
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Hortic., 06 June 2023
Sec. Sustainable Pest and Disease Management
Volume 2 - 2023 | https://doi.org/10.3389/fhort.2023.1175251
This article is part of the Research TopicEpidemiology and Management of Pome Fruit DiseasesView all 9 articles
Botrytis cinerea (gray mold) and Penicillium expansum (blue mold), are major pome fruit postharvest pathogens and their control relies heavily on the use of one postharvest fungicide (fludioxonil). This study aimed to evaluate the efficacy of the cyclolipopeptides (CLPs), in the form of fengycin and iturin A, in crude metabolite extracts from Bacillus amyloliquefaciens as an alternative biofungicide. The crude extract containing CLPs was applied with an edible coating (i.e., zein as a carrier) as a postharvest treatment against B. cinerea on “Packham’s Triumph” pears and P. expansum on “Cripps Pink” apples. Treatments were applied either as dips in combination with a zein edible coating at pH 2.0 or pH 8.0, or as sprays with a zein edible coating at pH 2.0 or pH 8.0. The efficacy of CLP applications was measured in comparison to the standard registered synthetic fungicide fludioxonil at a concentration of 299.0 mg/L. Treatments were applied to the fruit either preventatively (as sprays) or curatively (as dips or sprays). The lowest mean blue mold incidence (68.6%) was achieved when the edible coating containing the crude extract of B. amyloliquefaciens (CLP at pH 2.0) was applied as a curative dip treatment. B. cinerea infection was reduced by 92.6%, resulting in a 5.7% gray mold incidence for the curative application of CLPs at pH 2.0. This result was not significantly different from the inhibitory action of the fungicide fludioxonil. These results indicate that CLPs are an effective alternative biofungicide that can be used for the control of B. cinerea on pome fruit, especially if their formulation and application are improved and optimized.
A major constraint of pome fruit production worldwide is the occurrence of postharvest diseases, which can result in significant crop losses. Fruit is susceptible to damage by fungal diseases during cultivation, transportation, and storage, which results in 50% of the fruit harvested in countries with limited cold-chain capacity being lost due to decay (Qu et al., 2016). Botrytis cinerea Pers., causing gray mold, and Penicillium expansum Link, causing blue mold, are among the postharvest pathogens that extensively reduce the shelf and marketing life of apples and pears (Lutz et al., 2013; Spadaro and Droby, 2016).
Currently, postharvest fungal control is primarily conducted using synthetic fungicides, i.e., fludioxonil or pyrimethanil. However, owing to the pressure of consumer demands for reduced chemical residues in fruit because of perceived health risks, the increasing resistance of fungal pathogens to frequently used fungicides, and environmental concerns, there is a major drive toward the development of innovative non-chemical measures to control postharvest diseases (Conway et al., 2004; Zhang et al., 2007; Kefi et al., 2015; Spadaro and Droby, 2016). These growing concerns have resulted in the need to develop other methods to control postharvest decay, which can be complemented with antimicrobial agents and physical treatments (Nunes, 2012; Ali et al., 2015; Bordoh et al., 2020).
Cyclolipopeptides (CLPs) are secondary metabolites produced by microorganisms, primarily bacteria (e.g., Bacillus amyloliquefaciens Priest, Goodfellow, Shute & Berkeley, B. subtilis Cohn and B. vallismortis Roberts). They are a potential alternative to synthetic fungicides and can therefore both limit the overuse of postharvest synthetic fungicides and meet consumer demands for lower synthetic fungicide residue on fruit. Several studies report the use of these organisms as biocontrol agents; however, their variable efficacy when applied as living cells has hampered their application (Nunes, 2012). Instead of applying living bacteria as a fungicide, an alternative route is to produce the CLPs in fermentation, separate and purify the active component in the biocontrol agent, and apply them directly as biofungicides. Few studies have investigated methodologies to harvest significant quantities of CLPs, post fermentation, or investigated their efficacy when applied without the bacterial biocontrol agent present (Pretorius et al., 2015). Edible coatings, such as zein and carnauba waxes, have a positive influence on the quality and shelf life of fruit (Bai et al., 2003; Wibowo et al., 2021). Moreover, they can act as carriers of antimicrobials and antioxidants to protect produce from being attacked by pathogens. Zein is a protein-based coating that has been reported to be an excellent barrier that increases shelf life and prevents the weight loss of postharvest produce (Bai et al., 2003), whereas carnauba wax is a natural wax mainly used to improve fruit quality and appearance, provided it does not inhibit respiration, i.e., choose specific wax registered/used for pome fruit (Barman et al., 2011; Nascimento et al., 2016).
The use of CLPs, in the form of fengycin and iturin A, as potential biofungicidal applications has previously been reported (Ongena et al., 2005; Arrebola et al., 2010a). Although limited work has examined their application in plant pathogen control directly, CLPs have been found to be effective fungitoxic metabolites (Touré et al., 2004; Conway et al., 2004). The mode of action of fengycin and iturin A is thought to be the destruction of the fungal cell membrane, inhibition of spore germination, and inhibition of mycelial growth (Arrebola et al., 2010b; Qu et al., 2016; Calvo et al., 2017). Other studies have reported an indirect effect of CLPs as host defense inducers (Ongena et al., 2005; Waewthongrak et al., 2014). However, limited information has been published on the possibility of using CLP metabolites in the application of fruit fungicides (Dukare et al., 2018).
Furthermore, actual fungicide application to fruit requires that an application method be chosen, and each method differs in terms of its efficacy; some application methods that are widely used industrially include dipping, thermofogging, and spraying (Russouw et al., 2021). A consistent composition of CLPs can be achieved if metabolites are produced using a bioreactor (Tan et al., 2022). Further work is needed to verify the activity of each fungicidal lipopeptide component to achieve consistent results when usedas post harvest treatment.
This study aimed to evaluate the efficacy of the CLPs fengycin and iturin A from a crude extract from B. amyloliquefaciens fermentation broth against postharvest decay (gray or blue mold) on inoculated pome fruit either as a curative dip or spray application (preventatively or curatively).
B. amyloliquefaciens strain DSM 23117, obtained from Deutsche Sammlung von Mikroorganismen und Zellkulturen, was cultivated in growth media (as described in Pretorius et al., 2015) at 30°C for 48 h, at 150 rpm in an orbital shaker–incubator [Labcon model FSIM-SPO35); Labcon laboratory equipment (Pty.) Ltd., Krugersdorp, South Africa]. A 10% v/v of late-exponential-phase culture was transferred, axenically, to the liquid medium and incubated for 48 h at 30°C and 150 rpm. To remove bacterial cells, the broth containing the secondary metabolites was then centrifuged at 5,000 rpm for 35 minutes (Eppendorf 5702 R).
The cell-free culture supernatant was acidified to pH 2.0 through the drop-wise addition of 16% v/v hydrochloric acid (HCl) and the solution was stored for 16 h at 4°C so that complete precipitation could take place. The off-yellow colored acid precipitate, containing CLPs with some impurities, was concentrated, and recovered by centrifugation at 5,000 rpm for 15 minutes (Eppendorf 5702 R). The supernatant was discarded while the acid precipitate was collected from the pellet. The pellet was oven dried at 37°C for 24 h. The resulting dry acid precipitate was crushed into a powder using a pestle and mortar, weighed, and stored in a 50-mL Falcon tube at –18°C until required for further experimental use. The CLP extract was adjusted to pH 8.0 in 80% ethanol for the dip and spray application using 1 M sodium hydroxide (NaOH), with a final concentration corresponding to 10 times the dry weight of acid precipitate to culture supernatant (Mazibuko, 2018).
Analysis of the composition of the actives in the CLP extract was conducted using high-performance liquid chromatography (HPLC) to quantify fengycin and iturin A metabolites; this was undertaken at the Central Analytical Facilities (CAF) at Stellenbosch University. The CLPs were compared with pure technical grade standards (Sigma cat. Nr. I1774 and SMB00292) and the results corresponded to iturin A. However, the retention time of fengycin varied slightly between batches, likely indicating differing congeners.
Virulent strains of the postharvest pathogens B. cinerea (STE-U 9254) and P. expansum (STE-U 9255), obtained from the Department of Plant Pathology, Stellenbosch University culture collection (STEU), were maintained on potato dextrose agar (PDA) and periodically transferred to fresh fruit to induce infection and spore production.
“Cripps Pink” apples and “Packham’s Triumph” pears were collected from Western Cape pome fruit orchards. Unblemished, asymptomatic fruits were used in the trials. Prior to each treatment, fruits were surface sterilized by dipping in 250 mg.L-1 chlorine (HTH Chlorine; 50 mg.L-1 calcium hypochlorite) and tap water for 1 minute and air dried in a laminar flow cabinet overnight unless stated otherwise.
Sixty fruits (15 fruits per replicate, four replicates per treatment) were dipped for 30 seconds in one of the following treatments: (a) zein only (prepared as described above); (b) fludioxonil (299 mg/L; Teacher 230 SC, ICA, SA); (c) water only (untreated); (d) the “CLP in zein” treatment at pH 2.0; and (e) “CLP in zein” treatment at pH 8.0 (for B. cinerea only). Fruits were treated curatively (3 hours after inoculation). The trial was repeated once.
Zein-based edible coating was prepared by adding 1 g of zein powder (Sigma-Aldrich Cat. Nr. W555025, Merck KGaA, Darmstadt, Germany) with 10 mL of ethanol (80%) and left to dissolve in a 37 °CC water bath for 20 min. CLP extracts were added to both the zein coating solutions at a pH of either 2.0 (unadjusted, directly from acid precipitation product) or 8.0 (adjusted using 1 M NaOH). The dip application CLP extract contained 2,900.5 mg/L of fengycin and 191.3 mg/L of iturin A. The commercial standard fungicide fludioxonil was applied at the recommended dose of 299 mg/L as a positive control. Untreated fruit to which only water was applied) was included as a negative control.
Two wounds (3 mm wide × 5 mm deep) were made using a wounding tool and were inoculated with a 1 × 105 spores/mL spore suspension of B. cinerea or P. expansum. All fruits were incubated at 25°C ± 2°C in plastic-covered crates with moist paper towels. Lesion sizes were measured on days 4, 6, and 8 after inoculation (DAI). All fruits were incubated at 25°C ± 2°C in plastic-covered crates with moist paper towels. Fruit decay incidence (i.e., percentage of wounds infected per fruit) and severity (i.e., lesion size in mm) were evaluated on days 4, 6, and 8 after inoculation.
Twelve fruits were used for each treatment, and each treatment was repeated three times in each trial. The spray application was either applied preventatively (3 hours before inoculation) or curatively (3 hours after inoculation) by spraying 110 µL of the treatment on each fruit, followed by gentle brushing for uniform distribution. For the positive control treatment, fruits were dipped in fludioxonil for 30 seconds. The treatments tested were the zein treatment (alone), and the two different CLP applications, that is, the CLPs in zein treatment at a pH of 2.0 and “CLPs in zein” at a pH of 8.0. An additional control treatment combining fludioxonil (299 mg/L) with zein was included for the curative trial only (spray application as described above). The trial was repeated once. The CLP extracts contained 80.7 mg/L of fengycin and 1.1 mg/L of iturin A in the spray application. Inoculation and incubation were carried out as described above for the curative application, and the preventative application was applied in an additional trial; the fruit was wound inoculated as described above 3 hours after the preventative fungicide spray application.
The mean incidence and severity of fruit decay were calculated as a percentage for each treatment replicate. All statistical analysis was carried out in Statistica v. 13.5 (TIBCO, Software, CA, USA) at the Center of Statistical Analysis, Stellenbosch University. Data (pooled for days 4, 6, and 8) were analyzed for incidence (number of wounds infected) and severity (lesion size), using formulas (i) (D/T) × 100, where D = total number of decayed fruit wounds, and T = total number of fruit wounds for the incidence, and (ii) (lesion size from each treatment/lesion size from the inoculated-untreated fruit) × 100, respectively (Russouw et al., 2021). A two-way ANOVA was used to compare means between treatments with treatment and day as factors and Levene’s test was used to determine the homogeneity of variances. A probability level of 5% was considered significant for all significance tests. In some cases, homogeneity was strongly rejected (p < 0.01); therefore, a Welch test was added as a post hoc analysis.
Curative fludioxonil treatment showed the lowest blue mold and gray mold incidence in all trials, with complete inhibition being observed for B. cinerea when fludioxonil was administered as a dip. The CLPs in the zein treatment at pH 2.0 was also highly effective, resulting in 5.7% gray mold incidence and 3.8% severity (not significantly different from fludioxonil control; Table 1, Figure 1), whereas CLPs in the zein treatment at pH 8.0 was less effective, resulting in 34.4% gray mold incidence and 32.9% severity. The application of zein only slightly reduced gray mold incidence (85.4%) and significantly lowered gray mold severity (36.9%), whereas untreated and inoculated fruits had high gray mold incidence (98.3%) and severity (98.2%). The CLPs in the zein treatment at pH 2.0 reduced blue mold incidence to 68.6%, which was a significant reduction compared with the incidence for the untreated control (95.0%). The CLPs in the zein treatment at pH 2.0 also reduced the severity of P. expansum to 37.5% (compared with 95.8% in the untreated inoculated control). Zein dip application alone also reduced the incidence (89.9%) and severity (57.0%) of blue mold.
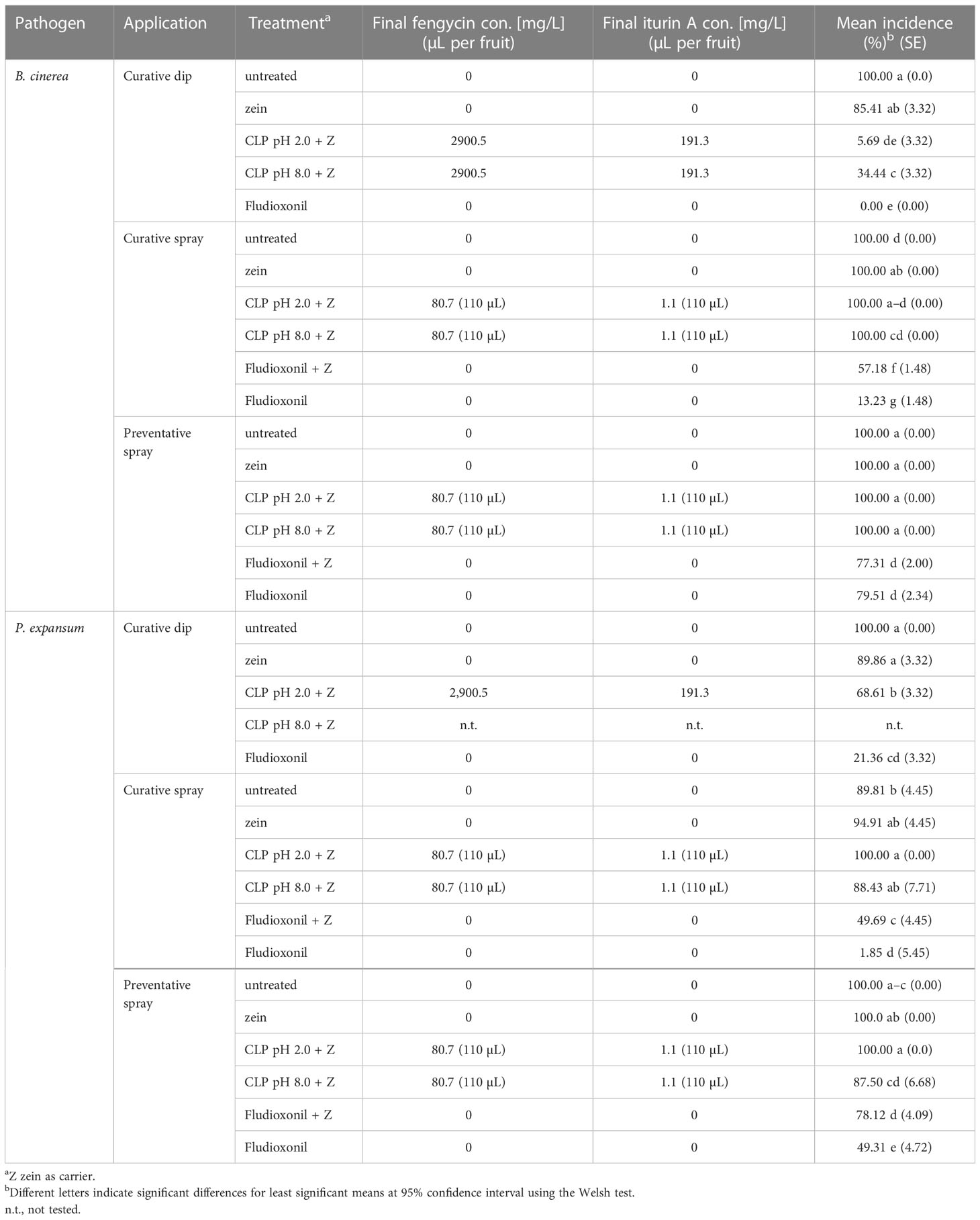
Table 1 Mean incidence of blue mold and gray mold infection after dip and spray trials with CLPs at pH 2.0 or pH 8.0.
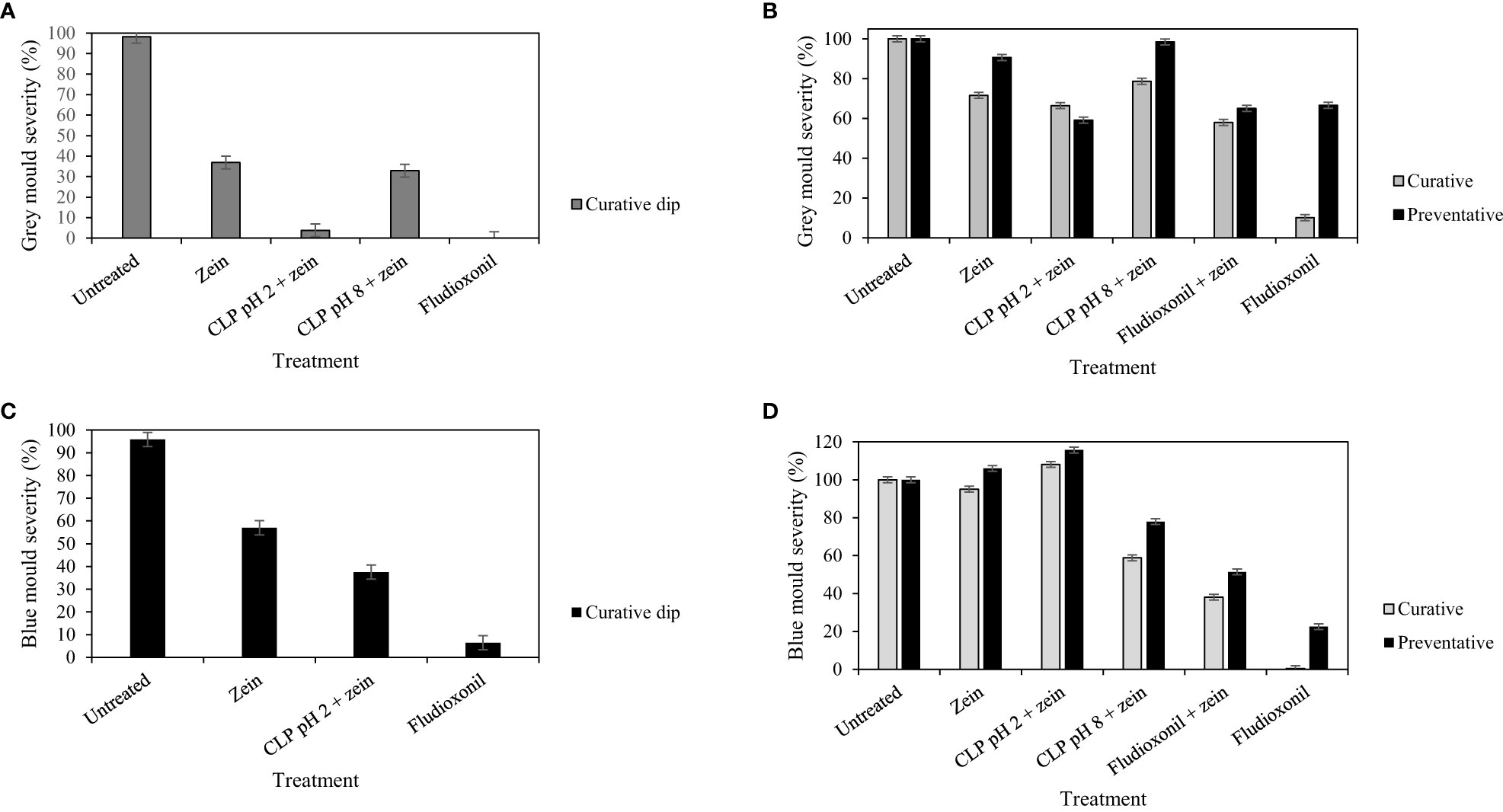
Figure 1 Comparison of decay severity for all treatments in the curative dip (A, C) or decay severity after treatment application either curatively or preventatively showing means across all 3 days (B, D) to control B. cinerea on pears (A, B) or P. expansum on apples (C, D). Error bars show standard error (SE) at a 95% confidence interval.
The most effective blue mold control was achieved with fludioxonil as a curative application (1.85% incidence; Table 1). Only the CLPs in the zein treatment at pH 8.0 applied preventatively marginally reduced blue mold incidence (87.5%), but not gray mold incidence. Nevertheless, the severity of blue mold was reduced in the CLPs in zein treatment at pH 8.0 (78.0%), compared with the untreated control (94.0%). Fludioxonil alone applied preventatively did not effectively protect the fruit from blue mold or gray mold infection with the very aggressive inoculation method used in this study, possibly owing to the wound that was created in the inoculation process that broke the film of the protective layer of edible coating.
An adverse effect increasing blue mold infection incidence in the protective and curative application of zein was found (Table 1); zein application resulted in higher incidence when fludioxonil control treatment was applied combined with zein, in particular when it was applied curatively as a spray (49.69% incidence vs. 21.36% when applied as a dip treatment). Blue mold severity also increased by 6% with preventative zein application alone, or by 15.7% if combined with CLPs in zein at pH 2.0 (Figure 1). On the other hand, the severity of P. expansum infection was significantly reduced when CLPs in zein at pH 8.0 were applied curatively, with a mean lesion diameter of 11.0 mm compared with the untreated control at 18.2 mm. This is a 40% reduction in severity compared with the untreated control (Figure 1).
CLPs are known for their strong antifungal activity against postharvest disease, and among them, iturin A and fengycin have been recognized as inhibitors of most pathogens (Ambrico and Trupo, 2017). This study set out to determine the efficacy of B. amyloliquefaciens CLPs as in vivo postharvest treatments on pome fruit. The CLPs were applied in a carrier of edible coatings, that is, zein for better efficacy and to improve the ease of their application. The CLPs have not been combined with zein in previous studies; however, zein has been found to maintain the overall health of fruit well compared with other commercial coatings (Bai et al., 2003). This study aimed to find better ways to apply the CLPs to the fruit to achieve greater efficacy in controlling postharvest pathogens. The CLPs in the zein treatment at pH 2.0 controlled blue mold when applied preventatively, whereas the CLPs in the zein treatment at pH 8.0 were most effective in controlling blue mold curatively. Conversely, previous studies have not found biofungicides to have eradicative activity and claimed that they would have a reduced spectrum of activity compared with synthetic fungicides (Conway et al., 2004). Calvo et al. (2017) demonstrated that B. amyloliquefaciens had a limited curative effect against B. cinerea, P. expansum, P. italicum, and P. digitatum, and higher disease inhibition was achieved with a preventative treatment. As the two fungi used in the current study are latent pathogens and the treatments used in packhouses are normally applied post harvest, a curative application was tested.
In the dip application, the fruit treated with CLP with zein as a carrier at pH 2.0 was most effective in reducing B. cinerea lesion size compared with the other application methods, which could also be due to the higher concentration of CLPs applied in the dip trial. Similarly, Calvo et al. (2017) reported that B. amyloliquefaciens was more effective at a lower pH (i.e., pH 5.0) compared with pH 7.5, which they explained was beneficial for the survival of the biocontrol agent in a more acidic fruit environment. In a parallel study, the thermofogging application of CLPs at pH 8.0 did not effectively reduce B. cinerea and P. expansum incidence, although it reduced the severity of the P. expansum infection. Additional studies to evaluate the thermofogging application using a higher treatment volume and CLP concentration are needed, in addition to a more in-depth evaluation of the effect of pH on the infection capabilities of B. cinerea and P. expansum.
Quantification of lipopeptides from crude extracts showed that the non-fungitoxic CLP surfactin produced higher concentrations than the CLPs of interest, that is, iturin A and fengycin (Ongena and Jacques, 2008; Pérez-García et al., 2011). Furthermore, the activity of other components of the CLP extract, such as mycosubtilin alone or in combination with surfactin, and their efficacy in controlling decay, will have to be tested (Kourmentza et al., 2021).
The adverse effects seen for zein as a spray application and with lower concentrations of fengycin and iturin A in the spray, and also for zein combined with fludioxonil in the same trial, could have been a result of micro-abrasions caused by brushing of the fruit to distribute the edible coating. Other (gentler) methods, such as atomizer application and the use of rollers instead of brushes to distribute the coating will have to be investigated. Further trials should also be done to optimize the formulation of zein in combination with propylene glycol as suggested by Bai et al. (2003). In addition, a preventative application should be carried out after wounding, that is, by pipetting the spore suspension into the treated wound.
B. amyloliquefaciens CLPs have the potential to control postharvest pathogens when used in a zein edible coating as a carrier. Delivery methods to achieve consistent amounts of active compounds present, and at a larger scale, are needed to improve the ease of CLP application on fruit. New commercial products containing antagonist metabolites or with B. amyloliquefaciens as an active ingredient are needed to control postharvest pathogens, as available products are mostly used for preharvest applications and are not formulated to effectively control the pathogens at the postharvest stage.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
SM conducted the experiments and drafted the manusript. JM-H, RP and CL acquired funding and edited the document. All authors contributed to the article and approved the submitted version.
The work was supported by grant number PO-17-USP-PH41 Hortgro Sciences and Stellenbosch University and a MSc bursary from the National Research Foundation (NRF).
We would like to thank Ms. Elveresha Davids and Ms. Michell Leibrandt for their technical assistance. We are also grateful to Prof. Martin Kidd from the Center for Statistical Analysis at Stellenbosch University for statistical data analysis. Thanks to Ms Sebenzile Mazibuko for optimizing the B. amyloliquefaciens culture conditions. Many thanks also to ICA Chemicals and all participating pome fruit producers and packhouses.
The author JM-H declared that she was an editorial board member of Frontiers, at the time of submission. This had no impact on the peer-review process and the final decision.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fhort.2023.1175251/full#supplementary-material
Ali A., Wei Y. Z., Mustafa M. A. (2015). Exploiting propolis as an antimicrobial edible coating to control post-harvest anthracnose of bell pepper. Packaging Technol. Sci. 28, 173–179. doi: 10.1002/pts.2088
Ambrico A., Trupo M. (2017). Efficacy of cell free supernatant from Bacillus subtilis ET-1, an iturin a producer strain, on biocontrol of green and grey mold. Postharvest Biol. Technol. 134, 5–10. doi: 10.1016/j.postharvbio.2017.08.001
Arrebola E., Sivakumar D., Bacigalupo R., Korsten L. (2010a). Combined application of antagonist bacillus amyloliquefaciens and essential oils for the control of peach postharvest diseases. Crop Protect 29 (4), 369–377. doi: 10.1016/j.cropro.2009.08.001
Arrebola E., Jacobs R., Korsten L. (2010b). Iturin A is the principal inhibitor in the biocontrol activity of Bacillus amyloliquefaciens PPCB004 against postharvest fungal pathogens. Journal of Applied Microbiology 108, 386–395. doi: 10.1111/j.1365-2672.2009.04438.x
Bai J., Alleyne V., Hagenmaier R. D., Mattheis J. P., Baldwin E. A. (2003). Formulation of zein coatings for apples (Malus domestica borkh). Postharvest Biol. Technol. 28 (2), 259–268. doi: 10.1016/S0925-5214(02)00182-5
Barman K., Asrey R., Pal R. K. (2011). Putrescine and carnauba wax pre-treatments alleviate chilling injury, enhance shelf life and preserve pomegranate fruit quality during cold storage. Scientia Hortic. 130 (4), 795–800. doi: 10.1016/j.scienta.2011.09.005
Bordoh P. K., Ali A., Dickinson M., Siddiqui Y. (2020). Antimicrobial effect of rhizome and medicinal herb extract in controlling postharvest anthracnose of dragon fruit and their possible phytotoxicity. Scientia Hortic. 265, 109249. doi: 10.1016/j.scienta.2020.109249
Calvo H., Blanco D., Oria R., Venturini M. E. (2017). Potential of a new strain of Bacillus amyloliquefaciens BUZ-14 as a biocontrol agent of postharvest fruit diseases. Food Microbiol. 63, 101–110. doi: 10.1016/j.fm.2016.11.004
Conway W. S., Leverentz B., Janisiewicz W. J., Blodgett A. B., Saftner R. A., Camp M. J. (2004). Integrating heat treatment, biocontrol and sodium bicarbonate to reduce postharvest decay of apple caused by Colletotrichum acutatum and Penicillium expansum. Postharvest Biol. Technol. 34 (1), 11–20. doi: 10.1016/j.postharvbio.2004.05.011
Dukare A. S., Paul S., Nambi E., Gupta R. K., Sharma R., K. and Vishwakarma K. R. (2018). Exploitation of microbial antagonists for the control of postharvest diseases of fruits: a review. Crit. Rev. Food Sci. Nutr. 1, 1–16. doi: 10.1080/10408398.2017.1417235
Kefi A., Slimene I. B., Karkouch I., Rihouey C., Azaeiz S., Bejaoui M., et al. (2015). Characterization of endophytic Bacillus strains from tomato plants (Lycopersicon esculentum) displaying antifungal activity against Botrytis cinerea pers. World J. Microbiol. Biotechnol. 31 (12), 1967–1976. doi: 10.1007/s11274-015-1943-x
Kourmentza K., Gromada X., Michael N., Degraeve C., Vanier G., Ravallec R., et al. (2021). Antimicrobial activity of lipopeptide biosurfactants against foodborne pathogen and food spoilage microorganisms and their cytotoxicity. Front. Microbiol. 11. doi: 10.3389/fmicb.2020.561060
Lutz M. C., Lopes C. A., Rodriguez M. E., Sosa M. C., Sangorrín M. P. (2013). Efficacy and putative mode of action of native and commercial antagonistic yeasts against postharvest pathogens of pear. Int. J. Food Microbiol. 164 (2-3), 166–172. doi: 10.1016/j.ijfoodmicro.2013.04.005
Mazibuko S. (2018). Purification of bacillus amyloliquefaciens lipopeptides for postharvest disease control (Stellenbosch University MSc thesis). Available at: http://hdl.handle.net/10019.1/103613.
Nascimento F. V., Almeida G. K., Silva S. J. N., Bender R. J. (2016). “Coatings based on chitosan and carnauba wax for postharvest use on 'Rocha' pears,” in VIII International Postharvest Symposium: Enhancing Supply Chain and Consumer Benefits-Ethical and Technological Issues, Vol. 1194. 283–288. doi: 10.17660/ActaHortic.2018.1194.41
Nunes C. A. (2012). Biological control of postharvest diseases of fruit. Eur. J. Plant Pathol. 133, 181–196. doi: 10.1007/s10658-011-9919-7
Ongena M., Jacques P. (2008). Bacillus cyclolipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol. 16, 115–125. doi: 10.1016/j.tim.2007.12.009
Ongena M., Jacques P., Touré Y., Destain J., Jabrane A., Thonart P. (2005). Involvement of fengycin-type cyclolipopeptides in the multifaceted biocontrol potential of Bacillus subtilis. appl. Microbiol. Biotechnol. 69 (1), 29–38. doi: 10.1007/s00253-005-1940-3
Pérez-García A., Romero D., de Vincente A. (2011). Plant protection and growth stimulation by microorganisms: biotechnological applications of bacilli in agriculture. Curr. Opin. Biotechnol. 22, 187–193. doi: 10.1016/j.copbio.2010.12.003
Pretorius D., Van Rooyen J., Clarke K. G. (2015). Enhanced production of antifungal lipopeptides by Bacillus amyloliquefaciens for biocontrol of postharvest disease’. N. Biotechnol. 32 (2), 243–252. doi: 10.1016/j.nbt.2014.12.003
Qu H., Zhao L., Zhao F., Liu Y., Yang Z. (2016). Biocontrol of gray mold decay in pear by Bacillus amyloliquefaciens strain BA3 and its effect on postharvest quality parameters. Pol. J. Microbiol. 65 (2), 171–176. doi: 10.5604/17331331.1204476
Russouw A., Meitz-Hopkins J., Den Breeyen A., Lennox C. (2021). Postharvest applications of fludioxonil and pyrimethanil to control Phlyctema vagabunda on apple in south Africa. Crop Protect. 141, 105451. doi: 10.1016/j.croppro.2020.105451
Spadaro D., Droby S. (2016). Development of biocontrol products for postharvest diseases of fruit: the importance of elucidating the mechanisms of action of yeast antagonists. Trends Food Sci. Tech. 47, 39–49. doi: 10.1016/j.tifs.2015.11.003
Tan W., Yin Y., Wen J. (2022). Increasing fengycin production by strengthening the fatty acid synthesis pathway and optimising fermentation conditions. Biochem. Eng. J. 177, 108235. doi: 10.1016/j.bej.2021.108235
Touré Y., Ongena M. A. R. C., Jacques P., Guiro A., Thonart P. (2004). Role of lipopeptides produced by Bacillus subtilis GA1 in the reduction of grey mould disease caused by Botrytis cinerea on apple. J. Appl. Microbiol. 96 (5), 1151–1160. doi: 10.1111/j.1365-2672.2004.02252
Waewthongrak W., Leelasuphakul W., McCollum G. (2014). Cyclic lipopeptides from Bacillus subtilis ABS–S14 elicit defense-related gene expression in citrus fruit. PloS One 9, (10). doi: 10.1371/journal.pone.0109386
Wibowo C., Haryanti P., Wicaksono R. (2021). “Effect of edible coating application by spraying method on the quality of red chili during storage,” in IOP Conference Series: Earth and Environmental Science, Vol. 74. 012004. doi: 10.1088/1755-1315/746/1/012004
Keywords: Bacillus amyloliquefaciens, alternative control, fruit decay, fungicides, postharvest treatment
Citation: Magwebu S, Meitz-Hopkins JC, Pott RWM and Lennox CL (2023) Efficacy of the cyclolipopeptides fengycin and iturin A against postharvest pome fruit pathogens. Front. Hortic. 2:1175251. doi: 10.3389/fhort.2023.1175251
Received: 27 February 2023; Accepted: 19 May 2023;
Published: 06 June 2023.
Edited by:
Mathias Twizeyimana, AgBiome Inc., United StatesReviewed by:
Asgar Ali, University of Nottingham Malaysia Campus, MalaysiaCopyright © 2023 Magwebu, Meitz-Hopkins, Pott and Lennox. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julia C. Meitz-Hopkins, anVsaWFtQHN1bi5hYy56YQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.