- 1Pediatric Immunohematology and Bone Marrow Transplantation Unit, IRCCS San Raffaele Scientific Institute, Milan, Italy
- 2Vita-Salute San Raffaele University, Milan, Italy
- 3San Raffaele Telethon Institute for Gene Therapy (SR-Tiget), IRCCS San Raffaele Scientific Institute, Milan, Italy
- 4Clinic of Infectious Diseases, Division of Immunology, Transplantation and Infectious Diseases, IRCCS San Raffaele Scientific Institute, Milan, Italy
- 5Laboratory of Medical Microbiology and Virology, IRCCS San Raffaele Hospital, Milan, Italy
- 6Pediatric Cardiology, Cardio-thoraco-vascular Department, IRCCS San Raffaele Scientific Institute, Milan, Italy
- 7Myocarditis Unit, Department of Cardiac Electrophysiology and Clinical Arrhythmology, IRCCS San Raffaele Scientific Institute, Milan, Italy
- 8Department of Clinical Biochemistry, IRCCS San Raffaele Scientific Institute, Milan, Italy
- 9Clinical and Experimental Radiology Unit, Experimental Imaging Center, IRCCS San Raffaele Institute, Milan, Italy
- 10Department of Anesthesia and Critical Care, IRCCS San Raffaele Scientific Institute, Milan, Italy
- 11Department of Pediatrics, HematoOncology Unit, Institute of Maternal and Child Health Burlo Garofolo, Trieste, Italy
- 12National Center for Global Health, Istituto Superiore di Sanità, Rome, Italy
- 13Department of Biomedical and Clinical Sciences “L. Sacco”, University of Milan, Milan, Italy
- 14Viral Evolution and Transmission Unit, IRCCS San Raffaele Scientific Institute, Milan, Italy
- 15Beta Cell Biology Unit, Diabetes Research Institute, IRCCS San Raffaele Scientific Institute, Milan, Italy
- 16Division of Genetics and Cell Biology, IRCCS San Raffaele Scientific Institute, Milan, Italy
In this work we present the case of SARS-CoV-2 infection in a 1.5-year-old boy affected by severe Wiskott-Aldrich Syndrome with previous history of autoinflammatory disease, occurring 5 months after treatment with gene therapy. Before SARS-CoV-2 infection, the patient had obtained engraftment of gene corrected cells, resulting in WASP expression restoration and early immune reconstitution. The patient produced specific immunoglobulins to SARS-CoV-2 at high titer with neutralizing capacity and experienced a mild course of infection, with limited inflammatory complications, despite pre-gene therapy clinical phenotype.
Introduction
Data about severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) infection in children with primary immunodeficiencies (PIDs) are limited (1). In the general pediatric population, this infection is known to be generally milder than in adults (2, 3). However, multisystem inflammatory syndrome, temporally associated to SARS-CoV-2 infection, has been increasingly reported in children and adolescents. Clinical spectrum ranges from general inflammatory syndromes to incomplete and complete forms of Kawasaki-like disease, leading to serious illness with wide cardiovascular involvement, suggestive of a systemic immune-mediated disease (4, 5).
Here we present the first case of a 1.5-year-old boy affected by severe Wiskott-Aldrich Syndrome (WAS), who experienced SARS-CoV-2 infection five months after treatment with gene therapy (GT). WAS is a rare, X-linked, life-threatening PID, caused by mutations in the gene encoding for the WAS protein (WASP), a key regulator of actin polymerization. WASP deficiency in platelets results in micro-thrombocytopenia, while in immune cells it mainly compromises immunological synapse formation, cell migration and cytotoxicity. Thus, WAS is characterized by bleeding episodes, development of recurrent or severe infections, eczema and increased risk of autoimmunity, autoinflammation and malignancies (6). Supportive treatment is based on immunogloblulin replacement therapy, antimicrobial prophylaxis and immunosuppressants. Allogeneic hematopoietic stem/progenitor cell (HSPC) transplantation is a recognized curative treatment for WAS, even if it may be hampered by complications such as graft-versus-host disease, rejection and autoimmunity. Moreover, donor availability may be limited. Investigational autologous gene therapy (GT) represents a safe and effective therapeutic alternative, according to available data from recent GT clinical trials using lentiviral vectors encoding for the human WAS gene (7–10).
Case Presentation
Our patient was diagnosed with WAS (WAS gene mutation: c.1384_1385delAG; p.S461Lfs*32) at 3 months of age, due to severe thrombocytopenia, eczema and early-onset steroid-refractory autoinflammatory manifestations (fever, vasculitis, increased inflammatory indexes, Zhu score 5A), treated with IL-1 soluble receptor antagonist. After diagnosis, anti-infective prophylaxis and immunoglobulin replacement therapy were also started. At 5 months of age, he experienced severe hypereosinophilia with mild cardiac injury, which required treatment with steroids. In the following months, he also developed chronic CMV infection with multiple reactivations, requiring specific antiviral treatment.
In 2019, at 1 year of age, the patient underwent at our Unit a reduced-intensity conditioning with mAb anti-CD20 (rituximab, 375 mg/sqm), busulfan [weight and area under the curve (AUC)-targeted, target AUC 48,000 ± 10% ng/ml*h] and fludarabine (total dose: 60 mg/sqm), followed by GT with OTL-103 [autologous CD34+ cell-enriched population containing HSPC transduced ex vivo using a lentiviral vector encoding the human WAS gene] in a clinical trial (OTL-103-4; EudraCT number: 2018-003842-18; NCT03837483) (10). Sinusoidal obstruction syndrome with suspected thrombotic microangiopathy occurred early after GT, possibly linked to an unexpected high busulfan exposure (AUC 80,988 ng/ml*h), and then resolved without sequelae. Neutrophil engraftment was achieved on day +15 post-GT. Multilineage engraftment of gene corrected cells resulted restoration of WASP expression in lymphocytes and platelets, improvement of in vitro T-cell functions, in particular anti-CD3i mediated response (11), and main lymphocyte subsets’ count normalization (T, B and NK cells) by 4 months post-GT (Figure 1, Supplementary Figure 1 and data not shown). At the same timepoint, platelets ranged between 21 and 29 *109/L.
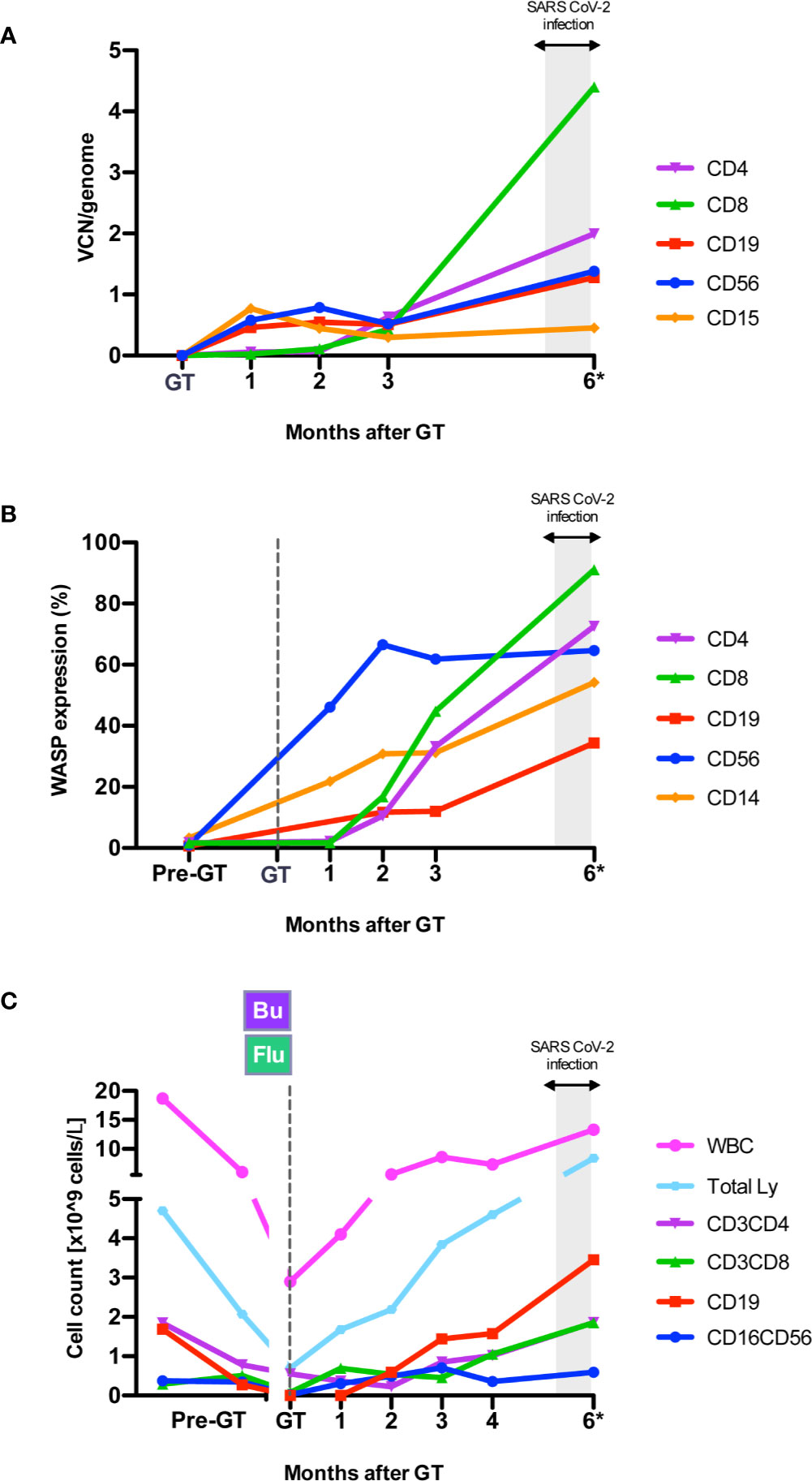
Figure 1 Immune reconstitution after gene therapy (GT). (A) Engraftment of gene corrected cells expressed as VCN/genome in sorted subpopulations from peripheral blood (PB), measured by Real Time-PCR (10) during follow-up after GT. VCN, Vector Copy Number. (B) WASP expression (% of WASP+ cells) by flow cytometry (10) in PB cell subpopulations. WASP, WAS protein. (C) Peripheral blood cell counts at different time points before GT and during follow-up. Bu, Busulfan; Flu, fludarabine. *The 6 month-follow up visit was performed between 6.4 and 6.9 months after GT, after second negative swab for SARS-CoV2.
In March 2020 (5 months after GT), our patient tested positive by RT-PCR for SARS-CoV-2 at nasopharyngeal and rectal swabs after his mildly symptomatic mother tested positive (Figure 2A). Although the patient was asymptomatic, due to the high risk of SARS-CoV-2-related complications based on his previous clinical history, off-label home-treatment with hydroxychloroquine (HCQ, 2.5 mg/kg BID) and lopinavir/ritonavir (LPV/r, 12 mg/kg BID) was administered for 2 weeks. Blood tests and nasopharyngeal swabs were monitored weekly, showing mild increase in inflammatory indexes (Figure 2A), without lymphopenia. During infection period platelets ranged between 21 and 55 *109/L (patient on ongoing TPO-agonist treatment) and showed normal volume, as expected based on previous studies (7, 12). There was no evidence of pro-coagulant status, hyperferritinemia or dyslipidemia. Nasopharyngeal swab RT-PCR became weakly positive 16 days after the first positivity detection, and two consecutive negative nasopharyngeal swabs were obtained within a total of 40 days.
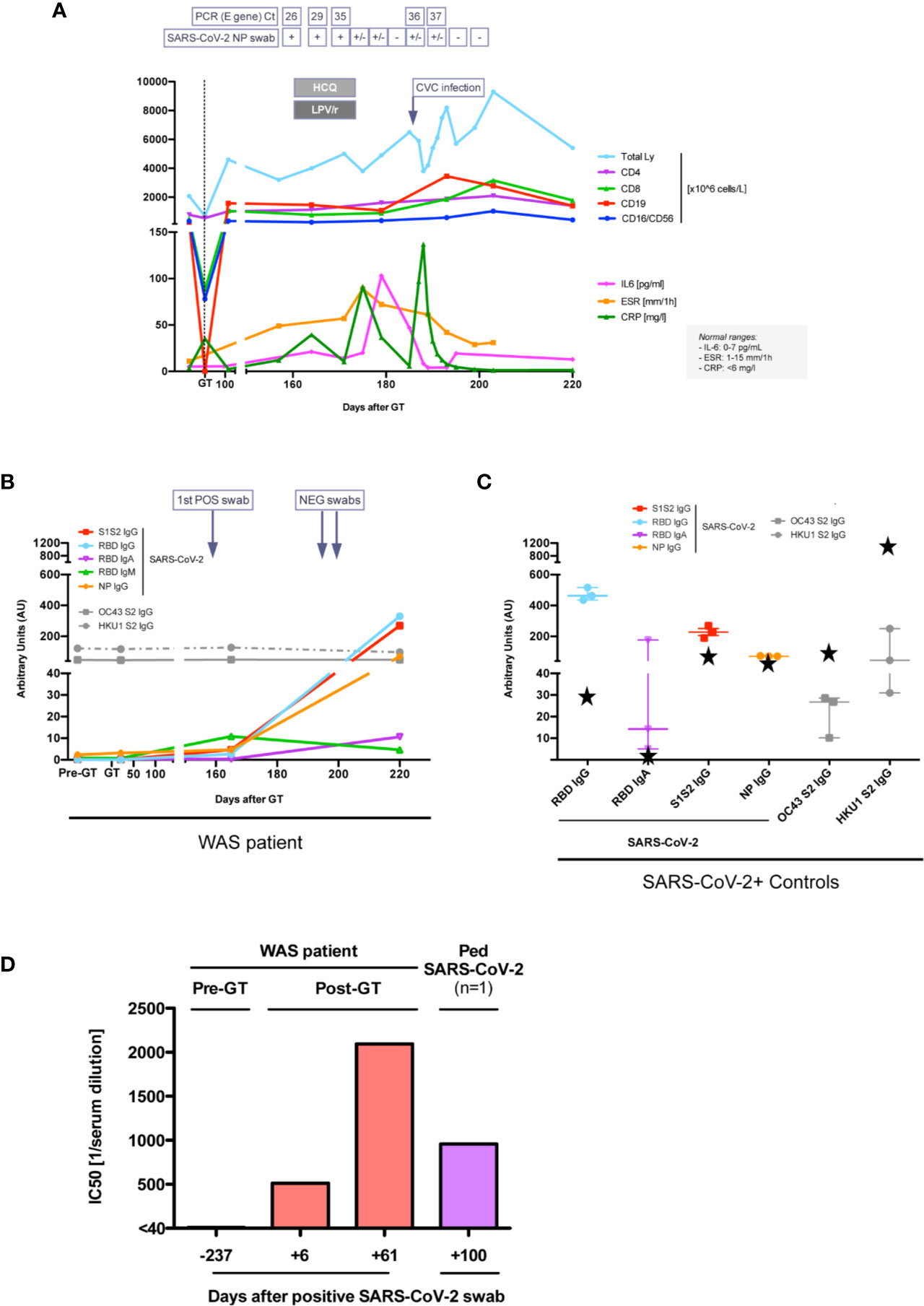
Figure 2 Immune response to SARS-CoV-2 infection. (A) Lymphocyte subpopulations counts and inflammatory marker levels during SARS-CoV-2 infection. SARS-CoV-2 E gene real time (RT)-PCR cycle threshold (Ct) on nasopharyngeal (NP) swabs are reported, when available. +, positive; +/−, weakly positive; –, negative. HCQ, hydroxychloroquine; LPV/r, lopinavir/ritonavir. CVC, central venous catheter. Units of measure of data reported on y axis are specified in the legend on the right. (B, C) Anti-SARS-CoV-2 and anti-OC43/HKU1 Coronaviruses antibody serum level in our patient (B), in three children and in patient’s mum (black stars) after SARS-CoV-2 infection (C). (D) 50% neutralization (IC50) (expressed as reciprocal of serum dilution) of SARS-CoV-2 with the serum of our patient and of another child after SARS-CoV-2 infection. The first dilution tested was 1/40.
One month after the first SARS-CoV-2 finding, the patient experienced a concomitant central venous catheter (CVC)-related infection from Staphylococcus epidermidis with fever and an increase in inflammatory indexes, which rapidly resolved with antibiotic treatment and CVC removal. At this time, repolarization anomalies at electrocardiogram (ECG) and increase of cardiac biomarkers (troponin T, pro-BNP, CK-MB) suggested ongoing myocardial inflammation (Supplementary Table 1). Thus, high-dose intravenous immunoglobulins (IVIg) (1 g/kg), but not steroids, were administered. Notably, transthoracic echocardiogram and 24-h Holter ECG were unremarkable. Furthermore, completely normal findings at delayed-enhanced cardiac magnetic resonance allowed to rule out myocarditis.
Specific antibodies to different SARS-CoV2 antigens were tested by a luciferase immunoprecipitation system (LIPS) assay developed in house (13). Serum IgG to Nucleocapsid protein (NP), Receptor binding domain (RBD) and Spike proteins (S1S2) were positive by day 6 after first positive swab and at day 61 (Figure 2B), reaching levels comparable to those of children who experienced SARS-CoV2 infection (n=3) and higher than the mother (Figure 2C). Serum IgA and IgM to RBD were also detected in the patient. Notably, at all time points the patient had a stable positive signal for antibodies against two coronaviruses associated with common cold (HKU1 and OC43) (Figure 2B). Since the patient was still on monthly IVIg supplementation, we tested eight children receiving Ig supplementation, all with negative SARS-CoV-2 swabs, and followed at our Unit in the same period. They all resulted negative for specific SARS-CoV2 antibodies, indicating their absence in the immunoglobulin preparations and the lack of interference with the LIPS assay (data not shown). Remarkably, the patient’s serum also showed a neutralizing capacity to SARS-CoV2 using a Spike-pseudotyped lentiviral vector in a VERO E6 cell assay (Figure 2D) (manuscript in preparation). After complete resolution of SARS-CoV-2 infection, molecular and immunological tests showed persistence of engraftment of gene corrected cells, with marked rise in CD8+ transduced cells, increase in WASP-expressing cells and stable lymphocyte count (Figure 1). IL-6 increased transiently during acute SARS-CoV-2 infection and then returned to normal values (Figure 1A). As compared to pre-GT values, plasma TNF-alpha and IL-1beta as well as cytokines which have been associated with symptomatic Coronavirus Disease 19 (COVID-19) (14) (IL-2, IL-7, IL-10, IFN-g, IL-8) were stable or decreased post-SARS-Cov2 infection. IL-1-alpha, eotaxin and VEGF were increased (data not shown).
Discussion
SARS-CoV-2 infection is known to cause a wide-spectrum of clinical presentations in adult population, varying from asymptomatic course to severe hyper inflammatory syndromes requiring intensive care (15). It is known that children generally have a milder course, possibly due to a different immunological status or different distribution of receptors compared to adults (3). Outbreaks of Kawasaki and Kawasaki-like syndromes have been described during SARS-CoV-2 pandemic (5), but the pathogenesis and predisposing factors have not yet been identified.
At present, little is known about SARS-CoV-2 infection in children with PID. A recent retrospective survey that included this patient (16) indicates that risk factors predisposing to severe disease and mortality in patients with congenital immune disorders were similar to the general population, but a significant number of younger patients were affected. In a small series in transplant centers in Spain, three immunodeficient patients with SARS-CoV-2 infection were reported post-transplantation, suggesting an increased risk for these patients (17).
WAS is a PID associated with micro-thrombocytopenia, recurrent or severe infections, eczema and increased risk to develop autoimmune and autoinflammatory manifestations, which represented the main clinical features of our patient at disease onset (7). While antiviral treatment (HCQ + LPV/r) was started precociously in our patient, it was not sufficient to induce SARS-CoV-2 viral clearance, with specific swabs remaining positive for 40 days, at the higher range of asymptomatic patients (14).
Although no data are available on early antiviral treatment in asymptomatic/mildly symptomatic subjects, recent work has shown lack of efficacy of HCQ and LPV/r in hospitalized adult with COVID-19 (18, 19).
Concluding Remarks
Immune reconstitution after GT allowed the patient to present a mild clinical course after infection with SARS-CoV-2, as observed in most children. He did not show lymphopenia, known to associate with longer duration of viremia (15), and controlled the infection with early development of immunity with specific antibody response to viral antigens and neutralizing activity. It is unlikely that the limited inflammatory complications, despite its underlying auto-inflammatory disease, is due to an incomplete immune-hematological reconstitution since at the time of presumptive SARS-CoV2 infection the patient had normal leukocyte counts and subsequently produced a prompt inflammatory response to the concomitant bacterial infection. Although we cannot rule out that our patient experienced a very mild form of COVID-19 related inflammatory syndrome (20), his cardiac biomarkers showed only a mild increase (Supplementary Table 1). In addition, he did not fall into the criteria of the Multisystem Inflammatory Syndrome of Children (MISC) and he was discharged after resolution of the bacterial infection. In conclusion, despite previous clinical history of inflammatory manifestations, early immune reconstitution after GT favored the development of specific, neutralizing IgG antibodies against SARS-CoV-2 and mild SARS-CoV-2 disease course without severe autoinflammatory complications.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
For the assessment of antibody response to SARS-CoV-2 serum level and neutralization capacity and cytokine analysis, we obtained peripheral blood samples from our patient and healthy controls, in accordance with the Declaration of Helsinki. Written informed consent forms were approved by the institutional ethics committee of the San Raffaele Hospital (Tiget06, Tiget09, ImmCOVID-19 and GENE-COVID protocols). Gene therapy protocol (OTL-103-4, NCT03837483) and other trial-related materials were approved by the independent ethics committee of the San Raffaele Scientific Institute and the Italian regulatory authority (Agenzia Italiana del Farmaco [AIFA]). Written informed consent was provided by patient's parents before initiation of study specific procedures.
Author Contributions
SC is an OTL-103-4 clinical trial investigator, provided clinical care for the patient, participated to data collection and analysis and wrote the manuscript. VC, FB, MB, CO, MM, VG, FT, FFr, EF, GC, PS, and MR provided clinical care for the patient. EA contributed to data collection. SG, FD, and CS performed molecular and immunological studies on patient’s samples. SR performed RT-PCR for SARS-CoV-2 on patient’s swabs. CC, GP, AE, and FC were involved in the cardiological assessments and data interpretation. RD performed inflammatory marker and cytokine analyses. AC, SD, and GS developed and performed SARS-CoV-2 neutralization assay. DT performed cytokine profiling experiments. LP and VL developed and performed an assay for the detection of specific antibodies to different SARS-CoV2 antigens. MC is an OTL-103-4 clinical trial investigator, contributed to patient’s care and data interpretation. AA is the OTL-103-4 clinical trial principal investigator, designed the study and contributed to data interpretation and manuscript writing. FFe is an OTL-103-4 clinical trial investigator, coordinated data collection, analysis and interpretation, provided clinical care for the patient, and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Supported by Fondazione Telethon, European Community (E-rare EUROCID), and Program Project COVID-19 OSR-UniSR. Study NCT03837483 is sponsored by Orchard Therapeutics (OTL).
Conflict of Interest
The San Raffaele Telethon Institute for Gene Therapy (SR-Tiget) is a joint venture between the Italian Telethon Foundation and Ospedale San Raffaele (OSR). WAS gene therapy was licensed to GlaxoSmithKline in 2014 and then transferred to Orchard Therapeutics (OTL) in April 2018. AA, FFe, MC and SC are investigators of trial # NCT03837483.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past co-authorship with several of the authors VG, FB, MC, FFe, and AA.
Acknowledgments
We thank for their support the health care personnel of the Pediatric Immunohematology and San Raffaele Stem Cell Program, SR-Tiget Clinical Trial office (TCTO) and SR-Tiget Clinical Lab. Several authors of this publication are members of the European Reference Network for Rare Immunodeficiency, Autoinflammatory and Autoimmune Diseases -Project ID No 739543; EBMT Inborn Errors Working Party and Italian Association of Pediatric Hematology and Oncology - Italian Network of Primary Immunodedeficiencies (AIEOP-IPINet). AA is the recipient of the Else Kröner Fresenius Prize for Medical Research 2020.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2020.603428/full#supplementary-material
References
1. Quinti I, Lougaris V, Milito C, Cinetto F, Pecoraro A, Mezzaroma I, et al. A possible role for B cells in COVID-19? Lesson from patients with agammaglobulinemia. J Allergy Clin Immunol (2020) 146:211–13.e4. doi: 10.1016/j.jaci.2020.04.013
2. Liguoro I, Pilotto C, Bonanni M, Ferrari ME, Pusiol A, Nocerino A, et al. SARS-COV-2 infection in children and newborns: a systematic review. Eur J Pediatr (2020) 179:1029–46. doi: 10.1007/s00431-020-03684-7
3. Midulla F, Cristiani L, Mancino E. Will children reveal their secret? The coronavirus dilemma. Eur Respir J (2020) 55(6):2001617. doi: 10.1183/13993003.01617-2020
4. Jiang L, Tang K, Levin M, Irfan O, Morris SK, Wilson K, et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis (2020) 20(11):e276–88. doi: 10.1016/S1473-3099(20)30651-4
5. Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet (London England) (2020) 395:1771–8. doi: 10.1016/S0140-6736(20)31103-X
6. Candotti F. Clinical Manifestations and Pathophysiological Mechanisms of the Wiskott-Aldrich Syndrome. J Clin Immunol (2017) 38:13–27. doi: 10.1007/s10875-017-0453-z
7. Ferrua F, Marangoni F, Aiuti A, Roncarolo MG. Gene therapy for Wiskott-Aldrich syndrome: History, new vectors, future directions. J Allergy Clin Immunol (2020) 146:262–5. doi: 10.1016/j.jaci.2020.06.018
8. Hacein-Bey Abina S, Gaspar HB, Blondeau J, Caccavelli L, Charrier S, Buckland K, et al. Outcomes Following Gene Therapy in Patients With Severe Wiskott-Aldrich Syndrome. JAMA (2015) 313:1550–63. doi: 10.1001/jama.2015.3253
9. Labrosse R, Chu J, Armant M, van der Spek J, Miggelbrink A, Fong J, et al. Outcome of Hematopoietic Stem Cell Gene Therapy for Wiskott-Aldrich Syndrome. Blood (2019) 134:4629. doi: 10.1182/blood-2019-126161
10. Ferrua F, Cicalese MP, Galimberti S, Giannelli S, Dionisio F, Barzaghi F, et al. Lentiviral haemopoietic stem/progenitor cell gene therapy for treatment of Wiskott-Aldrich syndrome: interim results of a non-randomised, open-label, phase 1/2 clinical study. Lancet Haematol (2019) 6:e239–53. doi: 10.1016/S2352-3026(19)30021-3
11. Massaad MJ, Ramesh N, Geha RS. Wiskott-Aldrich syndrome: a comprehensive review. Ann N Y Acad Sci (2013) 1285:26–43. doi: 10.1111/nyas.12049
12. Sereni L, Castiello MC, Di Silvestre D, Della Valle P, Brombin C, Ferrua F, et al. Lentiviral gene therapy corrects platelet phenotype and function in patients with Wiskott-Aldrich syndrome. J Allergy Clin Immunol (2019) 144:825–38. doi: 10.1016/j.jaci.2019.03.012
13. Secchi M, Piemonti L, Lampasona V. COVID-19 survival associates with the immunoglobulin response to the SARS-CoV-2 spike Receptor Binding Domain. J Clin Invest (2020) 142804. doi: 10.1172/JCI142804
14. Long QX, Tang XJ, Shi QL, Li Q, Deng HJ, Yuan J, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med (2020) 26:1200–4. doi: 10.1038/s41591-020-0965-6
15. Ciceri F, Castagna A, Rovere-Querini P, De Cobelli F, Ruggeri A, Galli L, et al. Early predictors of clinical outcomes of COVID-19 outbreak in Milan, Italy. Clin Immunol (2020) 217:108509. doi: 10.1016/j.clim.2020.108509
16. Meyts I, Bucciol G, Quinti I, Neven B, Fischer A, Seoane E, et al. Coronavirus Disease 2019 in patients with inborn errors of immunity: an international study. J Allergy Clin Immunol (2020) S0091-6749(20):31320–8. doi: 10.1016/j.jaci.2020.09.010
17. Vicent MG, Martinez AP, Trabazo del Castillo M, Molina B, Sisini L, Morón G, et al. COVID-19 in pediatric hematopoietic stem cell transplantation: The experience of Spanish Group of Transplant (GETMON/GETH). Pediatr Blood Cancer (2020) 67:e28514. doi: 10.1002/pbc.28514
18. Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, Avezum A, et al. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N Engl J Med (2020) NEJMoa2019014. doi: 10.1056/nejmoa2019014
19. Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N Engl J Med (2020) 382:1787–99. doi: 10.1056/NEJMoa2001282
Keywords: Wiskott-Aldrich Syndrome, primary immunodeficiencies, gene therapy, immune reconstitution, severe acute respiratory syndrome Coronavirus 2 (2019-nCoV)
Citation: Cenciarelli S, Calbi V, Barzaghi F, Bernardo ME, Oltolini C, Migliavacca M, Gallo V, Tucci F, Fraschetta F, Albertazzi E, Fratini ES, Consiglieri G, Giannelli S, Dionisio F, Sartirana C, Racca S, Camesasca C, Peretto G, Daverio R, Esposito A, De Cobelli F, Silvani P, Rabusin M, Cara A, Trabattoni D, Dispinseri S, Scarlatti G, Piemonti L, Lampasona V, Cicalese MP, Aiuti A and Ferrua F (2020) Mild SARS-CoV-2 Infection After Gene Therapy in a Child With Wiskott-Aldrich Syndrome: A Case Report. Front. Immunol. 11:603428. doi: 10.3389/fimmu.2020.603428
Received: 06 September 2020; Accepted: 19 October 2020;
Published: 24 November 2020.
Edited by:
Giuliana Giardino, University of Naples Federico II, ItalyReviewed by:
Sujal Ghosh, Heinrich Heine University of Düsseldorf, GermanyHans Dieter Ochs, University of Washington, United States
Copyright © 2020 Cenciarelli, Calbi, Barzaghi, Bernardo, Oltolini, Migliavacca, Gallo, Tucci, Fraschetta, Albertazzi, Fratini, Consiglieri, Giannelli, Dionisio, Sartirana, Racca, Camesasca, Peretto, Daverio, Esposito, De Cobelli, Silvani, Rabusin, Cara, Trabattoni, Dispinseri, Scarlatti, Piemonti, Lampasona, Cicalese, Aiuti and Ferrua. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alessandro Aiuti, YWl1dGkuYWxlc3NhbmRyb0Boc3IuaXQ=
 Sabina Cenciarelli
Sabina Cenciarelli Valeria Calbi1,3
Valeria Calbi1,3 Federica Barzaghi
Federica Barzaghi Maria Ester Bernardo
Maria Ester Bernardo Chiara Oltolini
Chiara Oltolini Stefania Giannelli
Stefania Giannelli Francesca Dionisio
Francesca Dionisio Claudia Sartirana
Claudia Sartirana Andrea Cara
Andrea Cara Daria Trabattoni
Daria Trabattoni Gabriella Scarlatti
Gabriella Scarlatti Lorenzo Piemonti
Lorenzo Piemonti Vito Lampasona
Vito Lampasona Maria Pia Cicalese
Maria Pia Cicalese Alessandro Aiuti
Alessandro Aiuti Francesca Ferrua
Francesca Ferrua