
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol. , 02 August 2021
Sec. Inflammation
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.708101
This article is part of the Research Topic C-Reactive Protein in Age-Related Disorders, Volume II View all 10 articles
Background: Plasma levels of C-reactive protein (CRP), induced by Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) triggering COVID-19, can rise surprisingly high. The increase of the CRP concentration as well as a certain threshold concentration of CRP are indicative of clinical deterioration to artificial ventilation. In COVID-19, virus-induced lung injury and the subsequent massive onset of inflammation often drives pulmonary fibrosis. Fibrosis of the lung usually proceeds as sequela to a severe course of COVID-19 and its consequences only show months later. CRP-mediated complement- and macrophage activation is suspected to be the main driver of pulmonary fibrosis and subsequent organ failure in COVID-19. Recently, CRP apheresis was introduced to selectively remove CRP from human blood plasma.
Case Report: A 53-year-old, SARS-CoV-2 positive, male patient with the risk factor diabetes type 2 was referred with dyspnea, fever and fulminant increase of CRP. The patient’s lungs already showed a pattern enhancement as an early sign of incipient pneumonia. The oxygen saturation of the blood was ≤ 89%. CRP apheresis using the selective CRP adsorber (PentraSorb® CRP) was started immediately. CRP apheresis was performed via peripheral venous access on 4 successive days. CRP concentrations before CRP apheresis ranged from 47 to 133 mg/l. The removal of CRP was very effective with up to 79% depletion within one apheresis session and 1.2 to 2.14 plasma volumes were processed in each session. No apheresis-associated side effects were observed. It was at no point necessary to transfer the patient to the Intensive Care Unit or to intubate him due to respiratory failure. 10 days after the first positive SARS-CoV-2 test, CRP levels stayed below 20 mg/l and the patient no longer exhibited fever. Fourteen days after the first positive SARS-CoV-2 test, the lungs showed no sign of pneumonia on X-ray.
Conclusion: This is the first report on CRP apheresis in an early COVID-19 patient with fulminant CRP increase. Despite a poor prognosis due to his diabetes and biomarker profile, the patient was not ventilated, and the onset of pneumonia was reverted.
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and has evolved into a pandemic causing a public health crisis (1, 2). It is diagnosed by detection of virus in respiratory secretions using PCR analysis. The course of disease is mild in the majority of patients with symptoms including sore throat, cough, breathlessness, fever, and fatigue among others. In some patients, however, the disease may progress to viral pneumonia and acute respiratory distress syndrome (ARDS), finally leading to multi-organ failure. In this small percentage, SARS-CoV-2 induces lung fibrosis and cardiac complications (3–5). So far, primary therapy is symptomatic and focuses on ARDS. Given it is a viral disease, plasma levels of C-reactive protein (CRP), a common bacterial infection marker, are surprisingly high. Further, CRP levels were consistently shown to correlate with the severity of the disease, the overall clinical situation and the risk for the patient to need mechanical ventilation (6–13).
CRP is an ancient antibody, being part of the innate immune response and increasing rapidly during the acute phase reaction. Increasing evidence suggests that CRP causally promotes tissue destruction during the inflammatory response under certain pathological circumstances (14). Intraalveolar edema and hemorrhage are a common observation in the lungs of COVID-19 patients, resulting in ischemic alveolar tissue. CRP itself is reported to trigger tissue damage by binding to these ischemic cells and thus is also causally involved in the enlargement of the destroyed tissue, contributing to irreversible tissue destruction (14). Two well-described immunological CRP functions are the activation of the classical complement pathway via C1q binding (15) and the binding to human immunoglobulin Fcγ-receptors (mainly FcγRIIa) after opsonization of biological particles for macrophages (16–18). The opsonization of affected endogenous cells significantly contributes to the enlargement of organ damage (19). In the case of progressive, severe COVID-19 with high CRP plasma concentrations, pulmonary tissue is irreversibly disposed by the action of CRP (20).
So far, there is no pharmaceutical option to reduce the extremely high synthesis/level of CRP during an acute phase response, but the rapid reduction of high CRP levels in medium and severe courses of COVID-19 was proposed early on during the pandemic (21–23) and also recently (14, 24).
Selective, extracorporeal CRP apheresis with a CRP adsorber (25) lowers the CRP concentration drastically within a few hours, and the repeatable treatment is safe and efficient (26, 27). Clear evidence has been shown in previous clinical studies, investigating CRP apheresis after myocardial infarction, that CRP depletion reduces systemic inflammation and cardiac tissue damage (27). We used this therapy in the early phase of incipient pulmonary fibrosis in a patient diagnosed with SARS-CoV-2 infection.
A 53-year-old male Caucasian with diabetes type 2 was tested positive for SARS-CoV-2 with a rapid antigen test. Diagnosis was substantiated with PCR testing afterwards. Moderate symptoms started roughly 48 h after the first positive test, including increasing fever, shivering, listlessness, hyperhidrosis, lack of exercise tolerance, mild hypoxia, shallow respiration and severe coughing. The patient was first examined 5 days after the positive antigen test. He showed an overall poor physical condition. Oxygen saturation was measured continuously and fluctuated above 90%, however it momentarily reached 89%, which led to referral to a chest x-ray. As the oxygen saturation improved during the first day of treatment, possibly due to rehydration, no oxygen supplementation was performed. Symptoms and CRP plasma levels were monitored twice daily. 5 days after the initial positive test, CRP concentrations increased rapidly. Based on the rate of increase in CRP concentration, which is a prognostic marker for the potential onset of ventilation requirement (7), treatment with CRP apheresis was decided. First CRP apheresis was initiated 5 days after positive SARS-CoV-2 test (3 days after symptom onset) and four apheresis treatments were performed on consecutive days with the PentraSorb CRP adsorber (Table 1).
Of note, a bacterial infection was excluded by measuring Procalcitonin (0.22 µg/L 3 days after symptom onset).
CRP concentration kinetics showed a rapid increase in plasma levels between 96 and 132 h after positive SARS-CoV-2 antigen test and 1st apheresis was then initiated. Apheresis treatments were performed as described elsewhere in detail (27) and the blood was anticoagulated with ACD-A (1st apheresis) or ACD-A and additional heparin (2nd - 4th apheresis). Treated plasma volume was dependent on patient condition and CRP concentration. Apheresis parameters are listed in Table 2.
CRP plasma concentrations declined rapidly during apheresis with a maximal depletion of 79% during the last apheresis session (Figure 1). Maximum CRP concentration of 133 mg/l was reached 7 days after positive antigen test and before 3rd apheresis. Massive CRP synthesis was observed during the first two CRP aphereses. CRP levels stayed below 50 mg/l after the fourth apheresis treatment and normalized (< 10 mg/l) 12 days after the first positive antigen test.
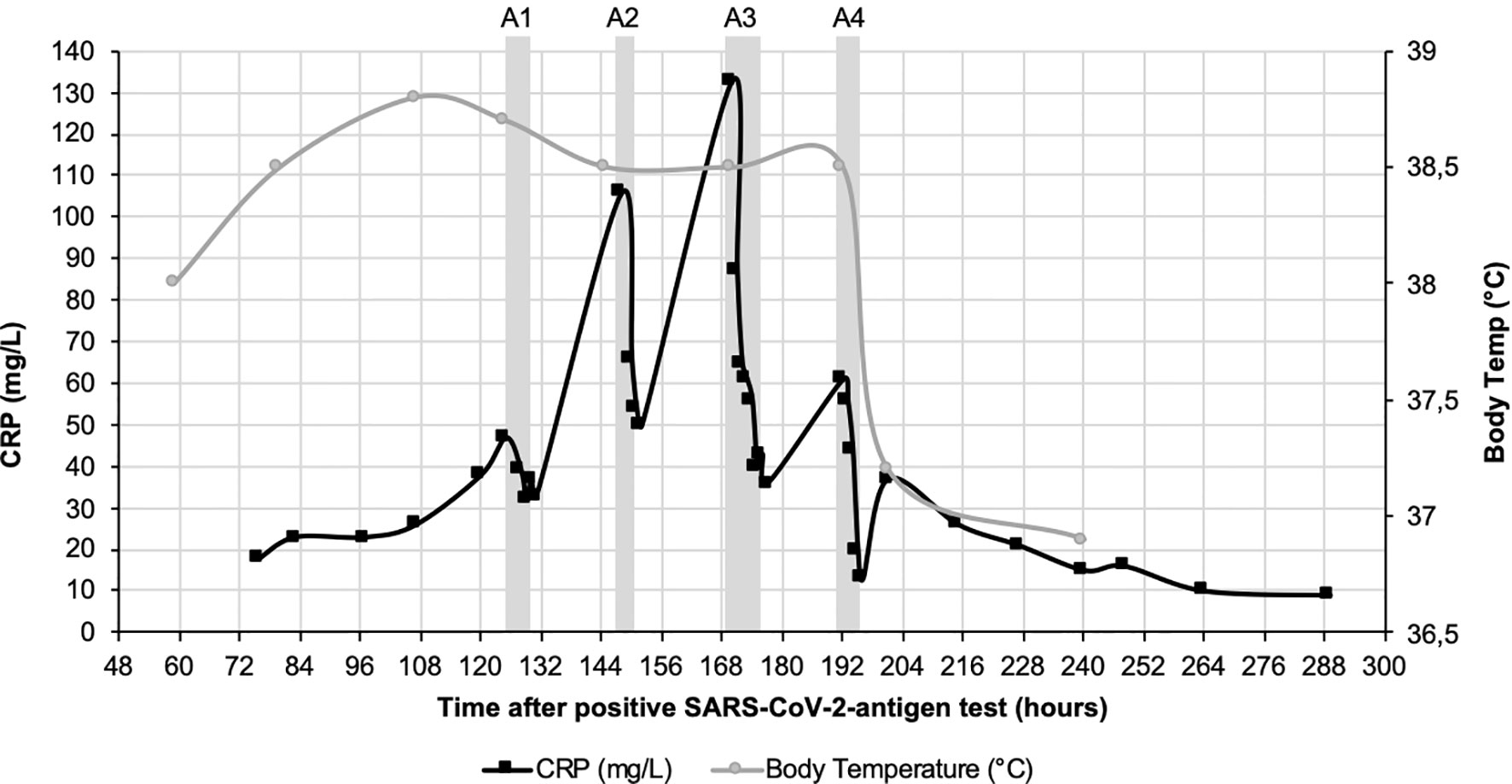
Figure 1 CRP course (black) and course of body temperature (grey) over 12 days after positive SARS-CoV-2-antigen test. CRP concentration was measured in plasma. Fever decreased 8 days after positive SARS-CoV-2 test during the last CRP apheresis. Grey boxes indicate the four apheresis treatments (A1-4).
Body temperature was measured daily and stayed consistently above 38.0°C until 8 days after positive antigen testing, when it decreased to a physiological 37.2°C during the last CRP apheresis, simultaneously reflecting the better condition of the patient (Figure 1).
During apheresis treatments, general cardiovascular parameters were measured at least hourly and did not show severe abnormalities. Blood pressure and pulse remained stable albeit the pulse was elevated over all four treatments. Sodium, Potassium, Calcium, Lactate and Glucose measurements showed normal concentrations without large changes during apheresis sessions. Venous O2 saturation improved during all four treatments. The patient’s hematocrit was influenced by fluid substitution during aphereses and decreased slightly during the treatments.
Chest X-ray was performed before 1st apheresis (day 5 after positive antigen test) and showed streaky compressions of the lung in the lower field/retrocardial left side. The abnormalities were consistent with the expected pattern of atypical pneumonia. The 2nd X-ray was performed 6 days after the last apheresis session and showed no more pathological findings (Figure 2).
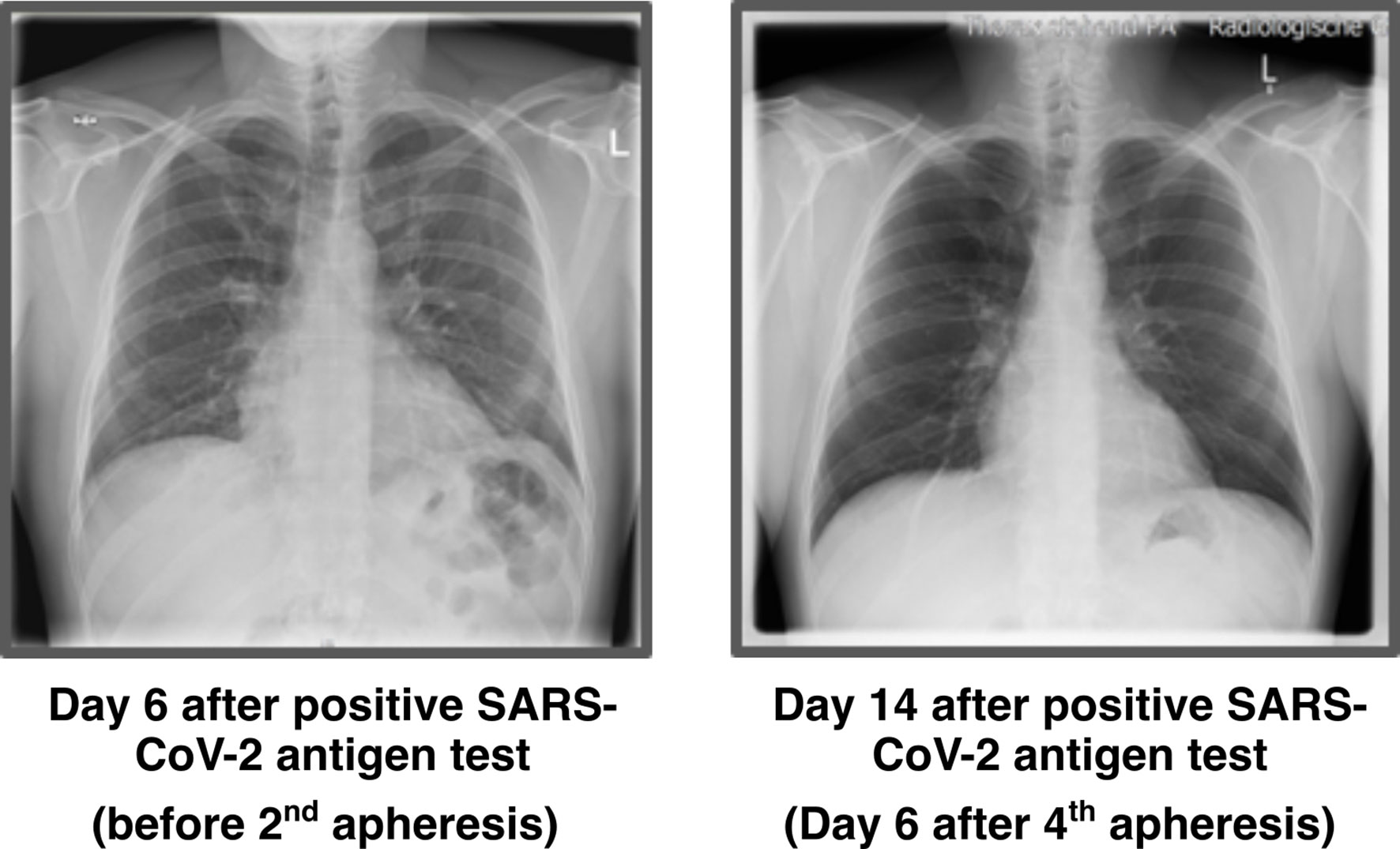
Figure 2 Chest X-ray images. X-ray thorax in 2 planes left adjacent. 6 days: Streaky compressions of the lung in the lower field/retrocardial left side. A low-grade concomitant effusion on the left. Heart normal size, no congestion. Upper mediastinum slender. No pneumothorax. Bony thorax intact. 14 days: No compressions and effusions.
Transthoracic Echocardiography was performed 27 days after last apheresis session with sinus rhythm. It exhibited no pathological findings. Left and right ventricle displayed normal size and healthy systolic and diastolic function. No hypertrophy or pericardial effusion was detected. Both atriums displayed normal size and cardiac valves exhibited regular function. Resting echocardiography showed no pathological findings as well.
Cardiac MRI was performed 41 days after last apheresis session and showed normal, healthy cardiac function. Specifically, there was no indication for exercise-induced myocardial ischemia, cardiomyopathy, acute/chronic peri/myocarditis or cardiac amyloidosis. There was also no evidence for myocardial fibrosis or acute/chronic inflammatory remodeling of the pericard.
This successful treatment of a fulminant COVID-19 course with poor prognosis indicates that the use of CRP apheresis should be considered a therapeutic option in moderate to severe COVID-19 courses with a pronounced increase in CRP plasma levels. Specifically, in patients belonging to the high-risk group because of pre-existing conditions. When initiated in the early phase, CRP apheresis could prevent the deterioration of the patient to mechanical ventilation or to the ICU. Patients who generate more than 100 mg/l CRP otherwise invariably end up on ventilation with 30% mortality (7). The reported patient did not have to be admitted to the hospital and was treated as an outpatient despite a rather low blood oxygen saturation and his pre-existing diabetes type 2.
The described therapy option is not tailored specifically to treat COVID-19, but can be used in every condition that is marked by a sharp increase in CRP levels and in which CRP contributes to accelerated tissue destruction (28). CRP apheresis in this patient efficiently reduced CRP levels even in the initial high synthesis phase. After two apheresis sessions, CRP levels peaked at ~130 mg/l 7 days after the positive antigen test despite CRP depletion on the two past days and then gradually declined with the substantial support of two subsequent apheresis procedures. We can only hypothesize how high CRP levels would have increased without apheresis. The initial rise and numerous other reports support the assumption that CRP would have risen to > 300 mg/l in this patient without this CRP apheresis (7, 10, 12). The patient also received 6 mg dexamethasone from day 7 onwards (after the third apheresis), despite being contraindicated in nonventilated patients according to the guideline. Although it is established that this treatment affects CRP levels by inhibiting the inflammatory response overall, this effect usually needs several days and is not fast (29). In fact, the rationale for the use of dexamethasone was to curb a potential, renewed rise in CRP the following week. The pronounced reduction of CRP during treatments and the overall achieved decrease in the area under the concentration curve should be attributed to CRP apheresis.
The severe and often fatal course of COVID-19 is not caused by the virus itself, but by a massive and excessive immune response, responsible for tissue destructive processes (30). The often-described cytokine storm presents a major responsibility for ARDS and CRP has been established as one of the key markers of this phenomenon, no matter of the underlying disease (22, 31–33). Reducing the decisive factor CRP may cause beneficial effects and slow down immunological self-destruction of the lung and other organs.
CRP apheresis has been used once before in a COVID-19 patient, who was already ventilated and progressed far during the deleterious course of SARS-CoV-2 infection (34). Here, CRP apheresis potentially improved laboratory parameters indicating failure of the heart, kidney and liver. However, CRP plasma levels already reached > 200 mg/l before 1st apheresis and treatment was probably started too late and interrupted for four days to save the patient.
CRP obviously mediates important immune defense mechanisms, which should not be suppressed during an ongoing bacterial infection. However, in this patient who was free of a bacterial infect, CRP levels never declined below 10 mg/l during the apheresis treatment. Rather, the dramatic rise in CRP concentration, mediating pathological tissue destroying functions, was inhibited by CRP apheresis.
Experimental therapies should be realistically considered, especially in the current situation where there are still no satisfying specific therapies for COVID-19 patients and where it is just becoming apparent what the long-term consequences (long COVID) will be in recovered patients.
Cyclooxygenase inhibitors, antibiotics, corticosteroids and other anti-inflammatory therapies do mildly affect CRP as a side effect (35, 36). However, these therapies needed several days in order to affect CRP levels. This is not feasible in the setting of an acute phase response, in which CRP levels rise dramatically within a day or two and need to be reduced in a short time window after the initial rise of the CRP amount. CRP apheresis is selective and efficient and only affects the negative effects of high CRP concentrations but not the rest of the inflammatory response.
While this patient successfully improved with CRP apheresis, a definitive cause and effect relationship needs to be analyzed in a larger group of patients. A randomized clinical trial using CRP apheresis in early-phase COVID-19 patients is planned. For now, CRP apheresis can be considered a safe and potentially effective treatment in patients with a moderate to severe COVID-19 course, specifically when started right after the initial increase of plasma CRP levels or early after the start of ventilation.
Through my daily work, I have already known about CRP apheresis and its positive effects. The theoretical and clinical approach for the treatment of my diagnosed COVID-19 was comprehensible to me and I was very happy that my colleagues treated me and averted a bad course. I had a good feeling throughout the therapy.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
AS drafted the manuscript. JR and CB reviewed and edited the manuscript. JR, AR, and CB collected all data. AR, CB, and AS coordinated CRP apheresis. All authors contributed to the article and approved the submitted version.
AS is Founder and Shareholder of Pentracor GmbH. CB and AR are employees of Pentracor GmbH.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We are grateful for the very kind support and the dedicated assistance of the team at the DIAMEDIKUM Potsdam.
1. Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, et al. Covid-19 in Critically Ill Patients in the Seattle Region - Case Series. N Engl J Med (2020) 382(21):2012–22. doi: 10.1056/NEJMoa2004500
2. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients With COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet (2020) 395(10229):1054–62. doi: 10.1016/S0140-6736(20)30566-3
3. Aghagoli G, Gallo Marin B, Soliman LB, Sellke FW. Cardiac Involvement in COVID-19 Patients: Risk Factors, Predictors, and Complications: A Review. J Card Surg (2020) 35:1302–5. doi: 10.1111/jocs.14538
4. Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, et al. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol (2020) 5(7):819–24. doi: 10.1001/jamacardio.2020.1096
5. Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the Cardiovascular System. Nat Rev Cardiol (2020) 17(5):259–60. doi: 10.1038/s41569-020-0360-5
6. Liu F, Li L, Xu M, Wu J, Luo D, Zhu Y, et al. Prognostic Value of Interleukin-6, C-Reactive Protein, and Procalcitonin in Patients With COVID-19. J Clin Virol (2020) 127:104370. doi: 10.1016/j.jcv.2020.104370
7. Mueller AA, Tamura T, Crowley CP, DeGrado JR, Haider H, Jezmir JL, et al. Inflammatory Biomarker Trends Predict Respiratory Decline in COVID-19 Patients. Cell Rep Med (2020) 1(8):100144. doi: 10.1016/j.xcrm.2020.100144
8. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical Predictors of Mortality Due to COVID-19 Based on an Analysis of Data of 150 Patients From Wuhan, China. Intensive Care Med (2020) 46(5):846–8. doi: 10.1007/s00134-020-05991-x
9. Shang W, Dong J, Ren Y, Tian M, Li W, Hu J, et al. The Value of Clinical Parameters in Predicting the Severity of COVID-19. J Med Virol (2020) 92:2188–92. doi: 10.1002/jmv.26031
10. Smilowitz NR, Kunichoff D, Garshick M, Shah B, Pillinger M, Hochman JS, et al. C-Reactive Protein and Clinical Outcomes in Patients With COVID-19. Eur Heart J (2021) 42(23):2270–9. doi: 10.1093/eurheartj/ehaa1103
11. Smilowitz NR, Nguy V, Aphinyanaphongs Y, Newman JD, Xia Y, Reynolds HR, et al. Multiple Biomarker Approach to Risk Stratification in COVID-19. Circulation (2021) 143(13):1338–40. doi: 10.1161/CIRCULATIONAHA.120.053311
12. Tan C, Huang Y, Shi F, Tan K, Ma Q, Chen Y, et al. C-Reactive Protein Correlates With Computed Tomographic Findings and Predicts Severe COVID-19 Early. J Med Virol (2020) 92:856–62. doi: 10.1002/jmv.25871
13. Velavan TP, Meyer CG. Mild Versus Severe COVID-19: Laboratory Markers. Int J Infect Dis (2020) 95:304–7. doi: 10.1016/j.ijid.2020.04.061
14. Sheriff A, Kayser S, Brunner P, Vogt B. C-Reactive Protein Triggers Cell Death in Ischemic Cells. Front Immunol (2021) 12:630430(273). doi: 10.3389/fimmu.2021.630430
15. Kaplan MH, Volanakis JE. Interaction of C-Reactive Protein Complexes With the Complement System. I. Consumption of Human Complement Associated With the Reaction of C-Reactive Protein With Pneumococcal C-Polysaccharide and With the Choline Phosphatides, Lecithin and Sphingomyelin. J Immunol (1974) 112(6):2135–47.
16. Bharadwaj D, Stein MP, Volzer M, Mold C, Du Clos TW. The Major Receptor for C-Reactive Protein on Leukocytes is Fcgamma Receptor II. J Exp Med (1999) 190(4):585–90. doi: 10.1084/jem.190.4.585
17. Manolov DE, Rocker C, Hombach V, Nienhaus GU, Torzewski J. Ultrasensitive Confocal Fluorescence Microscopy of C-Reactive Protein Interacting With FcgammaRIIa. Arterioscler Thromb Vasc Biol (2004) 24(12):2372–7. doi: 10.1161/01.ATV.0000147407.17137.02
18. Zwaka TP, Hombach V, Torzewski J. C-Reactive Protein-Mediated Low Density Lipoprotein Uptake by Macrophages: Implications for Atherosclerosis. Circulation (2001) 103(9):1194–7. doi: 10.1161/01.cir.103.9.1194
19. Sheriff A, Schindler R, Vogt B, Abdel-Aty H, Unger JK, Bock C, et al. Selective Apheresis of C-Reactive Protein: A New Therapeutic Option in Myocardial Infarction? J Clin Apher (2015) 30(1):15–21. doi: 10.1002/jca.21344
20. Mosquera-Sulbaran JA, Pedreañez A, Carrero Y, Callejas D. C-Reactive Protein as an Effector Molecule in Covid-19 Pathogenesis. Rev Med Virol (2021), e2221. doi: 10.1002/rmv.2221
21. Kayser S, Kunze R, Sheriff A. Selective C-Reactive Protein Apheresis for Covid-19 Patients Suffering From Organ Damage. Ther Apher Dial (2020) 25(2):251–2. doi: 10.1111/1744-9987.13532
22. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: Consider Cytokine Storm Syndromes and Immunosuppression. Lancet (2020) 395(10229):1033–4. doi: 10.1016/S0140-6736(20)30628-0
23. Risitano AM, Mastellos DC, Huber-Lang M, Yancopoulou D, Garlanda C, Ciceri F, et al. Complement as a Target in COVID-19? Nat Rev Immunol (2020) 20:343–4. doi: 10.1038/s41577-020-0320-7
24. Pepys MB. C-Reactive Protein Predicts Outcome in COVID-19: Is It Also a Therapeutic Target? Eur Heart J (2021) 42(23):2280–3. doi: 10.1093/eurheartj/ehab169
25. Mattecka S, Brunner P, Hähnel B, Kunze R, Vogt B, Sheriff A. PentraSorb C-Reactive Protein: Characterization of the Selective C-Reactive Protein Adsorber Resin. Ther Apheresis Dialysis (2019) 23(5):474–81. doi: 10.1111/1744-9987.12796
26. Ries W, Heigl F, Garlichs C, Sheriff A, Torzewski J. Selective C-Reactive Protein-Apheresis in Patients. Ther Apher Dial (2019) 23(6):570–4. doi: 10.1111/1744-9987.12804
27. Ries W, Torzewski J, Heigl F, Pfluecke C, Kelle S, Darius H, et al. C-Reactive Protein Apheresis as Anti-Inflammatory Therapy in Acute Myocardial Infarction: Results of the CAMI-1 Study. Front Cardiovasc Med (2021) 8:591714(155). doi: 10.3389/fcvm.2021.591714
28. Kayser S, Brunner P, Althaus K, Dorst J, Sheriff A. Selective Apheresis of C-Reactive Protein for Treatment of Indications With Elevated CRP Concentrations. J Clin Med (2020) (9):2947. doi: 10.3390/jcm9092947
29. Selvaraj V, Dapaah-Afriyie K, Finn A, Flanigan TP. Short-Term Dexamethasone in Sars-CoV-2 Patients. R I Med J (2013) (2020) 103(6):39–43.
30. Nienhold R, Ciani Y, Koelzer VH, Tzankov A, Haslbauer JD, Menter T, et al. Two Distinct Immunopathological Profiles in Autopsy Lungs of COVID-19. Nat Commun (2020) 11(1):5086. doi: 10.1038/s41467-020-18854-2
31. Fajgenbaum DC, June CH. Cytokine Storm. N Engl J Med (2020) 383(23):2255–73. doi: 10.1056/NEJMra2026131
32. Nadeem R, Elhoufi AM, Iqbal NE, Obaida ZA, Elgohary DM, Singh MK, et al. Prediction of Cytokine Storm and Mortality in Patients With COVID-19 Admitted to ICU: Do Markers Tell the Story? Dubai Med J (2021) 4:142–50. doi: 10.1159/000514406
33. Cappanera S, Palumbo M, Kwan SH, Priante G, Martella LA, Saraca LM, et al. When Does the Cytokine Storm Begin in COVID-19 Patients? A Quick Score to Recognize It. J Clin Med (2021) 10(2):297. doi: 10.3390/jcm10020297
34. Torzweski J, Heigl F, Zimmermann O, Wagner F, Schumann C, Hettich R, et al. First-In-Man: Case Report of Selective C-Reactive Protein Apheresis in a Patient With SARS-CoV-2 Infection. Am J Case Rep (2020) 21:e925020. doi: 10.12659/AJCR.925020
35. Copaescu A, James F, Mouhtouris E, Vogrin S, Smibert OC, Gordon CL, et al. The Role of Immunological and Clinical Biomarkers to Predict Clinical COVID-19 Severity and Response to Therapy-A Prospective Longitudinal Study. Front Immunol (2021) 12:646095. doi: 10.3389/fimmu.2021.646095
Keywords: COVID-19, C-reactive protein, SARS-CoV-2, apheresis - therapeutic, pulmonary fibrosis (MeSH)
Citation: Ringel J, Ramlow A, Bock C and Sheriff A (2021) Case Report: C-Reactive Protein Apheresis in a Patient With COVID-19 and Fulminant CRP Increase. Front. Immunol. 12:708101. doi: 10.3389/fimmu.2021.708101
Received: 11 May 2021; Accepted: 20 July 2021;
Published: 02 August 2021.
Edited by:
Mark Slevin, Manchester Metropolitan University, United KingdomReviewed by:
Shampa Chatterjee, University of Pennsylvania, United StatesCopyright © 2021 Ringel, Ramlow, Bock and Sheriff. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmed Sheriff, YWhtZWQuc2hlcmlmZkBjaGFyaXRlLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.