
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet. , 06 February 2025
Sec. Evolutionary and Population Genetics
Volume 16 - 2025 | https://doi.org/10.3389/fgene.2025.1552024
 Naoki Tani1,2*
Naoki Tani1,2* Chin Hong Ng3
Chin Hong Ng3 Soon Leong Lee3
Soon Leong Lee3 Chai Ting Lee3
Chai Ting Lee3 Norwati Muhammad3
Norwati Muhammad3 Toshiaki Kondo4
Toshiaki Kondo4 Yoshihiko Tsumura2
Yoshihiko Tsumura2 Saori Sugiyama5†
Saori Sugiyama5† Kaoru Niiyama6
Kaoru Niiyama6 Azizi Ripin7
Azizi Ripin7 Abdul Rahman Kassim8‡
Abdul Rahman Kassim8‡ Samsudin Musa8
Samsudin Musa8Introduction: The role of syngameons in adaption to microgeographical environmental heterogeneity is important and could be one of the sources of rich species diversity in tropical forests. In addition, negative frequency- or density-dependent selection is one of the major processes contributing to the maintenance of genetic diversity.
Methods: To assess genetic factors that affect the fitness of seedlings of Rubroshorea curtisii, a dominant canopy tree species in hill dipterocarp forests, the inter- and intra-population genetic structure of individuals from natural populations and individuals at two permanent plots in a hill dipterocarp forest with reproductive stage was studied. Further, a total of 460 seedlings derived from six mother trees in the plot were raised in a nursery, and their pollen donors were identified using genetic marker based paternity assignment. Seed weight, bi-parental genetic relatedness, and bi-parental genetic heterogeneity based on the clustering analysis were used to analyze their effects on seedling fitness.
Results: A Bayesian based clustering analysis revealed that three genetically distinct clusters were observed in almost all populations throughout the distributional range of the species in Malay Peninsula and provided the optimum explanation for the genetic structure of 182 mature individuals in the plots. The two clusters showed larger genetic differentiation from the ancestral admixture population, but the other one was not differentiated. The bi-parental larger genetic heterogeneity was associated with a significantly higher probability of seedling survivorship, and likewise, higher performance of vertical growth of the seedlings; but the seed weight and genetic relatedness did not significantly affect those.
Discussion: This evidence suggests that fitter seedlings derived from mating between parents with different genetic clusters contribute to maintaining genetic diversity through negative frequency-dependent selection and may have an important role in adaptation in the tropical forest plant community.
Stochastic and deterministic ecological mechanisms affect species co-existence in populations with high diversity and can allow the maintenance of a high diversity of species (Connell, 1961; Janzen, 1970; Hubbell, 1997; Webb and Peart, 1999; Wright, 2002; Usinowicz et al., 2017). The unified neutral theory of biodiversity and biogeography has provided an ecological null-model against which to test patterns of species diversity and composition within populations (Hubbell, 2001). However, species richness in tropical forests has been discussed as being the result of highly specialized adaptation to the biotic and physical environment, especially caused by forest disturbance (Ashton, 1969; Molino and Sabatier, 2001; Schnitzer and Carson, 2001; Alroy, 2017). Mechanisms to maintain species diversity in tropical forests have been studied; however, the adaptative potential of genetic diversity within tropical tree species and its effect on species diversity are poorly understood, despite the fact that genetic diversity is the basis of species diversity. It is frequently observed that tree species do not act as discrete evolutionary units (Cronk and Suarez-Gonzalez, 2018; Cannon and Petit, 2020). Closely-related and sympatric species often maintain some level of inter-specific fertility (Seehausen, 2004; Givnish, 2010; Schley et al., 2022), which can generate genotypes that facilitate further ecological diversification (the syngameon hypothesis; (reviewed in Seehausen, 2004). Therefore, the role of syngameons in adaption to microgeographical environmental heterogeneity is important and could be one of the sources of rich species diversity in tropical forests. In addition, negative frequency- or density-dependent selection is one of the major processes contributing to the maintenance of genetic diversity. At the species level, the high richness and co-existence of species in tropical forests has been partly maintained by density-dependent effects, appearing as reduced seed and seedling survival in areas with high densities or close proximity to conspecifics (the Jansen-Cornell hypothesis) (Janzen, 1970; Connell, 1971; Bagchi et al., 2010a; Bagchi et al., 2010b; Levi et al., 2019; Jia et al., 2020). This effect should also operate at syngameon and intra-species genetic diversity scales. Although negative-frequency dependent selection has theoretically been shown to be one of the mechanisms that maintains genetic diversity within populations, through the relative advantage of rare genotypes (Haldane, 1949; Ayala and Campbell, 1974; Antonovics, 1976), our understanding of the impact of frequency-dependent selection at syngameon and intra-species scales has focused on its maintenance of genetic and species diversity (Browne and Karubian, 2016; 2018; Eck et al., 2019; Cannon and Petit, 2020).
Tree species in the Dipterocarpaceae are highly diversified and form major components of the rainforest in Southeast Asia, in particular, the tribe Shoreae sensu Ashton (1979), which comprises five genera, Dryobalanops, Hopea, monotypic Neobalanocarpus, Parashorea and the large genus Shorea consisting of around 360 species. The genus Shorea has been traditionally classified based on timber characteristics (Symington, 1943) and Rubroshorea (= red meranti) is the largest among the six sections of Shorea (Maury-Lechon, 1978). Molecular phylogenetic approaches using various types of markers, such as PCR-RFLP (Tsumura et al., 1996; Indrioko et al., 2006) and plastid DNA sequences (Kajita et al., 1998; Dayanandan et al., 1999; Tsumura et al., 2011), have been used to examine these taxa. However, phylogenetic relationships have remained unclear, especially within the Rubroshorea due to low resolution of plastid DNA sequences (Tsumura et al., 2011). On the other hand, high-density genome wide DNA polymorphisms may be able to deliver high resolution of the species in the tribe Shoreae, similar to the traditional classification (Heckenhauer et al., 2018). Finally, Rubroshorea was proposed to dissected from genus Shorea based on molecular phylogenetic evidences (Ashton and Heckenhauer, 2022). Therefore, the highly diversified and closely related species belonging to Rubroshorea may be ideal models to study the evolutionary significance of syngameon complexes. Rubroshorea curtisii is commonly found in coastal and inland ridge forests throughout the Malay Peninsula (Symington, 1943) and is also confined to well-drained and fairly low nutrient soils along the northern coastal area in Borneo (Ashton et al., 1982). A low level of genetic differentiation between populations on the Malay Peninsula has been revealed by DNA polymorphisms in the chloroplast genome. However, chloroplast capture occurred through ancient hybridization events between R. curtisii and some unknown related species (Kamiya et al., 2012), and DNA sequences of two nuclear genes and the chloroplast have shown that hybridization occurs commonly between R. curtisii and R. leprosula and rarely between R. curtisii and R. parvifolia in natural populations (Kamiya et al., 2011). These species are morphologically distinct and, it has been suggested, are highly specifically adapted to their biotic and physical environments (Ashton, 1969; Brown and Whitmore, 1992; Potts et al., 2002; Dent and Burslem, 2016; Kenzo et al., 2019; Kenzo et al., 2023). Therefore, the genetic diversity and structure of R. curtisii are the result of stochastic processes, population history and hybridization events with extant (and ancient) closely related species.
We hypothesized that the multiple historical population and hybridization processes in R. curtisii caused the complex pattern of genetic diversity, which probably played an important role in adaption to the biotic and physical environments. To examine the hypothesis, the pattern of intra-specific genetic structure of R. curtisii in Malay Peninsula was assessed and the fitness of the seedlings categorized according to bi-parental genetic relationship and the pattern of genetic diversity. This approach can help in the understanding of the evolutionally and ecological importance of syngameons through detection of negative frequency dependent selection during mating and the subsequent regeneration of the forest ecosystem.
Leaf or inner bark tissue was collected from 134 R. curtisii individuals, representing nine natural populations throughout the natural distribution across Malay Peninsula (Table 1; Figure 1). The tissues were collected from R. curtisii individuals that were at least 20 m apart, to avoid collecting samples from genetically related individuals, regardless of age or size of trees. Samples were stored at −20°C, prior to DNA extraction. Semangkok Forest Reserve, a designated hill dipterocarp forest conservation area, is in and governed by the Selangor state; it is 60 km north of Kuala Lumpur, on the Malay Peninsula and is a designated hill dipterocarp forest conservation area. In 1993, Niiyama et al. (1999) established a 6-ha permanent plot (200 m × 300 m) in an undisturbed forest on a narrow ridge and steep slope, ranging from 340 to 450 m above sea level (3°37′07″N, 101°44′15″E). Another ca. 4-ha (100 m × 400 m) permanent plot was established within a selectively logged area of forest in 1994 and was extended to about 5.4-ha (ca. 140 m × 400 m) in 2007 (3°37′23″N, 101°44′15″E); this is only 200 m away from the 6-ha permanent plot (Yagihashi et al., 2010). Leaf or inner bark samples were collected from 144 to 38 R. curtisii individuals (with dbh more than 20 cm) from the undisturbed plot and the logged plot, respectively, of which 17 and three trees were growing in areas of the study plots adjacent to the undisturbed and the logged plots, respectively. A total of 17 and three trees growing in areas of the study plots adjacent to the undisturbed plot have previously been identified as the candidate pollen donors (Tani et al., 2012; Tani et al., 2015). Samples were also stored at −20°C, prior to DNA extraction.
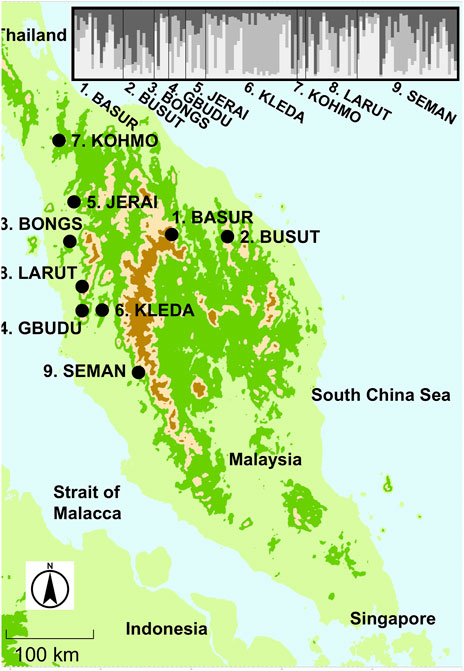
Figure 1. The locations of the sampled population of Rubroshorea curtisii trees on the Malay Peninsula and bar plot of Q values from STRUCTURE analysis for sampled individuals from nine populations.
A sporadic synchronized flowering event was observed in Semangkok Forest Reserve in October 2011, and fruit set occurred around February 2012. Seeds were collected from six selected mother trees in the undisturbed plot. After removal of wings from the seeds, seed weight was measured, then the seeds were placed on seedbeds consisting of river sand on 20 February 2012 to investigate germination. The result of the germination test is presented in Table 2. The germinated seedlings were potted in a mixture of river sand and soil without fertilizer on 26 March 2012 and maintained in the nursery of the Forest Research Institute Malaysia (FRIM, 3°14′01″N, 101°38′00″E) under 50% shade, with a water sprinkler system for irrigation. Height and survival of seedlings were monitored weekly before transplantation and monthly after transplantation. The data of seedling survival and growth were deposited in Dryad (https://doi.org/10.5061/dryad.nzs7h451m).
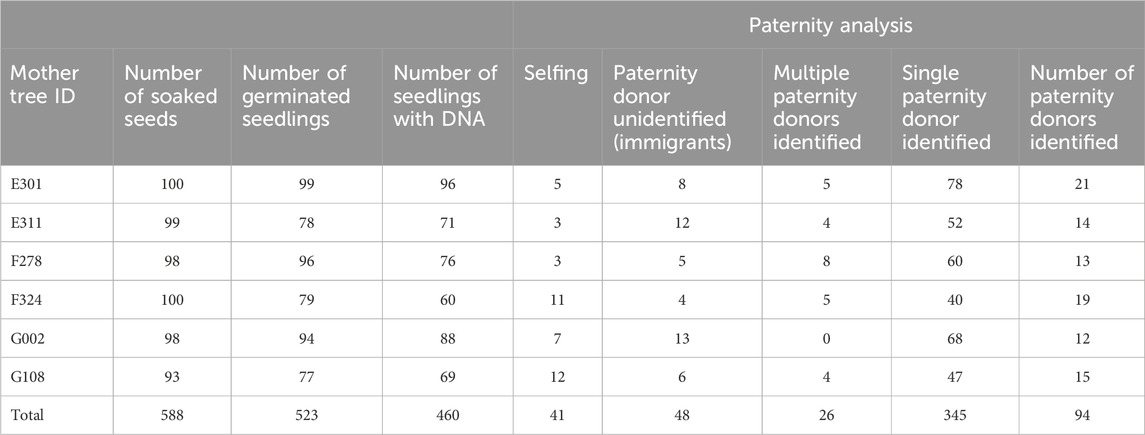
Table 2. Germination test for Rubroshorea curtsii seeds collected from six mother trees and result of paternity analysis for germinated seedlings with DNA retrievable.
Genomic DNA was extracted, using the method described by Murray and Thompson (1980). The material analyzed came from inner bark tissue samples of the adult trees from the research plots, material from either the inner bark or leaf tissues for the population samples, and from the leaves of seedlings. The extracted DNA from the adult trees and population samples was further purified using a High Pure PCR Template Preparation Kit (Roche). After RNA digestion, the DNA was diluted to a concentration of about 2 ng/μL. All samples were genotyped on the basis of ten microsatellite markers (shc04, shc07, and shc09 from Ujino et al. (1998), sle074, sle384, sle392, sle562, and sle566 from Lee et al. (2004), and slu044, and slu175 from Lee et al. (2006), see these references for details of microsatellite markers). Polymerase chain reaction (PCR) amplifications were carried out in total reaction volumes of 10 μL using a GeneAmp 9700 (Applied Biosystems). The PCR mixture contained 0.2 μM of each primer, 1x QIAGEN Multiplex PCR Master Mix (Qiagen), and 0.5–3 ng of template DNA. The temperature profile used was: 15 min at 95°C, then 30–35 cycles of 30 s at 94°C, 90 s at 50ºC–57°C and 90 s at 72°C, with a 10 min final extension step at 72°C. Amplified PCR fragments were electrophoretically separated using a 3100 genetic analyzer (Applied Biosystems) with a calibrated internal size standard (GeneScan ROX 400HD). The genotype of each individual was determined from the resulting electropherograms using GeneMarker (SoftGenetics). The microsatellite genotypic data from population samples, adult trees from the plots and seedlings from the nursery are referred as population genotype, plot genotype and seedling genotype, respectively. Due to fine-scale population structure and family structure in genotypes from adult trees and seedlings, quality of the microsatellite markers was checked using population sample in Semangkok forest reserve (abbreviated as SEMAN in Figure 1). No null alleles were detected in the Semangkok population, indicating a Hardy-Weinberg equilibrium state (Supplementary Table S1) by Micro-Checker analysis (Van Oosterhout et al., 2004). The plot genotypes were obtained in previous studies (Tani et al., 2012; Tani et al., 2015), and were already deposited in Dryad (http://dx.doi.org/10.5061/dryad.7k434). The population and seedling genotypes were separately deposited in Dryad (https://doi.org/10.5061/dryad.nzs7h451m).
The Bayesian model-based clustering method implemented in STRUCTURE 2.3.4 (Pritchard et al., 2000) was used to estimate the optimal number of genetic clusters and probability of individuals belonging to each cluster (k) for population and adult data separately. A burn-in of 5 × 104 steps followed by 105 steps of MCMC (Markov chain Monte Carlo) simulations for both genotypes were performed using the admixture and allele frequency correlated model without the LocPrior option. Each analysis was run five times for the range k = 1 - 6 for both sets of data. In order to evaluate the likelihood of K, we uploaded the structure-generated results to a web-based program structure harvester (Earl and von Holdt, 2012) and obtained plots of mean likelihood value (Ln PrX/K) and Delta K for successive values of K. We then determined the optimum values of K, following Evanno et al. (2005). For the selected K values, replicated results were aligned using CLUMPP version 1.1.2 (Jakobsson and Rosenberg, 2007), and visualized using distruct (Rosenberg, 2004) for population data.
We used categorical allocation in combination with an exclusion procedure to identify candidate paternal trees. The paternity of each offspring was determined based on likelihood ratios, and their confidence levels (greater than 95%) were derived using CERVUS ver. 3.0 (Marshall et al., 1998; Kalinowski et al., 2007). To conduct likelihood tests in CERVUS, we created 100,000 simulated offspring genotypes from 600 potential paternal candidates, with a mistyping rate of 0.1% in the categorical allocation. However, if the paternal candidates identified by the likelihood procedure had more than two loci mismatches in the simple exclusion procedure, we assumed that the paternal tree of the offspring was located outside the plot. Electropherograms were double-checked to confirm mismatches between the offspring and paternal candidates to minimize genotyping errors.
We estimated the genetic relatedness (rij) between the ith mother tree and the jth pollen donor within plots based on the microsatellite genotypes, using ML-Relate software (Kalinowski et al., 2006). Besides genetic relatedness, we also calculated two parameters to reflect the genetic heterogeneity between the parents of each seedling based on cluster analysis. As the probability of adult trees being assigned to the kth cluster was estimated as the Qk value, the attributional difference of the STRUCTURE cluster between the ith mother tree and the jth pollen donor using Q values (referred to as the ‘qdis’ statistic) was calculated, which is an application of Rogers’ statistic for genetic distance between populations (Rogers, 1972), as follows,
We also calculated another statistic whereby the attributional difference between the STRUCTURE cluster (Qk),and the genetic differentiation between the kth cluster and assumed ancestral admixed populations (fstk) were considered. The estimates of fstk from STRUCTURE analysis were: 0.10552, 0.00044 and −0.10342 for 1st, 2nd and 3rd cluster (kth), respectively. This was used to calculate the so-called “fdis” statistic, calculated as follows,
After these calculations, we assigned values of these statistics (r, qdis and fdis) to the parental pair of each seedling, based on the result of the paternity analysis.
In the seedling survival model, the status of the sth seedling at census time t, Ns(t), was coded as 0 until the seedling was found dead, at which point it was set to Ni(t) = 1 and no subsequent changes were considered. We used a proportional hazards model in the R statistical software using the package survival (Andersen and Gill, 1982; R Core Team, 2014; Therneau and Lumley, 2015) to fit maternal (seed weight represented as w) and biparental effects (r, qdis and fdis) to the survival of seedlings. As qdis and fdis were analyzed separately, we did not consider interaction between the explanatory variables. To select a best fit model among the combinations of the explanatory variables, and fixed or mixed models, we compared log-likelihood scores between candidate and null models and report the AICs of the models here.
We analyzed the height-based relative growth rate for each seedling in response to the maternal (w) and bi-parental effects (r, qdis and fdis) using a generalized linear mixed model (GLMM) with normal distribution error structure. Although we monitored the seedling height growth every month, the vertical growth during each 6-month period was used for the estimation of relative growth rate (RGR) after transplantation into individual pots. RGR from t-1 to t observation dates for the sth surviving seedling was calculated as follows:
where Hst is the seedling height in centimeters at Tt observation date and Hst-1 is the seedling height at Tt-1 observation date (about 6 months before Tt, the observation dates are shown in Table 4). A normal distribution was assumed for RGRst for the GLMM. As the survival analysis, qdis and fdis were analyzed separately, no interaction between the explanatory variables was considered. Uninformative priors were given for the parameters’ initial distribution, then the distribution was re-parameterized with a Hamiltonian Monte Carlo sampler using the No-U-Turns algorithm. These models were implemented in stan 2.5 (Carpenter et al., 2017) using the package “Rstan” in R 3.1.1 (R Core Team, 2014; Gelman et al., 2015; Stan Development Team, 2020) and glmmstan function (Shimizu, 2016). Three chains were run for each survival and growth model with explanatory variables. All models were run for 150,000 iterations, discarding the first 30,000 as a burn-in period. We used the Rhat statistic, together with a visual inspection of the chains, to assess convergence (Gelman and Rubin, 1992). The effect of the explanatory variables on the growth of seedlings was evaluated according to whether the 95% posterior credibility intervals of the estimated coefficients included the value of zero.
The software structure applying a Bayesian estimate without prior population information produced the highest Delta K when the number of populations was set at three clusters (K1 to 3) for population genotype (Supplementary Table S2). We applied three clusters for the subsequent analyses. Although the genetic differentiation between the ancestral admixed population and clustered populations showed that two of the clusters were well differentiated (Fst1 and Fst3 were 0.16698 and 0.16244, respectively), the genetic component of the second cluster was similar to that of the admixed population (Fst2 was 0.0006) when the population genotype was considered. On the other hand, the genetic differentiation between populations was lower (Fst = 0.082), which suggested that genetic components of the three clusters were distributed among most of the populations (Figure 1). This genetic clustering was also identified in adult tree populations in the plots. However, only the KLEDA population had a lower proportion for the K2 cluster and was like the genetic composition of the admixture.
At the fine scale, STRUCTURE analysis was also conducted using the plot genotypes in Semangkok Forest Reserve (the population genotypes of 9. Seman were collected from the vicinity of the forest monitoring plots, not within the plots). The adult trees in the plots also separated into three clusters at the fine scale. The fine-scale geographical distribution of Qk showed some aggregation of large Qk value individuals in all k clusters (Figure 2). Genetic differentiation between the ancestral admixed population and clustered populations showed the same tendency as the population genotypes, with Fstk values as follows, Fst1: 0.10552, Fst2: 0.00044, and Fst3: 0.10552. These Fstk values were used for the calculation of fdis.
Figure 2. The distribution of adult trees of Rubroshorea curtisii (with dbh greater than 20 cm) in the 6-ha undisturbed plot at Semangkok Forest Reserve, Selangor, Malay Peninsula. The diameters of red, green and blue circles of the adult trees represent Q values for cluster 1, cluster 2 and cluster 3, respectively, from structure analysis.
We collected nearly 100 seeds from each mother tree, which we soaked and placed on a seedbed of river sand on 30–31 January 2012. Seeds from three mother trees (E301, F278, and G002) showed almost 100% germination, while the other three mother trees (E311, F324, and G108) produced seeds with a lower germination rate, about 80%. After transplantation to pots, DNA was collected from leaf tissues of 60–96 individuals from each of the six mother trees, and this was used for paternity analysis (Table 2). Although the high exclusion power of the microsatellite markers was generally capable of assigning paternity to a single candidate or immigrant pollen from outside of the plot, 26 seedlings were excluded from subsequent analysis because there was no significant difference between the first and second candidate pollen donors. The paternity analysis showed 48 and 41 seedlings, respectively, derived from immigrant paternal donors and self-fertilization, and these were also excluded from subsequent analysis. As a result, 345 seedlings with single paternal donors inside the plots were used for further analysis (Table 2). Based on the paternity analysis, we evaluated the genetic relatedness between parents of the seedling (r), the attributional difference of clusters between the parents of seedling (qdis) and attributional difference of clusters with genetic differentiation (Fst) between the parents of the seedling (fdis). The most of seedlings were the result of genetically unrelated mating between paternal donors and mother trees; therefore, the distribution of rij was skewed to the left (Figure 3A). However, the distribution of qdisij was almost normal, with the exception of many mating pairs between parents that belonged to distinct clusters (less than 0.1 qdisij, Figure 3B). On the other hand, the effect of multiplying Fst changed the distribution of fdis. In particular, the difference in the probability of being assigned to the k2 cluster (Q2) between the parents of a seedling was masked by an almost zero value of Fst2 and differences in the probability of being assigned to the k1 and k3 clusters were offset by subtraction of the difference for cluster k1 from cluster k3 (Figure 3C).
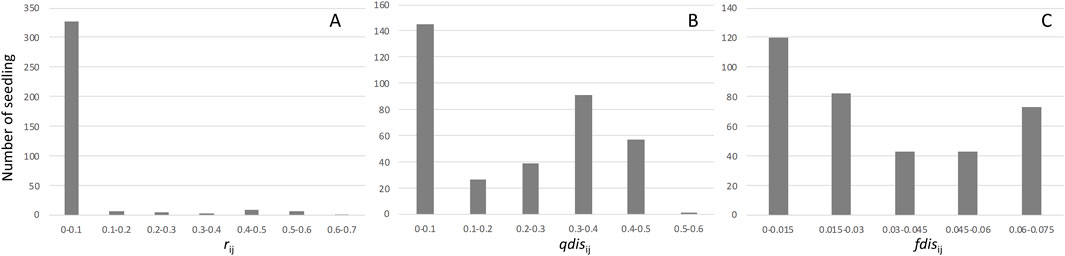
Figure 3. Frequencies of the genetic relationship between mother trees and pollen donors. Histogram (A) represents the distribution of relatedness between mother trees and pollen donors. Histogram (B) represents the distribution of qdis between mother trees and pollen donors. Histogram (C) represents the distribution of fdis between mother trees and pollen donors.
Based on paternity assignment, maternal and biparental effects on seedling survival and growth were investigated using the proportional hazards model (“cox” in the R package) and GLMM using a Bayesian approach, respectively. For survival analysis, the p value of the estimated coefficient of w was slightly significant in Models 1 and 5, and the p value of the estimated coefficient of r was not significant in Models 2 and 5 in the “cox” analysis. However, the values of the qdis and fdis statistics consistently had a high level of significance throughout the models (Table 3). Among the models including fdis and qdis, the ones with w and fdis or qdis parameters showed the best prediction based on AIC and BIC (Models 6 and 7). In the comparison between qdis and fdis, the residual deviance of Model 7 (involving w and qdis parameters) was slightly smaller than that of Model 6 (involving w and fdis), but there was slight difference of 3.37 between them (Table 3). The biparental effect, represented by the attributional difference of clusters between the parents of seedling (qdis) and the attributional difference of clusters with genetic differentiation (Fst) between the parents of the seedling (fdis), showed the best predictive ability to explain survival of R. curtisii seedlings in the nursery. The negative estimate of the qdis parameter represents the lower survival rate of seedlings with parents in the same cluster (based on the Q value in STRUCTURE). The positive estimate of the fdis parameter represents higher survival rates for seedlings resulting from mating between k1 and k3 type parents or mating between k2 type parents.
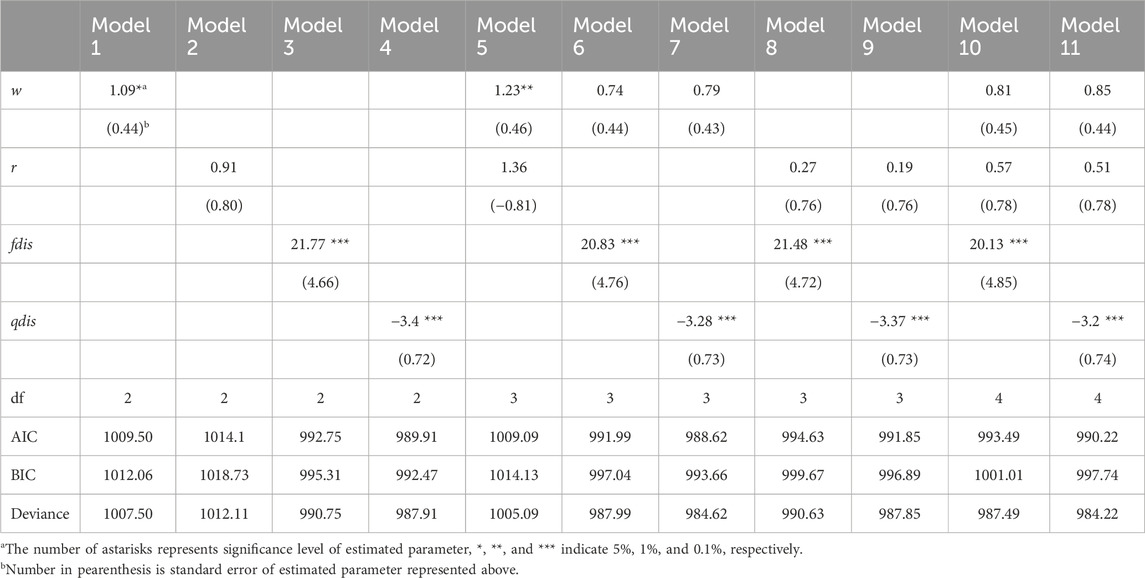
Table 3. Effects of seed weight, genetic biparental relatedness and difference of clustering attribution between parents on mortality of seedlings using COX proportional hazard model.
The observation period (about 2 years and 8 months) during which seedling growth was measured was separated into the period of growth in the seedbed and five subsequent periods (each lasting 6 months) in the nursery after transplantation. The 95% credibility intervals of estimated parameters relating to seed weight and genetic relatedness between parents (rij) included zero in all terms for both the qdis (Table 4) and fdis models (Table 5). However, the 95% credibility intervals of the estimated parameters for qdis were positively skewed from zero in the first and second 6-month periods in the nursery (Table 4). Those for fdis were negatively skewed from zero in the first and second 6-month periods in the nursery (Table 5). Any significance disappeared in the subsequent observation periods (Tables 4, 5). Only in the model involving fdis, was seed weight (w) positively correlated with seedling growth up to the third of the 6-month periods (Table 5), but the model involving qdis did not indicate any positive effect of seed weight (w) during any observation period (Table 4).
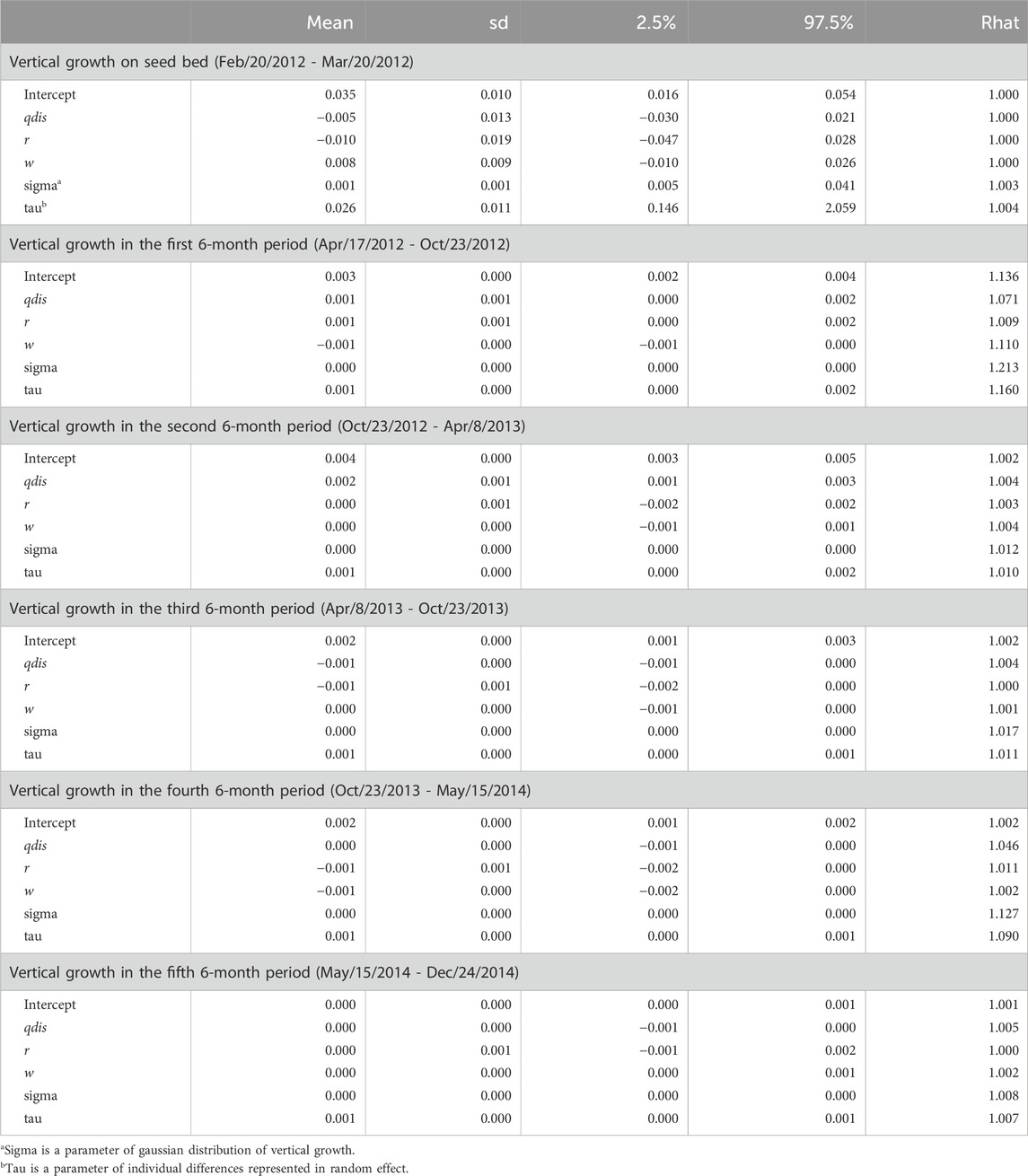
Table 4. Effects of attributional difference of clusters between the parents of seedling (qdis), relatedness (r) and seed weight (w) on relative vertical growth of seedlings in every 6 month period after transplantation.
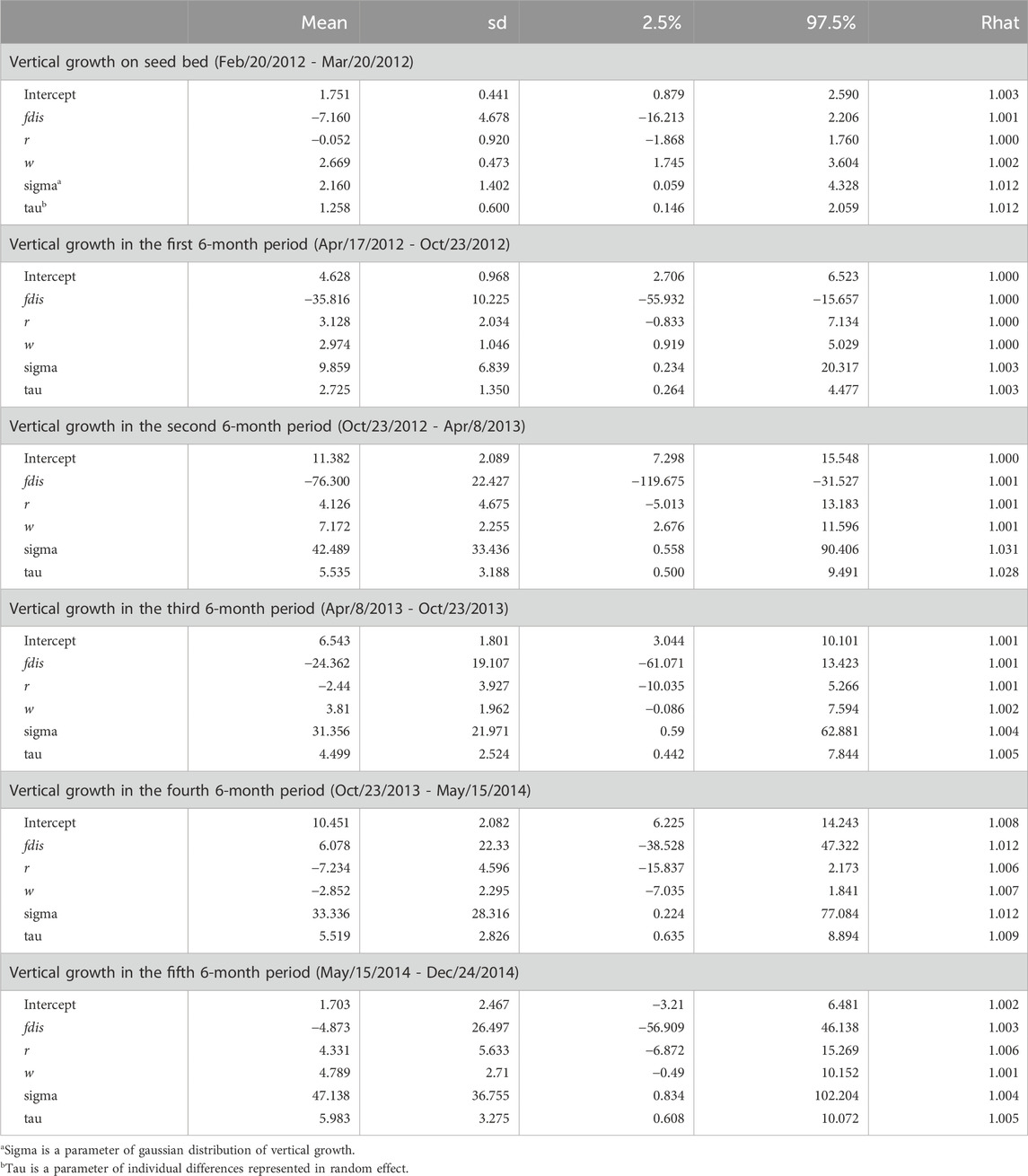
Table 5. Effects of attributional difference of clusters with genetic differentiation (Fst) between the parents of the seedling (fdis), relatedness (r) and seed weight (w) on relative vertical growth of seedlings in every 6 month period after transplantation.
The pattern of genetic structure of forest tree species has been shown to be related to only a few traits, such as mating system for the nuclear genome and seed dispersal mode or geographic range size for organelle markers (Duminil et al., 2007). The majority of dipterocarps adopt a mixed mating system (Kitamura et al., 1994; Lee et al., 2000c; Nagamitsu et al., 2001; Tani et al., 2009) and this is the case for R. curtisii (Obayashi et al., 2002; Tani et al., 2012; Tani et al., 2015). These species exhibit weak genetic differentiation between populations when there is no long-term geographic isolation, as is the case with other tree species in Malay Peninsula (Lee et al., 2000a; Lee et al., 2000b; Ng et al., 2017; Ng et al., 2019; Ng et al., 2024). On the other hand, comprehensive studies on the genetic structure of tropical tree species have suggested that limited seed dispersal enhances the genetic structure of populations (Hamrick et al., 1993; Lowe et al., 2018). Tropical tree species in Malay Peninsula with limited seed dispersal because of having wingless fruits also exhibit distinct genetic structure even though these species employ a mixed mating system (Ng et al., 2016; Lee et al., 2018; Ng C. H. et al., 2020). In the whole distributional scale, R. curtisii shows only two major distinct genetic clusters laying on northern major part and on southern tip of Malay Peninsula possibly due to the relatively larger genetic distinction at the southern populations (Ng et al., 2024). On the other hand, our analyzed population is within the range of main cluster of recent genetic diversity analysis. Although weak genetic differentiation was detected for the study species in Malay Peninsula, the Fst estimates of k1 and k3 in the Bayesian clustering analysis indicated large differentiation from the ancestral admixed population, which was the case in all studied populations except KLEDA (Figure 1), contributing to low genetic differentiation between populations. To date, there is no definitive evidence showing large genetic differentiation between the ancestral admixed population and the two clusters. However, a phylogenetic study using nucleotide variation of the chloroplast genome revealed that two distinct cpDNA haplogroups existed, differentiating the Malay peninsula and Borneo populations of R. curtisii, with several closely related species lying between the two lineages (Kamiya et al., 2012). Further, a nuclear PgiC phylogeny showed that samples from Malaysia Peninsula and Borneo were clustered together and were distinct from other closely related species (Kamiya et al., 2005). The incongruence between cpDNA and nuclear DNA-based phylogenies may be caused by organelle capture during historical hybridization events with closely related species. Currently, natural hybridization events have been reported between R. crtisii and R. leprosula especially in the coastal distribution area of Malay Peninsula (Kenzo et al., 2019; Ng K. K. S. et al., 2020). Dipterocarp species may include large groups of species that exchange genes forming syngameon complexes or interfertile species (Cannon and Lerdau, 2015). The large differentiation of k1 and k3 clusters from the ancestral admixed population (which is close to the k2 cluster) may have possibility resulted from historical interspecific gene exchange between ancestral closely related species constituting a syngaemon. However, this would not be the result of current inter-specific hybridization between R. curtisii and R. leprosula, because the hybrids can be distinguished from the parent species by several leaf morphological characters, and some characters of the hybrids resemble R. leprosula more closely (Kamiya et al., 2012; Kenzo et al., 2019). None of our seedlings exhibited the intermediate type of leaf morphology (observation in nursery). On the other hand, a subspecies has been reported (R. curtisii Dyer ex King ssp. grandis P. S. Ashton), although this is rare and has been found only in two limited areas close to the KLEDA population. The wide distributional ranges of the three clusters, covering most of the studied populations, may indicate the maintenance of a syngaemon formed by ancestral genetic differentiation within species and among closely-related species. Further studies are required to gain an understanding of the population history and evolution of R. curtisii.
Two evolutionary episodes were assumed to explain the effect of the putative syngameon on seedling fitness. Seedlings with large qdis were the offspring of parents with very different Qk estimates from each other. The fdis was designed to express the fact that the k2 cluster was intermediate between the k1 and k3 clusters. For example, the large Q1 value for ith mother tree of a seedling is neutralized by a large Q3 for jth paternal donor of the seedling when estimating fdis. In other words, a hybrid derived from mating between an individual with a high likelihood of belonging to the k1 cluster and another with a high likelihood of belonging to the k3 cluster is assumed to resemble the k2 cluster showing, with less genetic differentiation from the genetic make-up of the ancestral admixed population. The analyses to test the effects on survival showed that the absolute values of the qdis and fdis estimates are always larger than those of the other estimates, r, w (Table 3). Comparing qdis and fdis, deviances of models including qdis were always a little smaller than the corresponding models with fdis, indicating that the models with qdis explained survival of seedlings slightly better than those with fdis. Similarly, the early stages of growth were affected more by qdis and fdis than r and w, however, this tendency declined in the later stage of growth (Tables 4, 5). Thus, these variables indicating genetic differentiation between the parents of seedlings (qdis and fdis) can provide a sound explanation for the survival and initial growth of seedlings. This result helps us to understand the advantages of genetic differentiation, including the syngameon hypothesis, which may be linked to the maintenance of high tree species diversity in tropical rainforests. Seed dispersal limitation is typically associated with a spatially aggregated distribution pattern in tropical rainforest (Condit et al., 2000), which prevents tree species from recolonization and facilitates further aggregation of species complexes with intercrossing-ability (Cannon and Lerdau, 2015). The proximity of these mixed offspring could effectively allow them to back-cross, thus increasing mate choice with congeners and potentially re-establishing the local population (Baskett and Gomulkiewicz, 2011). This mechanism may enhance the fitness of seedlings from mother trees sired by pollen donors with different genetic backgrounds.
In the mating and seedling establishment processes, inbred offspring (both selfing and biparental inbreeding) tend to be less successful due to inbreeding depression during fruit maturation (Tani et al., 2015) and the early stage of seedling establishment (Naito et al., 2005); therefore, r contributes less to survival and growth of seedlings in the subsequent stages of establishment. In the early stage of seedling development, ca. 40% of seedlings were derived from mating between parents with a similar probability of being assigned to clusters with less inbred mating (Figures 3A,B). These outcrossed seedlings produced by mating between parents with similar probabilities of being assigned to a particular cluster exhibited poorer survival throughout the observation period (Figure 4) and lower growth rates during the first year after transplantation (Table 4). This selection process can maintain genetic diversity through effects of overdominance and negative frequency-dependent selection (Ayala and Campbell, 1974; Asmussen and Basnayake, 1990). These effects have been noted as a mechanism to maintain genetic diversity in tropical forest tree species (Browne and Karubian, 2016; 2018). In natural stands, genetic diversity and the effects of overdominance and negative frequency-dependent selection may have positive effects with respect to escaping pathogens and herbivores of other trees. The Janzen-Connell hypothesis states that seeds or seedlings occurring at high densities or close to adult conspecifics are more vulnerable to attack from natural enemies such as pathogens and herbivores (Janzen, 1970; Connell, 1971). If a difference of compatibility between the enemies and genotypes exists, this may be another reason to maintain genetic diversity. Our seedings were grown in river sand in the seed bed and a mixture of river sand and organic soil in pots, produced by FRIM, so it was impossible to show that negative frequency dependent selection (represented by higher fitness of seedlings sired by a father with a different type of genetic diversity) protects offspring from enemies present on the mother trees. Therefore, it is very important that, in the future, we study survival and growth of seedlings in natural stands in relation to the genotypes of the seedlings and nearby adult trees.
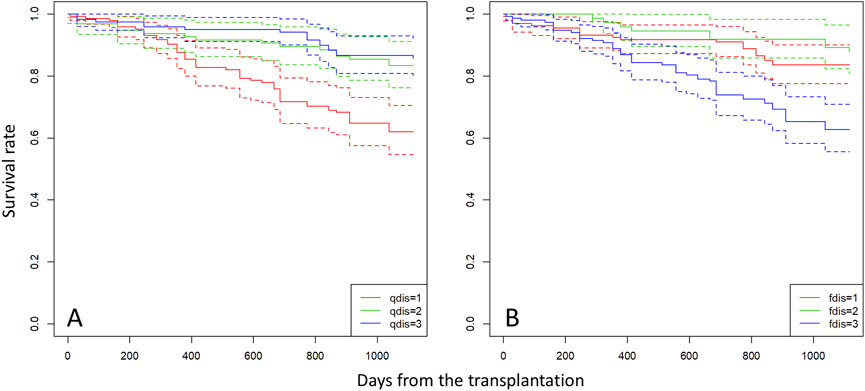
Figure 4. Survival curves for seedlings categorized into three groups based on qdis (A) and fdis (B) of each individual. Group “1” comprises seedlings with qdis < 0.1 and fdis < 0.02, group “2” comprises seedlings with 0.1 < qdis < 0.35 and 0.02 < fdis < 0.035, and group “3” comprises seedlings with qdis > 0.35 and fdis > 0.035.
We observed maintenance of strong genetic differentiation of the clusters in adult trees in the plots, the Semangkok population and at other levels (Figures 1, 2), which affected fitness of their seedlings. However, our seedling monitoring was conducted in a nursery, which can provide an ideal environment, including controlled light intensity and sufficient water. In natural stands, many dipterocarp species spend decades or more in the forest understory as seedlings (Delissio et al., 2002) and their ability to persist in the face of multiple stresses (e.g., pests, light, water and nutrient limitation) determines their chances of reaching the reproductive stage (Comita and Engelbrecht, 2014). Many studies have shown that drought is a major stress causing mortality of dipterocarp seedlings (e.g., Turner, 1990; Delissio and Primack, 2003; Bebber et al., 2004). Therefore, survival and growth of seedlings in relation to qdis and fdis statistics may represent different consequences under natural conditions. To understand the maintenance and evolutionary significance of the genetic differentiation pattern of clustering, the demographic analysis of natural seedlings with clear genetic structure is required. El Niño causes drought in Southeast Asian tropical forests and a greater possibility of drought is projected as a result of climate change in the near future (Amnuaylojaroen and Chanvichit, 2019; Rifai et al., 2019); this has the potential to cause failure of seedling recruitment and adversely affect seedling growth (Curran et al., 1999; Bebber et al., 2004; Condit et al., 2004; Itoh et al., 2012). Therefore, understanding adaptation of the species under various conditions, such as in managed and natural stands, is essential for conservation of tropical forests, especially in relation to climate change.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
NT: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. CN: Data curation, Investigation, Resources, Writing–original draft, Writing–review and editing. SL: Investigation, Project administration, Resources, Writing–original draft, Writing–review and editing. CL: Data curation, Investigation, Resources, Writing–original draft, Writing–review and editing. NM: Project administration, Supervision, Writing–original draft, Writing–review and editing. TK: Data curation, Investigation, Resources, Writing–original draft, Writing–review and editing. YT: Project administration, Supervision, Writing–original draft, Writing–review and editing. SS: Data curation, Investigation, Writing–original draft, Writing–review and editing. KN: Data curation, Investigation, Resources, Writing–original draft, Writing–review and editing. AR: Data curation, Investigation, Resources, Writing–original draft, Writing–review and editing. AK: Data curation, Resources, Writing–original draft, Writing–review and editing. SM: Project administration, Resources, Writing–original draft, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was partly supported by a JIRCAS-FRIM joint project entitled “Evaluation of genetic resources for strengthening productivity and adaptability of tropical forests” (a1A403a) funded by Agriculture, Forestry and Fisheries Research Council (AFFRC), and Japan and a Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Sciences (No. 24405034).
The authors wish to acknowledge Drs. S. Ibrahim, I. Harun, T. Okuda, T. Gotoh, R. Tabuchi, I. Noda, M. Saito and Y. Yamamoto for project management, Mr. G. Jaafar, Y. Marhani, R. Ponyoh, Y. Baya and A. G. Mohd-Afendi for field assistance and Ms. D. Mariam, Ms. A. W. Nor Salwah and Ms. M. Nurul Hudaini for their assistance with DNA extraction. We also thank Ms. K. Obayashi and Dr. T. Katsuki for providing some of the DNA material, Drs. T. Yagihashi and D. Hoshino for plot management in Semangkok Forest Reserve, and Ms. M. Koshiba, Ms. K. Ito and Ms. R. Yamada for their laboratory and administrative support. The Selangor State Forest Department is acknowledged for granting permission to conduct research in Semangkok Forest Reserve.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2025.1552024/full#supplementary-material
Alroy, J. (2017). Effects of habitat disturbance on tropical forest biodiversity. Proc. Natl. Acad. Sci. 114 (23), 6056–6061. doi:10.1073/pnas.1611855114
Amnuaylojaroen, T., and Chanvichit, P. (2019). Projection of near-future climate change and agricultural drought in Mainland Southeast Asia under RCP8. 5. Clim. Change 155 (2), 175–193. doi:10.1007/s10584-019-02442-5
Andersen, P. K., and Gill, R. D. (1982). Cox's regression model for counting processes: a large sample study. Ann. statistics 10, 1100–1120. doi:10.1214/aos/1176345976
Antonovics, J. (1976). The input from population genetics: the new ecological genetics. Syst. Bot. 1 (3), 233–245. doi:10.2307/2418718
Ashton, P., and Heckenhauer, J. (2022). Tribe Shoreae (Dipterocarpaceae subfamily dipterocarpoideae) finally dissected. Kew Bull. 77 (4), 885–903. doi:10.1007/s12225-022-10057-w
Ashton, P. S. (1969). Speciation among tropical forest trees: some deductions in the light of recent evidence. Biol. J. Linn. Soc. 1 (1-2), 155–196. doi:10.1111/j.1095-8312.1969.tb01818.x
Ashton, P. S. (1979). “Final discussion,” in Dipterocarpaceae: taxonomie-phylogénie-ecologie. Editor G. Maury-Lechon (Paris: Editions du Museum), 159.
Ashton, P. S. (1982). “Dipterocarpaceae,” in Flora malesiana. Editor C. G. G. J. Van Steenis (Hague, Netherland: Series I.).
Asmussen, M., and Basnayake, E. (1990). Frequency-dependent selection: the high potential for permanent genetic variation in the diallel, pairwise interaction model. Genetics 125 (1), 215–230. doi:10.1093/genetics/125.1.215
Ayala, F. J., and Campbell, C. A. (1974). Frequency-dependent selection. Annu. Rev. Ecol. Syst. 5 (1), 115–138. doi:10.1146/annurev.es.05.110174.000555
Bagchi, R., Press, M. C., and Scholes, J. D. (2010a). Evolutionary history and distance dependence control survival of dipterocarp seedlings. Ecol. Lett. 13 (1), 51–59. doi:10.1111/j.1461-0248.2009.01397.x
Bagchi, R., Swinfield, T., Gallery, R. E., Lewis, O. T., Gripenberg, S., Narayan, L., et al. (2010b). Testing the Janzen-Connell mechanism: pathogens cause overcompensating density dependence in a tropical tree. Ecol. Lett. 13 (10), 1262–1269. doi:10.1111/j.1461-0248.2010.01520.x
Baskett, M. L., and Gomulkiewicz, R. (2011). Introgressive hybridization as a mechanism for species rescue. Theor. Ecol. 4 (2), 223–239. doi:10.1007/s12080-011-0118-0
Bebber, D. P., Brown, N. P., and Speight, M. R. (2004). Dipterocarp seedling population dynamics in Bornean primary lowland forest during the 1997-8 El Niño-Southern Oscillation. J. Trop. Ecol. 20 (1), 11–19. doi:10.1017/s0266467404006133
Brown, N. D., and Whitmore, T. C. (1992). Do dipterocarp seedlings really partition tropical rain forest gaps? Philos. Trans. Biol. Sci. 335, 369–378. doi:10.1098/rstb.1992.0028
Browne, L., and Karubian, J. (2016). Frequency-dependent selection for rare genotypes promotes genetic diversity of a tropical palm. Ecol. Lett. 19 (12), 1439–1447. doi:10.1111/ele.12692
Browne, L., and Karubian, J. (2018). Rare genotype advantage promotes survival and genetic diversity of a tropical palm. New Phytol. 218 (4), 1658–1667. doi:10.1111/nph.15107
Cannon, C. H., and Lerdau, M. (2015). Variable mating behaviors and the maintenance of tropical biodiversity. Front. Genet. 6, 183. doi:10.3389/fgene.2015.00183
Cannon, C. H., and Petit, R. J. (2020). The oak syngameon: more than the sum of its parts. New Phytol. 226 (4), 978–983. doi:10.1111/nph.16091
Carpenter, B., Gelman, A., Hoffman, M. D., Lee, D., Goodrich, B., Betancourt, M., et al. (2017). Stan: a probabilistic programming language. J. Stat. Softw. 76 (1), 1. doi:10.18637/jss.v076.i01
Comita, L. S., and Engelbrecht, B. M. (2014). Drought as a driver of tropical tree species regeneration dynamics and distribution patterns. For. Glob. change, 261–308. doi:10.1017/cbo9781107323506.013
Condit, R., Aguilar, S., Hernandez, A., Perez, R., Lao, S., Angehr, G., et al. (2004). Tropical forest dynamics across a rainfall gradient and the impact of an El Niño dry season. J. Trop. Ecol. 20 (1), 51–72. doi:10.1017/s0266467403001081
Condit, R., Ashton, P. S., Baker, P., Bunyavejchewin, S., Gunatilleke, S., Gunatilleke, N., et al. (2000). Spatial patterns in the distribution of tropical tree species. Science 288 (5470), 1414–1418. doi:10.1126/science.288.5470.1414
Connell, J. H. (1961). The influence of interspecific competition and other factors on the distribution of the barnacle Chthamalus stellatus. Ecology 42 (4), 710–723. doi:10.2307/1933500
Connell, J. H. (1971). “On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forest trees,”. Dynamics of populations. Editors P. J. Den Boer, and G. R. Gradwell (Wageningen, the Netherlands: Centre for Agricultural Publishing and Documentation), 312.
Cronk, Q. C., and Suarez-Gonzalez, A. (2018). The role of interspecific hybridization in adaptive potential at range margins. Mol. Ecol. 27 (23), 4653–4656. doi:10.1111/mec.14927
Curran, L. M., Caniago, I., Paoli, G. D., Astianti, D., Kusneti, M., Leighton, M., et al. (1999). Impact of El Niño and logging on canopy tree recruitment in Borneo. Science 286 (5447), 2184–2188. doi:10.1126/science.286.5447.2184
Dayanandan, S., Ashton, P. S., Williams, S. M., and Primack, R. B. (1999). Phylogeny of the tropical tree family Dipterocarpaceae based on nucleotide sequences of the chloroplast rbcL gene. Am. J. Bot. 86 (8), 1182–1190. doi:10.2307/2656982
Delissio, L. J., and Primack, R. B. (2003). The impact of drought on the population dynamics of canopy-tree seedlings in an aseasonal Malaysian rain forest. J. Trop. Ecol. 19 (5), 489–500. doi:10.1017/s0266467403003547
Delissio, L. J., Primack, R. B., Hall, P., and Lee, H. (2002). A decade of canopy-tree seedling survival and growth in two Bornean rain forests: persistence and recovery from suppression. J. Trop. Ecol. 18 (5), 645–658. doi:10.1017/s0266467402002420
Dent, D. H., and Burslem, D. F. (2016). Leaf traits of dipterocarp species with contrasting distributions across a gradient of nutrient and light availability. Plant Ecol. and Divers. 9 (5-6), 521–533. doi:10.1080/17550874.2016.1265018
Duminil, J., Fineschi, S., Hampe, A., Jordano, P., Salvini, D., Vendramin, G. G., et al. (2007). Can population genetic structure be predicted from life-history traits? Am. Nat. 169 (5), 662–672. doi:10.1086/513490
Earl, D. A., and von Holdt, B. M. (2012). STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 4 (2), 359–361. doi:10.1007/s12686-011-9548-7
Eck, J. L., Stump, S. M., Delavaux, C. S., Mangan, S. A., and Comita, L. S. (2019). Evidence of within-species specialization by soil microbes and the implications for plant community diversity. Proc. Natl. Acad. Sci. 116 (15), 7371–7376. doi:10.1073/pnas.1810767116
Evanno, G., Regnaut, S., and Goudet, J. (2005). Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol. Ecol. 14 (8), 2611–2620. doi:10.1111/j.1365-294X.2005.02553.x
Gelman, A., Lee, D., and Guo, J. (2015). Stan: a probabilistic programming language for Bayesian inference and optimization. J. Educ. Behav. Statistics 40 (5), 530–543. doi:10.3102/1076998615606113
Gelman, A., and Rubin, D. B. (1992). Inference from iterative simulation using multiple sequences. Stat. Sci. 7, 457–511. doi:10.1214/ss/1177011136
Haldane, J. B. S. (1949). Disease and evolution. Suppl. La Ricerca Sci. 19, 68–76. doi:10.1111/j.1601-5223.1949.tb03339.x
Hamrick, J. L., Murawski, D. A., and Nason, J. D. (1993). The influence of seed dispersal mechanisms on the genetic-structure of tropical tree populations. Vegetatio 108, 281–297. doi:10.1007/978-94-011-1749-4_20
Heckenhauer, J., Samuel, R., Ashton, P. S., Salim, K. A., and Paun, O. (2018). Phylogenomics resolves evolutionary relationships and provides insights into floral evolution in the tribe Shoreeae (Dipterocarpaceae). Mol. phylogenetics Evol. 127, 1–13. doi:10.1016/j.ympev.2018.05.010
Hubbell, S. P. (1997). A unified theory of biogeography and relative species abundance and its application to tropical rain forests and coral reefs. Coral Reefs 16 (1), S9–S21. doi:10.1007/s003380050237
Hubbell, S. P. (2001). The unified theory of biodiversity and biogeography. Princeton, NJ: Princeton University Press.
Indrioko, S., Gailing, O., and Finkeldey, R. (2006). Molecular phylogeny of Dipterocarpaceae in Indonesia based on chloroplast DNA. Plant Syst. Evol. 261 (1), 99–115. doi:10.1007/s00606-006-0435-8
Itoh, A., Nanami, S., Harata, T., Ohkubo, T., Tan, S., Chong, L., et al. (2012). The effect of habitat association and edaphic conditions on tree mortality during El Niño-induced drought in a Bornean dipterocarp forest. Biotropica 44 (5), 606–617. doi:10.1111/j.1744-7429.2012.00867.x
Jakobsson, M., and Rosenberg, N. A. (2007). CLUMPP: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics 23 (14), 1801–1806. doi:10.1093/bioinformatics/btm233
Janzen, D. H. (1970). Herbivores and the number of tree species in tropical forests. Am. Nat. 104 (940), 501–528. doi:10.1086/282687
Jia, S., Wang, X., Yuan, Z., Lin, F., Ye, J., Lin, G., et al. (2020). Tree species traits affect which natural enemies drive the Janzen-Connell effect in a temperate forest. Nat. Commun. 11 (1), 286. doi:10.1038/s41467-019-14140-y
Kajita, T., Kamiya, K., Nakamura, K., Tachida, H., Wickneswari, R., Tsumura, Y., et al. (1998). Molecular phylogeny of Dipetrocarpaceae in southeast Asia based on nucleotide sequences of matK, trnL Intron, and trnL-trnF intergenic spacer region in chloroplast DNA. Mol. Phylogenetics Evol. 10 (2), 202–209. doi:10.1006/mpev.1998.0516
Kalinowski, S. T., Taper, M. L., and Marshall, T. C. (2007). Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 16 (5), 1099–1106. doi:10.1111/j.1365-294X.2007.03089.x
Kalinowski, S. T., Wagner, A. P., and Taper, M. L. (2006). ML-RELATE: a computer program for maximum likelihood estimation of relatedness and relationship. Mol. Ecol. Notes 6 (2), 576–579. doi:10.1111/j.1471-8286.2006.01256.x
Kamiya, K., Gan, Y. Y., Lum, S. K., Khoo, M. S., Chua, S. C., and Faizu, N. N. (2011). Morphological and molecular evidence of natural hybridization in Shorea (Dipterocarpaceae). Tree Genet. and Genomes 7 (2), 297–306. doi:10.1007/s11295-010-0332-8
Kamiya, K., Harada, K., Tachida, H., and Ashton, P. S. (2005). Phylogeny of PgiC gene in Shorea and its closely related genera (Dipterocarpaceae), the dominant trees in Southeast Asian tropical rain forests. Am. J. Bot. 92 (5), 775–788. doi:10.3732/ajb.92.5.775
Kamiya, K., Nanami, S., Kenzo, T., Yoneda, R., Diway, B., Chong, L., et al. (2012). Demographic history of Shorea curtisii (Dipterocarpaceae) inferred from chloroplast DNA sequence variations. Biotropica 44 (5), 577–585. doi:10.1111/j.1744-7429.2011.00834.x
Kenzo, T., Ichie, T., Norichika, Y., Kamiya, K., Inoue, Y., Ngo, K. M., et al. (2023). Drought tolerance in dipterocarp species improved through interspecific hybridization in a tropical rainforest. For. Ecol. Manag. 548, 121388. doi:10.1016/j.foreco.2023.121388
Kenzo, T., Kamiya, K., Ngo, K. M., Faizu, N., Lum, S. K. Y., Igarashi, S., et al. (2019). Overlapping flowering periods among Shorea species and high growth performance of hybrid seedlings promote hybridization and introgression in a tropical rainforest of Singapore. For. Ecol. Manag. 435, 38–44. doi:10.1016/j.foreco.2018.12.038
Kitamura, K., Rahman, M., Ochiai, Y., and Yoshimaru, H. (1994). Estimation of the outcrossing rate on Dryobalanops aromatica Gaertn. f. in primary and secondary forests in Brunei, Borneo, Southeast Asia. Plant species Biol. 9 (1), 37–41. doi:10.1111/j.1442-1984.1994.tb00080.x
Lee, C. T., Tnah, L. H., Ng, K. K. S., Nurul-Farhanah, Z., Ng, C. H., Lau, K. H., et al. (2018). Plant material transfer guidelines for tropical forest species. Kepong, Selangor, Malaysia: Kepong, Selengor, Malaysia: Forest Research Institute Malaysia.
Lee, S. L., Ang, K. C., and Norwati, M. (2000a). Genetic diversity of Dryobalanops aromatica Gaertn. f. (Dipterocarpaceae) in Peninsular Malaysia and its pertinence to genetic conservation and tree improvement. For. Genet. 7 (3), 209–217.
Lee, S. L., Ng, K. K. S., Saw, L. G., Lee, C. T., Muhammad, N., Tani, N., et al. (2006). Linking the gaps between conservation research and conservation management of rare dipterocarps: a case study of Shorea lumutensis. Biol. Conserv. 131 (1), 72–92. doi:10.1016/j.biocon.2006.02.005
Lee, S. L., Tani, N., Ng, K. K. S., and Tsumura, Y. (2004). Isolation and characterization of 20 microsatellite loci for an important tropical tree Shorea leprosula (Dipterocarpaceae) and their applicability to S. parvifolia. Mol. Ecol. Notes 4 (2), 222–225. doi:10.1111/j.1471-8286.2004.00623.x
Lee, S. L., Wickneswari, R., Mahani, M. C., and Zakri, A. H. (2000b). Genetic diversity of a tropical tree species, Shorea leprosula miq. (Dipterocarpaceae), in Malaysia: implications for conservation of genetic resources and tree improvement. Biotropica 32 (2), 213–224. doi:10.1646/0006-3606(2000)032[0213:gdoatt]2.0.co;2
Lee, S. L., Wickneswari, R., Mahani, M. C., and Zakri, A. H. (2000c). Mating system parameters in a tropical tree species, Shorea leprosula miq. (Dipterocarpaceae), from Malaysian lowland dipterocarp forest. Biotropica 32 (4a), 693–702. doi:10.1646/0006-3606(2000)032[0693:mspiat]2.0.co;2
Levi, T., Barfield, M., Barrantes, S., Sullivan, C., Holt, R. D., and Terborgh, J. (2019). Tropical forests can maintain hyperdiversity because of enemies. Proc. Natl. Acad. Sci. 116 (2), 581–586. doi:10.1073/pnas.1813211116
Lowe, A. J., Breed, M. F., Caron, H., Colpaert, N., Dick, C., Finegan, B., et al. (2018). Standardized genetic diversity-life history correlates for improved genetic resource management of Neotropical trees. Divers. Distributions 24 (6), 730–741. doi:10.1111/ddi.12716
Marshall, T. C., Slate, J., Kruuk, L. E. B., and Pemberton, J. M. (1998). Statistical confidence for likelihood-based paternity inference in natural populations. Mol. Ecol. 7 (5), 639–655. doi:10.1046/j.1365-294x.1998.00374.x
Maury-Lechon, G. (1978). Diptérocarpacées: du fruit à la plantule. Universite Paul Sabatier. PhD Dissertation.
Molino, J.-F., and Sabatier, D. (2001). Tree diversity in tropical rain forests: a validation of the intermediate disturbance hypothesis. Science 294 (5547), 1702–1704. doi:10.1126/science.1060284
Murray, M. G., and Thompson, W. F. (1980). Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 8 (19), 4321–4325. doi:10.1093/nar/8.19.4321
Nagamitsu, T., Ichikawa, S., Ozawa, M., Shimamura, R., Kachi, N., Tsumura, Y., et al. (2001). Microsatellite analysis of the breeding system and seed dispersal in Shorea leprosula (Dipterocarpaceae). Int. J. Plant Sci. 162 (1), 155–159. doi:10.1086/317902
Naito, Y., Konuma, A., Iwata, H., Suyama, Y., Seiwa, K., Okuda, T., et al. (2005). Selfing and inbreeding depression in seeds and seedlings of Neobalanocarpus heimii (Dipterocarpaceae). J. Plant Res. 118 (6), 423–430. doi:10.1007/s10265-005-0245-z
Ng, C. H., Lee, S. L., Tnah, L. H., Ng, K. K., Lee, C. T., Diway, B., et al. (2019). Genetic diversity and demographic history of an upper hill dipterocarp (Shorea platyclados): implications for conservation. J. Hered. 110 (7), 844–856. doi:10.1093/jhered/esz052
Ng, C. H., Lee, S. L., Tnah, L. H., Ng, K. K. S., Lee, C. T., Diway, B., et al. (2017). Geographic origin and individual assignment of Shorea platyclados (Dipterocarpaceae) for forensic identification. PloS one 12 (4), e0176158. doi:10.1371/journal.pone.0176158
Ng, C. H., Ng, K. K. S., Lee, S. L., Alias, N. N., Humaira' Zakaria, H., Tnah, L. H., et al. (2024). Genetic diversity and population structure of Rubroshorea curtisii, a hill dipterocarp species: implications for conservation. Tree Genet. and Genomes 20 (3), 16. doi:10.1007/s11295-024-01649-7
Ng, C. H., Ng, K. K. S., Lee, S. L., Tnah, L. H., Lee, C. T., and Zakaria, N.-F. (2020a). A geographical traceability system for Merbau (Intsia palembanica Miq.), an important timber species from peninsular Malaysia. Forensic Sci. Int. Genet. 44, 102188. doi:10.1016/j.fsigen.2019.102188
Ng, K. K. S., Lee, S. L., Tnah, L. H., Ng, C. H., Lee, C. T., Nurul-Farhanah, Z., et al. (2020b). Confirmation of natural hybrid of Shorea leprosula and S. curtisii in pasir panjang forest reserve, negeri sembilan, Malaysia. J. Trop. For. Sci. 32 (1), 92–96. doi:10.26525/jtfs32.1.92
Ng, K. K. S., Lee, S. L., Tnah, L. H., Nurul-Farhanah, Z., Ng, C. H., Lee, C. T., et al. (2016). Forensic timber identification: a case study of a CITES listed species, Gonystylus bancanus (Thymelaeaceae). Forensic Sci. Int. Genet. 23, 197–209. doi:10.1016/j.fsigen.2016.05.002
Niiyama, K., Rahman, K. A., Iida, S., Kimura, K., Azizi, R., and Appanah, S. (1999). Spatial patterns of common tree species relating to topography, canopy gaps and understory vegetation in a hill dipterocarp forest at Semangkok forest reserve, Peninsular Malaysia. J. Trop. For. Sci. 11, 731–745.
Obayashi, K., Tsumura, Y., Ihara-Ujino, T., Niiyama, K., Tanouchi, H., Suyama, Y., et al. (2002). Genetic diversity and outcrossing rate between undisturbed and selectively logged forests of Shorea curtisii (Dipterocarpaceae) using microsatellite DNA analysis. Int. J. Plant Sci. 163 (1), 151–158. doi:10.1086/324549
Potts, M. D., Ashton, P. S., Kaufman, L. S., and Plotkin, J. B. (2002). Habitat patterns in tropical rain forests: a comparison of 105 plots in northwest Borneo. Ecology 83 (10), 2782–2797. doi:10.2307/3072015
Pritchard, J. K., Stephens, M., and Donnelly, P. (2000). Inference of population structure using multilocus genotype data. Genetics 155 (2), 945–959. doi:10.1093/genetics/155.2.945
R Core Team (2014). R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Rifai, S. W., Li, S., and Malhi, Y. (2019). Coupling of El Niño events and long-term warming leads to pervasive climate extremes in the terrestrial tropics. Environ. Res. Lett. 14 (10), 105002. doi:10.1088/1748-9326/ab402f
Rogers, J. S. (1972). “Measures of genetic similarity and genetic distance,” in Studies in genetics VII (Austin, Texas: University of Texas Publication), 145–153.
Rosenberg, N. A. (2004). DISTRUCT: a program for the graphical display of population structure. Mol. Ecol. Notes 4 (1), 137–138. doi:10.1046/j.1471-8286.2003.00566.x
Schley, R. J., Twyford, A. D., and Pennington, R. T. (2022). Hybridization: a ‘double-edged sword’for Neotropical plant diversity. Botanical J. Linn. Soc. 199 (1), 331–356. doi:10.1093/botlinnean/boab070
Schnitzer, S. A., and Carson, W. P. (2001). Treefall gaps and the maintenance of species diversity in a tropical forest. Ecology 82 (4), 913–919. doi:10.2307/2679891
Seehausen, O. (2004). Hybridization and adaptive radiation. Trends Ecol. and Evol. 19 (4), 198–207. doi:10.1016/j.tree.2004.01.003
Symington, C. F. (1943). “Foresters’ manual of dipterocarps,” in Malayan forest record no. 16 (Kuala Lumpur, Malaysia: University of Malaya Press).
Tani, N., Tsumura, Y., Fukasawa, K., Kado, T., Taguchi, Y., Lee, S. L., et al. (2012). Male fecundity and pollen dispersal in hill dipterocarps: significance of mass synchronized flowering and implications for conservation. J. Ecol. 100, 405–415. doi:10.1111/j.1365-2745.2011.01929.x
Tani, N., Tsumura, Y., Fukasawa, K., Kado, T., Taguchi, Y., Lee, S. L., et al. (2015). Mixed mating system are regulated by fecundity in Shorea curtisii (Dipterocarpaceae) as revealed by comparison under different pollen limited conditions. Plos one 10 (5), e0123445. doi:10.1371/journal.pone.0123445
Tani, N., Tsumura, Y., Kado, T., Taguchi, Y., Lee, S. L., Muhammad, N., et al. (2009). Paternity analysis-based inference of pollen dispersal patterns, male fecundity variation and influence of flowering tree density and general flowering magnitude in two dipterocarp species. Ann. Bot. 104 (7), 1421–1434. doi:10.1093/aob/mcp252
Tsumura, Y., Kado, T., Yoshida, K., Abe, H., Ohtani, M., Taguchi, Y., et al. (2011). Molecular database for classifying Shorea species (Dipterocarpaceae) and techniques for checking the legitimacy of timber and wood products. J. plant Res. 124 (1), 35–48. doi:10.1007/s10265-010-0348-z
Tsumura, Y., Kawahara, T., Wickneswari, R., and Yoshimura, K. (1996). Molecular phylogeny of Dipterocarpaceae in Southeast Asia using RFLP of PCR-amplified chloroplast genes. Theor. Appl. Genet. 93 (1), 22–29. doi:10.1007/BF00225722
Turner, I. (1990). The seedling survivorship and growth of three Shorea species in a Malaysian tropical rain forest. J. Trop. Ecol. 6 (4), 469–478. doi:10.1017/s0266467400004879
Ujino, T., Kawahara, T., Tsumura, Y., Nagamitsu, T., Yoshimaru, H., and Ratnam, W. (1998). Development and polymorphism of simple sequence repeat DNA markers for Shorea curtisii and other Dipterocarpaceae species. Heredity 81, 422–428. doi:10.1046/j.1365-2540.1998.00423.x
Usinowicz, J., Chang-Yang, C.-H., Chen, Y.-Y., Clark, J. S., Fletcher, C., Garwood, N. C., et al. (2017). Temporal coexistence mechanisms contribute to the latitudinal gradient in forest diversity. Nature 550 (7674), 105–108. doi:10.1038/nature24038
Van Oosterhout, C., Hutchinson, W. F., Wills, D. P., and Shipley, P. (2004). MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Mol. Ecol. notes 4 (3), 535–538. doi:10.1111/j.1471-8286.2004.00684.x
Webb, C. O., and Peart, D. R. (1999). Seedling density dependence promotes coexistence of Bornean rain forest trees. Ecology 80 (6), 2006–2017. doi:10.1890/0012-9658(1999)080[2006:sddpco]2.0.co;2
Wright, J. S. (2002). Plant diversity in tropical forests: a review of mechanisms of species coexistence. Oecologia 130 (1), 1–14. doi:10.1007/s004420100809
Keywords: dipterocarp, syngameon, genetic structure, fitness, frequency-dependent selection, tropical rainforest
Citation: Tani N, Ng CH, Lee SL, Lee CT, Muhammad N, Kondo T, Tsumura Y, Sugiyama S, Niiyama K, Ripin A, Kassim AR and Musa S (2025) Negative frequency-dependent selection through variations in seedling fitness due to genetic differentiation of parents’ pair in a tropical rainforest tree, Rubroshorea curtisii (Dipterocarpaceae). Front. Genet. 16:1552024. doi: 10.3389/fgene.2025.1552024
Received: 27 December 2024; Accepted: 17 January 2025;
Published: 06 February 2025.
Edited by:
Jiasheng Wu, Zhejiang Agriculture and Forestry University, ChinaReviewed by:
Xia Liu, Chongqing University of Arts and Sciences, ChinaCopyright © 2025 Tani, Ng, Lee, Lee, Muhammad, Kondo, Tsumura, Sugiyama, Niiyama, Ripin, Kassim and Musa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Naoki Tani, dGFuaW4wMjgzQGppcmNhcy5nby5qcA==
†Present address: Saori Sugiyama, National Institute of Informatics, Hitotsubashi, Chiyoda-ku, Tokyo, Japan
‡Deceased
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.