
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol. , 17 March 2022
Sec. Cancer Immunity and Immunotherapy
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.815598
This article is part of the Research Topic Factors Determining Long Term Anti-Tumor Responses to Immune Checkpoint Blockade Therapy View all 21 articles
There is currently a lack of effective systemic treatment for patients with advanced pleomorphic rhabdomyosarcoma (PRMS). Although programmed death protein 1 (PD-1) inhibitors have shown efficacy in various solid tumors, their effects on PRMS have not been well established. Here, we present a case of a 12-year-old Chinese male adolescent with metastatic PRMS who benefited from the PD-1 inhibitor nivolumab. The patient initially underwent primary tumor resection but failed to respond to subsequent first-line chemotherapy and second-line pazopanib treatment. Pathological examination showed positive PD-L1 expression and tumor-infiltrating lymphocytes in the tumor tissue, and the patient was administered nivolumab as a posterior-line treatment. After attaining a clinically partial response (PR), surgical resection was performed, which was followed by adjuvant nivolumab. At the time of the submission of this manuscript, the patient achieved recurrence-free survival (RFS) lasting 45 months and counting. This is the first clinical evidence that a patient with refractory PRMS was controlled by anti-PD-1 antibody, with an RFS lasting more than 3 years. This case suggests that PD-L1 expression and T-cell infiltration could be used as potential biomarkers for PRMS immunotherapy.
Rhabdomyosarcoma (RMS), which originates from the mesenchymal tissue, is the most common type of soft tissue sarcoma (STS) that occurs in childhood and adolescence (1). According to the World Health Organization classification of soft tissue tumors, RMS can be classified into four histological subtypes: alveolar RMS (ARMS), embryonal RMS (ERMS), spindle cell/sclerosing RMS (SRMS), and pleomorphic RMS (PRMS) (2). In comparison to the other RMS subtypes, PRMS is associated with a poor response to standard chemotherapy and a poor prognosis for both local and metastatic disease (3). Nonetheless, anthracycline-based chemotherapy remains the first-line standard treatment for advanced PRMS (4, 5). Pazopanib and regorafenib are multitarget tyrosine kinase inhibitors (TKIs) that are recommended as second-line treatment options for nonspecific STS, including PRMS (6).
Monoclonal antibodies against programmed death-1 (PD-1) and programmed death-ligand 1 (PD-L1) could help activate cytolytic T lymphocytes by blocking the PD-L1/PD-1 signaling pathway to prevent tumors from achieving immune evasion. Immune checkpoint inhibitor (ICI)-based immunotherapy has shown promising efficacy in various malignancies (7–9). Evidence regarding ICI efficacy in RMS remains scarce (10). A multicenter phase 2 clinical trial showed that ICIs exhibited promising efficacy and an acceptable safety profile in advanced STS (11). However, a retrospective study revealed that RMS showed no response to the anti-PD-1 antibody pembrolizumab (12). A phase I/II study (NCT02304458) is underway to explore the use of nivolumab as a single agent or in combination with ipilimumab in refractory solid tumors, including RMS. Predictive biomarkers should be investigated to identify the subset of patients who respond to immunotherapy.
Here, we present a case of a patient with advanced PRMS who failed to respond to chemotherapy and antiangiogenic therapy but achieved a partial response (PR) with nivolumab treatment. Subsequently, the patient underwent R0 resection on the recurrent lesions, followed by nivolumab treatment as adjuvant and maintenance immunotherapy. At the time of submission of this manuscript, the patients achieved recurrence-free survival (RFS) lasting 45 months and counting (Figure 1A).
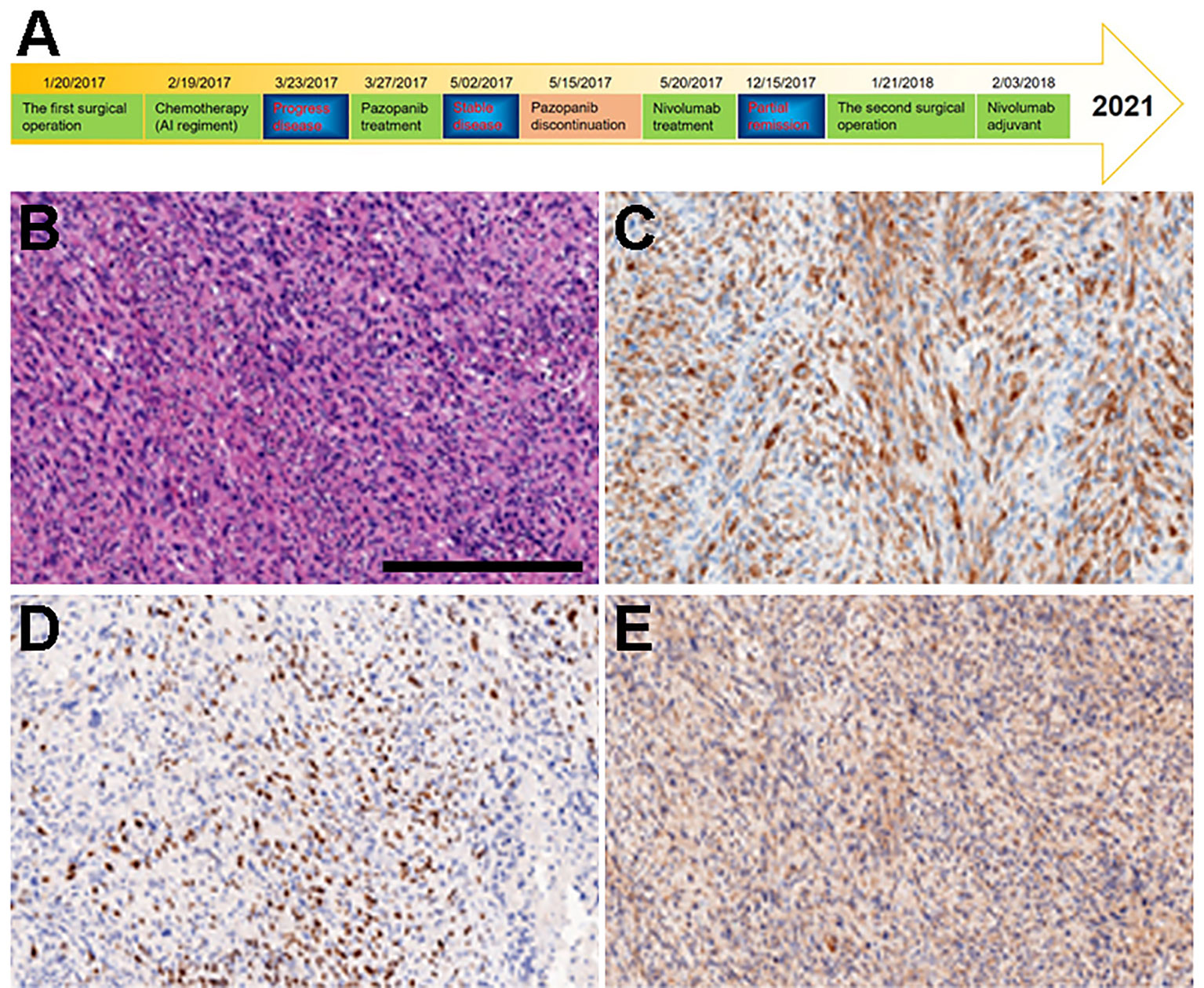
Figure 1 Diagnosis and treatment schematic plot and IHC of primary tumor tissue obtained from surgery. (A) Diagnosis and treatment schematic plot. (B) H&E. (C) DESMIN. (D) MYOD1. (E) MYGLB.
A 12-year-old Chinese male adolescent was referred due to a mass in his left elbow. In January 2017, surgical treatment was performed due to the rapid growth of the mass. Immunohistochemistry (IHC) results of the tumor specimens showed highly positive expression of DESMIN, MYOD1, and MYOGLB (Figures 1B–E) . PAX3-FOXO gene fusion was not detected by using fluorescence in situ hybridization (FISH). In February 2017, computed tomography (CT) of the chest and an enhanced CT scan of the left elbow revealed recurrence, showing two locoregionally relapsed lesions and multiple lung metastases (Figures 2A1–A3). Accordingly, the patient was diagnosed with poorly differentiated PRMS, stage IV (pT2N0M1).
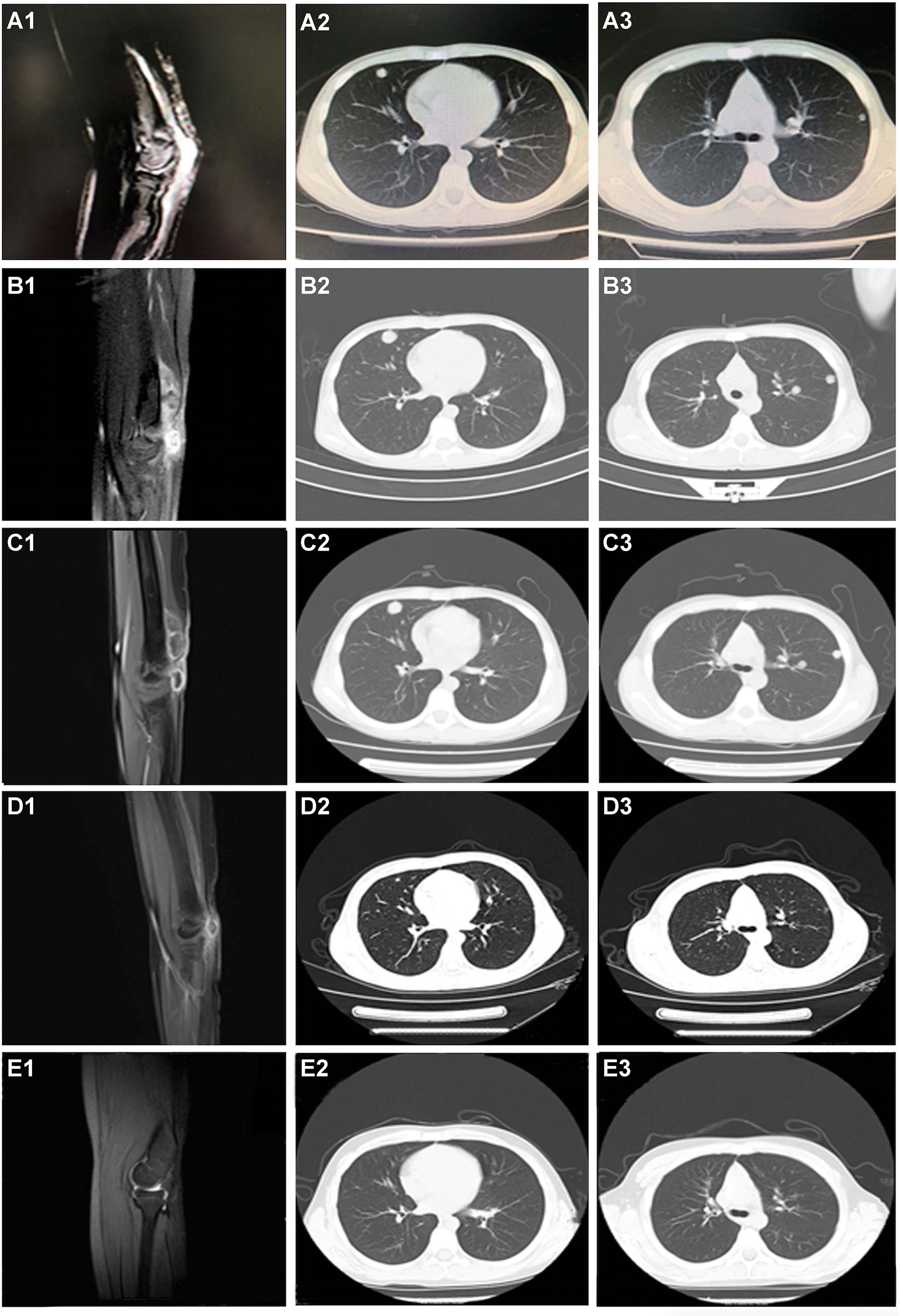
Figure 2 CT scanning during the whole treatment. (A1–A3) Before any systemic treatment. (B1–B3) After one cycle of chemotherapy and endostar. (C1–C3) After 1 month of pazopanib. (D1–D3) After four cycles of nivolumab. (E1–E3) The last followed up scanning. The first column was the limb viewport; the middle and last column was the lung viewport.
Given his good physical condition, the patient was treated with endosarc (a recombinant human endostatin) in combination with chemotherapy (doxorubicin and ifosfamide). Progressive disease (PD) and serious treatment-related adverse events, such as nausea and myelosuppression, occurred after one cycle of chemotherapy (Figures 2B1–B3). This indicated a failure of the first-line chemotherapy. Starting on March 27, 2017, pazopanib monotherapy was administered following a multidisciplinary discussion. Although stable disease (SD) was achieved 1 month later (Figures 2C1–C3), the patient experienced severe adverse effects, including asthenia, myelosuppression, and nausea. The use of pazopanib was discontinued.
Under the consent of the patient’s guardian, the surgical tissue sample was tested for the feasibility of immunotherapy. More than 30% of the tumor cells expressed PD-L1 (as tested by the SP263 assay on the Ventana platform), and tumor-infiltrating T cells appeared in the tumor region (Figure 3). Whole exon sequencing (WES) revealed a microsatellite-stable (MSS) status and HLA-I locus heterozygosity. The tumor mutation burden (TMB) was 2.05 mut/Mb (Table 1). Based on these findings, nivolumab monotherapy (100 mg Q2W) was initiated on May 20, 2017. CT scans exhibited a significant remission of the lung lesions after four courses of nivolumab treatment. One of the primary lesions had PR, but the other had little change (Figures 2D1–D3). Taken together, the total tumor volume was reduced by 87%, demonstrating that PR was achieved.
On January 21, 2018, the patient underwent R0 resection after 19 cycles of nivolumab immunotherapy. Another WES was performed on the surgical tumor tissue obtained during the second surgery and showed a TMB of 4.79 mut/Mb (Table 1). Upon submission of this manuscript, the patient continued to receive maintenance monotherapy with nivolumab. The most recent CT scan indicated no recurrence or metastasis. The patient has achieved an RFS of 45 months and counting (Figures 2E1–E3). Moreover, the toxicity associated with nivolumab treatment was tolerable through the whole course of immunotherapy.
Unlike other RMS subtypes, PRMS is a rare subtype that is noted more commonly in adults rather than in children and teenagers (1). There are no effective treatments and accurate prognostic biomarkers for PRMS, especially for refractory disease. Here, we reviewed the clinical courses and prognosis of 15 patients with metastatic or recurrent PRMS (Table 2) and found that surgical resection was still the best option. Adjuvant therapy, such as chemotherapy and immunotherapy, would benefit the patients further. The majority of patients died within a year of diagnosis. Nonetheless, long-term event-free survival has been achieved in two patients with adjuvant immunotherapy following surgical resection or immunotherapy alone (15). These two patients, in particular, were younger than the others. This indicates that ICI-based immunotherapy could be a promising treatment option for refractory PRMS, especially in teenagers. According to WHO classification, PRMS was not classified as a separate disease diagnosis in pediatric patients but was categorized as incorporated in ERMS diffuse anaplasia (23, 24). This highlights that age is a vital risk stratification factor for RMS.
The expression of PD-L1 in tumor cells correlates with inferior prognosis in STS patients, suggesting that the PD-1/PD-L1 axis could be a promising therapeutic biomarker (25). In many advanced malignant tumors, including STS (11, 26), monoclonal antibodies targeting the PD-1/PD-L1 axis have demonstrated considerable antitumor effects with a controllable toxicity profile. One of the essential approaches to improve the therapeutic benefit is to stratify patients using reliable biomarkers (27, 28).
In many solid tumors, such as non-small-cell lung cancer (NSCLC), PD-L1 expression in tumor cells and tumor-infiltrating lymphocytes (TILs) have strong predictive effects for anti-PD-1/L1 therapy (8). Little is known about the predictive significance of PD-L1 expression in STS. In a phase 2 study of pembrolizumab in advanced STS and bone sarcoma, three patients (4%) were identified as PD-L1 positive (TPS >1%) (9). Two of the three PD-L1-positive patients achieved an evaluable response: one complete response (CR) and one PR. Moreover, in another phase 2 clinical trial evaluating the efficacy and safety of cyclophosphamide plus pembrolizumab in 57 STS patients (29), only one patient presented a high level of PD-L1 expression (immune proportion score >10%) and achieved PR. A favorable response was also observed in a 17-year-old PD-L1-positive patient (TPS = 75%) with metastatic histiocytic sarcoma treated with nivolumab (30). Moreover, Tlemsani et al. reported the first case of a patient with PRMS (TPS = 60%), high levels of PD-L1 expression, and mismatch repair deficiency (dMMR). This patient was administered nivolumab monotherapy and achieved a long-term CR (15). Given the above data, PD-L1 expression may serve as a predictive biomarker for anti-PD-1/L1 treatment in STS patients.
The PD-L1 expression in STS patients varied among the studies. According to Perisano et al., 68.3% of high-grade sarcomas displayed positive PD-L1 expression (31). Chowdhury’s study revealed that RMS had the highest (86%) PD-L1-positive expression (TPS > 5%) when compared to other common pediatric tumors (32). Similar to the clinical result in NSCLC (33), our study also found that the proportion of CD8+ TIL seemed to be positively correlated with PD-L1 expression, implying that CD8+ TIL infiltration could become another effective indicator of immune response (32). The patient in our case presented with positive PD-L1 expression (TPS = 30%) and increased infiltration of CD8+ T cell (Figure 3). Given the impressive clinical response, we hypothesized that PD-L1-positive expression and/or TILs could be useful biomarkers for predicting the response to PD-1 blockade in PRMS.
Deficient mismatch repair/microsatellite instability-high (dMMR/MSI-H) has been identified as a powerful predictor of ICIs in multiple solid tumors, particularly colorectal and gastric cancer (8–11). The prevalence of dMMR/MSI-H in STS is 2.3% (34). In Doyle’s study, no response was observed in three dMMR PRMS patients treated with pembrolizumab. Lewin et al. reported that two dMMR cases with alveolar soft part sarcoma responded to durvalumab (anti-PD-L1) alone or in combination with tremelimumab (anti-CTLA-4) (35). The patient in our study was microsatellite stable (MSS) but benefited from nivolumab. Thus, the predictive value of dMMR/MSI-H for STS with anti-PD-1/L1 treatment remains debatable. In Doyle’s study, dMMR patients exhibited significantly higher TMB than proficient mismatch repair (pMMR) patients, consistent with previous clinical studies (36). High TMB has been reported as a potential biomarker to screen patients who may benefit from ICI therapy (37). However, TMB has no predictive ability for STS with anti-PD-1/L1 treatment due to the small patient population (35). Studies with larger sample sizes are needed to assess the predictive effects of dMMR/MSI-H and TMB in STS and PRMS.
Moreover, prior pazopanib treatment may have reinforced the brilliant response to nivolumab. The combination of atezolizumab (PD-L1 antibody) and bevacizumab [vascular endothelial growth factor (VEGF) antibody] has been recommended as the preferred first-line systemic therapy regimen in hepatocellular carcinoma (HCC) based on the favorable objective response rate (ORR) and survival benefit reported (38). Similarly, the clinical study of lenvatinib plus pembrolizumab demonstrated an impressive ORR. By blocking the FGFR4-GSK3β axis, lenvatinib reshaped Treg differentiation and reduced tumor PD-L1 levels, resulting in improved anti-PD-1 efficacy (39). In this case, the patient achieved SD after receiving pazopanib treatment (Figures 2C1–C3). Although the TKI was terminated due to severe adverse effects 1 month later, it is rational to speculate that pazopanib may have contributed to refining the tumor immune microenvironment and paved the way for the effects of nivolumab. Our findings suggest that TKI plus immunotherapy might be an effective option for PRMS.
Here, we described a Chinese male adolescent PRMS patient with positive PD-L1 expression and TILs who achieved a remarkable response to nivolumab following TKI therapy. The PD-L1 and CD8 statuses were informative in guiding therapy decisions for this patient. Our findings could pave the way for a better understanding of the predictive biomarkers of anti-PD-1 therapies in PRMS.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Peking University Cancer Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
JL conceived the study. PL Liu took care of the patient. YL supervised patient care. FG, YT, and XZ from 3DMed Clinical Laboratory Inc. performed WES and immunohistochemistry on tumor tissue. JL evaluated and prepared images for the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 81802689).
Authors FG, YT and XZ are employed by 3DMed Clinical Laboratory Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We gratefully acknowledge the patient and his family for allowing us to publish this clinical case, and appreciate Ting Bei’s (3D Medicines Inc.) amendment opinions and revising work.
CT, computed tomography; dMMR, mismatch repair deficiency; ICI, immune checkpoint inhibitor; IHC, immunohistochemistry; MSI-H, microsatellite instability-high; MSS, microsatellite-stable status; MTI, medication time interval; PD, progressive disease; PD-1, programmed death protein 1; PR, partial response; PRMS, pleomorphic rhabdomyosarcoma; RMS, rhabdomyosarcoma; SD, stable disease; STS, soft tissue sarcoma; TIL, tumor-infiltrating lymphocytes; TMB, tumor mutation burden; WES, whole exon sequencing.
1. Miwa S, Yamamoto N. Recent Advances and Challenges in the Treatment of Rhabdomyosarcoma. Cancers (Basel) (2020) 12(7):1758. doi: 10.3390/cancers12071758
2. Jo VY, Fletcher CD. WHO Classification of Soft Tissue Tumours: An Update Based on the 2013 (4th) Edition. Pathology (2014) 46(2):95–104. doi: 10.1097/pat.0000000000000050
3. Noujaim J, Thway K, Jones RL, Miah A, Khabra K, Langer R, et al. Adult Pleomorphic Rhabdomyosarcoma: A Multicentre Retrospective Study. Anticancer Res (2015) 35(11):6213–7.
4. Ruiz-Mesa C, Goldberg JM, Coronado Munoz AJ, Dumont SN, Trent JC. Rhabdomyosarcoma in Adults: New Perspectives on Therapy. Curr Treat Options Oncol (2015) 16(6):27. doi: 10.1007/s11864-015-0342-8
5. Chen C, Dorado Garcia H, Scheer M, Henssen AG. Current and Future Treatment Strategies for Rhabdomyosarcoma. Front Oncol (2019) 9:1458. doi: 10.3389/fonc.2019.01458
6. van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, et al. Pazopanib for Metastatic Soft-Tissue Sarcoma (PALETTE): A Randomised, Double-Blind, Placebo-Controlled Phase 3 Trial. Lancet (2012) 379(9829):1879–86. doi: 10.1016/s0140-6736(12)60651-5
7. Levi J, Lam T, Goth SR, Yaghoubi S, Bates J, Ren G. Imaging of Activated T Cells as an Early Predictor of Immune Response to Anti-PD-1 Therapy. Cancer Res (2019) 79(13):3455–65. doi: 10.1158/0008-5472.can-19-0267
8. Dyson KA, Stover BD, Grippin A, Mendez-Gomez HR, Lagmay J, Mitchell DA, et al. Emerging Trends in Immunotherapy for Pediatric Sarcomas. J Hematol Oncol (2019) 12(1):78. doi: 10.1186/s13045-019-0756-z
9. Silva MA, Triltsch N, Leis S, Kanchev I, Tan TH, Van Peel B, et al. Biomarker Recommendation for PD-1/PD-L1 Immunotherapy Development in Pediatric Cancer Based on Digital Image Analysis of PD-L1 and Immune Cells. J Pathol Clin Res (2020) 6(2):124–37. doi: 10.1002/cjp2.152
10. Salvador-Coloma C, Saigí M. Identification Of Actionable Genetic Targets In Primary Cardiac Sarcomas. Onco Targets Ther (2019) 12:9265–75. doi: 10.2147/ott.s214319
11. Tawbi HA, Burgess M, Bolejack V, Van Tine BA, Schuetze SM, Hu J, et al. Pembrolizumab in Advanced Soft-Tissue Sarcoma and Bone Sarcoma (SARC028): A Multicentre, Two-Cohort, Single-Arm, Open-Label, Phase 2 Trial. Lancet Oncol (2017) 18(11):1493–501. doi: 10.1016/s1470-2045(17)30624-1
12. Paoluzzi L, Cacavio A, Ghesani M, Karambelkar A, Rapkiewicz A, Weber J, et al. Response to Anti-PD1 Therapy With Nivolumab in Metastatic Sarcomas. Clin Sarcoma Res (2016) 6:24. doi: 10.1186/s13569-016-0064-0
13. Ansai S, Takeda H, Koseki S, Hozumi Y, Kondo S. A Patient With Rhabdomyosarcoma and Clear Cell Sarcoma of the Skin. J Am Acad Dermatol (1994) 31(5 Pt 2):871–6. doi: 10.1016/s0190-9622(94)70249-7
14. Ashley CW, Da Cruz Paula A, Ferrando L, Gularte-Mérida R. Genetic Characterisation of Adult Primary Pleomorphic Uterine Rhabdomyosarcoma and Comparison With Uterine Carcinosarcoma. Histopathology (2021) 79(2):176–86. doi: 10.1111/his.14346
15. Tlemsani C, Leroy K, Gimenez-Roqueplo AP, Mansuet-Lupo A, Pasmant E, Larousserie F, et al. Chemoresistant Pleomorphic Rhabdomyosarcoma: Whole Exome Sequencing Reveals Underlying Cancer Predisposition and Therapeutic Options. J Med Genet (2020) 57(2):104–8. doi: 10.1136/jmedgenet-2018-105594
16. Miracco C, Materno M, De Santi MM, Pirtoli L, Ninfo V. Unusual Second Malignancies Following Radiation Therapy: Subcutaneous Pleomorphic Rhabdomyosarcoma and Cutaneous Melanoma. Two Case Reports. J Cutan Pathol (2000) 27(8):419–22. doi: 10.1034/j.1600-0560.2000.027008419.x
17. Stock N, Chibon F, Binh MB, Terrier P, Michels JJ, Valo I, et al. Adult-Type Rhabdomyosarcoma: Analysis of 57 Cases With Clinicopathologic Description, Identification of 3 Morphologic Patterns and Prognosis. Am J Surg Pathol (2009) 33(12):1850–9. doi: 10.1097/PAS.0b013e3181be6209
18. Marburger TB, Gardner JM, Prieto VG, Billings SD. Primary Cutaneous Rhabdomyosarcoma: A Clinicopathologic Review of 11 Cases. J Cutan Pathol (2012) 39(11):987–95. doi: 10.1111/cup.12007
19. Scatena C, Massi D, Franchi A, De Paoli A, Canzonieri V. Rhabdomyosarcoma of the Skin Resembling Carcinosarcoma: Report of a Case and Literature Review. Am J Dermatopathol (2012) 34(1):e1–6. doi: 10.1097/DAD.0b013e31822381fas
20. Sabater-Marco V, Zapater Latorre E, Martorell Cebollada M. Postradiation Cutaneous Pleomorphic Rhabdomyosarcoma With Extracellular Collagen Deposits Reminiscent of So-Called Amianthoid Fibers. J Cutan Pathol (2014) 41(3):316–21. doi: 10.1111/cup.12282
21. Li JJ, Forstner D, Henderson C. Cutaneous Pleomorphic Rhabdomyosarcoma Occurring on Sun-Damaged Skin: A Case Report. Am J Dermatopathol (2015) 37(8):653–7. doi: 10.1097/dad.0000000000000191
22. Watanabe M, Ansai SI, Iwakiri I, Fukumoto T, Murakami M. Case of Pleomorphic Rhabdomyosarcoma Arising on Subcutaneous Tissue in an Adult Patient: Review of the Published Works of 13 Cases Arising on Cutaneous or Subcutaneous Tissue. J Dermatol (2017) 44(1):59–63. doi: 10.1111/1346-8138.13549
23. Rudzinski ER, Anderson JR, Hawkins DS, Skapek SX, Parham DM, Teot LA. The World Health Organization Classification of Skeletal Muscle Tumors in Pediatric Rhabdomyosarcoma: A Report From the Children’s Oncology Group. Arch Pathol Lab Med (2015) 139(10):1281–7. doi: 10.5858/arpa.2014-0475-OA
24. Skapek SX, Ferrari A, Gupta AA, Lupo PJ, Butler E, Shipley J, et al. Rhabdomyosarcoma. Nat Rev Dis Primers (2019) 5(1):1. doi: 10.1038/s41572-018-0051-2
25. Kim C, Kim EK, Jung H, Chon HJ, Han JW, Shin KH, et al. Prognostic Implications of PD-L1 Expression in Patients With Soft Tissue Sarcoma. BMC Cancer (2016) 16:434. doi: 10.1186/s12885-016-2451-6
26. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab Versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med (2016) 375(19):1823–33. doi: 10.1056/NEJMoa1606774
27. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors With Mismatch-Repair Deficiency. N Engl J Med (2015) 372(26):2509–20. doi: 10.1056/NEJMoa1500596
28. Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-Driven Biomarkers to Guide Immune Checkpoint Blockade in Cancer Therapy. Nat Rev Cancer (2016) 16(5):275–87. doi: 10.1038/nrc.2016.36
29. Toulmonde M, Penel N, Adam J, Chevreau C, Blay JY, Le Cesne A, et al. Use of PD-1 Targeting, Macrophage Infiltration, and IDO Pathway Activation in Sarcomas: A Phase 2 Clinical Trial. JAMA Oncol (2018) 4(1):93–7. doi: 10.1001/jamaoncol.2017.1617
30. Bose S, Robles J. Favorable Response to Nivolumab in a Young Adult Patient With Metastatic Histiocytic Sarcoma. Pediatr Blood Cancer (2019) 66(1):. doi: 10.1002/pbc.27491
31. Perisano C, Vitiello R, Sgambato A, Greco T, Cianni L, Ragonesi G, et al. Evaluation of PD1 and PD-L1 Expression in High-Grade Sarcomas of the Limbs in the Adults: Possible Implications of Immunotherapy. J Biol Regul Homeost Agents (2020) 34(4 Suppl. 3):289–94. Congress of the Italian Orthopaedic Research Society.
32. Chowdhury F, Dunn S, Mitchell S, Mellows T, Ashton-Key M, Gray JC. PD-L1 and CD8+PD1+ Lymphocytes Exist as Targets in the Pediatric Tumor Microenvironment for Immunomodulatory Therapy. OncoImmunology (2015) 4(10):e1029701. doi: 10.1080/2162402X.2015.1029701
33. Kim H, Kwon HJ, Han YB, Park SY, Kim ES, Kim SH, et al. Increased CD3+ T Cells With a Low FOXP3+/CD8+ T Cell Ratio can Predict Anti-PD-1 Therapeutic Response in Non-Small Cell Lung Cancer Patients. Mod Pathol (2019) 32(3):367–75. doi: 10.1038/s41379-018-0142-3
34. Doyle LA, Nowak JA, Nathenson MJ, Thornton K, Wagner AJ. Characteristics of Mismatch Repair Deficiency in Sarcomas. Mod Pathol (2019) 32(7):977–87. doi: 10.1038/s41379-019-0202-3
35. Lewin J, Davidson S, Anderson ND, Lau BY, Kelly J, Tabori U, et al. Response to Immune Checkpoint Inhibition in Two Patients With Alveolar Soft-Part Sarcoma. Cancer Immunol Res (2018) 6(9):1001–7. doi: 10.1158/2326-6066.cir-18-0037
36. Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 Human Cancer Genomes Reveals the Landscape of Tumor Mutational Burden. Genome Med (2017) 9(1):34. doi: 10.1186/s13073-017-0424-2
37. Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, et al. Nivolumab Plus Ipilimumab in Lung Cancer With a High Tumor Mutational Burden. N Engl J Med (2018) 378(22):2093–104. doi: 10.1056/NEJMoa1801946
38. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab Plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med (2020) 382(20):1894–905. doi: 10.1056/NEJMoa1915745
Keywords: pleomorphic rhabdomyosarcoma, immunotherapy, pazopanib, PD-L1, CD8 T cell
Citation: Liu J, Liu P, Gong F, Tian Y and Zhao X (2022) Case Report: A PD-L1-Positive Patient With Pleomorphic Rhabdomyosarcoma Achieving an Impressive Response to Immunotherapy. Front. Immunol. 13:815598. doi: 10.3389/fimmu.2022.815598
Received: 15 November 2021; Accepted: 18 February 2022;
Published: 17 March 2022.
Edited by:
Alison Taylor, University of Leeds, United KingdomReviewed by:
Jiyang Yu, St. Jude Children’s Research Hospital, United StatesCopyright © 2022 Liu, Liu, Gong, Tian and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiayong Liu, liujiayong_doc@163.com
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.