- 1Department of Neurology, Mayo Clinic, Rochester, MN, United States
- 2Center for Multiple Sclerosis and Autoimmune Neurology, Mayo Clinic, Rochester, MN, United States
- 3Department of Radiology, Mayo Clinic, Rochester, MN, United States
- 4Women’s Health Research Center, Mayo Clinic, Rochester, MN, United States
Clear sex differences are observed in clinical and imaging phenotypes of multiple sclerosis (MS), which evolve significantly over the age spectrum, and more specifically, during reproductive milestones such as pregnancy and menopause. With neuroimaging being an outcome measure and also a key subclinical biomarker of subsequent clinical phenotype in MS, this comprehensive review aims to provide an overview of sex and hormone differences in structural and functional imaging biomarkers of MS, including lesion burden and location, atrophy, white matter integrity, functional connectivity, and iron distribution. Furthermore, how therapies aimed at altering sex hormones can impact imaging of women and men with MS over the lifespan is discussed. This review also explores the key intersection between age, sex, and race/ethnicity in MS, and how this intersection may affect imaging biomarkers of MS.
1 Introduction
There are clinical sex differences affecting aspects of multiple sclerosis (MS) from susceptibility to disease course, and from relapse recovery to progression. MS is more common in women (1) and women often have an earlier disease onset than men (2). Women have more frequent relapses early in the disease course (3), but with better relapse recovery potential than men (4). Although men have fewer relapses (3), they usually have faster disability worsening early on (5) due to lower relapse recovery potential with higher likelihood of entering the progressive phase earlier (6), possibly partly associated with age-related decline of androgens in men (7). However, once women enter the progressive phase (around the fifth decade), they accumulate disability faster and consequently can catch up to men (8). Along with aging, menopause results in a more dramatic sex hormone drop compared to men and thus likely contributes to this alteration in the MS disease course.
In addition to sex, race/ethnicity is also closely related to MS susceptibility, course, and progression. There is a similar prevalence of African Americans to White Americans with MS in California (9). African American women have a higher risk of MS than White American women (9). African Americans have more aggressive clinical disease than White Americans (10) and African American men are more likely to have primary progressive MS (PPMS) than White American men (11). Little to no work has been conducted looking at how sex and race/ethnicity impact imaging findings, individually or together, in MS.
It is imperative to incorporate the role of aging into sex and racial/ethnic differences in MS imaging as aging is a key determinant of the phenotypic and radiological variability in MS (12, 13). Central nervous system (CNS) reserve decreases with aging, not only in the general population, but also in MS (14). With aging, inflammatory activity and therefore new relapse and lesion formation frequency tend to decrease in MS, but the recovery potential from relapses decreases with aging as well (15). Most importantly, transition to the progressive phase of MS increases with aging (16), which often overlaps with changes in age-related sex hormone levels during menopause and andropause.
Imaging biomarkers in those of diverse races/ethnicities differ from those in White persons. African Americans have earlier brain atrophy, lower cortical thickness, and higher WM lesion load than White Americans (17, 18). Latin Americans with MS have higher T2 lesion volume, and lower brain volume, white matter volume, and cortex volume than non-Latin American White persons with MS (19). Japanese persons with MS have greater T2 lesion volume per lesion, and lower total brain volume, white matter volume, thalamic volume, and deep grey matter volume compared to White persons with MS (20). Magnetic resonance imaging (MRI) differences between diverse racial/ethnic groups worldwide with MS has been comprehensively reviewed (21). Less is known about sex differences in diverse populations. However, men generally have greater brain atrophy than women (22) with a higher rate of decreasing cortical thickness (23).
Similarly, there are sex and racial/ethnic differences in laboratory biomarkers of MS such as vitamin D, cerebrospinal fluid (CSF) kappa free light chain, oligoclonal bands, and neurofilament light chain (NfL). Vitamin D supplementation seems to be more effective at reducing CD4+ T-cell proliferation in women than in men with MS (24). With respect to diverse racial/ethnic groups, African Americans have lower vitamin D levels than non-Latin American White Americans and Mexican Americans in the general population (25). In persons with MS, vitamin D levels were found to be higher in White Americans compared to Black Americans and Hispanic Americans, with the association of higher vitamin D levels with reduced risk of MS being significant only in White Americans (26). On the other hand, the CSF biomarkers of kappa free light chain (27) and oligoclonal bands (28) do not appear to differ between the sexes, while some studies have shown that men are more likely to have negative CSF oligoclonal bands (29–31). Moreover, CSF neurofilament light chain levels are higher in men than women (29, 32, 33). Regarding biomarkers in diverse racial/ethnic groups, Black persons with MS are more likely to have CSF oligoclonal bands than White persons with MS (34). However, overall, knowledge of the interaction of sex and race in laboratory biomarkers remains limited, similar to what has been observed in imaging metrics of MS.
As the focus of this review, imaging metrics can serve both as an outcome and a biomarker. To better understand and explain sex and racial/ethnic differences in MS clinical findings, identification of sex and racial/ethnic differences in imaging biomarkers of MS in the age spectrum is essential. As many of the differences on imaging are expected to precede differences observed clinically, evaluating the impact of sex and race/ethnicity on imaging phenotypes provides an opportunity to intervene and optimize MS management in a timely manner. Investigating the interaction of sex with race/ethnicity in imaging in MS is important to target and reduce disparities.
This comprehensive review provides an overview of sex and hormone differences in imaging biomarkers of MS, and highlights how reproductive milestones (pregnancy, menopause) along with hormone therapy (HT) may impact MS imaging differences in the age spectrum. The review also focuses on the key interactions of age, sex, and race/ethnicity in MS and how this intersection may affect imaging biomarkers of MS.
2 How do sex and hormones impact lesion load and location in MS?
Women generally have a greater number of gadolinium-enhancing lesions compared to men (35–37), though a few studies have not found a significant sex difference (38, 39). More gadolinium-enhancing lesions in women is indicative of a more inflammatory phenotype, related either to abnormally low testosterone levels in women (36) or due to high estradiol and low progesterone levels (40). A small study of eight women showed that the ratio of progesterone/17-beta-estradiol during the luteal phase was associated with the number and volume of gadolinium-enhancing lesions (41). These MRI results align with a large study including over 6,000 women and 3,000 men with MS, demonstrating that women have more relapses up to menopause than men, indicative of more inflammatory disease in premenopausal women than men (42), likely related to changes associated with sex hormone levels and aging. Aging in MS is generally associated with decreased inflammatory activity regarding relapses (8) and new enhancing and/or T2 lesions on MRI (43), and thus, interactions between sex and age warrant further investigation to determine the relative contribution of each variable to inflammatory activity in MS.
With respect to T1 hypointense lesions related to more severe axonal/neuronal damage, men with progressive MS have higher T1 lesion volume and higher T1/T2 ratio compared to women (44), which has been replicated in another study showing higher T1/T2 ratio in men compared with women in both relapsing-remitting MS (RRMS) and secondary progressive MS (SPMS) (35). T2 hyperintense lesion area was increased in women compared to men with MS as well (45).
Regarding lesion location, men with RRMS had a much greater likelihood of having exclusively infratentorial lesions than women, a relationship that did not hold in progressive MS (39). This study did not find any difference between sexes regarding spinal cord lesions (39), though another study showed that men have more spinal cord lesions than women (46). Men also have more cortical GM lesions than women (47) which has been confirmed by a neuropathological study (48).
3 How do sex and hormones impact atrophy in MS?
Brain atrophy occurs with aging in the general population, but the atrophy rate is faster in those with MS (14). Sex differences contribute to clinical phenotypic variability in MS, both independently and in association with aging (49). Sex differences also impact imaging biomarkers of atrophy in MS across the lifespan.
Although CNS atrophy occurs in both sexes in MS, men show more significant whole brain and GM atrophy compared to women, especially during the early and midlife periods of the disease. Regional GM atrophy, including localized cortical thinning and deep GM atrophy, independent of age and disease duration, occurs more extensively in men (50–52). In parallel, higher bifrontal GM atrophy in men compared to women with MS persisted even after the groups were matched for IQ, education level, cognitive performance and physical disability in addition to age and disease duration (53). Moreover, men showed more prominent central atrophy, with larger third and lateral ventricle volumes than age-matched women, indirectly reflecting deep GM damage (38, 50). There is also a stronger association between thalamic atrophy and clinical metrics such as 9-hole peg test (52) and cognitive function (51) in men than women with MS.
Data on sex differences in spinal cord atrophy in MS is limited. In a study of early RRMS patients, although not statistically significant, women exhibited a smaller cervical spinal cord cross-sectional area than men (54). In another study, women had smaller cervical spinal cord areas compared to men in the control group, whereas cervical spinal cord areas were similar between women and men in the MS group (55). In parallel, a postmortem pathology study found similar lateral column cross-sectional areas at C3 and T2 between the sexes, but that the nerve fiber layer density was significantly lower in men, suggesting greater axonal damage in men than women (56).
In contrast to multiple unfavorable structural imaging findings in men, one study found that women had more advanced WM atrophy in the brain compared to men with MS (38). This could relate to higher inflammatory activity with a higher number of WM lesions in women, discussed in the previous section, leading to more accelerated WM loss.
4 How do sex and hormones impact non-conventional imaging metrics in MS?
Sex differences in WM integrity, functional connectivity, microglia, and iron deposition warrant attention since these advanced imaging techniques could enlighten the underlying mechanisms better and may correlate more strongly with clinical outcomes.
A diffusion tensor imaging (DTI) study showed that diffuse and regional WM damage was significantly higher in men, while disease duration, disability, and WM lesion load were similar between sexes with MS (57). The normal appearing WM was the main driver of more extensive and severe WM integrity loss in men. The region-wise WM integrity loss was specifically more severe in the thalamus, which was associated with faster deterioration in cognition in men (57). Another DTI study found a significant difference in the microstructural change rate of chronic stable demyelinating WM lesions, with men having a faster rate of ongoing inflammation, demyelination, and axonal loss in lesions compared to women, which was associated with progressive brain atrophy (58).
In a resting-state functional MRI (fMRI) study on early-stage MS, men exhibited greater GM atrophy but also increased functional connectivity compared to women (53). However, in a similar group of patients with MS, in the caudate, men had lower functional connectivity to the posterior cingulate cortex compared to women (59). In another fMRI study, MS patients had impaired functional connectivity within the male group, whereas no difference was found between MS patients and controls in the female group (60). Additionally, a decline in functional connectivity and network efficiency was associated with a decline in visuospatial memory only in men with MS (60).
Women with MS have a more clustered hippocampal network organization with an increase in hippocampal connectivity, despite more widespread hippocampal atrophy than men with MS (61). It is hypothesized that in men, increased functional connectivity seen earlier in the disease course may be due to a compensatory mechanism aiming to overcome increased structural tissue damage, but it seems to evolve into a more maladaptive mechanism as the disease progresses. In contrast, women start to demonstrate greater functional connectivity and re-organization as the disease continues since women may have better functional preservation and reserve, resulting in lower rates of disability worsening (29, 53). However, how this relates to aging and menopause remains unknown.
Quantitative susceptibility mapping (QSM) has been used to identify chronic active lesions in MS with one study determining that men are more likely to have QSM-visible lesions with rims, indicative of chronic active inflammation compared to women (62). This finding has been replicated in neuropathology studies that showed an increase in smoldering lesions (63) and more mixed active/inactive lesions in men than women with MS (48).
Positron emission tomography (PET) is an emerging advanced imaging technique in MS targeting various underlying mechanisms such as demyelination and neuroinflammation, based on which radioligand is used (64, 65). For example, microglia can be evaluated using radioligands that bind to 18kDA translocator protein (TSPO). In a recent study, men showed higher TSPO binding on PET compared to women, both in MS and healthy individuals, and this sex difference in TSPO-expressing microglia was suggested to contribute to the higher likelihood of progression in men with MS (66).
5 How do reproductive milestones affect imaging biomarkers in MS?
With respect to reproductive milestones for women, studies in MS using MRI have been conducted during pregnancy, in the postpartum period, and with the transition to menopause. The impact of pregnancy on new MRI activity has been demonstrated even at the earliest phase of MS, radiologically isolated syndrome (RIS), a form of asymptomatic MS, where a significant increase in the number of T2 lesions and T2 lesion volume was seen in individuals with RIS who became pregnant compared to those who did not (67).
In the same vein, a study conducted on women with MS with 2 MRIs completed before pregnancy and 2 MRIs completed after delivery demonstrated higher T2 lesion volume and greater annualized T2 lesion volume increase as compared to the pre-pregnancy period (68). Of note, in this study, MS was deemed to be mild, with only 6% of participants on moderate to high efficacy DMTs and 81% on low efficacy DMT (68). This study paradigm was interesting in that each MS patient served as their own internal control with multiple scans, enabling for comparisons within a single individual over 4 MRIs. An increase in brain T2 lesion volume postpartum has been replicated by other studies (69), with one of these studies also finding an increase in brain T1 lesion volume (69).
Other studies have compared MRI gadolinium-enhancing lesions before and after pregnancy, which have shown a significant increase in the number of gadolinium-enhancing lesions on brain MRI postpartum compared to pre-pregnancy (70, 71), even in the absence of clinical attacks (70, 71).
Regarding breastfeeding, one study noted a protective effect of breastfeeding on MRI activity (70), while another did not (71). Most of the aforementioned studies did not include spinal cord MRIs, or if they did, did not provide separate analyses for spinal cord. This is noteworthy, as the development of new spinal cord lesions are more likely to be symptomatic than new brain lesions and thus to contribute to disability worsening (72). Interestingly, in the postpartum period, breastfeeding duration of >6 months was associated with lower WM volume, though this could be linked to increased inflammatory disease activity in the postpartum period rather than the independent effect of breastfeeding (73). The postpartum inflammatory activity was also associated with shorter breastfeeding duration (73).
Upon entering menopause, the MS disease course and MRI features change for women into a less inflammatory form. Menopausal women have lower annualized relapse rate and MRI activity than women not in menopause (74). Although women have more benign volumetric outcomes and men have faster atrophy rates early in the MS disease course, this trend starts to change with aging and possibly with menopause.
In an MS cohort with a mean age of 30 years, while the initial normalized deep GM volumes were greater in men, the follow-up volumes became similar between two sexes after 5 years (75). Moreover, compared to men, greater total brain, cortical and brainstem volumes were observed in women with MS onset before menopause, whereas no difference was found in women with MS onset after menopause (76). In parallel, another study found greater GM and central atrophy rates in men compared to age-matched women in earlier decades of life, but this difference was nullified after age 60 (50). This suggests a potential role of menopause and change in sex hormone levels contributing to increased atrophy rates in women, resulting in women catching up to men. This aligns with what is observed clinically; after progressive MS onset, disability worsening rate increases in women, catching up to men (8). Additionally, women with an earlier age at menopause onset tend to transition to the progressive phase earlier (77) and disability worsening increases after menopause (78).
Anti-Mullerian hormone (AMH) can be used as a biomarker of ovarian aging, as plasma AMH levels associate with oocyte and leukocyte telomere lengths as well as antral follicle counts and start to decrease with ovarian aging (79). In contrast, the levels of gonadal sex hormones such as estrogen and progesterone often start to drop later on during the perimenopausal transition. Although AMH may not necessarily have similar pleiotropic effects on the brain like gonadal sex hormones, in a study on women with MS, lower AMT levels correlated with greater GM atrophy and disability independent of age and disease duration in women with MS (80). The impact of this decline in reproductive hormone levels on brain atrophy is also seen in the general population. Premenopausal women who underwent bilateral salpingo-oophorectomy had smaller amygdala volumes, thinner parahippocampal-entorhinal cortex, and lower entorhinal WM integrity compared to controls (81). Whether abrupt or relatively gradual, reproductive hormone changes may lead to regional structural abnormalities in the brain, possibly preceding cognitive decline in cognitively unimpaired women (81) and disability worsening in women with MS (80).
6 How does hormone therapy affect imaging biomarkers in MS?
The above arguments point to clear sex differences in MS, suggesting a potential for reversal of these trends with HT in both men and women. As deficiency in sex hormones is associated with deterioration of imaging metrics in MS, patients may benefit from HT.
In a pilot study, the effect of testosterone supplementation was evaluated in 10 men with relapsing-remitting MS (82). Patients first had a 6-month pretreatment period, followed by a 12-month period of 100 mg daily testosterone gel treatment. After one year of treatment, participants showed an increase in lean body mass without any significant adverse effects, as well as a significant improvement in Paced Auditory Serial Addition Task (PASAT) scores. There was no significant change in the number or volume of gadolinium-enhancing lesions with treatment. However, compared to the first half of the study (6 months of pretreatment, 3 months of testosterone treatment), in the second half of the study (9 more months of testosterone treatment), the annualized rate of brain volume loss was reduced by 67%. Therefore, in addition to the improvement in cognition, men with MS experienced a slowing in brain atrophy after using testosterone treatment for 12 months. Although a potential anti-inflammatory effect of testosterone was not detected in this group of patients with low level of baseline inflammatory activity, the findings of this small study suggested a potential neuroprotective impact of testosterone supplementation in men with MS, which would merit further exploration (82).
In women with MS <50 years, after 24-months of estriol treatment (along with glatiramer acetate), the voxel-based morphometry showed localized GM sparing, particularly in the frontal cortex, correlating with cognitive improvement (83). This is supported by animal studies demonstrating an increase in remyelination and decrease in microglial activation with estrogen treatment (84). This is also consistent with HT study findings in healthy women, such as the Kronos Early Estrogen Prevention Study (KEEPS) (85). In recently menopausal women treated with transdermal estradiol or oral conjugated equine estrogen (CEE), the WM hyperintensity volume increased in both groups, which was different from the rate of WM hyperintensity increase in the placebo group in the oral CEE group, but not in the transdermal estradiol group. Furthermore, the transdermal estradiol group had preservation of prefrontal cortex volume over 7 years of longitudinal MRI compared to the placebo group (85). However, the increase in WM hyperintensity in the oral CEE group compared to the placebo group did not persist 10 years after the end of KEEPS in the KEEPS continuation study. No differences in WMH was identified when the treatment groups (transdermal estradiol, oral CEE) were compared to placebo 10 years after the end of hormone therapies (14 years after randomization) (86).
Few studies have used therapies aimed at altering a woman's hormones, either as oral contraceptive pill or HT, with MRI lesion load as an outcome measure. One study used a combination of interferon beta-1a and oral contraceptive pill (containing ethinylestradiol and desogestrel), finding that more patients did not develop gadolinium-enhancing lesions compared to those treated with interferon beta-1a alone (87). Similarly, another study showed a longer time to the next gadolinium-enhancing lesion in women on continuous oral contraception compared to women who were not (88). There was also a randomized clinical trial using either the combination of glatiramer acetate and estriol or glatiramer acetate and placebo in women ages 18–50 with MS, finding a decrease in relapse rate but no change to MRI lesions (89). Lastly, one study (POPARTMUS) used a combination of nomegestrol acetate and 17-beta-estradiol in post-partum women with MS, finding no difference in annualized relapse rate compared to placebo at 12 weeks, and no difference between groups with respect to volume or number of gadolinium-enhancing or T2 lesions on MRI (90).
In a study on 14 peri/postmenopausal women with MS and 13 controls, the use of HT (estradiol and cyclical dydrogesterone) for 12 months improved vasomotor and depressive symptoms at 3 and 12 months in both groups and showed no change to MRI lesion burden with respect to gadolinium-enhancing or T2-FLAIR lesions at 12-months (91). In a follow-up study on 16 peri/postmenopausal women with MS, lower baseline estradiol correlated with lower whole brain volume on MRI independent of age. Lower baseline estradiol also correlated with higher brain white matter lesion load and higher serum NfL (sNfL) and serum glial fibrillary acidic protein (sGFAP) levels (92). Over one year of menopausal HT, there was no significant change in white matter lesion load, whole brain volumes, sNfL and sGFAP. In another pilot study on 24 peri/postmenopausal women with MS treated with bazedoxifene plus conjugated estrogen for 2 months, hot flashes were improved (93). Of the 12 participants who underwent MRI, only one in the placebo group, who was not on DMT, developed new gadolinium enhancing lesions in 8 weeks, whereas none of the 8 in the hormone treatment group, who were all on DMTs, developed new lesions (93). Other than the aforementioned studies, there is a dearth of studies on HT use in menopausal women, which would be important given the higher propensity for disability worsening upon entering menopause.
Transgender individuals also warrant mention here, though data is very limited. One study has shown that MS risk is higher in transgender individuals having undergone male-to-female transition (94). The specific effects of HT on clinical and imaging outcomes in these individuals with MS is unknown. However, in transgender individuals without MS, those undergoing male-to-female transition receiving estradiol and anti-androgen treatment developed volume decreases in total brain (95), hypothalamus (95), and hippocampus (96), as well as reduction in cortical thickness (97).
7 How does the intersection of sex and race/ethnicity affect imaging biomarkers in MS?
Most studies above do not report on the racial/ethnic makeup of the participants, nor do they analyze differences between diverse racial/ethnic groups in conjunction with age and sex. This is an unmet need in MS, and of great importance given African Americans tend to have more aggressive disease, both clinically and radiologically, than White Americans (21, 98, 99).
Few studies exist on international populations with MS looking at sex differences on imaging. A study using the Argentine MS Registry (RelevarEM) (39) did not report on the race/ethnicity makeup of their participants. Other registries with diverse racial/ethnic groups with MS include the National African Americans with MS Registry (NAAMSR) (100) and the North American Research Committee on Multiple Sclerosis (NARCOMS) (101). These are promising avenues for further exploration of the interactions between sex and race/ethnicity and MS, including MRI outcomes.
With the lack of MS studies looking at the intersection between sex and race/ethnicity, one can turn to other systemic autoimmune conditions where this has been studied more extensively, including systemic lupus erythematosus (SLE) and sarcoidosis, for insight. In SLE, African American men fare worse than African American women with a higher likelihood of end organ damage and death (102). Similar to MS, the prevalence of SLE in African American women is higher than that in White American women (103) as is the prevalence in Latin American women compared to non-Latin American White women (104). Furthermore, African Americans with SLE have more severe disease compared to White Americans (105). The presence of focal brain lesions in SLE is associated with African American ethnicity, with analysis of sex not revealing an additional association (106).
Sarcoidosis is more common in African Americans than White Americans, with African Americans having earlier age of onset and being more likely to die from the disease (107). In neurosarcoidosis specifically, African Americans are less likely to show resolution of abnormalities on MRI than other races/ethnicities (108).
The extent of interaction between race/ethnicity and sex in other disorders is not limited to the immune activation and its measures. As the other component of pathobiology of MS is neurodegeneration, one can investigate such interactions in other neurodegenerative disorders. In dementia, age-standardized incidence of Alzheimer's disease (AD) was found to be higher in women than men, and AD risk was higher in African Americans and Native Hawaiians, whereas the risk was similar in Latin Americans, and lower in Asian Americans compared to White Americans (109). High exposure to statins correlated with a lower risk of AD among White women, White men, Latin American women, Latin American men and Black women, but not in Black men (110). Given the clinical and imaging interactions between sex and race/ethnicity noted in these studies of other systemic autoimmune and neurodegenerative diseases, more work needs to be done using imaging biomarkers as an outcome measure to study the intersection of sex and race/ethnicity in the clinical, imaging, and laboratory immunophenotypes of MS in the age spectrum.
8 Discussion
There are clear sex differences in MS seen with imaging, with women tending to have a higher number of T2 hyperintense and gadolinium-enhancing lesions, as well as greater WM atrophy. Both T2 hyperintense and gadolinium-enhancing lesions tend to increase in the postpartum period but decrease after menopause. In contrast, men are likely to have more T1 hypointense, cortical GM, infratentorial, and spinal cord lesions. Men also demonstrate lower diffuse and regional WM integrity as well as decreased functional connectivity and re-organization in the brain than women, which suggests better functional preservation and CNS reserve in women. Moreover, men have a higher number of chronic active WM lesions with rims and lower WM integrity in chronic stable WM lesions than women. Men exhibit greater whole brain and GM atrophy than women, especially early in the disease course. However, with aging, and potentially with menopause, no significant difference in brain volume is seen between the sexes, especially after around the sixth decade. In parallel, a decrease in T2 hyperintense and gadolinium-enhancing lesions along with decrease in brain atrophy rates were observed in women who received HT (Figure 1).
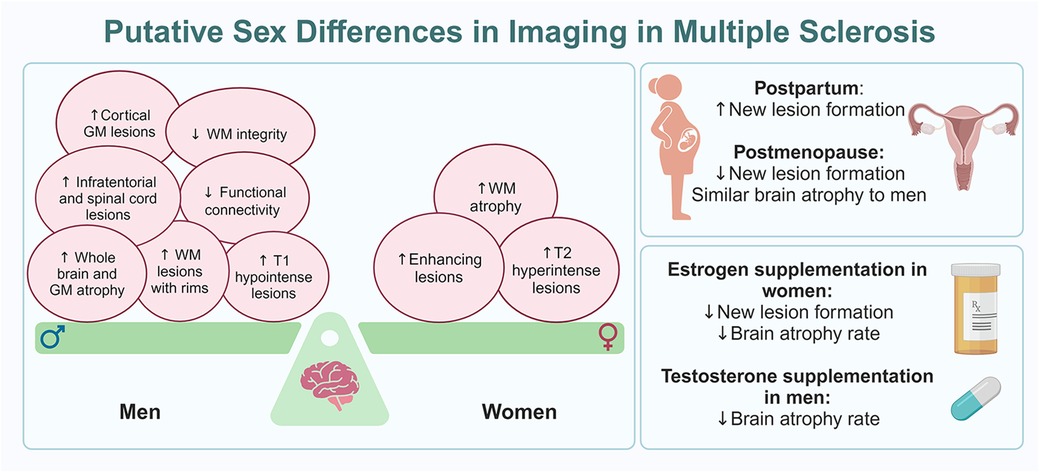
Figure 1 Putative sex differences in imaging in MS. The panel on the left shows the balance of imaging findings between women and men with MS, with women having more MRI markers of inflammatory disease while men have more MRI markers of neurodegeneration, often early in the disease course. The panels on the right outline MRI changes seen at reproductive milestones for women with MS (top) and how hormone therapy can impact MRI findings in both women and men with MS (bottom).
Based on imaging study findings, neuronal and axonal loss is overall more extensive in men with MS, leading to a more neurodegenerative disease process early on (49, 111) in a region-specific manner (52). Chromosomal differences between sexes and how they affect the nervous and immune systems are one of the main drivers of sex differences observed in CNS atrophy metrics of MS. The XX genotype exhibits a more proinflammatory immune response (112) whereas the XY genotype exhibits a more neurodegenerative response to an immune system attack (49, 111).
Differences in sex hormone patterns also play a main role in the sex variability in imaging metrics through their relationship with nervous and immune systems, as sex hormones have both neuroprotective and anti-inflammatory effects (113–115). The gradual decline in sex hormones with aging and menopause is associated with immuno-senescence and decreased neuronal repair, and appears to result in enhancement of neurodegenerative outcomes including increase in brain atrophy in MS.
From the work reviewed here, while there is a higher number of studies looking at impact of sex on clinical and imaging phenotypes of MS, such studies are less common with race/ethnicity. Similarly, with imaging biomarkers, the interactions with race/ethnicity have not been studied. The paucity of studies with race/ethnicity as opposed to sex is somewhat understandable given the easier definition of sex as a variable rather than race/ethnicity in studies, along with the fact that many centers may not have enough representation of different ethnicities across the globe.
While single variable studies are helpful in answering focused questions in MS, genetic and hereditary variables such as sex and race/ethnicity, along with the impact of socioeconomic status and disparities directly tied into these variables, cannot be separated into single variable silo studies. Our review highlights the significant unmet need in studying how age, sex and race/ethnicity interact in predicting imaging and clinical outcomes in MS. Such studies need to be conducted with significant effort across multiple centers with sufficient power to come up with better predictive models to individualize health care in discrepant MS populations.
Author contributions
NNa: Conceptualization, Writing – original draft, Writing – review & editing. NNe: Data curation, Visualization, Writing – review & editing. OK: Conceptualization, Writing – review & editing. BZ: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
BZ has been the recipient of research funding from the National Institutes of Health [U54 AG044170 and K12 AR084222]; is supported by the Mayo Clinic Eugene and Marcia Applebaum Award and the Mayo Clinic Radiology Research Grant.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Koch-Henriksen N, Sorensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. (2010) 9(5):520–32. doi: 10.1016/S1474-4422(10)70064-8
2. Harbo HF, Gold R, Tintore M. Sex and gender issues in multiple sclerosis. Ther Adv Neurol Disord. (2013) 6(4):237–48. doi: 10.1177/1756285613488434
3. Kalincik T, Vivek V, Jokubaitis V, Lechner-Scott J, Trojano M, Izquierdo G, et al. Sex as a determinant of relapse incidence and progressive course of multiple sclerosis. Brain. (2013) 136(Pt 12):3609–17. doi: 10.1093/brain/awt281
4. Bove R, Chitnis T. The role of gender and sex hormones in determining the onset and outcome of multiple sclerosis. Mult Scler. (2014) 20(5):520–6. doi: 10.1177/1352458513519181
5. Confavreux C, Vukusic S. Age at disability milestones in multiple sclerosis. Brain. (2006) 129(Pt 3):595–605. doi: 10.1093/brain/awh714
6. Kantarci O, Siva A, Eraksoy M, Karabudak R, Sutlas N, Agaoglu J, et al. Survival and predictors of disability in turkish MS patients. Turkish multiple sclerosis study group (TUMSSG). Neurology. (1998) 51(3):765–72. doi: 10.1212/WNL.51.3.765
7. Ysrraelit MC, Correale J. Impact of andropause on multiple sclerosis. Front Neurol. (2021) 12:766308. doi: 10.3389/fneur.2021.766308
8. Soldan MM P, Novotna M, Abou Zeid N, Kale N, Tutuncu M, Crusan DJ, et al. Relapses and disability accumulation in progressive multiple sclerosis. Neurology. (2015) 84(1):81–8. doi: 10.1212/WNL.0000000000001094
9. Langer-Gould A, Brara SM, Beaber BE, Zhang JL. Incidence of multiple sclerosis in multiple racial and ethnic groups. Neurology. (2013) 80(19):1734–9. doi: 10.1212/WNL.0b013e3182918cc2
10. Cree BA, Khan O, Bourdette D, Goodin DS, Cohen JA, Marrie RA, et al. Clinical characteristics of African Americans vs Caucasian Americans with multiple sclerosis. Neurology. (2004) 63(11):2039–45. doi: 10.1212/01.WNL.0000145762.60562.5D
11. Wallin MT, Culpepper WJ, Maloni H, Kurtzke JF. The gulf war era multiple sclerosis cohort: 3. Early clinical features. Acta Neurol Scand. (2018) 137(1):76–84. doi: 10.1111/ane.12810
12. Zeydan B, Kantarci OH. Progressive forms of multiple sclerosis: distinct entity or age-dependent phenomena. Neurol Clin. (2018) 36(1):163–71. doi: 10.1016/j.ncl.2017.08.006
13. Zeydan B, Kantarci OH. Impact of age on multiple sclerosis disease activity and progression. Curr Neurol Neurosci Rep. (2020) 20(7):24. doi: 10.1007/s11910-020-01046-2
14. Zivadinov R, Reder AT, Filippi M, Minagar A, Stuve O, Lassmann H, et al. Mechanisms of action of disease-modifying agents and brain volume changes in multiple sclerosis. Neurology. (2008) 71(2):136–44. doi: 10.1212/01.wnl.0000316810.01120.05
15. Conway BL, Zeydan B, Uygunoglu U, Novotna M, Siva A, Pittock SJ, et al. Age is a critical determinant in recovery from multiple sclerosis relapses. Mult Scler. (2019) 25(13):1754–63. doi: 10.1177/1352458518800815
16. Tutuncu M, Tang J, Zeid NA, Kale N, Crusan DJ, Atkinson EJ, et al. Onset of progressive phase is an age-dependent clinical milestone in multiple sclerosis. Mult Scler. (2013) 19(2):188–98. doi: 10.1177/1352458512451510
17. Caldito NG, Saidha S, Sotirchos ES, Dewey BE, Cowley NJ, Glaister J, et al. Brain and retinal atrophy in African-Americans versus Caucasian-Americans with multiple sclerosis: a longitudinal study. Brain. (2018) 141(11):3115–29. doi: 10.1093/brain/awy245
18. Turney IC, Lao PJ, Renteria MA, Igwe KC, Berroa J, Rivera A, et al. Brain aging among racially and ethnically diverse middle-aged and older adults. JAMA Neurol. (2023) 80(1):73–81. doi: 10.1001/jamaneurol.2022.3919
19. Perez CA, Salehbeiki A, Zhu L, Wolinsky JS, Lincoln JA. Assessment of racial/ethnic disparities in volumetric MRI correlates of clinical disability in multiple sclerosis: a preliminary study. J Neuroimaging. (2021) 31(1):115–23. doi: 10.1111/jon.12788
20. Nakamura Y, Gaetano L, Matsushita T, Anna A, Sprenger T, Radue EW, et al. A comparison of brain magnetic resonance imaging lesions in multiple sclerosis by race with reference to disability progression. J Neuroinflammation. (2018) 15(1):255. doi: 10.1186/s12974-018-1295-1
21. Nathoo N, Zeydan B, Neyal N, Chelf C, Okuda DT, Kantarci OH. Do magnetic resonance imaging features differ between persons with multiple sclerosis of various races and ethnicities? Front Neurol. (2023) 14:1215774. doi: 10.3389/fneur.2023.1215774
22. Xu J, Kobayashi S, Yamaguchi S, Iijima K, Okada K, Yamashita K. Gender effects on age-related changes in brain structure. AJNR Am J Neuroradiol. (2000) 21(1):112–8.10669234
23. Christova P, Georgopoulos AP. Differential reduction of gray matter volume with age in 35 cortical areas in men (more) and women (less). J Neurophysiol. (2023) 129(4):894–9. doi: 10.1152/jn.00066.2023
24. Correale J, Ysrraelit MC, Gaitan MI. Gender differences in 1,25 dihydroxyvitamin D3 immunomodulatory effects in multiple sclerosis patients and healthy subjects. J Immunol. (2010) 185(8):4948–58. doi: 10.4049/jimmunol.1000588
25. Ginde AA, Liu MC, Camargo CA Jr. Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. (2009) 169(6):626–32. doi: 10.1001/archinternmed.2008.604
26. Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. (2006) 296(23):2832–8. doi: 10.1001/jama.296.23.2832
27. Hegen H, Berek K, Cavalla P, Christiansen M, Emersic A, Di Filippo M, et al. Diagnostic value of kappa free light chain index in patients with primary progressive multiple sclerosis—a multicentre study. Front Immunol. (2023) 14:1327947. doi: 10.3389/fimmu.2023.1327947
28. Castellazzi M, Ferri C, Tecilla G, Huss A, Crociani P, Desina G, et al. The sexual dimorphism in cerebrospinal fluid protein content does not affect intrathecal IgG synthesis in multiple sclerosis. J Pers Med. (2022) 12(6):1–13. doi: 10.3390/jpm12060977
29. Alvarez-Sanchez N, Dunn SE. Potential biological contributers to the sex difference in multiple sclerosis progression. Front Immunol. (2023) 14:1175874. doi: 10.3389/fimmu.2023.1175874
30. Karrenbauer VD, Bedri SK, Hillert J, Manouchehrinia A. Cerebrospinal fluid oligoclonal immunoglobulin gamma bands and long-term disability progression in multiple sclerosis: a retrospective cohort study. Sci Rep. (2021) 11(1):14987. doi: 10.1038/s41598-021-94423-x
31. Mero IL, Gustavsen MW, Saether HS, Flam ST, Berg-Hansen P, Sondergaard HB, et al. Oligoclonal band status in scandinavian multiple sclerosis patients is associated with specific genetic risk alleles. PLoS One. (2013) 8(3):e58352. doi: 10.1371/journal.pone.0058352
32. Bridel C, van Wieringen WN, Zetterberg H, Tijms BM, Teunissen CE, the NFLGet al., Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: a systematic review and meta-analysis. JAMA Neurol. (2019) 76(9):1035–48. doi: 10.1001/jamaneurol.2019.1534
33. Momtazmanesh S, Shobeiri P, Saghazadeh A, Teunissen CE, Burman J, Szalardy L, et al. Neuronal and glial CSF biomarkers in multiple sclerosis: a systematic review and meta-analysis. Rev Neurosci. (2021) 32(6):573–95. doi: 10.1515/revneuro-2020-0145
34. da Gama PD, Machado Ldos R, Livramento JA, Gomes HR, Adoni T, Morales Rde R, et al. Oligoclonal bands in cerebrospinal fluid of black patients with multiple sclerosis. Biomed Res Int. (2015) 2015:217961. doi: 10.1155/2015/217961
35. Pozzilli C, Tomassini V, Marinelli F, Paolillo A, Gasperini C, Bastianello S. ‘Gender gap’ in multiple sclerosis: magnetic resonance imaging evidence. Eur J Neurol. (2003) 10(1):95–7. doi: 10.1046/j.1468-1331.2003.00519.x
36. Tomassini V, Onesti E, Mainero C, Giugni E, Paolillo A, Salvetti M, et al. Sex hormones modulate brain damage in multiple sclerosis: MRI evidence. J Neurol Neurosurg Psychiatry. (2005) 76(2):272–5. doi: 10.1136/jnnp.2003.033324
37. Weatherby SJ, Mann CL, Davies MB, Fryer AA, Haq N, Strange RC, et al. A pilot study of the relationship between gadolinium-enhancing lesions, gender effect and polymorphisms of antioxidant enzymes in multiple sclerosis. J Neurol. (2000) 247(6):467–70. doi: 10.1007/s004150070179
38. Antulov R, Weinstock-Guttman B, Cox JL, Hussein S, Durfee J, Caiola C, et al. Gender-related differences in MS: a study of conventional and nonconventional MRI measures. Mult Scler. (2009) 15(3):345–54. doi: 10.1177/1352458508099479
39. Luetic GG, Menichini ML, Vrech C, Pappolla A, Patrucco L, Cristiano E, et al. Clinical and demographic characteristics of male MS patients included in the national registry-RelevarEM. Does sex or phenotype make the difference in the association with poor prognosis? Mult Scler Relat Disord. (2022) 58:103401. doi: 10.1016/j.msard.2021.103401
40. Bansil S, Lee HJ, Jindal S, Holtz CR, Cook SD. Correlation between sex hormones and magnetic resonance imaging lesions in multiple sclerosis. Acta Neurol Scand. (1999) 99(2):91–4. doi: 10.1111/j.1600-0404.1999.tb00663.x
41. Pozzilli C, Falaschi P, Mainero C, Martocchia A, D'Urso R, Proietti A, et al. MRI in multiple sclerosis during the menstrual cycle: relationship with sex hormone patterns. Neurology. (1999) 53(3):622–4. doi: 10.1212/WNL.53.3.622
42. Magyari M, Koch-Henriksen N. Quantitative effect of sex on disease activity and disability accumulation in multiple sclerosis. J Neurol Neurosurg Psychiatry. (2022) 93(7):716–22. doi: 10.1136/jnnp-2022-328994
43. Filippi M, Wolinsky JS, Sormani MP, Comi G. European/Canadian glatiramer acetate study G. Enhancement frequency decreases with increasing age in relapsing-remitting multiple sclerosis. Neurology. (2001) 56(3):422–3. doi: 10.1212/WNL.56.3.422
44. van Walderveen MA, Lycklama ANGJ, Ader HJ, Jongen PJ, Polman CH, Castelijns JA, et al. Hypointense lesions on T1-weighted spin-echo magnetic resonance imaging: relation to clinical characteristics in subgroups of patients with multiple sclerosis. Arch Neurol. (2001) 58(1):76–81. doi: 10.1001/archneur.58.1.76
45. Li DK, Zhao GJ, Paty DW. University of British Columbia MSMRIARGTSSG. Randomized controlled trial of interferon-beta-1a in secondary progressive MS: MRI results. Neurology. (2001) 56(11):1505–13. doi: 10.1212/WNL.56.11.1505
46. Zeydan B, Neyal N, Son J, Atkinson EJ, Port JD, Kantarci K, et al., editors. Sex and age differences in MS imaging biomarkers. Americas Committee for Treatment and Research in Multiple Sclerosis. West Palm Beach, FL, USA: Multiple Sclerosis Journal (2022). p. 112.
47. Calabrese M, De Stefano N, Atzori M, Bernardi V, Mattisi I, Barachino L, et al. Detection of cortical inflammatory lesions by double inversion recovery magnetic resonance imaging in patients with multiple sclerosis. Arch Neurol. (2007) 64(10):1416–22. doi: 10.1001/archneur.64.10.1416
48. Luchetti S, Fransen NL, van Eden CG, Ramaglia V, Mason M, Huitinga I. Progressive multiple sclerosis patients show substantial lesion activity that correlates with clinical disease severity and sex: a retrospective autopsy cohort analysis. Acta Neuropathol. (2018) 135(4):511–28. doi: 10.1007/s00401-018-1818-y
49. Voskuhl RR, Gold SM. Sex-related factors in multiple sclerosis susceptibility and progression. Nat Rev Neurol. (2012) 8(5):255–63. doi: 10.1038/nrneurol.2012.43
50. Jakimovski D, Zivadinov R, Bergsland N, Ramasamy DP, Hagemeier J, Weinstock-Guttman B, et al. Sex-specific differences in life span brain volumes in multiple sclerosis. J Neuroimaging. (2020) 30(3):342–50. doi: 10.1111/jon.12709
51. Schoonheim MM, Popescu V, Rueda Lopes FC, Wiebenga OT, Vrenken H, Douw L, et al. Subcortical atrophy and cognition: sex effects in multiple sclerosis. Neurology. (2012) 79(17):1754–61. doi: 10.1212/WNL.0b013e3182703f46
52. Voskuhl RR, Patel K, Paul F, Gold SM, Scheel M, Kuchling J, et al. Sex differences in brain atrophy in multiple sclerosis. Biol Sex Differ. (2020) 11(1):49. doi: 10.1186/s13293-020-00326-3
53. Sanchis-Segura C, Cruz-Gómez AJ, Belenguer A, Fittipaldi Márquez MS, Ávila C, Forn C. Increased regional gray matter atrophy and enhanced functional connectivity in male multiple sclerosis patients. Neurosci Lett. (2016) 630:154–7. doi: 10.1016/j.neulet.2016.07.028
54. Rashid W, Davies GR, Chard DT, Griffin CM, Altmann DR, Gordon R, et al. Upper cervical cord area in early relapsing-remitting multiple sclerosis: cross-sectional study of factors influencing cord size. J Magn Reson Imaging. (2006) 23(4):473–6. doi: 10.1002/jmri.20545
55. Daams M, Weiler F, Steenwijk MD, Hahn HK, Geurts JJ, Vrenken H, et al. Mean upper cervical cord area (MUCCA) measurement in long-standing multiple sclerosis: relation to brain findings and clinical disability. Mult Scler. (2014) 20(14):1860–5. doi: 10.1177/1352458514533399
56. Ganter P, Prince C, Esiri MM. Spinal cord axonal loss in multiple sclerosis: a post-mortem study. Neuropathol Appl Neurobiol. (1999) 25(6):459–67. doi: 10.1046/j.1365-2990.1999.00205.x
57. Schoonheim MM, Vigeveno RM, Rueda Lopes FC, Pouwels PJ, Polman CH, Barkhof F, et al. Sex-specific extent and severity of white matter damage in multiple sclerosis: implications for cognitive decline. Hum Brain Mapp. (2014) 35(5):2348–58. doi: 10.1002/hbm.22332
58. Klistorner A, Wang C, Yiannikas C, Graham SL, Parratt J, Barnett MH. Progressive injury in chronic multiple sclerosis lesions is gender-specific: a DTI study. PLoS One. (2016) 11(2):e0149245. doi: 10.1371/journal.pone.0149245
59. Koenig KA, Lowe MJ, Lin J, Sakaie KE, Stone L, Bermel RA, et al. Sex differences in resting-state functional connectivity in multiple sclerosis. AJNR Am J Neuroradiol. (2013) 34(12):2304–11. doi: 10.3174/ajnr.A3630
60. Schoonheim MM, Hulst HE, Landi D, Ciccarelli O, Roosendaal SD, Sanz-Arigita EJ, et al. Gender-related differences in functional connectivity in multiple sclerosis. Mult Scler. (2012) 18(2):164–73. doi: 10.1177/1352458511422245
61. Ciolac D, Gonzalez-Escamilla G, Radetz A, Fleischer V, Person M, Johnen A, et al. Sex-specific signatures of intrinsic hippocampal networks and regional integrity underlying cognitive status in multiple sclerosis. Brain Commun. (2021) 3(3):fcab198. doi: 10.1093/braincomms/fcab198
62. W Z BT, S C HC, Dm H XL. Sex-specific differences in rim appearance of multiple sclerosis lesions on quantitative susceptibility mapping. Mult Scler Relat Disord. (2020) 45:102317. doi: 10.1016/j.msard.2020.102317
63. Frischer JM, Weigand SD, Guo Y, Kale N, Parisi JE, Pirko I, et al. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann Neurol. (2015) 78(5):710–21. doi: 10.1002/ana.24497
64. Bodini B, Tonietto M, Airas L, Stankoff B. Positron emission tomography in multiple sclerosis—straight to the target. Nat Rev Neurol. (2021) 17(11):663–75. doi: 10.1038/s41582-021-00537-1
65. Zeydan B, Lowe VJ, Schwarz CG, Przybelski SA, Tosakulwong N, Zuk SM, et al. Pittsburgh compound-B PET white matter imaging and cognitive function in late multiple sclerosis. Mult Scler. (2018) 24(6):739–49. doi: 10.1177/1352458517707346
66. Laaksonen S, Saraste M, Nylund M, Hinz R, Snellman A, Rinne J, et al. Sex-driven variability in TSPO-expressing microglia in MS patients and healthy individuals. Front Neurol. (2024) 15:1352116. doi: 10.3389/fneur.2024.1352116
67. Lebrun C, Le Page E, Kantarci O, Siva A, Pelletier D, Okuda DT, et al. Impact of pregnancy on conversion to clinically isolated syndrome in a radiologically isolated syndrome cohort. Mult Scler. (2012) 18(9):1297–302. doi: 10.1177/1352458511435931
68. Uher T, Kubala Havrdova E, Vodehnalova K, Krasensky J, Capek V, Vaneckova M, et al. Pregnancy-induced brain magnetic resonance imaging changes in women with multiple sclerosis. Eur J Neurol. (2022) 29(5):1446–56. doi: 10.1111/ene.15245
69. Khalid F, Healy BC, Dupuy SL, Chu R, Chitnis T, Bakshi R, et al. Quantitative MRI analysis of cerebral lesions and atrophy in post-partum patients with multiple sclerosis. J Neurol Sci. (2018) 392:94–9. doi: 10.1016/j.jns.2018.06.025
70. Anderson A, Krysko KM, Rutatangwa A, Krishnakumar T, Chen C, Rowles W, et al. Clinical and radiologic disease activity in pregnancy and postpartum in MS. Neurol Neuroimmunol Neuroinflamm. (2021) 8(2):1–11. doi: 10.1212/NXI.0000000000000959
71. Houtchens M, Bove R, Healy B, Houtchens S, Kaplan TB, Mahlanza T, et al. MRI activity in MS and completed pregnancy: data from a tertiary academic center. Neurol Neuroimmunol Neuroinflamm. (2020) 7(6):1–10. doi: 10.1212/NXI.0000000000000890
72. Ruggieri S, Prosperini L, Petracca M, Logoteta A, Tinelli E, De Giglio L, et al. The added value of spinal cord lesions to disability accrual in multiple sclerosis. J Neurol. (2023) 270(10):4995–5003. doi: 10.1007/s00415-023-11829-5
73. Lorefice L, Fronza M, Fenu G, Frau J, Coghe G, D'Alterio MN, et al. Effects of pregnancy and breastfeeding on clinical outcomes and MRI measurements of women with multiple sclerosis: an exploratory real-world cohort study. Neurol Ther. (2022) 11(1):39–49. doi: 10.1007/s40120-021-00297-6
74. Lorefice L, Fenu G, Fronza M, Murgia F, Frau J, Coghe G, et al. Menopausal transition in multiple sclerosis: relationship with disease activity and brain volume measurements. Front Neurol. (2023) 14:1251667. doi: 10.3389/fneur.2023.1251667
75. Dolezal O, Gabelic T, Horakova D, Bergsland N, Dwyer MG, Seidl Z, et al. Development of gray matter atrophy in relapsing-remitting multiple sclerosis is not gender dependent: results of a 5-year follow-up study. Clin Neurol Neurosurg. (2013) 115(Suppl 1):S42–8. doi: 10.1016/j.clineuro.2013.09.020
76. Rojas JI, Sánchez F, Patrucco L, Miguez J, Funes J, Cristiano E. Structural sex differences at disease onset in multiple sclerosis patients. Neuroradiol J. (2016) 29(5):368–71. doi: 10.1177/1971400916666560
77. Zeydan B, Atkinson EJ, Weis DM, Smith CY, Gazzuola Rocca L, Rocca WA, et al. Reproductive history and progressive multiple sclerosis risk in women. Brain Commun. (2020) 2(2):fcaa185. doi: 10.1093/braincomms/fcaa185
78. Baroncini D, Annovazzi PO, De Rossi N, Mallucci G, Torri Clerici V, Tonietti S, et al. Impact of natural menopause on multiple sclerosis: a multicentre study. J Neurol Neurosurg Psychiatry. (2019) 90(11):1201–6. doi: 10.1136/jnnp-2019-320587
79. Depmann M, Broer SL, van der Schouw YT, Tehrani FR, Eijkemans MJ, Mol BW, et al. Can we predict age at natural menopause using ovarian reserve tests or mother’s age at menopause? A systematic literature review. Menopause. (2016) 23(2):224–32. doi: 10.1097/GME.0000000000000509
80. Graves JS, Henry RG, Cree BAC, Lambert-Messerlian G, Greenblatt RM, Waubant E, et al. Ovarian aging is associated with gray matter volume and disability in women with MS. Neurology. (2018) 90(3):e254–e60. doi: 10.1212/WNL.0000000000004843
81. Zeydan B, Tosakulwong N, Schwarz CG, Senjem ML, Gunter JL, Reid RI, et al. Association of bilateral salpingo-oophorectomy before menopause onset with medial temporal lobe neurodegeneration. JAMA Neurol. (2019) 76(1):95–100. doi: 10.1001/jamaneurol.2018.3057
82. Sicotte NL, Giesser BS, Tandon V, Klutch R, Steiner B, Drain AE, et al. Testosterone treatment in multiple sclerosis: a pilot study. Arch Neurol. (2007) 64(5):683–8. doi: 10.1001/archneur.64.5.683
83. MacKenzie-Graham A, Brook J, Kurth F, Itoh Y, Meyer C, Montag MJ, et al. Estriol-mediated neuroprotection in multiple sclerosis localized by voxel-based morphometry. Brain Behav. (2018) 8(9):e01086. doi: 10.1002/brb3.1086
84. Kim RY, Mangu D, Hoffman AS, Kavosh R, Jung E, Itoh N, et al. Oestrogen receptor β ligand acts on CD11c+ cells to mediate protection in experimental autoimmune encephalomyelitis. Brain. (2018) 141(1):132–47. doi: 10.1093/brain/awx315
85. Kantarci K, Tosakulwong N, Lesnick TG, Zuk SM, Lowe VJ, Fields JA, et al. Brain structure and cognition 3 years after the end of an early menopausal hormone therapy trial. Neurology. (2018) 90(16):e1404–e12. doi: 10.1212/WNL.0000000000005325
86. Faubion L, Mak FK, Tosakulwong N, Lesnick T, Reid R, Kara F, et al. Long-term effects of short-term menopausal hormone therapy on white matter integrity. Menopause. (2023) 30(12):1276.
87. Pozzilli C, De Giglio L, Barletta VT, Marinelli F, Angelis FD, Gallo V, et al. Oral contraceptives combined with interferon beta in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. (2015) 2(4):e120. doi: 10.1212/NXI.0000000000000120
88. Chen CS, Krishnakumar T, Rowles W, Anderson A, Zhao C, Do L, et al. Comparison of MS inflammatory activity in women using continuous versus cyclic combined oral contraceptives. Mult Scler Relat Disord. (2020) 41:101970. doi: 10.1016/j.msard.2020.101970
89. Voskuhl RR, Wang H, Wu TC, Sicotte NL, Nakamura K, Kurth F, et al. Estriol combined with glatiramer acetate for women with relapsing-remitting multiple sclerosis: a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. (2016) 15(1):35–46. doi: 10.1016/S1474-4422(15)00322-1
90. Vukusic S, Ionescu I, Cornu C, Bossard N, Durand-Dubief F, Cotton F, et al. Oral nomegestrol acetate and transdermal 17-beta-estradiol for preventing post-partum relapses in multiple sclerosis: the POPARTMUS study. Mult Scler. (2021) 27(9):1458–63. doi: 10.1177/1352458520978218
91. Juutinen L, Ahinko K, Tinkanen H, Rosti-Otajarvi E, Sumelahti ML. Menopausal symptoms and hormone therapy in women with multiple sclerosis: a baseline-controlled study. Mult Scler Relat Disord. (2022) 67:104098. doi: 10.1016/j.msard.2022.104098
92. Juutinen L, Ahinko K, Hagman S, Basnyat P, Jaaskelainen O, Herukka SK, et al. The association of menopausal hormone levels with progression-related biomarkers in multiple sclerosis. Mult Scler Relat Disord. (2024) 85:105517. doi: 10.1016/j.msard.2024.105517
93. Bove R, Anderson A, Rowles W, Rankin KA, Hills NK, Carleton M, et al. A hormonal therapy for menopausal women with MS: a phase Ib/IIa randomized controlled trial. Mult Scler Relat Disord. (2022) 61:103747. doi: 10.1016/j.msard.2022.103747
94. Pakpoor J, Wotton CJ, Schmierer K, Giovannoni G, Goldacre MJ. Gender identity disorders and multiple sclerosis risk: a national record-linkage study. Mult Scler. (2016) 22(13):1759–62. doi: 10.1177/1352458515627205
95. Pol H, Cohen-Kettenis P, Van Haren N, Peper J, Brans R, Cahn W, et al. Changing your sex changes your brain: influences of testosterone and estrogen on adult human brain structure. Eur J Endocrinol. (2006) 155:S107–S14. doi: 10.1530/eje.1.02248
96. Seiger R, Hahn A, Hummer A, Kranz GS, Ganger S, Woletz M, et al. Subcortical gray matter changes in transgender subjects after long-term cross-sex hormone administration. Psychoneuroendocrinology. (2016) 74:371–9. doi: 10.1016/j.psyneuen.2016.09.028
97. Zubiaurre-Elorza L, Junque C, Gomez-Gil E, Guillamon A. Effects of cross-sex hormone treatment on cortical thickness in transsexual individuals. J Sex Med. (2014) 11(5):1248–61. doi: 10.1111/jsm.12491
98. Moog TM, McCreary M, Stanley T, Wilson A, Santoyo J, Wright K, et al. African Americans experience disproportionate neurodegenerative changes in the medulla and upper cervical spinal cord in early multiple sclerosis. Mult Scler Relat Disord. (2020) 45:102429. doi: 10.1016/j.msard.2020.102429
99. Okuda DT, Stanley T, McCreary M, Smith A, Wilson A, Pinho MC, et al. Selective vulnerability of brainstem and cervical spinal cord regions in people with non-progressive multiple sclerosis of black or African American and European ancestry. Mult Scler. (2023) 29(6):691–701. doi: 10.1177/13524585221139575
100. Okai AF, Howard AM, Williams MJ, Brink JD, Chen C, Stuchiner TL, et al. Advancing care and outcomes for African American patients with multiple sclerosis. Neurology. (2022) 98(24):1015–20. doi: 10.1212/WNL.0000000000200791
101. Marrie RA, Cutter GR, Fox RJ, Vollmer T, Tyry T, Salter A. NARCOMS and other registries in multiple sclerosis: issues and insights. Int J MS Care. (2021) 23(6):276–84. doi: 10.7224/1537-2073.2020-133
102. Tan TC, Fang H, Magder LS, Petri MA. Differences between male and female systemic lupus erythematosus in a multiethnic population. J Rheumatol. (2012) 39(4):759–69. doi: 10.3899/jrheum.111061
103. Bae SC, Fraser P, Liang MH. The epidemiology of systemic lupus erythematosus in populations of African ancestry: a critical review of the “prevalence gradient hypothesis”. Arthritis Rheum. (1998) 41(12):2091–9. doi: 10.1002/1529-0131(199812)41:12%3C2091::AID-ART2%3E3.0.CO;2-D
104. Alarcon GS, Roseman J, Bartolucci AA, Friedman AW, Moulds JM, Goel N, et al. Systemic lupus erythematosus in three ethnic groups: II. Features predictive of disease activity early in its course. LUMINA study group. Lupus in minority populations, nature versus nurture. Arthritis Rheum. (1998) 41(7):1173–80. doi: 10.1002/1529-0131(199807)41:7%3C1173::AID-ART5%3E3.0.CO;2-A
105. Fernandez M, Alarcon GS, Calvo-Alen J, Andrade R, McGwin G Jr, Vila LM, et al. A multiethnic, multicenter cohort of patients with systemic lupus erythematosus (SLE) as a model for the study of ethnic disparities in SLE. Arthritis Rheum. (2007) 57(4):576–84. doi: 10.1002/art.22672
106. Petri M, Naqibuddin M, Carson KA, Wallace DJ, Weisman MH, Holliday SL, et al. Brain magnetic resonance imaging in newly diagnosed systemic lupus erythematosus. J Rheumatol. (2008) 35(12):2348–54. doi: 10.3899/jrheum.071010
107. Rybicki BA, Maliarik MJ, Major M, Popovich J Jr, Iannuzzi MC. Epidemiology, demographics, and genetics of sarcoidosis. Semin Respir Infect. (1998) 13(3):166–73.9764947
108. Affan M, Mahajan A, Rehman T, Kananeh M, Schultz L, Cerghet M. The effect of race on clinical presentation and outcomes in neurosarcoidosis. J Neurol Sci. (2020) 417:117073. doi: 10.1016/j.jns.2020.117073
109. Lim U, Wang S, Park SY, Bogumil D, Wu AH, Cheng I, et al. Risk of Alzheimer’s disease and related dementia by sex and race/ethnicity: the multiethnic cohort study. Alzheimers Dement. (2022) 18(9):1625–34. doi: 10.1002/alz.12528
110. Zissimopoulos JM, Barthold D, Brinton RD, Joyce G. Sex and race differences in the association between statin use and the incidence of Alzheimer disease. JAMA Neurol. (2017) 74(2):225–32. doi: 10.1001/jamaneurol.2016.3783
111. Du S, Itoh N, Askarinam S, Hill H, Arnold AP, Voskuhl RR. XY sex chromosome complement, compared with XX, in the CNS confers greater neurodegeneration during experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. (2014) 111(7):2806–11. doi: 10.1073/pnas.1307091111
112. Itoh Y, Golden LC, Itoh N, Matsukawa MA, Ren E, Tse V, et al. The X-linked histone demethylase Kdm6a in CD4+ T lymphocytes modulates autoimmunity. J Clin Invest. (2019) 129(9):3852–63. doi: 10.1172/JCI126250
113. Gold SM, Voskuhl RR. Estrogen and testosterone therapies in multiple sclerosis. Prog Brain Res. (2009) 175:239–51. doi: 10.1016/S0079-6123(09)17516-7
114. Spence RD, Voskuhl RR. Neuroprotective effects of estrogens and androgens in CNS inflammation and neurodegeneration. Front Neuroendocrinol. (2012) 33(1):105–15. doi: 10.1016/j.yfrne.2011.12.001
Keywords: aging, hormone therapy, magnetic resonance imaging, multiple sclerosis, race, sex
Citation: Nathoo N, Neyal N, Kantarci OH and Zeydan B (2024) Imaging phenotypic differences in multiple sclerosis: at the crossroads of aging, sex, race, and ethnicity. Front. Glob. Womens Health 5:1412482. doi: 10.3389/fgwh.2024.1412482
Received: 5 April 2024; Accepted: 11 June 2024;
Published: 28 June 2024.
Edited by:
Riley Bove, University of California, San Francisco, United StatesReviewed by:
Nkiru Nwamaka Ezeama, Nnamdi Azikiwe University, NigeriaChristine Lebrun Frenay, Centre Hospitalier Universitaire de Nice, France
© 2024 Nathoo, Neyal, Kantarci and Zeydan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Burcu Zeydan, emV5ZGFuLmJ1cmN1QG1heW8uZWR1
 Nabeela Nathoo
Nabeela Nathoo Nur Neyal
Nur Neyal Orhun H. Kantarci
Orhun H. Kantarci Burcu Zeydan
Burcu Zeydan