- 1School of Studies in Botany, Jiwaji University, Gwalior, India
- 2Pharmacognosy Division, CSIR—National Botanical Research Institute, Lucknow, India
Marigold (Tagetes erecta L.), a popular ornamental plant of the family Asteraceae, is commonly cultivated in many countries, including India, for its decorative flowers. The plants grow easily in a variety of soil and climatic conditions and have been reported to damage the nematode population of soil and control indirectly harmful microbes. High-performance thin-layer chromatography (HPTLC) was utilized in the present study, with a view to identify some important biologically active compounds in the flowers and leaves of two cultivars of marigold, Pusa Narangi Gainda (PNG) and Pusa Basanti Gainda (PBG). Quantitative analyses were carried out using silica gel thin-layer chromatography (TLC) plates and toluene–ethyl acetate–formic acid (T-E-F) (13:11:2 v/v/v) as the mobile phase used. Bands of gallic acid, caffeic acid, quercetin, p-coumaric acid, and kaempferol were observed. The results revealed a greater number of compounds in leaves than in flowers, and that the cultivar PNG accumulated a greater number of compounds than PBG. Gallic acid was found in leaves and flowers of both cultivars; however, it was found maximum in the flowers of cultivar PBG. Caffeic acid and quercetin were detected in the leaves of both cultivars, whereas p-coumaric acid was detected only in the leaves and kaempferol only in the flowers of cultivar PNG. The information generated in this report may be meaningfully used for the furtherance of research on marigolds as a natural source of antioxidants, insecticides, herbicides, etc.
Introduction
Marigolds (Tagetes erecta L.), locally known as ‘Gainda’ in India belonging to the family Asteraceae, are native to Mexico, but numerous varieties of T. erecta have naturalized in both the tropics and subtropics of the world, including in India and Bangladesh (Nikkon et al., 2009). T. erecta has several medicinal properties: the leaves are reported to be effective against piles, kidney problems, muscular pain, ulcers, wounds, etc. (Priyanka et al., 2013), and the flowers are reported to have astringent and carminative properties and to be effective against epileptic fits, fever, scabies, stomach and liver troubles, and eye problems (Kirtikar and Basu, 1987; Priyanka et al., 2013). Studies have revealed the presence of polyphenolic compounds in Tagetes have pharmacological properties, such as antifungal, antibacterial, antioxidant, and anticoagulative effects (Dasgupta et al., 2012; Gong et al., 2012; Dasgupta et al., 2016), and potentially with cytotoxic properties (Burlec et al., 2021). The dietary lutein obtained from marigold extract improves immune function (Chew et al., 1996), reducing the risk of many life-threatening diseases such as age-related muscular degeneration, cancer, and diseases related to the heart (Wang et al., 2006).
The cultivars of T. erecta used in the present study are Pusa Narangi Gainda (PNG) and Pusa Basanti Gainda (PBG), which are locally developed at the Indian Agricultural Research Institute (IARI) in New Delhi (Raghava et al., 2013). The PNG cultivar, whose plants have dark-green foliage and orange flowers, is a cross between ‘Cracker Jack’ and ‘Golden Jubilee’, whereas the PBG cultivar is a cross between ‘Golden Yellow’ and ‘Sun Giant’, having plants with dark-green foliage and sulfur yellow flowers (Raghava et al., 2013). The chemical composition and estimated antioxidant and antimicrobial activities of essential oils in the PNG cultivar have been reported by Tripathi et al. (2012). However, there are hardly any reports available on the analysis of polyphenolic compounds using high-performance thin-layer chromatography (HPTLC) in these two local cultivars of T. erecta.
In addition to its pharmacological importance, in India the marigold is commercially used in all kinds of rituals, such as the decoration of mandaps, temples, marriage parties, and cars, because of its colorful flowers of different sizes (Mir et al., 2019; Mir et al., 2021). Currently, an area of 191,000 hectares is under horticulture, with an annual production of 1,031,000 metric tonnes of loose flowers and 69,027 lakh cut flowers in India (Kumar et al., 2011), and marigold ranks first among the loose flowers. The area used for marigold cultivation is 43,000 ha, with a production of 360,000 metric tonnes (Tiwari et al., 2013). The flowers after use in various social and religious occasions may be disposed of in agricultural land. The phytochemicals released after the degradation of the plant tissue may affect the crops grown/growing there. It is against this background that the present study was designed to analyze polyphenolic compounds/phytochemicals in different tissues of Tagetes erecta L.
HPTLC is a popular analytical technique with the capability of analyzing several samples at a time. In addition, it offers high sample throughput at low operating cost, easy preparation of samples, and a short analysis time (Dixit et al., 2017; Hakim et al., 2017; Panigrahi et al., 2017). Hence, the present study was performed to evaluate certain important polyphenolic compounds, that is, gallic acid, caffeic acid, quercetin, p-coumaric acid, and kaempferol, in the leaves and flowers of T. erecta (cultivars PNG and PBG) using HPTLC. This study could be advantageous to the pharmaceutical industries, while screening and selecting materials for specific uses and identifying compounds of allelopathic importance.
Materials and methods
Plant materials, chemicals and reagents
Certified seeds of two marigold (T. erecta) cultivars, PNG and PBG, were obtained from IARI, New Delhi, and grown in the botanical garden of the School of Studies in Botany, Jiwaji University, Gwalior, India. Soil (sandy clay loam) was prepared by the addition of 30 tonnes of well-decomposed farmyard manure (FYM), 120 kg of nitrogen, and 80 kg each of phosphorus and potash per hectare (Raghava et al., 2013). Subsequently, seed beds (0.762 m × 1.066 m) were prepared and seeds sown. After 1 month the seedlings were transplanted in pots, with an area of 0.0962 m2 one seedling in each pot. At flowering stage [110 days after sowing (DAS)] plant material (leaves and flowers) of both cultivars was collected and dried at 70°C for 48 hours. The dried plant material was ground into a fine powder using a mechanical grinder. The reference markers gallic acid, caffeic acid, quercetin, p-coumaric acid, and kaempferol were procured from Sigma-Aldrich (Mumbai, India). The analytical-grade reagents and solvents used in the present study were procured from SD Fine Chemicals (Mumbai, India).
Sample preparation
The sample preparation was done following Dixit et al. (2017). Four independent replicates of each cultivar were processed and analyzed. Leaves and flowers of both cultivars were ground separately into a coarse powder, and 5 g of each ground sample was placed in a 100-mL volumetric flask, to which 25 mL of 50% hydroethanol was then added; thereafter the flask was shaken for 2 hours at shaker speed and left overnight. Whatman No 1 filter paper was used to filter the extracts. The procedure was repeated three times at room temperature (i.e., 25°C ± 2°C). The collected extracts were evaporated/concentrated at 50°C. Each concentrated/dried extract (10 mg) was dissolved in 1.0 mL of methanol and filtered using a 0.45-μm filter membrane, and the filtrates were used as sample solutions. The 1.0-mg chemical reference substance (i.e., gallic acid, caffeic acid, quercetin, p-coumaric acid, and kaempferol) was dissolved in 1 mL of methanol to obtain a stock solution for thin-layer chromatography (TLC) analysis.
Chromatography
HPTLC was performed using 0.2-mm silica gel TLC plates (20 cm × 10 cm) containing ultraviolet (UV) 254 fluorescent indicators (Aluchrosep nanosilica, G/UV254; SD Fine Chemicals Ltd, Mumbai, India) and 100-μL Hamilton syringes with Linomat 5 applicators (CAMAG®, Muttenz, Switzerland) under N2 gas flow for sample applications. Scanning was done using a TLC Scanner 3 (CAMAG) at a λmax wavelength of 300 nm in UV absorbance mode, which was operated by winCATS software (version 3.2.1; CAMAG). Each sample of plant extract (10 μL), along with a mixture of different standard markers (10 μL), was applied to the TLC plate. The plate was developed with 20 mL of mobile phase [toluene–ethyl acetate–formic acid (T-E-F) (13:11:2 v/v/v] in a CAMAG twin-trough chamber to a distance of 8 cm from the lower edge of the plate. The chamber was already saturated with mobile phase vapor for 30 minutes, maintaining a temperature of 25°C ± 2°C and a relative humidity of 40%. The plate was dried after the removal from the chamber at room temperature. Densitometric scanning was performed at a wavelength of 300 nm, and photographs were captured using a CAMAG Reprostar 3 video documentation unit with light of wavelengths 254 and 366 nm.
Standard markers (0.1 μg/mL), that is, caffeic acid, gallic acid, quercetin, kaempferol (each 100–500 ng per spot), and p-coumaric acid (200–600 ng per spot), were applied on TLC plates and chromatographed, and linear ascending development was carried out.
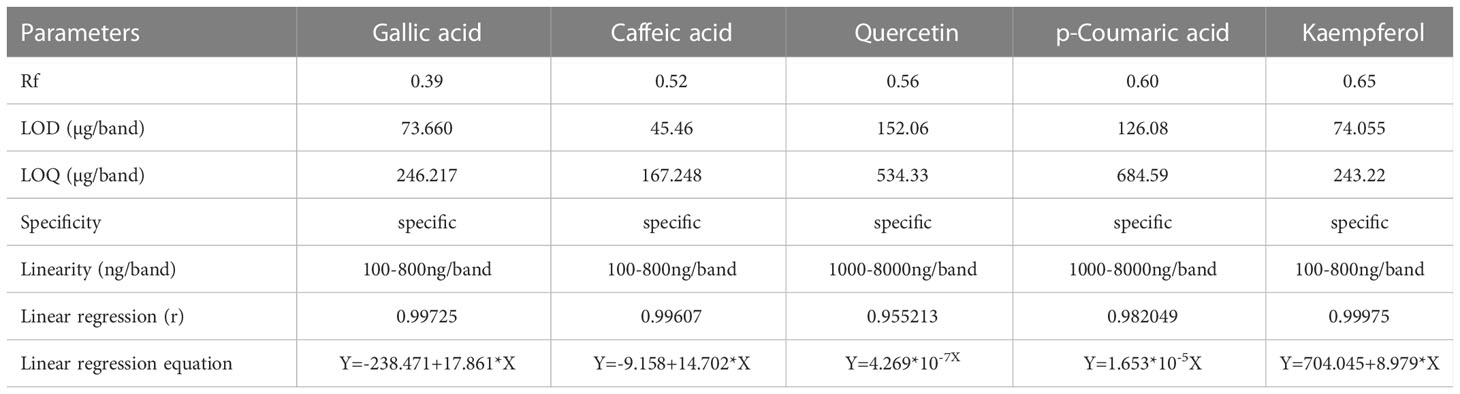
The International Conference on Harmonization guidelines (Anonymous, 2005) were followed for the validation parameters of the quantitative HPTLC analysis, that is, precision, linearity, and specificity, limit of detection (LOD), and limit of quantification (LOQ).
Statistical analysis
To ensure reproducibility, each experiment was conducted three times. Data are presented as the mean ± standard error of the three values. The average content was calculated as the mean of three different readings in the extracts obtained from two different cultivars and plant parts of Tagetes erecta, and the results were subjected to Pearson correlation analyses to identify relationships among the quantified markers.
Results and discussion
Chromatographic studies
In the HPTLC analysis, all compounds were resolved with reproducibility of peaks with the optimized mobile phase of T-E-F (13:11:2 v/v/v). Marker compounds, that is, gallic acid, caffeic acid, quercetin, p-coumaric acid, and kaempferol (Figure S1) in plant samples were identified on the basis of similarity of colors and the Rf values of the bands. Rf values are a prerequisite for HPTLC analysis of phytochemicals and indicate whether the analytes prefer stationary or mobile phases. The values are used to determine the polarity, relative mass, and relative solubility of different analytes: high Rf values indicate low polarity and vice versa. In the present study, gallic acid, caffeic acid, quercetin, p-coumaric acid, and kaempferol were resolved at an Rf value of 0.39, 0.52, 0.56, 0.60, and 0.65, respectively (Figure 1). Densitometric scans of the extract samples and of the biomarkers are shown in Figure 2. Quantification of the polyphenolic compounds was carried out by calculating the area of the resolved peaks, using the calibration curve (Figure 3). The peak purity of gallic acid, caffeic acid, quercetin, p-coumaric acid, and kaempferol was assessed by spiking and comparing the spectra of samples with the standard at three different points on the peak, namely the start (S), the maximum (M), i.e., the apex, and the end (E). The quantity of the compounds detected in sample extracts of different parts (leaves and flowers) of the two marigold cultivars is shown in Table 1, and the optimization and validation of the parameters are shown in Table 2.
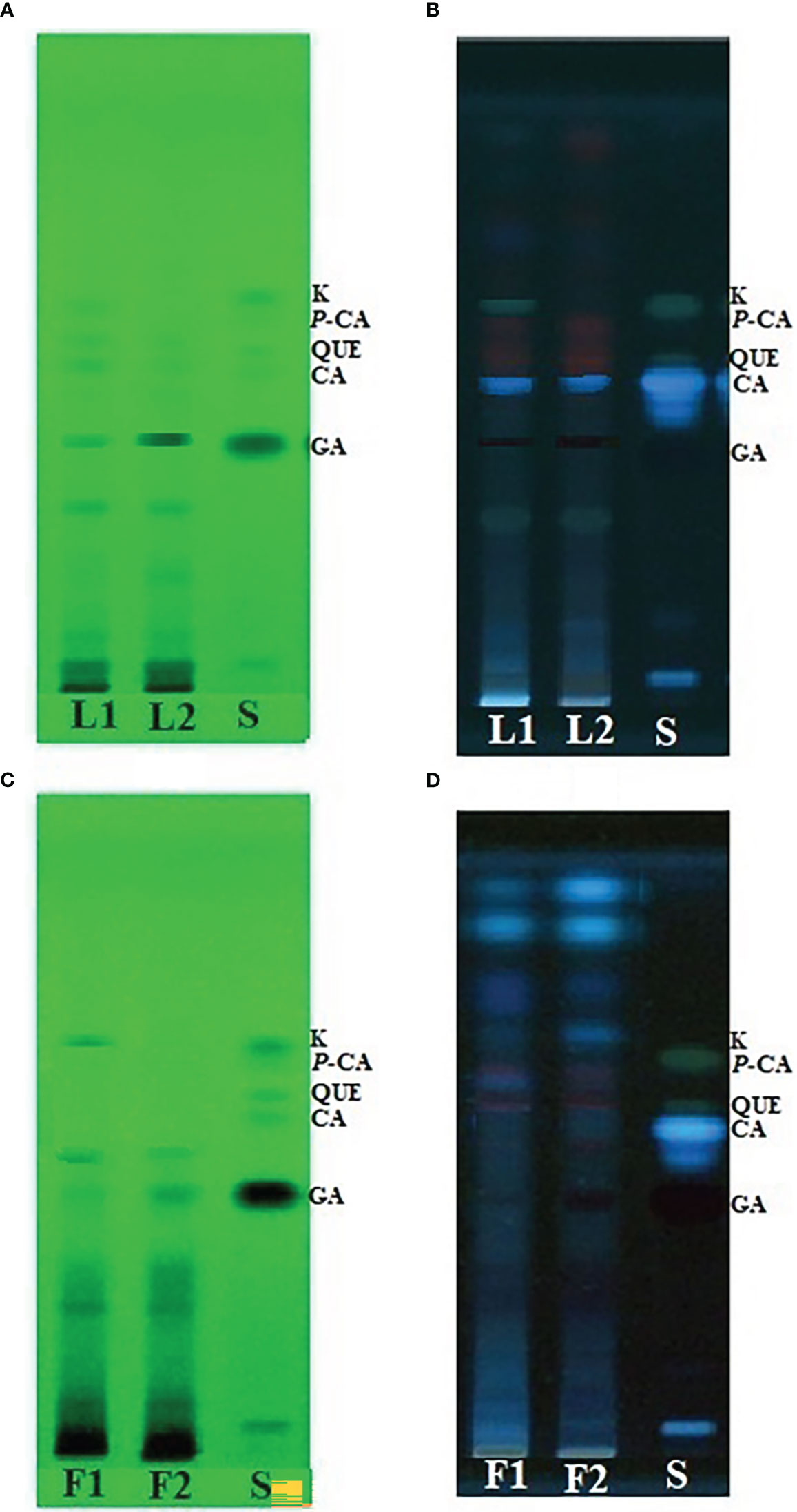
Figure 1 High-performance thin-layer chromatography (HPTLC) fingerprint profile of two cultivars (PNG and PBG) of Tagetes erecta L. visualized at (A, C) UV 254 nm and (B, D) at UV 366 nm. Abbreviations: PBG, Pusa Basanti Gainda; PNG, Pusa Narangi Gainda. L1, leaf extract of PNG; L2, leaf extract of PBG; F1, flower extract of PNG; F2, flower extract of PBG; S, mixture of gallic acid (GA), caffeic acid (CA), quercetin (Q), p-coumaric acid (P-CA), and kaempferol (K) standard markers.
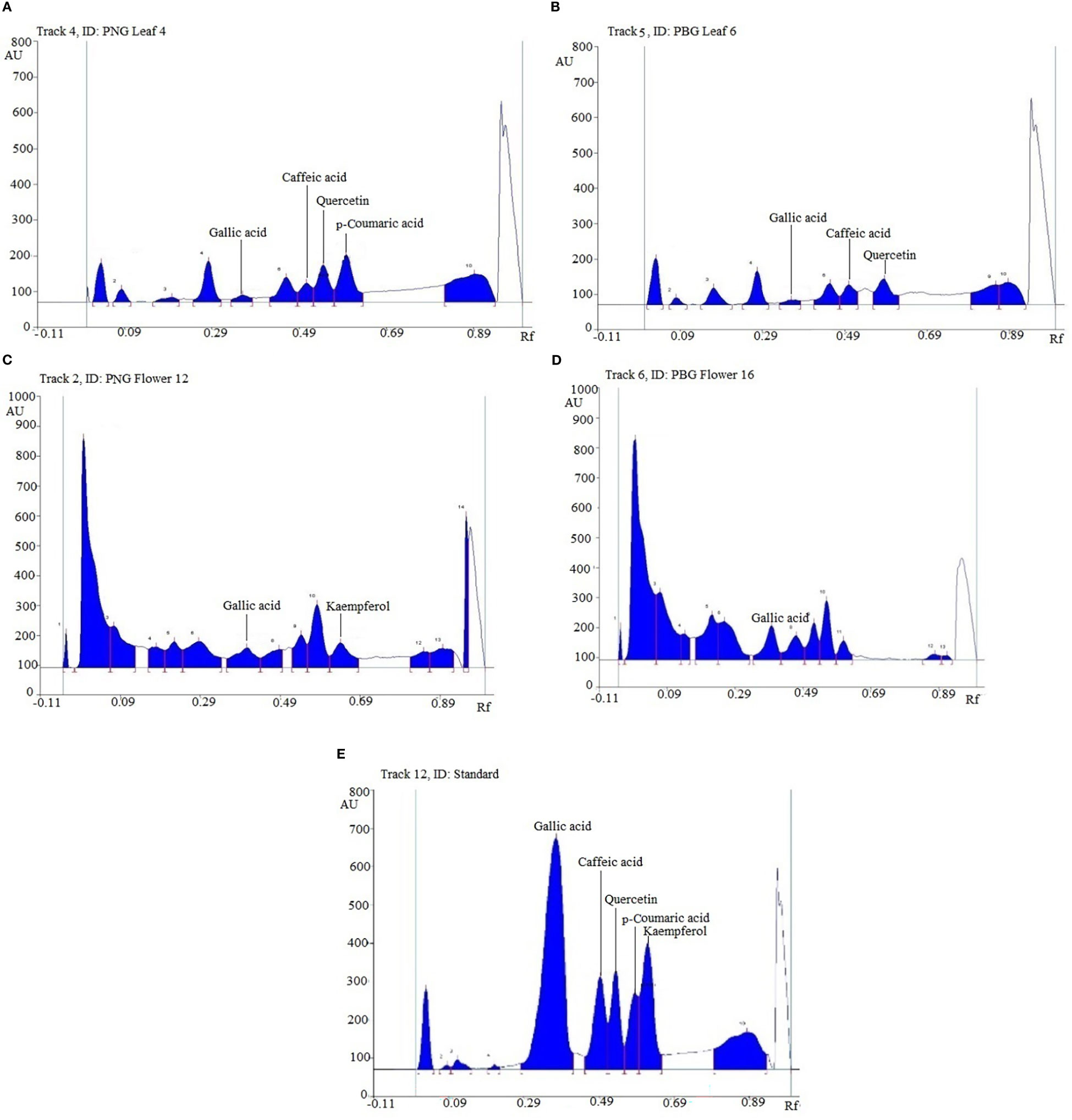
Figure 2 Densitometric scanning profile at the wavelength of 300 nm of Tagetes erecta L. (A) Leaf extract of PNG cultivar, (B) leaf extract of PBG cultivar, (C) flower extract of PNG cultivar, (D) flower extract of PBG cultivar, and (E) standards. PBG, Pusa Basanti Gainda; PNG, Pusa Narangi Gainda.
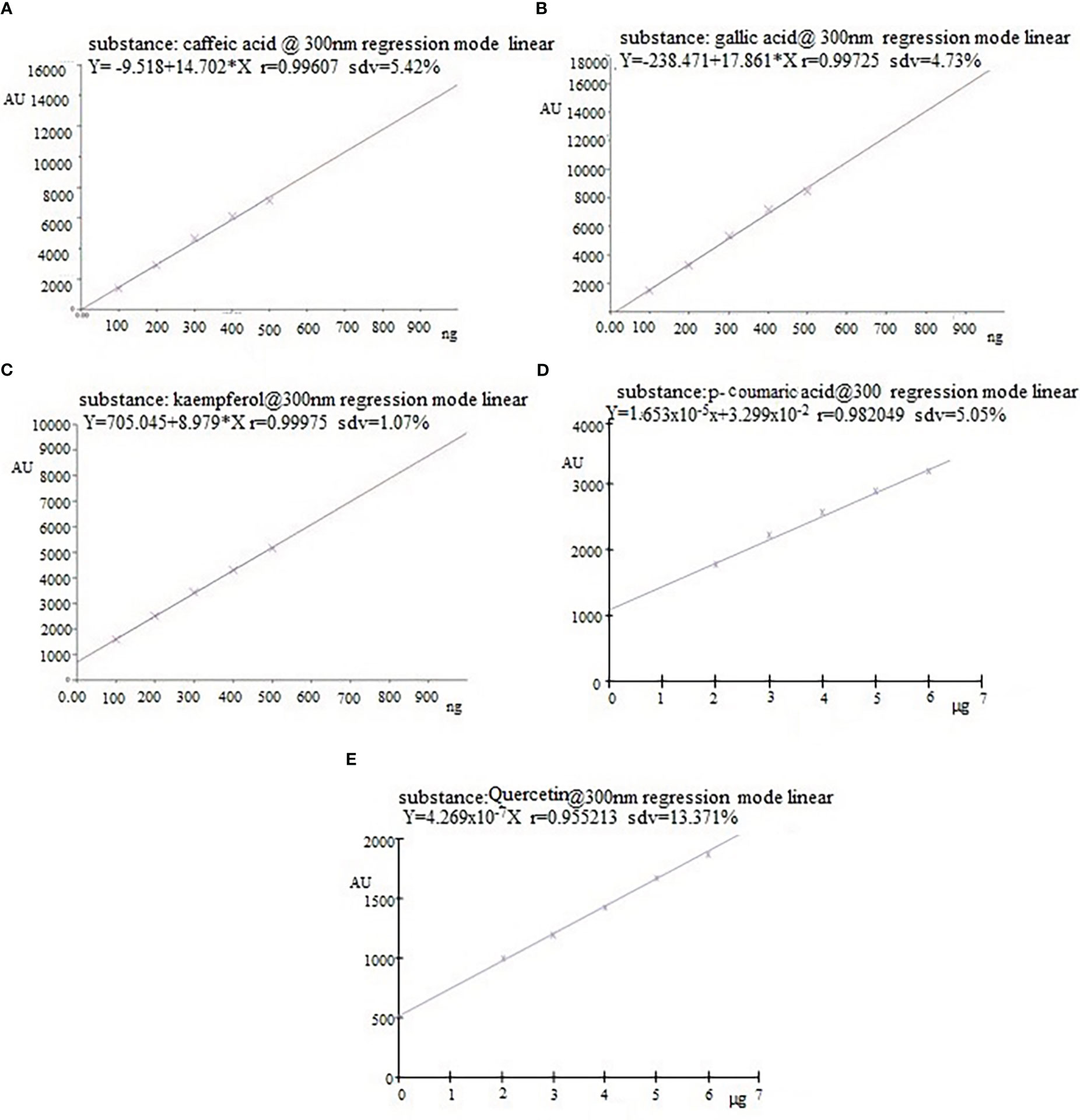
Figure 3 Linear regression graph of phenolic compounds: (A) caffeic acid, (B) gallic acid, (C) kaempferol, (D) p-coumaric acid, and (E) quercetin.
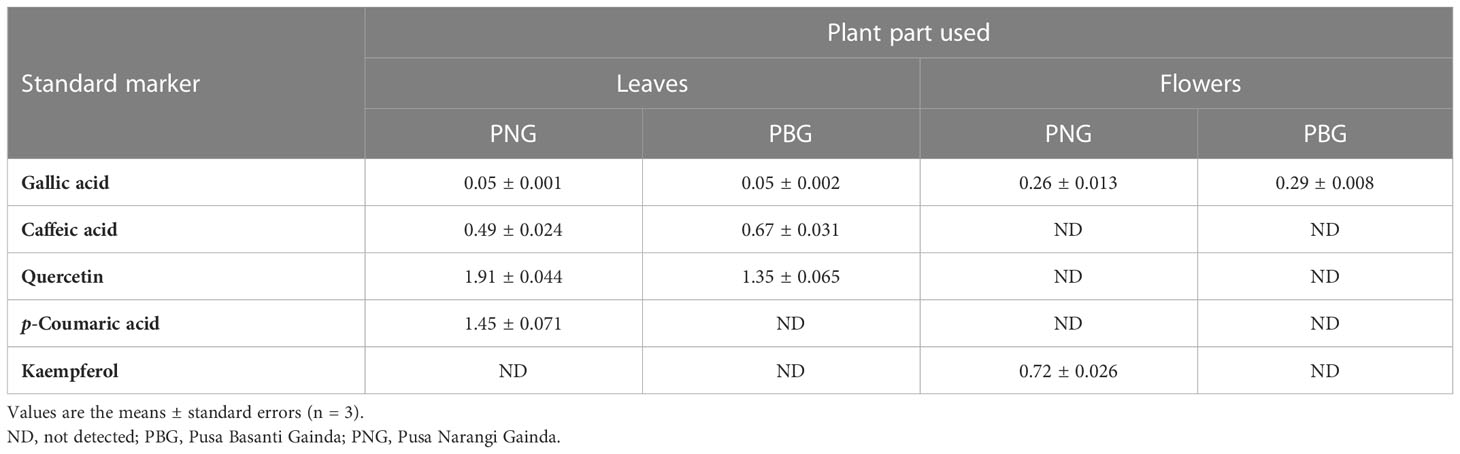
Table 1 Polyphenolic compounds (mg/g dry weight) in different plant parts of Tagetes erecta L. cultivars PNG and PBG.
Chemical variation in different cultivars and parts of Tagetes erecta L.
The HPTLC fingerprint profile showed quantitative differences in polyphenolic compounds among cultivars and different plant parts of T. erecta. Such variations have also been reported previously in other plants, such as the leaves, stem bark, and fruits of Juglans regia L. (Khatoon et al., 2016); different species of Leucas (Dixit et al., 2017); the root, stem, and leaves of marigold (Mir et al., 2018; Mir et al., 2021); and the flowers of Tagetes patula ‘Petite Gold’ and ‘Petite Orange’ (Krzymińska et al., 2022). In plants, phenolic compounds are responsible for defense and acceleration of pollination and coloring for camouflage (Lin et al., 2016). A large number of plant phenolics have been investigated in different plant species as antioxidants and free radical scavengers (Orcic et al., 2011; Youssef et al., 2020). In humans, phenolics play important roles in antioxidant, anti-aging, anti-inflammatory, and antiproliferative processes, reducing the incidence of various life-threatening diseases such as diabetes, cancers, and cardiovascular disease (Lin et al., 2016).
Flowers of PBG showed greater quantities of gallic acid (see Table 1) than those of PNG. Gallic acid is a naturally occurring polyphenolic compound/secondary metabolite that is widely distributed in plants, vegetables, and fruits, and it possesses antioxidant, anticarcinogenic, antimicrobial, and anti-inflammatory properties. It also has multiple industrial uses, e.g., as a food supplement and preservative (Yang et al., 2020). Caffeic acid, quercetin, and p-coumaric acid were observed in the leaves of both PNG and PBG marigold cultivars (Table 1). Caffeic acid, quercetin, and p-coumaric acid are known to have many health benefits because of their capacity to scavenge free radicals (i.e., their antioxidant properties). Caffeic and p-coumaric acid also have potential pharmacological uses because of their anti-inflammatory, antimicrobial, and neuroprotective effects (Ferreira et al., 2018; Cizmarova et al., 2020). Similarly, quercetin shows beneficial effects against Alzheimer’s disease and also possesses antioxidant, antifungal, anticancer, hepatoprotective, and cytotoxic properties (Batiha et al., 2020). Kaempferol was found only in the floral extracts of the PNG cultivar (Table 1). Kaempferol is a plant-derived flavonoid (antioxidant) that has several pharmacological benefits, which include anticancer, antidiabetic, cardioprotective, neuroprotective, and antimicrobial properties (Cid-Ortega and Monroy-Rivera, 2018). Plant phenolics have also been reported to play a role in allelopathy; there is some correlation between plant allelopathy and polyphenols, especially gallic acid and p-coumaric acid (Li et al., 2010; Kulbat, 2016). The Pearson correlation results indicated that there is a moderately positive but non-significant relationship between PNG and PBG; the five quantified markers with (r) = 0.478, (P) = 0.415 are shown in Figure S2. The polyphenolic compounds analyzed in the present study are considered to be of medicinal as well as allelopathic importance.
Conclusion
This article describes HPTLC analysis for the concurrent estimations of gallic acid, caffeic acid, quercetin, p-coumaric acid, and kaempferol from the leaves and flowers of two cultivars of T. erecta. The method used is relatively simple, sensitive, economical, and suitable for rapid screening. We found that the quantities of the polyphenolic compounds we analyzed in the study showed variation between plant parts (leaves and flowers) and between cultivars (PNG and PBG), which may account for differences in the medicinal properties of these plant parts and cultivars. These compounds may also contribute to the allelopathic activity of T. erecta. The study will help further explore the medicinally potential cultivar(s) of Tagetes for pharmacological activities. The information generated in this study could be very important because the application of naturally occurring bioactive compounds is currently attracting a great deal of attention.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
RA designed the experiment. RM carried out the experiment and wrote the first draft of the manuscript. SA helped in the literature survey and the experimentation. SI helped in manuscript preparation by revising critically for some important content. SK helped in the analysis and interpretation of data and the final approval of the version to be submitted. All authors contributed to the article and approved the submitted version.
Acknowledgments
Authors are highly thankful to Head, School of Studies in Botany, Jiwaji University, Gwalior, for providing the necessary facilities and Director, CSIR-National Botanical Research Institute, Lucknow, for the HPTLC facilities. Grateful thanks are also due to IARI, New Delhi, for providing certified seeds of Tagetes erecta L. cultivars.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fhort.2023.1120267/full#supplementary-material
Supplementary Figure 1 | The chemical structure of (A) caffeic acid, (B) gallic acid, (C) kaempferol, (D) p-coumaric acid, and (E) quercetin.
Supplementary Figure 2 | Linear correlation graph of two marigold cultivars—Pusa Narangi Gainda (PNG) and Pusa Basanti Gainda (PBG).
References
1. Anonymous (2022). International Conference on Harmonization (ICH) Guidelines, Q2 (R1) Validation of Analytical Procedures: Text and Methodology, ICH Harmonized Tripartite Guideline, Geneva.
Batiha G. E., Beshbishy A. M., Ikram M., Mulla Z. S., El-Hack M. E. A., Taha A. E., et al. (2020). The pharmacological activity, biochemical properties, and pharmacokinetics of the major natural polyphenolic flavonoid: Quercetin. Foods 9, 374. doi: 10.3390/foods9030374
Burlec A. F., Pecio L., Kozachok S., Mircea C., Corciova A., Vereştiuc L., et al. (2021). Phytochemical profile, antioxidant activity, and cytotoxicity assessment of Tagetes erecta l. flowers. Molecules 26 (5), 1201. doi: 10.3390/molecules26051201
Chew B. P., Wong M. W., Wong T. S. (1996). Effects of lutein from marigold extract on immunity and growth of mammary tumors in mice. Anticancer Res. 16, 3689–3694.
Cid-Ortega S., Monroy-Rivera J. A. (2018). Extraction of kaempferol and its glycosides using supercritical fluids from plant sources: a review. Food Technol. Biotechnol. 56, 480–493. doi: 10.17113/ftb.56.04.18.5870
Cizmarova B., Hubkova B., Bolerazska B., Marekova M., Birkova A. (2020). Caffeic acid: A brief overview of its presence, metabolism, and bioactivity. Bioact. Compd. Health Dis. 3, 74–81. doi: 10.31989/bchd.v3i4.692
Dasgupta N., Ranjan S., Saha P., Jain R., Malhotra S., Saleh M. M. (2012). Antibacterial activity of leaf extract of Mexican marigold (Tagetes erecta) against different gram positive and gram negative bacterial strains. J. Pharm. Res. 5, 4201–4203.
Dasgupta N., Ranjan S., Shree M., Saleh M. A. A. M., Ramalingam C. (2016). Blood coagulating effect of marigold (Tagetes erecta l.) leaf and its bioactive compounds. Orient. Pharm. Exp. Med. 16, 67–75. doi: 10.1007/s13596-015-0200-z
Dixit V., Irshad S., Singh H., Agnihotri P., Husain T., Khatoon S. (2017). HighPerformance thin-layer chromatographic determination of three therapeutic phenolic components in Leucas species. J. Planar. Chromatogr. Mod. TLC. 30, 25–31. doi: 10.1556/1006.2017.30.1.3
Ferreira P. S., Victorelli F. D., Fonseca-Santos B., Chorilli M. (2018). A review of analytical methods for p-coumaric acid in plant-based products, beverages, and biological matrices. Crit. Rev. Anal. Chem. 49, 21–31. doi: 10.1080/10408347.2018.1459173
Gong Y., Liu X., He W. H., Xu H. G., Yuan F., Gao Y. X. (2012). Investigation into the antioxidant activity and chemical composition of alcoholic extracts from defatted marigold (Tagetes erecta l.) residue. Fitoterapia 83, 481–489. doi: 10.1016/j.fitote.2011.12.013
Hakim M., Rathod D., Panigrahi J., Gantait S., Trivedi D. A., Patel I. C. (2017). High performance thinlayer chromatographic quantification of key cholesterol reducing compound (β-sitosterol) fromleaf, bark, fruit and root of Terminalia arjuna, T. bellerica and T. chebula. Med. Plants 9, 272–278. doi: 10.5958/0975-6892.2017.00044.2
Khatoon S., Singh S., Singh A. P., Singh N., Rawat A. K. S. (2016). Chemical evaluation of different parts of Juglans regia l. and simultaneous determination of important polyphenols by thin layer chromatography. Int. J. Res. Pharm. Chem. 6, 773–781.
Krzymińska A., Frąszczak B., Gąsecka M., Magdziak Z., Kleiber T. (2022). The content of phenolic compounds and organic acids in two Tagetes patula cultivars flowers and its dependence on light colour and substrate. Molecules 27 (2), 527. doi: 10.3390/molecules27020527
Kulbat K. (2016). The role of phenolic compounds in plant resistance. Biotechnol. Food. Sci. 80, 97–108.
Kumar B., Mistry N. C., Singh B., Gandhi C. P. (2011). Indian Horticulture Database- National Horticulture Board (NHB), Gurgaon. (Department of Agriculture and Cooperation, Govt. of India). 1–296. Available at: http://www.nhb.gov.in.
Li Z. H., Wang Q., Jiang D. A. (2010). Phenolics and plant allelopathy. Molecules 15, 8933–8952. doi: 10.3390/molecules15128933
Lin D., Xiao M., Zhao J., Li Z., Xing B., Li X., et al. (2016). An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 21, 1–19. doi: 10.3390/molecules21101374
Mir R. A., Ahanger M. A., Agarwal R. M. (2019). Marigold: From mandap to medicine and from ornamentation to remediation. Am. J. Plant Sci. 10, 309–338. doi: 10.4236/ajps.2019.102024
Mir R. A., Argal S., Agarwal R. M. (2018). Accumulation of secondary metabolites and osmotica in different parts of Tagetes erecta l. and its ecophysiological relevance. Int. J. Sci. Res. Rev. 7, 198–209.
Mir R. A., Argal S., Ahanger M. A., Tomar N. S., Agarwal R. M. (2021). Variation in phenolic compounds, antioxidant activity and osmotica of different cultivars of Tagetes erecta l. at different growth stages and effect of its leachates on germination and growth of wheat (Triticum aestivum l.). J. Plant Growth Regul. 41, 907–921. doi: 10.1007/s00344-021-10348-9
Nikkon F., Habib M. R., Saud Z. A. (2009). Toxicological evaluation of chloroform fraction of flower of Tagetes erecta l. @ on rats. Int. J. Drug Dev. Res. 1, 161–165.
Orcic D. Z., Mimica-Dukic N. M., Franciskovic M. M., Petrovic S. S., Jovin E. D. (2011). Antioxidant activity relationship of phenolic compounds in Hypericum perforatum l. Chem. Cent. J. 5, 1–8. doi: 10.1186/1752-153X-5-34
Panigrahi J., Gantait S., Patel I. C. (2017). Concurrent production and relative quantification of vasicinone from in vivo and in vitro plant parts of malabar nut (Adhatoda vasica nees). 3 Biotech. 7, 280. doi: 10.1007/s13205-017-0882-7
Priyanka D., Shalini T., Navneet V. K. (2013). A brief study on marigold (Tagetes species): A review. Int. Res. J. Pharm. 4, 3–48.
Raghava S. P. S., Singh K. P., Dantuluri V. S. R. (2013). “Marigold,” in Ornamental plants for gardening. Eds. Chopra ,. V. L., Singh M. (New Delhi) India: Scientific publishers), 257–267.
Tiwari R. K., Mistry N. C., Singh B., Gandhi C. P. (2013). Indian Horticulture Database- National Horticulture Board, Gurgaon, Ministry of Agriculture, Govt of India. IG Printer Pvt. Ltd. DSIDC, Okhla Phase-I, New Delhi. Available at: http://www.nhb.gov.in.
Tripathi B., Bhatia R., Walia S., Kumar B. (2012). Chemical composition and evaluation of Tagetes erecta (Var. pusa narangi genda) essential oil for its antioxidant and antimicrobial activity. Biopestic. Int. 8, 138–146.
Wang M. C., Tsao R., Zhang S. F., Dong Z. M., Yang R., Gong J. H., et al. (2006). Antioxidant activity, mutagenicity/anti-mutagenicity and clastogenicity/anti-clastogenicity of lutein from marigold flowers. Food Chem. Toxicol. 44, 1522–1529. doi: 10.1016/j.fct.2006.04.005
Yang K., Zhang L., Liao P., Xiao Z., Zhang F., Sindaye D., et al. (2020). Impact of Gallic acid on gut health: Focus on gut microbiome, immune response, and mechanism of action. Front. Immunol. 11, 580208. doi: 10.3389/fimmu.2020.580208
Keywords: allelopathy, high-performance thin-layer chromatography, medicinal property, polyphenols, Pusa Basanti Gainda (PBG), Pusa Narangi Gainda (PNG)
Citation: Mir RA, Irshad S, Argal S, Agarwal RM and Khatoon S (2023) Quantitative analysis of polyphenolic compounds in two different cultivars of marigold (Tagetes erecta L.) using high-performance thin-layer chromatography. Front. Hortic. 2:1120267. doi: 10.3389/fhort.2023.1120267
Received: 09 December 2022; Accepted: 03 February 2023;
Published: 27 February 2023.
Edited by:
Daniela Romano, University of Catania, ItalyReviewed by:
Jitendriya Panigrahi, Shri Alpesh N Patel PG Institute of Science and Research Anand, IndiaMarco Devecchi, University of Turin, Italy
Copyright © 2023 Mir, Irshad, Argal, Agarwal and Khatoon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rayees Ahmad Mir, cmF5ZWVzbWlyODlAZ21haWwuY29t
 Rayees Ahmad Mir
Rayees Ahmad Mir Saba Irshad2
Saba Irshad2 Surendra Argal
Surendra Argal