- 1Department of Preventive and Community Dentistry, Faculty of Dentistry, Osaka Dental University, Hirakata, Japan
- 2Department of Oral and Molecular Microbiology, Graduate School of Dentistry, Osaka University, Suita, Japan
- 3Department of Biomaterials, Faculty of Dentistry, Osaka Dental University, Hirakata, Japan
- 4Division of Clinical Vaccinology, International Research and Development Center for Mucosal Vaccines, The Institute of Medical Science, The University of Tokyo, Tokyo, Japan
- 5Department of Pediatric Dentistry, School of Dentistry, The University of Alabama at Birmingham, Birmingham, AL, United States
Our previous studies showed that a combination of a DNA plasmid encoding Flt3 ligand (pFL) and CpG oligodeoxynucleotides 1826 (CpG ODN) (FL/CpG) as a nasal adjuvant provoked antigen-specific immune responses. In this study, we investigated the efficacy of a nasal vaccine consisting of FimA as the structural subunit of Porphyromonas gingivalis (P. gingivalis) fimbriae and FL/CpG for the induction of FimA-specific antibody (Ab) responses and their protective roles against nasal and lung infection by P. gingivalis, a keystone pathogen in the etiology of periodontal disease. C57BL/6 mice were nasally immunized with recombinant FimA (rFimA) plus FL/CpG three times at weekly intervals. As a control, mice were given nasal rFimA alone. Nasal washes (NWs) and bronchoalveolar lavage fluid (BALF) of mice given nasal rFimA plus FL/CpG resulted in increased levels of rFimA-specific secretory IgA (SIgA) and IgG Ab responses when compared with those in controls. Significantly increased numbers of CD8- or CD11b-expressing mature-type dendritic cells (DCs) were detected in the respiratory inductive and effector tissues of mice given rFimA plus FL/CpG. Additionally, significantly upregulated Th1/Th2-type cytokine responses by rFimA-stimulated CD4+ T cells were noted in the respiratory effector tissues. When mice were challenged with live P. gingivalis via the nasal route, mice immunized nasally with rFimA plus FL/CpG inhibited P. gingivalis colonization in the nasal cavities and lungs. In contrast, controls failed to show protection. Of interest, when IgA-deficient mice given nasal rFimA plus FL/CpG were challenged with nasal P. gingivalis, the inhibition of bacterial colonization in the respiratory tracts was not seen. Taken together, these results show that nasal FL/CpG effectively enhanced DCs and provided balanced Th1- and Th2-type cytokine response-mediated rFimA-specific IgA protective immunity in the respiratory tract against P. gingivalis. A nasal administration with rFimA and FL/CpG could be a candidate for potent mucosal vaccines for the elimination of inhaled P. gingivalis in periodontal patients.
Introduction
Secretory IgA (SIgA) antibody (Ab) is the major isotype at the mucosal surface. SIgA Abs are mainly secreted as dimeric or polymeric forms and play roles as the first line of defense by neutralizing viruses and toxins as well as by inhibiting bacterial adherence to host mucosal surfaces (1). Hence, mucosal immunization can be a major strategy for the induction of antigen (Ag)-specific SIgA Ab responses (1). For example, nasal vaccination effectively induces Ag-specific SIgA Ab responses in various mucosal tissues including the respiratory tract (2), the oral cavity (3), and the reproductive tract (4). An additional unique feature of mucosal immunization is to elicit Ag-specific IgG Ab responses in the systemic compartment (2–5). Despite these advantages, mucosal immunization requires adjuvants or a delivery system for the induction and regulation of Ag-specific immune responses. In this regard, we have previously shown that nasal application of a DNA plasmid encoding Flt3 ligand cDNA (pFL) as a mucosal adjuvant and ovalbumin as an Ag preferentially expands CD8+ CD11c+ dendritic cells (DCs) and subsequently induces IL-4-producing CD4+ T cell-mediated Ag-specific mucosal immune responses (6). Further, we have shown that the combination of pFL and CpG oligodeoxynucleotides (FL/CpG) as a DC-targeting nasal adjuvant enhances Ag-specific mucosal and systemic immunity with a balanced Th1/Th2-type cytokine response that protects from bacterial and viral infection (7–9).
Periodontitis is one of the most prevalent infectious diseases worldwide and is characterized by gingival inflammation and bone loss following periodontal-pathogenic bacterial infection and disruption of host immunity. Porphyromonas gingivalis (P. gingivalis) is a keystone pathogen that is responsible for the progression of periodontitis (10) and various systemic diseases including aspiration pneumonia (11), despite being detected in healthy people (12). Fimbriae on the cell surfaces of P. gingivalis are known to be adhesins primarily composed of polymers of FimA protein (fimbrillin) encoded by the gene fimA (13). Thus, this protein is considered to be a virulence factor and plays an important role in initial attachment or colonization through its association with salivary proteins and other bacteria on the surfaces of oral mucosa or teeth (14, 15). For example, it has been reported that fimA-inactivated mutants have lessened the ability to adhere to human gingival fibroblasts and epithelial cells (16). In addition, it has also been proven that the FimA protein elicits inflammatory responses via the TLR4/NF-κB signaling pathway in human peripheral blood mononuclear cells (17). Our previous study also showed that recombinant FimA (rFimA) protein specifically and rigidly binds to human salivary proteins such as salivary statherin or proline-rich protein (15).
In this study, we investigated the efficacy of a nasal vaccine consisting of FimA and FL/CpG for the induction of FimA-specific Ab responses and their functional properties against nasal and pulmonary infection with P. gingivalis. Our results showed an essential role of rFimA-specific SIgA Abs for the prevention of P. gingivalis colonization in the upper and lower airways.
Materials and Methods
DNA Adjuvants
The plasmid pUNO1-mFlt3L (pFL) consists of the pUNO1-mcs vector plus the full-length murine FL cDNA gene (InvivoGen, San Diego, CA, USA). This plasmid was purified using the EndoFree Plasmid purification Giga kit (QIAGEN, Valencia, CA, USA). The Limulus amebocyte lysate assay (BioWhittaker, Walkersville, MD, USA) resulted in <0.1 endotoxin units of LPS per 1 µg of plasmid. A synthetic ODN containing CpG motif 1826 (CpG ODN) (FASMAC Co., Ltd., Kanagawa, Japan) was synthesized artificially.
Nasal Vaccination
Specific pathogen-free 6- to 8-week-old female C57BL/6 (IgA+/+) mice were purchased from SLC Japan, and 6- to 8-week-old IgA-deficient (IgA−/−) mice (genetic C57BL/6 background) (17) were kindly provided by Dr. Tomoko Kurita-Ochiai from the Department of Microbiology and Immunology, Nihon University School of Dentistry at Matsudo and used in this study. Upon arrival, these mice were transferred to microisolators and maintained in horizontal laminar flow cabinets, and provided with sterile food and water as part of a specific pathogen-free facility at Osaka Dental University. Mice were immunized three times at weekly intervals nasally with 6 µl/nostril PBS containing 5 µg of rFimA plus 50 µg of pFL and 10 µg of CpG ODN as mucosal adjuvants (7–9). As controls, mice were immunized nasally with 5 µg of rFimA alone under the combination anesthesia with medetomidine, midazolam, and butorphanol. All experiments were conducted in accordance with the guidelines provided by Osaka Dental University. All of the mice used in these assays were free of bacterial and viral pathogens. This study conformed with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guideline for preclinical animal studies.
Recombinant FimA (rFimA)
The DNA plasmid vector PYT1245 expressing whole FimA protein was kindly provided by Dr. Yutaka Terao at Niigata University (18). Escherichia coli BL21 competent cells (BioDynamics Laboratory Inc., Tokyo, Japan) were transformed with PYT1245 by the heat-shock method and were cultured in Luria-Bertani medium supplemented with ampicillin (100 µg/ml). The supernatants from ultrasonicated E. coli BL21 transformants carrying the PYT1245 plasmid were applied to a GST affinity column (Cytiva, Sheffield, UK). The rFimA protein was eluted by cleaving the GST-rFimA fusion protein with PreScission protease™ (Cytiva, Sheffield, UK). The recovered protein was applied to the affinity column (JNC CORPORATION, Tokyo, Japan) to remove endotoxin, and the elution was employed as the purified rFimA protein. The Limulus amebocyte lysate assay (BioWhittaker, Walkersville, MD, USA) resulted in <0.1 endotoxin units of LPS per 1 µg of rFimA.
ELISA and ELISPOT Assays for rFimA-Specific Ab Responses
To assess rFimA-specific Ab levels, NWs, BALF, and plasma samples were collected 7 days after the last immunization and were then subjected to ELISA as described previously (19). Briefly, 96-well microtest assay plates (BD Biosciences, Oxnard, CA, USA) were coated with 1 µg/ml of rFimA in PBS. After incubating serial dilutions of samples, horseradish peroxidase-conjugated goat anti-mouse IgA or IgG Ab (Southern Biotechnology Associates Inc., Birmingham, AL, USA) were added to the wells. The color reaction was developed using 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) substrate buffer for 15 min at room temperature. Endpoint titers were expressed as the reciprocal log2 of the last dilution that gave an OD415 of 0.1 greater than the background. Mice were euthanized 1 week after the final immunization by cervical spine fracture-dislocation under inhaled isoflurane anesthesia. Mononuclear cells were isolated from nasopharyngeal-associated lymphoid tissues (NALT), nasal passages (NPs), cervical lymph nodes (CLNs), lungs, mediastinal lymph nodes (MeLNs), and spleen and were then subjected to an enzyme-linked immunospot (ELISPOT) assay to enumerate the numbers of rFimA-specific IgA or IgG Ab-forming cells (AFCs) (19).
Preparation of IgA-Enriched Samples
IgA-enriched samples were prepared by removing IgG and IgM Abs from NWs and BALF. NWs and BALF from mice immunized nasally with rFimA and FL/CpG or rFimA alone were applied on a protein G affinity mini-column (Protein G HP SpinTrap; Cytiva, Sheffield, UK), and the eluted solutions were further subjected to an IgM purification kit (Thermo Fisher Scientific, Tokyo, Japan). Approximately 800 µl of NWs and BALF were recovered by these procedures. These samples were serially diluted by PBS and subjected to live P. gingivalis cell-aggregation assays.
Live P. gingivalis Cell-Aggregation Assay
P. gingivalis (500 µl; 3 × 108 cells) in PBS were incubated with the serially-diluted IgA-enriched solutions (500 µl) from NWs or BALF in cuvettes. Five min later, the absorbance of the mixture was measured by a spectrophotometer (Eppendorf AG, Hamburg, Germany; OD 600 nm).
Flow Cytometric Analysis
To characterize the phenotype of DCs, mononuclear cells were isolated from various mucosal tissues and lymph nodes 1 week after the last immunization with rFimA and FL/CpG or rFimA alone. The cells (0.2–1.0 × 106 cells) were stained with Brilliant Violet 421-conjugated anti-mouse CD11c, and PE-labeled anti-mouse CD11b, CD8, or B220 mAbs (BioLegend, San Diego, CA, USA). In some experiments, mononuclear cells were incubated with Brilliant Violet 421-conjugated anti-mouse CD11c, and PE-labeled anti-mouse I-Ab, CD40, CD80, or CD86 mAbs (BioLegend, San Diego, CA). These samples were then subjected to flow cytometry analysis (FACSVerse and Flow Jo; BD Biosciences, San Jose, CA, USA) (6).
Cytokine Production by rFimA-Stimulated CD4+ T Cells
CD4+ T cells from NPs, CLNs, lungs, MeLNs, and spleens were purified by using an automatic cell sorter (AutoMACS®) system (Miltenyi Biotec B.V. & Co. KG, Bergisch Gladbach, Germany) as described previously (6, 20). The purified CD4+ T cell fraction (>97% CD4+ and >99% viable) was resuspended in RPMI 1640 (Sigma-Aldrich) supplemented with HEPES buffer (10 mM), L-glutamine (2 mM), nonessential amino acid solution (10 µl/ml), sodium pyruvate (10 mM), penicillin (100 U/ml), streptomycin (100 µg/ml), gentamycin (80 µg/ml), and 10% FCS (complete medium; 4 × 106 cells/ml), and cultured in the presence of T cell-depleted, complement-, and mitomycin-treated splenic Ag-presenting cells taken from naïve BALB/c mice with or without rFimA (2 µg/ml). The culture supernatants were collected on day five and analyzed by using IFN-γ-, IL-2-, IL-4-, IL-5-, and IL-6-specific ELISA kits (Invitrogen, Carlsbad, CA, USA). The detection limits for each cytokine were: 5.3 pg/ml for IFN-γ, 2.0 pg/ml for IL-2, 4 pg/ml for IL-4, 3.3 pg/ml for IL-5, and 6.5 pg/ml for IL-6.
P. gingivalis Clearance in the Upper and Lower Respiratory Tracts
One week after the last immunization, IgA+/+ and IgA−/− (genetic background C57BL/6) mice were nasally challenged with the P. gingivalis 381 strain at a dose of 1 × 108 cfu (100 µl). Two days after the bacterial challenge, NWs were harvested aseptically by flushing with 1 ml of sterile PBS from the choana. After the lungs were removed with the tracheae, BALF was collected by gentle injection of 1 ml sterile PBS into the lungs from the tracheae. One hundred µl of NWs and BALF were spread on agar medium including kanamycin and were cultivated for 7 days at 37°C under anaerobic conditions.
Statistical Analysis
The data are expressed as the mean ± standard error of the mean (SEM). All mouse groups were compared to control mice with an unpaired Mann-Whitney U test using GraphPad Prism version 7 (GraphPad Software, Inc., La Jolla, CA, USA). p values of <0.05 were considered statistically significant.
Results
Induction of rFimA-Specific Ab Responses in Mucosal and Systemic Tissues With FL/CpG Adjuvant
We initially examined whether nasal administration of FL/CpG as a mucosal adjuvant would enhance rFimA-specific Ab responses. Mice given nasal rFimA plus FL/CpG had significantly increased levels of rFimA-specific IgA Ab responses in NWs when compared with mice given nasal rFimA alone (Figure 1A). Further, significantly elevated levels of rFimA-specific IgA and IgG Abs were seen in BALF of mice given nasal rFimA plus FL/CpG when compared with Ab levels in the controls (Figure 1B). These findings were further confirmed at the cellular level by using enzyme-linked immunospot (ELISPOT) assays. Elevated numbers of rFimA-specific IgA antibody-forming cells (AFCs) were detected in the mucosal inductive and effector tissues as well as their draining lymph nodes of mice given nasal rFimA plus FL/CpG (Figures 2A–E). In addition, increased numbers of anti-rFimA-specific IgG AFCs were seen in cervical lymph nodes (CLNs) and lungs (Figures 2C, D). Since nasal immunization is known to induce systemic immune responses in addition to mucosal immunity, rFimA-specific Ab responses in plasma and spleen were examined. Nasal administration with FL/CpG as a mucosal adjuvant successfully enhanced rFimA-specific IgG and IgA Ab responses in plasma (Figure 3A). Thus, significantly higher numbers of rFimA-specific IgG and IgA AFCs were also seen in spleens of mice given FL/CpG than in mice given rFimA alone (Figure 3B). Of importance, 6 months after the last immunization with rFimA plus FL/CpG, IgA Ab responses in NWs were maintained (Reciprocal Log2 titer 7.2). Further, rFimA-specific IgA and IgG Ab responses in BALF as well as those in plasma are essentially the same as those responses detected at 4 weeks after the initial immunization. When rFimA-specific IgG subclass Ab levels were examined, increased anti-rFimA IgG1, IgG2a, and IgG2b Abs were noted in mice given nasal rFimA plus FL/CpG when compared with those Ab responses in mice given rFimA alone (Figure 3C). Essentially no IgG3 Ab response against rFimA was detected (data not shown). Taken together, these findings show that the nasal FL/CpG system effectively upregulated rFimA-specific Ab responses in both mucosal and systemic immune compartments.

Figure 1 P. gingivalis rFimA-specific Ab responses in the external secretions. C57BL/6 (6–8 weeks) mice were nasally immunized three times at weekly intervals with rFimA (10 µg) plus pFL (50 µg) and CpG ODN (10 µg) (filled bars), or rFimA (10 µg) alone (open bars). Seven days after the last immunization, the levels of rFimA-specific IgA and IgG Abs in NWs (A) and BALF (B), were determined by rFimA-specific ELISA. The values shown are the mean ± SE (n = 20). *p < 0.05 when compared with mice given rFimA alone.
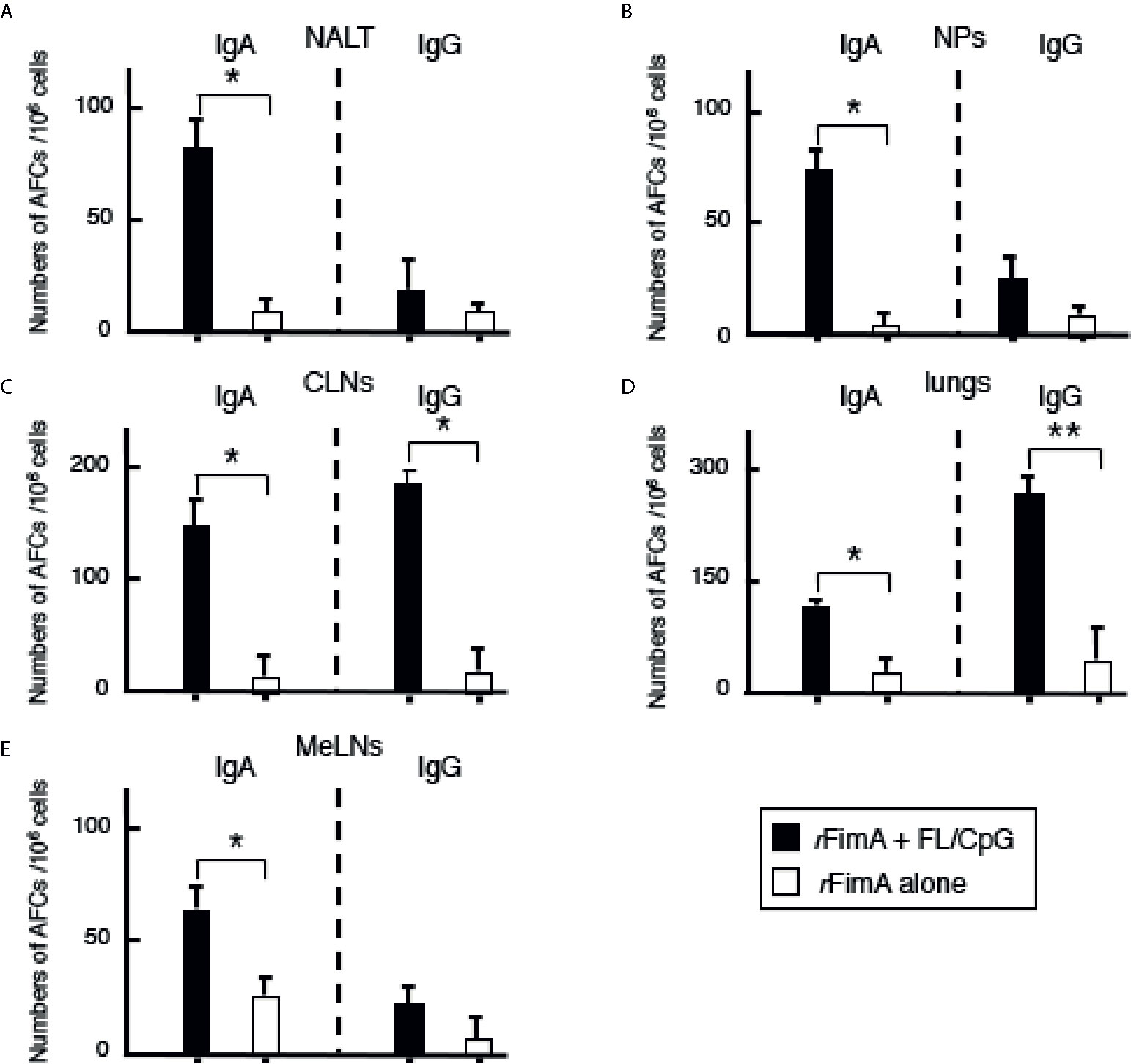
Figure 2 Antibody forming cells in the respiratory tracts, Mice were nasally immunized as described in Figure 1 legend. Seven days after the last immunization, mononuclear cells were isolated from NALT (A), NPs (B), CLNs (C), lungs (D), and MeLNs (E), and were then subjected to ELISPOT assays to enumerate the numbers of Ag-specific IgG and IgA AFCs. The values shown are the mean ± SE (n = 20). *p < 0.05 and **p < 0.01 when compared with mice given rFimA alone.
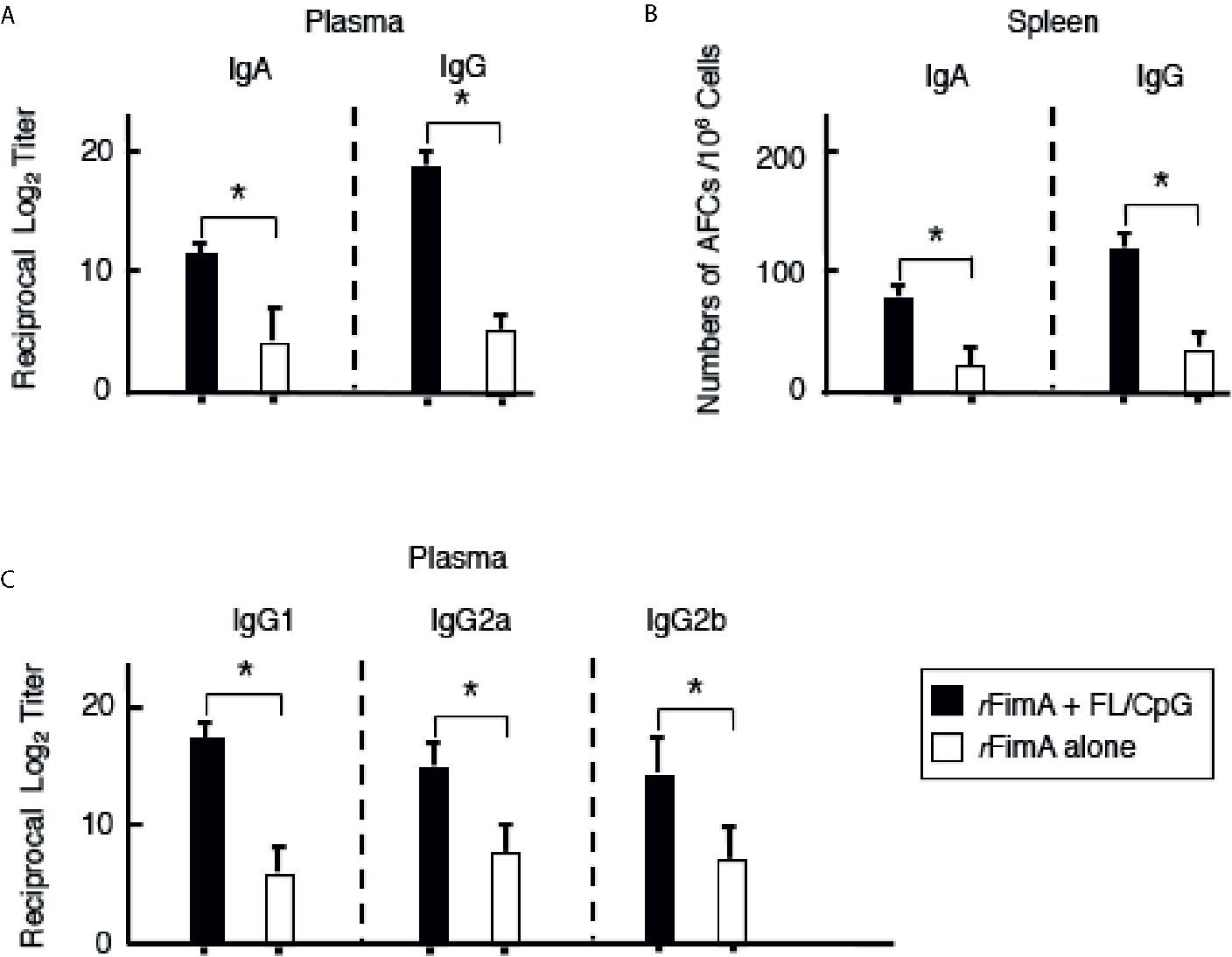
Figure 3 P. gingivalis rFimA-specific Ab responses in plasma and spleens. Mice were nasally immunized as described in Figure 1 legend. Seven days after the last immunization, the levels of rFimA-specific IgA, IgG, and IgG subclass Abs in plasma (A, C) were determined by rFimA-specific ELISA. In some experiments, mononuclear cells were isolated from spleen (B), and were then subjected to ELISPOT assays to enumerate the numbers of Ag-specific IgG and IgA AFCs. The values shown are the mean ± SE (n = 20). *p < 0.05 when compared with mice given rFimA alone.
Nasal rFimA Plus pFL and CpG ODN Induce CD11b+ and CD8+ DCs in the Respiratory Tract
Since our previous studies reported that nasal administration with FL/CpG as a mucosal adjuvant resulted in increased numbers of matured-type CD11c+ DCs, which contribute to the induction of specific immune responses to ovalbumin (7), recombinant pneumococcal surface protein A (PspA) (8), or hemagglutinin of influenza virus (9), we next characterized CD11c+ DCs in the various mucosal tissues of mice given rFimA plus FL/CpG or rFimA alone. Nasal immunization of rFimA plus FL/CpG significantly increased the frequency of CD11c+ cells in the NALT, NPs, MeLNs, lungs, and CLNs when compared with mice given rFimA alone (Table 1, Supplementary Figure 1). The numbers of CD8+- and CD11b+-DC subsets were significantly increased in mucosal tissues of mice given FL/CpG as a nasal adjuvant when compared with mice given nasal rFimA alone (Table 1). Of interest, the expanded CD11c+ DCs in NALT, NPs, MeLNs, lungs, and CLNs expressed higher levels of MHC II, CD40, CD80, and CD86 molecules (Table 1). Taken together, these results indicate that nasal vaccination with rFimA plus FL/CpG preferentially expands the numbers of matured-type CD8+- and CD11b+-DC populations, which are most likely involved in the induction of rFimA-specific T and B cell responses.
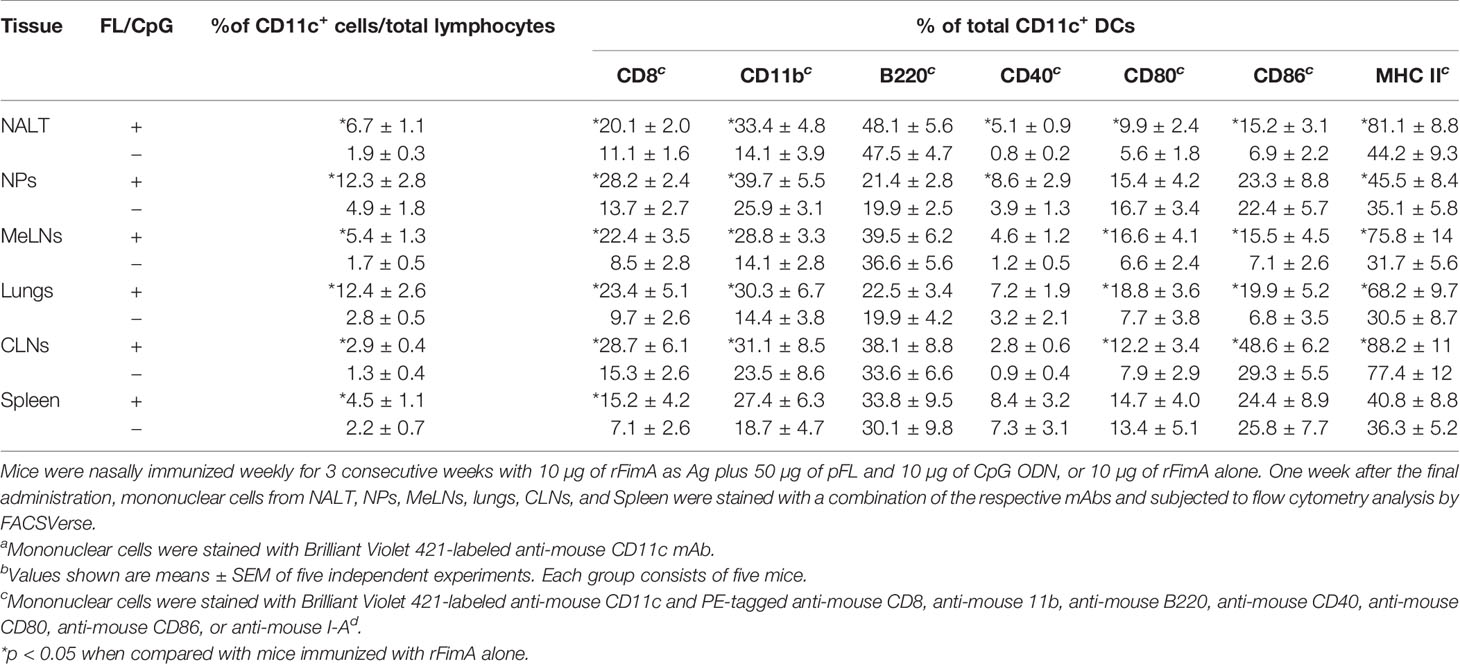
Table 1 Frequencies of CD11c+ DCs and CD8, CD11b, and B220, and costimulatory molecule expressions by CD11c+ DCs in mucosal inductive and effector tissues of mice given nasal rFimA with or without FL/CpGb.
Th1- and Th2-Type Cytokine Responses by rFimA-Stimulated Mucosal CD4+ T Cells
We next assessed cytokines production by rFimA-stimulated CD4+ T cells in NPs, MeLNs, lungs, CLNs, and spleens of mice given nasal rFimA plus FL/CpG or rFimA alone. rFimA-stimulated CD4+ T cells from NPs and lungs of mice given FL/CpG as a nasal adjuvant exhibited significantly higher levels of IFN-γ, IL-2, IL-4, and IL-5 production than in control mice, though the levels of IL-6 synthesis were unchanged (Table 2). rFimA-stimulated CD4+ T cells from MeLNs and CLNs of mice given rFimA plus FL/CpG displayed significantly higher levels of IFN-γ, IL-2, and IL-4 production. In addition, splenic CD4+ T cells isolated from mice given nasal FL/CpG showed increased levels of Th1- and Th2-type cytokine responses when compared with those in mice given rFimA alone. These results show that FL/CpG as a nasal adjuvant provokes a balanced Th1- and Th2-type cytokine response in the lower and upper respiratory mucosa as well as in the spleens.

Table 2 Th1- and Th2-type cytokine responses by CD4+ T cells after in vitro restimulation with rFimA.
Interactions Between NWs/BALF SIgA Abs and Live P. gingivalis Cells
Thus far, our results showed that nasal FL/CpG as a mucosal adjuvant effectively activated mucosal DCs for the induction of Th1- and Th2-type cytokine-mediated rFimA-specific Ab responses. We next assessed the functional property of rFimA-specific IgA Abs by the aggregation of live P. gingivalis cells. SIgA Abs in NWs and BALF were enriched by removing IgG and IgM Abs using respective affinity columns. When live P. gingivalis (1 × 108) were incubated with enriched SIgA Abs from NWs and BALF of mice given FL/CpG as a nasal adjuvant, a significant aggregation of P. gingivalis was induced in the Ab concentration-dependent manner (Figures 4A, B). In contrast, the enriched SIgA Ab from mice given Ag alone exhibited essentially no live P. gingivalis cell aggregation (Figures 4A, B). We also performed that the P. gingivalis aggregation assay using unpurified NW and BALF. The levels of the aggregation are essentially the same when purified NW and BALF were employed (data not shown). These results indicate that rFimA-specific SIgA SIgA, but not IgG nor IgM Abs in NW and BLAF play a key role in the aggregation of P. gingivalis.
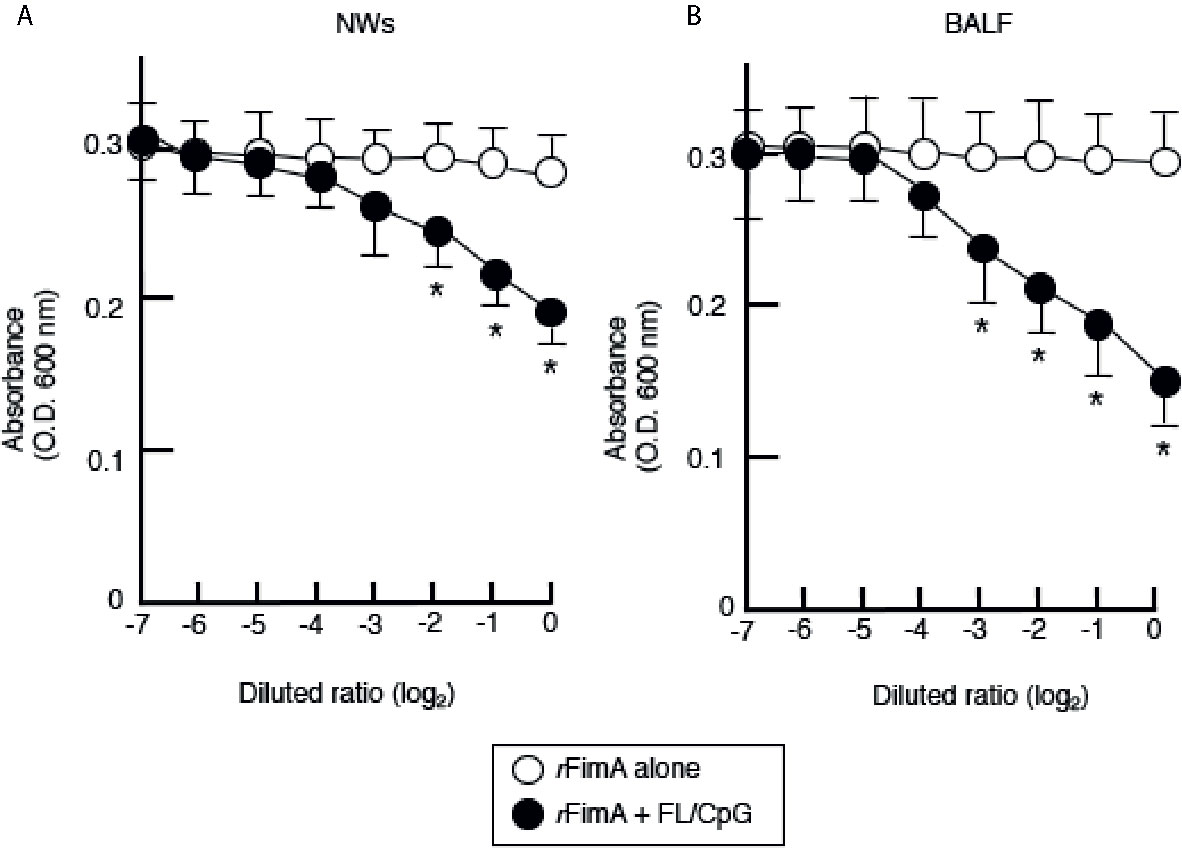
Figure 4 Aggregation of live P. gingivalis cells by SIgA Abs. Enriched SIgA Abs from NWs (A) and BALF (B) of mice given nasal rFimA plus FL/CpG (filled circles) or rFimA alone (open circles) were serially diluted with PBS. The serial-diluted IgA-enriched solutions (500 µl) from NWs or BALF were added to 3 × 108 P. gingivalis cells (500 µl) in cuvettes for 5 min. The optical density of the mixture was measured by a spectrophotometer (OD 600 nm). The values shown are the means ± SE (n = 5). *p < 0.05, when compared with mice given rFimA alone.
An Essential Role for SIgA Abs in the Protection Against Respiratory P. gingivalis Infection
To further determine the essential roles of rFimA-specific SIgA Abs induced by nasal vaccination with rFImA plus FL/CpG ODN, both IgA-deficient (IgA−/−) and the genetic background control (IgA+/+) C57BL/6 mice were nasally challenged with the P. gingivalis 381 strain (0.2 × 108 cfu/20 µl per shot, 1 × 108 cfu consecutive inoculation of a total of five shots) 1 week after the last immunization. Two days after the bacterial challenge, NWs and BALF samples were collected, and the samples were cultured anaerobically on kanamycin-supplemented blood agar plates to enumerate the recovered CFU. IgA+/+ mice given nasal rFimA plus FL/CpG showed significantly low numbers of bacterial CFUs (Figure 5). Conversely, NWs and BALF of IgA+/+ mice given rFimA alone contained high numbers of P. gingivalis (Figure 5, Supplementary Figure 2). These results show that nasal rFimA plus FL/CpG provides effective protection against P. gingivalis colonization in the lungs and nasal cavity. Of importance, when IgA−/− mice given rFimA plus FL/CpG ODN were challenged nasally with the P. gingivalis 381 strain, high numbers of bacterial colonies were noted in NWs and BALF, which were comparable to those seen in NWs and BALF of IgA+/+ mice given rFimA alone (Figure 5).
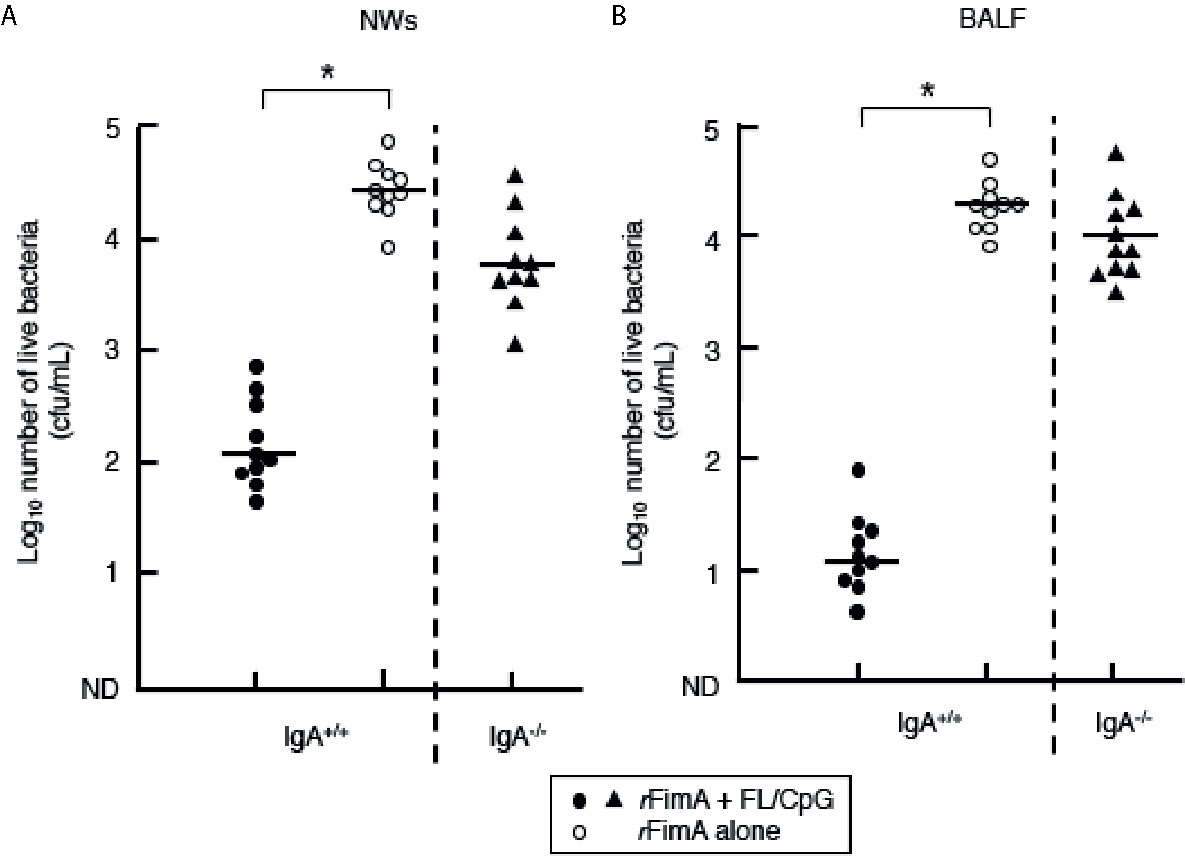
Figure 5 Clearance of P. gingivalis cells in the upper and lower respiratory tracts. IgA+/+ and IgA−/− mice given nasal FL/CpG ODN-based rFimA vaccine (filled circles or triangles). As a control, mice were nasally immunized with rFimA alone (open circles). One week after the last immunization, all groups of mice were nasally challenged with P. gingivalis cells (1 × 108 cfu). Two days after the bacterial challenge, NWs (A) and BALF (B) samples were collected. The samples (100 µl) were cultured anaerobically on kanamycin-supplemented blood agar plates to enumerate the recovered CFU. The values shown are the means ± SE (n = 10). Each line represents the median log10 CFU/mouse. *p < 0.05 when compared with mice given rFimA alone.
Discussion
In this study, we investigated whether nasal pFL and CpG ODN (FL/CpG) as a mucosal adjuvant could elicit bacterial Ag (rFimA)-specific Ab responses to protect against P. gingivalis respiratory infection. Our results showed that the nasal FL/CpG adjuvant system successfully provoked rFimA-specific mucosal and systemic immune responses through the expansion of CD8- and CD11b-expressing DC populations and the induction of Th1- and Th2-type cytokine responses by CD4+ T cells. In addition, anti-rFimA SIgA Abs induced by nasal FL/CpG effectively purged P. gingivalis in the lower and upper respiratory mucosa. It is well known that pathogen-specific SIgA Ab plays key roles in the protection and homeostatic regulation of mucosal epithelia, including the oral cavity and the respiratory tracts, of humans and many other mammals (21). Indeed, our previous study showed that pFL as a nasal adjuvant elicits pneumococcal surface protein A (PspA)-specific SIgA Ab responses in the nasal cavity, which prevents nasal carriage of S. pneumoniae (22). In addition, it has been shown that hemagglutinin-specific SIgA Abs are essential for the prevention of influenza virus infection in the nasal mucosa (9). These results indicate that Ag-specific SIgA Abs are required for protection against the initial step of bacterial and viral infection, which mainly occurs in the nasal mucosa. To this end, previous studies have shown that the clearance of bacterial or viral infections in the lower respiratory tract can be achieved without pathogen-specific SIgA Ab responses (23, 24). According to these findings, our current study is the first to show that P. gingivalis-specific functional SIgA Abs play an indispensable role in the clearance of oral pathogenic bacteria in the lower respiratory tract in addition to the nasal cavity.
The induction of Ag-specific IgG Ab is essential for the prevention of systemic infection. In this study, increased levels of rFimA-specific IgG Ab responses were detected in the respiratory tract. Unfortunately, these rFimA-specific IgG Abs did not show the protective activity, since IgA−/− mice given nasal FL/CpG vaccine failed to eliminate P. gingivalis from the respiratory tract. However, we anticipated that rFimA-specific IgG Abs could play essential roles when P. gingivalis invades into the systemic circulation of the host. Indeed, FL/CpG as combined mucosal adjuvant elicited a balanced Th1- and Th2-type cytokine responses, we predict that rFimA-specific IgG1, IgG2a, and IgG2b Ab responses were induced in plasma. Thus, it is possible that these IgG Abs could participate in the opsonization and/or antibody-dependent cellular cytotoxicity (ADCC) activities.
Since populations are aging all over the world, including in Japan, it is important to maintain healthy conditions in the elderly. However, it has been reported that the number of pneumonia patients is increasing in Japan in step with the growing aged population due to poor oral hygiene or a decrease in salivary clearance (25). Furthermore, it has been reported that 5–15% of cases of pneumonia in the hospitalized population are aspiration pneumonia (26). In this regard, many people aged 65 or older die of pneumonia, including aspiration pneumonia (27). Since some pathogenic bacteria causing aspiration pneumonia are anaerobes, including periodontal pathogens such as P. gingivalis that originate in the oral cavity (11, 28), antimicrobial agents targeting anaerobic bacteria are prescribed to aspiration pneumonia patients in order to control and treat inflammation and infection. For prophylaxis of aspiration pneumonia, oral cleaning and oral healthcare of the elderly can promote quality of life or prolong life expectancy (29), since good oral hygiene leads to the prevention of aspiration pneumonia (30). Based upon these reports, it is possible that the effective inhibition of oral, nasopharyngeal, and pulmonary bacterial colonization may lead to a drastic reduction of bacterial growth and subsequently prevent aspiration pneumonia by P. gingivalis.
Our current study clearly showed that a nasal vaccine consisting of rFimA and FL/CpG elicited functional rFimA-specific SIgA Ab responses in the nasal cavity and lungs, and thus vaccinated mice exhibited complete protection in the lower and upper respiratory tracts when nasally challenged with a large amount (1.0 × 108 cfu) of P. gingivalis strain 381. In contrast, mice given nasal rFimA alone showed significantly increased numbers of bacteria in the BALF and NWs after being challenged nasally with P. gingivalis compared to mice given rFimA and FL/CpG. In addition, we showed that this nasal vaccination strategy induced rFimA-specific IgA Ab responses in the saliva (3), that blocked P. gingivalis binding to a salivary protein (statherin) on the hydroxyapatite beads. Therefore, rFimA-specific salivary IgA could reduce the number of P. gingivalis in the oral cavity that contributes to the prevention of aspiration pneumonia by rFimA-specific IgA Ab in the respiratory tracts. Furthermore, the nasal FL/CpG system has been shown to elicit pathogen-specific SIgA Ab responses in aged mice (3, 8, 9). To this end, one can predict that nasal rFimA plus FL/CpG is a potent strategy to prevent aspiration pneumonia not only in young adults but also in aged patients with periodontitis. Therefore, we are currently testing whether the nasal vaccine with rFimA and FL/CpG can induce protective rFimA-specific SIgA Ab responses in aged mice.
A combination of pFL and CpG ODN has been employed as a DC-targeting nasal adjuvant for the induction of functional CD4+ T cells and pathogen-specific mucosal immunity (8, 9, 31). Thus, increased numbers of mature-type CD8- or CD11b-expressing DC subsets that co-express MHC II, CD40, CD80, and CD86 molecules have been noted in NALT, NPs, and CLNs (8, 9). Furthermore, others have also reported that the induction of CD8+ or CD11b+ DC subsets is essential for protection against respiratory Bordetella pertussis infection (32) or influenza virus infection (33). The current study clearly agrees with these previous findings. Thus, a nasal vaccine composed of FL/CpG and rFimA significantly elicited mature-type CD8+ or CD11b+ DCs for the subsequent induction of P. gingivalis-specific protective immunity in the respiratory tract. Our previous study showed that pFL as nasal adjuvant resulted in CD8+ DCs-mediated Th2-type cytokine responses. In the present study, when CpG was added to pFL as a nasal adjuvant, both Th1-and Th2-type cytokine responses and increased number of CD8+ DCs and CD11b+ DCs were noted. Thus, it is possible that nasal CpG is known as Th1-mediated nasal adjuvant (34) preferentially elicit CD11b+ DCs. To support this view, nasal adenovirus expressing FL induced increased numbers of CD11b+ DCs with humoral and cellular immunity (35). Further, this study showed that the migration of DCs from NALT to CLNs, NP, and SMGs (35). Thus, increased numbers of certain subsets of DCs in the various lymphoid tissues of mice given nasal rFimA plus FL/CpG could be attributed to DC migration from NALT to these tissues. Similarly, CD11b+ DCs in the lamina propria of the small intestine contribute both humoral and cellular immunity (36), while CD103 expressing CD8+ DCs in the lamina propria are prone to induce Th1-type responses with CTL activity (37). Taken together, a vaccine containing FL/CpG as nasal adjuvant potentially induce CD8+ and CD11b+ DCs for facilitating the induction of Th1- and Th2-type cytokine-mediated humoral and cellular immune responses. Since it has been reported that interactions between DCs and CD4+ T cells play an important role in the induction of pulmonary immunity (38), we next investigated Th1- and Th2-type cytokine responses by rFimA-stimulated CD4+ T cells in various mucosal tissues of mice given nasal rFimA and FL/CpG. rFimA-stimulated CD4+ T cells from NPs, lungs, MeLNs, and CLNs of mice given rFimA and FL/CpG exhibited significantly higher levels of Th1- and Th2-type cytokine production when compared with those in control mice (Table 2). These results also agree with our previous studies that showed that a balanced Th1- and Th2-type cytokine response occurred in mice given nasal FL/CpG as a mucosal adjuvant (7–9). It has been shown that both Th1- and Th2-type responses contribute to the induction of Ag-specific mucosal IgA Ab responses (39, 40). However, it has been also suggested that polarized either Th1- or Th2-type response could elicit undesired inflammation or allergy (41). Thus, a balanced Th1- and Th2-type response by DC-targeting pFL/CpG nasal adjuvant is essential for the development of safe vaccines. Taken together, the nasal FL/CpG system with rFimA as Ag provoked mucosal and systemic immunity, which were mediated by the upregulation of CD8- and CD11b-expressing DC populations and Th1-/Th2-type cytokine-producing CD4+ T cells.
In summary, our present study clearly showed that nasal rFimA and FL/CpG as a mucosal adjuvant provoked CD8- or CD11b-expressing DCs and Th1- and Th2-type cytokine-producing CD4+ T cells for the induction anti-rFimA SIgA Abs, which play an indispensable role in protection against P. gingivalis infection in the respiratory mucosa. These findings are the first to show that nasal vaccine-induced bacterial Ag (FimA)-specific SIgA Abs are potent for the prevention of P. gingivalis-mediated aspiration pneumonia.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by Osaka Dental University animal experimental committee.
Author Contributions
KKa and KF contributed to the conception, design, data acquisition, analysis, and interpretation of the study, and drafted and critically revised the manuscript. SK contributed to the conception, design, data acquisition, analysis, and interpretation of the study, and critically revised the manuscript. YH contributed to the study conception and critically revised the manuscript. KKo and TM contributed to the data acquisition and analysis, and critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work is supported by the Japan Society for the Promotion of Science (JEPS) KAKENHI Grant Numbers JP17H04424 (B) and JP17K12034 (C) to KKa, as well as JP20H03856 (B) and JP20K20495 to KF from the Ministry of Education, Science, Sports, and Culture of Japan. KKa is a recipient of a grant from the Central R&D Laboratory, Kobayashi Pharmaceutical Co., Ltd.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial and financial relationships that could be construed as a potential conflict of interest.
The reviewer SM declared a shared affiliation with one of the authors, KF, to the handling editor at the time of review.
The reviewer ZM declared a shared affiliation with one of the authors, KF, to the handling editor at the time of review.
Acknowledgments
We are grateful to Dr. Kenjiro Kobuchi in the Department of Periodontology, Osaka Dental University for technical assistance in measuring antibody values. In addition, our study was in part performed in the Central Research Center of Osaka Dental University, and we thank Mr. Naoya Kawade and Ms. Keiko Azuma for excellent technical assistance.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.634923/full#supplementary-material
Supplementary Figure 1 | Typical FACS plot and gating strategy for NALT and lungs. Mononuclear cells in NALT and lungs were gated by using the forward- and side-scatter properties, and were subsequently analyzed for CD11c+ cells.
Supplementary Figure 2 | Agar medium plates incubated with NWs or BALF of IgA+/+ mice given nasal rFimA and FL/CpG or rFimA alone and IgA−/− mice given nasal rFimA and FL/CpG. One hundred µl of NWs and BALF were spread on agar medium including kanamycin and were cultivated for 144 h at 37°C under anaerobic conditions.
Abbreviations
FL, Flt3 ligand; pFL, a DNA plasmid encoding; CpG ODN, CpG oligodeoxynucleotide; FL/CpG, pFL and CpG ODN; Ab, antibody; Ag, antigen; rFimA, recombinant FimA; NWs, Nasal Washes; BALF, bronchoalveolar lavage fluid; SIgA, secretory IgA; DCs, dendritic cells; NALT, nasopharyngeal-associated lymphoid tissues; NPs, nasal passages; CLNs, cervical lymph nodes; MLNs, mediastinal lymph nodes; P. gingivalis, Porphyromonas gingivalis.
References
1. Mestecky J. Innovation for preventing infectious diseases. In: Kiyono H, Pascual DW, editors. Mucosal vaccines, 2nd edition. London: Academic Press (2019). p. 71–84. Chapter 4.
2. Kataoka K, Fujihashi K, Oma K, Fukuyama Y, Hollingshead SK, Sekine S, et al. The Nasal dendritic cell-targeting Flt3 ligand as a safe adjuvant elicits effective protection against fatal pneumococcal pneumonia. Infect Immun (2011) 79:2819–28. doi: 10.1128/IAI.01360-10
3. Kobuchi K, Kataoka K, Taguchi Y, Miyake T, Umeda M. Nasal double DNA adjuvant induces salivary FimA-specific secretory IgA antibodies in young and aging mice and blocks Porphyromonas gingivalis binding to a salivary protein. BMC Oral Health (2019) 19:188–96. doi: 10.1111/1348-0421.12487
4. Joo S, Suwanto A, Sato A, Nakahashi-Ouchida R, Mori H, Uchida Y, et al. A role for the CCR5-CCL5 interaction in the preferential migration of HSV-2-specific effector cells to the vaginal mucosa upon nasal immunization. Mucosal Immunol (2019) 12:1391–403. doi: 10.1038/s41385-019-0203-z
5. Azegami T, Yuki Y, Kiyono H. Challenges in mucosal vaccines for the control of infectious diseases. Int Immunol (2014) 26:517–28. doi: 10.1093/intimm/dxu063
6. Kataoka K, McGhee JR, Kobayashi R, Fujihashi K, Shizukuishi S, Fujihashi K. Nasal Flt3 ligand cDNA elicits CD11c+ CD8+ dendritic cells for enhanced mucosal immunity. J Immunol (2004) 172:3612–19. doi: 10.4049/jimmunol.172.6.3612
7. Fukuiwa T, Sekine S, Kobayashi R, Suzuki H, Kataoka K, Gilbert RS, et al. A combination of Flt3 ligand cDNA and CpG ODN as nasal adjuvant elicits NALT dendritic cells for prolonged mucosal immunity. Vaccine. (2008) 26:4849–59. doi: 10.1016/j.vaccine.2008.06.091
8. Fukuyama Y, King JD, Kataoka K, Kobayshi R, Gilbert RS, Hollingshead SK, et al. A combination of Flt3 ligand cDNA and CpG oligodeoxynucleotide as nasal adjuvant elicits protective secretory-IgA immunity to Streptococcus pneumoniae in aged mice. J Immunol (2011) 186:2454–61. doi: 10.4049/jimmunol.1002837
9. Asanuma H, Zamri NB, Sekine S, Fukuyama Y, Tokuhara D, Gilbert RS, et al. A novel combined adjuvant for nasal delivery elicits mucosal immunity to influenza in aging. Vaccine. (2012) 30(4):803–12. doi: 10.1016/j.vaccine.2011.10.093
10. Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL Jr. Microbial complexes in subgingival plaque. J Clin Periodontol (1998) 25:134–44. doi: 10.1111/j.1600-051x.1998.tb02419.x
11. Benedyk M, Mydel PM, Delaleu N, Plaza K, Gawron K, Milewska A, et al. Gingipains: Critical factors in the development of aspiration pneumonia caused by Porphyromonas gingivalis. J Innate Immun (2016) 8:185–98. doi: 10.1159/000441724
12. Haffajee AD, Cugini MA, Tanner A, Pollack RP, Smith C, Kent RL Jr, et al. Subgingival microbiota in healthy, well-maintained elder and periodontitis subjects. J Clin Periodontol (1998) 25:346–53. doi: 10.1111/j.1600-051x.1998.tb02454.x
13. How KY, Song KP, Chan KG. Porphyromonas gingivalis: an overview of periodontopathic pathogen below the gum line. Front Microbiol (2016) 7:53. doi: 10.3389/fmicb.2016.00053
14. Hospenthal MK, Costa TRD, Waksman G. A comprehensive guide to pilous biogenesis in gram-negative bacteria. Nat Rev Microbiol (2017) 15:365–79. doi: 10.1038/nrmicro.2017.40
15. Kataoka K, Amano A, Kuboniwa M, Horie H, Nagata H, Shizukuishi S. Active sites of salivary proline-rich protein for binding to Porphyromonas gingivalis fimbriae. Infect Immun (1997) 65:3159–64. doi: 10.1128/IAI.65.8.3159-3164.1997
16. Hamada S, Amano A, Kimura S, Nakagawa I, Kawabata S, Morisaki I. The importance of fimbriae in the virulence and ecology of some oral bacteria. Oral Microbiol Immunol (1998) 13:129–38. doi: 10.1111/j.1399-302x.1998.tb00724.x
17. Harriman GR, Bogue M, Rogers P, Finegold M, Pacheco S, Bradley A, et al. Targeted deletion of the IgA constant region in mice leads to IgA deficiency with alternations in expression of other Ig isotypes. J Immunol (1999) 162:2521–9.
18. Fujiwara T, Morishima S, Takahashi I, Hamada S. Molecular cloning and sequencing of fimbrillin gene of Porphyromonas gingivalis strains and characterization of recombinant proteins. Biochem Biophys Res Commun (1993) 197:241–7. doi: 10.1006/bbrc.1993.2467
19. Takahashi E, Kataoka K, Fujii K, Chida J, Mizuno D, Fukui M, et al. Attenuation of inducible respiratory immune responses by oseltamivir treatment in mice infected with influenza A virus. Microbes Infect (2010) 12:778–83. doi: 10.1016/j.micinf.2010.04.013
20. Kataoka K, Fujihashi K, Sekine S, Fukuiwa T, Kobayashi R, Suzuki H, et al. Nasal cholera toxin elicits IL-5 and IL-5 receptor α-chain expressing B-1a B cells for innate mucosal IgA antibody responses. J Immunol (2007) 178:6058–65. doi: 10.4049/jimmunol.178.10.6058
21. Corthesy B. Multi-faceted functions of secretory IgA at mucosal surfaces. Front Immunol (2013) 4:185. doi: 10.3389/fimmu.2013.00185
22. Fukuyama Y, King JD, Kataoka K, Kobayshi R, Gilbert RS, Oishi K, et al. Secretory-IgA play an important role in the immunity to Streptococcus pneumoniae. J Immunol (2010) 185(3):1755–62. doi: 10.4049/jimmunol.1000831
23. Ferreira DM, Darrieux M, Silva DA, Leite LC, Ferreira JM Jr, Ho PL, et al. Characterization of protective mucosal and systemic immune responses elicited by pneumococcal surface protein PspA and PspC nasal vaccines against a respiratory pneumococcal challenge in mice. Clin Vaccine Immunol (2009) 16:636–45. doi: 10.1128/CVI.00395-08
24. Mbawuike IN, Pacheco S, Acuna CL, Switzer KC, Zhang Y, Harriman GR. Mucosal immunity to influenza without IgA: An IgA knockout mouse model. J Immunol (1999) 162:2530–37.
25. Ministry of Health, Labor, Welfare of Japan. Summary of vital statistics; Trends in leading causes of death.; Ministry of Health, Labor, Welfare of Japan, Tokyo (2018). Available at: http://www.mhlw.go.jp/toukei/saikin/hw/jinkou/kakutei18/index.html (Accessed Oct, 28, 2020).
26. DiBardino DM, Wunderink RG. Aspiration pneumonia: a review of modern trends. J Crit Care (2015) 30:40–8. doi: 10.1056/NEJMoa1500245
27. Teramoto S, Fukuchi Y, Sasaki H, Sato K, Sekizawa K, Matsuse T. Japanese Study Group on Aspiration Pulmonary Disease. High incidence of aspiration pneumonia in community- and hospital-acquired pneumoniae in hospitalized patients: A multicenter, prospective study in Japan. J Am Geriatr Soc (2008) 56:577–9. doi: 10.1111/j.1532-5415.2008.01597.x
28. Cesar L, Gonzalez C, Calia FM. Bacteriologic flora of aspiration-induced pulmonary infections. Arch Intern Med (1975) 135:711–4. doi: 10.1001/archinte.135.5.711
29. Yoneyama T, Yoshida M, Matsui T, Sasaki H. Oral care and pneumonia. Oral Care Working Group Lancet (1999) 354:515. doi: 10.1016/s0140-6736(05)75550-1
30. Terpenning MS, Taylor GW, Lopatin DE, Kerr CK, Dominguez BL, Loeshe WJ. Aspiration pneumonia: Dental and oral risk factors in older veteran population. J Am Geriatr Soc (2001) 49:557–63. doi: 10.1046/j.1532-5415.2001.49113.x
31. Kataoka K, Fukuyama Y, Briles DE, Miyake T, Fujihashi K. Dendritic cell-argeting DNA-based nasal adjuvants for protective mucosal immunity to Streptococcus pneumoniae. Microbiol Immunol (2017) 61:195–205. doi: 10.1111/s1348-0421.12487
32. Dunne PJ, Moran B, Cummins RC, Mills KHG. CD11c+ CD8+ dendritic cells promote protective immunity to respiratory infection with Bordetella pertussis. J Immunol (2009) 183:400–10. doi: 10.4049/jimmunol.0900169
33. GeurtsvanKessel CH, Willart AM, van Rijt LS, Muskens F, Kool M, Baas C, et al. Clearance of influenza virus from the lung depends on migratory langerin+ CD11b- but not plasmacytoid dendritic cells. J Exp Med (2008) 205:1621–34. doi: 10.1084/jem.20071365
34. Moldoveanu Z, Love-Homan L, Huang WQ, Kreig AM. CpG DNA, a novel immune enhancer for systemic and mucosal immunization with influenza virus. Vaccine. (1998) 16:1216–24. doi: 10.1016/s0264-410x(98)80122-9
35. Sekine S, Kataoka K, Fukuyama Y, Adachi Y, Davydova J, Yamamoto M, et al. A novel adenovirus expressing Flt3 ligand enhances mucosal immunity by inducing mature nasopharyngeal-associated tissue dendritic cell migration. J Immunol (2008) 180:8126–34. doi: 10.4049/jimmunol.180.12.8126
36. Uematsu S, Fujimoto K, Jang MH, Yang B-G, Jung Y-J, Nishiyama M, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol (2008) 9:769–76. doi: 10.1038/ni.1622
37. Fujimoto K, Karuppuchamy T, Takemura N, Shimohigoshi M, Machida T, Haseda Y, et al. A new subset of CD103+ CD8alpha+ dendritic cells in the small intestine expresses TLR3, TLR7 and TLR9 and induces Th1 response and CTL activity. J Immunol (2011) 186:6287–95. doi: 10.4049/jimmunol.1004036
38. Bakocevic N, Worbs T, Davalos-Misslitz A, Forster R. T cell-dendritic cell interaction dynamics during the induction of respiratory tolerance and immunity. J Immunol (2010) 184:1317–27. doi: 10.4049/jimmunol.0902277
39. Xu-Amano J, Kiyono H, Jackson RJ, Staats HF, Fujihashi K, Burrows PD, et al. Helper T cell subsets for immunoglobulin A responses: Oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa associated tissues. J Exp Med (1993) 178:1309–20. doi: 10.1084/jem.178.4.1309
40. VanCott JL, Staats HF, Pascual DW, Roberts M, Chatfield SN, Yamamoto M, et al. Regulation of mucosal and systemic antibody responses by T helper cell subsets, macrophages, and derived cytokines following oral immunization with live recombinant Salmonella. J Immnol (1996) 156:1504–14.
Keywords: nasal vaccine, recombinant FimA (rFimA), dendritic cells (DCs), double DNA adjuvant, mucosal IgA, Porphyromonas gingivalis
Citation: Kataoka K, Kawabata S, Koyanagi K, Hashimoto Y, Miyake T and Fujihashi K (2021) Respiratory FimA-Specific Secretory IgA Antibodies Upregulated by DC-Targeting Nasal Double DNA Adjuvant Are Essential for Elimination of Porphyromonas gingivalis. Front. Immunol. 12:634923. doi: 10.3389/fimmu.2021.634923
Received: 29 November 2020; Accepted: 18 January 2021;
Published: 25 February 2021.
Edited by:
Marcela Pasetti, University of Maryland, United StatesReviewed by:
Suzanne Michalek, University of Alabama at Birmingham, United StatesZina Moldoveanu, University of Alabama at Birmingham, United States
Copyright © 2021 Kataoka, Kawabata, Koyanagi, Hashimoto, Miyake and Fujihashi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kosuke Kataoka, kataoka-k@cc.osaka-dent.ac.jp; Kohtaro Fujihashi, kohtarof@ims.u-tokyo.ac.jp
 Kosuke Kataoka
Kosuke Kataoka