- 1Taunton and Somerset NHS Foundation Trust, Taunton, United Kingdom
- 2School of Medicine and Biological Sciences Research Complex, University of St. Andrews, St. Andrews, Scotland
Natural killer cell deficiency (NKD) is a primary immunodeficiency where the main defect lies in CD56+CD3− natural killer (NK) cells which mediate cytotoxicity against tumors. Most cases are observed in children and adolescents with recurrent viral infections and cancer. GATA2 and MCM4 mutations are found in NKD patients with cancer. However, the question remains unclear whether NKD increases the risk of cancer. Mutations in the second zinc finger of GATA2 cause both NKD and haematopoietic malignancies. MCM4 splice site mutations are found in NKD patients and they increase susceptibility to DNA instability during replication. IRF8, RTEL1, and FCGR3A mutations are associated with NKD but their associations with cancer are unknown. Based on the studies, it is hypothesized that genetic mutations alone are sufficient to cause cancer. However, a number of NKD patients developed oncogenic viral infections which progressed into cancer. Here, we review the evidence of genetic mutations responsible for both NKD and cancer to identify whether NKD contributes to development of cancer. The findings provide insights into the role of NK cells in the prevention of cancer and the significance of assessing NK cell functions in susceptible individuals.
Introduction
NK cells mediate cytotoxicity against tumor and viral infected cells. NK cells have activating and inhibitory receptors (1) which allow NK cells to selectively target pathogenic cells while preventing autoimmunity against autologous cell (2). The inhibitory receptors are activated by MHC-I while activating receptors are activated by activating ligands on pathogenic cells (2). NK cells play important role in cancer immunity as they mediate cytotoxicity against tumor cells that evade CD8+ T cells by up-regulating activating ligands and failing to express MHC-I (3). In addition, NK cells interact with dendritic cells and T cells to mediate cytotoxicity against tumor cells. Dendritic cells secrete cytokines which activate NK cells, resulting in NK cell secretion of perforin and expression of TNF ligands such as FASL and TRAIL that are responsible for tumor cell apoptosis (4). Conversely, NK cells present antigen debris that activate dendritic cells and they secrete IFNγ that activate T cells, resulting in cytotoxicity against tumor cells (4).
The role of NK cells in cancer immunity has been demonstrated from NK cell deficient mice and humans. NK1.1+CD3− cell deficient transgenic mice showed poor tumor cell removal and increased metastasis compared to wild type in vivo (5). NK cells suppress spontaneous and MCA carcinogen induced tumor formation in mice (3). Furthermore, cancer patients with low peripheral or intratumoral NK cell activity have poorer survival rates (6) and low NK cell cytotoxicity facilitates the progression of precancerous disease into cancer (7) as well as metastases (8). An 11-year cohort study of Japanese residents illustrated that participants in the lowest tertile of natural cytotoxicity were more likely to develop cancer (9).
In humans, NKD has been defined as a primary defect in the number or function of CD56+CD3− NK cell in peripheral blood which is caused by genetic abnormalities that persist over time (10). NKD can be subdivided into classical natural killer cell deficiency (CNKD) and functional natural killer cell deficiency (FNKD) (10). CNKD is defect in both number and function of NK cells in peripheral blood whereas FNKD results in only a functional defect (10). The mutations in GATA2, MCM4, IRF8, and RTEL1 are associated with CNKD and substitutions in FCGR3A with FNKD (11).
The observational studies of NK cell deficient mice and humans have illuminated that NK cells contribute to tumor surveillance. The cancers have been observed in NKD patients with GATA2 and MCM4 mutations although not in patients with IRF8, RTEL1, and FCGR3A mutations (11). Despite these aspects, it is unclear whether NKD is a risk factor of cancer due to a difficulty in isolating NK cell defects as NK cells share receptors and cytokines with other immune cells. This poses the question of whether genetically defined NKDs weaken cancer immunity which cannot be overcome by other immune cells. If NKD increases susceptibility to at least some cancers, it would be worth assessing the number and cytotoxicity of NK cells to prevent such cancers. This review explores whether NKD increases the risk of cancer, based on existing literature.
GATA2 Mutations are Associated With AML-MDS
GATA2 regulates the proliferation and survival of multi-potent haematopoietic stem cells (12). GATA2 mutations selectively affect NK cells, B cells, dendritic cells, and monocytes (12). GATA2 mutations have been observed in CNKD patients with haematopoietic malignancies, especially myelodysplastic syndrome, and acute myeloid leukemia (MDS-AML) (12). DNA sequencing of four MDS-AML families identified missense and frameshift mutations at Thr354 and Thr355 in the second zinc finger of GATA2 (13). Both mutations resulted in the production of abnormal GATA2 proteins that lacked an affinity to the consensus DNA and PU.1 transcription factor (13). When the mutant GATA2 was transfected to promyelocytes, they proliferated substantially and failed to undergo apoptosis when treated with all-trans retinoic acid (ATRA) in vitro unlike those transfected with wild-type GATA2 (14). The same GATA2 mutations have been identified in Emberger syndrome (14), MonoMac syndrome (15) and dendritic cell, monocyte, B and NK lymphoid (DCML) deficiency (16), which accompany both MDS-AML and deficiency in NK cells. The observations reinforced that GATA2 controls haematopoiesis and therefore its disruption results in cancer in affected cell lineages.
Mace et al. found that patients with GATA2 mutations had CD56bright subset loss and consequently increased proportion of CD56dim subset, regardless of total NK cell count (12). In addition, there was significant downregulation of NKG2D and reduced cytotoxicity per NK cell in these patients, indicating that GATA2 may be crucial for producing functional NK cells as well as maintaining CD56bright NK cells (12). The downregulation of NKG2D compromises NK cell cytotoxicity in MDS and facilitates progression to AML (17). Furthermore, leukemic cells shed activating ligands to evade NK cell cytotoxicity, indicating role of NK cells in leukemia (18). Therefore, NKD caused by GATA2 mutations is associated with increased risk of MDS-AML.
MCM4 Mutation Increases the Risk of Cancer
The oncogenic mechanism of MCM4 mutation was revealed by the genetic mapping of mice erythrocytes, which showed that mutant MCM4Chaos3 allele causes T to A substitution in the downstream of the MCM4 zinc finger, resulting in amino acid change in F345I (19). When mouse embryonic fibroblasts (MEFs) were transfected with DNAs containing MCM4Chaos3 allele, they were more susceptible to breakage when their DNAs were treated with aphidicolin. This suggested that MCM4 mutations produce non-functional MCM4 protein that has increased DNA instability upon replication stress.
Similarly, the genetic mapping of MCM4 mutation of the four Irish children with CNKD identified premature stop codon, resulting in shortened MCM4 proteins that lacked N-terminal domain containing F345I as observed in mice (20, 21). The affected children had reduced number of CD56dim NK cell as well as reduced total number of NK cells and they had immature CD56bright NK cells (12). One of them had lymphoproliferative disorder which progressed to EBV induced lymphoma (20). When the fibroblasts of the patients were stimulated with cytokines produced by CD56bright NK cells, there were defects in their DNA replication (12). Therefore, high chromosomal breakage may have contributed to development of EBV induced lymphoma in this patient. The mutant alleles may not always lead to cancer even if they increase DNA instability as the complex interplay of genes and environmental factors determines occurrence of cancer (3). This explains why three out of four Irish CNKD children (20) did not develop cancer despite having the same MCM4 mutations (22). Therefore, MCM4 mutations are associated with both NKD and increased susceptibility to DNA instability upon stress.
NKD Increases Susceptibility to Virus Related Cancer
Most solid tumors observed in NKD patients are associated with oncogenic viral infections, especially Epstein Barr Virus (EBV), and Human Papilloma Virus (HPV). However, there are no reported cases of hepatitis B and C, HTLV-1, and KSHV induced cancers in NKD patients so far (23). The role of NK cells in virus driven malignancies is unclear although NK cells may indirectly suppress tumor growth as tumor cells have mechanisms of evading NK cell cytotoxicity (16). As with MDS-AML, NK activating receptors are downregulated in HPV-16 driven cervical cancer patients, indicating dysfunction of NK cells in these patients (24). The compromised NK cell cytotoxicity against oncogenic viral infection results in viral gene transfection (25) and inflammatory responses (26), both of which predispose to oncogenic changes, as observed in an Irish NKD patient with EBV-related lymphoma (20, 22) (Figure 1).
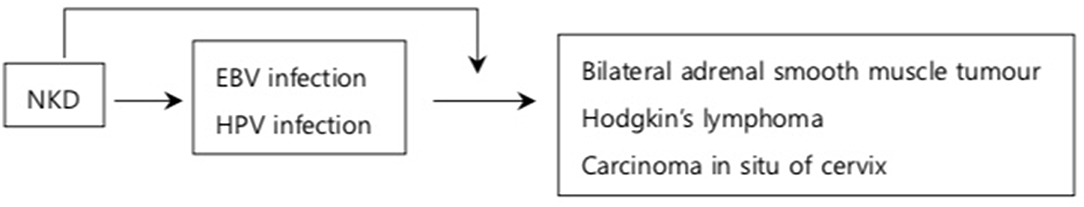
Figure 1. NKD and viral induced cancer. NKD results in reduced number of NK cells (11) and downregulation of activating receptors against oncogenic viruses (24). This increases the susceptibility to EBV and HPV infections which subsequently progress into cancer (27–31). In addition, NKD compromises cytotoxicity against viruses during the infection allowing viral genes to be transfected into infected cells which transform into cancerous cells (23, 25). The arrows indicate the increased risk of developing conditions on the box that it is pointing to.
The effect of NKD on non-viral induced cancer remains unknown. A 56-year-old patient with FNKD developed multiple cutaneous squamous cell carcinoma (cSCC) unresponsive to chemoradiotherapy (32). However, her HPV serology was negative, hence cSCC was not induced by HPV (32). Similarly, NKD has been observed in a few patients with breast cancer and solid tumors but the mechanism of oncogenesis is unknown (33). Therefore, while NKD increases the risk of certain virus induced cancer, its effect on non-viral induced cancer not associated with GATA2 and MCM4 mutations is unknown.
The viral driven malignancies have been observed in patients with normal NK cells as well as in NKD patients (9, 33). While incidence of cancer in both groups cannot be compared due to small number of studies conducted across different periods of time among patients of various demographics, Spinner et al. (33) found that patients with normal NK cell counts were less likely to have major complications including severe infection and cancer than NKD patients. Also, such patients were likely to have fewer number of complications than those with low NK cell counts. Similarly, Imati et al. (9) reported higher relative risk of cancer incidence in patients with low natural cytotoxicity (1.0) in comparison to those with higher natural cytotoxicity (0.6). While the results were not specific to NK cells as natural cytotoxicity is exerted by several innate cells, the study illustrated the significance of natural cytotoxicity in increased incidence of cancer.
NKD and Hodgkin's Lymphoma
The first case of viral-induced cancer in an NKD patient was observed in a 12-year-old girl with isolated CNKD who developed EBV related smooth muscle tumors (SMTs) in the bilateral adrenal glands (27). The patient had longstanding latent EBV infection, during which EBV transfects its gene into host DNA, facilitating development of cancer (27) (Figure 1). Therefore, the case suggests that NKD may have increased the risk of SMTs during latent EBV infection. Similar cases have been reported in a 6-year-old Taiwanese girl (28) and one of the three siblings (29, 30) with isolated NKDs who developed Hodgkin's lymphoma in the latent phase of EBV infection which may have been facilitated by NKD. While EBV downregulates NKG2D expression on NK cells to evade NK mediated cytotoxicity (23), NKD was present before primary EBV infection and persisted after complete recovery from the lymphoma in these patients (29, 30). Therefore, NKD was not transiently caused by EBV but it rather increased the risk of lymphoma (29, 30). In addition, NK cells have cytotoxicity against EBV infected B cells and they prevent transformation of EBV infected B cells into cancer in the acute EBV infection (23). Therefore, NKD increases the risk of EBV induced lymphoma by facilitating progression into cancer in acute and latent EBV infection. Furthermore, considering its antiviral immunity, NK cells may increase the risk of lymphoma by increasing the risk of developing EBV infection which subsequently progresses into lymphoma.
NKD and Carcinoma in situ of Cervix
Finally, HPV driven cervical cancer is one of the most common virus driven malignancies in NKD patients with GATA2 mutations. In a retrospective study of 57 patients with GATA2 mutations, more than one third of patients had HPV driven malignancies (33). Similar to GATA2 deficient patients with MDS-AML, patients with HPV driven malignancies had low number of CD56bright NK cells (11). Spinner et al. found that almost all GATA2 deficient patients with one or more complications of severe infections, MDS-AML, and pulmonary alveolar proteinosis had significantly decreased NK cell counts (33). This was in contrast to patients without such complications, whose median NK cell count fell within the normal range (33). This implied the crucial role of NK cells in preventing GATA2 associated fatal diseases including HPV driven malignancies. Similarly, a 23-year-old girl diagnosed with recurrent carcinoma in situ of cervix (31) had peripheral blood mononuclear cells (PBMCs) which responded poorly against K562 on several occasions compared to the controls' PBMCs. The poor natural cytotoxicity was attributed to NK cell numbers, as the proportion of NK cells defined as CD3−CD16+ and CD3−NKH1+ cells was very low unlike CD3+ T cells. The NK cell selective markers were used to conclude that cytotoxicity of NKT and T cells was not sufficient to defense against HPV in the absence of NK cells. The observations imply the crucial role of NK cells on immunity against HPV driven malignancies (11).
Conclusion
In conclusion, most NKD cases with cancer have been observed in children and adolescents who have compromised immunity. The relatively small cohort size of NKD patients inevitably limits somewhat the generalization of the findings. Nevertheless, GATA2 related autosomal dominant syndromes provide strong evidence that substitution and frameshift GATA2 mutations at N-terminal to and within the second zinc finger cause both CNKD and MDS-AML. Both mice and human studies suggest that frameshift mutation at MCM4 splice site results in the production of non-functional MCM4 protein which increases susceptibility to DNA instability upon replication stress. IRF8, RTEL1, and FCGR3A mutations have been observed in NKD patients but their association with cancer is unknown. NKD may compromise anti-EBV immunity during acute and latent infections, facilitating the development of EBV induced cancer. However, the precise mechanisms of NK cell mediated EBV cytotoxicity need to be elucidated to consolidate such findings. The HPV induced cancer observed in CNKD patients imply the potential role of NK cells on HPV induced cancer. Therefore, NKD may increase the risk of cancers associated with certain viruses and mutations while future studies on mechanisms of cancer in NKD patients would highlight the role of NK cells in cancer immunity (Figure 2).
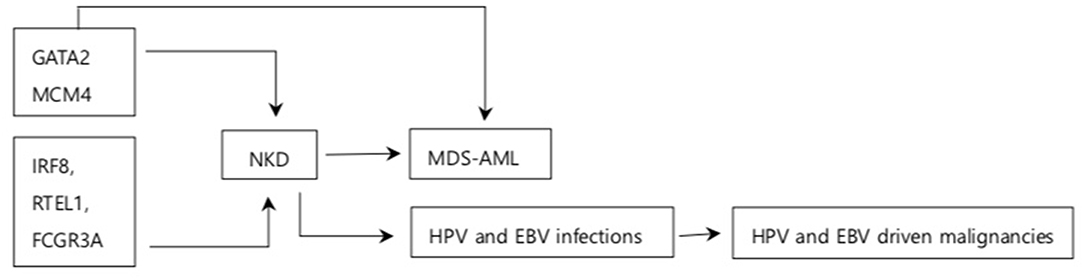
Figure 2. Summary of association between NKD and cancer. Arrows indicate association between the box it is emerging from and the box it is pointing to. GATA2, MCM4, IRF8, RTEL1, and FCGR3A are the genes responsible for NKD. GATA2 increases the risk of MDS-AML MCM4 is associated with increased NDA instability. NKD is associated with increased susceptibility to MDS-AML and some oncogenic viral infections. NKD has been observed in other solid tumors and breast cancer although association on between them is unknown.
Author Contributions
WM and SP: conception and design of the study, manuscript revision, and approval of the manuscript to be published. WM: literature review and drafting the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. (2008) 9:503–10. doi: 10.1038/ni1582
2. Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene. (2008) 27:5932–43. doi: 10.1038/onc.2008.267
3. Wu J, Lanier LL. Natural killer cells and cancer. Adv Cancer Res. (2003) 90:127–56. doi: 10.1016/S0065-230X(03)90004-2
4. Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer. (2002) 2:850–61. doi: 10.1038/nrc928
5. Kim S, Iizuka K, Aguila HL, Weissman IL, Yokoyama WM. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc Natl Acad Sci USA. (2000) 97:2731–6. doi: 10.1073/pnas.050588297
6. Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Che X, Iwashige H, et al. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer. (2000) 88:577–83. doi: 10.1002/(SICI)1097-0142(20000201)88:3<577::AID-CNCR13>3.3.CO;2-M
7. Nakajima T, Mizushima N, Kanai K. Relationship between natural killer activity and development of hepatocellular carcinoma in patients with cirrhosis of the liver. Jpn J Clin Oncol. (1987) 17:327–32.
8. Chiossone L, Dumas P, Vienne M, Vivier E. Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol. (2018) 18:671–88. doi: 10.1038/s41577-018-0061-z
9. Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. (2000) 356:1795–9. doi: 10.1016/S0140-6736(00)03231-1
10. Orange JS. Natural killer cell deficiency. J Allergy Clin Immunol. (2013) 132:515–25. doi: 10.1016/j.jaci.2013.07.020
11. Mace EM, Orange JS. Genetic causes of human NK cell deficiency and their effect on NK cell subsets. Front. Immunol. (2016) 545:1–8. doi: 10.3389/fimmu.2016.00545
12. Mace EM, Hsu AP, Monaco-Shawver L, Makedonas G, Rosen JB, Dropulic L, et al. Mutations in GATA2 cause human NK cell deficiency with specific loss of the CD56(bright) subset. Blood. (2013) 121:2669–77. doi: 10.1182/blood-2012-09-453969
13. Hahn CN, Chong C, Carmichael CL, Wilkins EJ, Brautigan PJ, Li X, et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat Genet. (2011) 43:1012–7. doi: 10.1038/ng.913
14. Ostergaard P, Simpson MA, Connell FC, Steward CG, Brice G, Woollard WJ, et al. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome). Nat Genet. (2011) 43:929–31. doi: 10.1038/ng.923
15. Hsu AP, Sampaio EP, Khan J, Calvo KR, Lemieux JE, Patel SY, et al. Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood. (2011) 118:2653–5. doi: 10.1182/blood-2011-05-356352
16. Dickinson RE, Griffin H, Bigley V, Reynard LN, Hussain R, Haniffa M, et al. Exome sequencing identifies GATA-2 mutation as the cause of dendritic cell, monocyte, B and NK lymphoid deficiency. Blood. (2011) 118:2656–8. doi: 10.1182/blood-2011-06-360313
17. Epling-Burnette PK, Bai F, Painter JS, Rollison DE, Salih HR, Krusch M, et al. Reduced natural killer (NK) function associated with high-risk myelodysplastic syndrome (MDS) and reduced expression of activating NK receptors. Blood. (2007) 109:4816–24. doi: 10.1182/blood-2006-07-035519
18. Hilpert J, Grosse-Hovest L, Grunebach F, Buechele C, Neubling T, Raum T, et al. Comprehensive analysis of NKG2D ligand expression and release in leukemia: implications for NKG2D-mediated NK cell responses. J. Immunol. (2012) 189:1360–71. doi: 10.4049/jimmunol.1200796
19. Shima N, Alcaraz A, Liachko I, Buske TR, Andrews CA, Munroe RJ, et al. A viable allele of Mcm4 causes chromosome instability and mammary adenocarcinomas in mice. Nat Genet. (2007) 39:93–8. doi: 10.1038/ng1936
20. Eidenschenk C, Dunne J, Jouanguy E, Fourlinnie C, Gineau L, Bacq D, et al. A novel primary immunodeficiency with specific natural-killer cell deficiency maps to the centromeric region of chromosome 8. Am J Hum Genet. (2006) 78:721–7. doi: 10.1086/503269
21. Hughes CR, Guasti L, Meimaridou E, Chuang CH, Schimenti JC, King PJ, et al. MCM4 mutation causes adrenal failure, short stature, and natural killer cell deficiency in humans. J Clin Invest. (2012) 122:814–20. doi: 10.1172/JCI60224
22. Gineau L, Cognet C, Kara N, Lach FP, Dunne J, Veturi U, et al. Partial MCM4 deficiency in patients with growth retardation, adrenal insufficiency, and natural killer cell deficiency. J Clin Invest. (2012) 122:821–32. doi: 10.1172/JCI61014
23. Mishra R, Welsh RM, Szomolanyi-Tsuda E. NK cells and virus-related cancers. Crit Rev Oncog. (2014) 19:107–19. doi: 10.1615/CritRevOncog.2014010866
24. Garcia-Iglesias T, Toro-Arreola ADT, Albarran-Somoza B, Toro-Arreola SD, Sanchez-Hernandez PE, Ramirez-Dueñas MG, et al. Low NKp30, NKp46 and NKG2D expression and reduced cytotoxicity activity on NK cells in cervical cancer and precursor lesions. BMC Cancer. (2009) 9:186. doi: 10.1186/1471-2407-9-186
25. Thompson MP, Kurzrock R. Epstein-Barr virus and cancer. Clin Cancer Res. (2004) 10:803–21. doi: 10.1158/1078-0432.CCR-0670-3
26. Philip M, Rowley DA, Schreiber H. Inflammation as a tumor promoter in cancer induction. Semin Cancer Biol. (2004) 14:433–9. doi: 10.1016/j.semcancer.2004.06.006
27. Shaw RK, Issekutz AC, Fraser R, Schmit P, Morash B, Monaco-Shawver L, et al. Bilateral adrenal EBV-associated smooth muscle tumors in a child with a natural killer cell deficiency. Blood. (2012) 119:4009–12. doi: 10.1182/blood-2011-10-385377
28. Yang C, Yang Y, Lin Y, Lu M, Chiang B. Natural Killer cell deficiency associated with Hodgkin's lymphoma: a case report. J Formosan Med Assoc. (2002) 101:73–5.
29. Komiyama A, Kawai H, Yamada S, Kato M, Yanagisawa M, Miyagawa Y, et al. A killing defect of natural killer cells with the absence of natural killer cytotoxic factors in a child with Hodgkin's disease. Blood. (1987) 69:1686–90.
30. Komiyama A, Kawai H, Yabuhara A, Yanagisawa M, Miyagawa Y, Ota M, et al. Natural killer cell immunodeficiency in siblings: defective killing in the absence of natural killer cytotoxic factor activity in natural killer and lymphokine-activated killer cytotoxicities. Pediatrics. (1990) 85:323–30.
31. Ballas ZK, Turner JM, Turner DA, Goetzman EA, Kemp JD. A patient with simultaneous absence of “classical” natural killer cells (CD3−, CD16+, and NKH1+) and expansion of CD3+, CD4−, CD8−, NKH1+ subset. J Allergy Clin Immunol. (1990) 85:453–9. doi: 10.1016/0091-6749(90)90155-W
32. Ilyas M, Costello CM, Sharma A. Exploring the relationship between natural killer cells and cutaneous squamous cell carcinoma development. JAAD Case Rep. (2017) 3:364–6. doi: 10.1016/j.jdcr.2017.04.006
Keywords: NKD, NK cells, oncogenic viral infections, CD56bright NK cells, MDS-AML
Citation: Moon WY and Powis SJ (2019) Does Natural Killer Cell Deficiency (NKD) Increase the Risk of Cancer? NKD May Increase the Risk of Some Virus Induced Cancer. Front. Immunol. 10:1703. doi: 10.3389/fimmu.2019.01703
Received: 09 January 2019; Accepted: 08 July 2019;
Published: 19 July 2019.
Edited by:
José Mordoh, Leloir Institute Foundation (FIL), ArgentinaReviewed by:
Emily Mace, Columbia University, United StatesEstrella Mariel Levy, National Council for Scientific and Technical Research (CONICET), Argentina
Copyright © 2019 Moon and Powis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Won Young Moon, wonyoungmoon@doctors.org.uk
 Won Young Moon
Won Young Moon