
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 02 March 2022
Sec. Autoimmune and Autoinflammatory Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.823863
This article is part of the Research Topic The Innate Immune System in Rheumatoid Arthritis View all 6 articles
The aryl hydrocarbon receptor (AHR) signaling pathway participates in immune regulation of multiple autoimmune diseases, including rheumatoid arthritis (RA). We conducted this study to investigate the association of AHR signaling pathway genes (AHR, ARNT, AHRR) single nucleotide polymorphisms (SNPs), as well as their methylation levels, with RA susceptibility. Nine SNPs (AHR gene rs2066853, rs2158041, rs2282885, ARNT gene rs10847, rs1889740, rs11204735, AHRR gene rs2292596, rs2672725, rs349583) were genotyped via improved multiple ligase detection reaction (iMLDR) in 479 RA patients and 496 healthy controls. We used the Illumina Hiseq platform to detect methylation levels of these genes in 122 RA patients and 123 healthy controls. A significant increase in rs11204735 C allele frequency was observed in RA patients when compared to controls. Further, rs11204735 polymorphism was associated with a decreased risk of RA under the dominant model. ARNT CCC haplotype frequency was significantly increased in RA patients in comparison to controls. In the AHRR gene, rs2672725 GG genotype, G allele frequencies were significantly related to an increased risk of RA and rs2292596, rs2672725 polymorphism were significantly associated with an increased risk of RA under the dominant model, recessive model, respectively. However, no significant association was identified between AHR gene polymorphism and RA susceptibility. The AHR methylation level in RA patients was significantly higher than the controls, while AHRR methylation level was abnormally reduced in RA patients. In addition, AHRR rs2672725 genotype distribution was significantly associated with the AHRR methylation level among RA patients. In summary, ARNT rs11204735, AHRR rs2292596, and rs2672725 polymorphisms were associated with RA susceptibility and altered AHR, AHRR methylation levels were related to the risk of RA.
Rheumatoid arthritis (RA) is a common, chronic autoimmune disease that affects approximately 1% of the general population (1). The main clinical features of RA patients are synovial inflammation, bone and cartilage erosion, and symmetric polyarthritis with joint swelling (2), however, the pathogenesis of this disease is not fully understood. In previous studies, the immunological dysfunctions of RA were identified in various immune cells, such as T cells, B cells, macrophages, and abnormal expression levels of inflammatory cytokines, including IL-1, IL-6, IL-10, TNF-α, were observed in RA patients (3). In addition to immunological findings, many studies have also found an influence of genomic and epigenomic features, and environmental factors on disease activity, prognosis, and therapy prediction of RA (4–6). Genome wide association study and candidate gene studies have identified a large number of RA related genes/loci in different ethnic groups, however, these genes/loci only account for a small part of RA phenotypic variation (7, 8). DNA methylation is a widely studied form of epigenetic modification, and some prior studies have suggested that an association between DNA methylation and inflammation-regulated immune pathways may play an important role in the pathogenesis of RA (9).
Previous studies confirmed that environmental pollutants from smoking and hydrocarbon burning were associated with RA disease risk, as both pollutants contained agonists or exogenous ligands for aryl hydrocarbon receptor (AHR) that may promote AHR activation (10). As a ligand-activated transcription factor, AHR is involved in regulating the gene activity in response to hydrophobic halogenated aromatic hydrocarbons in order to promote the metabolism, and elimination of xenobiotics. The role of AHR in cellular responses against a variety of endogenous and physiological ligands have been extensively studied, while increasing evidence indicates that AHR is involved in the regulation of innate and adaptive immune responses, and immunologic processes in autoimmune disease, including multiple sclerosis (MS) (11, 12). For example, AHR expression is abnormally elevated in synovial tissues of RA patients and could regulate the expression of cytokines such as growth factors (13). Over-activation of AHR signaling may promote inflammation and bone destruction in RA by activating macrophages, osteoclasts, dendritic cells, and inhibiting osteoblasts (10).
In the regulation of the AHR signaling pathway, ligand binding to AHR causes conformational changes that induce this receptor to migrate to the nucleus where it can generate a hetero dimer with AHR nuclear translocation protein (ARNT) to form aryl hydrocarbon receptor complex, inducing the transcription of relevant target genes (14). In addition, AHR repressor (AHRR) serves as a negative regulator within this pathway through inhibiting AHR-mediated signal transduction (15). Recent studies have investigated the association of common, potentially functional single nucleotide polymorphisms (SNPs) in AHR signaling pathway genes with MS, essential hypertension (14, 16). Given the importance of the AHR signaling pathway in the development of RA, this present study aimed to evaluate the association between SNPs in the AHR signaling pathway genes (AHR, ARNT, AHRR) and RA susceptibility. The methylation levels of these genes were also detected in these patients because DNA methylation is considered to be an important factor for RA.
In this study, the participant included 479 RA patients and 496 healthy controls that were recruited to explore the association between AHR, ARNT, AHRR genes SNP and RA susceptibility. Of which 122 RA patients and 123 healthy controls were selected to simultaneously detect DNA methylation levels of AHR, ARNT, AHRR genes (Table 1). All patients were diagnosed by experienced clinicians according to the 1987 American College of Rheumatology revised criteria (17), and patients with other autoimmune diseases, infectious diseases, tumors were excluded from this study. Volunteers with no history of inflammatory/autoimmune diseases, cancers, etc. were recruited as healthy controls. The DNA samples, demographic data of all subjects were obtained from Anhui Provincial Laboratory of Inflammatory and Immune Diseases, and the clinical data of RA patients including anti-cyclic citrullinate peptide (anti-CCP) and rheumatoid factor (RF) was also recorded.
First, we used the public database (CHBS_1000G, Ensembl Genome Browser 85) to obtain the genotype data of the Han population in Beijing, China. Then, we screened tag SNPs capturing all the common SNPs located in the chromosomal locations of AHR signaling pathway genes (AHR, ARNT, AHRR) and the 2.0 kbp region of their flank. The selection was conducted using the Haploview 4.0 software (Cambridge, MA, USA). Next, we searched for the SNP in AHR, ARNT and AHRR genes that were significantly associated with other diseases, and other potentially functional SNP. Finally, our study selected three tagSNPs (rs2066853, rs2158041, rs2282885) in AHR, three tagSNPs (rs10847, rs1889740, rs11204735) in ARNT, three tagSNPs (rs2292596, rs2672725, rs349583) in AHRR for genotyping. All SNPs were consistent with MAF ≥ 0.05 in CHB and r2 threshold > 0.8.
Genotyping was performed with improved multiple ligase detection reaction (iMLDR) under the technical support from the Center for Genetic & Genomic Analysis, Genesky Biotechnologies (Inc., Shanghai, China) (18). The detailed experimental steps were as follows: (1) 1μl DNA sample was extracted, and the quality of the sample was checked and the concentration was estimated by 1% agarose electrophoresis. Then the DNA sample was diluted to 5-10 ng/μl according to the estimated concentration. (2) Multiplex PCR reaction was carried out with 20μl reaction system included 1x HotStarTaq bufer, 3.0 mMMg2+, 0.3mM dNTP, 1U HotStarTaq polymerase (Qiagen Inc.), 1μl sample DNA and 1μl multiple PCR primers. (3) Purifcation of multiple PCR products: 5U SAP enzyme and 2U Exonuclease I enzyme were added to 20 μl PCR product, 37°C warm bath for 1h, then 75°C inactivated for 15min. (4) Ligating reaction system: 1 μl 10x ligating bufer, 0.25 μl high temperature ligase, 0.4 μl 5’ ligating primer mixture (1μM), 0.4 μl primer 3’ ligating primer mixture (2 μM), 2 μl purified multiple PCR products, 6 μl ddH2O mixing. (5) The 0.5 μl diluted product was mixed with 0.5 μl Liz500 SIZE STANDARD, 9 μl Hi-Di, denatured at 95°C for 5 min, then placed on the ABI3730XL sequencer. (6) The raw data collected on the ABI3730XL sequencer are analyzed by GeneMapper 4.1 (AppliedBiosystems, USA). The subjects with 100% genotyping success rate for all SNPs were included for final analysis.
The methylation levels of AHR, ARNT, AHRR genes were detected by MethylTarget® with technical support from the Center for Genetic & Genomic Anal-ysis, Genesky Biotechnologies (Inc., Shanghai, China) (19). We sequenced the CpG islands in the promoter region of AHR, ARNT, AHRR genes using the Illumina Hiseq platform. Primers were designed to amplify the specific sites of interest from the bisulfite-converted DNA (Table S1), and the mean methylation level of all CpG sites on the fragment was calculated as the methylation level of the specific sites of each gene.
The detailed experimental steps were as follows: (1) Genomic DNA (400ng) was subjected to sodium bisulfite treatment using EZ DNA Methylation™-GOLD Kit (Zymo Research) according to manufacturer’s protocols. (2) 2 μl bisulfite DNA was prepared for multiplex PCR reaction in 20 μl reaction mixture, included 1x reaction buffer (Takara), 1 U HotStarTaq polymerase (Takara), 3 mM Mg2+, 0.2 mM dNTP, and 0.1 µM of each primer for PCR amplification. The cycling program was as follows: 95°C for 2 min; 11 cycles of 94°C for 20 s, 63°C for 40s with a decreasing temperature step of 0.5°C per cycle, and 72°C for 1 min; subsequently 24 cycles of 94°C for 20 s, 65°C for 30 s, and 72°C for 1 min; and 72°C for 2 min. (3) 1 μl diluted PCR amplicons were used for index PCR reaction in 20 μl mixture, containing 1x reaction buffer (NEB Q5™), 1 U Q5™ DNA polymerase (NEB), 0.3 mM dNTP, 0.3 mM of F primer, and 0.3 μM of index primer. The cycling program was 98°C for 30 s, 11 cycles of 98°C for 10 s, 65°C for 30 s, and 72°C for 30 s; and 72°C for 5 min. (4) PCR amplicons (170bp-270bp) were separated by agarose electrophoresis and purified using QIAquick Gel Extraction kit (QIAGEN) and then loaded onto Illumina NextSeq 500 (Illumina, San Diego, CA, USA) according to the manufacturer’s protocols.
Statistical analysis was conducted with the SPSS 23.0 (Armonk, NY: IBM Corp, USA). Hardy–Weinberg equilibrium (HWE) was calculated in RA patients and healthy controls by using Chi-square (χ2). Genotype, allele distribution frequencies were compared using logistic regression analysis, and two genetic models (dominant, recessive) were also tested. The following statistical indexes, including P values, odds ratios (OR), and 95% confidence intervals (CI), were analyzed as measures of associations. Haplotype analysis for each gene was calculated using the SHEsis software. The methylation levels of candidate genes were expressed as mean and standard deviation, and the differences of methylation levels between two groups and three groups were conducted using T-test and variance analysis, respectively. P values < 0.05 were considered as statistically significant for all tests. The Bonferroni correction was used for multiple testing.
In this study, the mean age of the 479 RA patients was 52.71 ± 12.42 years, including 391 females and 88 males, and the average age of the 496 healthy controls was 50.61 ± 14.76 years, including 384 females and 112 males. The genotype distributions of all SNPs in RA patients were in accordance with HWE (rs2066853, P = 0.997; rs2158041, P = 0.792; rs2282885, P = 0.555; rs10847, P = 0.747; rs1889740, P = 0.530; rs11204735, P = 0.914; rs2292596, P = 0.713; rs2672725, P = 0.346; rs349583, P = 0.913). In healthy controls, AHR rs2158041 did not conform to HWE (P = 0.023), while other SNPs conformed to HWE (rs2066853, P = 0.249; rs2282885, P = 0.401; rs10847, P = 0.871; rs1889740, P = 0.461; rs11204735, P = 0.603; rs2292596, P = 0.373; rs2672725, P = 0.918; rs349583, P = 0.855). Therefore, this study excluded rs2158041, and the allele and genotype frequency distributions of other SNPs in RA patients and healthy controls were shown in Tables 2, 3.
The genotype frequencies of AHR rs2066853, rs2282885 polymorphism were similar between RA patients and healthy controls with no significant association, moreover there was no significant difference in allele distributions of the AHR rs2066853, rs2282885 between RA patients and healthy controls. In the ARNT gene, we determined that the rs11204735 C allele frequency was significantly increased in RA patients when compared to healthy controls (C versus T: P = 0.037), while rs11204735 polymorphism was found to have a decreased risk of RA under the dominant model (TT versus CT+CC: P = 0.023). The rs11204735 CT genotype frequency was also increased in RA patients, while the significant association disappeared after multiple testing by Bonferroni correction (CT versus TT: P > 0.0167). In addition, no significant association was identified with AHR rs10847, rs1889740 in RA patients.
When investigating the genotype and allele frequencies of rs2292596, rs2672725, rs349583 in AHRR, we demonstrated that the rs2292596 polymorphism was significantly associated with an increased risk of RA under dominant model (CC versus GC+GG: P = 0.032). The rs2292596 GC genotype frequency appeared to be lower in RA patients than that in healthy controls, however, the difference was not statistically significant by Bonferroni correction (GC versus CC: P > 0.0167). The results also showed that rs2672725 GG genotype, G allele frequencies in RA patients were significantly higher than the healthy controls (GG versus CC: P = 0.011, G versus C: P = 0.010). Moreover, an increased risk of rs2672725 was found under the recessive model (GG versus GC+CC: P = 0.016). However, there was no significant difference in genotype, allele distributions of AHRR rs349583 between RA patients and healthy controls.
Our study also analyzed the potential association between AHR, ARNT, AHRR genes polymorphisms and anti-CCP, RF status in RA patients (Table S2). None of the SNPs exhibited significant differences between anti-CCP-positive RA patients and anti-CCP-negative RA patients, as well as RA patients with RF-positive and with RF-negative.
This study constructed the haplotypes of AHR, ARNT, AHRR genes by SHEsis software, and three main haplotypes (AA, GA, GG) for AHR gene, four main haplotypes (CCC, CTC, CTT, TCC) for ARNT gene, six main haplotypes (CCA, CCG, CGA, CGG, GCA, GCG) for AHRR gene were detected by SHEsis (Table 4). We found that the ARNT CCC haplotype frequency was significantly increased in RA patients when compared to healthy controls (P = 0.048).
We enrolled 122 RA patients and 123 healthy controls from the subjects in the genotyping experiment for detecting methylation. The RA group included 100 females and 22 males, with an average age of 52.61 ± 13.05 years, and the control group included 82 females and 41 males with an average age of 46.93 ± 14.29 years. The final aspect of this study was to measure the methylation levels of two specific sites in AHR gene, two specific sites in ARNT gene, and a specific site in AHRR gene. As shown in Table 5, AHR_2 methylation level significantly increased in RA patients (P = 0.012), while AHRR_1 methylation level was significantly lower (P = 0.049), when compared to controls.
We further determined the cumulative methylation levels of each gene by calculating the mean methylation levels of all CpG sites on the included specific sites. The results revealed that the methylation level of AHR gene was significantly higher in RA patients (0.0076 ± 0.0006) than the healthy controls (0.0073 ± 0.0007) (P = 0.020). Our study only examined methylation levels of a specific site in the AHRR gene. Consistent with AHRR_1, AHRR methylation level was abnormally reduced in RA patients (0.9632 ± 0.0058) when compared to healthy controls (0.9644 ± 0.0038) (P = 0.049). There was no significant difference in ARNT methylation level between RA patients (0.0102 ± 0.0015) and healthy controls (0.0101 ± 0.0016) (P = 0.538) (Figure 1). The results also suggested that ARNT methylation level was significantly increased in anti-CCP-positive RA patients (0.0104 ± 0.0015) in comparison to anti-CCP-negative RA patients (0.0090 ± 0.0013) (P = 0.002). In addition, ARNT_1 methylation level in anti-CCP-positive RA patients than that in anti-CCP-negative RA patients (0.0116 ± 0.0024) (P = 0.002). However, AHR, AHRR methylation levels were not associated with anti-CCP, RF in RA patients (Table 6).
This study explored the associations between AHR, ARNT, AHRR genes methylation levels and their different genotype in 122 RA patients (Table 7). The results demonstrated a significant difference in methylation level of AHRR among different genotypes of AHRR rs2672725 polymorphism in RA patients (P = 0.012). After multiple comparisons, the AHRR methylation level in RA patients with rs2672725 GC (0.9615 ± 0.0076) was significantly lower than RA patients with rs2672725 CC (0.9649 ± 0.0026) (P < 0.0167). No statistical difference in the methylation level of AHR, ARNT in RA patients with their disparate genotypes was recorded (Table 7).
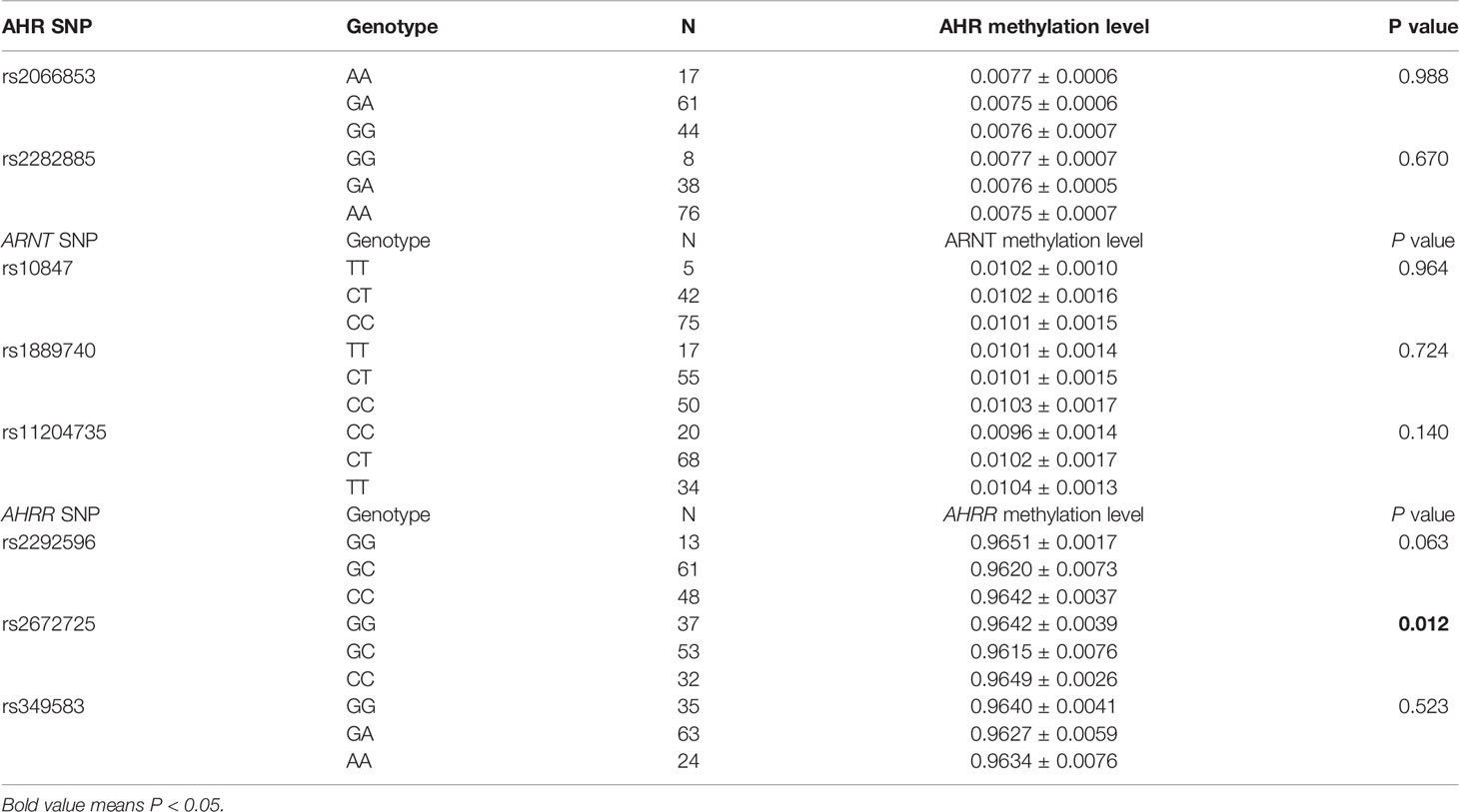
Table 7 Associations between AHR, ARNT, AHRR genes polymorphisms with their methylation levels in RA patients.
The impact of environmental factors on the pathogenesis of autoimmune diseases has attracted increasing attention (20). AHR is considered to be a crucial regulator of host-environment interactions, especially in immune and inflammatory responses (21, 22). Proper regulation of AHR-mediated signal transduction might be critical for maintaining immune cell function in autoimmunity, while abnormal AHR signaling has been closely related to the pathogenesis of autoimmune diseases (13). Clarifying the role of AHR in disease and health is crucial in understanding the disease occurrence process and individual differences in treatment response, both of which might contribute to the development of novel therapies (23). RA is a common autoimmune disease and AHR plays a role in regulating the response to the environment factors and may contribute to disease development. Clinical studies had demonstrated that AHR expression was approximately two-fold higher in RA patients than in controls (24). AHRR expression of the synovia was significantly increased from RA patients who smoke cigarettes, but not from the patients who do not smoke, which indicated that there was a potential interaction between AHR activation and cigarette smoking in RA patients (25). The murine models of RA suggested that AHR activation with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) could contribute to RA disease severity, disease progression, osteoclasts differentiation, and increase the numbers of IL17-expressing cells in the inflamed joints (10). In addition, previous studies had shown that several SNPs located near AHR-regulated genes might contribute to AHR-dependent disease mechanisms (26). Therefore, we investigated the association between multiple SNPs in AHR signaling pathway genes (AHR, ARNT, AHRR) and RA genetic risk, and detected the methylation levels of these genes in RA patients.
A large number of studies had discussed the association of the genetic polymorphism of AHR, ARNT, AHRR genes and human diseases such as Crohn’s disease (CD), lung cancer, male infertility, and autoimmune diseases (27–30). Zorlu et al. evaluated the relationship between multiple SNPs in the AHR signaling pathway genes, including AHR, ARNT, AHRR and MS susceptibility, and their results inferred that the AHR signaling pathway genetic variation might contribute to MS pathogenesis (14). However, our study did not indicate a significant association of AHR rs2066853, rs2282885 polymorphism with RA susceptibility, and rs2158041 was excluded as it did not satisfy HWE. Consistent with our findings, Cheng et al. demonstrated that rs2066853 polymorphism did not have any effect on RA susceptibility in Han Chinese populations (31). These results suggested that AHR gene variation might not be associated with RA, however, other functional SNPs required further investigation.
Our study also determined that ARNT rs11204735 polymorphism was significantly associated with an increased risk of RA, and that a decreased risk of rs11204735 polymorphism was observed under the dominant model. This result was similar to Schurman’s study (30), and further reflected the importance of rs11204735 polymorphism in the pathogenesis of autoimmune diseases. Haplotype analysis demonstrated that ARNT CCC haplotype frequency significantly increased in RA patients in comparison to controls. Therefore, this present study provided additional evidence that ARNT gene was an important susceptibility gene in RA, and reproducibility studies were needed to verify our results. A previous study demonstrated that rs2292596 genotype frequency was significant with genetic susceptibility to RA patients (31). Their study also demonstrated that the rs2292596 G allele might have a dangerous effect on RA (31), while we identified an increased frequency of rs2292596 G allele with no statistical significance. This difference might be due to sample size and experimental methods. Our result was also the first to show that AHRR rs2672725 GG genotype, G allele frequencies in RA patients were significantly increased, and that this SNP could be a novel susceptibility locus to RA.
In addition to DNA sequence, epigenetic variations also contained important genetic information, and the key role of epigenetic variation in the pathogenesis of human disease could not be ignored (32, 33). For example, DNA hypomethylation was associated with differentiation and proliferation of inflammatory processes, resulting in the increased transcription and secretion of inflammatory proteins (34). At present, studying the role of DNA methylation in autoimmune diseases offers an interesting perspective. One study detected the methylation status of lymphocytes in patients with systemic lupus erythematosus, RA, and found a significant hypomethylation in T cells (35). Due to the possible role of AHR, ARNT, AHRR genes in RA pathogenesis, we also examined the methylation status of these genes in RA patients. The results revealed that AHR methylation level was significantly increased, while AHRR methylation level was abnormally reduced in RA patients. These data confirmed that AHR, AHRR genes were involved in the development of RA, and provided valuable information for further revealing RA pathogenesis. RA patients could be divided into different subgroups according to the status of autoantibodies, including RF and anti-CCP, which was of great significance when selecting appropriate treatments. Our study also determined that ARNT methylation level was related to the anti-CCP status of RA patients. In addition, we also revealed that AHRR rs2672725 polymorphism was significantly associated with AHRR methylation level in RA patients. We assumed that rs2672725 might be involved in the pathogenesis of RA by affecting AHRR methylation level, however further verification was needed.
Our study had several limitations. First, the sample size was insufficient and might affect the power of this study. Second, this study failed to analyze the influence of the interaction between environmental factors and gene variation on RA occurrence due to a lack of information on environmental factors. Undoubtedly, a complete understanding of the precise role of AHR signaling pathway genes in RA pathogenesis will require additional experiments and clinical studies with larger sample sizes.
In conclusion, our results provided strong evidence that the ARNT gene rs11204735, AHRR gene rs2292596, rs2672725 polymorphisms were related to RA susceptibility in the Chinese population, while AHR genetic variation might not be associated with RA risk. These three SNPs might modify individual genetic susceptibility to RA, which could provide a basis for early detection of RA susceptible individuals. Furthermore, an increased methylation level of AHR, as well as a decreased methylation level of AHRR, was identified in RA patients, and ARNT methylation level was related to anti-CCP in RA patients. We can assume that AHR, AHRR methylation level might be used as novel auxiliary biomarkers for RA and ARNT methylation level could be used to distinguish the different serotypes of RA.
The data presented in the study are deposited in the dbSNP repository, accession number 1063327. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Ethical Committee of the First Affiliated Hospital of USTC (Hefei, Anhui, China). The patients/participants provided their written informed consent to participate in this study.
X-ML and G-SW designed the study. T-PZ and H-ML conducted the experiment. RL performed the statistical analyses. H-ML performed the statistical analyses. NX and ZT participated in the collection of samples. T-PZ drafted the manuscript. X-ML, G-SW and RL contributed to manuscript revision. All authors contributed to the article and approved the submitted version.
This work was supported by the Fundamental Research Funds for the Central Universities (WK9110000180, WK9110000148), Anhui Provincial Natural Science Foundation (2108085QH362), and National Natural Science Foundation of China (81871271).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We sincerely thank all the participants in this project.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.823863/full#supplementary-material
1. Rossini M, Rossi E, Bernardi D, Viapiana O, Gatti D, Idolazzi L, et al. Prevalence and Incidence of Rheumatoid Arthritis in Italy. Rheumatol Int (2014) 34:659–64. doi: 10.1007/s00296-014-2974-6
2. Croia C, Bursi R, Sutera D, Petrelli F, Alunno A, Puxeddu I. One Year in Review 2019: Pathogenesis of Rheumatoid Arthritis. Clin Exp Rheumatol (2019) 37(3):347–57.
3. Nguyen NT, Nakahama T, Kishimoto T. Aryl Hydrocarbon Receptor and Experimental Autoimmune Arthritis. Semin Immunopathol (2013) 35:637–44. doi: 10.1007/s00281-013-0392-6
4. Klein K, Gay S. Epigenetics in Rheumatoid Arthritis. Curr Opin Rheumatol (2015) 27:76–82. doi: 10.1097/BOR.0000000000000128
5. Zhang TP, Li R, Huang Q, Pan HF, Ye DQ, Li XM. Association of NCF2, NCF4, and CYBA Gene Polymorphisms With Rheumatoid Arthritis in a Chinese Population. J Immunol Res (2020) 2020:8528976. doi: 10.1155/2020/8528976
6. Adami G, Viapiana O, Rossini M, Orsolini G, Bertoldo E, Giollo A, et al. Association Between Environmental Air Pollution and Rheumatoid Arthritis Flares. Rheumatol (Oxf) (2021) 60:4591–97. doi: 10.1016/j.bonr.2021.100797
7. Kwon YC, Lim J, Bang SY, Ha E, Hwang MY, Yoon K, et al. Genome-Wide Association Study in a Korean Population Identifies Six Novel Susceptibility Loci for Rheumatoid Arthritis. Ann Rheum Dis (2020) 79:1438–45. doi: 10.1136/annrheumdis-2020-217663
8. Zhang TP, Zhang Q, Wu J, Zhao YL, Wang JB, Leng RX, et al. The Expression Levels of Long Noncoding RNAs Lnc0640 and Lnc5150 and Its Gene Single-Nucleotide Polymorphisms in Rheumatoid Arthritis Patients. J Cell Biochem (2018) 119:10095–106. doi: 10.1002/jcb.27346
9. Liebold I, Grützkau A, Göckeritz A, Gerl V, Lindquist R, Feist E, et al. Peripheral Blood Mononuclear Cells Are Hypomethylated in Active Rheumatoid Arthritis and Methylation Correlates With Disease Activity. Rheumatol (Oxf) (2021) 60:1984–95. doi: 10.1093/rheumatology/keaa649
10. Fu J, Nogueira SV, Drongelen VV, Coit P, Ling S, Rosloniec EF, et al. Shared Epitope-Aryl Hydrocarbon Receptor Crosstalk Underlies the Mechanism of Gene-Environment Interaction in Autoimmune Arthritis. Proc Natl Acad Sci USA (2018) 115:4755–60. doi: 10.1073/pnas.1722124115
11. Wheeler MA, Rothhammer V, Quintana FJ. Control of Immune-Mediated Pathology via the Aryl Hydrocarbon Receptor. J Biol Chem (2017) 292:12383–9. doi: 10.1074/jbc.R116.767723
12. Hui W, Dai Y. Therapeutic Potential of Aryl Hydrocarbon Receptor Ligands Derived From Natural Products in Rheumatoid Arthritis. Basic Clin Pharmacol Toxicol (2020) 126:469–74. doi: 10.1111/bcpt.13372
13. Wang XS, Cao F, Zhang Y, Pan HF. Therapeutic Potential of Aryl Hydrocarbon Receptor in Autoimmunity. Inflammopharmacology (2020) 28:63–81. doi: 10.1007/s10787-019-00651-z
14. Zorlu N, Hoffjan S, Haghikia A, Deyneko IV, Epplen JT. Evaluation of Variation in Genes of the Arylhydrocarbon Receptor Pathway for an Association With Multiple Sclerosis. J Neuroimmunol (2019) 334:576979. doi: 10.1016/j.jneuroim.2019.576979
15. Vogel CFA, Haarmann-Stemmann T. The Aryl Hydrocarbon Receptor Repressor More Than a Simple Feedback Inhibitor of AhR Signaling: Clues for Its Role in Inflammation and Cancer. Curr Opin Toxicol (2017) 2:109–19. doi: 10.1016/j.cotox.2017.02.004
16. Polonikov AV, Bushueva OY, Bulgakova IV, Freidin MB, Churnosov MI, Solodilova MA, et al. A Comprehensive Contribution of Genes for Aryl Hydrocarbon Receptor Signaling Pathway to Hypertension Susceptibility. Pharmacogenet Genomics (2017) 27:57–69. doi: 10.1097/FPC.0000000000000261
17. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 Revised Criteria for the Classification of Rheumatoid Arthritis. Arthritis Rheum (1988) 31:315–24. doi: 10.1002/art.1780310302
18. Qi Y, Wei Y, Yu F, Lin Q, Yin J, Fu J, et al. Association Study of a Genetic Variant in the Long Intergenic Noncoding RNA (Linc01080) With Schizophrenia in Han Chinese. BMC Psychiatry (2021) 21(1):613. doi: 10.1186/s12888-021-03623-2
19. Chen M, Wu M, Hu X, Yang J, Han R, Ma Y, et al. Ankylosing Spondylitis Is Associated With Aberrant DNA Methylation of IFN Regulatory Factor 8 Gene Promoter Region. Clin Rheumatol (2019) 38(8):2161–9. doi: 10.1007/s10067-019-04505-5
20. Zhao CN, Xu Z, Wu GC, Mao YM, Liu LN, Dan YL, et al. Emerging Role of Air Pollution in Autoimmune Diseases. Autoimmun Rev (2019) 18:607–14. doi: 10.1016/j.autrev.2018.12.010
21. Stockinger B, Di Meglio P, Gialitakis M, Duarte JH. The Aryl Hydrocarbon Receptor: Multitasking in the Immune System. Annu Rev Immunol (2014) 32:403–32. doi: 10.1146/annurev-immunol-032713-120245
22. Beischlag TV, Luis Morales J, Hollingshead BD, Perdew GH. The Aryl Hydrocarbon Receptor Complex and the Control of Gene Expression. Crit Rev Eukaryot Gene Expr (2008) 18:207–50. doi: 10.1615/CritRevEukarGeneExpr.v18.i3.20
23. Thomas R. RelB and the Aryl Hydrocarbon Receptor: Dendritic Cell Tolerance at the Epithelial Interface. Immunol Cell Biol (2013) 91:543–4. doi: 10.1038/icb.2013.51
24. Vogel CF, Khan EM, Leung PS, Gershwin ME, Chang WL, Wu D, et al. Cross-Talk Between Aryl Hydrocarbon Receptor and the Inflammatory Response: A Role for Nuclear Factor κb. J Biol Chem (2014) 289:1866–75. doi: 10.1074/jbc.M113.505578
25. Kazantseva MG, Highton J, Stamp LK, Hessian PA. Dendritic Cells Provide a Potential Link Between Smoking and Inflammation in Rheumatoid Arthritis. Arthritis Res Ther (2012) 14:R208. doi: 10.1186/ar4046
26. Neavin DR, Liu D, Ray B, Weinshilboum RM. The Role of the Aryl Hydrocarbon Receptor (AHR) in Immune and Inflammatory Diseases. Int J Mol Sci (2018) 19. doi: 10.3390/ijms19123851
27. Wu CQ, Lin QR, Ying SJ, Luo JK, Hong WJ, Lin ZJ, et al. Association of Crohn's Disease With Aryl Hydrocarbon Receptor Gene Polymorphisms in Patients From Southeast China. Immunol Invest (2019) 48:809–21. doi: 10.1080/08820139.2019.1569677
28. Budhwar S, Bahl C, Sharma S, Singh N, Behera D. Role of Sequence Variations in AhR Gene Towards Modulating Smoking Induced Lung Cancer Susceptibility in North Indian Population: A Multiple Interaction Analysis. Curr Genomics (2018) 19:313–26. doi: 10.2174/1389202918666170915160606
29. Safarinejad MR, Shafiei N, Safarinejad S. Polymorphisms in Aryl Hydrocarbon Receptor Gene Are Associated With Idiopathic Male Factor Infertility. Reprod Sci (2013) 20:1423–32. doi: 10.1177/1933719113488451
30. Schurman SH, O'Hanlon TP, McGrath JA, Gruzdev A, Bektas A, Xu H, et al. Transethnic Associations Among Immune-Mediated Diseases and Single-Nucleotide Polymorphisms of the Aryl Hydrocarbon Response Gene ARNT and the PTPN22 Immune Regulatory Gene. J Autoimmun (2020) 107:102363. doi: 10.1016/j.jaut.2019.102363
31. Cheng L, Qian L, Wang GS, Li XM, Li XP. Genetic Association of Aromatic Hydrocarbon Receptor and Its Repressor Gene Polymorphisms With Risk of Rheumatoid Arthritis in Han Chinese Populations. Med (Baltimore) (2017) 96:e6392. doi: 10.1097/MD.0000000000006392
32. Manjrekar J. Epigenetic Inheritance, Prions and Evolution. J Genet (2017) 96:445–56. doi: 10.1007/s12041-017-0798-3
33. Shnorhavorian M, Schwartz SM, Stansfeld B, Sadler-Riggleman I, Beck D, Skinner MK. Differential DNA Methylation Regions in Adult Human Sperm Following Adolescent Chemotherapy: Potential for Epigenetic Inheritance. PloS One (2017) 12(2):e0170085. doi: 10.1371/journal.pone.0170085
34. Burmester GR, Feist E, Dorner T. Emerging Cell and Cytokine Targets in Rheumatoid Arthritis. Nat Rev Rheumatol (2014) 10:77–88. doi: 10.1038/nrrheum.2013.168
Keywords: autoimmune disease, aryl hydrocarbon receptor, methylation, single nucleotide polymorphisms, rheumatoid arthritis
Citation: Zhang T-P, Li R, Li H-M, Xiang N, Tan Z, Wang G-S and Li X-M (2022) The Contribution of Genetic Variation and Aberrant Methylation of Aryl Hydrocarbon Receptor Signaling Pathway Genes to Rheumatoid Arthritis. Front. Immunol. 13:823863. doi: 10.3389/fimmu.2022.823863
Received: 28 November 2021; Accepted: 01 February 2022;
Published: 02 March 2022.
Edited by:
Ali Abdul-Sater, York University, CanadaReviewed by:
Jose Artur Chies, Federal University of Rio Grande do Sul, BrazilCopyright © 2022 Zhang, Li, Li, Xiang, Tan, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-Mei Li, bGl4aWFvbWVpQHVzdGMuZWR1LmNu; bGl4aWFvbWVpLmZzbXlAYWxpeXVuLmNvbQ==; Guo-Sheng Wang, Z3N3YW5nMDU1MUB1c3RjLmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.