- 1Department of Postgraduate, Jiangxi University of Chinese Medicine, Nanchang, Jiangxi, China
- 2Department of Respiration, Affiliated Hospital of Jiangxi University of Traditional Chinese Medicine, Nanchang, Jiangxi, China
Background: A plethora of observational studies has established a significant correlation between Obstructive Sleep Apnea (OSA) and Telomere Length (TL). Nevertheless, a universal consensus on precise causal association and its directionality has not yet been achieved. To shed light on this, we employed Mendelian Randomization (MR) to investigate the bidirectional causal association between OSA and TL.
Method: Utilizing publicly accessible Genome-Wide Association Studies (GWAS) datasets, we procured genetic data pertinent to MR analysis. The study incorporated samples from both the OSA (n = 217,955) and TL (n = 472,174) cohorts. In the forward MR analysis, OSA served as the exposure variable and TL as the outcome. Conversely, the reverse MR analysis treated TL as the exposure and OSA as the outcome. We employed the Inverse variance weighted (IVW) as the primary methodology for MR analysis. To ensure the robustness of our MR findings, multiple sensitivity analyses were performed.
Results: In the forward MR analysis, a negative correlation was indicated between OSA and TL (IVW: odds ratio (OR) = 0.964, 95% confidence interval (CI): 0.939–0.980, P = 0.006 < 0.05). However, no significant association was identified between TL and the risk of OSA in the reverse MR analysis (IVW: OR = 0.965, 95% CI: 0.870–1.070, P = 0.499 > 0.05).
Conclusion: Our study indicated a potential association between OSA and the increased risk of shorter TL, offering vital academic support for future clinical studies on this association.
1 Introduction
OSA is a sleep-associated respiratory disorder (Schütz et al., 2021). Its prevalence has surged recently (Peppard et al., 2013; Ghavami et al., 2023), affecting approximately 100 million adults worldwide (Benjafield et al., 2019). OSA primarily arises from the obstruction of the upper respiratory pathway, which restricts airflow. Those afflicted with OSA often experience apnea or hypoventilation during sleep, leading to consistent intermittent hypoxia and repeated sleep interruptions (Wallace et al., 2022). This sequence of pathophysiological reactions can induce chronic inflammation and oxidative stress, potentially resulting in cellular damage and early cellular aging (Gaspar et al., 2017; Turkiewicz et al., 2021). Persistent exposure to these conditions might compromise the integrity of various physiological systems, and in severe instances, pose mortal threats (Kapur et al., 2017; Bandi et al., 2021). Therefore, comprehensive research into OSA’s implications for human health is of paramount importance.
Telomeres, situated at the termini of eukaryotic chromosomes, are specific structures whose length is commonly assessed in white blood cells (O'Sullivan and Karlseder, 2010). Their fundamental role is to maintain chromosome integrity and stability. Owing to intrinsic constraints of cellular replication, telomeres aren't comprehensively replicated with each cellular division, resulting in their consistent attrition. Upon reaching a specific diminutive length, cells might suspend division, potentially precipitating premature senescence or apoptosis (Blackburn et al., 2015; Wang et al., 2018). Consequently, telomeres are recognized as pivotal biological markers of cellular aging, often termed the aging “timer” (Fumagalli et al., 2012). Existing research underscores the profound correlation between telomere attrition and elevated disease incidence and mortality (Aung et al., 2023; Wang et al., 2023; Zhu S. et al., 2023). Thus, preserving telomere stability is imperative for disease prevention and the deceleration of cellular aging.
In recent years, the relationship between OSA and TL has garnered considerable attention, particularly the contentious debate over OSA as a potential risk factor for telomere shortening (Turkiewicz et al., 2021). The majority of studies highlighted a negative correlation between OSA severity and TL (Barceló et al., 2010; Bhatt et al., 2021; Pinilla et al., 2021). Contrarily, a few studies suggested that children diagnosed with OSA might experience telomere elongation instead of reduction (Kim et al., 2010). Another study proposed a J-shaped relationship between TL and the severity of OSA, suggesting that patients with moderate to severe OSA might have longer telomeres (Polonis et al., 2017). Interestingly, research indicated shorter TL in high-risk female OSA patients, and this trend was independent of income, age, obesity, smoking, hypertension, alcohol consumption, and education level, but such a trend was absent in their male OSA counterparts (Riestra et al., 2017). In contrast, another study emphasized OSA as a significant factor leading to telomere shortening in middle-aged men (Boyer et al., 2016). Clarifying the exact relationship between OSA and TL is crucial for a deeper understanding of OSA and its impact on human health. However, given the conflicting findings on TL variations in OSA patients and the absence of definitive evidence on whether TL changes occur before or after OSA onset, discerning a clear causal relationship remains elusive.
MR analysis is an analytical approach that leverages genetic variations (single nucleotide polymorphisms, SNP) as instrumental variables (IVs) to infer the causal relationship between exposure and outcome (Xu et al., 2022). Given its capacity to circumvent confounding factors and reverse causality, the findings derived from MR are deemed more credible. In this study, we employed a bidirectional two-sample MR strategy to probe the potential causal association between OSA and TL.
2 Methods
2.1 Research design
In alignment with the guidelines of the STROBE checklist for MR studies (STROBE-MR), we undertook a bidirectional MR analysis to assess the bidirectional association between OSA and TL (Skrivankova et al., 2021). For the validity of this analysis, it was essential that three core assumptions be met (Davies et al., 2018): ① IVs must be strongly associated with the exposure; ② IVs must be independent of any confounders that could affect the outcome; ③ IVs affect the outcome only through their association with the exposure, and not through any other pathways.
2.2 Data source
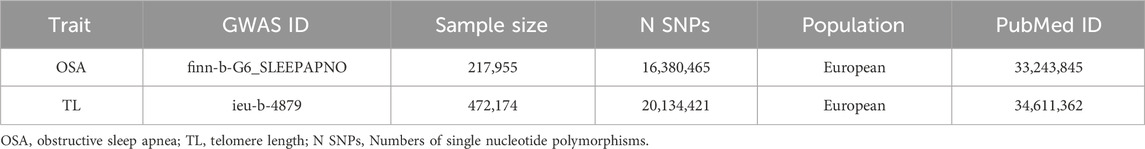
For this study, we utilized summary data from two GWAS (https://gwas.mrcieu.ac.uk/) of European ancestry. Genetic information for OSA was sourced from the publicly available GWAS data in the FinnGen database, featuring 16,761 cases and 201,194 controls (Table 1). The diagnosis of OSA relied on ICD codes (ICD-10: G47.3; ICD-9: 3472A), which were obtained from the Finnish National Hospital Discharge Registry and the Cause of Death Registry. This diagnosis was based on subjective symptoms, clinical examination, and sleep registration, with a threshold of AHI ≥5 events·h-1 or a respiratory event index ≥5 events·h-1 serving as key indicators for confirmation (Strausz et al., 2021). The GWAS data for TL was derived from the United Kingdom Biobank (UKB), comprising 472,174 adults with specific traits (Codd et al., 2021) (Table 1). This research strictly relied on samples of European ancestry, effectively eliminating confounding factors associated with racial variations. As the data we employed are publicly available, no supplementary ethical approval was required.
2.3 Selection of IVs
In accordance with the objective of identifying SNPs significantly correlated with exposure, we adopted a genome-wide significance threshold of P < 5 × 10−8. However, under this criterion, many SNPs related to OSA were not identified. Thus, we adjusted our criterion to a more lenient P < 5 × 10−7 for isolating OSA-associated SNPs (Zhu Q. et al., 2023). Employing the PLINK clustering technique, we excluded SNPs in linkage disequilibrium (r2 > 0.001; aggregation window: 10,000 KB), retaining only the SNPs with the most significant P-values. During data integration, we discarded palindromic SNPs. To ascertain potential biases within weak IVs, the strength of IVs was assessed using the F-statistic. Previous research indicated that an F-statistic exceeding 10 signifies a reduced likelihood of IV bias (Zhu Q. et al., 2023).
2.4 Statistical analysis
In version 4.3.0 of R software, the “TwoSampleMR” package was utilized for causality assessment. Four MR methods were selected for analysis: IVW, Weighted Median (WM), weighted mode, and MR-Egger regression. IVW evaluates the variance of each SNP’s effect estimation and assigns more weight to the SNPs considered to be more stable and precise, enhancing the statistical robustness of the overall effect estimation (Burgess et al., 2013). Thus, we primarily adopted IVW for our MR analysis. However, caution is required as associations between certain genetic variants and non-exposed confounders may introduce bias into IVW results (Bowden et al., 2017). To ensure the stability of our results, we also implemented MR-Egger regression, WM, and weighted mode methodologies. MR-Egger regression can adjust for potential confounders and is somewhat tolerant to weaker IVs, but it generally requires a large sample size (Burgess and Thompson, 2017). WM yields consistent estimates even when up to 50% of the genetic variations prove ineffective (Bowden et al., 2016). Additionally, the weighted mode has a relatively lenient assumption regarding the efficacy of genetic variations.
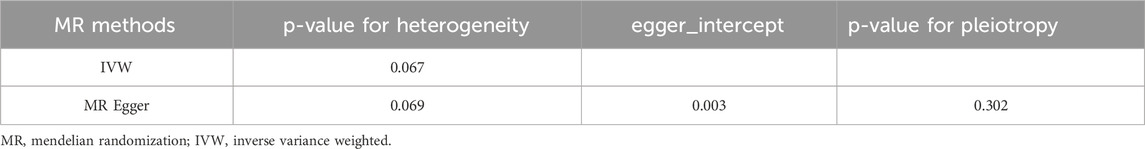
In MR analysis, when a specific SNP affects the outcome but this influence is independent of the exposure’s causal relationship, it’s termed horizontal pleiotropy. This phenomenon could introduce biases in MR results. To comprehensively assess potential horizontal pleiotropy, we employed several analytical methodologies: Firstly, we used MR- Pleiotropy RESidual Sum and Outlier (MR-PRESSO) to detect outliers that might violate the causal effect (Ong and MacGregor, 2019). Subsequently, MR-Egger regression was conducted. A substantial deviation of its intercept from zero indicates horizontal pleiotropy. Lastly, by adopting a“leave-one-out” study, we systematically eliminated each SNP to compare the MR findings of the residual SNPs with the aggregate MR results. Furthermore, given the potential varying impact of distinct SNPs on exposure, such variations can lead to heterogeneity. Thus, Cochrane’s Q value was utilized to gauge the heterogeneity among SNPs.
3 Results
3.1 Forward MR analysis: causality of OSA on TL
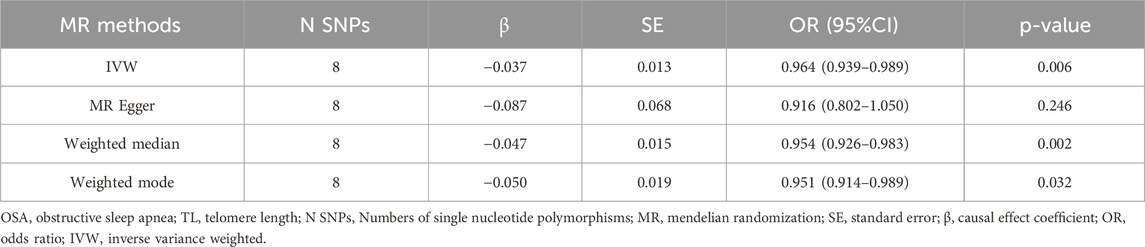
In our forward MR analysis, we adopted 8 SNPs linked with OSA (P < 5.00 × 10−7) to explore their latent effects on TL. The F-statistic for each SNP exceeded 10, with values ranging from 26.162 to 66.586. Details were provided in Supplementary Material: Supplementary Table S1. Based on the IVW method, we found a significant negative causal relationship between OSA and TL (OR = 0.964, 95%CI: 0.939–0.989, P = 0.006 < 0.05, Table 2). This relationship was reinforced by results from both WM (OR = 0.954, 95%CI: 0.926–0.983, P = 0.002 < 0.05, Table 2) Weighted Mode (OR = 0.951, 95%CI: 0.914–0.989, P = 0.032 < 0.05, Table 2). Yet, findings from the MR-Egger regression did not confirm a distinct causal association between OSA and TL (OR = 0.916, 95%CI: 0.802–1.050, P = 0.246 > 0.05, Table 2). We have visualized these causal estimates in a scatter plot (Figure 1A).
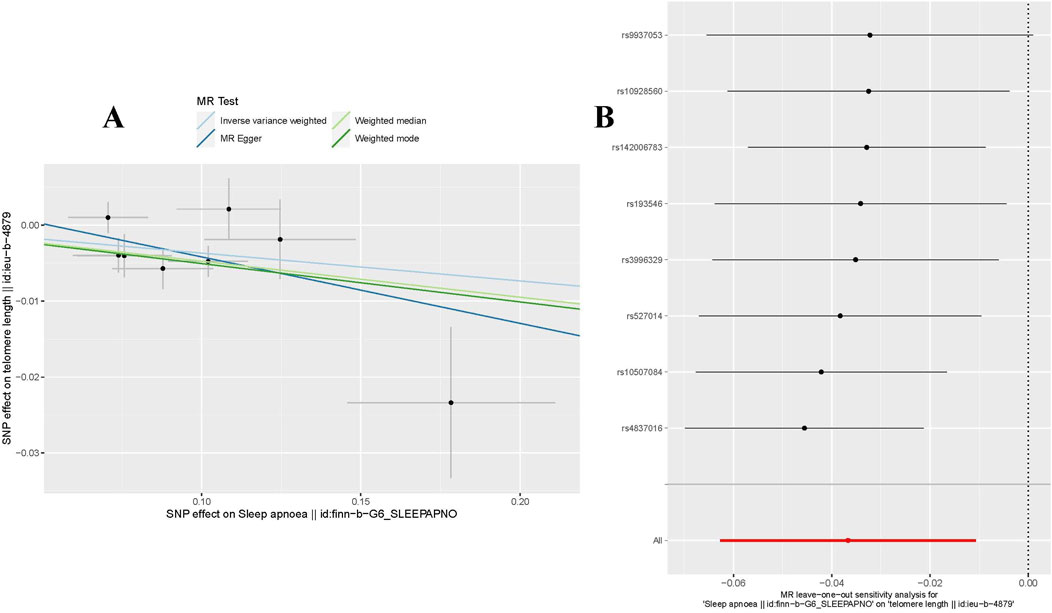
Figure 1. MR analysis for OSA on TL. (A) The scatter plot illustrated an intuitive depiction of the relationship between OSA-related IVs and TL. (B) The “Leave-one-out” sensitivity analysis enables the identification of bias-inducing SNPs, further elucidating their potential impact on the overall causal estimation. OSA, Obstructive Sleep Apnea; TL, Telomere Length; instrumental variables, IVs; SNPs, single nucleotide polymorphisms.
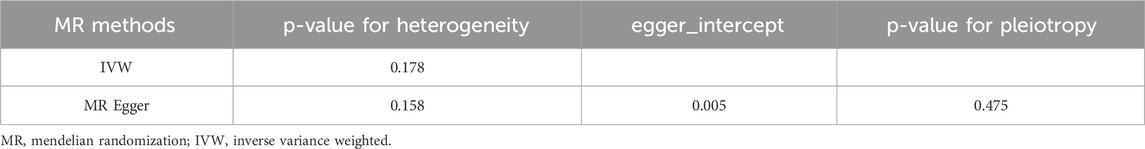
To evaluate the stability of the aforementioned findings, we conducted sensitivity analyses and heterogeneity tests. The MR-PRESSO test revealed no outliers that could disrupt the causal relationship (P = 0.241 > 0.05). The MR-Egger regression further confirmed that the study results were uninfluenced by horizontal pleiotropy (P = 0.475 > 0.05, Table 3). Using the “Leave-one-out” technique for sensitivity analysis revealed that the stepwise exclusion of individual SNPs exerted no substantial influence on the causal relationship estimates (Figure 1B). Additionally, Cochran’s Q test demonstrated no evidence of heterogeneity in either IVW (P = 0.178 > 0.05, Table 3) or MR-Egger regression (P = 0.158 > 0.05, Table 3).
3.2 Reverse MR analysis: causality of TL on OSA
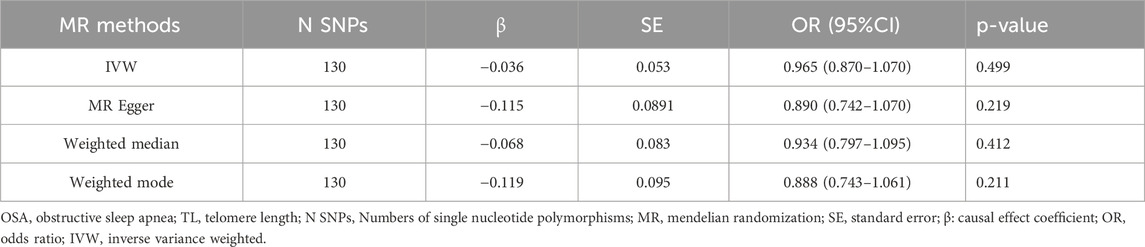
In reverse MR analysis, SNPs associated with TL were employed as the IVs to evaluate their influence on OSA. After data consolidation, four palindromic SNPs (rs2276182, rs2306646, rs56178008, and rs670180) were excluded, and 130 validated SNPs were chosen as IVs (P < 5.00 × 10−8). See Supplementary Material for specifics: Supplementary Table S2. According to the IVW results, there was no significant causal relationship between TL and OSA. This finding was corroborated by other MR analyses (Table 4). Moreover, no horizontal pleiotropy or heterogeneity was observed (Table 5).
4 Discussion
In this study, we utilized an open-access GWAS dataset and bidirectional two-sample MR methods to comprehensively evaluate the relationship between OSA and TL. The results of forward MR analysis, using IVW, WM, and weighted mode MR methodologies, demonstrated a negative association between OSA and TL. This suggests a close association between OSA and telomere depletion. Although the MR-Egger findings were not statistically significant, possibly due to the method accommodating horizontal pleiotropy, resulting in wider confidence intervals and potential biases (Burgess and Thompson, 2017; Rees et al., 2017). This underscores the importance of considering methodological limitations when making causal inferences. On the other hand, reverse MR analysis substantiated the absence of a causal relationship between TL and OSA risk, indicating that a reduction in telomere length does not directly cause the onset of OSA. Sensitivity analyses and heterogeneity tests further affirmed the robustness of our findings. In summary, this study suggests that while OSA may expedite the depletion of TL, no causal association exists between TL and OSA risk.
Based on the literature search to date, this research is the inaugural study employing MR techniques to scrutinize the causal relationship between OSA and TL. Our results substantiated a negative causal linkage between OSA and TL, which aligns with previous research outcomes. For instance, a meta-analysis incorporating seven case-control studies along with one cohort study, involving a total of 2,639 participants, revealed that individuals with OSA have significantly shorter TL compared to their healthy counterparts (mean difference: −0.03; 95%CI: −0.06 to −0.00; P = 0.003) (Huang et al., 2018). Subgroup analyses based on age and sample size further reinforce this observation. Moreover, after accounting for demographic and lifestyle factors, cross-sectional studies demonstrated a significant association between severe OSA symptoms and reduced TL (P = 0.007) (Carroll et al., 2019). Additionally, a pilot study revealed that after 6 months of Continuous Positive Airway Pressure (CPAP) treatment, significant alleviation of hypoxia symptoms was observed in OSA patients, accompanied by an increase in TL (P = 0.03) (Madaeva et al., 2022).
While the mechanisms underlying the association between OSA and TL remain to be conclusively elucidated, extant research supports the hypothesis that oxidative stress and inflammation serve as foundational elements in establishing a negative causal relationship between the two (Kim et al., 2016; Turkiewicz et al., 2021). As such, this reinforces our findings that OSA serves as a risk factor for TL shortening. Specifically, the prevalent conditions of chronic intermittent hypoxia and sleep fragmentation in OSA patients disrupt the oxygen equilibrium in the bloodstream, thus precipitating oxidative stress. This sequence of events culminates in the production of a plethora of Reactive Oxygen Species (ROS), which inflict harm upon proteins, lipids, and DNA, thereby accelerating the shortening of TL (Cadet and Wagner, 2013). Moreover, heightened levels of various inflammatory indicators like Tumor Necrosis Factor-α, Interleukin-6, and C-Reactive Protein are frequently detected in the bloodstream of OSA patients, possibly triggering systemic inflammation and establishing a biological nexus between OSA and TL (Zhang et al., 2016). Concurrently, OSA is often accompanied by endocrine imbalances, notably fluctuations in cortisol levels, which may further modulate telomerase activity, thus indirectly affecting TL. Research by TempAku PF also suggested that OSA may affect telomerase activity by inhibiting the expression of KLOTHO protein, thereby connecting OSA and TL (Tempaku et al., 2021). TL serves as a key biomarker for biological aging and is connected to various age-related diseases, whereas OSA is intimately linked with a multitude of health challenges, including but not limited to cardiovascular disorders (Labarca et al., 2018; Huang et al., 2020; Ooi and Rajendran, 2023). Given that these phenomena may interact through complex biochemical mechanisms and genetic regulations, it becomes particularly crucial to gain a deeper understanding of the impact of OSA on biological aging. Consequently, our research sheds light on the potential negative causal relationship between OSA and TL, providing new perspectives for comprehending their interplay. This suggests that alleviating OSA symptoms may be significant for delaying cellular aging and maintaining telomere stability. Timely treatment of OSA may not only emerge as a vital strategy for combating aging but also afford novel insights for the prevention of age-related diseases.
The present study is characterized by multiple noteworthy strengths. First, we employed a two-sample MR design based on large-scale GWAS data, effectively minimizing the bias introduced by unobserved confounding variables and thus more accurately establishing the causal link between OSA and TL. Second, we conducted a bidirectional causality analysis to comprehensively ensure the exclusion of misleading causal effects when exploring the relationship between OSA and TL. Lastly, to holistically evaluate the causal effects, we used a variety of advanced statistical methods, including IVW, WM, Weighted Mode, and MR-Egger regression.
This study is subject to several limitations. The reliance on GWAS data from European populations limited the global applicability of our findings. Further MR studies involving diverse ethnic groups are warranted to corroborate these findings. Second, there may be differential effects on TL among OSA patients based on gender, age, and severity level. Unfortunately, the absence of stratified GWAS data precludes a more comprehensive analysis. Third, the IVs currently available for causal inference were relatively limited. However, as GWAS research evolves, we expect to identify a greater number of genetic markers strongly associated with OSA. To summarize, the study did shed light on the relationship between OSA and diminished TL. Nonetheless, the exact mechanisms contributing to this correlation warrant further in-depth investigation.
5 Conclusion
Overall, our study definitively demonstrated that OSA substantially hastens the deterioration of telomeres, which bears significant implications for clinical practice, particularly given that accelerated telomere degradation is linked to numerous ailments and shortened lifespan. Nevertheless, we found no causal relationship between TL and the risk of OSA onset. This insight offers a novel direction for subsequent studies, implying that TL may not be a dependable indicator for assessing the risk of OSA.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
RX: Conceptualization, Formal Analysis, Methodology, Writing–original draft. SC: Data curation, Software, Writing–original draft. XL: Data curation, Writing–review and editing. ZL: Writing–review and editing, Resources.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The work was supported by the National Natural Science Foundation of China (No. 82060841, No. 82260913); Jiangxi Province Key Laboratory of Traditional Chinese Medicine—Pulmonary Science (No. 2024SSY06321); Jiangxi University of Chinese Medicine Science and Technology Innovation Team Development Program (No. CXTD22011).
Acknowledgments
The authors express sincere appreciation to all contributors from the United Kingdom Biobank(UKB) for their invaluable contributions to genetic research on telomere length(TL). We also extend our deepest gratitude to researchers and participants in the FinnGen database for their role in advancing genetic studies on Obstructive Sleep Apnea (OSA). The dissemination of this data has greatly facilitated the progress of our research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2025.1294105/full#supplementary-material
References
Aung, N., Wang, Q., van Duijvenboden, S., Burns, R., Stoma, S., Raisi-Estabragh, Z., et al. (2023). Association of longer leukocyte telomere length with cardiac size, function, and heart failure. JAMA Cardiol. 8 (9), 808–815. doi:10.1001/jamacardio.2023.2167
Bandi, P. S., Panigrahy, P. K., Hajeebu, S., Ngembus, N. J., and Heindl, S. E. (2021). Pathophysiological mechanisms to review association of atrial fibrillation in heart failure with obstructive sleep apnea. Cureus 13 (7), e16086. doi:10.7759/cureus.16086
Barceló, A., Piérola, J., López-Escribano, H., de la Peña, M., Soriano, J. B., Alonso-Fernández, A., et al. (2010). Telomere shortening in sleep apnea syndrome. Respir. Med. 104 (8), 1225–1229. doi:10.1016/j.rmed.2010.03.025
Benjafield, A. V., Ayas, N. T., Eastwood, P. R., Heinzer, R., Ip, M. S. M., Morrell, M. J., et al. (2019). Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir. Med. 7 (8), 687–698. doi:10.1016/s2213-2600(19)30198-5
Bhatt, S. P., Guleria, R., and Vikram, N. K. (2021). The effect of the severity of obstructive sleep apnea on leukocyte telomere length, 25 hydroxy vitamin D, and parathyroid hormonal concentrations in asian Indians. Front. Neurol. 12, 682739. doi:10.3389/fneur.2021.682739
Blackburn, E. H., Epel, E. S., and Lin, J. (2015). Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science 350 (6265), 1193–1198. doi:10.1126/science.aab3389
Bowden, J., Davey Smith, G., Haycock, P. C., and Burgess, S. (2016). Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 40 (4), 304–314. doi:10.1002/gepi.21965
Bowden, J., Del Greco, M. F., Minelli, C., Davey Smith, G., Sheehan, N., and Thompson, J. (2017). A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat. Med. 36 (11), 1783–1802. doi:10.1002/sim.7221
Boyer, L., Audureau, E., Margarit, L., Marcos, E., Bizard, E., Le Corvoisier, P., et al. (2016). Telomere shortening in middle-aged men with sleep-disordered breathing. Ann. Am. Thorac. Soc. 13 (7), 1136–1143. doi:10.1513/AnnalsATS.201510-718OC
Burgess, S., Butterworth, A., and Thompson, S. G. (2013). Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 37 (7), 658–665. doi:10.1002/gepi.21758
Burgess, S., and Thompson, S. G. (2017). Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 32 (5), 377–389. doi:10.1007/s10654-017-0255-x
Cadet, J., and Wagner, J. R. (2013). DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb. Perspect. Biol. 5 (2), a012559. doi:10.1101/cshperspect.a012559
Carroll, J. E., Irwin, M. R., Seeman, T. E., Diez-Roux, A. V., Prather, A. A., Olmstead, R., et al. (2019). Obstructive sleep apnea, nighttime arousals, and leukocyte telomere length: the Multi-Ethnic Study of Atherosclerosis. Sleep 42 (7), zsz089. doi:10.1093/sleep/zsz089
Codd, V., Wang, Q., Allara, E., Musicha, C., Kaptoge, S., Stoma, S., et al. (2021). Polygenic basis and biomedical consequences of telomere length variation. Nat. Genet. 53 (10), 1425–1433. doi:10.1038/s41588-021-00944-6
Davies, N. M., Holmes, M. V., and Davey Smith, G. (2018). Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. Bmj 362, k601. doi:10.1136/bmj.k601
Fumagalli, M., Rossiello, F., Clerici, M., Barozzi, S., Cittaro, D., Kaplunov, J. M., et al. (2012). Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat. Cell Biol. 14 (4), 355–365. doi:10.1038/ncb2466
Gaspar, L. S., Álvaro, A. R., Moita, J., and Cavadas, C. (2017). Obstructive sleep apnea and hallmarks of aging. Trends Mol. Med. 23 (8), 675–692. doi:10.1016/j.molmed.2017.06.006
Ghavami, T., Kazeminia, M., Ahmadi, N., and Rajati, F. (2023). Global prevalence of obstructive sleep apnea in the elderly and related factors: a systematic review and meta-analysis study. J. Perianesth Nurs. 38, 865–875. doi:10.1016/j.jopan.2023.01.018
Huang, H. Y., Lin, S. W., Chuang, L. P., Wang, C. L., Sun, M. H., Li, H. Y., et al. (2020). Severe OSA associated with higher risk of mortality in stage III and IV lung cancer. J. Clin. Sleep. Med. 16 (7), 1091–1098. doi:10.5664/jcsm.8432
Huang, P., Zhou, J., Chen, S., Zou, C., Zhao, X., and Li, J. (2018). The association between obstructive sleep apnea and shortened telomere length: a systematic review and meta-analysis. Sleep. Med. 48, 107–112. doi:10.1016/j.sleep.2017.09.034
Kapur, V. K., Auckley, D. H., Chowdhuri, S., Kuhlmann, D. C., Mehra, R., Ramar, K., et al. (2017). Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American academy of sleep medicine clinical practice guideline. J. Clin. Sleep. Med. 13 (3), 479–504. doi:10.5664/jcsm.6506
Kim, J., Lee, S., Bhattacharjee, R., Khalyfa, A., Kheirandish-Gozal, L., and Gozal, D. (2010). Leukocyte telomere length and plasma catestatin and myeloid-related protein 8/14 concentrations in children with obstructive sleep apnea. Chest 138 (1), 91–99. doi:10.1378/chest.09-2832
Kim, K. S., Kwak, J. W., Lim, S. J., Park, Y. K., Yang, H. S., and Kim, H. J. (2016). Oxidative stress-induced telomere length shortening of circulating leukocyte in patients with obstructive sleep apnea. Aging Dis. 7 (5), 604–613. doi:10.14336/ad.2016.0215
Labarca, G., Reyes, T., Jorquera, J., Dreyse, J., and Drake, L. (2018). CPAP in patients with obstructive sleep apnea and type 2 diabetes mellitus: systematic review and meta-analysis. Clin. Respir. J. 12 (8), 2361–2368. doi:10.1111/crj.12915
Madaeva, I. M., Kurashova, N. A., Ukhinov, E. B., Berdina, O. N., Semenova, N. V., Madaev, V. V., et al. (2022). Changes in the telomeres length in patients with obstructive sleep apnea after continuous positive airway pressure therapy: a pilot study. Zh Nevrol. Psikhiatr Im. S S Korsakova 122 (5. Vyp. 2), 52–57. doi:10.17116/jnevro202212205252
Ong, J. S., and MacGregor, S. (2019). Implementing MR-PRESSO and GCTA-GSMR for pleiotropy assessment in Mendelian randomization studies from a practitioner's perspective. Genet. Epidemiol. 43 (6), 609–616. doi:10.1002/gepi.22207
Ooi, E. L., and Rajendran, S. (2023). Obstructive sleep apnea in coronary artery disease. Curr. Probl. Cardiol. 48 (8), 101178. doi:10.1016/j.cpcardiol.2022.101178
O'Sullivan, R. J., and Karlseder, J. (2010). Telomeres: protecting chromosomes against genome instability. Nat. Rev. Mol. Cell Biol. 11 (3), 171–181. doi:10.1038/nrm2848
Peppard, P. E., Young, T., Barnet, J. H., Palta, M., Hagen, E. W., and Hla, K. M. (2013). Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 177 (9), 1006–1014. doi:10.1093/aje/kws342
Pinilla, L., Santamaria-Martos, F., Benítez, I. D., Zapater, A., Targa, A., Mediano, O., et al. (2021). Association of obstructive sleep apnea with the aging process. Ann. Am. Thorac. Soc. 18 (9), 1540–1547. doi:10.1513/AnnalsATS.202007-771OC
Polonis, K., Somers, V. K., Becari, C., Covassin, N., Schulte, P. J., Druliner, B. R., et al. (2017). Moderate-to-severe obstructive sleep apnea is associated with telomere lengthening. Am. J. Physiol. Heart Circ. Physiol. 313 (5), H1022-H1030–h1030. doi:10.1152/ajpheart.00197.2017
Rees, J. M. B., Wood, A. M., and Burgess, S. (2017). Extending the MR-Egger method for multivariable Mendelian randomization to correct for both measured and unmeasured pleiotropy. Stat. Med. 36 (29), 4705–4718. doi:10.1002/sim.7492
Riestra, P., Gebreab, S. Y., Xu, R., Khan, R. J., Quarels, R., Gibbons, G., et al. (2017). Obstructive sleep apnea risk and leukocyte telomere length in African Americans from the MH-GRID study. Sleep. Breath. 21 (3), 751–757. doi:10.1007/s11325-016-1451-8
Schütz, S. G., Dunn, A., Braley, T. J., Pitt, B., and Shelgikar, A. V. (2021). New frontiers in pharmacologic obstructive sleep apnea treatment: a narrative review. Sleep. Med. Rev. 57, 101473. doi:10.1016/j.smrv.2021.101473
Skrivankova, V. W., Richmond, R. C., Woolf, B. A. R., Davies, N. M., Swanson, S. A., VanderWeele, T. J., et al. (2021). Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration. Bmj 375, n2233. doi:10.1136/bmj.n2233
Strausz, S., Ruotsalainen, S., Ollila, H. M., Karjalainen, J., Kiiskinen, T., Reeve, M., et al. (2021). Genetic analysis of obstructive sleep apnoea discovers a strong association with cardiometabolic health. Eur. Respir. J. 57 (5), 2003091. doi:10.1183/13993003.03091-2020
Tempaku, P. F., D'Almeida, V., da Silva, S. M. A., Andersen, M. L., Belangero, S. I., and Tufik, S. (2021). Klotho genetic variants mediate the association between obstructive sleep apnea and short telomere length. Sleep. Med. 83, 210–213. doi:10.1016/j.sleep.2021.01.015
Turkiewicz, S., Ditmer, M., Sochal, M., Białasiewicz, P., Strzelecki, D., and Gabryelska, A. (2021). Obstructive sleep apnea as an acceleration trigger of cellular senescence processes through telomere shortening. Int. J. Mol. Sci. 22 (22), 12536. doi:10.3390/ijms222212536
Wallace, E. S., Carberry, J. C., Toson, B., and Eckert, D. J. (2022). A systematic review and meta-analysis of upper airway sensation in obstructive sleep apnea - implications for pathogenesis, treatment and future research directions. Sleep. Med. Rev. 62, 101589. doi:10.1016/j.smrv.2022.101589
Wang, Q., Zhan, Y., Pedersen, N. L., Fang, F., and Hägg, S. (2018). Telomere length and all-cause mortality: a meta-analysis. Ageing Res. Rev. 48, 11–20. doi:10.1016/j.arr.2018.09.002
Wang, W., Huang, N., Zhuang, Z., Song, Z., Li, Y., Dong, X., et al. (2023). Identifying potential causal effects of telomere length on health outcomes: a phenome-wide investigation and Mendelian randomization study. J. Gerontol. A Biol. Sci. Med. Sci. 79, glad128. doi:10.1093/gerona/glad128
Xu, J., Li, M., Gao, Y., Liu, M., Shi, S., Shi, J., et al. (2022). Using Mendelian randomization as the cornerstone for causal inference in epidemiology. Environ. Sci. Pollut. Res. Int. 29 (4), 5827–5839. doi:10.1007/s11356-021-15939-3
Zhang, J., Rane, G., Dai, X., Shanmugam, M. K., Arfuso, F., Samy, R. P., et al. (2016). Ageing and the telomere connection: an intimate relationship with inflammation. Ageing Res. Rev. 25, 55–69. doi:10.1016/j.arr.2015.11.006
Zhu, Q., Hua, L., Chen, L., Mu, T., Dong, D., Xu, J., et al. (2023a). Causal association between obstructive sleep apnea and gastroesophageal reflux disease: a bidirectional two-sample Mendelian randomization study. Front. Genet. 14, 1111144. doi:10.3389/fgene.2023.1111144
Keywords: obstructive sleep apnea, telomere length, causality, genetic association, mendelian randomization
Citation: Xie R, Chen S, Li X and Lan Z (2025) Assessment of the causal association between obstructive sleep apnea and telomere length: a bidirectional mendelian randomization study. Front. Genet. 16:1294105. doi: 10.3389/fgene.2025.1294105
Received: 14 September 2023; Accepted: 13 February 2025;
Published: 04 March 2025.
Edited by:
Rene Cortese, University of Kansas Medical Center, United StatesReviewed by:
Nancy Monroy-Jaramillo, National Institute of Neurology and Neurosurgery., MexicoDavid Sanz Rubio, Hospital Universitario Miguel Servet, Spain
Copyright © 2025 Xie, Chen, Li and Lan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhihui Lan, bHpoaWh1aTA5MTJAMTI2LmNvbQ==
 Rongfang Xie
Rongfang Xie Shiyu Chen1
Shiyu Chen1 Xiaojian Li
Xiaojian Li Zhihui Lan
Zhihui Lan