
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol., 09 December 2022
Sec. Viral Immunology
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1053914
This article is part of the Research TopicWomen In Viral ImmunologyView all 5 articles
The infection of Nocardia gipuzkoensis is a relatively uncommon form of pulmonary nocardiosis seen in clinical patients. In general, nocardiosis tends to occur in patients with immune deficiency. Here, we report a 23-year-old female who was admitted to the hospital due to cough and sputum production over 10 years, diagnosed with bronchiectasis. The N. gipuzkoensis infection was identified by metagenomic next-generation sequencing and whole genome sequencing. Imipenem/cilastatin and compound sulfamethoxazole tablets were used to control the infection and the pulmonary inflammation subsided gradually.
● N. gipuzkoensis can be responsible for opportunistic infections in immunocompetent humans.
● The mNGS combined with WGS is reliable for identifying and typing strains of species.
● Identification and elimination of Nocardia might be the way for preventing bronchiectasis caused due to recurrent infections.
Nocardia is a genus of rod-shaped bacteria with the characteristics of weakly staining Gram-positive and catalase-positive. It contains a total of approximately 134 species according to Wikipedia (last revised on August 24, 2022), and an increasing number of species have been recognized as human pathogens. The common clinical presentations contain pulmonary and cutaneous nocardiosis, which are acquired by inhalation of the bacteria or through traumatic introduction (1, 2). Nocardia is considered a major opportunistic pathogen, especially affecting patients with impaired immune systems. However, Nocardia infections in immunocompetent patients have been increasingly reported (3, 4). In immunocompetent patients, chronic obstructive pulmonary disease and bronchiectasis could be considered to represent risk factors for pulmonary Nocardia (5–7). It is worth mentioning that a novel drug-resistant community-acquired Nocardia spp was identified in a patient with bronchiectasis (8).
Metagenomic NGS (mNGS) is an incredibly sensitive detection method to understand which microbes are present and in what proportions via running all nucleic acids in a sample to the reference genomes (9, 10). Compared with traditional culture methods, mNGS provides a faster detection turnaround time and identifies theoretically all infectious pathogens in clinical specimens by sequencing DNA or RNA fragments; the detection rate would not be reduced while the patients had received antibiotic treatment (11). On the other hand, cultivating cannot classify specific species; however, mNGS was more efficient and sensitive in detecting different Nocardia (12). Its possible clinical applications are tremendous, especially in the discovery of pathogens from respiratory samples, blood, and other body fluid.
Whole genome sequencing (WGS) is a testing method that determines the order of nucleotide bases in the genome of an organism, therefore assessing the bacterial genomic features and possible origins. WGS procedures have qualified significant contributions in virology areas, especially for the rapid identification and classification of pathogens (13). So far, WGS has been used for revealing the genetic diversity, taxonomic structure, and the evolution relationship of the Nocardia (14).
In this report, we present a case of an immunocompetent patient suffering from recurrent lung infections caused by Nocardia gipuzkoensis, which was identified through the mNGS combined with WGS; its diagnosis and treatment processes were reported as well.
A 23-year-old female was admitted to the Respiratory Department of The First Affiliated Hospital, Guangzhou University of Chinese Medicine, with recurrent cough and phlegm that had been present for more than 10 years. The timeline during hospitalization is shown in Figure 1, and the complete case progress record in detail is reported as follows.
The patient was diagnosed with bronchiectasis on May 29, 2021, according to the symptoms of chronic cough and sputum production as well as chest computed tomography (CT), which demonstrated pneumonia with bronchiectasis in the right middle lobe (RML) and left lower lobe (LLL) principally. The patient had not received further diagnosis and treatment systematically. By asking for medical history and consulting the patient’s medical record, we noticed the patient had been hospitalized for the same illness on February 16, 2022. The IgM test of respiratory tract pathogens showed that Legionella pneumophila and mycoplasma pneumoniae were positive. The patient got better and was discharged after being given antibiotics containing Nemonoxacin, Cefoperazone-sulbactam, and Piperacillin-sulbactam. However, the patient did not undergo fiberoptic bronchoscopy to obtain bronchoalveolar lavage fluid (BALF) for mNGS but only identified the potential infectious pathogens by the IgM test.
Approximately 10 days before this admission, the patient developed an aggravated cough with sputum production and even a little hemoptysis, so the patient was admitted to the respiratory department for treatment on July 20, 2022. No obvious abnormalities were observed in vital signs. Chest CT performed at an external hospital on June 26, 2022, showed more serious pneumonia with bronchiectasis in the RML and LLL. The laboratory investigations after hospital admission prompted normal C-reactive protein, blood sedimentation rate, and normal kidney and liver function. However, blood analysis on the day of admission showed that the total white blood cells (WBC) were 9.44*10^9/L, the total number of neutrophils (NEU) was 6.79*10^9/L, the total lymphocyte count (LYM) was 2.03*10^9/L, and neutrophil percentage (NEU%) was 71.9%. Levofloxacin injection was the initial empiric antimicrobial therapy administered.
To identify the pathogen as soon as possible, fiberoptic bronchoscopy was performed on hospital day 2 to obtain BALF for mNGS. Airway secretions can be found via fiberoptic bronchoscopy; the lesions of the bronchus are presented in Figure 2. The N. gipuzkoensis infection was identified by mNGS (results of this analysis are shown in Table 1). The antibiotic regimen was then adjusted to Imipenem/cilastatin, 0.5g Q8H IV, and compound sulfamethoxazole tablets (SMZ), 2# Q6H PO (composed of Trimethoprim 80 mg and sulfamethoxazole 0.4 g per tablet). WGS technology was arranged for genome-wide profiling.
On hospital day 7, drug susceptibility tests according to the standards of U.S. Clinical and Laboratory Standards Institute (CLSI) (15) demonstrated sensitivity to Ceftriaxone, Linezolid, and Trimethoprim/Sulfamethoxazole (the antibiotic susceptibility of Nocardia from the BALF is shown in Table 2). Therefore, this patient achieved unchanged antibiotic treatment. On July 30 (day 10 after admission), the repeat blood routine examination results, WBC, NEU, LYM, and NEU% were 4.93*10^9/L, 2.60*10^9/L, 1.68*10^9/L, and 52.7%, respectively, decreased compared with before, indicating that the condition had improved. A chest CT scan demonstrated that bronchiectasis was evident in the RML and LLL with multiple nodular, flocculent shadow, and tree-in-bud signs. Imaging changes over time showed progressive improvement in pneumonia with bronchiectasis (Figure 3). Finally, she was discharged on August 1, 2022, and 7 days of SMZ treatment was continued.
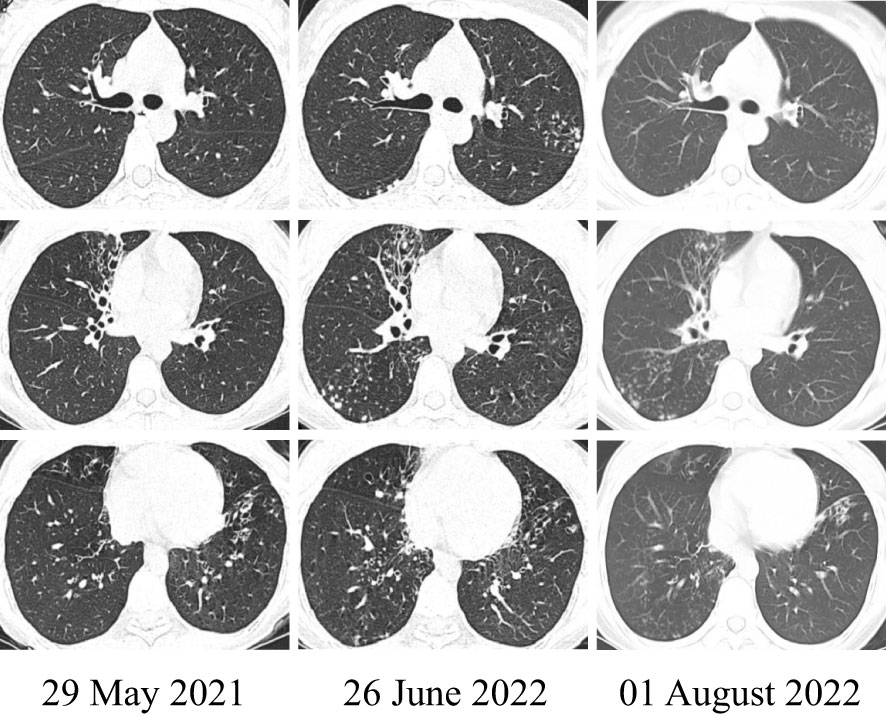
Figure 3 The representative image: changing in the patient’s chest CT. The CT on June 26, 2022, compared with May 29, 2021, showed more serious pneumonia with bronchiectasis in the RML and LLL. However, after the identification of Nocardia species and systemic treatment, the imaging on August 1, 2022, showed improvement.
After waiting approximately a month, we received the patient’s WGS results and identified the infection of N. gipuzkoensis. The coverage map of N. gipuzkoensis is shown in Figure 4. The sequences identified by the whole genome were 54,536. The phylogenetic trees were constructed based on the sequences of 16S rRNA gene and housekeeping genes and are shown as Figure 5.
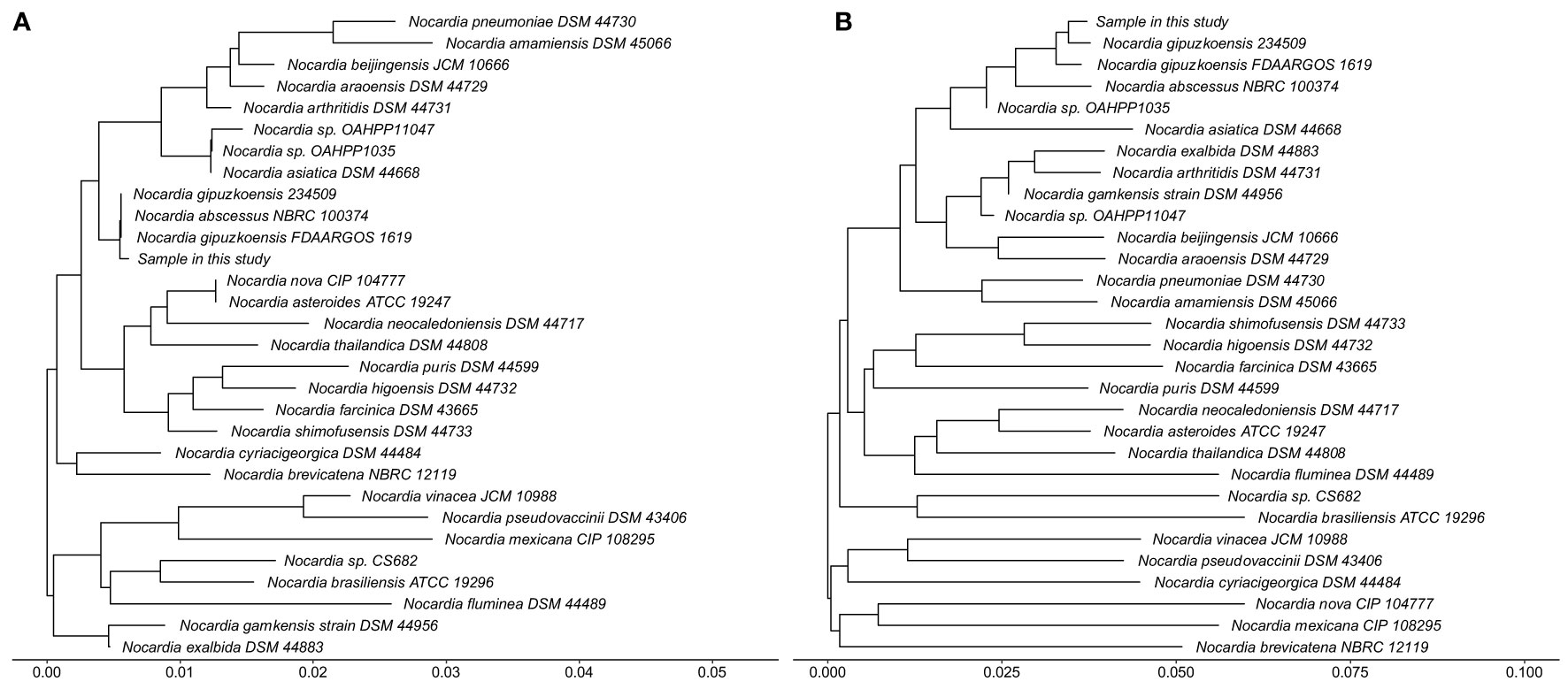
Figure 5 Phylogenetic trees based on the sequences of 16S rRNA gene (A) and housekeeping genes (B) show the phylogenetic position of the sample in this study within the evolutionary radiation of the genus Nocardia. The housekeeping genes contains hsp65, secA1, gyrB, and rpoB. The methods of determining phylogeny are following the steps: comparing multiple sequences by MAFFT, extracting conserved sequences by Gblocks, and then constructing the Neighbor-Joining by TreeBeST.
At the same time, the strain was validated by quantitative PCR (qPCR). The primers for N. gipuzkoensis used in qPCR detection were Forward, 5’- GGCACGACCGATAACCGAACG-3’, and Reverse, 5’-ATCCATCCGCTACCGCTCCTTC-3’. We designed the primers based on the N. gipuzkoensis sequences from the Basic Local Alignment Search Tool. We tested their amplification specificity with Primer-BLAST software. No target templates were found in the Refseq mRNA database (organism limited to homo sapiens). Using our qPCR assay, the BALF sample of three replicates was positive compared with the sample of another healthy adult.
Nocardiosis is an uncommon and opportunistic bacterial infection caused by inhalation or direct inoculation. This patient lived in an environment where mud for turtles was piled up at the bottom of the bed for a long time, and this is considered to be an important cause of Nocardia infection. It is especially interesting that N. gipuzkoensis was finally identified through mNGS combined with WGS.
N. gipuzkoensis was initially isolated from the sputum of an old female patient, who was diagnosed with bronchiectasis before, and expectoration was the main symptom (16). This seems to indicate that elderly patients with chronic lung diseases are more susceptible to N. gipuzkoensis, but no more cases have been reported.
The use of corticoid treatment in people with chronic lung disease, such as bronchiectasis, was a risk factor for pulmonary nocardiosis (7). A rare case of pulmonary nocardiosis has also been reported in a non-immunocompromised patient who had bronchiectasis without corticoid treatment (17). Lung consolidation, nodules, pleural involvement, and chest wall extension were the frequent CT findings of pulmonary nocardiosis (18). In this patient, the presentation of bronchiectasis with pneumonia progressed over the past year. The patient had recurrently suffered lower respiratory tract infections since the diagnosis of bronchiectasis in 2021 but improved with the treatment of SMZ after the identification of Nocardia. Before the nocardiosis diagnosis, the CT in 2022 compared with 2021 showed more serious pneumonia with bronchiectasis in the RML and LLL. However, after the identification of Nocardia and receiving systemic treatment, the next imaging showed an improvement. We speculated that infection of the Nocardia might be a significant etiology causing bronchiectasis. The elimination of Nocardia could be the way for preventing bronchiectasis caused due to recurrent infections. In the case of bronchiectasis with recurring pneumonia, BALF collected via fiberoptic bronchoscopy should be performed as soon as possible, and the pathogenic bacteria should be identified by high-throughput sequencing technology. The using of mNGS combined with WGS is reliable and practical for identifying and typing strains of species.
We have reported a case of an immunocompetent patient suffering from recurrent lung infections caused by the infection of N. gipuzkoensis. Nevertheless, we did no laboratory studies to evaluate these bacterial attributes in an attempt to clarify the pathogenesis of the disease. Next, an animal experiment could be considered to test the pathogenicity in vivo.
The original contributions presented in the study are included in the article/supplementary materials. Further inquiries can be directed to the corresponding authors.
Written informed consent was provided by the patient to allow the case details and images to be published. Our report was approved by ethics committee of The First Affiliated Hospital, Guangzhou University of Chinese Medicine (K-2022-108).
Article design and writing: CL. The collection and detection of clinical sample: JY. Patient management and treatment: HH, SZ and XX. All authors contributed to the article and approved the submitted version.
This work has been funded by grants from the “Double First-Class” and High-level University Discipline Collaborative Innovation Team Project of Guangzhou University of Chinese Medicine (Grant No. 2021XK16), and Special project for inheriting classics of clinical Chinese medicine initiated by Guangzhou University of Chinese Medicine (A1-2601-22-429-002Z02).
We would like to thank Vision Medicals Co., Ltd., Guangzhou, China for technical guidance in our research, and also assisting us with clinical sample collection and detection.
Author JY is employed by Vision Medicals Co., Ltd., Guangzhou, China.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ott SR, Meier N, Kolditz M, Bauer TT, Rohde G, Presterl E, et al. Pulmonary nocardiosis in Western Europe-clinical evaluation of 43 patients and population-based estimates of hospitalization rates. Int J Infect Dis (2019) 81:140–8. doi: 10.1016/j.ijid.2018.12.010
2. Laube H. Primary cutaneous nocardiosis. Deutsches Arzteblatt Int (2019) 116:362. doi: 10.3238/arztebl.2019.0362a
3. Abe S, Tanabe Y, Ota T, Fujimori F, Youkou A, Makino M. Case report: pulmonary nocardiosis caused by nocardia exalbida in an immunocompetent patient. BMC Infect Dis (2021) 21:776. doi: 10.1186/s12879-021-06416-w
4. Wintheiser GA, Venable ER, Temesgen Z. Disseminated nocardia in an immunocompetent host. Mayo Clinic Proc (2021) 96:847–8. doi: 10.1016/j.mayocp.2020.11.019
5. Menéndez R, Cordero PJ, Santos M, Gobernado M, Marco V. Pulmonary infection with nocardia species: a report of 10 cases and review. Eur Respir J (1997) 10:1542–6. doi: 10.1183/09031936.97.10071542
6. Garcia-Bellmunt L, Sibila O, Solanes I, Sanchez-Reus F, Plaza V. Pulmonary nocardiosis in patients with COPD: characteristics and prognostic factors. Archivos bronconeumol (2012) 48:280–5. doi: 10.1016/j.arbr.2012.06.006
7. Ercibengoa M, Càmara J, Tubau F, García-Somoza D, Galar A, Martín-Rabadán P, et al. A multicentre analysis of nocardia pneumonia in Spain: 2010-2016. Int J Infect Dis (2020) 90:161–6. doi: 10.1016/j.ijid.2019.10.032
8. Li Z, Li Y, Li S, Li Z, Mai Y, Cheng J, et al. Identification of a novel drug-resistant community-acquired nocardia spp. in a patient with bronchiectasis. Emerging Microbes infections (2022) 11:1346–55. doi: 10.1080/22221751.2022.2069514
9. Bragg L, Tyson GW. Metagenomics using next-generation sequencing. Methods Mol Biol (Clifton N.J.) (2014) 1096:183–201. doi: 10.1007/978-1-62703-712-9_15
10. Miller S, Chiu C. The role of metagenomics and next-generation sequencing in infectious disease diagnosis. Clin Chem (2021) 68:115–24. doi: 10.1093/clinchem/hvab173
11. Miao Q, Ma Y, Wang Q, Pan J, Zhang Y, Jin W, et al. Microbiological diagnostic performance of metagenomic next-generation sequencing when applied to clinical practice. Clin Infect Dis (2018) 67:S231–s240. doi: 10.1093/cid/ciy693
12. Weng SS, Zhang HY, Ai JW, Gao Y, Liu YY, Xu B, et al. Rapid detection of nocardia by next-generation sequencing. Front Cell infection Microbiol (2020) 10:13. doi: 10.3389/fcimb.2020.00013
13. Quiñones-Mateu ME, Avila S, Reyes-Teran G, Martinez MA. Deep sequencing: becoming a critical tool in clinical virology. J Clin Virol (2014) 61:9–19. doi: 10.1016/j.jcv.2014.06.013
14. Xu S, Li Z, Huang Y, Han L, Che Y, Hou X, et al. Whole genome sequencing reveals the genomic diversity, taxonomic classification, and evolutionary relationships of the genus nocardia. PloS Negl Trop Dis (2021) 15:e0009665. doi: 10.1371/journal.pntd.0009665
15. Woods GL, Brown-Elliott BA, Conville PS, Desmond EP, Hall GS, Lin G, et al. CLSI standards: Guidelines for health care excellence, susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes. Wayne (PA: Clinical and Laboratory Standards Institute Copyright (2011).
16. Nouioui I, Cortés-Albayay C, Neumann-Schaal M, Vicente D, Cilla G, Klenk HP, et al. Genomic virulence features of two novel species nocardia barduliensis sp. nov. and nocardia gipuzkoensis sp. nov., isolated from patients with chronic pulmonary diseases. Microorganisms (2020) 8:1517. doi: 10.3390/microorganisms8101517
17. Cremades MJ, Menéndez R, Santos M, Gobernado M. Repeated pulmonary infection by nocardia asteroides complex in a patient with bronchiectasis. Respiration Int Rev Thorac Dis (1998) 65:211–3. doi: 10.1159/000029264
Keywords: nocardiosis, antibiotic susceptibility, pathogen identification, metagenomic next-generation sequencing, whole genome sequencing, case report
Citation: Liu C, Yang J, Huang H, Zhan S and Xia X (2022) Case report: Nocardia gipuzkoensis infection in an immunocompetent patient diagnosed by metagenomic next-generation sequencing and whole genome sequencing. Front. Immunol. 13:1053914. doi: 10.3389/fimmu.2022.1053914
Received: 26 September 2022; Accepted: 21 November 2022;
Published: 09 December 2022.
Edited by:
Susan Prockop, Boston Children’s Hospital and Harvard Medical School, United StatesReviewed by:
Catalina Lunca, Grigore T. Popa University of Medicine and Pharmacy, RomaniaCopyright © 2022 Liu, Yang, Huang, Zhan and Xia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaofeng Zhan, emhhbnNoYW9mZW5nMTY4MEBnenVjbS5lZHUuY24=; Xintian Xia, dGlhbjExMzI2NkAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.