- Department of Burn and Plastic Surgery, West China Hospital, Sichuan University, Chengdu, Sichuan, China
Melanoma is one of the most lethal tumors with highly aggressive and metastatic properties. Although immunotherapy and targeted therapy have certain therapeutic effects in melanoma, a significant proportion of patients still have drug resistance after treatment. Recent studies have shown that long noncoding RNAs (lncRNAs) are widely recognized as regulatory factors in cancer. They can regulate numerous cellular processes, including cell proliferation, metastasis, epithelial-mesenchymal transition (EMT) progression and the immune microenvironment. The role of lncRNAs in malignant tumors has received much attention, whereas the relationship between lncRNAs and melanoma requires further investigation. Our review summarizes tumor suppressive and oncogenic lncRNAs closely related to the occurrence and development of melanoma. We summarize the role of lncRNAs in the immune microenvironment, immunotherapy and targeted therapy to provide new targets and therapeutic methods for clinical treatment.
1. Introduction
The incidence and mortality of melanoma have gradually increased over the past few decades (1). Currently, multiple therapeutic strategies, including surgical resection, radiotherapy and chemotherapy, immunotherapy, and biological and targeted therapy, have significantly improved the therapeutic effect of melanoma and prolonged the survival time of patients (2). Given a strong metastatic tendency, these treatments have very limited therapeutic efficacy in patients with advanced melanoma. Moreover, the occurrence and progression of melanoma have complex relationships with targeted genes and signaling pathways that influence the proliferation, migration, invasion and metastasis of tumor cells (3). Therefore, it is important to understand the molecular mechanism of melanoma progression to complement effective therapeutic strategies.
LncRNAs play regulatory roles in a variety of tumors and are widely involved in multiple biological processes, such as proliferation, migration, invasion, EMT process, cell cycle, apoptosis and chemoresistance. Compared with benign nevi and melanocytes, lncRNA-ATB is upregulated in human skin melanoma tissues and cells. It regulates cell proliferation, metastasis, cell cycle arrest and apoptosis by regulating miR-590-5p and YAP1 (4). In addition, lncRNA-XIST promotes the proliferation and migration of melanoma cells by decreasing the expression of PI3KRI and AKT and increasing the expression of Bcl-2 and Bax, which are considered key regulators of oxaliplatin resistance in melanoma progression (5). Additionally, lncRNA-LINC00518 could significantly promote the invasion, migration, proliferation, clonogenicity and metastasis of malignant melanoma cells and induce radioresistance by regulating the miR-33a-3p/HIF-1α negative feedback pathway (6). Therefore, there is an urgent need to find new breakthroughs, such as specific lncRNAs, to improve melanoma therapeutic effects.
The communication between cancer cells and their surrounding microenvironment is very important in many tumors. LncRNA-NEAT1 promoted cell proliferation and migration by regulating the miR‐495‐3p/E2F3 axis and activated the EMT process and immune responses through the miR-200b-3p/SMAD2 pathway in melanoma (7, 8). Moreover, in melanoma cells with low FOXF1-AS1 expression, the expression of immune-related genes was downregulated, and the activity of inflammation and Wnt signaling pathways were also changed (9). Additionally, the expression of lncRNA-SNHG15 can be modulated by palbociclib and alleviate temozolomide resistance by regulating the CDK6/miR-627 pathway and reducing M2 polarization of glioma-associated microglia, providing evidence for treatment with temozolomide resistance with the use of CDK6 inhibitors (10).
Immune checkpoint inhibitors, especially anti-PD-1 (programmed death protein 1) antibodies, target the dysfunctional immune system and induce CD8-positive T cells to kill tumor cells, completely altering the treatment of various cancers, including advanced melanoma. In addition, targeted therapy for melanoma is primarily an appropriate treatment based on BRAF and NRAS mutational status. However, there are currently no highly sensitive and specific biomarkers to evaluate the therapeutic efficacy of immunotherapy and targeted therapy in patients with advanced melanoma. Therefore, lncRNAs may play an important role in melanoma immunotherapy and targeted therapy, serving as new therapeutic targets or drug sensitivity assessment markers. lncRNA-CRNDE (colon rectal neoplasia differentially expressed) promoted the cell invasion and apoptosis of melanoma by targeting CCL18, which was correlated with the expression of PD-L1 (programmed death ligand 1) and induced immunosuppression (11). Moreover, lncRNA-SNHG14 is upregulated in diffuse large B-cell lymphoma, and the SNHG14/miR-5590-3p/ZEB1 axis can also regulate the PD-1/PD-L1 checkpoint to promote the progression and immune evasion of tumor cells, which indicates that targeting SNHG14 may be a potential target to improve the immunotherapeutic effect in tumors (12). Thus, it is of great clinical importance to elucidate the therapeutic effect and molecular mechanism of lncRNAs in melanoma immunotherapy and targeted therapy.
In this review, we summarized the regulatory mechanisms by which lncRNAs exert oncogenic and tumor suppressive functions in tumor progression, particularly in melanoma. Furthermore, we screened lncRNAs involved in the regulation of the tumor immune microenvironment, provided relevant evidence for their efficacy in promoting immunotherapy and targeted therapy, and discussed their potential therapeutic prospects.
2. Oncogenesis of melanoma
Most of malignant tumors have complex etiologies, poor treatment effects and short survival times. Their overall incidence in the world is rising every year and seriously threatening human health. According to the statistics of cancer incidence and mortality rate of 38 cancer sites and 185 countries or regions in the world, it is estimated that there were 19.3 million new cancer cases and approximately 10 million cancer deaths around the world in 2020 (13). In recent years, almost 75% of patients with malignant melanoma have relapsed one year after treatment, and the 3-year overall survival rate of patients with advanced malignant melanoma is less than 30% (14). The incidence and mortality rates of melanoma were 324,635 and 57,043, respectively (15). Therefore, there is an urgent need to find an effective treatment for advanced melanoma.
The two of the most critical factors in reducing melanoma mortality are early detection and prompt treatment (16). Surgical resection is considered the primary treatment for early-stage melanoma, but it still has the possibility of metastasis and affects long-term survival outcomes (17). The US Food and Drug Administration (FDA)-approved treatments for metastatic melanoma, including immune checkpoint blocking antibodies (such as anti-CTLA-4 and anti-PD-1), have an effect on reducing population mortality (18). The anti-CTLA-4 drugs (including Ipilimumab) and anti-PD-1 drugs (including Nivolumab and Pembrolizumab) have therapeutic effects in advanced metastatic melanoma and are used as adjuvant therapy after surgery (19). In a study of 945 patients with stage III or IV melanoma, the overall survival after treatment with Nivoluma and Ipilimumad was 36.9 months and 19.9 months respectively, while the overall survival of Nivoluma and Ipilimumad combination was more than 60 months (20). The immune responses induced by anti-CTLA-4 and anti-PD-1 checkpoint blockade are driven by distinct cellular mechanisms. Anti-PD-1 mainly induces an increase in specific tumor-infiltrating exhausted-like CD8 T-cell populations, while anti-CTLA-4 predominantly induces the expansion of an ICOS+ Th1-like CD4 effector cell subset and binds to specific subsets of exhausted-like CD8 T cells (21). PD-1 inhibitors have become an adjuvant treatment for stage III or IV melanoma patients after surgical resection, and immune checkpoint therapy may become an extremely effective treatment in the future (22).
Transcriptome sequencing analysis of tissue samples from melanoma patients indicated that mutations closely related to melanoma progression mainly included BRAF mutation, NRAS mutation and NF1 mutation (23). These three mutations are found in most skin melanomas (about 94%) and can activate the downstream Ras/Raf/MEK/ERK axis (MAPK signal pathway) (24). Among them, BRAF mutation exists in more than 60% of skin melanomas and promotes the occurrence and development of tumors (25). ATF-3, a cyclic APM-dependent transcription factor, is significantly decreased in human metastatic melanoma cell lines. Overexpression of this gene downregulates the ERK and AKT signaling pathways, upregulates apoptosis-related genes, and reduces melanoma metastasis (26). In addition, silencing MED27 (as a potential melanoma target) leads to a decrease in iNOS expression by inhibiting the activity of a series of key proteins in the NF-κB signaling pathway and is accompanied by the inhibition of melanoma cell proliferation, induction of apoptosis and regulation of the cell cycle by changing the activity of the PI3K/AKT, MAPK/ERK and Bax/Cyto-C/Caspase-dependent apoptotic pathways (27). Moreover, some genes were found to improve the therapeutic effect of chemotherapy drugs on metastatic melanoma by regulating these target genes and signaling pathways. The expression of SEMA6A protein was higher in melanoma tissues from BRAF-mut patients than in melanoma tissues from BRAF-wt patients. In addition, SEMA6A regulates actin cytoskeleton remodeling through RhoA-dependent activation of YAP in BRAF-mut melanoma cells. Dabrafenib/trametinib treatment helps melanoma cells escape from the microenvironment, which may be a predictor of the effectiveness of dual BRAF/MEK (mitogen-activated protein kinase kinase) inhibitors in treating melanoma (28). Thus, the in-depth study of target genes and signaling pathways can improve the therapeutic effect in melanoma.
3. LncRNAs in carcinogenesis
More than 90% of transcripts have not been translated into proteins in the human genome (known as noncoding RNA). Noncoding RNAs are a class of regulatory molecules that play a crucial role in regulating gene expression and are closely related to the progression of multiple diseases, especially different types of cancer. Both lncRNAs and miRNAs belong to the non-coding RNA. The difference is that the lncRNAs is longer than 200nt. They all regulate the expression of target genes. In particular, they are expected to be combined in the diagnosis and treatment of melanoma (29). Numerous studies have shown that lncRNAs play a key role in the initiation and development of cancer, participating in biological processes such as tumor cell proliferation, metastasis, EMT process, stemness, angiogenesis, chemotherapy resistance, and regulation of the tumor microenvironment (Figure 1).
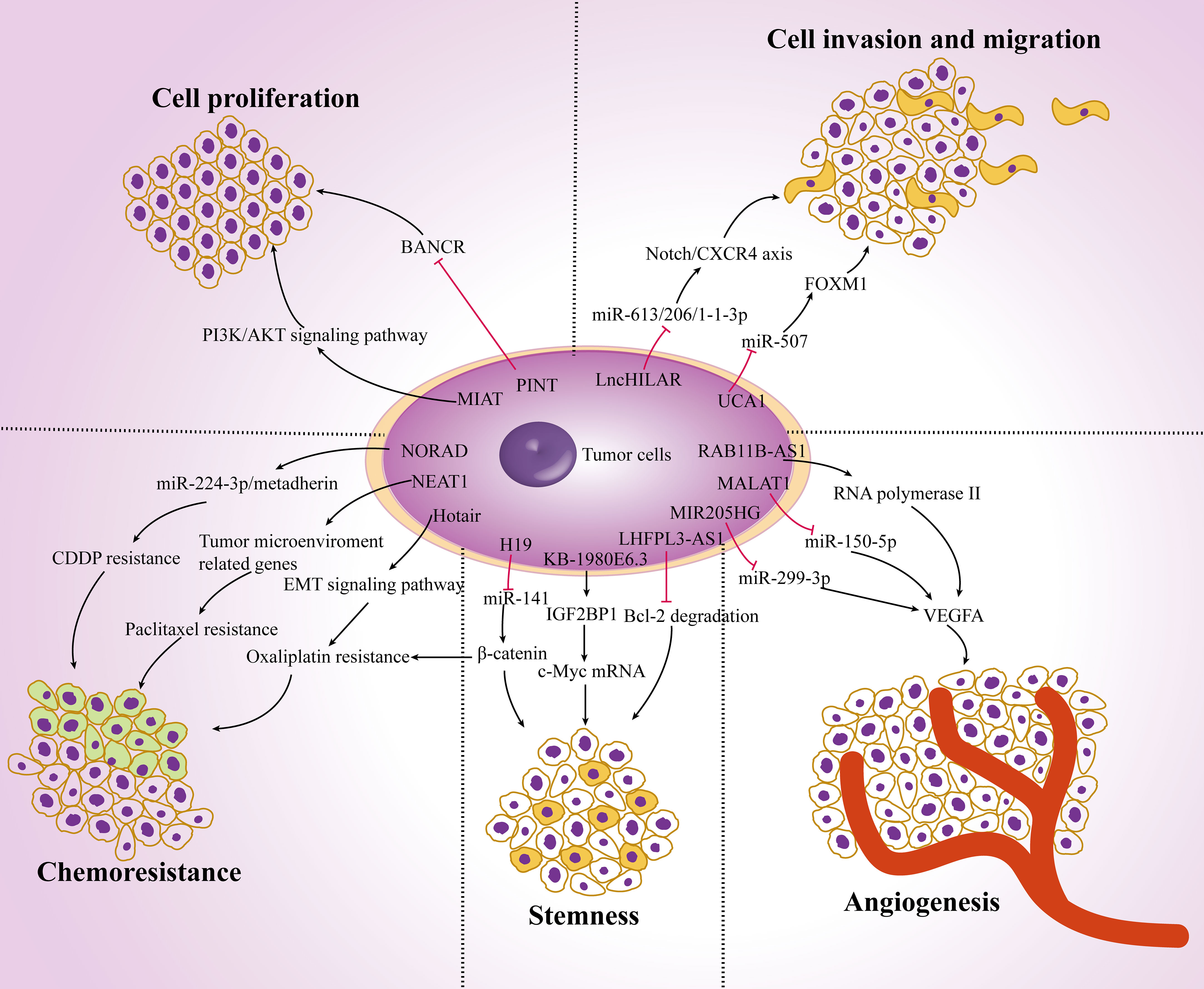
Figure 1 The role of lncRNAs in carcinogenesis. Dysregulation of lncRNAs in melanoma cells affects tumor cell proliferation, invasion and migration, angiogenesis, stemness and chemoresistance by targeting multiple genes and ultimately regulate tumor progression.
LncRNAs can regulate a few target proteins related to tumor proliferation and metastasis. Hypoxia-induced lncRNA lncHILAR promotes cell invasion and migration by acting as a ceRNA for miR-613/206/1-1-3p, thus resulting in the upregulation of the Notch/CXCR4 axis (30). The expression of lncRNA-BASP1-AS1 was up-regulated in melanoma tissues. BASP1-AS1 interacts with YBX1 and recruit it into the promoter of Notch3 to activate the transcription of multiple oncogenes, including c-MYC, PCNA and CDK4, and promote the proliferation, migration and invasion of A375 and SK-MEL-2 cells (31). LncRNA-MALAT1 knockdown downregulates the expression of vascular endothelial growth factor A (VEGFA), enhances the expression of miR-150-5p and changes the proliferation and migration ability of vascular endothelial cells (32). Tumor stem cells also promote tumor proliferation and metastasis. For example, the expression of lncRNA-NR2F1-AS1 is increased in dormant mesenchymal-like stem cells. It mediates the translation of NR2F1 and inhibits the transcription of ΔNp63, thereby reducing the tumorigenicity and enhancing the dormancy of cancer cells (33). Moreover, overexpression of lncRNA-KB-1980E6.3 maintains the stemness of cells and enhances c-Myc mRNA stability by interacting with IGF2BP1, promoting tumorigenesis in a hypoxic microenvironment (34).
Accumulating studies have shown that lncRNAs play an important role in chemoresistance. lncRNA-MALAT1 increased drug resistance and promoted tumor cell proliferation by affecting the expression of cyclin D1, p-PI3K and p-Akt, as well as regulating the EMT process by targeting ZEB2, YAP, Vimentin and E-cadherin (35). In addition, lncRNA-H19 delivered by exosomes from carcinoma-associated fibroblasts (CAFs) can competitively bind miR-141 and activate the expression of β-catenin protein, thereby promoting the stemness and chemoresistance (36). LncRNAs change the therapeutic effects of multiple drugs on cancer. LncRNA-Hotair regulates the EMT-related signaling pathway by altering hypoxia-induced oxaliplatin resistance (37). Overexpression of lncRNA-NORAD affects the EMT process by interacting with hsa-miR-125a-3p, thus promoting the invasion and migration in vitro and in vivo (38). Meanwhile, researchers also found that NORAD regulates the miR-224-3p/metadherin axis to increase the expression of β-catenin, thereby enhancing the CDDP resistance in tumor cells (39). In addition, lncRNA-NEAT1 directly targets the expression of many prometastatic genes and tumor microenvironment-related genes (such as STAT3, WNT7A and VEGF-A) by interacting with miR-361, while miR-361 can inhibit tumor proliferation, invasion, stemness and paclitaxel resistance (40). Moreover, lncRNA-SAMMSON is highly expressed in doxorubicin-resistant cancer cells, resulting in metabolic recombination, reduced production of mitochondrial ROS, increased mitochondrial replication, transcription, and translation, and reduced resistance to chemotherapy (41).
LncRNAs interact with the tumor microenvironment and affect cancer progression. LncRNA-H19 is considered to be a key lncRNA in CAFs. It is an important component of the tumor microenvironment. It can affect proliferation, migration and glycolysis by regulating miR-675-5p and PFKFB3 (42). Most importantly, lncRNAs can affect the function of a variety of immune cells. LncRNA-LINC00301 changes the amount of regulatory T cells and CD8+ T cells by regulating TGF-β, promotes cell proliferation, cell migration and invasion, releases cell cycle arrest, and reduces cell apoptosis in tumor cells (43). LncRNA-SATB2-AS1 is downregulated in tumor tissues and suppresses metastasis by regulating the expression of Th1-type chemokines and the number of immune cells (44). Recent studies have found that macrophages can also be regulated by lncRNAs in the tumor microenvironment. LncRNA-LNMAT1 induces the upregulation of CCL2 and recruits a large number of macrophages into tumor cells, which then promotes the secretion of VEGF-C and enhances tumor metastasis (45). LncRNA-HOMER3-AS1 modulates proliferation, migration, invasion and apoptosis in tumor cells and enhances M2 macrophage recruitment by activating the Wnt/β-Catenin signaling pathway and CSF-1 expression (46). Moreover, lncRNA-CRNDE can promote M2 macrophage polarization and indirectly modulate angiogenesis-related proteins such as VEGF, VEGFR2, Notch1 and Dll4, which is consistent with the regulatory mechanism in the tumor immune microenvironment (47). Taken together, these data indicate that lncRNAs are involved in carcinogenesis and may become a potential diagnostic target for malignant tumors.
4. LncRNAs as regulators in melanoma
4.1. Tumor suppressor lncRNAs in melanoma
Some lncRNAs have become tumor suppressors because they can affect the proliferation and metastasis of melanoma by competitively binding miRNAs and regulating downstream related signaling pathways, such as the Wnt and Hippo signaling pathways (Table 1). CASC2 is a lncRNA downregulated in a variety of cancer types, including endometrial cancer, lung cancer, gastric cancer and colorectal cancer, which exerts tumor suppressor effects through various mechanisms, such as inhibiting the Wnt/β-Catenin signaling pathway (79). Zhang Y et al. found that the overexpression of CASC2 in melanoma cells inhibited cell proliferation, migration and invasion by regulating miR-18a-5p and its target gene RUNX1 (48). LncRNA-linc00961 is also downregulated in cutaneous melanoma tissues compared to benign nevi. It restrains proliferation and promotes apoptosis in melanoma cells by regulating the miR‐367/PTEN axis (49). As reduced lncRNA-HOXA11-AS expression regulates the miR-152-3p/ITGA9 axis and inhibits the proliferation, metastasis, apoptosis and EMT of melanoma cells, it can be used as a biomarker for the diagnosis and treatment of cutaneous melanoma (50).
In addition to exerting tumor suppressor functions, lncRNAs also play a crucial role in influencing drug sensitivity via ceRNA regulation. LncRNA-MEG3 affects the differentiation of cancer stem cells and the metastasis of melanoma by inhibiting miR-206 and SOX4. MEG3 also regulates the expression of miR-499-5 and CYLD. Thus, it affects proliferation, invasion, migration, apoptosis and cell cycle processes and enhances the chemosensitivity of melanoma cells to cisplatin and 5-FU treatment (51, 52). When the expression of lncRNA-TINCR is decreased in metastatic melanoma, its downregulation promotes the expression level of proliferation-, migration- and invasion-related marker genes and increases its resistance to drugs such as BRAF and MEK inhibitors in melanoma progression (80). In addition, TINCR regulates the expression of LATS1 (a target of miR-424-5p) to activate the Hippo signaling pathway and to inhibit the activity of Yes-1-related transcriptional regulators, thus playing a tumor suppressor role in the development of cutaneous melanoma (81).
Tumor suppression-associated lncRNAs suppress melanoma progression through multiple mechanisms, such as the regulation of downstream target proteins and affecting other long noncoding RNAs. LncRNA-GAS5 plays an antitumor role by regulating gelatinases A and B in melanoma metastasis and promotes the proliferation of melanoma cells by regulating the G1/S cell cycle, apoptosis, reactive oxygen species and redox balance (53, 54). LncRNA-NKILA plays a role in preventing tumor growth and inhibiting metastasis in melanoma, breast cancer and other types of solid tumors, while the expression of NKILA is enhanced by the nuclear factor NF-κB (55). Recent studies have found that lncRNA-CPS1-IT1 is recognized as a tumor suppressor factor in several cancers, including melanoma. The competitive binding of CPS1-IT1 to BRG1 inhibits the expression of Cyr61 (an angiogenic factor involved in tumor metastasis) and works together to control the EMT and angiogenesis of melanoma cells (56). Moreover, the expression of lncRNA p53-induced transcript (LINC-PINT) was decreased in melanoma tissues compared to adjacent tissues, while LINC-PINT overexpression downregulated the expression of lncRNA-BANCR in melanoma cells to regulate cell proliferation (57). Additionally, LINC-PINT inhibits the growth and metastasis of melanoma by regulating the epigenetics of target genes, including PCNA, CDK1, CCNA2 and AURKA (58).
4.2. Oncogenic LncRNAs in melanoma
The other lncRNAs may serve as oncogenic lncRNAs because they regulate a variety of signaling pathways related to melanoma progression. LncRNA-H19 was upregulated in melanoma tissues compared to adjacent normal tissues. Furthermore, its expression in metastatic melanoma tissues was higher than that in orthotopic tumor tissues (59). Knockdown of H19 affects melanoma cell growth, invasion, migration, apoptosis, G0/G1 phase arrest and sensitivity to cisplatin (60). Functionally, downregulation of H19 mediates the inhibition of the PI3K/AKT signaling pathway and NF-κB signaling pathway, thereby inhibiting the progression of melanoma (82). LncRNA-BANCR (BRAF-activated long noncoding RNA) participates in the occurrence and development of melanoma by reducing the interaction with miR-204 and activating the Notch2 signaling pathway and promotes its expression in melanoma tissues and cell lines (83). The overexpression of lncRNA-MIAT obviously promotes the proliferation, migration and invasion of melanoma cells by regulating the PI3K/AKT signaling pathway and can also strengthen the interaction between TCF12 and the NFAT5 promoter region to promote the progression of melanoma (61, 62).
In oncogenic lncRNAs promoting melanoma development, microRNAs play a regulatory role. Accumulating studies have shown that lncRNA-PVT1 (named plasmacytoma variant translocation 1) is upregulated in melanoma tissues compared to adjacent normal tissues, and PVT1 levels are significantly higher in the serum of melanoma patients than in healthy individuals (63). In terms of molecular regulation, PVT1 promotes the occurrence and metastasis of melanoma by regulating the expression of EZH2 and miR−200c (64). The level of lncRNA-MALAT1 in melanoma was significantly higher than that in paired adjacent normal tissues, which affects the expression of c-Myc/Met by regulating a competing endogenous RNA of miR-34a and regulates cell proliferation, migration, invasion and cell apoptosis by miR-23a (65, 66). Additionally, the expression of lncRNA-UCA1 was upregulated in melanoma tissues compared to normal tissues, while the downregulation of UCA1 was controlled by direct binding with miR-507, resulting in cell proliferation, invasion, migration, metastasis and cell cycle arrest inhibition (67). Moreover, integrative transcriptome analysis demonstrated that lncRNA-Gm31932 has definite effects on cell cycle arrest and melanoma differentiation through the miR-344d-3-5p/Prc1 (and Nuf2) axis (68). LncRNA-HnRNPK (heterogeneous nuclear ribonucleoprotein K) acts as a ceRNA for miR-147a and regulates LINC00263, thus accelerating malignant capabilities by targeting CAPN2 (84).
LncRNAs affect the occurrence and development of melanoma by regulating a variety of biological processes, such as stemness, angiogenesis, autophagy and drug resistance. SRA, known as the steroid receptor RNA activator, is a lncRNA encoding the conserved protein SRAP. Its expression is upregulated in melanoma tissues compared to normal tissues. In melanoma cells, the deletion of SRA induces the activation of p38 and inhibits EMT process and distal metastasis by increasing the expression of CCL21 and reducing the expression of β-catenin and N-cadherin (69). Moreover, lncRNA-LHFPL3-AS1 was screened out by analyzing differentially expressed genes between stem cells and nonstem cells in melanoma, which encouraged the stemness of melanoma stem cells by inhibiting the degradation of Bcl-2 (70). The expression levels of lncRNA-MIR205HG were significantly upregulated in melanoma tissues and cells compared to normal skin tissues and cells. In addition, MIR205HG directly binds to miR-299-3p, and miR-299-3p then interacts with the 3’UTR of VEGFA mRNA to promote angiogenesis in melanoma (71). The direct interaction of lncRNA-PURPL (p53 upregulated regulator of p53 levels) with mTOR and ULK1 promotes the phosphorylation of ULK1 at Ser757 to inhibit autophagy and reduce cell death, while the inhibition of PURPL induces autophagy and inhibits melanoma progression by regulating the AMPK signaling pathway and leading to the phosphorylation of ULK1 at Ser555 and Ser317 (72). In addition, it was recently reported that the expression of lncRNA-TUG1 was negatively correlated with prognosis in patients with gastrointestinal tumors, urinary system tumors and gynecological tumors, independent of overall survival in patients with head and neck tumors or melanoma (85). However, knockdown of TUG1 inhibited the growth and metastasis of melanoma cells by regulating miR-29c-3p and its target gene RGS1, as well as inducing apoptosis (73). Moreover, inhibition of TUG1 expression can downregulate Bcl-2, MMP-9 and cyclin D1 protein, reduce the growth of tumors in melanoma and improve the chemosensitivity of A375 cells to cisplatin and 5-FU (74).
5. LncRNAs in the immune microenvironment
Immune-related lncRNAs can predict the prognosis of multiple tumors. They have the potential to become therapeutic targets for multiple tumors, including melanoma. Recently, researchers have analyzed the expression data of melanoma in the TCGA database and established a prediction model between immune-related lncRNAs and the survival status of melanoma (86, 87). The analysis in TCGA database indicated that 6 differentially expressed m7G-related lncRNAs have been identified, and a prognostic model was constructed for predicting the tumor growth, metastasis and survival status of patients (88). Another study found that a number of glycolysis-correlated lncRNAs show pivotal clinical effects by oncogenic pathways such as EMT and immune-related regulation (89). Based on next-generation sequencing technology, Yang et al. constructed a new immune-related lncRNA model and clarified that the high-risk group with low survival and low PD-L1 expression was associated with plasma B cell, monocyte, M2 macrophage, and neutrophil levels (90). The testis-specific lncRNA-RFPL3S was significantly downregulated in testicular germ cell tumors and correlated with the infiltration of immune cells such as T cells, B cells, NK cells and Th cells to predict the effect of immunotherapy (91). LncRNA-HSD11B1-AS1 was highly expressed in melanoma cells and promoted tumor proliferation, migration and invasion by targeting IL-2/STAT-5 and IL-6/JAK/STAT-3 signaling pathways. At the same time, immune invasion analysis showed that HSD11B1-AS1 affected the activation of T cells, Th cells, dendritic cells and B cells (92). And the overexpression of lncRNA-LINC02249 is associated with a shorter survival time in melanoma patients, and affects the immune infiltration of dendritic cells, Treg cells and macrophages (93). LncRNA-SNHG16 regulates mitochondrial function, cell metabolism and the immune infiltration of Th cells and NK cells by competitively binding with let-7b-5p and targeting TUB4A (94). Moreover, lncRNAs in immune cells also play important roles in the occurrence and development of cancer. Transcriptome sequencing analysis of lncRNAs in immune cells showed that the lncRNA expression profiles of T cells and monocytes differed between normal human and melanoma patients. These results provide new possibilities for the regulatory mechanisms of different immune cells, helping to accelerate the immunotherapy of specific cell types in melanoma (95) (Figure 2).
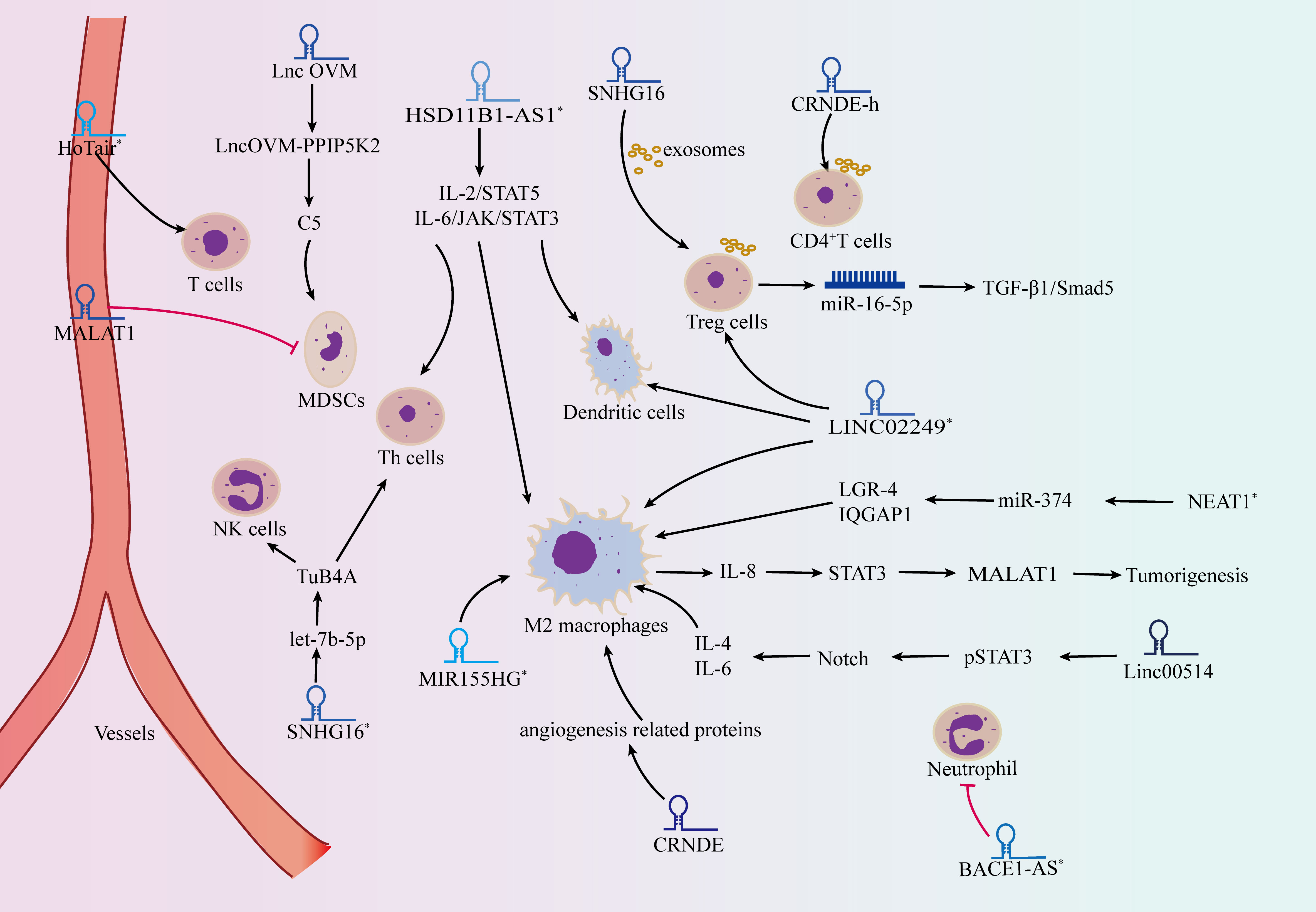
Figure 2 lncRNAs act as modulators in the tumor microenvironment. lncRNAs affect the occurrence and development of tumors through regulating the activity of immune cells, including CD8+ T cells, regulatory T cells (Tregs), T helper cells (Th cells), macrophages, myeloid-derived suppressor cells (MDSCs) and neutrophils. * refers to melanoma associated lncRNAs.
A multiomic integrative assessment including lncRNAs was performed to identify key molecular characteristics for the transcriptomic status of melanoma cells, which was significantly correlated with the therapeutic efficacy of checkpoint inhibitors and adoptive T cells (96). The detection of lncRNA-HOTAIR in the serum and intratumoral lymphocytes of metastatic patients suggested that it was involved in the regulation of the tumor microenvironment and could be used for the treatment of malignant melanoma (97). LncRNA-SNHG16 isolated from exosomes of tumor cells can increase the expression of CD73 in γδ1 Treg cells by regulating the TGF-β1/Smad5 signaling pathway (98). LncRNA-CRNDE-h is also abundant in tumor exosomes and participates in tumor progression by mediating ubiquitination and degradation of RORγt, promoting Th17-cell differentiation and affecting the activity of the IL-17 promoter (99). Single-cell sequencing analysis has identified noncoding IL-4 RNA (IL4nc), which can promote the production of IL-4 protein in Th2 cells by posttranscriptional regulation (100).
M2 macrophages can stimulate IL-8 secretion and promote the STAT3 signaling pathway in tumor progression. Subsequently, STAT3 binds to the lncRNA-MALAT1 promoter region and transcriptionally activates MALAT1 expression, inhibiting cell proliferation, invasion and tumorigenesis (101). Overexpression of lncRNA-linc00514 promotes the phosphorylation of the transcription factor STAT3, activates the Notch signaling pathway, facilitates the secretion of IL-4 and IL-6, and finally induces M2 polarization of macrophages (102). Furthermore, lncRNAs, including SNHG12, PACERR and HITT, function as key regulators of tumor-associated macrophages, regulating tumor cell proliferation, invasion and migration by altering the number of M2-polarized cells and contributing to immune escape (103–105). LncRNA-MIR155HG regulates the infiltration of macrophages and the balance of M1/M2 macrophages in tumor microenvironment to affect cell cycle and apoptosis as well as promoting melanoma progression (106). And lncRNA-NEAT1 derived from exosomes can inhibit miR-374, promote the expression of LGR4 and induce the recruitment of M2 macrophages to accelerate melanoma (107).
Neutrophils are also involved in the immunomodulatory process of lncRNA in tumors. While lncRNA-BACE1-AS is an immune-related factor in the tumorigenesis of melamona, its expression levels are negatively correlated with the neutrophil content (108). Neutrophil extracellular traps (NETs) generated in the tumor microenvironment promote the EMT process and metastasis by promoting the expression of lncRNA-MIR503HG and activating the downstream NF-κB/NLRP3 signaling pathway (109). Therefore, lncRNAs play an important role in the regulation of the immune microenvironment, thus affecting tumor progression.
Myeloid-derived suppressor cells (MDSCs) are precursors of dendritic cells, macrophages and granulocytes, which can inhibit the immune response of tumors and facilitate the formation of the tumor microenvironment. LncRNA-MALAT1 resulted in a significant reduction in MDSC numbers and decreased peripheral blood mononuclear cells in patients with malignant tumors (110). LncRNA-LncOVM maintains the stability of PPIP5K2 by inhibiting ubiquitination degradation and promoting the secretion of complement C5, thus allowing complement C5 to attract MDSC infiltration in the tumor microenvironment and promote tumor metastasis (111). Recent studies have found that lncRNAs also play a role in regulating the development and function of polymorphonuclear bone marrow-derived suppressor cells (PMN-MDSCs). For example, lncRNA-AK036396 is highly expressed in PMN-MDSCs, and its downregulation can weaken the stability of Fcnb protein through the ubiquitin−proteasome pathway, thereby affecting the maturation and immunosuppressive function of PMN-MDSCs in tumors (112). Although the interaction between lncRNAs and immune cells (include T cells, macrophages, neutrophils) has been reported in several studies, the interaction between lncRNAs and MDSCs is still unclear. The function and mechamism of lncRNAs in regulating the immune microenvironment in melanoma still needs further research.
6. LncRNAs as potential therapeutic targets
6.1. Immunotherapy
The use of immune checkpoint inhibitors to enhance the T-cell immune response holds great promise in tumor immunotherapy. However, the effect of immune checkpoint inhibition in patients with solid tumors is very limited, and the mechanism and efficacy of this treatment of solid tumors remain unclear. Computational analysis indicated that lncRNAs play an important role in evaluating the tumor immunotherapy response, and their binding to specific immune checkpoint factors can serve as biomarkers of the immune checkpoint inhibitor response (113). Therefore, it is necessary to elucidate the mechanism of action of lncRNAs and explore new combined strategies for immunotherapy.
LncRNAs transcribed from PD-L1 gene sites also affect the effectiveness of tumor immunotherapy. PD-L1-lnc, a long noncoding RNA subtype produced by alternative splicing of PD-L1 mRNA, can promote the progression of tumor cells by enhancing the transcriptional activity of c-Myc in human lung adenocarcinoma. Its depletion coupled to PD-L1 blockade may be used for tumor suppression (114). LncRNA-INCR1 (interferon-stimulated noncoding RNA 1) also transcribed from the PD-L1 locus promotes the expression of PD-L1, JAK2, and several IFNγ-related genes, which can regulate the sensitivity of tumor cells to cytotoxic T-cell-mediated killing and affect the therapeutic effect of CAR T-cell therapy (115).
As a monoclonal therapy, PD-1 has been used in the treatment of multiple tumors, including melanoma, and can predict the survival of patients. Recent studies have described the characteristics of tumor infiltrating immune-related lncRNAs (Ti-lncRNAs) and have found a better efficacy of anti-PD-1 treatment in melanoma patients with a low Ti-lncRNA score (116). WGCNA indicated that 15 lncRNAs, such as NARF-AS1 and LINC01126, were identified to predict the prognosis of melanoma patients treated with anti-PD-1 (117). By regulating the expression of miR-33a-5p and miR-330b-5p, lncRNA-LINC01140 promotes c-Myc expression, suppresses cisplatin-induced apoptosis and promotes cell proliferation and metastasis. In addition, this lncRNA directly decreased the expression of miR-377-3p and miR-155-5p, resulting in increased PD-L1 expression. Knockdown of lncRNA-LINC01140 in combination with CIK treatment can inhibit the expression of PD-L1 in severe combined immunodeficiency mice and has the potential to become a more effective target for tumor growth inhibition (118). Additionally, Q Hu et al. found that the level of lncRNA-LINK-A was elevated and that the antigen peptide-loading complex was downregulated in triple-negative breast cancer patients with PD-1 blockade tolerance, which may provide a basis for the development of new combined immunotherapies and effective early prevention strategies (119). In addition, lncRNA-SNHG29 inhibits PD-L1 expression under treatment with simvastatin (considered a novel inhibitor of PD-L1) by mediating YAP activation and promoting the antitumor immune process, which clarifies the therapeutic implications of SNHG29 in an antitumor immune response (120). In the cytoplasm, lncRNA-IFITM4P directly binds to SASH1 and phosphorylates TAK1 (Thr187) to increase the phosphorylation of NF-κB (ser536), thus inducing the expression of PD-L1, inhibiting the activation of the immune system and increasing the immune escape of tumor cells. IFITM4P enhances the interaction of KDM5A with the PTEN promoter, resulting in reduced transcription of PTEN and upregulated PD-L1 expression, thus activating the therapeutic sensitivity of PD-1 in the nucleus of tumor cells (121). LncRNA-NORAD reduces the expression of miR-199a-5p in exosomes by inhibiting the expression of pri-miR-199a1, and pri-miR 199a1 suppresses the ATR/Chk1 pathway by targeting EEPD1, enabling cells to better respond to radiotherapy. At the same time, inhibiting the expression of NORAD can reduce the ubiquitination of PD-L1, thereby increasing sensitivity to radiation and anti-PD-1 therapy in a mouse model (122). Thus, lncRNAs are involved in PD-1/PD-L1-related immunotherapy and may become a target of combined therapy.
The method of editing lncRNAs in T cells has the potential to become a new antitumor immunotherapy. Recent studies have found that knockdown of lncRNA-NKILA in cytotoxic T lymphocytes regulates the sensitivity of T cells to activation-induced cell death by reducing the expression of NF-κB, thereby effectively inhibiting the growth of patient-derived xenografts from breast cancer in mice (123). Melanoma-overexpressed antigen 1 (MELOE-1), which is encoded by a long noncoding RNA in tumor cells and can specifically improve the tumor antigen of MELOE-1 through thapsigargin drug stimulation, enhances the ability of T cells to recognize melanoma cells (124). Additionally, exosome-related therapy can inhibit tumor progression by regulating the immune system of the organism. M1 macrophage-derived exosomal lncRNA-HOTTIP and M2 macrophage-derived exosomal lncRNA-AFAP1-AS1 affect tumor metastasis by modulating the miR-26a/ATF2 axis and miR-19a/b-3p/TLR5/NF-κB signaling pathway, respectively, which provides a potential strategy for tumor immunotherapy (125, 126). In summary, targeting tumors with immune checkpoint inhibitors (especially anti- PD-1/PD-L1) combined with lncRNA modulation is a promising method for tumor therapy.
6.2. Targeted therapy
Approximately 66% of malignant melanomas have BRAF (B-RAF proto-oncogene) mutations, which lead to an increase in constitutive BRAF kinase activity and the MEK-ERK1/2 pathway and are necessary for proliferation, invasion and survival in melanoma cells (127). Recent studies have found that lncRNAs are associated with BRAF mutation and the growth of melanoma cells (Figure 3). LncRNA-ZEB1-AS1 is upregulated in melanoma cells and is related to the mutations of BRAF and RAS family genes, which can affect the invasion and migration of melanoma by activating the expression of ZEB1 (128). Moreover, a p53-induced long intergenic noncoding RNA (named LINC-PINT) affects the proliferation, migration and invasion of melanoma by interacting with the BRAF-activated noncoding RNA/MAPK pathway (129). Additionally, lncRNA-orilncl (the genetic target of RAS) was upregulated in BRAF mutant melanoma and promoted tumor cell proliferation and growth by regulating the RAS-RAF-MEK-ERK signaling pathway (75).
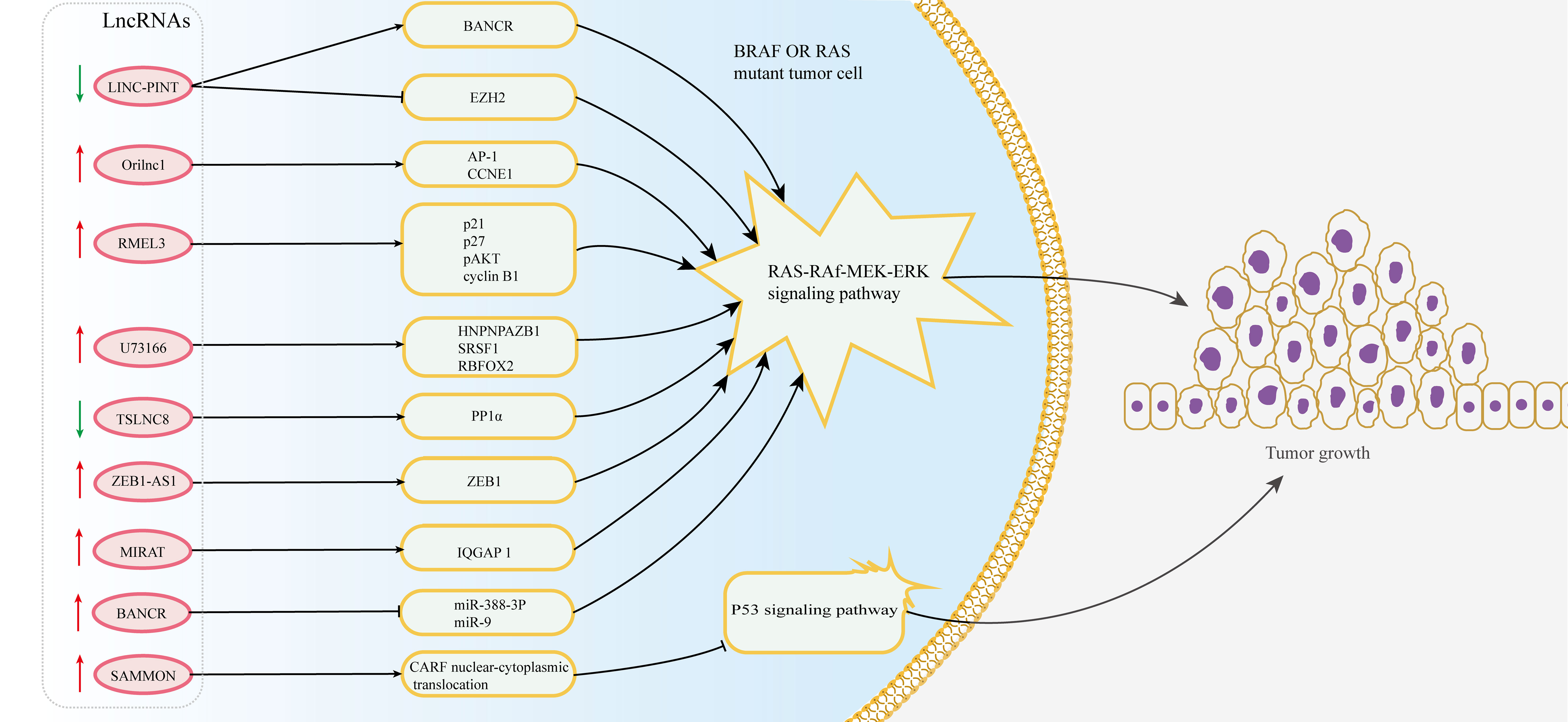
Figure 3 Multiple lncRNAs regulate resistance to BRAF inhibitors in melanoma. The dysregulation of lncRNAs targets the MAPK and p53 signaling pathway by regulating downstream target proteins in BRAF or RAS mutant melanoma cells, thus affecting the resistance of melanoma to BRAF inhibitors.
Although BRAF inhibitors have made great progress in the treatment of melanoma, the generation of drug resistance in tumor cells limits their efficacy. The experiment identified 11 lncRNA loci that induce resistance to BRAF inhibitors through genome-scale CRISPR activation screening and characterization (130). The expression of lncRNA-RMEL3 is significantly increased in BRAF V600E mutant melanoma cells and can be regulated by BRAF and MEK inhibitors. And its expression can promote colony formation in melanoma cells and the growth of subcutaneous xenografts in mice by inducing protein levels of p21, p27, pAKT and cyclin B1 (131). LncRNA-SAMMSON is a target of the transcription factor Sox10 and can interact with p32 to strengthen its role in targeting mitochondria and promoting cancer progression (132). The overexpression of SAMMSON made melanoma cells tolerant to the cytotoxicity induced by vemurafenib (functioning as an inhibitor of mutant BRAF kinase) by modulating the CARF/p53 axis (76, 77). Additionally, the intergenic lncRNA-U73166 changes the proliferation, migration and invasion abilities of melanoma cells and is also related to vemurafenib chemotherapy resistance (133). In addition, the expression level of lncRNA-TSLNC8 is downregulated in BRAF inhibitor-resistant melanoma cells, and its low expression attenuates the toxicity response of tumor cells to PLX4720 (a type of BRAF inhibitor). Mechanistically, TSLNC8 activates the MAPK signaling pathway by regulating the accumulation of PP1α in the cytoplasm and promotes the sensitivity of tumor cells to PLX4720, which enables melanoma patients to benefit from the combined treatment of PLX4720 and TSLNC8 (134). MIRAT, a novel cytoplasmic intergenic lncRNA, is upregulated in NRAS mutant melanoma and regulates the MEK scaffold protein IQGAP1 and MAPK signaling pathways to influence the drug resistance of tumor cells (78). Thus, it has the prospect to better explore the mechanism of drug resistance and improve the response to BRAF inhibitors.
Regulating the expression of lncRNAs by common drugs or oligonucleotides may be a potential way to inhibit the occurrence and development of melanoma. Researchers found that lncRNA-SLNCR interacts with AR and regulates the combination of AR- and EGR1-specific genomic sites, which cooperate with growth-related downstream regulatory genes to promote the proliferation of melanoma (135). Taking advantage of oligonucleotides binding to the AR N-terminal domain or AR RNA motif to block the interaction between SLNCR and AR represents a feasible therapeutic strategy in the process of melanoma (136). In addition, lncRNA-ZCCHC4 inhibits DNA damage-induced apoptosis by interacting with lncRNA-AL133467.2, and knockdown of this gene can enhance chemosensitivity to DDA in hepatocellular carcinoma cells, which is a potential target to improve the chemotherapeutic effect (137). Additionally, the lncRNA-POU3F3 expression level was elevated in dacarbazine-resistant melanoma cells, and knockdown of POU3F3 restored the sensitivity of cells to dacarbazine by secreting miR-650 and upregulating the expression of MGMT protein (138). Another novel therapeutic strategy, reprogramming abnormal lncRNA-ANRIL in gene clusters at chromosome 9p21, can significantly reduce the ability of tumor growth and metastasis (139). Therefore, targeting lncRNAs is a feasible way to inhibit the progression of melanoma.
7. Conclusion
Melanoma is one of the most rapidly progressing tumors with strong metastatic potential. Although a large number of genes involved in the tumor process have been found in melanoma, the specific molecular targets for their occurrence and development still need further research. Accumulating studies have found that lncRNAs play a key role in biological processes, including tumor proliferation, migration, invasion, cell cycle, apoptosis, stemness, EMT and chemoresistance. Based on the role of lncRNAs in melanoma, it can be used as a biomarker or a therapeutic target for the early diagnosis, prognosis and treatment of melanoma patients. Because lncRNAs can be secreted into the body fluids, the early diagnosis and speculated prognosis of melanoma can be performed painless by the lncRNAs analysis in the body fluids, compared with the invasive biohistopathological biopsies. For treatment, if the tumor suppressor lncRNA is downregulated, we might return it to normal function or even overexpression. Conversely, if the oncogenic lncRNA is upregulated in melanoma, we may suppress the oncogenic lncRNA. ASOs(antisense oligonucleotides), RNAi(RNA interference) and CRISPR(clustered regularly interspaced short palindromic repeats) are the main methods of downregulating lncRNAs. However, because the molecular mechanism of many lncRNAs is unclear and the interaction with functional partners, including proteins, is still uncertain, the development of lncRNA therapy is limited to a certain extent and needs further research.
Immunotherapy (such as immune checkpoint inhibitors) and targeted therapies (such as BRAF inhibitors) can exert certain therapeutic effects, but their effectiveness is usually limited by drug resistance. Reassuringly, a few lncRNAs can influence the therapeutic effect of immune checkpoint inhibitors in melanoma, and it is a feasible method to target tumors in combination with immune checkpoint inhibitors and lncRNA regulators. Furthermore, lncRNAs also play a regulatory role in alleviating the drug resistance caused by the use of BRAF inhibitors. Moreover, it is effective to eliminate the point mutation of the binding site between lncRNA and protein or use oligonucleotides to block the invasion of melanoma, which indicates that targeting lncRNA and its protein complexes has therapeutic prospects in melanoma. However, it is still unclear whether other novel lncRNAs are involved in immunotherapy and targeted therapy and whether they can be applied to clinical treatment. Therefore, elucidating the mechanism of lncRNAs in immunotherapy and targeted therapy and applying it to improve the effectiveness of drug therapy remain to be studied.
Author contributions
Literature search and manuscript preparation: WZ. Concepts and paper design: XX, YC and JC. Manuscript review: JC. Authors contributed to the article and approved the submitted version.
Funding
This study was funded by 1•3•5 project for disciplines of excellence, West China Hospital, Sichuan University, No. ZYPY20001 and ZYPY20002.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Berwick M, Buller DB, Cust A, Gallagher R, Lee TK, Meyskens F, et al. Melanoma epidemiology and prevention. Melanoma (2016) 167:17–49. doi: 10.1007/978-3-319-22539-5_2
2. Rozeman EA, Dekker TJ, Haanen JB, Blank CU. Advanced melanoma: current treatment options, biomarkers, and future perspectives. Am J Clin Dermatol (2018) 19(3):303–17. doi: 10.1007/s40257-017-0325-6
3. Luan W, Ding Y, Yuan H, Ma S, Ruan H, Wang J, et al. Long non-coding RNA LINC00520 promotes the proliferation and metastasis of malignant melanoma by inducing the miR-125b-5p/EIF5A2 axis. J Exp Clin Cancer Res (2020) 39(1):1–16. doi: 10.1186/s13046-020-01599-7
4. Mou K, Liu B, Ding M, Mu X, Han D, Zhou Y, et al. lncRNA-ATB functions as a competing endogenous RNA to promote YAP1 by sponging miR-590-5p in malignant melanoma. Int J Oncol (2018) 53(3):1094–104. doi: 10.3892/ijo.2018.4454
5. Pan B, Lin X, Zhang L, Hong W, Zhang Y. Long noncoding RNA X-inactive specific transcript promotes malignant melanoma progression and oxaliplatin resistance. Melanoma Res (2019) 29(3):254–62. doi: 10.1097/CMR.0000000000000560
6. Liu Y, He D, Xiao M, Zhu Y, Zhou J, Cao K. Long noncoding RNA LINC00518 induces radioresistance by regulating glycolysis through an miR-33a-3p/HIF-1α negative feedback loop in melanoma. Cell Death Dis (2021) 12(3):1–19. doi: 10.1038/s41419-021-03523-z
7. Xia Y, Zhou Y, Han H, Li P, Wei W, Lin N. lncRNA NEAT1 facilitates melanoma cell proliferation, migration, and invasion via regulating miR-495-3p and E2F3. J Cell Physiol (2019) 234(11):19592–601. doi: 10.1002/jcp.28559
8. Zhou W-J, Wang H-Y, Zhang J, Dai HY, Yao ZX, Zheng Z, et al. NEAT1/miR-200b-3p/SMAD2 axis promotes progression of melanoma. Aging (Albany NY) (2020) 12(22):22759. doi: 10.18632/aging.103909
9. Munteanu MC, Sethuraman SN, Singh MP, Singh MP, Malayer J, Ranjan A, et al. LncRNA FENDRR expression correlates with tumor immunogenicity. Genes (2021) 12(6):897. doi: 10.3390/genes12060897
10. Li Z, Zhang J, Zheng H, Li C, Xiong J, Wang W, et al. Modulating lncRNA SNHG15/CDK6/miR-627 circuit by palbociclib, overcomes temozolomide resistance and reduces M2-polarization of glioma associated microglia in glioblastoma multiforme. J Exp Clin Cancer Res (2019) 38(1):1–13. doi: 10.1186/s13046-019-1371-0
11. Xu L, Zhang Y, Zhao Z, Chen Z, Wang Z, Xu S, et al. The long non-coding RNA CRNDE competed endogenously with miR-205 to promote proliferation and metastasis of melanoma cells by targeting CCL18. Cell Cycle (2018) 17(18):2296–308. doi: 10.1080/15384101.2018.1526602
12. Zhao L, Liu Y, Zhang J, Liu Y, Qi Q. LncRNA SNHG14/miR-5590-3p/ZEB1 positive feedback loop promoted diffuse large b cell lymphoma progression and immune evasion through regulating PD-1/PD-L1 checkpoint. Cell Death Dis (2019) 10(10):1–15. doi: 10.1038/s41419-019-1886-5
13. Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Pineros M, Znaor A, et al. Cancer statistics for the year 2020: An overview. Int J Cancer (2021) 149(4):778–89. doi: 10.1002/ijc.33588
14. Schadendorf D, van Akkooi AC, Berking C, Griewank KG, Gutzmer R, Hauschild A, et al. Melanoma. Lancet (2018) 392(10151):971–84. doi: 10.1016/S0140-6736(18)31559-9
15. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
16. Wang M, Gendreau JL, Gemelas J, Capulong D, Lau C, Mata-Diaz S, et al. Diagnosis and management of malignant melanoma in store-and-forward teledermatology. Telemed e-Health (2017) 23(11):877–80. doi: 10.1089/tmj.2017.0009
17. Cummins DL, Cummins JM, Pantle H, Silverman MA, Leonard AL, Chanmugam A. Cutaneous malignant melanoma. Mayo Clin Proc (2006) 81:500–07. doi: 10.4065/81.4.500
18. Berk-Krauss J, Stein JA, Weber J, Polsky D, Geller AC. New systematic therapies and trends in cutaneous melanoma deaths among US whites, 1986–2016. Am J Public Health (2020) 110(5):731–33. doi: 10.2105/AJPH.2020.305567
19. Willsmore ZN, Coumbe BG, Crescioli S, Reci S, Gupta A, Harris RJ, et al. Combined anti-PD-1 and anti-CTLA-4 checkpoint blockade: Treatment of melanoma and immune mechanisms of action. Eur J Immunol (2021) 51(3):544–56. doi: 10.1002/eji.202048747
20. Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol (2018) 19(11):1480–92. doi: 10.1016/S1470-2045(18)30700-9
21. Wei SC, Levine JH, Cogdill AP, Zhao Y, Anang N, Andrews MC, et al. Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1 checkpoint blockade. Cell (2017) 170(6):1120–33.e17. doi: 10.1016/j.cell.2017.07.024
22. Carlino MS, Larkin J, Long GV. Immune checkpoint inhibitors in melanoma. Lancet (2021) 398(10304):1002–14. doi: 10.1016/S0140-6736(21)01206-X
23. Akbani R, Akdemir KC, Aksoy BA, Albert M, Ally A, Amin SB, et al. Genomic classification of cutaneous melanoma. Cell (2015) 161(7):1681–96. doi: 10.1016/j.cell.2015.05.044
24. Tao Q, Ming J, Lan Y. The mutational landscape of mucosal melanoma. In: Semin Cancer Biol 61:139–48. doi: 10.1016/j.semcancer.2019.09.013
25. Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WJ, et al. Braf(V600E) cooperates with pten silencing to elicit metastatic melanoma. Nat Genet (2009) 41:544–52. doi: 10.1038/ng.356
26. Zu T, Wang D, Xu S, Lee C, Zhen E, Yoon CH, et al. ATF-3 expression inhibits melanoma growth by downregulating ERK and AKT pathways. Lab Invest (2021) 101(5):636–47. doi: 10.1038/s41374-020-00516-y
27. Tang R, Xu X, Yang W, Yu W, Hou S, Xuan Y, et al. MED27 promotes melanoma growth by targeting AKT/MAPK and NF-κB/iNOS signaling pathways. Cancer Lett (2016) 373(1):77–87. doi: 10.1016/j.canlet.2016.01.005
28. Loria R, Laquintana V, Scalera S, Fraioli R, Caprara V, Falcone I, et al. SEMA6A/RhoA/YAP axis mediates tumor-stroma interactions and prevents response to dual BRAF/MEK inhibition in BRAF-mutant melanoma. J Exp Clin Cancer Res (2022) 41(1):1–18. doi: 10.1186/s13046-022-02354-w
29. Tang L, Liang Y, Xie H, Yang X, Zheng G. Long non-coding RNAs in cutaneous biology and proliferative skin diseases: Advances and perspectives. Cell Proliferation (2020) 53(1):e12698. doi: 10.1111/cpr.12698
30. Hu G, Ma J, Zhang J, Chen Y, Liu H, Huang Y, et al. Hypoxia-induced lncHILAR promotes renal cancer metastasis via ceRNA for the miR-613/206/1-1-3p/Jagged-1/Notch/CXCR4 signaling pathway. Mol Ther (2021) 29(10):2979–94. doi: 10.1016/j.ymthe.2021.05.020
31. Li Y, Gao Y, Niu X, Tang M, Li J, Song B, et al. LncRNA BASP1-AS1 interacts with YBX1 to regulate notch transcription and drives the malignancy of melanoma. Cancer Sci (2021) 112(11):4526. doi: 10.1111/cas.15140
32. Vimalraj S, Subramanian R, Dhanasekaran A. LncRNA MALAT1 promotes tumor angiogenesis by regulating MicroRNA-150-5p/VEGFA signaling in osteosarcoma: In-vitro and in-vivo analyses. Front Oncol (2021) 11:742789. doi: 10.3389/fonc.2021.742789
33. Liu Y, Zhang P, Wu Q, Fang H, Wang Y, Xiao Y, et al. Long non-coding RNA NR2F1-AS1 induces breast cancer lung metastatic dormancy by regulating NR2F1 and ΔNp63. Nat Commun (2021) 12(1):1–14. doi: 10.1038/s41467-021-25552-0
34. Zhu P, He F, Hou Y, Tu G, Li Q, Jin T, et al. A novel hypoxic long noncoding RNA KB-1980E6. 3 maintains breast cancer stem cell stemness via interacting with IGF2BP1 to facilitate c-myc mRNA stability. Oncogene (2021) 40(9):1609–27. doi: 10.1038/s41388-020-01638-9
35. Mao T-L, Fan M-H, Dlamini N, Liu CL. LncRNA MALAT1 facilitates ovarian cancer progression through promoting chemoresistance and invasiveness in the tumor microenvironment. Int J Mol Sci (2021) 22(19):10201. doi: 10.3390/ijms221910201
36. Ren J, Ding L, Zhang D, Shi G, Xu Q, Shen S, et al. Carcinoma-associated fibroblasts promote the stemness and chemoresistance of colorectal cancer by transferring exosomal lncRNA H19. Theranostics (2018) 8(14):3932. doi: 10.7150/thno.25541
37. Weng X, Liu H, Ruan J, Du M, Wang L, Mao J, et al. HOTAIR/miR-1277-5p/ZEB1 axis mediates hypoxia-induced oxaliplatin resistance via regulating epithelial-mesenchymal transition in colorectal cancer. Cell Death Discovery (2022) 8(1):1–10. doi: 10.1038/s41420-022-01096-0
38. Li H, Wang X, Wen C, Huo Z, Wang W, Zhan Q, et al. Long noncoding RNA NORAD, a novel competing endogenous RNA, enhances the hypoxia-induced epithelial-mesenchymal transition to promote metastasis in pancreatic cancer. Mol Cancer (2017) 16(1):1–14. doi: 10.1186/s12943-017-0738-0
39. Jia Y, Tian C, Wang H, Yu F, Lv W, Duan Y, et al. Long non-coding RNA NORAD/miR-224-3p/MTDH axis contributes to CDDP resistance of esophageal squamous cell carcinoma by promoting nuclear accumulation of β-catenin. Mol Cancer (2021) 20(1):1–22. doi: 10.1186/s12943-021-01455-y
40. Dong P, Xiong Y, Yue J, Xu D, Ihira K, Konno Y, et al. Long noncoding RNA NEAT1 drives aggressive endometrial cancer progression via miR-361-regulated networks involving STAT3 and tumor microenvironment-related genes. J Exp Clin Cancer Res (2019) 38(1):1–15. doi: 10.1186/s13046-019-1306-9
41. Orre C, Dieu X, Guillon J, Gueguen N, Ahmadpour ST, Dumas JF, et al. The long non-coding RNA SAMMSON is a regulator of chemosensitivity and metabolic orientation in MCF-7 doxorubicin-resistant breast cancer cells. Biology (2021) 10(11):1156. doi: 10.3390/biology10111156
42. Yang J, Shi X, Yang M, Luo J, Gao Q, Wang X, et al. Glycolysis reprogramming in cancer-associated fibroblasts promotes the growth of oral cancer through the lncRNA H19/miR-675-5p/PFKFB3 signaling pathway. Int J Oral Sci (2021) 13(1):1–11. doi: 10.1038/s41368-021-00115-7
43. Sun C-C, Zhu W, Li S-J, Hu W, Zhang J, Zhuo Y, et al. FOXC1-mediated LINC00301 facilitates tumor progression and triggers an immune-suppressing microenvironment in non-small cell lung cancer by regulating the HIF1α pathway. Genome Med (2020) 12(1):1–27. doi: 10.1186/s13073-020-00773-y
44. Xu M, Xu X, Pan B, Chen X, Lin K, Zeng K, et al. LncRNA SATB2-AS1 inhibits tumor metastasis and affects the tumor immune cell microenvironment in colorectal cancer by regulating SATB2. Mol Cancer (2019) 18(1):1–16. doi: 10.1186/s12943-019-1063-6
45. Chen C, He W, Huang J, Wang B, Li H, Cai Q, et al. LNMAT1 promotes lymphatic metastasis of bladder cancer via CCL2 dependent macrophage recruitment. Nat Commun (2018) 9(1):1–18. doi: 10.1038/s41467-018-06152-x
46. Pu J, Li W, Wang A, Zhang Y, Qin Z, Xu Z, et al. Long non-coding RNA HOMER3-AS1 drives hepatocellular carcinoma progression via modulating the behaviors of both tumor cells and macrophages. Cell Death Dis (2021) 12(12):1–13. doi: 10.1038/s41419-021-04309-z
47. Han C, Yang Y, Sheng Y, Wang J, Li W, Zhou X, et al. The mechanism of lncRNA-CRNDE in regulating tumour-associated macrophage M2 polarization and promoting tumour angiogenesis. J Cell Mol Med (2021) 25(9):4235–47. doi: 10.1111/jcmm.16477
48. Zhang Y, Qian W, Feng F, Cao Q, Li Y, Hou Y, et al. Upregulated lncRNA CASC2 may inhibit malignant melanoma development through regulating miR-18a-5p/RUNX1. Oncol Res (2019) 27(3):371. doi: 10.3727/096504018X15178740729367
49. Mu X, Mou KH, Ge R, Han D, Zhou Y, Wang LJ, et al. Linc00961 inhibits the proliferation and invasion of skin melanoma by targeting the miR−367/PTEN axis. Int J Oncol (2019) 55(3):708–20. doi: 10.3892/ijo.2019.4848
50. Xu Y, Zhang J, Zhang Q, Xu H, Liu L. Long non-coding RNA HOXA11-as modulates proliferation, apoptosis, metastasis and EMT in cutaneous melanoma cells partly via miR-152-3p/ITGA9 axis. Cancer Manage Res (2021) 13:925. doi: 10.2147/CMAR.S281920
51. Yang Y, Jin L, He J, Wang R, Wang Y, Bai J, et al. Upregulation LncRNA MEG3 expression suppresses proliferation and metastasis in melanoma via miR-208/SOX4. Mol Cell Biochem (2022), 1–8. doi: 10.1007/s11010-022-04515-z
52. Long J, Pi X. lncRNA-MEG3 suppresses the proliferation and invasion of melanoma by regulating CYLD expression mediated by sponging miR-499-5p. BioMed Res Int (2018) 2018:2086564. doi: 10.1155/2018/2086564
53. Chen L, Yang H, Xiao Y, Tang X, Li Y, Han Q. LncRNA GAS5 is a critical regulator of metastasis phenotype of melanoma cells and inhibits tumor growth in vivo. OncoTargets Ther (2016) 9:4075. doi: 10.2147/OTT.S98203
54. Chen L, Yang H, Yi Z, Jiang L, Li Y, Han Q, et al. LncRNA GAS5 regulates redox balance and dysregulates the cell cycle and apoptosis in malignant melanoma cells. J Cancer Res Clin Oncol (2019) 145(3):637–52. doi: 10.1007/s00432-018-2820-4
55. Hussen BM, Azimi T, Hidayat HJ, Taheri M, Ghafouri-Fard S. NF-KappaB interacting LncRNA: review of its roles in neoplastic and non-neoplastic conditions. Biomed Pharmacother (2021) 139:111604. doi: 10.1016/j.biopha.2021.111604
56. Zhou X, Rao Y, Sun Q, Liu Y, Chen J, Bu W. Long noncoding RNA CPS1-IT1 suppresses melanoma cell metastasis through inhibiting Cyr61 via competitively binding to BRG1. J Cell Physiol (2019) 234(12):22017–27. doi: 10.1002/jcp.28764
57. Huang Q, Zhang D, Diao Q, Lin M. lncRNA LINC−PINT is downregulated in melanoma and regulates cell proliferation by downregulating lncRNA BANCR. Oncol Lett (2019) 18(3):2917–22. doi: 10.3892/ol.2019.10631
58. Xu Y, Wang H, Li F, Heindl LM, He X, Yu J, et al. Long non-coding RNA LINC-PINT suppresses cell proliferation and migration of melanoma via recruiting EZH2. Front Cell Dev Biol (2019) 7:350. doi: 10.3389/fcell.2019.00350
59. Shi G, Li H, Gao F, Tan Q. Lncrna h19 predicts poor prognosis in patients with melanoma and regulates cell growth, invasion, migration and epithelial–mesenchymal transition in melanoma cells. OncoTargets Ther (2018) 11:3583. doi: 10.2147/OTT.S160143
60. An L-f, Huang J-w, Han X, Wang J. Downregulation of lncRNA H19 sensitizes melanoma cells to cisplatin by regulating the miR-18b/IGF1 axis. Anti-Cancer Drugs (2020) 31(5):473–82. doi: 10.1097/CAD.0000000000000888
61. Yang Y, Zhang Z, Wu Z, Lin W, Yu M. Downregulation of the expression of the lncRNA MIAT inhibits melanoma migration and invasion through the PI3K/AKT signaling pathway. Cancer Biomarkers (2019) 24(2):203–11. doi: 10.3233/CBM-181869
62. Lu F, Song Y, Cui S, Zhao H, Chen Y, Du H. LncRNA MIAT promotes the proliferation, migration, and invasion of melanoma cells through recruiting TCF12 and activating NFAT5. Am J Trans Res (2021) 13(11):12588.
63. Chen X, Gao G, Liu S, Yu L, Yan D, Yao X, et al. Long noncoding RNA PVT1 as a novel diagnostic biomarker and therapeutic target for melanoma. BioMed Res Int (2017) 2017:7038579. doi: 10.1155/2017/7038579
64. Chen L, Ma D, Li Y, Li X, Zhao L, Zhang J, et al. Effect of long non-coding RNA PVT1 on cell proliferation and migration in melanoma. Int J Mol Med (2018) 41(3):1275–82. doi: 10.3892/ijmm.2017.3335
65. Li F, Li X, Qiao L, Liu W, Xu C, Wang X, et al. MALAT1 regulates miR-34a expression in melanoma cells. Cell Death Dis (2019) 10(6):1–11. doi: 10.1038/s41419-019-1620-3
66. Wang P, Hu L, Fu G, Lu J, Zheng Y, Li Y, et al. LncRNA MALAT1 promotes the proliferation, migration, and invasion of melanoma cells by downregulating miR-23a. Cancer Manage Res (2020) 12:6553. doi: 10.2147/CMAR.S249348
67. Wei Y, Sun Q, Zhao L, Wu J, Chen X, Wang Y, et al. LncRNA UCA1-miR-507-FOXM1 axis is involved in cell proliferation, invasion and G0/G1 cell cycle arrest in melanoma. Med Oncol (2016) 33(8):1–9. doi: 10.1007/s12032-016-0804-2
68. Wang D, Chen J, Li B, Jiang Q, Liu L, Xia Z, et al. A noncoding regulatory RNA Gm31932 induces cell cycle arrest and differentiation in melanoma via the miR-344d-3-5p/Prc1 (and Nuf2) axis. Cell Death Dis (2022) 13(4):1–12. doi: 10.1038/s41419-022-04736-6
69. Hong C-H, Ho J-C, Lee C-H. Steroid receptor RNA activator, a long noncoding RNA, activates p38, facilitates epithelial-mesenchymal transformation, and mediates experimental melanoma metastasis. J Invest Dermatol (2020) 140(7):1355–63.e1. doi: 10.1016/j.jid.2019.09.028
70. Zhang S, Wan H, Zhang X. LncRNA LHFPL3-AS1 contributes to tumorigenesis of melanoma stem cells via the miR-181a-5p/BCL2 pathway. Cell Death Dis (2020) 11(11):1–16. doi: 10.1038/s41419-020-03141-1
71. Guo J, Gan Q, Gan C, Zhang X, Ma X, Dong M. LncRNA MIR205HG regulates melanomagenesis via the miR-299-3p/VEGFA axis. Aging (Albany NY) (2021) 13(4):5297. doi: 10.18632/aging.202450
72. Han S, Li X, Wang K, Zhu D, Meng B, Liu J, et al. PURPL represses autophagic cell death to promote cutaneous melanoma by modulating ULK1 phosphorylation. Cell Death Dis (2021) 12(11):1–13. doi: 10.1038/s41419-021-04362-8
73. Wang Y, Liu G, Ren L, Wang K, Liu A. Long non-coding RNA TUG1 recruits miR−29c−3p from its target gene RGS1 to promote proliferation and metastasis of melanoma cells. Int J Oncol (2019) 54(4):1317–26. doi: 10.3892/ijo.2019.4699
74. Long J, Menggen Q, Wuren Q, Shi Q, Pi X. Long noncoding RNA taurine-upregulated gene1 (TUG1) promotes tumor growth and metastasis through TUG1/Mir-129-5p/astrocyte-elevated gene-1 (AEG-1) axis in malignant melanoma. Med Sci monitor: Int Med J Exp Clin Res (2018) 24:1547. doi: 10.12659/msm.906616
75. Zhang D, Zhang G, Hu X, Wu L, Feng Y, He S, et al. Oncogenic RAS regulates long noncoding RNA Orilnc1 in human CancerRAS regulates lncRNA in human cancer. Cancer Res (2017) 77(14):3745–57. doi: 10.1158/0008-5472.CAN-16-1768
76. Han S, Yan Y, Ren Y, Hu Y, Wang Y, Chen L, et al. LncRNA SAMMSON mediates adaptive resistance to RAF inhibition in BRAF-mutant melanoma cells. Cancer Res (2021) 81(11):2918–29. doi: 10.1158/0008-5472.can-20-3145
77. Goding CR. Targeting the lncRNA SAMMSON reveals metabolic vulnerability in melanoma. Cancer Cell (2016) 29(5):619–21. doi: 10.1016/j.ccell.2016.04.010
78. Sanlorenzo M, Vujic I, Esteve-Puig R, Lai K, Vujic M, Lin K, et al. The lincRNA MIRAT binds to IQGAP1 and modulates the MAPK pathway in NRAS mutant melanoma. Sci Rep (2018) 8(1):1–9. doi: 10.1038/s41598-018-27643-3
79. Yu X, Zheng H, Tse G, Zhang L, Wu W. CASC 2: An emerging tumour-suppressing long noncoding RNA in human cancers and melanoma. Cell Proliferation (2018) 51(6):e12506. doi: 10.1111/cpr.12506
80. Melixetian M, Bossi D, Mihailovich M, Punzi S, Barozzi I, Marocchi F, et al. Long non-coding RNA TINCR suppresses metastatic melanoma dissemination by preventing ATF4 translation. EMBO Rep (2021) 22(3):e50852. doi: 10.15252/embr.202050852
81. Han X, Jia Y, Chen X, Sun C, Sun J. lncRNA TINCR attenuates the proliferation and invasion, and enhances the apoptosis of cutaneous malignant melanoma cells by regulating the miR−424−5p/LATS1 axis. Oncol Rep (2021) 46(5):1–11. doi: 10.3892/or.2021.8189
82. Liao Z, Zhao J, Yang Y. Downregulation of lncRNA H19 inhibits the migration and invasion of melanoma cells by inactivating the NF−κB and PI3K/Akt signaling pathways. Mol Med Rep (2018) 17(5):7313–18. doi: 10.3892/mmr.2018.8782
83. Cai B, Zheng Y, Ma S, Xing Q, Wang X, Yang B, et al. BANCR contributes to the growth and invasion of melanoma by functioning as a competing endogenous RNA to upregulate Notch2 expression by sponging miR−204. Int J Oncol (2017) 51(6):1941–51. doi: 10.3892/ijo.2017.4173
84. Lee WJ, Shin CH, Ji H, Jeong SD, Park MS, Won HH, et al. hnRNPK-regulated LINC00263 promotes malignant phenotypes through miR-147a/CAPN2. Cell Death Dis (2021) 12(4):1–18. doi: 10.1038/s41419-021-03575-1
85. Huang Q, Wu J, Wang H, Li N, Yang Z, Zhang M. LncRNA taurine upregulated gene 1 as a potential biomarker in the clinicopathology and prognosis of multiple malignant tumors: A meta-analysis. Dis Markers (2021) 2021:8818363. doi: 10.1155/2021/8818363
86. Wang Y, Ba H-J, Wen X-Z, Zhou M, Kucuk C, Tamagnone L, et al. A prognostic model for melanoma patients on the basis of immune-related lncRNAs. Aging (Albany NY) (2021) 13(5):6554. doi: 10.18632/aging.202730
87. Ma Y, Wang N, Yang S. Skin cutaneous melanoma properties of immune-related lncRNAs identifying potential prognostic biomarkers. Aging (Albany NY) (2022) 14(7):3030. doi: 10.18632/aging.203982
88. Rong J, Wang H, Yao Y, Wu Z, Chen L, Jin C, et al. Identification of m7G-associated lncRNA prognostic signature for predicting the immune status in cutaneous melanoma. Aging (Albany NY) (2022) 14(12):5233. doi: 10.18632/aging.204151
89. Ho K-H, Huang T-W, Shih C-M, Lee YT, Liu AJ, Chen PH, et al. Glycolysis-associated lncRNAs identify a subgroup of cancer patients with poor prognoses and a high-infiltration immune microenvironment. BMC Med (2021) 19(1):1–17. doi: 10.1186/s12916-021-01925-6
90. Yang W, Qiu Z, Zhang J, Zhi X, Yang L, Qiu M, et al. Correlation between immune cell infiltration and PD-L1 expression and immune-related lncRNA determination in triple-negative breast cancer. Front Genet (2022) 662:878658. doi: 10.3389/fgene.2022.878658
91. Guo J, Wang S, Jiang Z, Tang L, Liu Z, Cao J, et al. Long non-coding RNA RFPL3S functions as a biomarker of prognostic and immunotherapeutic prediction in testicular germ cell tumor. Front Immunol (2022) 13:859730. doi: 10.3389/fimmu.2022.859730
92. Liu K, Zhang L, Li X, Zhao J. High expression of lncRNA HSD11B1−AS1 indicates favorable prognosis and is associated with immune infiltration in cutaneous melanoma. Oncol Lett (2022) 23(2):1–14. doi: 10.3892/ol.2021.13172
93. Du M, Han L, Shen P, Wu D, Tu S. Long noncoding RNA LINC02249 is a prognostic biomarker and correlates with immunosuppressive microenvironment in skin cutaneous melanoma. J Oncol (2022) 2022:2054901. doi: 10.1155/2022/2054901
94. Chen G, Yan J. Dysregulation of SNHG16 (lncRNA)-hsa-let-7b-5p (miRNA)-TUBB4A (mRNA) pathway fuels progression of skin cutaneous melanoma. Curr Protein Pept Sci (2022) 23:791–809. doi: 10.2174/1389201023666220928120902
95. Wang L, Felts SJ, Van Keulen VP, Scheid AD, Block MS, Markovic SN, et al. Integrative genome-wide analysis of long noncoding RNAs in diverse immune cell types of melanoma PatientsCharacterization of long noncoding RNAs in melanoma. Cancer Res (2018) 78(15):4411–23. doi: 10.1158/0008-5472.CAN-18-0529
96. Andrews MC, Oba J, Wu C-J, Zhu H, Karpinets T, Creasy CA, et al. Multi-modal molecular programs regulate melanoma cell state. Nat Commun (2022) 13(1):1–18. doi: 10.1038/s41467-022-31510-1
97. Cantile M, Scognamiglio G, Marra L, Aquino G, Botti C, Falcone MR, et al. HOTAIR role in melanoma progression and its identification in the blood of patients with advanced disease. J Cell Physiol (2017) 232(12):3422–32. doi: 10.1002/jcp.25789
98. Ni C, Fang Q-Q, Chen W-Z, Jiang JX, Jiang Z, Ye J, et al. Breast cancer-derived exosomes transmit lncRNA SNHG16 to induce CD73+ γδ1 treg cells. Signal transduction targeted Ther (2020) 5(1):1–14. doi: 10.1038/s41392-020-0129-7
99. Sun J, Jia H, Bao X, Wu Y, Zhu T, Li R, et al. Tumor exosome promotes Th17 cell differentiation by transmitting the lncRNA CRNDE-h in colorectal cancer. Cell Death Dis (2021) 12(1):1–14. doi: 10.1038/s41419-020-03376-y
100. Yin W, Song Y, Chang X. Single-cell RNA-seq analysis identifies a noncoding interleukin 4 (IL-4) RNA that post-transcriptionally up-regulates IL-4 production in T helper cells. J Biol Chem (2019) 294(1):290–98. doi: 10.1074/jbc.ra118.004111
101. Zheng T, Ma G, Tang M, Li Z, Xu R. IL-8 secreted from M2 macrophages promoted prostate tumorigenesis via STAT3/MALAT1 pathway. Int J Mol Sci (2018) 20(1):98. doi: 10.3390/ijms20010098
102. Tao S, Chen Q, Lin C, Dong H. Linc00514 promotes breast cancer metastasis and M2 polarization of tumor-associated macrophages via Jagged1-mediated notch signaling pathway. J Exp Clin Cancer Res (2020) 39(1):1–17. doi: 10.1186/s13046-020-01676-x
103. Zhao K, Wang X, Zhao D, Lin Q, Zhang Y, Hu Y. lncRNA HITT inhibits lactate production by repressing PKM2 oligomerization to reduce tumor growth and macrophage polarization. Research (2022) 2022:9854904. doi: 10.34133/2022/9854904
104. Liu Y, Shi M, He X, Cao Y, Liu P, Li F, et al. LncRNA-PACERR induces pro-tumour macrophages via interacting with miR-671-3p and m6A-reader IGF2BP2 in pancreatic ductal adenocarcinoma. J Hematol Oncol (2022) 15(1):1–18. doi: 10.1186/s13045-022-01272-w
105. Qian M, Ling W, Ruan Z. Long non-coding RNA SNHG12 promotes immune escape of ovarian cancer cells through their crosstalk with M2 macrophages. Aging (Albany NY) (2020) 12(17):17122. doi: 10.18632/aging.103653
106. Liu R, Sun X, Hu Z, Peng C, Wu . Knockdown of long non-coding RNA MIR155HG suppresses melanoma cell proliferation, and deregulated MIR155HG in melanoma is associated with M1/M2 balance and macrophage infiltration. Cells Dev (2022) 170:203768. doi: 10.1016/j.cdev.2022.203768
107. Yang Y, Ma S, Ye Z, Zheng Y, Zheng Z, Liu X, et al. NEAT1 in bone marrow mesenchymal stem cell-derived extracellular vesicles promotes melanoma by inducing M2 macrophage polarization. Cancer Gene Ther (2022) 29:1–12. doi: 10.1038/s41417-021-00392-8
108. Wang M, Chen D, Xu Y, Qiu M, Jiang X, Xiong Z. Identification and validation of the lncRNA BACE1-AS as immune-related influencing factor in tumorigenesis following pan-carcinoma analysis. J Immunol Res (2021) 2021:1589864. doi: 10.1155/2021/1589864
109. Wang Y, Liu F, Chen L, Fang C, Li S, Yuan S, et al. Neutrophil extracellular traps (NETs) promote non-small cell lung cancer metastasis by suppressing lncRNA MIR503HG to activate the NF-κB/NLRP3 inflammasome pathway. Front Immunol (2022) 13:867516. doi: 10.3389/fimmu.2022.867516
110. Zhou Q, Tang X, Tian X, Tian J, Zhang Y, Ma J, et al. LncRNA MALAT1 negatively regulates MDSCs in patients with lung cancer. J Cancer (2018) 9(14):2436. doi: 10.7150/jca.24796
111. Li Y, Zhang Q, Wu M, Zhang P, Huang L, Ai X, et al. Suppressing MDSC infiltration in tumor microenvironment serves as an option for treating ovarian cancer metastasis. Int J Biol Sci (2022) 18(9):3697–713. doi: 10.7150/ijbs.70013
112. Tian X, Zheng Y, Yin K, Ma J, Tian J, Zhang Y, et al. LncRNA AK036396 inhibits maturation and accelerates immunosuppression of polymorphonuclear myeloid–derived suppressor cells by enhancing the stability of ficolin BLncRNA AK036396/Fcnb accelerates PMN-MDSC immunosuppression. Cancer Immunol Res (2020) 8(4):565–77. doi: 10.1158/2326-6066.CIR-19-0595
113. Sun J, Zhang Z, Bao S, Yan C, Hou P, Wu N, et al. Identification of tumor immune infiltration-associated lncRNAs for improving prognosis and immunotherapy response of patients with non-small cell lung cancer. J immunother Cancer (2020) 8(1). doi: 10.1136/jitc-2019-000110
114. Qu S, Jiao Z, Lu G, Yao B, Wang T, Rong W, et al. PD-L1 lncRNA splice isoform promotes lung adenocarcinoma progression via enhancing c-myc activity. Genome Biol (2021) 22(1):1–24. doi: 10.1186/s13059-021-02331-0
115. Mineo M, Lyons SM, Zdioruk M, von Spreckelsen N, Ferrer-Luna R, Ito H, et al. Tumor interferon signaling is regulated by a lncRNA INCR1 transcribed from the PD-L1 locus. Mol Cell (2020) 78(6):1207–23. doi: 10.1016/j.molcel.2020.05.015
116. Ma B, Jiang H, Luo Y, Liao T, Xu W, Wang X, et al. Tumor-infiltrating immune-related long non-coding RNAs indicate prognoses and response to PD-1 blockade in head and neck squamous cell carcinoma. Front Immunol (2021) 12:692079. doi: 10.3389/fimmu.2021.692079
117. Zhou J-G, Liang B, Liu J-G, Jin SH, He SS, Frey B, et al. Identification of 15 lncRNAs signature for predicting survival benefit of advanced melanoma patients treated with anti-PD-1 monotherapy. Cells (2021) 10(5):977. doi: 10.3390/cells10050977
118. Xia R, Geng G, Yu X, Xu Z, Guo J, Liu H, et al. LINC01140 promotes the progression and tumor immune escape in lung cancer by sponging multiple microRNAs. J immunother Cancer (2021) 9(8). doi: 10.1136/jitc-2021-002746
119. Hu Q, Ye Y, Chan L-C, Li Y, Liang K, Lin A, et al. Oncogenic lncRNA downregulates cancer cell antigen presentation and intrinsic tumor suppression. Nat Immunol (2019) 20(7):835–51. doi: 10.1038/s41590-019-0400-7
120. Ni W, Mo H, Liu Y, Xu Y, Qin C, Zhou Y, et al. Targeting cholesterol biosynthesis promotes anti-tumor immunity by inhibiting long noncoding RNA SNHG29-mediated YAP activation. Mol Ther (2021) 29(10):2995–3010. doi: 10.1016/j.ymthe.2021.05.012
121. Shi L, Yang Y, Li M, Li C, Zhou Z, Tang G, et al. LncRNA IFITM4P promotes immune escape by up-regulating PD-L1 via dual mechanism in oral carcinogenesis. Mol Ther (2022) 30(4):1564–77. doi: 10.1016/j.ymthe.2022.01.003
122. Sun Y, Wang J, Ma Y, Li J, Sun X, Zhao X, et al. Radiation induces NORAD expression to promote ESCC radiotherapy resistance via EEPD1/ATR/Chk1 signalling and by inhibiting pri-miR-199a1 processing and the exosomal transfer of miR-199a-5p. J Exp Clin Cancer Res (2021) 40(1):1–22. doi: 10.1186/s13046-021-02084-5
123. Huang D, Chen J, Yang L, Ouyang Q, Li J, Lao L, et al. NKILA lncRNA promotes tumor immune evasion by sensitizing T cells to activation-induced cell death. Nat Immunol (2018) 19(10):1112–25. doi: 10.1038/s41590-018-0207-y
124. Charpentier M, Dupré E, Fortun A, Briand F, Maillasson M, Com E, et al. hnRNP-A1 binds to the IRES of MELOE-1 antigen to promote MELOE-1 translation in stressed melanoma cells. Mol Oncol (2022) 16(3):594–606. doi: 10.1002/1878-0261.13088
125. Jiang H, Zhou L, Shen N, Ning X, Wu D, Jiang K, et al. M1 macrophage-derived exosomes and their key molecule lncRNA HOTTIP suppress head and neck squamous cell carcinoma progression by upregulating the TLR5/NF-κB pathway. Cell Death Dis (2022) 13(2):1–15. doi: 10.1038/s41419-022-04640-z
126. Mi X, Xu R, Hong S, Xu T, Zhang W, Liu M. M2 macrophage-derived exosomal lncRNA AFAP1-AS1 and MicroRNA-26a affect cell migration and metastasis in esophageal cancer. Mol Therapy-Nucleic Acids (2020) 22:779–90. doi: 10.1016/j.omtn.2020.09.035
127. Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature (2002) 417(6892):949–54. doi: 10.1038/nature11412
128. Siena ÁDD, Plaça JR, Araújo LF, de Barros II, Peronni K, Molfetta G, et al. Whole transcriptome analysis reveals correlation of long noncoding RNA ZEB1-AS1 with invasive profile in melanoma. Sci Rep (2019) 9(1):1–11. doi: 10.1038/s41598-019-47363-6
129. He T, Yuan C, Zhao C. Long intragenic non-coding RNA p53-induced transcript (LINC-PINT) as a novel prognosis indicator and therapeutic target in cancer. Biomed Pharmacother (2021) 143:112127. doi: 10.1016/j.biopha.2021.112127
130. Joung J, Engreitz JM, Konermann S, Abudayyeh OO, Verdine VK, Aguet F, et al. Genome-scale activation screen identifies a lncRNA locus regulating a gene neighbourhood. Nature (2017) 548(7667):343–46. doi: 10.1038/nature23451
131. Cardoso C, Serafim RB, Kawakami A, Goncalves PC, Roszik J, Valente V, et al. The lncRNA RMEL3 protects immortalized cells from serum withdrawal-induced growth arrest and promotes melanoma cell proliferation and tumor growth. Pigment Cell melanoma Res (2019) 32(2):303–14. doi: 10.1111/pcmr.12751
132. Leucci E, Vendramin R, Spinazzi M, Laurette P, Fiers M, Wouters J, et al. Melanoma addiction to the long non-coding RNA SAMMSON. Nature (2016) 531(7595):518–22. doi: 10.1038/nature17161
133. Siena ÁDD, Barros I, Storti CB, de Biagi JC, Da CCL, Maria-Engler SS, et al. Upregulation of the novel lncRNA U731166 is associated with migration, invasion and vemurafenib resistance in melanoma. J Cell Mol Med (2022) 26(3):671–83. doi: 10.1111/jcmm.16987
134. Han Y, Fang J, Xiao Z, Deng J, Zhang M, Gu L. Downregulation of lncRNA TSLNC8 promotes melanoma resistance to BRAF inhibitor PLX4720 through binding with PP1α to re-activate MAPK signaling. J Cancer Res Clin Oncol (2021) 147(3):767–77. doi: 10.1007/s00432-020-03484-4
135. Schmidt K, Carroll JS, Yee E, Thomas DD, Wert-Lamas L, Neier SC, et al. The lncRNA SLNCR recruits the androgen receptor to EGR1-bound genes in melanoma and inhibits expression of tumor suppressor p21. Cell Rep (2019) 27(8):2493–507.e4. doi: 10.1016/j.celrep.2019.04.101
136. Schmidt K, Weidmann CA, Hilimire TA, Yee E, Hatfield BM, Schneekloth JJ, et al. Targeting the oncogenic long non-coding RNA SLNCR1 by blocking its sequence-specific binding to the androgen receptor. Cell Rep (2020) 30(2):541–54.e5. doi: 10.1016/j.celrep.2019.12.011
137. Zhu H, Chen K, Chen Y, Liu J, Zhang X, Zhou Y, et al. RNA-Binding protein ZCCHC4 promotes human cancer chemoresistance by disrupting DNA-damage-induced apoptosis. Signal Transduct Target Ther (2022) 7:240. doi: 10.1038/s41392-022-01033-8
138. Wu K, Wang Q, Liu Y-L, Xiang Z, Wang QQ, Yin L, et al. LncRNA POU3F3 contributes to dacarbazine resistance of human melanoma through the MiR-650/MGMT axis. Front Oncol (2021) 11:643613. doi: 10.3389/fonc.2021.643613
Keywords: lncRNAs, melanoma, immunotherapy, tumor microenvironment, targeted therapy
Citation: Zhou WC, Xu XW, Cen Y and Chen JJ (2022) The role of lncRNAs in the tumor microenvironment and immunotherapy of melanoma. Front. Immunol. 13:1085766. doi: 10.3389/fimmu.2022.1085766
Received: 31 October 2022; Accepted: 05 December 2022;
Published: 19 December 2022.
Edited by:
Mark Andrew Lindsay, University of Bath, United KingdomReviewed by:
Isabella Parolini, National Institute of Health (ISS), ItalyMireia Castillo-Martin, Champalimaud Foundation, Portugal
Copyright © 2022 Zhou, Xu, Cen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junjie Chen, Y2pqZW1haWxAMTYzLmNvbQ==
 Wencheng Zhou
Wencheng Zhou Xuewen Xu
Xuewen Xu Junjie Chen
Junjie Chen