyReviewed by:
Lindsey Kennedy, Indiana University Bloomington, United StatesLinda Beenet, University of California, Los Angeles, United States
Copyright © 2022 Bauer and Habior. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alicja Bauer, alicja.bauer@cmkp.edu.pl
 Alicja Bauer
Alicja Bauer