
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 08 December 2022
Sec. Vaccines and Molecular Therapeutics
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1040027
Quercetin (QCT) is a naturally occurring phenolic flavonoid compound with inbuilt characteristics of antioxidant, anti-inflammatory, and immune protection. Several recent studies have shown that QCT and QCTits nanoparticles have therapeutic potential against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection. Novel therapeutics also include the implication of extracellular vesicles (EVs) to protect from SARS-CoV-2 viral infection. This article highlighted the therapeutic/prophylactic potential of engineered EVs loaded with QCT against SARS-CoV-2 infection. Several biotechnological engineering approaches are available to deliver EVs loaded with QCT nanoparticles. Among these biotechnological advances, a specific approach with significantly higher efficiency and yield has to be opted to fabricate such drug delivery of nano molecules, especially to combat SARS-CoV-2 infection. The current treatment regime protects the human body from virus infection but has some limitations including drugs and long-term steroid side effects. However, the vaccine strategy is somehow effective in inhibiting the spread of coronavirus disease-19 (COVID-19) infection. Moreover, the proposed exosomal therapy met the current need to repair the damaged tissue along with inhibition of COVID-19-associated complications at the tissue level. These scientific findings expand the possibilities and predictability of developing a novel and cost-effective therapeutic approach that combines the dual molecule, EVs and QCT nanoparticles, to treat SARS-CoV-2 infection. Therefore, the most suitable engineering method to fabricate such a drug delivery system should be better understood before developing novel therapeutics for clinical purposes.
QCT is a plant flavonoid, also known as 3,3′,4′,5,7-pentahydroxyflavone, and is present in naturally occurring vegetables, grains, leaves, and seeds in the form of QCT glycosides bounded with residual sugars (1). QCT exhibits various biological properties, including antioxidant (2), immunoprotective (3), anti-inflammatory, and anti-viral (4). QCT, being a promising anti-viral, showed its effect by inhibition of biological enzymes including protease (5), polymerase (6), reverse transcriptase (7), DNA gyrase and proteins of viral capsids (8, 9). Furthermore, QCT functions as a protein kinase inhibitor, a phytoestrogen, a quinone, a chelator, a free radical scavenger, and an Aurora kinase inhibitor (10). Previously published studies showed that neutrophils upon treatment with QCT flavonoids demonstrated suppression of pro-inflammatory gene mRNA along with mi-RNA modulation (11, 12). QCT application in COVID-19 infection occurs significant therapeutic effect if used in combination with standard care of treatment. Moreover, recently two studies demonstrated effectiveness of using QCT with remdesivir and favipiravir in hospitalized patients with severe SARS-CoV-2 infection. The patients were given 1000 mg QCT along with anti-viral drugs daily and found to reduced serum levels of ALP, q-CRP, and LDH in the intervention group compared to those who were on only standard care of treatment (13). Similarly in another randomized, open-label, and controlled clinical study, the add-on supplement of the QCT particles with standard care of treatment showed viral clearance within 1 week of the infection (14). Such studies favoured the utility of using QCT nanoparticles either alone or in combination with standard care of treatment for effective management and treatment of COVID-19 infection.
QCT showed its abundance in many plants, including apples, grapes, green tea, citrus fruits, cherries, onions, coffee, red wine, and others (15). However, QCT exhibits low bioavailability and hence the need to supplement with other supplements such as catechins, resveratrol, and genistein to increase the high absorption within the intestinal cells (16–19). Moreover, QCT has several limitations in pharmaceuticals, including instability, low solubility, poor permeability, and low bioavailability. Within the past decades, numerous nanotechnology-based approaches were designed to combat such limitations. Some of the delivery approaches of QCT to overcome such limitations include liposomes, inclusion complexes, micelles, and nanoparticles. These nanotechnology-based approaches have proved beneficial in the treatment of several human diseases, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections.
QCT possessed anti-viral characteristics and thereby hampered the life cycle of SARS-CoV-2 by interfering with viral replication cycles and lowering the inflammation responses. Recently published studies demonstrated that QCT interferes with the viral replication cycle of SARS-CoV-2 and thereby offers numerous opportunities to explore QCT as a supplement in the form of nanoparticles as therapeutics in COVID-19 infection (20–22). A recent study also claimed that QCT supplementation offers 87% improvement in the form of survival outcomes in patients with SARS-CoV-2 infection and may be more beneficial during the early stages of COVID-19 infection (21–24). In another randomized controlled trial (RCT), QCT showed prophylaxis among COVID-19- infected patients (25) Some of the pioneer studies related to QCT therapy in COVID-19 infection are presented in Figure 1 (26).
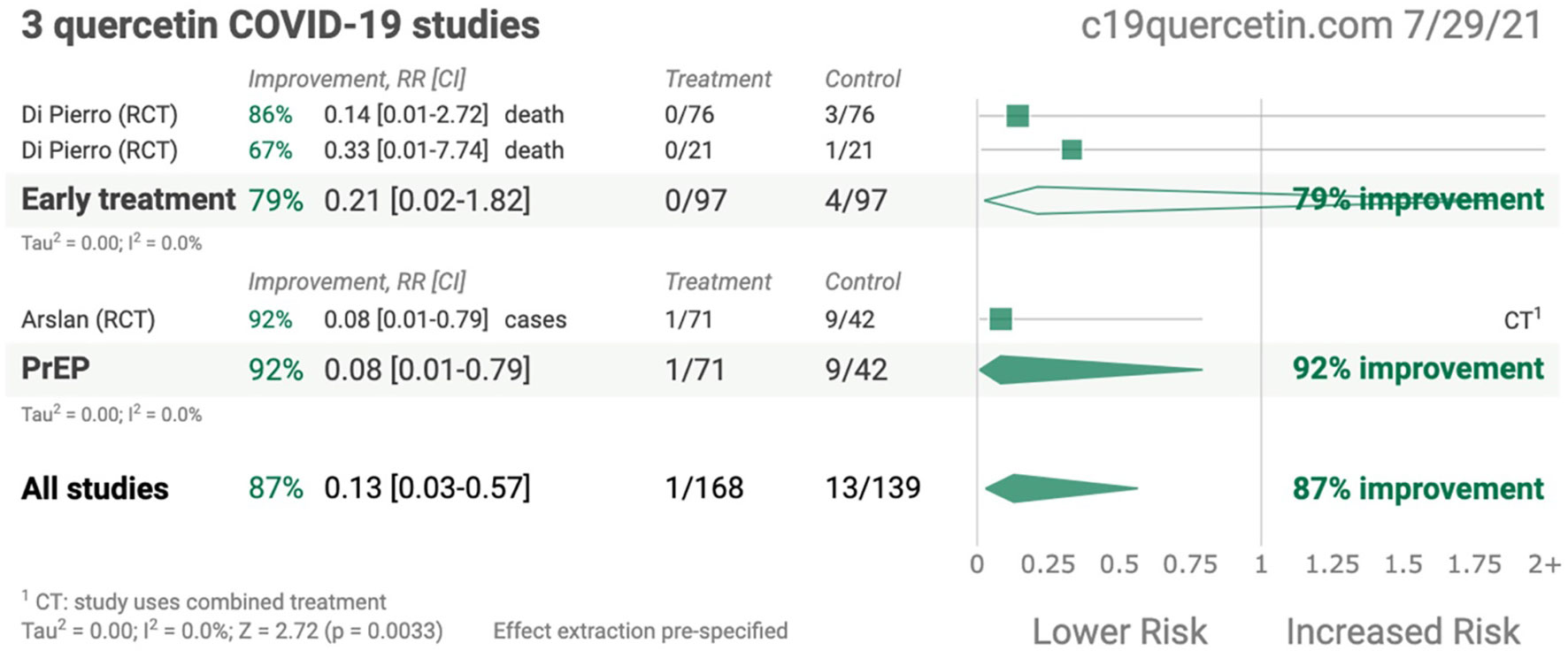
Figure 1 Summary of quercetin COVID-19 studies (Adopted under Creative Commons Attribution-Non Commercial v4.0 License from Ref. 24).
It is still a debatable matter that vaccines are associated with a risk of local and systemic inflammatory immune responses along with systemic toxicity, and hence supplementation of QCT nanoparticles may mitigate such effects. The present study evaluates the characteristics of QCT nanoparticles and extracellular vesicle-based delivery along with the immunomodulation mechanism of these nanoparticles in COVID-19 infection.
The chemical name of QCT is C15H10O7 or 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one or 3,3,4,5,7-pentahydroxyflavone, which tends to present conjugation with glucose, xylose, and rutinose via hydroxyl (OH) functional groups present in its structure, which imparts the antioxidant tendency because it is composed of five hydroxyl groups as demonstrated in Figure 2. QCT is of nutritional importance and is majorly present in the form of QCT-3-O-glycoside rather than aglycones (27–31). Substitution of OH functional groups at positions C3, C5, and C7, as well as H atom substitution at C3’ and OCH3 substitution at C4’, proved to be beneficial and protective to cells (32). Furthermore, QCT has a chemical composition that includes 3′, 4′-OH groups (B ring) and 2, 3-double bond conjugation with a 4-oxo functional group present in the C ring, which helps to reduce oxidative stress (33). Another study found that the orthodihydroxy, 4-carbonyl, and 3′, 4-OH group substitutions on the B, C, and A-rings, respectively, exhibit metal ion chelator activity of QCT (34).
QCT has significant solubility in lipids and alcohols but shows poor solubility in water. A previous study observed that QCT glycoside has a high affinity for water due to the presence of several glycosyl functional groups (35) (Table 1). QCT is usually present in two forms, namely glycosides and glycones, and follows passive diffusion and an anion-transporting pathway for absorption into the small intestine. QCT metabolism occurs in the liver, intestines, and kidneys with a half-life ranging from 11 to 28 h and an average terminal half-life of 3.5 h (36, 37). A study showed that QCT glucoside showed better solubility than rutinosides in the small intestine upon hydrolysis by glucosidases (38, 39). The enzymes of the intestinal mucosa and epithelial cells transform QCT and its derivatives into diverse metabolites, including phenolic acids. These metabolites are excreted majorly by the kidney through urine in the form of benzoic acids (40).
Fabrication of nanoparticle-based drug delivery systems has been validated and tested in several pre-clinical and clinical trials for the treatment and diagnosis of several diseases in the past decades (Table 2). The therapeutic potential of QCT nanoparticles includes antioxidant, anti-inflammatory, anti-bacterial and anti-neoplastic activities (Figures 3, 4) (41). These nanoparticle delivery agents such as polymeric micelles, liposomes, quantum dots, chitosan, Polylactic/glycolic acid (PLGA) and PLGA-based nanoparticles, polymeric micelles, dendrimers, and inorganic nanoparticles have been explored well for targeted delivery of desired drugs, proteins, peptides, and nucleic acids (42). Entrapment of the desired drug into the vicinity of nanoparticles enhances its bioavailability and stability with prolonged circulation time, which helps in improving therapeutic outcomes (43). These nanoparticles enable drug delivery by exhibiting functional and structural modifications like ligand linkage within a single delivery partner.
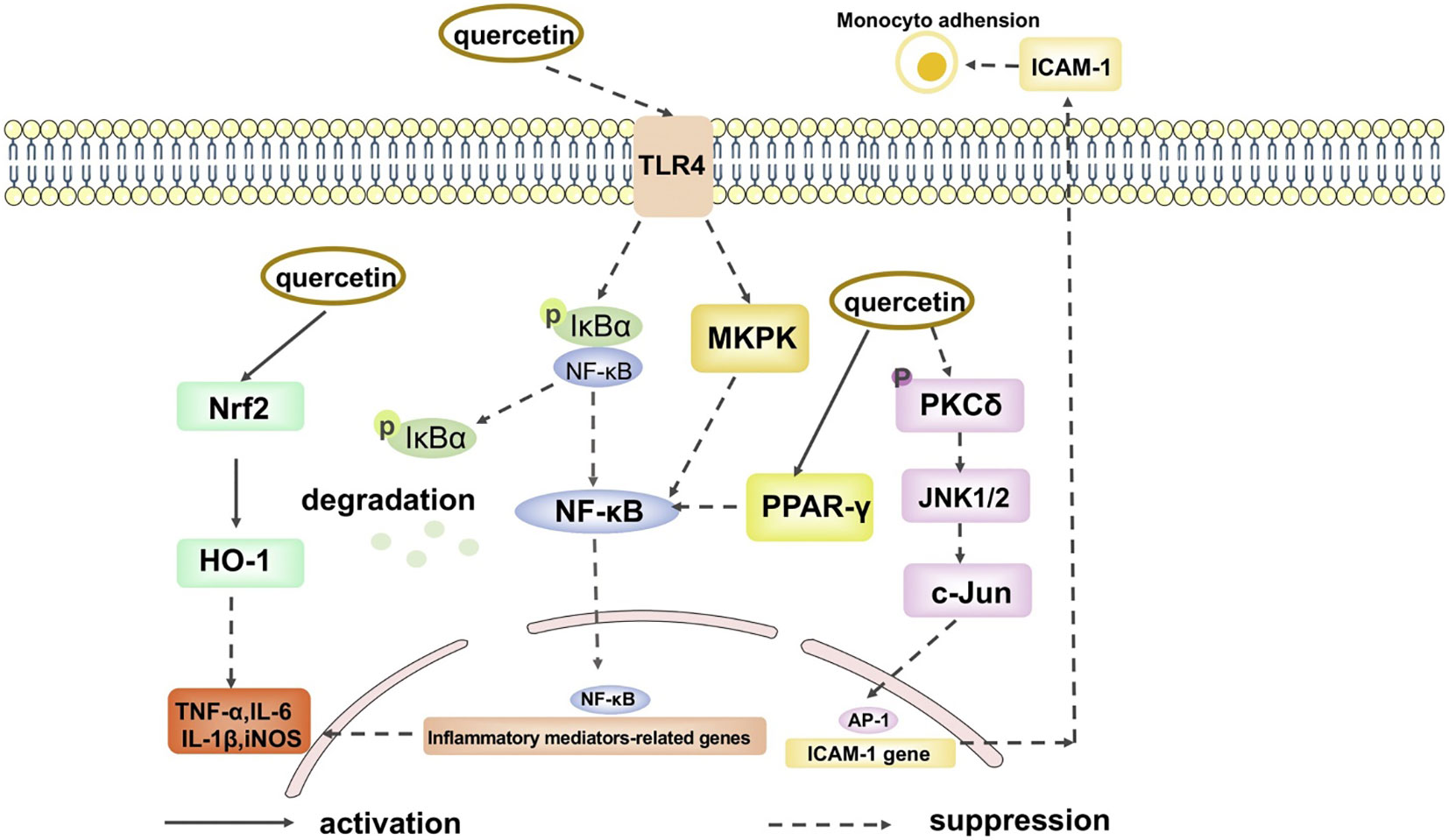
Figure 4 The anti-inflammation signal pathways of quercetin. (Adopted under Creative Commons Attribution-Non-Commercial v4.0 License from Ref. 39).
Moreover, several nanoparticles, including liposomes, offer opportunities to deliver hydrophobic drug molecules like QCT to explore its improved encapsulation efficiency, targeted delivery with extended circulation time within the body with controlled release, and high therapeutic efficiency. In the past decades, significant advances have been made in developing engineered nanoparticles containing QCT as therapeutics. To achieve this, researchers have explored liposomes, PLGA nanoparticles, polymeric micelles, metal-organic frameworks (MOFS), inorganic molecules, and other delivery systems for biomacromolecule-based nanoparticles. Some limitations associated with these nanoparticles are systemic toxicity and adverse effects, and fewer tendencies to cross the blood-brain barrier (BBB). To combat such limitations, EVs have been a preferred choice nowadays for delivering nanoparticles, including QCT.
Extracellular vesicles (EVs) are nano-sized lipid bilayer vesicles secreted by metabolically active cells that have the potential for functional and structural modifications for drug delivery to targeted organs. Compared to other delivery agents, EVs offer several advantages, including biocompatibility, negligible systemic toxicity and adverse effects, increased bio-distribution, and high transmission efficiency with a tendency to deliver biomolecules including proteins, peptides, lipids, and nucleic acids (44). Previously, EVs demonstrated promising potential for delivering a wide range of drugs and biomolecules as carriers with significantly improved bioavailability and high transmission across the BBB. A study showed plasma-derived exosome-loaded QCT nanoparticles showed improved bioavailability with therapeutic effect in Alzheimer’s disease by inhibiting cyclin-dependent kinase 5 (CDK5) facilitated phosphorylation of protein Tau (45). The atomic force microscopic image of exosome loaded QCT was shown in Figure 5. In another study, authors engineered exosomes containing QCT nanoparticles and monoclonal antibodies against GAP43 (mAb GAP43) and found a therapeutic effect in cerebral ischemia by decreasing reactive oxygen species (ROS) (46).

Figure 5 Morphology determination by atomic force microscopy (A) Exosome and (B) Exosome loaded with quercetin. (Adopted under Creative Commons Attribution-Non-Commercial v4.0 License from Ref. 43).
The authors of a recently published study (47) assessed the nutraceutical properties of EVs containing QCT and saponin. Fabricated EVs loaded with QCT and saponins extracted from black bean extract (Phaseolus vulgaris L.) along with three more phytochemicals at a single time to deliver them all at once to the target site or recipient cells. The study concluded that EVs loaded with nanoparticles have increased bioactivity compared to the phytochemicals used alone with EVs (47). Above, studies favor the development of new products containing EVs containing nutraceuticals for the treatment of several diseases as EVs serve various therapeutic functions and roles (48) (Figure 6).
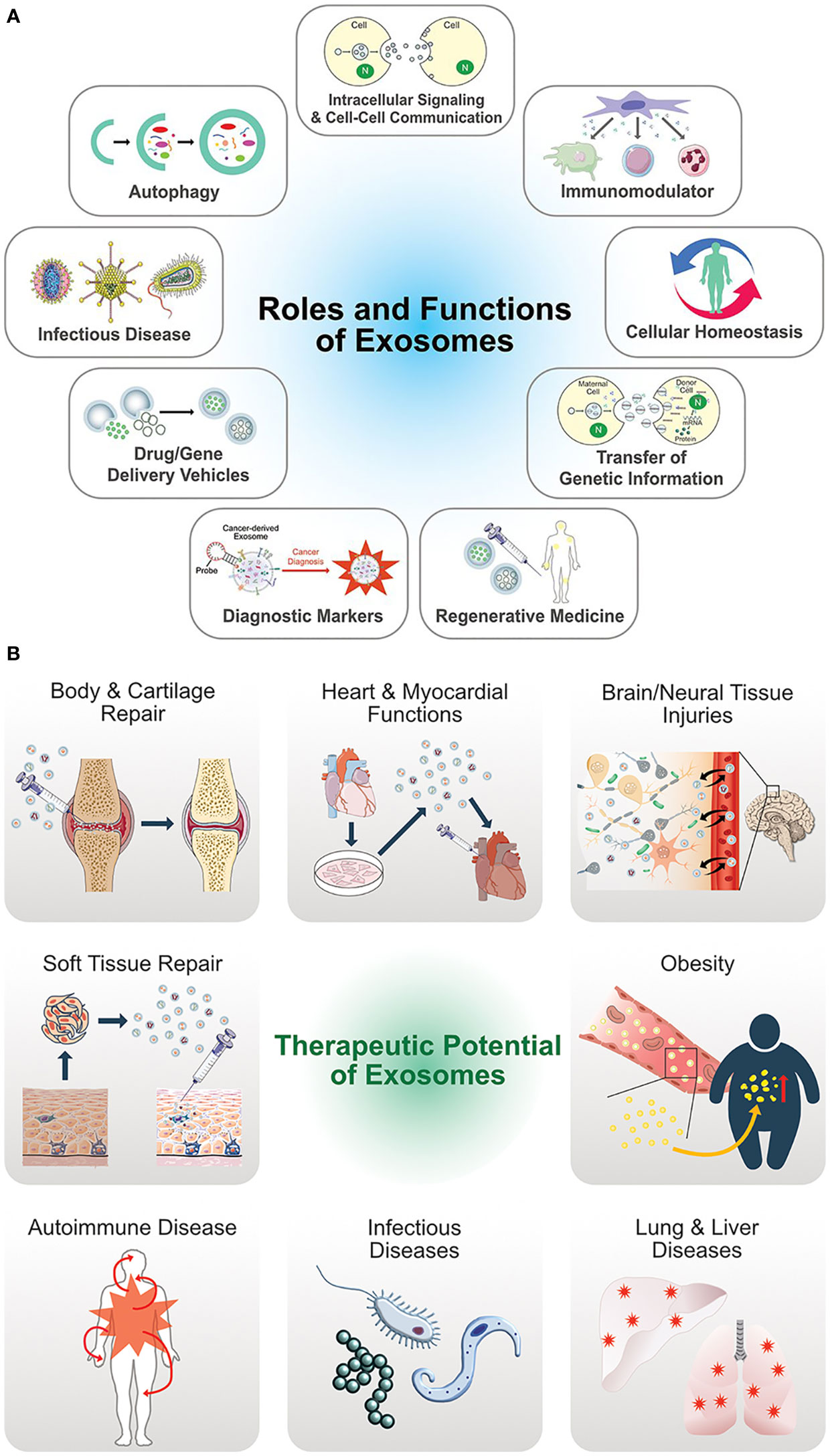
Figure 6 (A) Multifunctional aspects biological functions of exosomes. (B) Therapeutic potential and versatile clinical implications of exosomes. (Adopted under Creative Commons Attribution-Non-Commercial v3.0 License from Ref. 46).
EV modification and bioengineering have gained increased attention in recent years. Due to their ability to manipulate for delivering multiple biological molecules such as proteins, drugs, lipids, and nucleic acids (coding or non-coding), studies have demonstrated the beneficial role of EVs as drug carriers, proving their therapeutic role in several diseases (49). Previously published literature supported our study as they claimed that EVs exhibits immunomodulation characteristics and hence can be effectively used in the treatment of lungs affected with SARS-CoV-2 infection (48). Moreover, EVs also have therapeutics role in the treatment of cardiovascular diseases associated with COVID-19 by repairing ischemic myocardial injury by inducing neovascularization (49). Moreover, study also claimed protective role of EVs against acute kidney injury and acute liver injury (48). The modification approach of EVs is further classified into two types: I. direct modification and II. indirect modifications. The direct modification approach refers to EV engineering that is further classified into physical and chemical modifications (50).
EVs membrane and indigenous content can be modified using different physical approaches which are mentioned below;
Conventional surface modifications of EVs exploit the attachment of proteins, lipids, and polypeptides on their outer membrane using specific methods. Such EV modification was found to be beneficial in the treatment of leukaemia and tumor growth suppression (51). Because EVs are composed of a lipid bilayer, they can be easily conjugated or modified using liposomes, as demonstrated by previous studies (52, 53). In one of the previously published studies, authors fused exosomes derived from bone marrow mesenchymal stromal cells (BMSCs) with liposomes containing polypyrrole nanoparticles for the treatment of diabetic peripheral neuropathy (53). Moreover, liposomes were fused with the MSC-derived EVs to incorporate miR-34a and this system was tested against breast cancer progression (54).
Several studies were conducted on EV surface modification and demonstrated that the linkage of glycosyl phosphatidylinositol (GPI) on the EV surface imparts stability to deliverable EVs and also protects such EVs from hydrolytic degradation (55, 56). Another study on the SARS-CoV-2 virus discovered that EVs contain the enzyme ACE-2, and their levels can be estimated using protein palmitoylation (57). Two enzyme systems, namely zinc finger DHHC-Type Palmitoyl transferase 3 (ZDHHC3) and acyl protein thioesterase 1 (LYPLA1), performed the mechanism of palmitoylation and de-palmitoylation of such proteins. The authors fabricated engineered EVs with S-palmitoylation targeted sequences as a therapeutic and prophylactic approach against SARS-CoV-2 and thereby protect the lungs from inflammation (57).
The efficiency of the EVs for targeted drug delivery can also be fairly improved by doing engineering with EVs content through specific approaches including incubation, sonication, electroporation, and others that are helpful in loading many drugs, proteins, peptides, miRNAs and long-non-coding RNAs into the EVs as also suggested by previously published study (50, 58).
This engineering method involves the simple incubation of EVs with the desired drug or biological molecules, which is still known as “passive loading of cargo.” The driving force behind the loading of desired cargo in the EVs is the interplay between the concentration of drugs inside and outside the EV medium that allows the transfer of drugs or desired cargo within the lipid bilayer of the EVs. Some studies implicated the incubation approach for loading anti-cancerous drugs including paclitaxel and doxorubicin into the EVs and achieved significant chemotherapeutic effects (59, 60). In another study, the authors used this method for loading curcumin nanoparticles into the EVs and evaluated its effect as an anti-inflammatory approach (61). This is a cost-effective and simple method that does not require high throughput facilities but with the major limitation of low yield and transport of hydrophobic cargoes only.
This engineering approach relies on sound waves for the generation of mild shearing forces that are needed for the disruption of lipid bilayers of EVs so that desired drug or biological molecules mediate transport into them. Kim and their coworkers successfully loaded paclitaxel and doxorubicin into the EVs and exploited them for cancer treatment (62). It is reported that the sonication approach decreases the viscosity of the EVs membrane and thereby allows the passage of cargo inside it (62). This method exhibits high loading efficiency compared to the simple incubation approach, with some limitations in shearing forces that are not suitable to deliver labile biological molecules such as miRNAs. This approach resulted in improved drug loading efficiency with better biocompatibility and controlled release of desired drugs with incubation and electroporation (63).
This engineering approach is a commonly used method for EV modification using the electric field for cargo loading. An electric field disrupts the lipid bilayer of the EVs, thereby facilitating the entry of the desired drug or biomolecule inside it. This method is most suitable for loading hydrophilic cargo like miRNA, siRNA, and other nucleic acids (64, 65). Johnsen et al. reported that electroporation tends to aggregate the EVs without altering their functions (66). For maintaining the structural conformation of the EVs, the authors used trehalos-containing buffer during the electroporation (66). Another study used electroporation for the loading of doxorubicin drug with hydrophobically modified miRNA 159 into the EVs for the treatment of tumors (67). This approach, however, facilitates the rapid entry of the desired drug into the EVs but simultaneously damages the structural integrity of such EVs, thereby reducing the efficiency of drug/molecule loading (68–70). Furthermore, one study used electroporation to load miRNA 155 into the EVs with good efficiency at a voltage of 0.13–0.2 kV (concentration of EVs 500–1000 mg/mL) (71).
This engineering approach involves the temporary generation of pores on the EVs’ membrane upon multiple freezing and subsequent thawing cycles that facilitate the entry of drugs inside EVs. A freeze cycle consists of -800C while thawing at 370C, repeated several times. Some studies have reported the formation of aggregates following this approach while loading drugs into EVs (72, 73). Hanley and their co-workers successfully loaded the enzyme catalase into the EVs using this approach (72). This approach offers the benefit of mass production of engineered EVs compared to the ultrasonication method with improved drug loading efficiency (62, 74). Moreover, freeze-thaw process is associated with some disadvantages as evident by the previously published study (A). The repeated process of the freeze-thaw severely affected the membrane stability of the EVs (75). Author of another study demonstrated the decrease in EVs concentration up to 2 folds even after the single freeze-thaw cycle and also reported changes in the structural morphology of EVs post storage (75).
This engineering approach involves the use of a small-sized polycarbonate porous membrane for reversible disruption of the phospholipid bilayer of the EVs to facilitate the entry of desired drugs/cargo inside the EVs (72, 73). This method results in the production of uniform-sized EVs with efficient drug loading compared to incubation and electroporation under controlled conditions (74). Extrusion, however disrupt the membrane integrity of the EVs thereby causing leakage of loaded drugs. During the process of extrusion, the EVs membrane faces vigorous forces and as a resultant, disruption occurs. This is also evident with the changes in the membrane zeta potential and membrane protein disorganization. Fuhrmann and their coworkers proved that EVs prepared using the extrusion method exhibits cytotoxicity compared to non-extruded EVs (76). The authors compared the loading of siRNA into EVs using the natural mode of EV secretion from cells with EV mimics prepared by multiple sequential extrusions of MCF10A cells and discovered that EV mimics had the highest drug loading (77, 78).
Chemical-based modification or engineering of EVs refers to the transformation of EVs’ surfaces. This can be further classified as covalent (reaction between EVs and chemical linkers) and non-covalent (electrostatic interaction) based modifications.
Covalent modification, or click chemistry, involves the chemical conjugation of ligands with EV surfaces. Several amino acids can be effectively conjugated on EV surfaces through azide-alkyne cyclo-addition click chemistry (79, 80). Another study fabricated azide-labeled c-RGD peptide on an EV surface using alkyne-azide cyclo-addition with dibenzo cyclooctyne to treat ischemic brain injury (81). The Click chemistry-based modification exhibits high specificity, selectivity, and high compatibility without affecting the structural and functional integrity of the EVs.
Non-covalent-based EVs’ modification mostly involves electrostatic and ligand-based alteration to EVs. The presence of a lipid bilayer on EVs’ surfaces imparts a negative charge with a zeta potential of approximately-8.82 mV, making them suitable to add cations on their surfaces (82). Authors have fabricated EVs-based immune blockers to promote the phagocytosis of tumor cells by macrophages through a non-covalent-based modification approach (83, 84). However, ligand-receptor-based modification of EVs involves hydrophobic ligand interactions on the lipid component present on the surface of EVs. For therapeutic and diagnostic purposes, liposomes modified using polyethylene glycol (PEG) is commonly used for EV modification to load a desired molecule of interest.
Most of the cells secrete EVs in the medium that can be further exploited for therapeutic and diagnostic purposes. The EV-producing parental cells can be genetically and metabolically engineered to produce modified EVs with enhanced drug-loading capacity.
Genetic engineering approaches for the modification of EVs producing parental cells to achieve specific and targeted drug/biomolecule loaded EVs have become sophisticated within the past decades. Membrane proteins can be efficiently linked with EVs using this method. This involves transfection (via viral or non-viral invasion/infection) of the EVs producing parental cells using specific gene targets that allow the manufacturing of cargo-loaded EVs during the biogenesis process. Jiang et al., reported two types of viral vectors for modification of EVs using a genetic engineering approach, i.e., retroviral and adenoviral, for delivery of tumor necrosis factor (TNF)-stimulated gene-6 (TSG-6) (85). A study reported use of MSCs-derived modified EVs using a genetic engineering approach with miRNAs delivery (86). In another study, authors fabricated engineered EVs expressing Lamp2b conjugated integrin-specific iRGD peptide for the treatment of breast cancer (87). Similarly, in another study, engineered EVs were synthesized derived from HEK293T cells expressing Lamp2b conjugated with IL-3 fragments for the treatment of chronic myeloid leukaemia (CML) (88).
Rivoltini and their co-workers transfected the K562 cell line with lentiviral human membrane TRAIL (TNF-Related Apoptosis-Inducing Ligand) for fabrication of TRAIL (+) EVs for apoptosis of cancerous cells (89). In another study, dendritic cells (DCs) were transfected using a genetic engineering approach with a Lamp2b-modified pEGFP-C1 vector to produce RVG-modified EVs (90). The major limitation associated with this approach is that it affects the efficiency of the drug/molecule loading to EVs loading of foreign impurities that result in a decrease in EV purity.
The metabolic engineering approach for modification of EVs delivers metabolites or biological molecules including amino acids, lipids, and sugar moieties to the growth medium of parental cells for promotion of biosynthesis. These biomolecules can be integrated into the proteome, liposomes, and glycoproteins present in the EVs (91). This engineering approach used click conjugation to attach glycans and glycoproteins with chemically active azide functional groups and bio-orthogonal moieties to produce metabolically engineered EVs (92). In another study, authors replaced the naturally occurring methionine amino acid with L-azidohomoalanine (AHA), an azide-bearing amino acid (which is an analogue of natural methionine) inside EVs (93). In continuation with this study, the introduction of AHA into the exosomes offers an additional azide active site for bio-conjugation, thereby providing an additional free site for binding of desired cargo/molecule of interest for therapeutic and diagnostic purposes (94).
The spread of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is a coronavirus disease (COVID-19) progressing as a worldwide pandemic targeting the lungs of infected patients. The SARS-CoV-2 virus is an RNA virus that recognizes human cells through angiotensin-converting enzyme 2 receptors (ACE-2) present on the epithelial cells for its entry (95–97). Virus infection triggers inflammatory responses such as cytokine storm, oxidative stress, and acute respiratory distress syndrome (98, 99). Studies demonstrated the anti-SARS-CoV-2 activity of QCT nanoparticles by inhibiting the binding of viruses with ACE-2 receptors, lowering the pro-inflammatory cytokines, and down-regulating the expression of the RdRp gene (100–102).
Docking studies conducted recently showed the anti-SARS-CoV2 effect of QCT molecules (101). The study showed that QCT inhibits the expression of the NLRP3 inflammasome through various regulatory proteins (101). The study also concluded that QCT nanoparticles can be prospectively performed as antioxidant, anti-inflammatory, and analgesic characteristics and, thereby, can be used to treat severe inflammation associated with SARS-COV-2 infection in COVID-19 (101).
Studies demonstrated that QCT inhibits the expression of the NLRP3 inflammasome mediated through TXNIP (103). In an animal study conducted on a spinal cord injury model, QCT nanoparticles demonstrated anti-inflammatory and antioxidant properties along with inhibition of pro-inflammatory cytokine generation (103). A study quoted said QCT showed antioxidant and anti-inflammatory roles that further protected the cells from apoptosis (103). In-silico finding of SARS-CoV-2 protease proteins (PDB ID: 6LU7) showed an inhibitory effect of QCT by forming new hydrogen bonds with some amino acid residues (His164, Glu166, Asp187, Gln192, and Thr190) of 6LU7 (100, 102). Moreover, another preclinical study of a mouse model conducted on asthma showed that QCT therapy lowers the count of white blood cells (WBCs) along with eosinophils in the blood, lung parenchyma, and bronchoalveolar lavage fluid (104). Previously published study showed that, QCT particles mediate inhibition of p38 mitogen-activated protein kinase (MAPK) and NF-k (105). Another author demonstrated that QCT nanoparticles downregulate the expression of histamine, prostaglandin D2, leukotriene, and granulocyte-macrophage colony-stimulating factors (106). In one of the recently published study, the authors have reported the role of quercetin as antituberculosis, antioxidant and cytotoxicity and therefore favored this study (107).
Several studies focused on the immune-modulatory and anti-inflammatory roles of MSC-derived EVs similar to their parental cell source (108, 109). In a preclinical study, MSC-derived EVs showed therapeutic effects in Acute Respiratory Distress Syndrome (ARDS) (110). Another study showed that EVs initiate anti-inflammatory responses that further reduce the severity of lung injury through the maintenance of alveolar epithelium (111, 112). It has been seen from previous literature that the use of EVs or nano decoys slows down the progression of viral infection through binding with viral cells (113–115). Studies also showed that these nano-molecules entrap the viral pathogens and also mediate their clearance from body fluids, preventing the spread of infections (113–115). The mechanism of viral entrapment by lung-derived EVs involves binding of SARS-CoV-2 viral spike protein (S protein) with the ACE-2 receptor available on these EVs, so that viral S protein does not participate in binding with the human ACE-2 receptor, but binds with EVs-ACE-2 receptors, thereby protecting the spread of SARS-CoV-2 infection and reducing the associated respiratory complications (116, 117).
SARS-CoV-2 is a highly diverse enveloped positive-sense single-stranded RNA virus that follows the entry into the human via angiotensin-converting enzyme 2 receptors (hACE2) present on the epithelial cells. The initial entry step for entry involve binding of its spike (S) protein to these receptors including human amino peptidase N (APN; HCoV-229E), angiotensin-converting enzyme 2 (ACE2; HCoV-NL63, SARS-CoV and SARS-CoV-2) and dipeptidyl peptidase 4 (DPP4; MERS-CoV) (118). The S proteins of the coronavirus are glycoproteins that exhibit S1 and S2 domains (119, 120). Moreover, the S1 domain comprehends the receptor-binding domain (RBD) that specifically recognizes host epithelial cell receptors. S2 domain exhibits heptad repeat sequences along with fusion peptides which assist fusion of viral and host cell membranes undergoing rearrangements mechanism (121). QCT is a widely known flavonoid and exhibits anti-COVID-19 activity and protective mechanism against SARS-CoV-2 through inhibition and triggering down regulation of hACE-2 along with discharge of pro-inflammatory chemokines.
Furthermore, QCT-3-b-D-glucoside also known to inhibit the expression of 3CLpro (also referred to as the main protease) and papain- like protease (PLpro) as quoted by authors of recently published study (122). The molecular docking study revealed that anti-SARS activity of QCT is demonstrated by the inhibition of SARS-CoV-2 protease through fabricating new hydrogen bonds with amino acid residues including His164, Glu166, Asp187, Gln192, and Thr190) of 6LU7 (123, 124). QCT known to exhibit >80.0% inhibition in an in-vitro experiment, with IC50 value of 73 μM, on the recombinant 3CLpro protein expressed in Pichia pastoris as reported by authors of previously published study (125). Another molecular docking observation revealed that QCT interacts with Asp38 of hACE-2 and inhibit the entry into the epithelial cells by preventing attachment of viral spike S1 protein. Some previously published studies showed the therapeutic role of EVs as anti-viral in many diseases (126–128).
Altogether, it is evident that QCT and EV supplementation significantly help to combat inflammatory responses, including SARS-CoV-2 infection. Here we show that QCT can be prospectively used in the form of nanoparticles after loading into the EVs to address the therapeutic potential in the COVID-19 pandemic. QCT nanoparticles can be loaded into the EVs using a suitable method of engineering after optimization of the efficiency and yield among the several physical and chemical methods available for engineering EVs. The quercetin nanoparticles loaded EVs act in two ways: (a) QCT will aid in combating the associated complications with SARS-CoV-2, such as inflammation, reactive oxygen species, and the regulation of genes involved in the release of cytokines and pro-inflammatory cytokines; and (b) the presence of ACE-2 receptors on the lungs derived EVs will show strong binding affinity with the S protein of the SARS-CoV-2 virus and thus As a result, developing QCT-loaded EVs as a therapeutic drug delivery approach is a better option to investigate for the treatment of viral and inflammatory diseases, such as SARS-CoV-2 infection. Several pre-clinical and clinical trials within this domain are needed to make this prospective therapy into a translational aspect within clinics. QCT is a GRAS molecule as per the USFDA guidelines and accordingly it may be exploited along with the EVs as antivirals and as drug constituent to control the symptoms of the COVID-19. In future research is needed to further explore the different types of cell sources derived EVs containing the QCT nanoparticles for effective treatment of comorbidities associated with the COVID-19. Further translational therapeutics can be developing in the form of oral and also inhaler medicine using this therapeutics.
Conceptualization, AR, G-BJ, RG, and SK. Methodology, AR and G-BJ. Software, AR. Validation, G-BJ, RG, and SK. Formal analysis, G-BJ, RG, and SK. Investigation, AR. Resources, SK. Data curation, AR. Writing—original draft preparation, AR. Writing—review and editing, RG, SA, G-BJ. Visualization, RG and SK. Supervision: RG, G-BJ, and SK. Project administration, SK. All authors contributed to the article and approved the submitted version.
The authors would like to thank the National Research Foundation and Gachon University for providing assistance in the form of salary to Dr. Alok Raghav and research facilities to perform current study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Li Y, Yao J, Han C, Yang J, Chaudhry MT, Wang S, et al. Quercetin inflammation and immunity. Nutrients (2016) 8(3):167. doi: 10.3390/nu8030167
2. Robaszkiewicz A, Balcerczyk A, Bartosz G. Antioxidative and prooxidative effects of quercetiq on A549 cells. Cell Biol Int (2007) 31:1245–50. doi: 10.1016/j.cellbi.2007.04.009
3. Uchide N, Toyoda H. Antioxidant therapy as a potential approach to severe influenza-associated complications. Molecules (2011) 16:2032–52. doi: 10.3390/molecules16032032
4. Nair MP, Kandaswami C, Mahajan S, Chadha KC, Chawda R, Nair H, et al. The flavonoid, quercetin, differentially regulates Th-1 (IFNgamma) and Th-2 (IL4) cytokine gene expression by normal peripheral blood mononuclear cells. Biochim Biophys Acta (2002) 1593:29–36. doi: 10.1016/S0167-4889(02)00328-2
5. Shinozuka K, Kikuchi Y, Nishino C, Mori A, Tawata S. Inhibitory effect of flavonoids on DNA-dependent DNA and RNA polymerases. Experientia (1988) 44:882–5. doi: 10.1007/BF01941188
6. Bachmetov L, Gal-Tanamy M, Shapira A, Vorobeychik M, Giterman-Galam T, Sathiyamoorthy P, et al. Suppression of hepatitis c virus by the flavonoid quercetin is mediated by inhibition of NS3 protease activity. J Viral Hepat (2012) 19:e81–8. doi: 10.1111/j.1365-2893.2011.01507.x
7. Spedding G, Ratty A. Middleton E jr. inhibition of reverse transcriptases by flavonoids. Antiviral Res (1989) 12:99–110. doi: 10.1016/0166-3542(89)90073-9
8. Cushnie TP, Lamb AJ. Antimicrobial activity of flavonoids. Int J Antimicrob Agents (2005) 26:343–56. doi: 10.1016/j.ijantimicag.2005.09.002
9. Debiaggi M, Tateo F, Pagani L, Luini M, Romero E. Effects of propolis flavonoids on virus infectivity and replication. Microbiologica (1990) 13:207–13.
10. NIH. (2021) (Accessed May 27, 2021). PubChem., quercetinpubchem.ncbi.nlm.nih.gov/compound/quercetin, published.
11. Chuammitri P, Srikok S, Saipinta D, Boonyayatra S. The effects of quercetin on microRNA and inflammatory gene expression in lipopolysaccharide-stimulated bovine neutrophils. Vet World (2017) 10(4):403–10. doi: 10.14202/vetworld.2017.403-410
12. Dostal Z, Modriansky M. The effect of quercetin on microRNA expression: a critical review. BioMed Pap Med Fac Univ Palacky Olomouc (2019) 163(2):95–106. doi: 10.5507/bp.2019.030
13. Di Pierro F, Iqtadar S, Khan A, Ullah Mumtaz S, Chaudhry MM, Bertuccioli A, et al. Potential clinical benefits of QCT in the early stage of COVID-19: Results of a second, pilot, randomized, controlled and open-label clinical trial. Int J Gen Med (2021) 14:2807–16. doi: 10.2147/IJGM.S318949
14. Shohan M, Nashibi R, Mahmoudian-Sani MR, Abolnezhadian F, Ghafourian M, Alavi SM, et al. The therapeutic efficacy of QCT in combination with antiviral drugs in hospitalized COVID-19 patients: A randomized controlled trial. Eur J Pharmacol (2022) 914:174615. doi: 10.1016/j.ejphar.2021.174615
15. Xu D, Hu MJ, Wang YQ, Cui YL. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules (2019) 24(6):1123. doi: 10.3390/molecules24061123
16. Terao J. Factors modulating bioavailability of quercetin -related flavonoids and the consequences of their vascular function. Biochem Pharmacol (2017) 139:15–23. doi: 10.1016/j.bcp.2017.03.021
17. Kaşıkcı MB, Bağdatlıoğlu N. Bioavailability of quercetin. Curr Res Nutr Food Sci (2016) 4:146–51. doi: 10.12944/CRNFSJ.4
18. Pignatelli P, Pulcinelli FM, Celestini A, Lenti L, Ghiselli A, Gazzaniga PP, et al. The flavonoids quercetin and catechin synergistically inhibit platelet function by antagonizing the intracellular production of hydrogen peroxide. Am J Clin Nutr (2000) 72(5):1150–5. doi: 10.1093/ajcn/72.5.1150
19. Scheepens A, Tan K, Paxton JW. Improving the oral bioavailability of beneficial polyphenols through designed synergies. Genes Nut (2010) 5(1):75–87. doi: 10.1007/s12263-009-0148-z
20. Derosa G, Maffioli P, D’Angelo A, Di Pierro F. A role for quercetin in coronavirus disease 2019 (COVID-19). Phytother Res (2021) 35(3):1230–6. doi: 10.1002/ptr.6887
21. Bastaminejad S, Bakhtiyari S. Quercetin and its relative therapeutic potential against COVID-19: a retrospective review and prospective overview. Curr Mol Med (2021) 21(5):385–91. doi: 10.2174/1566524020999200918150630
22. Agrawal PK, Agrawal C, Blunden G. Quercetin : antiviral significance and possible COVID-19 integrative considerations. Nat Prod Commun (2020) 15(12):1–10. doi: 10.1177/1934578X20976293
23. Di Pierro F, Derosa. G, Maffioli P, Bertuccioli A, Togni S, Riva A, et al. Possible therapeutic effects of adjuvant quercetin supplementation against early-stage COVID-19 infection: a prospective, randomized, controlled, and open-label study. Int J Gen Med (2021) 14:2359–66. doi: 10.2147/IJGM.S318720
24. Önal H, Arslan B, Üçüncü Ergun N, Topuz Ş, Yilmaz Semerci S, Kurnaz ME, et al. Treatment of COVID-19 patients with quercetin: a prospective, single center, randomized, controlled trial. Turk J Biol. (2021) 45(4):518–29. doi: 10.3906/biy-2104-16
25. Arslan B, Ergun NU, Topuz S, Semerci SY, Suner N, Kocatas A, et al. A synergistic effect of quercetin and vitamin c against COVID-19: is a possible guard for front liners. SSRN Preprints (2020). doi: 10.2139/ssrn.3682517
26. Boretti A. Quercetin supplementation and COVID-19. Natural Product Commun (2021) 16(9):1–3. doi: 10.1177/1934578X211042763
27. Available at: https://pubchem.ncbi.nlm.nih.gov (Accessed 01/June/2022).
28. Available at: https://clinicaltrials.gov/ (Accessed 01/June/2022).
29. Sytar O, Kosyan A, Taran N, Smetanska I. Anthocyanin’s as marker for selection of buckwheat plants with high rutin content. Gesunde Pflanzen (2014) 66:165–9. doi: 10.1007/s10343-014-0331-z
30. Miles S,L, McFarland M, Niles RM. Molecular and physiological actions of quercetin : need for clinical trials to assess its benefits in human diseases. Nutr Rev (2014) 72:720–34. doi: 10.1111/nure.12152
31. Guo Y, Bruno RS. Endogenous and exogenous mediators of quercetin bioavailability. J Nutr Biochem (2015) 26:201–10. doi: 10.1016/j.jnutbio.2014.10.008
32. Echeverry C, Arredondo F, Abin-Carriquiry JA, Midiwo JO, Ochieng C, Kerubo L, et al. Pretreatment with natural flavones and neuronal cell survival after oxidative stress: a structure– activity relationship study. J Agric Food Chem (2010) 58(4):2111–5. doi: 10.1021/jf902951v
33. Wang H, Joseph JA. Structure–activity relationships of quercetin in antagonizing hydrogen peroxide-induced calcium dysregulation in PC12 cells. Free Radic Biol Med (1999) 27(5):683–94. doi: 10.1016/S0891-5849(99)00119-7
34. Barreca D, Bellocco E, D’Onofrio G, Nabavi SF, Daglia M, Rastrelli L, et al. Neuroprotective effects of quercetin : from chemistry to medicine, CNS neurol. Disord Drug Targets (2016) 15:964–75. doi: 10.2174/1871527315666160813175406
35. Li Y, Yao J, Han C, Yang J, Chaudhry MT, Wang S, et al. Quercetin , inflammation and immunity. Nutrients (2016) 8(3 167):1–14. doi: 10.3390/nu8030167
36. Manach C, Mazur A, Scalbert A. Polyphenols and prevention of cardiovascular diseases. Curr Opin Lipidol (2005) 16:77–84. doi: 10.1097/00041433-200502000-00013
37. Konrad M, Nieman DC. Chapter 10 Evaluation of quercetin as a countermeasure to exercise-induced physiological stress. In: Antioxidants in sport nutrition. Boca Raton (FL: CRC Press/Taylor & Francis (2015) 2015. Available at: https://www.ncbi.nlm.nih.gov/books/NBK299055/.
38. Scholz S, Williamson G. Interactions affecting the bioavailability of dietary polyphenols in vivo. Int J Vitam Nutr Res (2007) 77:224–35. doi: 10.1024/0300-9831.77.3.224
39. Ader P, Wessmann A, Wolffram S. Bioavailability and metabolism of the flavonol quercetin in the pig. Free Radic Biol Med (2000) 28:1056–67. doi: 10.1016/S0891-5849(00)00195-7
40. Kim DH, Kim SY, Park SY, Han MJ. Metabolism of quercetin by human intestinal bacteria and its relation to some biological activities. Biol Pharm Bull (1999) 22:749–51. doi: 10.1248/bpb.22.749
41. Graefe EU, Derendorf H, Veit M. Pharmacokinetics and bioavailability of the flavonol quercetin in humans. Int J Clin Pharmacol Ther (1999) 37:219–33.
42. Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Laura SAT, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol (2018) 16:71. doi: 10.1186/s12951-018-0392-8
43. Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol (2015) 33:941–51. doi: 10.1038/nbt.3330
44. Fais S, O'Driscoll L, Borras FE, Buzas E, Camussi G, Cappello F, et al. Evidence-based clinical use of nanoscale extracellular vesicles in nanomedicine. ACS Nano (2016) 10:3886–99. doi: 10.1021/acsnano.5b08015
45. Qi Y, Guo L, Jiang Y, Shi Y, Sui H. Brain delivery of quercetin -loaded exosomes improved cognitive function in AD mice by inhibiting phosphorylated tau-mediated neurofibrillary tangles. Drug (2020) 1:745–55. doi: 10.1080/10717544.2020.1762262
46. Guo L, Huang Z, Huang L, Liang J, Wang P, Zhao L, et al. Surface-modified engineered exosomes attenuated cerebral ischemia/reperfusion injury by targeting the delivery of quercetin towards impaired neurons. J Nanobiotechnol (2021) 19:141. doi: 10.1186/s12951-021-00879-4
47. Donoso-Quezada J, Guajardo-Flores D, González-Valdez J. Enhanced exosome-mediated delivery of black bean phytochemicals (Phaseolus vulgaris l.) for cancer treatment applications. BioMed Pharmacother (2020) 131:110771. doi: 10.1016/j.biopha.2020.110771
48. Ovchinnikova LA, Terekhov SS, Ziganshin RH, Bagrov DV, Filimonova IN, Zalevsky AO, et al. Reprogramming extracellular vesicles for protein therapeutics delivery. Pharmaceutics (2021) 13(6):768. doi: 10.3390/pharmaceutics13060768
49. Andaloussi SEL, Mäger I, Breakefield XO, Wood MJA. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discovery (2013) 12(5):347–57. doi: 10.1038/nrd3978
50. Raghav A, Jeong GB. A systematic review on the modifications of extracellular vesicles: a revolutionized tool of nano-biotechnology. J Nanobiotechnol (2021) 19:459. doi: 10.1186/s12951-021-01219-2
51. Bellavia D, Raimondo S, Calabrese G, Forte S, Cristaldi M, Patinella A, et al. Interleukin 3- receptor targeted exosomes inhibit in vitro and in vivo chronic myelogenous leukemia cell growth. Theranostics (2017) 7:1333–45. doi: 10.7150/thno.17092
52. Rayamajhi S, Nguyen TDT, Marasini R, Aryal S. Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta Biomater (2019) 94:482–94. doi: 10.1016/j.actbio.2019.05.054
53. Singh A, Raghav A, Shiekh PA, Kumar A. Transplantation of engineered exosomes derived from bone marrow mesenchymal stromal cells ameliorate diabetic peripheral neuropathy under electrical stimulation. Bioact Mater (2021) 6(8):2231–49. doi: 10.1016/j.bioactmat.2021.01.008
54. Vakhshiteh F, Rahmani S, Ostad SN, Madjd Z, Dinarvand R, Atyabi F, et al. Exosomes derived from miR-34a-overexpressing mesenchymal stem cells inhibit in vitro tumor growth: a new approach for drug delivery. Life Sci (2021) 266:118871. doi: 10.1016/j.lfs.2020.118871
55. Williams C, Royo F, Aizpurua-Olaizola O, Pazos R, Boons GJ, Reichardt NC, et al. Glycosylation of extracellular vesicles: current knowledge, tools and clinical perspectives. J Extracell Vesicles (2018) 7:1442985. doi: 10.1080/20013078.2018.1442985
56. Hung ME, Leonard JN. Stabilization of exosome-targeting peptides via engineered glycosylation. J Biol Chem (2015) 290:8166–72. doi: 10.1074/jbc.M114.621383
57. Xie F, Su P, Pan T, Zhou X, Li H, Huang H, et al. Engineering extracellular vesicles enriched with palmitoylated ACE2 as COVID-19 therapy. Adv Mater (2021) 33(49):e2103471. doi: 10.1002/adma.202103471
58. Raghav A, Tripathi P, Mishra BK, Jeong GB, Banday S, Gautam KA, et al. Mesenchymal stromal cell-derived tailored exosomes treat bacteria-associated diabetes foot ulcers: A customized approach from bench to bed. Front Microbiol (2021) 12:712588. doi: 10.3389/fmicb.2021.712588
59. Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials (2014) 35:2383–90. doi: 10.1016/j.biomaterials.2013.11.083
60. Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R, et al. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in danio rerio. Pharm Res (2015) 32:2003–14. doi: 10.1007/s11095-014-1593-y
61. Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther (2010) 18:1606–14. doi: 10.1038/mt.2010.105
62. Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine (2016) 12:655–64. doi: 10.1016/j.nano.2015.10.012
63. Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, et al. Exosomes as drug delivery vehicles for parkinson’s disease therapy. J Controll Release (2015) 207:18–30. doi: 10.1016/j.jconrel.2015.03.033
64. Faruqu FN, Xu L, Al-Jamal KT. Preparation of exosomes for siRNA delivery to cancer cells. J Vis Exp (2018) 142:1–13. doi: 10.3791/58814
65. Kobayashi M, Sawada K, Miyamoto M, Shimizu A, Yamamoto M, Kinose Y, et al. Exploring the potential of engineered exosomes as delivery systems for tumor-suppressor microRNA replacement therapy in ovarian cancer. Biochem Biophys Res Commun (2020) 527:153–61. doi: 10.1016/j.bbrc.2020.04.076
66. Johnsen KB, Gudbergsson JM, Skov MN, Christiansen G, Gurevich L, Moos T, et al. Evaluation of electroporation-induced adverse effects on adipose-derived stem cell exosomes. Cytotechnology (2016) 68:2125–38. doi: 10.1007/s10616-016-9952-7
67. Gong C, Tian J, Wang Z, Gao Y, Wu X, Ding X, et al. Functional exosome-mediated co-delivery of doxorubicin and hydrophobically modified microRNA 159 for triple-negative breast cancer therapy. J Nanobiotechnol (2019) 17:93. doi: 10.1186/s12951-019-0526-7
68. Kim H, Kim D, Nam H, Moon S, Kwon YJ, Lee JB. Engineered extracellular vesicles and their mimetics for clinical translation. Methods (San Diego Calif) (2020) 177:80–94. doi: 10.1016/j.ymeth.2019.10.005
69. Lamichhane TN, Raiker RS, Jay SM. Exogenous DNA loading into extracellular vesicles via electroporation is size-dependent and enables limited gene delivery. Mol Pharm (2015) 12:3650–7. doi: 10.1021/acs.molpharmaceut.5b00364
70. Kooijmans SAA, Stremersch S, Braeckmans K, de, Smedt. SC, Hendrix A, Wood MJA, et al. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J Control Release (2013) 172:229–38. doi: 10.1016/j.jconrel.2013.08.014
71. Momen-Heravi F, Bala S, Bukong T, Szabo G. Exosome-mediated delivery of functionally active miRNA-155 inhibitor to macrophages. Nanomed Nanotechnol Biol Med (2014) 10:1517–27. doi: 10.1016/j.nano.2014.03.014
72. Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, Plotnikova EG, et al. Exosomes as drug delivery vehicles for parkinson’s disease therapy. J Control Release (2015) 207:18–30. doi: 10.1016/j.jconrel.2015.03.033
73. Le Saux S, Aarrass H, Lai-Kee-Him J, Bron P, Armengaud J, Miotello G, et al. Post-production modifications of murine mesenchymal stem cell (mMSC) derived extracellular vesicles (EVs) and impact on their cellular interaction. Biomaterials (2020) 231:119675. doi: 10.1016/j.biomaterials.2019.119675
74. Shearn AIU, Aday S, Ben-Aicha S, Carnell-Morris P, Siupa A, Angelini GD, et al. Analysis of neat biofluids obtained during cardiac surgery using nanoparticle tracking analysis: methodological considerations. Front Cell Dev Biol (2020) 8:367. doi: 10.3389/fcell.2020.00367
75. Sivanantham A, Jin Y. Impact of storage conditions on EV Integrity/Surface markers and cargos. Life (Basel) (2022) 12(5):697. doi: 10.3390/life12050697
76. Fuhrmann G, Serio A, Mazo M, Nair R, Stevens MM. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J Control Release (2015) 205:35–44. doi: 10.1016/j.jconrel.2014.11.029
77. Rayamajhi S, Aryal S. Surface functionalization strategies of extracellular vesicles. J Mater Chem B (2020) 8:4552–69. doi: 10.1039/d0tb00744g
78. Yang Z, Xie J, Zhu J, Kang C, Chiang C, Wang X, et al. Functional exosome-mimic for delivery of siRNA to cancer: in vitro and in vivo evaluation. J Control Release (2016) 243:160–71. doi: 10.1016/j.jconrel.2016.10.008
79. Kooijmans SAA, Gitz-Francois J, Schiffelers RM, Vader P. Recombinant phosphatidylserine-binding nanobodies for targeting of extracellular vesicles to tumor cells: a plug-and-play approach. Nanoscale (2018) 10:2413–26. doi: 10.1039/c7nr06966a
80. Smyth T, Petrova K, Payton NM, Persaud I, Redzic JS, Graner MW, et al. Surface functionalization of exosomes using click chemistry. Bioconjug Chem (2014) 25:1777–84. doi: 10.1021/bc500291r
81. Tian T, Zhang HX, He CP, Fan S, Zhu YL, Qi C, et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials (2018) 150:137–49. doi: 10.1016/j.biomaterials.2017.10.012
82. Kaddour H, Panzner TD, Welch JL, Shouman N, Mohan M, Stapleton JT, et al. Electrostatic surface properties of blood and semen extracellular vesicles: implications of sialylation and HIV-induced changes on EV internalization. Viruses (2020) 12(10):1117. doi: 10.3390/v12101117
83. Koh E, Lee EJ, Nam GH, Hong Y, Cho E, Yang Y, et al. Exosome-SIRPα, a CD47 blockade increases cancer cell phagocytosis. Biomaterials (2017) 121:121–9. doi: 10.1016/j.biomaterials.2017.01.004
84. Nie W, Wu G, Zhang J, Huang LL, Ding J, Jiang A, et al. Responsive exosome nano-bioconjugates for synergistic cancer therapy. Angew Chem Int Ed Engl (2020) 59:2018–22. doi: 10.1002/anie.201912524
85. Jiang L, Zhang Y, Liu T, Wang X, Wang H, Song H, et al. Exosomes derived from TSG-6 modified mesenchymal stromal cells attenuate scar formation during wound healing. Biochimie (2020) 177:40–9. doi: 10.1016/j.biochi.2020.08.003
86. Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, et al. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells (2012) 30:1556–64. doi: 10.1002/stem.1129
87. Wei H, Chen Q, Lin L, Sha C, Li T, Liu Y, et al. Regulation of exosome production and cargo sorting. Int J Biol Sci (2021) 17:163–77. doi: 10.7150/ijbs.53671
88. Bellavia D, Raimondo S, Calabrese G, Forte S, Cristaldi M, Patinella A. Interleukin 3-receptor targeted exosomes inhibit in vitro and in vivo chronic myelogenous leukemia cell growth. Theranostics (2017) 7:1333–45. doi: 10.7150/thno.17092
89. Rivoltini L, Chiodoni C, Squarcina P, Tortoreto M, Villa A, Vergani B, et al. TNF-related apoptosis-inducing ligand (TRAIL)-armed exosomes deliver proapoptotic signals to tumor site. Clin Cancer Res (2016) 22:3499–512. doi: 10.1158/1078-0432.ccr-15-2170
90. Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ, et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol (2011) 29:341–5. doi: 10.1038/nbt.1807
91. Du J, Che PL, Wang ZY, Aich U, Yarema KJ. Designing a binding interface for control of cancer cell adhesion via 3D topography and metabolic oligosaccharide engineering. Biomaterials (2011) 32:5427–37. doi: 10.1016/j.biomaterials.2011.04.005
92. Wang M, Altinoglu S, Takeda YS, Xu Q. Integrating protein engineering and bioorthogonal click conjugation for extracellular vesicle modulation and intracellular delivery. PloS One (2015) 10:e0141860. doi: 10.1371/journal.pone.0141860
93. Dieterich DC, Link AJ, Graumann J, Tirrell DA, Schuman EM. Selective identification of newly synthesized proteins in mammalian cells using bioorthogonal noncanonical amino acid tagging (BONCAT). Proc Natl Aca Sci U.S.A. (2006) 103(25):9482–7. doi: 10.1073/pnas.0601637103
94. Sletten EM, Bertozzi CR. Bioorthogonal chemistry: Fishing for selectivity in a Sea of functionality. Angew Chem Int Ed (2009) 48(38):6974–98. doi: 10.1002/anie.200900942
95. Imran M, Alshrari AS, Asdaq SMB, Abida. Trends in the development of remdesivir based inventions against COVID-19 and other disorders: A patent review. J Infect Public Health (2021) 14:1075–86. doi: 10.1016/j.jiph.2021.06.013
96. Imran M, Kumar AM, Asdaq SMB, Khan SA, Alaqel SI, Alshammari MK, et al. Discovery, development, and patent trends on molnupiravir: A prospective oral treatment for COVID-19. Molecules (2021) 26:5795. doi: 10.3390/molecules26195795
97. Hashemian SM, Farhadi T, Velayati AA. A review on favipiravir: The properties, function, and usefulness to treat COVID-19. Expert Rev Anti Infect Ther (2021) 19:1029–37. doi: 10.1080/14787210.2021.1866545
98. Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev (2020) 54:62–75. doi: 10.1016/j.cytogfr.2020.06.001
99. Markoulaki D, Iordanou S, Koukios D, Christoldoulou I, Papadopoulos P, Matsentidou CT, et al. Severe multisystem inflammatory syndrome associated with SARS-CoV-2 in a 31-Year-Old Male patient: The first clinical case report from the republic of Cyprus. Cureus (2022) 14:e22640. doi: 10.7759/cureus.22640
100. Rondanelli M, Perna S, Gasparri C, Petrangolini G, Allegrini P, Cavioni A, et al. Promising effects of 3-month period of quercetin phytosome® supplementation in the prevention of symptomatic COVID-19 disease in healthcare workers: A pilot study. Life (2022) 12:66. doi: 10.3390/life12010066
101. Saeedi-Boroujeni A, Mahmoudian-Sani MR. Anti-inflammatory potential of quercetin in COVID-19 treatment. J Inflamm Lond (2021) 18:3. doi: 10.1186/s12950-021-00268-6
102. Derosa G, Maffioli P, D’Angelo A, Di Pierro F. A role for quercetin in coronavirus disease (COVID-19). Phytother Res (2019) 35:1230–6. doi: 10.1002/ptr.6887
103. Jiang W, Huang Y, Han N, He F, Li M, Bian Z, et al. Quercetin suppresses NLRP3 NLRP3 inflammasome activation and attenuates histopathology in a rat model of spinal cord injury. Spinal Cord (2016) 54:592–6. doi: 10.1038/sc.2015.227
104. Rogerio ADP, Kanashiro A, Fontanari C, Da Silva EVG, Lucisano-Valim YM, Soares EG, et al. Anti-inflammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma. Inflamm Res (2007) 56:402–8. doi: 10.1007/s00011-007-7005-6
105. Min YD, Choi CH, Bark H, Son HY, Park HH, Lee S, et al. Quercetin inhibits expression of inflammatory cytokines through attenuation of NF-κB and p38 MAPK in HMC-1 human mast cell line. Inflamm Res (2007) 56:210–5. doi: 10.1007/s00011-007-6172-9
106. Kimata M, Shichijo M, Miura T, Serizawa I, Inagaki N, Nagai H, et al. Effects of luteolin, quercetin and baicalein on immunoglobulin e-mediated mediator release from human cultured mast cells. Clin Exp Allergy (2000) 30:501–8. doi: 10.1046/j.1365-2222.2000.00768.x
107. Swain SS, Rout SS, Sahoo A, Oyedemi SO, Hussain T. Antituberculosis, antioxidant and cytotoxicity profiles of quercetin: a systematic and cost-effective in silico and in vitro approach. Nat Prod Res (2022) 36(18):4763–7. doi: 10.1080/14786419.2021.2008387
108. Harding CV, Heuser JE, Stahl PD. Exosomes: Looking back three decades and into the future. J Cell Biol (2013) 200:367–71. doi: 10.1083/jcb.201212113
109. Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci BioMed Cent (2019) 9:9–19. doi: 10.1186/s13578-019-0282-2
110. Abraham A, Krasnodembskaya A. Mesenchymal stem cell-derived extracellular vesicles for the treatment of acute respiratory distress syndrome: Concise review. Stem Cells Transl Med (2019) 9:28–38. doi: 10.1002/sctm.19-0205
111. Wang M, Yuan Q, Xie L. Mesenchymal stem cell-based immunomodulation: properties and clinical application. Stem Cells Int (2018) 14:3057624. doi: 10.1155/2018/3057624
112. Worthington EN, Hagood JS. Therapeutic use of extracellular vesicles for acute and chronic lung disease. Int J Mol Sci (2020) 21:2318. doi: 10.3390/ijms21072318
113. Rao L, Tian R, Chen X. Cell-Membrane-Mimicking nanodecoys against infectious diseases. ACS Nano (2020) 14(3):2569–74. doi: 10.1021/acsnano.0c01665
114. Zhang Y, Chen Y, Lo C, Zhuang J, Angsantikul P, Wei X, et al. Inhibition of pathogen adhesion by bacterial outer membrane-coated nanoparticles. Angew Chem Int Ed Engl (2019) 58(33):11404–8. doi: 10.1002/anie.201906280
115. Rao L, Xia S, Xu W, Tian R, Yu G, Gu C, et al. Decoy nanoparticles protect against COVID-19 by concurrently adsorbing viruses and inflammatory cytokines. Proc Natl Acad Sci U S A (2020) 117(44):27141–7. doi: 10.1073/pnas.2014352117
116. Wei X, Zhang G, Ran D, Krishnan N, Fang RH, Gao W, et al. T-Cell-Mimicking nanoparticles can neutralize HIV infectivity. Adv Mater (2018) 30(45):e1802233. doi: 10.1002/adma.201802233
117. Popowski KD, Dinh PC, George A, Lutz H, Cheng K. Exosome therapeutics for COVID-19 and respiratory viruses.View Beijing (2021) 2(3):20200186. doi: 10.1002/VIW.20200186
118. V’kovski P, Kratzel A, Steiner S, Stalder H, Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol (2021) 19:155–70. doi: 10.1038/s41579-020-00468-6
119. Tortorici MA, Veesler D. Structural insights into coronavirus entry. Adv Virus Res (2019) 105:93–116. doi: 10.1016/bs.aivir.2019.08.002
120. Li F. Structure, function, and evolution of coronavirus spike proteins. Annu Rev Virol (2016) 3(1):237–61. doi: 10.1146/annurev-virology-110615-042301
121. Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage b betacoronaviruses. Nat Microbiol (2020) 5(4):562–9. doi: 10.1038/s41564-020-0688-y
122. Jo S, Kim H, Kim S, Shin DH, Kim MS. Characteristics of flavonoids as potent MERS-CoV 3C-like protease inhibitors. Chem Biol Drug Des (2019) 94(6):2023–30. doi: 10.1111/cbdd.13604
123. Derosa G, Maffioli P, D’Angelo A, Di Pierro F. A role for quercetin in coronavirus disease 2019 (COVID-19) phytother. Res (2021) 35:1230–6. doi: 10.1002/ptr.6887
124. Imran M, Thabet HK, Alaqel SI, Alzahrani AR, Abida A, Alshammari MK, et al. The Therapeutic and Prophylactic Potential of Quercetin against COVID-19: An Outlook on the Clinical Studies, Inventive Compositions, and Patent Literature. Antioxidants (2022) (Basel) 11(5):876. doi: 10.3390/antiox11050876
125. Nguyen TTH, Woo HJ, Kang HK, Kim YM, Kim DW, Kim DW, et al. Flavonoid-mediated inhibition of SARS coronavirus 3C-like protease expressed in pichia pastoris. Biotechnol Lett (2012) 34(5):831–8. doi: 10.1007/s10529-011-0845-8
126. Karn V, Ahmed S, Tsai LW, Dubey R, Ojha S, Singh HN, et al. Extracellular vesicle-based therapy for COVID-19: Promises, challenges and future prospects. Biomedicines (2021) 9(10):1373. doi: 10.3390/biomedicines9101373
127. Bian S, Zhang L, Duan L, Wang X, Min Y, Yu H, et al. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med (2014) 92:387–97. doi: 10.1007/s00109-013-1110-5
Keywords: Quercetin, extracellular vesicle, SARS CoV2, nanoparticle, therapeutic target
Citation: Raghav A, Giri R, Agarwal S, Kala S and Jeong G-B- (2022) Protective role of engineered extracellular vesicles loaded quercetin nanoparticles as anti-viral therapy against SARS-CoV-2 infection: A prospective review. Front. Immunol. 13:1040027. doi: 10.3389/fimmu.2022.1040027
Received: 08 September 2022; Accepted: 23 November 2022;
Published: 08 December 2022.
Edited by:
Bertrand Kaeffer, Institut National de recherche pour l’agriculture, l’alimentation et l’environnement (INRAE), FranceReviewed by:
Kaijian Hou, Shantou University, ChinaCopyright © 2022 Raghav, Giri, Agarwal, Kala and Jeong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Goo-Bo- Jeong, Z2JqZW9uZ0BnYWNob24uYWMua3I=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.