
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Genet., 24 February 2023
Sec. Cancer Genetics and Oncogenomics
Volume 14 - 2023 | https://doi.org/10.3389/fgene.2023.1110656
This article is part of the Research TopicRecent Advances in Genetic and Proteomic Biomarkers involved in the Early Detection of Solid TumorsView all 9 articles
Cancerous inhibitor of protein phosphatase 2A (CIP2A), initially reported as a tumor-associated antigen (known as p90), is highly expressed in most solid and hematological tumors. The interaction of CIP2A/p90, protein phosphatase 2A (PP2A), and c-Myc can hinder the function of PP2A toward c-Myc S62 induction, thus stabilizing c-Myc protein, which represents a potential role of CIP2A/p90 in tumorigeneses such as cell proliferation, invasion, and migration, as well as cancer drug resistance. The signaling pathways and regulation networks of CIP2A/p90 are complex and not yet fully understood. Many previous studies have also demonstrated that CIP2A/p90 can be used as a potential therapeutic cancer target. In addition, the autoantibody against CIP2A/p90 in sera may be used as a promising biomarker in the diagnosis of certain types of cancer. In this Review, we focus on recent advances relating to CIP2A/p90 and their implications for future research.
The sera of patients diagnosed with cancer contain antibodies that can react with a unique group of autologous cellular proteins called tumor-associated antigens (TAAs) (Chen et al., 2018). The immune system of cancer patients is a sensor of alterations in the structure and/or function of participants in tumorigenesis pathways and is capable of immune responses in the form of autoantibodies against these TAAs (Jhunjhunwala et al., 2021). Circulating autoantibodies have been used as ’probes’ in cancer patients to isolate TAAs, which have been shown to be cellular factors participating in known tumorigenesis pathways (Tan, 2001; Tan and Zhang, 2008; Zhang et al., 2022). The constitution of TAAs do not include all cellular antigens identified by autoantibodies in cancer sera as some autoantibodies may exist in conditions that pre-date malignancy. Thus, many approaches aimed at identifying and characterizing authentic TAAs have been identified by anti-TAA autoantibodies, which can be used as biomarkers for diagnosis or early detection only after extensive evaluation with cancer and non-cancer sera (Zhang and Tan, 2010; Li et al., 2021).
CIP2A was initially identified as a TAA and was named p90 due to its molecular weight of 90 kDa (Soo Hoo et al., 2002). Autoantibodies against p90 were found in 21% of sera from a group of patients with liver cancer. Sera with anti-p90 localized to the cytoplasm were detected by indirect immunofluorescent staining in fetal mouse liver but not in adult liver (Zhang et al., 2002). Full-length cDNA encoding p90 was successfully isolated from a T24 expression library, including a sequence coding for a 905-amino-acid protein, predicted to have a molecular mass of 102 kDa. In a subsequent study, p90 was found to be identical to cancerous inhibitor of protein phosphatase2A (CIP2A) by a research group from Finland (Junttila et al., 2007). The function of CIP2A/p90 is related to its binding with c-Myc and inhibiting dephosphorylation of S62 caused by PP2A (Farrington et al., 2020).
Many studies have focused on the function of CIP2A/p90 since the protein was identified by our study group. This review focuses on recent advances, which have primarily been associated with the determination of CIP2A/p90 function or its potential as a biomarker for the early detection of various types of cancer.
Protein kinase phosphorylation and protein phosphatase (PP) dephosphorylation are considered the most common mechanisms involved in intracellular protein regulation and signal transduction. Their imbalance is associated with cystic fibrosis, Alzheimer’s disease (AD), and other diseases, such as cancer (Ruvolo, 2019; Shentu et al., 2019; Mercier et al., 2020; Khan M M et al., 2021; Vainonen et al., 2021). According to the dephosphorylated amino acid residues, PP has been categorized into two families, the protein tyrosine phosphatase family and the serine threonine phosphatase family. PP2A is a widely conserved serine threonine phosphatase and has been defined as a kind of tumor suppressor protein (Chen et al., 2013; Perrotti and Neviani, 2013). PP2A is a trimeric holoenzyme, with a scaffold A subunit, a catalytic C subunit, and several different regulatory B subunits. The B subunits determine the subcellular localization and substrate specificity of the PP2A holoenzyme (Ruvolo, 2016). Although PP2A has multiple substrates, its anti-cancer function is mostly related to the dephosphorylation and stabilization of c-Myc (Pippa and Odero, 2020). Recent studies had shown that PP2A is widely involved in the regulation of cellular physiological and pathological processes, such as energy metabolism, cell cycle, DNA replication, proliferation, apoptosis, and inflammatory responses (Sangodkar et al., 2016; Baskaran and Velmurugan, 2018; Kauko and Westermarck, 2018; Remmerie and Janssens, 2019; Khan R et al., 2021). C-Myc is overexpressed in most cancers as a transcription factor with oncogenic capability that mediates cell proliferation, apoptosis, differentiation, adhesion, migration, metabolism, and DNA replication (Sun and Gao, 2017; Duffy et al., 2021; Dhanasekaran et al., 2022; Grieb and Eischen, 2022). As mentioned earlier, CIP2A, encoded by the KIAA1524 gene located on human chromosome 3q13.13, is a major endogenous PP2A-inhibiting protein. The interaction among CIP2A/p90, PP2A, and c-Myc can hinder the function of PP2A toward c-Myc S62 induction and therefore stabilize c-Myc protein, which represents a potential role of CIP2A/p90 in the promotion of cancer (Pippa and Odero, 2020; Scarpa et al., 2021).
CIP2A/p90 plays an important role in the proliferation, apoptosis, invasion, migration, epithelial–mesenchymal transition (EMT), cell cycle, and drug resistance of different tumor cells. CIP2A/p90 was overexpressed in 65%–90% of tissues in almost all human cancers, and this has been associated with poor survival (Tarek et al., 2021). The molecular mechanism of CIP2A/p90 in cancer has mostly been associated with the interaction among CIP2A/p90, PP2A, and c-Myc (Table 1). On the other hand, several studies have indicated that the silencing of CIP2A/p90 by small interfering RNAs (siRNA) inhibited the growth of xenografted tumors of various kinds of cancer cells (Table 1).
As shown in Table 1, silencing CIP2A/p90 with siRNA can further reduce the expression of c-Myc to inhibit cell proliferation and induce cell apoptosis (Yang et al., 2016; Zheng et al., 2016). In addition, siRNA inhibition of CIP2A transcription can make colorectal cancer cells sensitive to radiation and reduce their survival rate in vitro (Birkman et al., 2018). CIP2A/p90 can promote p27Kip1 phosphorylation at Ser10 by via inhibiting Akt-associated PP2A activity, which seems to relocalize p27Kip1 to the cytoplasm. On the other hand, CIP2A/p90 can also recruit c-Myc to mediate the transcriptional inhibition of p27Kip1 and induce cell cycle arrest at the G2/M phase (Liu H et al., 2017). In addition, in cells expressing human papillomavirus 16 oncoprotein E6, it can promote the transformation of the G1/S cell cycle through B-Myb (Tian et al., 2018). Furthermore, several studies have shown that CIP2A/P90 regulates STAT3 phosphorylation and IL-17 expression in Th17 cells by regulating the intensity of interaction between AGK and STAT3 (Chen et al., 2013; Khan et al., 2020a; Khan et al., 2020b). However, only a few studies on the molecular mechanism of the CIP2A/p90 regulating function are mentioned aboved. CIP2A/p90 also has a PP2A-independent function, which can directly interact with Polo-like kinase1 (PLK1) but not with mitosis gene A-related kinase 2 (NEK2), H-Ras, etc., to regulate cellular function. CIP2A/p90 can interact with PLK1 and enhance the stability and activity of PLK1, thereby promoting mitosis in human cancer cells (Kim et al., 2013). The depletion of CIP2A/p90 may also prolong cell division time. CIP2A/p90 interacts with NEK2 during the G2/M phase, and can facilitate centrosome separation and mitotic spindle dynamics in cell cycle progression (Jeong et al., 2014). CIP2A/p90, in association with the oncogene H-Ras and through the recruitment of the MEK/ERK signaling pathway and c-Myc dephosphorylation by PP2A, is required for EMT in the progression of cancer (Wu et al., 2015). Patients with both HOXB13 T and CIP2A T alleles have a higher risk of prostate cancer and invasive disease, earlier biochemical recurrence, and lower disease-specific life expectancy. HOXB13 protein binding to the CIP2A gene can functionally promote CIP2A transcription (Sipeky et al., 2018). Studies have confirmed that CIP2A is an essential gene in BRCA1 and BRCA2 mutant cells, finding that the CIP2A-TOPBP1 axis can protect chromosome stability, which is a synthetic lethal target for BRCA mutant cancer (Adam et al., 2021).
The regulation network of CIP2A/p90 was established through direct interactions of CIP2A/p90 or indirectly through interactions of CIP2A/PP2A with either multiple key cellular proteins/transcription factors or with oncogenic signaling pathways. Figure 1 shows the signaling pathways and regulation mechanisms mainly associated with CIP2A/p90.
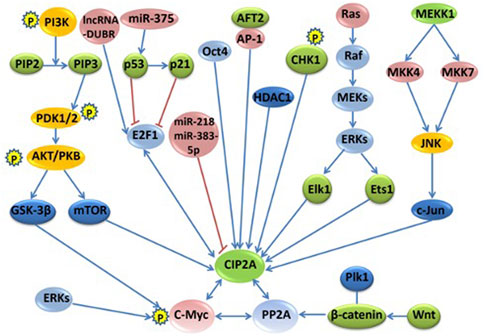
FIGURE 1. The signaling pathways and regulation networks of CIP2A/p90. Several signaling pathways, including the PI3K–AKT–mTOR pathway, the RAS–MEK–ERK pathway, the Wnt–β-catenin pathway, the MKK4/MKK7-JNK-c-Jun pathway, the p53-p21-E2F1-CIP2A/p90 pathway, and the phosphorylation and degradation of c-Myc, non-coding RNAs, and other regulation factors, such as Oct4, AFT2, CHK1, and HDAC1, are included in this figure. Bidirectional blue arrows indicate interactions between two entities; unidirectional blue arrows indicate a positive influence of an entity on another; red lines indicate a negative influence of one entity on another.
Phosphatidylinositol 3-kinase (PI3K) is a heterodimer consisting of a regulatory subunit (p85) and a catalytic subunit (p110). Activated PI3K can convert phosphatidylinositol 4,5-bisphosphate (PIP2) to PIP3, which is a second messenger through 3-phosphoinositide-dependent kinase1 (PDK1), indirectly activates AKT. The activated AKT acts on a variety of substrates, such as mTOR and glycogen synthase kinase-3β (GSK-3β), to regulate cell growth, proliferation, and other functions (Vogelstein et al., 2013). IL-10 phosphorylates cAMP response element-binding protein (CREB) through the PI3K/AKT signaling pathway, thereby regulating CIP2A/p90 gene expression (Sung et al., 2013). Based on our previous study, it was found that CIP2A/p90 can regulate AKT phosphorylation at S473 under growth factor stimulation. Our research also showed that CIP2A/p90 might promote cell proliferation through the AKT–mTOR signaling pathway (Lei et al., 2014). In addition, a new study further confirmed that the overexpression of CIP2A was a key contributory event of AKT phosphorylation in the correlation analysis of p-AKT and CIP2A in 220 clinical samples, and emphasized that the CIP2A-AKT axis is a promising therapeutic target for breast cancer (Luque et al., 2022).
Ras, which is stimulated by extracellular signals, recruits Raf to bind and activate it on the cell membrane. The activated Raf (MAPKKK) can reactivate MAPKK, which in turn activates extracellular protein kinases (ERKs) (also known as MAPK), and finally, the activated ERK can further activate a number of transcription factors, such as Elk-1, Ets1, ATF, NF-κB, and c-Myc, to trigger a variety of biological effects (De et al., 2014). Ets1, as the transcription factor, can mediate high CIP2A/p90 expression in human cancers through increased activity of the EGFR-MEK1/2-ERK pathway (Khanna et al., 2011). The binding of Ets1 and Elk1 together to the proximal CIP2A/p90 promoter is absolutely required for CIP2A/p90 expression in liver, endometrial, and cervical carcinoma cells (Pallai et al., 2012). Additionally, 17β-estradiol (E2) activates EGFR, thus stimulating the MEK1/2 and PI3K pathways and further increasing the expression of CIP2A/p90 through the MEK1/2-induced transcription factor Ets1 to enhance the proliferation of cancer cells (Choi et al., 2014).
JNK belongs to the mitogen-activated protein family (MAPK), which responds to certain stimuli, such as cytokines, UV radiation, heat, and osmotic shock. The activated JNK leads to cell migration, proliferation, and invasion in cancers. According to our research, we found that the overexpression of CIP2A/p90 is associated with increased JNK pathway through the phosphorylation of MKK4/MKK7-JNK-c-Jun signaling. However, the exact mechanism by which CIP2A/p90 modulates the JNK phosphorylation pathway is still unknown (Peng et al., 2015). Knockdown of CIP2A decreases JNK phosphorylation and the phosphorylation of downstream transcriptional factors ATF2 and c-Jun, the transcriptional activity of which is also decreased. Furthermore, the expression level of CIP2A also affects the phosphorylation of the upstream kinase of JNK, MKK4/MKK7 (Peng et al., 2015).
The overexpression of E2F1 leads to activated cell cycle and uncontrolled cellular proliferation in the majority of human cancers. Owing to the inactivation of p53 or p21, the overexpression of E2F1 promotes the expression of oncoprotein CIP2A/p90, which in turn increases stabilizing serine 364 phosphorylation of E2F1. The p53-p21-Rb pathway can negatively regulate the activity of E2F1 transcription (Lucas et al., 2015). Furthermore, research has shown that the positive feedback loop of E2F1-CIP2A/p90 is very important to the sensitivity of senescence and growth arrest induction in breast and cervical cancer cells (Laine et al., 2013; Wang et al., 2017). The CIP2A-AKT-mTOR pathway controls cell growth, apoptosis, and autophagy. Polyphyllin I (PPI) and polyphyllin VII (PPVII) are natural components extracted from Paris polyphylla that have anticancer properties. Examination of the mechanism revealed that PPI and PPVII significantly upregulate p53, induce caspase-dependent apoptosis, and suppress the CIP2A-AKT-mTOR pathway. The activation of autophagy is mediated through PPI and PPVII, which induce the inhibition of mTOR (Feng F. et al., 2019).
MicroRNA, with a length of 18–25 nucleotides, is a type of small single non-coding RNA that regulates gene post-transcriptional expression through binding with complementary sequences, which can degrade the target mRNA or inhibit its translation (Jung et al., 2013). miR-218 can bind to the 3'-UTR region of CIP2A/p90 in cutaneous melanoma cells to regulate the gene expression of CIP2A/p90. The upregulation of miR-218 inhibits the expression of CIP2A/p90 and meanwhile suppresses the functions of melanoma cells, such as migration, proliferation, invasion, and cell cycle (Lu et al., 2015). The study examined the effect of miR-218 on the expression of CIP2A in clear cell renal cell carcinoma (ccRCC). The results showed that the expression level of miR-218 in ccRCC was lower than that in adjacent non-tumor kidney tissues. The downregulation of CIP2A or the overexpression of miR-218 in ccRCC cells can inhibit cell proliferation and migration (Wei et al., 2019). miR-383-5p directly targets CIP2A/p90 to inhibit cell proliferation by G1 cell cycle phase arrest and promotes apoptosis in lung adenocarcinoma (Zhao et al., 2017). CIP2A/p90 is also targeted by miR-375, which stimulates the expression of p21 due to the promotion of its major transcriptional activator, p53, and consequently restrains the action of CIP2A/p90 and c-Myc in cell proliferation. These findings suggest that microRNA can act as a tumor suppressor of oncogenic elements, such as CIP2A/p90 (Jung et al., 2014). In addition, miR-548b-3p regulates proliferation, apoptosis, and mitochondrial function by targeting CIP2A in HCC (Lin and Wang, 2018). There is an automatic regulation feedback loop between CIP2A and miR-301a. Additionally, the feedback of miR-301a promotes the expression of CIP2A through ERK/CREB signal (Yin et al., 2019). A specificity protein 1 (SP1)-induced long non-coding RNA, DPPA2 upstream binding RNA (DUBR), upregulates CIP2A expression through E2F1-mediated transcription regulation, which also plays a role in upregulating CIP2A at the mRNA level by binding miR-520d-5p as a competing endogenous RNA (Liu et al., 2022). The knockdown of LINC00665 can also significantly decrease the cell proliferation, migration, and invasion of HCC, while overexpression of the short peptides of LINC00665 (CIP2A-BP) can markedly increase cell proliferation, invasion, and migration (Li et al., 2022).
The Wnt-β-catenin pathway: after the activation of Wnt, β-catenin is stabilized and bound to the T-cell factor (Tcf)/lymphoid enhancer factor (Lef) family transcription factors, thus leading to a transcriptional activation of target genes (Huang et al., 2019). Aberrant activation of the Wnt/β-catenin pathway is a common event in many types of cancers (Zhang and Wang, 2020). The upregulation of CIP2A/p90 might indirectly lead to reduced β-catenin levels via PP2A inactivation, reinforcing the polo-like kinases (Plk1)-dependent β-catenin inhibition (Li et al., 2015). Additionally, CIP2A/p90 enhances the stabilization of β-catenin to promote fibronectin-induced cancer cell proliferation (Gao et al., 2017).
Phosphorylation and degradation of c-Myc: ERK can phosphorylate c-Myc Ser62 to stabilize it. Then, GSK-3β further phosphorylates c-Myc Thr58, followed by prolyl isomerase (PIN-1), which can transform c-Myc (including both Ser62 and Thr58 phosphorylation sites) from a cis-structure to a trans-structure (Posternak and Cole, 2016). PP2A can catalyze the trans-structure of c-Myc Ser62 dephosphorylation to form the trans-structure of c-Myc (including the Thr58 phosphorylation site), which may be further ubiquitinated and degraded by protein ligase complex (containing FWB7) (Dang, 2012). CIP2A/p90 interacts directly with c-Myc and inhibits PP2A activity toward c-Myc Ser 62, thereby preventing c-Myc proteolytic degradation (Junttila et al., 2007).
Other regulation factors also exist. The expression of CIP2A/p90 in various tumor cells is regulated by other regulation factors with a certain complexity and cell specificity. Moreover, most of them are transcription factors. Octamer-binding transcription factor 4 (Oct4) positively regulates the expression of CIP2A/p90 both in embryonic stem cells and testicular cancer cell lines. The co-expression of Oct4 and CIP2A/p90 is also associated with the increased radio-resistance and aggressiveness in HNSCC cell lines (Ventelä et al., 2015). In addition, the study found that CIP2A can directly interact with TopBP1 and coordinate DNA damage-induced mitotic checkpoint and proliferation, thus driving the initiation and progression of basal breast cancer (Laine et al., 2021). In mouse embryonic fibroblasts, the transcription factor ATF2 binds to the AP-1 site in the promoter region of the CIP2A/p90 gene and initiates gene transcription (Mathiasen et al., 2012). Activated transcription factor 6 (ATF6) is one of the three major stress transduction factors of the endoplasmic reticulum and has been proven to promote chemotherapy resistance by changing the survival of cancer cells. Recent studies have shown that endoplasmic reticulum stress-related ATF6 upregulates CIP2A/p90, which helps to improve the prognosis of colon cancer (Liu X et al., 2018). The activity of checkpoint kinase 1 (CHK1) promotes the transcription of CIP2A/p90, thereby inhibiting the activity of PP2A, the tumor suppressor. In addition, the phosphorylation of CHK1 can upregulate the expression of the CIP2A/p90 gene through phosphorylation of serine 345 of CHK1 via DNA damage response kinases (DNA-PK) in human gastric cancer, ovarian cancer, colon cancer, and neuroblastoma (Khanna et al., 2013; Khanna et al., 2020). Histone deacetylase 1 (HDAC1) regulates CIP2A/p90 gene expression in colorectal cancer cells. The inhibition of HDAC1 by (S)-2 downregulated the transcription of CIP2A/p90 and unleashed PP2A activity, thereby inducing growth arrest and apoptosis in colorectal cancer cells (Balliu et al., 2016).
Compared with normal or para-cancerous tissues, CIP2A/p90 (protein or mRNA) is overexpressed or amplified at a high frequency in the vast majority of solid and hematological tumors (Tang et al., 2018). Recent studies have shown that the aberrant expression level of CIP2A/p90 is either significantly correlated with tumor stages or serves as a prognostic marker for overall survival (OS) and disease-free survival (DFS) (Table 2). According to numerous studies, the high expression of CIP2A/p90 in some cancers, such as cutaneous melanoma, breast cancer, colon cancer, cervical cancer, prostate cancer, and oral cancer, is associated with pathologic high-grade tumor and the progression of disease (Côme et al., 2009; Vaarala et al., 2010; Böckelman et al., 2011a; Böckelman et al., 2012; Shi et al., 2014; Velmurugan et al., 2019). As shown in our previous study, CIP2A/p90 is rarely expressed in non-cancerous/non-transformed cells, but is abundantly expressed in typically transformed cells (Soo Hoo et al., 2002).
As shown in Table 2, some controversial conclusions have been made in the same type of cancer by different research groups. Out of two studies (He et al., 2012; Huang C.Y et al., 2012), He et al. concluded that the high expression of CIP2A/p90 can predict poor outcome in patients with hepatocellular carcinoma, and therefore, this can be used as a significant prognostic factor for DFS and OS (He et al., 2012). Conversely, in the study by Huang et al., the expression of intratumoral CIP2A/p90 mRNA was not associated with prognosis, whereas non-cancerous CIP2A/p90 mRNA was shown to be an independent prognostic factor of OS and recurrence-free survival (RFS) (Huang L.P et al., 2012). Therefore, more extensive research evaluating both CIP2A/p90 protein and mRNA expression, with normal controls, is needed. As with hepatocellular carcinoma, the results from three investigations evaluating the prognostic value of CIP2A/p90 expression were contradictory (Böckelman et al., 2012; Teng et al., 2012; Wiegering et al., 2013). The investigations carried out by Wiegering et al. (2013) and Teng et al. (2012), examining 104 and 167 colon cancer specimens, respectively, both revealed that CIP2A/p90 expression is positively associated with prognosis. By contrast, Böckelman et al. (2012) analyzed 752 specimens and showed there was no significant association between CIP2A/p90 expression and prognosis. This disparity might be due to the different size of each sample or the different antibodies used for staining CIP2A/p90. In addition, the high expression of CIP2A/p90 has diagnostic significance in some cancers, such as papillary thyroid carcinoma, breast cancer, and chronic myeloid leukemia (Liu C Y et al., 2014; Chao et al., 2016; Xing et al., 2016; Clark et al., 2021).
As described above , CIP2A/p90 was initially isolated and characterized as a type of TAA (Soo Hoo et al., 2002). The immune system of certain cancer patients can recognize these aberrant TAA proteins as foreign antigens, thus producing antibodies, called autoantibodies in response. Therefore, anti-TAA autoantibodies might be regarded as biomarkers for the early detection of certain types of cancer (Tan, 2001; Tan and Zhang, 2008). According to our previous studies and others, the frequency of autoantibodies to CIP2A/p90 in sera is significantly higher than that of normal controls. When we selected a panel of TAAs, such as CIP2A/p90, the accumulative positive autoantibodies’ reactions in sera were much higher (Shi et al., 2005; Xie et al., 2011; Liu et al., 2014a). Some data showed the selected panel of TAAs had high specificity and sensitivity as immunodiagnostic biomarkers in both he test cohort and the validation cohort (Zhang et al., 2016; Hoshino et al., 2017). In addition, a few of the panel TAAs, including CIP2A/p90, had a high diagnostic performance in the detection of cancers, especially for the patients at early stage (Zhang et al., 2016; Wang X et al., 2019; Table 3).
The clinical value of the autoantibody responses to CIP2A/p90 and other TAAs might be further validated by more studies of different cancers. The more precise circumscriptions about whether the expression level of anti-TAA autoantibodies varies with disease progression or the response to treatment, and when autoantibodies against these TAAs appear as early predictors of cancers, also needs further investigation (Liu J et al., 2011).
The overexpression of CIP2A/p90 can upregulate the drug resistance of tumor cells to chemotherapy (Liu et al., 2022). Based on the pathophysiology of cancer cells, it can be suggested that effective therapeutic responses against them require simultaneous inhibition of kinase signaling pathways and the reactivation of their inhibitors, such as PP2A (Soofiyani et al., 2017; Westermarck, 2018). CIP2A/p90 siRNA and some small-molecule compounds can inhibit some tumor cell proliferation and corresponding nude mice xenografts. The inhibition was related to the downregulation of CIP2A/p90, the downstream molecules of which could increase PP2A activity and attenuate AKT phosphorylation (Table 4).
According to Table 4, the mechanism by which some small-molecule compounds downregulate CIP2A/p90 has been elucidated. Hypoglycemia and metformin impair the metabolic plasticity and growth of tumors by regulating the PP2A-GSK3b-MCL-1 axis (Elgendy et al., 2019). Lapatinib, erlotinib derivative TD52, and afatinib interfered transcription factor Elk1 combined with the CIP2A/p90 promoter further downregulate the expression of CIP2A/p90 separately in breast cancer cells, liver cancer cells, and NSCLC cells (Yu et al., 2014; Chao et al., 2015; Liu C Y et al., 2016; Liu et al., 2017a). Bortezomib, as a proteasome inhibitor, has an anti-tumor effect in HCC, HNSCC, leukemia, breast cancer, and colon cancer by inhibiting the CIP2A-PP2A-AKT signaling pathway (Chen et al., 2010; Lin et al., 2012; Tseng et al., 2012; Liu et al., 2013; Ding et al., 2014). Celastrol, bound to CIP2A/p90 in NSCLC cells, promotes the connection of CIP2A/p90 with the carboxyl terminus of Hsp70-interacting protein (CHIP) and induces the degradation of CIP2A/p90 (Liu et al., 2014b). Gambogenic acid induces the degradation of CIP2A/p90 through the ubiquitin–proteasome pathway in HCC cells (Yu et al., 2016). Notably, the direct and accurate antagonists of CIP2A/p90 are still unknown. There are multiple challenges in establishing direct CIP2A/p90-target drugs as effective clinical anticancer therapies.
CIP2A/p90 is overexpressed in most types of cancer and is positively correlated with the poor prognosis of many patients. The interaction among CIP2A/p90, PP2A, and c-Myc is an important mechanism of CIP2A/p90 in promoting cancer. Owing to the nature of CIP2A/p90, which can play important roles in the proliferation, apoptosis, invasion, migration, EMT, cell cycle, and drug resistance of tumor cells, it can be used as a potential diagnostic biomarker, as well as an antitumor drug target. However, there are still some important issues to be resolved: (1) the function of CIP2A/p90 in both cell proliferation and drug resistance suggests that it plays an important role in cancer stem cells, which have drug resistance and rapid proliferation. (2) The signaling pathways and regulation networks of CIP2A/p90 are complex. Genomic or systems-level analysis with new tools and technologies will reveal how the signaling pathways and regulators of CIP2A/p90 contribute to tumorigenesis. (3) The precise molecular structure of CIP2A/p90 has not yet been resolved. Therefore, the direct antagonists of CIP2A/p90 still need further investigation and additional application in clinical therapy. (4) The clinical value of autoantibody against CIP2A/p90 as biomarker in cancer needs to be further clinically validated. Overall, there is an urgent need for large studies that will clearly validate the clinical significance of CIP2A/p90, the potential benefit of which is huge.
XC conceived the study. BC and HH conducted the study and drafted the application sections. BC contributed to the writing and review of the manuscript. All authors read, revised, and approved the final manuscript.
This work was supported by the Medical Science and Technique Foundation of Henan Province (LHGJ20210172), the Science and Technique Foundation of Henan Province (222102310424), and the Project of international scientific and technological cooperation in Henan Province (182102410023).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adam, S., Rossi, S. E., Moatti, N., De Marco Zompit, M., Xue, Y., Ng, T. F., et al. (2021). The CIP2A-TOPBP1 axis safeguards chromosome stability and is a synthetic lethal target for BRCA-mutated cancer. Nat. Cancer 2, 1357–1371. doi:10.1038/s43018-021-00266-w
Alzahrani, R., Alrehaili, A. A., Gharib, A. F., Anjum, F., Ismail, K. A., and Elsawy, W. H. (2020). Cancerous inhibitor of protein phosphatase 2A as a molecular marker for aggressiveness and survival in oral squamous cell carcinoma. J. Cancer Prev. 25, 21–26. doi:10.15430/jcp.2020.25.1.21
Balliu, M., Cellai, C., Lulli, M., Laurenzana, A., Torre, E., Vannucchi, A. M., et al. (2016). HDAC1 controls CIP2A transcription in human colorectal cancer cells. Oncotarget 7, 25862–25871. doi:10.18632/oncotarget.8406
Barragán, E., Chillón, M. C., Castelló-Cros, R., Marcotegui, N., Prieto, M. I., Hoyos, M., et al. (2015). CIP2A high expression is a poor prognostic factor in normal karyotype acute myeloid leukemia. Haematologica 100, e183–e185. doi:10.3324/haematol.2014.118117
Baskaran, R., and Velmurugan, B. K. (2018). Protein phosphatase 2A as therapeutic targets in various disease models. Life Sci. 210, 40–46. doi:10.1016/j.lfs.2018.08.063
Birkman, E. M., Elzagheid, A., Jokilehto, T., Avoranta, T., Korkeila, E., Kulmala, J., et al. (2018). Protein phosphatase 2A (PP2A) inhibitor CIP2A indicates resistance to radiotherapy in rectal cancer. Cancer Med. 7, 698–706. doi:10.1002/cam4.1361
Böckelman, C., Hagström, J., Mäkinen, L. K., Keski-Säntti, H., Häyry, V., Lundin, J., et al. (2011a). High CIP2A immunoreactivity is an independent prognostic indicator in early-stage tongue cancer. Br. J. Cancer 104, 1890–1895. doi:10.1038/bjc.2011.167
Böckelman, C., Koskensalo, S., Hagström, J., Lundin, M., Ristimäki, A., and Haglund, C. (2012). CIP2A overexpression is associated with c-Myc expression in colorectal cancer. Cancer Biol. Ther. 13, 289–295. doi:10.4161/cbt.18922
Böckelman, C., Lassus, H., Hemmes, A., Leminen, A., Westermarck, J., Haglund, C., et al. (2011b). Prognostic role of CIP2A expression in serous ovarian cancer. Br. J. Cancer 105, 989–995. doi:10.1038/bjc.2011.346
Cai, F., Zhang, L., Xiao, X., Duan, C., Huang, Q., Fan, C., et al. (2016). Cucurbitacin B reverses multidrug resistance by targeting CIP2A to reactivate protein phosphatase 2A in MCF-7/adriamycin cells. Oncol. Rep. 36, 1180–1186. doi:10.3892/or.2016.4892
Cantini, L., Attaway, C. C., Butler, B., Andino, L. M., Sokolosky, M. L., and Jakymiw, A. (2013). Fusogenic-oligoarginine peptide-mediated delivery of siRNAs targeting the CIP2A oncogene into oral cancer cells. PLoS One 8, e73348. doi:10.1371/journal.pone.0073348
Celikden, S. G., Baspinar, S., Ozturk, S. A., and Karaibrahimoglu, A. (2020). CIP2A expression in high grade prostatic intraepithelial neoplasia and prostate adenocarcinoma: A tissue mıcroarray study. Malays J. Pathol. 42, 227–236.
Cha, G., Xu, J., Xu, X., Li, B., Lu, S., Nanding, A., et al. (2017). High expression of CIP2A protein is associated with tumor aggressiveness in stage I-III NSCLC and correlates with poor prognosis. Onco Targets Ther. 10, 5907–5914. doi:10.2147/ott.S148250
Chao, T. T., Maa, H. C., Wang, C. Y., Pei, D., Liang, Y. J., Yang, Y. F., et al. (2016). CIP2A is a poor prognostic factor and can be a diagnostic marker in papillary thyroid carcinoma. Apmis 124, 1031–1037. doi:10.1111/apm.12602
Chao, T. T., Wang, C. Y., Chen, Y. L., Lai, C. C., Chang, F. Y., Tsai, Y. T., et al. (2015). Afatinib induces apoptosis in NSCLC without EGFR mutation through Elk-1-mediated suppression of CIP2A. Oncotarget 6, 2164–2179. doi:10.18632/oncotarget.2941
Chao, T. T., Wang, C. Y., Lai, C. C., Chen, Y. L., Tsai, Y. T., Chen, P. T., et al. (2014). TD-19, an erlotinib derivative, induces epidermal growth factor receptor wild-type nonsmall-cell lung cancer apoptosis through CIP2A-mediated pathway. J. Pharmacol. Exp. Ther. 351, 352–358. doi:10.1124/jpet.114.215418
Chen, J. S., Wu, B. B., Bao, H. L., Du, J. M., Zhang, S. C., and Zheng, Y. H. (2015). Relationship between CIP2A expression, and prognosis and MDR-related proteins in patients with advanced gastric cancer. Int. J. Clin. Exp. Pathol. 8, 15007–15012.
Chen, K. F., Liu, C. Y., Lin, Y. C., Yu, H. C., Liu, T. H., Hou, D. R., et al. (2010). CIP2A mediates effects of bortezomib on phospho-Akt and apoptosis in hepatocellular carcinoma cells. Oncogene 29, 6257–6266. doi:10.1038/onc.2010.357
Chen, K. F., Pao, K. C., Su, J. C., Chou, Y. C., Liu, C. Y., Chen, H. J., et al. (2012). Development of erlotinib derivatives as CIP2A-ablating agents independent of EGFR activity. Bioorg Med. Chem. 20, 6144–6153. doi:10.1016/j.bmc.2012.08.039
Chen, K. F., Yen, C. C., Lin, J. K., Chen, W. S., Yang, S. H., Jiang, J. K., et al. (2015). Cancerous inhibitor of protein phosphatase 2A (CIP2A) is an independent prognostic marker in wild-type KRAS metastatic colorectal cancer after colorectal liver metastasectomy. BMC Cancer 15, 301. doi:10.1186/s12885-015-1300-3
Chen, N., Li, X., Chintala, N. K., Tano, Z. E., and Adusumilli, P. S. (2018). Driving CARs on the uneven road of antigen heterogeneity in solid tumors. Curr. Opin. Immunol. 51, 103–110. doi:10.1016/j.coi.2018.03.002
Chen, W., Liang, J. L., Zhou, K., Zeng, Q. L., Ye, J. W., and Huang, M. J. (2020). Effect of CIP2A and its mechanism of action in the malignant biological behavior of colorectal cancer. Cell Commun. Signal 18, 67. doi:10.1186/s12964-020-00545-6
Chen, W., Wang, Z., Jiang, C., and Ding, Y. (2013). PP2A-Mediated anticancer therapy. Gastroenterol. Res. Pract. 2013, 675429. doi:10.1155/2013/675429
Choi, Y. A., Koo, J. S., Park, J. S., Park, M. Y., Jeong, A. L., Oh, K. S., et al. (2014). Estradiol enhances CIP2A expression by the activation of p70 S6 kinase. Endocr. Relat. Cancer 21, 189–202. doi:10.1530/erc-13-0453
Clark, R. E., Basabrain, A. A., Austin, G. M., Holcroft, A. K., Loaiza, S., Apperley, J. F., et al. (2021). Validation of CIP2A as a biomarker of subsequent disease progression and treatment failure in chronic myeloid leukaemia. Cancers (Basel) 13, 2155. doi:10.3390/cancers13092155
Côme, C., Laine, A., Chanrion, M., Edgren, H., Mattila, E., Liu, X., et al. (2009). CIP2A is associated with human breast cancer aggressivity. Clin. Cancer Res. 15, 5092–5100. doi:10.1158/1078-0432.Ccr-08-3283
De, P., Carlson, J., Leyland-Jones, B., and Dey, N. (2014). Oncogenic nexus of cancerous inhibitor of protein phosphatase 2A (CIP2A): An oncoprotein with many hands. Oncotarget 5, 4581–4602. doi:10.18632/oncotarget.2127
Denk, S., Schmidt, S., Schurr, Y., Schwarz, G., Schote, F., Diefenbacher, M., et al. (2021). CIP2A regulates MYC translation (via its 5'UTR) in colorectal cancer. Int. J. Colorectal Dis. 36, 911–918. doi:10.1007/s00384-020-03772-y
Dhanasekaran, R., Deutzmann, A., Mahauad-Fernandez, W. D., Hansen, A. S., Gouw, A. M., and Felsher, D. W. (2022). The MYC oncogene - the grand orchestrator of cancer growth and immune evasion. Nat. Rev. Clin. Oncol. 19, 23–36. doi:10.1038/s41571-021-00549-2
Ding, Y., Wang, Y., Ju, S., Wu, X., Zhu, W., Shi, F., et al. (2014). Role of CIP2A in the antitumor effect of bortezomib in colon cancer. Mol. Med. Rep. 10, 387–392. doi:10.3892/mmr.2014.2173
Dong, Q. Z., Wang, Y., Dong, X. J., Li, Z. X., Tang, Z. P., Cui, Q. Z., et al. (2011). CIP2A is overexpressed in non-small cell lung cancer and correlates with poor prognosis. Ann. Surg. Oncol. 18, 857–865. doi:10.1245/s10434-010-1313-8
Duan, J., Zhan, J. C., Wang, G. Z., Zhao, X. C., Huang, W. D., and Zhou, G. B. (2019). The red wine component ellagic acid induces autophagy and exhibits anti-lung cancer activity in vitro and in vivo. J. Cell Mol. Med. 23, 143–154. doi:10.1111/jcmm.13899
Duffy, M. J., O'Grady, S., Tang, M., and Crown, J. (2021). MYC as a target for cancer treatment. Cancer Treat. Rev. 94, 102154. doi:10.1016/j.ctrv.2021.102154
Elgendy, M., Cirò, M., Hosseini, A., Weiszmann, J., Mazzarella, L., Ferrari, E., et al. (2019). Combination of hypoglycemia and metformin impairs tumor metabolic plasticity and growth by modulating the PP2A-gsk3β-MCL-1 Axis. Cancer Cell 35, 798–815.e5. doi:10.1016/j.ccell.2019.03.007
Fang, L., Zhang, Y., Zang, Y., Chai, R., Zhong, G., Li, Z., et al. (2019). HP-1 inhibits the progression of ccRCC and enhances sunitinib therapeutic effects by suppressing EMT. Carbohydr. Polym. 223, 115109. doi:10.1016/j.carbpol.2019.115109
Fang, Y., Li, Z., Wang, X., and Zhang, S. (2012). CIP2A is overexpressed in human ovarian cancer and regulates cell proliferation and apoptosis. Tumour Biol. 33, 2299–2306. doi:10.1007/s13277-012-0492-2
Farrington, C. C., Yuan, E., Mazhar, S., Izadmehr, S., Hurst, L., Allen-Petersen, B. L., et al. (2020). Protein phosphatase 2A activation as a therapeutic strategy for managing MYC-driven cancers. J. Biol. Chem. 295, 757–770. doi:10.1074/jbc.RA119.011443
Feng, F., Cheng, P., Wang, C., Wang, Y., and Wang, W. (2019a). Polyphyllin I and VII potentiate the chemosensitivity of A549/DDP cells to cisplatin by enhancing apoptosis, reversing EMT and suppressing the CIP2A/AKT/mTOR signaling axis. Oncol. Lett. 18, 5428–5436. doi:10.3892/ol.2019.10895
Feng, F. F., Cheng, P., Sun, C., Wang, H., and Wang, W. (2019b). Inhibitory effects of polyphyllins I and VII on human cisplatin-resistant NSCLC via p53 upregulation and CIP2A/AKT/mTOR signaling axis inhibition. Chin. J. Nat. Med. 17, 768–777. doi:10.1016/s1875-5364(19)30093-7
Flørenes, V. A., Emilsen, E., Dong, H. P., Førsund, M., Holm, R., and Slipicevic, A. (2015). Cellular localization of CIP2A determines its prognostic impact in superficial spreading and nodular melanoma. Cancer Med. 4, 903–913. doi:10.1002/cam4.425
Gao, D., Nyalali, A. M. K., Hou, Y., Xu, Y., Zhou, J., Zhao, W., et al. (2021). 2,5-Dimethyl celecoxib inhibits proliferation and cell cycle and induces apoptosis in glioblastoma by suppressing CIP2A/PP2A/akt signaling Axis. J. Mol. Neurosci. 71, 1703–1713. doi:10.1007/s12031-020-01773-8
Gao, F., Xu, T., Wang, X., Zhong, S., Chen, S., Zhang, M., et al. (2017). CIP2A mediates fibronectin-induced bladder cancer cell proliferation by stabilizing β-catenin. J. Exp. Clin. Cancer Res. 36, 70. doi:10.1186/s13046-017-0539-8
Gao, H., Li, Y., Lin, T., Cheng, Y., and Ma, Y. (2020). Downregulation of CIP2A inhibits cancer cell proliferation and vascularization in renal clear cell carcinoma. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 164, 196–202. doi:10.5507/bp.2019.031
Garner, E. F., Williams, A. P., Stafman, L. L., Aye, J. M., Mroczek-Musulman, E., Moore, B. P., et al. (2018). FTY720 decreases tumorigenesis in group 3 medulloblastoma patient-derived xenografts. Sci. Rep. 8, 6913. doi:10.1038/s41598-018-25263-5
Grieb, B. C., and Eischen, C. M. (2022). MTBP and myc: A dynamic duo in proliferation, cancer, and aging. Biol. (Basel) 11, 881. doi:10.3390/biology11060881
He, H., Wu, G., Li, W., Cao, Y., and Liu, Y. (2012). CIP2A is highly expressed in hepatocellular carcinoma and predicts poor prognosis. Diagn Mol. Pathol. 21, 143–149. doi:10.1097/PDM.0b013e318249fd8b
Hoshino, I., Nagata, M., Takiguchi, N., Nabeya, Y., Ikeda, A., Yokoi, S., et al. (2017). Panel of autoantibodies against multiple tumor-associated antigens for detecting gastric cancer. Cancer Sci. 108, 308–315. doi:10.1111/cas.13158
Hou, D. R., Huang, A. C., Shiau, C. W., Wang, C. Y., Yu, H. C., and Chen, K. F. (2013). Bortezomib congeners induce apoptosis of hepatocellular carcinoma via CIP2A inhibition. Molecules 18, 15398–15411. doi:10.3390/molecules181215398
Huang, C. Y., Wei, C. C., Chen, K. C., Chen, H. J., Cheng, A. L., and Chen, K. F. (2012). Bortezomib enhances radiation-induced apoptosis in solid tumors by inhibiting CIP2A. Cancer Lett. 317, 9–15. doi:10.1016/j.canlet.2011.11.005
Huang, J., Jia, J., Tong, Q., Liu, J., Qiu, J., Sun, R., et al. (2015). Knockdown of cancerous inhibitor of protein phosphatase 2A may sensitize metastatic castration-resistant prostate cancer cells to cabazitaxel chemotherapy. Tumour Biol. 36, 1589–1594. doi:10.1007/s13277-014-2748-5
Huang, L. P., Savoly, D., Sidi, A. A., Adelson, M. E., Mordechai, E., and Trama, J. P. (2012). CIP2A protein expression in high-grade, high-stage bladder cancer. Cancer Med. 1, 76–81. doi:10.1002/cam4.15
Huang, P., Qiu, J., You, J., Hong, J., Li, B., Zhou, K., et al. (2012). Expression and prognostic significance of CIP2A mRNA in hepatocellular carcinoma and nontumoral liver tissues. Biomarkers 17, 422–429. doi:10.3109/1354750x.2012.680608
Huang, P., Yan, R., Zhang, X., Wang, L., Ke, X., and Qu, Y. (2019). Activating Wnt/β-catenin signaling pathway for disease therapy: Challenges and opportunities. Pharmacol. Ther. 196, 79–90. doi:10.1016/j.pharmthera.2018.11.008
Jeong, A. L., Lee, S., Park, J. S., Han, S., Jang, C. Y., Lim, J. S., et al. (2014). Cancerous inhibitor of protein phosphatase 2A (CIP2A) protein is involved in centrosome separation through the regulation of NIMA (never in mitosis gene A)-related kinase 2 (NEK2) protein activity. J. Biol. Chem. 289, 28–40. doi:10.1074/jbc.M113.507954
Jhunjhunwala, S., Hammer, C., and Delamarre, L. (2021). Antigen presentation in cancer: Insights into tumour immunogenicity and immune evasion. Nat. Rev. Cancer 21, 298–312. doi:10.1038/s41568-021-00339-z
Ji, J., Zhen, W., Si, Y., Ma, W., Zheng, L., Li, C., et al. (2018). Increase in CIP2A expression is associated with cisplatin chemoresistance in gastric cancer. Cancer Biomark. 21, 307–316. doi:10.3233/cbm-170416
Jin, L., Si, Y., Hong, X., Liu, P., Zhu, B., Yu, H., et al. (2018). Ethoxysanguinarine inhibits viability and induces apoptosis of colorectal cancer cells by inhibiting CIP2A. Int. J. Oncol. 52, 1569–1578. doi:10.3892/ijo.2018.4323
Jung, H. M., Patel, R. S., Phillips, B. L., Wang, H., Cohen, D. M., Reinhold, W. C., et al. (2013). Tumor suppressor miR-375 regulates MYC expression via repression of CIP2A coding sequence through multiple miRNA-mRNA interactions. Mol. Biol. Cell 24, 1638–1648. doi:10.1091/mbc.E12-12-0891
Jung, H. M., Phillips, B. L., and Chan, E. K. (2014). miR-375 activates p21 and suppresses telomerase activity by coordinately regulating HPV E6/E7, E6AP, CIP2A, and 14-3-3ζ. Mol. Cancer 13, 80. doi:10.1186/1476-4598-13-80
Junttila, M. R., Puustinen, P., Niemelä, M., Ahola, R., Arnold, H., Böttzauw, T., et al. (2007). CIP2A inhibits PP2A in human malignancies. Cell 130, 51–62. doi:10.1016/j.cell.2007.04.044
Kauko, O., and Westermarck, J. (2018). Non-genomic mechanisms of protein phosphatase 2A (PP2A) regulation in cancer. Int. J. Biochem. Cell Biol. 96, 157–164. doi:10.1016/j.biocel.2018.01.005
Khan, M. M., Kalim, U. U., Khan, M. H., and Lahesmaa, R. (2021). PP2A and its inhibitors in helper T-cell differentiation and autoimmunity. Front. Immunol. 12, 786857. doi:10.3389/fimmu.2021.786857
Khan, M. M., Ullah, U., Khan, M. H., Kong, L., Moulder, R., Välikangas, T., et al. (2020a). CIP2A constrains Th17 differentiation by modulating STAT3 signaling. iScience 23, 100947. doi:10.1016/j.isci.2020.100947
Khan, M. M., Välikangas, T., Khan, M. H., Moulder, R., Ullah, U., Bhosale, S. D., et al. (2020b). Protein interactome of the Cancerous Inhibitor of protein phosphatase 2A (CIP2A) in Th17 cells. Curr. Res. Immunol. 1, 10–22. doi:10.1016/j.crimmu.2020.02.001
Khan, R., Kulasiri, D., and Samarasinghe, S. (2021). Functional repertoire of protein kinases and phosphatases in synaptic plasticity and associated neurological disorders. Neural Regen. Res. 16, 1150–1157. doi:10.4103/1673-5374.300331
Khanna, A., Böckelman, C., Hemmes, A., Junttila, M. R., Wiksten, J. P., Lundin, M., et al. (2009). MYC-dependent regulation and prognostic role of CIP2A in gastric cancer. J. Natl. Cancer Inst. 101, 793–805. doi:10.1093/jnci/djp103
Khanna, A., Kauko, O., Böckelman, C., Laine, A., Schreck, I., Partanen, J. I., et al. (2013). Chk1 targeting reactivates PP2A tumor suppressor activity in cancer cells. Cancer Res. 73, 6757–6769. doi:10.1158/0008-5472.Can-13-1002
Khanna, A., Okkeri, J., Bilgen, T., Tiirikka, T., Vihinen, M., Visakorpi, T., et al. (2011). ETS1 mediates MEK1/2-dependent overexpression of cancerous inhibitor of protein phosphatase 2A (CIP2A) in human cancer cells. PLoS One 6, e17979. doi:10.1371/journal.pone.0017979
Khanna, A., Rane, J. K., Kivinummi, K. K., Urbanucci, A., Helenius, M. A., Tolonen, T. T., et al. (2015). CIP2A is a candidate therapeutic target in clinically challenging prostate cancer cell populations. Oncotarget 6, 19661–19670. doi:10.18632/oncotarget.3875
Khanna, A., Thoms, J. A. I., Stringer, B. W., Chung, S. A., Ensbey, K. S., Jue, T. R., et al. (2020). Constitutive CHK1 expression drives a pSTAT3-cip2a circuit that promotes glioblastoma cell survival and growth. Mol. Cancer Res. 18, 709–722. doi:10.1158/1541-7786.Mcr-19-0934
Kim, J. S., Kim, E. J., Oh, J. S., Park, I. C., and Hwang, S. G. (2013). CIP2A modulates cell-cycle progression in human cancer cells by regulating the stability and activity of Plk1. Cancer Res. 73, 6667–6678. doi:10.1158/0008-5472.Can-13-0888
Kleszcz, R., Szymańska, A., Krajka-Kuźniak, V., Baer-Dubowska, W., and Paluszczak, J. (2019). Inhibition of CBP/β-catenin and porcupine attenuates Wnt signaling and induces apoptosis in head and neck carcinoma cells. Cell Oncol. (Dordr) 42, 505–520. doi:10.1007/s13402-019-00440-4
Laine, A., Nagelli, S. G., Farrington, C., Butt, U., Cvrljevic, A. N., Vainonen, J. P., et al. (2021). CIP2A interacts with TopBP1 and drives basal-like breast cancer tumorigenesis. Cancer Res. 81, 4319–4331. doi:10.1158/0008-5472.Can-20-3651
Laine, A., Sihto, H., Come, C., Rosenfeldt, M. T., Zwolinska, A., Niemelä, M., et al. (2013). Senescence sensitivity of breast cancer cells is defined by positive feedback loop between CIP2A and E2F1. Cancer Discov. 3, 182–197. doi:10.1158/2159-8290.Cd-12-0292
Lei, N., Peng, B., and Zhang, J. Y. (2014). CIP2A regulates cell proliferation via the AKT signaling pathway in human lung cancer. Oncol. Rep. 32, 1689–1694. doi:10.3892/or.2014.3375
Li, J., Karki, A., Hodges, K. B., Ahmad, N., Zoubeidi, A., Strebhardt, K., et al. (2015). Cotargeting polo-like kinase 1 and the wnt/β-catenin signaling pathway in castration-resistant prostate cancer. Mol. Cell Biol. 35, 4185–4198. doi:10.1128/mcb.00825-15
Li, J., Qin, B., Huang, M., Ma, Y., Li, D., Li, W., et al. (2021). Tumor-associated antigens (TAAs) for the serological diagnosis of osteosarcoma. Front. Immunol. 12, 665106. doi:10.3389/fimmu.2021.665106
Li, Y. R., Zong, R. Q., Zhang, H. Y., Meng, X. Y., and Wu, F. X. (2022). Mechanism analysis of LINC00665 and its peptides CIP2A-BP in hepatocellular carcinoma. Front. Genet. 13, 861096. doi:10.3389/fgene.2022.861096
Li, Y., Wang, M., Zhu, X., Cao, X., Wu, Y., and Fang, F. (2019). Prognostic significance of CIP2A in esophagogastric junction adenocarcinoma: A study of 65 patients and a meta-analysis. Dis. Markers 2019, 2312439. doi:10.1155/2019/2312439
Lin, L., and Wang, Y. (2018). miR-548b-3p regulates proliferation, apoptosis, and mitochondrial function by targeting CIP2A in hepatocellular carcinoma. Biomed. Res. Int. 2018, 7385426. doi:10.1155/2018/7385426
Lin, Y. C., Chen, K. C., Chen, C. C., Cheng, A. L., and Chen, K. F. (2012). CIP2A-mediated Akt activation plays a role in bortezomib-induced apoptosis in head and neck squamous cell carcinoma cells. Oral Oncol. 48, 585–593. doi:10.1016/j.oraloncology.2012.01.012
Liu, C. Y., Hsieh, F. S., Chu, P. Y., Tsai, W. C., Huang, C. T., Yu, Y. B., et al. (2017a). Carfilzomib induces leukaemia cell apoptosis via inhibiting ELK1/KIAA1524 (Elk-1/CIP2A) and activating PP2A not related to proteasome inhibition. Br. J. Haematol. 177, 726–740. doi:10.1111/bjh.14620
Liu, C. Y., Hsu, C. C., Huang, T. T., Lee, C. H., Chen, J. L., Yang, S. H., et al. (2018). ER stress-related ATF6 upregulates CIP2A and contributes to poor prognosis of colon cancer. Mol. Oncol. 12, 1706–1717. doi:10.1002/1878-0261.12365
Liu, C. Y., Hu, M. H., Hsu, C. J., Huang, C. T., Wang, D. S., Tsai, W. C., et al. (2016). Lapatinib inhibits CIP2A/PP2A/p-Akt signaling and induces apoptosis in triple negative breast cancer cells. Oncotarget 7, 9135–9149. doi:10.18632/oncotarget.7035
Liu, C. Y., Huang, T. T., Huang, C. T., Hu, M. H., Wang, D. S., Wang, W. L., et al. (2017b). EGFR-independent Elk1/CIP2A signalling mediates apoptotic effect of an erlotinib derivative TD52 in triple-negative breast cancer cells. Eur. J. Cancer 72, 112–123. doi:10.1016/j.ejca.2016.11.012
Liu, C. Y., Hung, M. H., Wang, D. S., Chu, P. Y., Su, J. C., Teng, T. H., et al. (2014). Tamoxifen induces apoptosis through cancerous inhibitor of protein phosphatase 2A-dependent phospho-Akt inactivation in estrogen receptor-negative human breast cancer cells. Breast Cancer Res. 16, 431. doi:10.1186/s13058-014-0431-9
Liu, C. Y., Shiau, C. W., Kuo, H. Y., Huang, H. P., Chen, M. H., Tzeng, C. H., et al. (2013). Cancerous inhibitor of protein phosphatase 2A determines bortezomib-induced apoptosis in leukemia cells. Haematologica 98, 729–738. doi:10.3324/haematol.2011.050187
Liu, H., Qiu, H., Song, Y., Liu, Y., Wang, H., Lu, M., et al. (2017). Cip2a promotes cell cycle progression in triple-negative breast cancer cells by regulating the expression and nuclear export of p27Kip1. Oncogene 36, 1952–1964. doi:10.1038/onc.2016.355
Liu, J., Wang, M., Zhang, X., Wang, Q., Qi, M., Hu, J., et al. (2016). CIP2A is associated with multidrug resistance in cervical adenocarcinoma by a P-glycoprotein pathway. Tumour Biol. 37, 2673–2682. doi:10.1007/s13277-015-4032-8
Liu, J., Wang, X., Zhou, G., Wang, H., Xiang, L., Cheng, Y., et al. (2011). Cancerous inhibitor of protein phosphatase 2A is overexpressed in cervical cancer and upregulated by human papillomavirus 16 E7 oncoprotein. Gynecol. Oncol. 122, 430–436. doi:10.1016/j.ygyno.2011.04.031
Liu, N., He, Q. M., Chen, J. W., Li, Y. Q., Xu, Y. F., Ren, X. Y., et al. (2014). Overexpression of CIP2A is an independent prognostic indicator in nasopharyngeal carcinoma and its depletion suppresses cell proliferation and tumor growth. Mol. Cancer 13, 111. doi:10.1186/1476-4598-13-111
Liu, P., Xiang, Y., Liu, X., Zhang, T., Yang, R., Chen, S., et al. (2019). Cucurbitacin B induces the lysosomal degradation of EGFR and suppresses the CIP2A/PP2A/akt signaling Axis in gefitinib-resistant non-small cell lung cancer. Molecules 24, 647. doi:10.3390/molecules24030647
Liu, S., Bu, X., Kan, A., Luo, L., Xu, Y., Chen, H., et al. (2022). SP1-induced lncRNA DUBR promotes stemness and oxaliplatin resistance of hepatocellular carcinoma via E2F1-CIP2A feedback. Cancer Lett. 528, 16–30. doi:10.1016/j.canlet.2021.12.026
Liu, W., Peng, B., Lu, Y., Xu, W., Qian, W., and Zhang, J. Y. (2011). Autoantibodies to tumor-associated antigens as biomarkers in cancer immunodiagnosis. Autoimmun. Rev. 10, 331–335. doi:10.1016/j.autrev.2010.12.002
Liu X, X., Chai, Y., Li, J., Ren, P., Liu, M., Dai, L., et al. (2014). Autoantibody response to a novel tumor-associated antigen p90/CIP2A in breast cancer immunodiagnosis. Tumour Biol. 35, 2661–2667. doi:10.1007/s13277-013-1350-6
Liu, X., Cao, W., Qin, S., Zhang, T., Zheng, J., Dong, Y., et al. (2017c). Overexpression of CIP2A is associated with poor prognosis in multiple myeloma. Signal Transduct. Target Ther. 2, 17013. doi:10.1038/sigtrans.2017.13
Liu, X., Duan, C., Ji, J., Zhang, T., Yuan, X., Zhang, Y., et al. (2017d). Cucurbitacin B induces autophagy and apoptosis by suppressing CIP2A/PP2A/mTORC1 signaling axis in human cisplatin resistant gastric cancer cells. Oncol. Rep. 38, 271–278. doi:10.3892/or.2017.5648
Liu, X., Sun, Z., Deng, J., Liu, J., Ma, K., Si, Y., et al. (2018). Polyphyllin I inhibits invasion and epithelial-mesenchymal transition via CIP2A/PP2A/ERK signaling in prostate cancer. Int. J. Oncol. 53, 1279–1288. doi:10.3892/ijo.2018.4464
Liu, Z., Ma, L., Wen, Z. S., Cheng, Y. X., and Zhou, G. B. (2014a). Ethoxysanguinarine induces inhibitory effects and downregulates CIP2A in lung cancer cells. ACS Med. Chem. Lett. 5, 113–118. doi:10.1021/ml400341k
Liu, Z., Ma, L., Wen, Z. S., Hu, Z., Wu, F. Q., Li, W., et al. (2014b). Cancerous inhibitor of PP2A is targeted by natural compound celastrol for degradation in non-small-cell lung cancer. Carcinogenesis 35, 905–914. doi:10.1093/carcin/bgt395
Lu, Y. F., Zhang, L., Waye, M. M., Fu, W. M., and Zhang, J. F. (2015). MiR-218 mediates tumorigenesis and metastasis: Perspectives and implications. Exp. Cell Res. 334, 173–182. doi:10.1016/j.yexcr.2015.03.027
Lucas, C. M., Harris, R. J., Holcroft, A. K., Scott, L. J., Carmell, N., McDonald, E., et al. (2015). Second generation tyrosine kinase inhibitors prevent disease progression in high-risk (high CIP2A) chronic myeloid leukaemia patients. Leukemia 29, 1514–1523. doi:10.1038/leu.2015.71
Luque, M., Cristóbal, I., Sanz-Álvarez, M., Santos, A., Zazo, S., Eroles, P., et al. (2022). CIP2A as a key regulator for AKT phosphorylation has partial impact determining clinical outcome in breast cancer. J. Clin. Med. 11, 1610. doi:10.3390/jcm11061610
Ma, L., Wen, Z. S., Liu, Z., Hu, Z., Ma, J., Chen, X. Q., et al. (2011). Overexpression and small molecule-triggered downregulation of CIP2A in lung cancer. PLoS One 6, e20159. doi:10.1371/journal.pone.0020159
Ma, W., Xiang, Y., Yang, R., Zhang, T., Xu, J., Wu, Y., et al. (2019). Cucurbitacin B induces inhibitory effects via the CIP2A/PP2A/C-KIT signaling axis in t(8;21) acute myeloid leukemia. J. Pharmacol. Sci. 139, 304–310. doi:10.1016/j.jphs.2018.12.010
Mathiasen, D. P., Egebjerg, C., Andersen, S. H., Rafn, B., Puustinen, P., Khanna, A., et al. (2012). Identification of a c-Jun N-terminal kinase-2-dependent signal amplification cascade that regulates c-Myc levels in ras transformation. Oncogene 31, 390–401. doi:10.1038/onc.2011.230
Mercier, C., Rousseau, M., and Geraldes, P. (2020). Growth factor deregulation and emerging role of phosphatases in diabetic peripheral artery disease. Front. Cardiovasc Med. 7, 619612. doi:10.3389/fcvm.2020.619612
Monga, J., Suthar, S. K., Rohila, D., Joseph, A., Chauhan, C. S., and Sharma, M. (2022). (+)-Cyanidan-3-ol inhibits epidermoid squamous cell carcinoma growth via inhibiting AKT/mTOR signaling through modulating CIP2A-PP2A axis. Phytomedicine 101, 154116. doi:10.1016/j.phymed.2022.154116
Pallai, R., Bhaskar, A., Barnett-Bernodat, N., Gallo-Ebert, C., Nickels, J. T., and Rice, L. M. (2015). Cancerous inhibitor of protein phosphatase 2A promotes premature chromosome segregation and aneuploidy in prostate cancer cells through association with shugoshin. Tumour Biol. 36, 6067–6074. doi:10.1007/s13277-015-3284-7
Pallai, R., Bhaskar, A., Sodi, V., and Rice, L. M. (2012). Ets1 and Elk1 transcription factors regulate cancerous inhibitor of protein phosphatase 2A expression in cervical and endometrial carcinoma cells. Transcription 3, 323–335. doi:10.4161/trns.22518
Pang, X., Fu, X., Chen, S., Zhu, X., Qi, H., Li, Y., et al. (2016). Overexpression of CIP2A promotes bladder cancer progression by regulating EMT. Clin. Transl. Oncol. 18, 289–295. doi:10.1007/s12094-015-1366-z
Peng, B., Chai, Y., Li, Y., Liu, X., and Zhang, J. (2015). CIP2A overexpression induces autoimmune response and enhances JNK signaling pathway in human lung cancer. BMC Cancer 15, 895. doi:10.1186/s12885-015-1899-0
Perrotti, D., and Neviani, P. (2013). Protein phosphatase 2A: A target for anticancer therapy. Lancet Oncol. 14, e229–e238. doi:10.1016/s1470-2045(12)70558-2
Pippa, R., and Odero, M. D. (2020). The role of MYC and PP2A in the initiation and progression of myeloid leukemias. Cells 9, 544. doi:10.3390/cells9030544
Posternak, V., and Cole, M. D. (2016). Strategically targeting MYC in cancer. F1000Res 5, 408. doi:10.12688/f1000research.7879.1
Qin, S., Li, J., Si, Y., He, Z., Zhang, T., Wang, D., et al. (2018). Cucurbitacin B induces inhibitory effects via CIP2A/PP2A/Akt pathway in glioblastoma multiforme. Mol. Carcinog. 57, 687–699. doi:10.1002/mc.22789
Qu, W., Li, W., Wei, L., Xing, L., Wang, X., and Yu, J. (2012). CIP2A is overexpressed in esophageal squamous cell carcinoma. Med. Oncol. 29, 113–118. doi:10.1007/s12032-010-9768-9
Rantanen, T., Kauttu, T., Åkerla, J., Honkanen, T., Krogerus, L., Salo, J., et al. (2013). CIP2A expression and prognostic role in patients with esophageal adenocarcinoma. Med. Oncol. 30, 684. doi:10.1007/s12032-013-0684-7
Remmerie, M., and Janssens, V. (2019). PP2A: A promising biomarker and therapeutic target in endometrial cancer. Front. Oncol. 9, 462. doi:10.3389/fonc.2019.00462
Ren, J., Li, W., Yan, L., Jiao, W., Tian, S., Li, D., et al. (2011). Expression of CIP2A in renal cell carcinomas correlates with tumour invasion, metastasis and patients' survival. Br. J. Cancer 105, 1905–1911. doi:10.1038/bjc.2011.492
Routila, J., Bilgen, T., Saramäki, O., Grénman, R., Visakorpi, T., Westermarck, J., et al. (2016). Copy number increase of oncoprotein CIP2A is associated with poor patient survival in human head and neck squamous cell carcinoma. J. Oral Pathol. Med. 45, 329–337. doi:10.1111/jop.12372
Ruvolo, P. P. (2019). Role of protein phosphatases in the cancer microenvironment. Biochim. Biophys. Acta Mol. Cell Res. 1866, 144–152. doi:10.1016/j.bbamcr.2018.07.006
Ruvolo, P. P. (2016). The broken "off" switch in cancer signaling: PP2A as a regulator of tumorigenesis, drug resistance, and immune surveillance. BBA Clin. 6, 87–99. doi:10.1016/j.bbacli.2016.08.002
Saafan, H., Alahdab, A., Michelet, R., Gohlke, L., Ziemann, J., Holdenrieder, S., et al. (2021). Constitutive cell proliferation regulating inhibitor of protein phosphatase 2A (CIP2A) mediates drug resistance to erlotinib in an EGFR activating mutated NSCLC cell line. Cells 10, 10040716. doi:10.3390/cells10040716
Sangodkar, J., Farrington, C. C., McClinch, K., Galsky, M. D., Kastrinsky, D. B., and Narla, G. (2016). All roads lead to PP2A: Exploiting the therapeutic potential of this phosphatase. Febs J. 283, 1004–1024. doi:10.1111/febs.13573
Scarpa, M., Singh, P., Bailey, C. M., Lee, J. K., Kapoor, S., Lapidus, R. G., et al. (2021). PP2A-activating drugs enhance FLT3 inhibitor efficacy through AKT inhibition-dependent GSK-3β-mediated c-myc and pim-1 proteasomal degradation. Mol. Cancer Ther. 20, 676–690. doi:10.1158/1535-7163.Mct-20-0663
Shentu, Y. P., Hu, W. T., Zhang, Q., Huo, Y., Liang, J. W., Liuyang, Z. Y., et al. (2019). CIP2A-promoted astrogliosis induces AD-like synaptic degeneration and cognitive deficits. Neurobiol. Aging 75, 198–208. doi:10.1016/j.neurobiolaging.2018.11.023
Shi, F., Ding, Y., Ju, S., Wu, X., and Cao, S. (2014). Expression and prognostic significance of CIP2A in cutaneous malignant melanoma. Biomarkers 19, 70–76. doi:10.3109/1354750x.2013.871752
Shi, F. D., Zhang, J. Y., Liu, D., Rearden, A., Elliot, M., Nachtsheim, D., et al. (2005). Preferential humoral immune response in prostate cancer to cellular proteins p90 and p62 in a panel of tumor-associated antigens. Prostate 63, 252–258. doi:10.1002/pros.20181
Sipeky, C., Gao, P., Zhang, Q., Wang, L., Ettala, O., Talala, K. M., et al. (2018). Synergistic interaction of HOXB13 and CIP2A predisposes to aggressive prostate cancer. Clin. Cancer Res. 24, 6265–6276. doi:10.1158/1078-0432.Ccr-18-0444
Soo Hoo, L., Zhang, J. Y., and Chan, E. K. (2002). Cloning and characterization of a novel 90 kDa 'companion' auto-antigen of p62 overexpressed in cancer. Oncogene 21, 5006–5015. doi:10.1038/sj.onc.1205625
Soofiyani, S. R., Hejazi, M. S., and Baradaran, B. (2017). The role of CIP2A in cancer: A review and update. Biomed. Pharmacother. 96, 626–633. doi:10.1016/j.biopha.2017.08.146
Sumazaki, M., Ogata, H., Nabeya, Y., Kuwajima, A., Hiwasa, T., and Shimada, H. (2021). Multipanel assay of 17 tumor-associated antibodies for serological detection of stage 0/I breast cancer. Cancer Sci. 112, 1955–1962. doi:10.1111/cas.14860
Sun, L., and Gao, P. (2017). Reproducibility in cancer biology: Small molecules remain on target for c-myc. Elife 6, e22915. doi:10.7554/eLife.22915
Sung, W. W., Wang, Y. C., Lin, P. L., Cheng, Y. W., Chen, C. Y., Wu, T. C., et al. (2013). IL-10 promotes tumor aggressiveness via upregulation of CIP2A transcription in lung adenocarcinoma. Clin. Cancer Res. 19, 4092–4103. doi:10.1158/1078-0432.Ccr-12-3439
Tan, E. M. (2001). Autoantibodies as reporters identifying aberrant cellular mechanisms in tumorigenesis. J. Clin. Invest. 108, 1411–1415. doi:10.1172/jci14451
Tan, E. M., and Zhang, J. (2008). Autoantibodies to tumor-associated antigens: Reporters from the immune system. Immunol. Rev. 222, 328–340. doi:10.1111/j.1600-065X.2008.00611.x
Tang, M., Shen, J. F., Li, P., Zhou, L. N., Zeng, P., Cui, X. X., et al. (2018). Prognostic significance of CIP2A expression in solid tumors: A meta-analysis. PLoS One 13, e0199675. doi:10.1371/journal.pone.0199675
Tang, Q., Wang, Q., Zeng, G., Li, Q., Jiang, T., Zhang, Z., et al. (2015). Overexpression of CIP2A in clear cell renal cell carcinoma promotes cellular epithelial-mesenchymal transition and is associated with poor prognosis. Oncol. Rep. 34, 2515–2522. doi:10.3892/or.2015.4217
Tarek, M. M., Yahia, A., El-Nakib, M. M., and Elhefnawi, M. (2021). Integrative assessment of CIP2A overexpression and mutational effects in human malignancies identifies possible deleterious variants. Comput. Biol. Med. 139, 104986. doi:10.1016/j.compbiomed.2021.104986
Teng, H. W., Yang, S. H., Lin, J. K., Chen, W. S., Lin, T. C., Jiang, J. K., et al. (2012). CIP2A is a predictor of poor prognosis in colon cancer. J. Gastrointest. Surg. 16, 1037–1047. doi:10.1007/s11605-012-1828-3
Tian, Y., Chen, H., Qiao, L., Zhang, W., Zheng, J., Zhao, W., et al. (2018). CIP2A facilitates the G1/S cell cycle transition via B-Myb in human papillomavirus 16 oncoprotein E6-expressing cells. J. Cell Mol. Med. 22, 4150–4160. doi:10.1111/jcmm.13693
Tseng, L. M., Liu, C. Y., Chang, K. C., Chu, P. Y., Shiau, C. W., and Chen, K. F. (2012). CIP2A is a target of bortezomib in human triple negative breast cancer cells. Breast Cancer Res. 14, R68. doi:10.1186/bcr3175
Vaarala, M. H., Väisänen, M. R., and Ristimäki, A. (2010). CIP2A expression is increased in prostate cancer. J. Exp. Clin. Cancer Res. 29, 136. doi:10.1186/1756-9966-29-136
Vainonen, J. P., Momeny, M., and Westermarck, J. (2021). Druggable cancer phosphatases. Sci. Transl. Med. 13, eabe2967. doi:10.1126/scitranslmed.abe2967
Velmurugan, B. K., Wang, H. K., Chung, C. M., Lee, C. H., Huang, L. R., Yeh, K. T., et al. (2019). CIP2A overexpression in Taiwanese oral cancer patients. Cancer Manag. Res. 11, 2589–2594. doi:10.2147/cmar.S201154
Ventelä, S., Sittig, E., Mannermaa, L., Mäkelä, J. A., Kulmala, J., Löyttyniemi, E., et al. (2015). CIP2A is an Oct4 target gene involved in head and neck squamous cell cancer oncogenicity and radioresistance. Oncotarget 6, 144–158. doi:10.18632/oncotarget.2670
Vogelstein, B., Papadopoulos, N., Velculescu, V. E., Zhou, S., Diaz, L. A., and Kinzler, K. W. (2013). Cancer genome landscapes. Science 339, 1546–1558. doi:10.1126/science.1235122
Wang, C. Y., Chao, T. T., Chang, F. Y., Chen, Y. L., Tsai, Y. T., Lin, H. I., et al. (2014). CIP2A mediates erlotinib-induced apoptosis in non-small cell lung cancer cells without EGFR mutation. Lung Cancer 85, 152–160. doi:10.1016/j.lungcan.2014.05.024
Wang, H. W., Yang, S. H., Huang, G. D., Lin, J. K., Chen, W. S., Jiang, J. K., et al. (2014). Temsirolimus enhances the efficacy of cetuximab in colon cancer through a CIP2A-dependent mechanism. J. Cancer Res. Clin. Oncol. 140, 561–571. doi:10.1007/s00432-014-1596-4
Wang, J., Huang, T., Sun, J., Yu, Y., Liu, Z., Li, W., et al. (2014). CIP2A is overexpressed and involved in the pathogenesis of chronic myelocytic leukemia by interacting with breakpoint cluster region-Abelson leukemia virus. Med. Oncol. 31, 112. doi:10.1007/s12032-014-0112-7
Wang, J., Li, W., Li, L., Yu, X., Jia, J., and Chen, C. (2011). CIP2A is over-expressed in acute myeloid leukaemia and associated with HL60 cells proliferation and differentiation. Int. J. Lab. Hematol. 33, 290–298. doi:10.1111/j.1751-553X.2010.01288.x
Wang, L., Gu, F., Ma, N., Zhang, L., Bian, J. M., and Cao, H. Y. (2013). CIP2A expression is associated with altered expression of epithelial-mesenchymal transition markers and predictive of poor prognosis in pancreatic ductal adenocarcinoma. Tumour Biol. 34, 2309–2313. doi:10.1007/s13277-013-0775-2
Wang, N., Zhou, F., Guo, J., Zhu, H., Luo, S., and Cao, J. (2018). Euxanthone suppresses tumor growth and metastasis in colorectal cancer via targeting CIP2A/PP2A pathway. Life Sci. 209, 498–506. doi:10.1016/j.lfs.2018.08.052
Wang, P., Qin, J., Ye, H., Li, L., Wang, X., and Zhang, J. (2019). Using a panel of multiple tumor-associated antigens to enhance the autoantibody detection in the immunodiagnosis of ovarian cancer. J. Cell Biochem. 120, 3091–3100. doi:10.1002/jcb.27497
Wang, X., Gao, P., Wang, M., Liu, J., Lin, J., Zhang, S., et al. (2017). Feedback between E2F1 and CIP2A regulated by human papillomavirus E7 in cervical cancer: Implications for prognosis. Am. J. Transl. Res. 9, 2327–2339.
Wang, X., Yang, R., Wang, Q., Wang, Y., Ci, H., and Wu, S. (2019). Aberrant expression of vasculogenic mimicry, PRRX1, and CIP2A in clear cell renal cell carcinoma and its clinicopathological significance. Med. Baltim. 98, e17028. doi:10.1097/md.0000000000017028
Wei, L., Qu, W., Sun, J., Wang, X., Lv, L., Xie, L., et al. (2014). Knockdown of cancerous inhibitor of protein phosphatase 2A may sensitize NSCLC cells to cisplatin. Cancer Gene Ther. 21, 194–199. doi:10.1038/cgt.2014.18
Wei, R., Ye, X., Zhao, Y., Jia, N., Liu, T., Lian, W., et al. (2019). MicroRNA-218 inhibits the cell proliferation and migration in clear cell renal cell carcinoma through targeting cancerous inhibitor of protein phosphatase 2A. Oncol. Lett. 17, 3211–3218. doi:10.3892/ol.2019.9986
Westermarck, J. (2018). Targeted therapies don't work for a reason; the neglected tumor suppressor phosphatase PP2A strikes back. Febs J. 285, 4139–4145. doi:10.1111/febs.14617
Wiegering, A., Pfann, C., Uthe, F. W., Otto, C., Rycak, L., Mäder, U., et al. (2013). CIP2A influences survival in colon cancer and is critical for maintaining Myc expression. PLoS One 8, e75292. doi:10.1371/journal.pone.0075292
Williams, A. P., Garner, E. F., Waters, A. M., Stafman, L. L., Aye, J. M., Markert, H., et al. (2019). Investigation of PP2A and its endogenous inhibitors in neuroblastoma cell survival and tumor growth. Transl. Oncol. 12, 84–95. doi:10.1016/j.tranon.2018.09.011
Wu, J., Ding, M., Mao, N., Wu, Y., Wang, C., Yuan, J., et al. (2017). Celastrol inhibits chondrosarcoma proliferation, migration and invasion through suppression CIP2A/c-MYC signaling pathway. J. Pharmacol. Sci. 134, 22–28. doi:10.1016/j.jphs.2016.12.007
Wu, Y., Gu, T. T., and Zheng, P. S. (2015). CIP2A cooperates with H-Ras to promote epithelial-mesenchymal transition in cervical-cancer progression. Cancer Lett. 356, 646–655. doi:10.1016/j.canlet.2014.10.013
Xie, C., Kim, H. J., Haw, J. G., Kalbasi, A., Gardner, B. K., Li, G., et al. (2011). A novel multiplex assay combining autoantibodies plus PSA has potential implications for classification of prostate cancer from non-malignant cases. J. Transl. Med. 9, 43. doi:10.1186/1479-5876-9-43
Xing, M. L., Lu, Y. F., Wang, D. F., Zou, X. Y., Zhang, S. X., and Yun, Z. (2016). Clinical significance of sCIP2A levels in breast cancer. Eur. Rev. Med. Pharmacol. Sci. 20, 82–91.
Xu, P., Huang, Q., Xie, F., Xu, X. L., and Shao, F. (2013). Increased expression of CIP2A in cholangiocarcinoma and correlation with poor prognosis. Hepatogastroenterology 60, 669–672.
Xu, P., Xu, X. L., Huang, Q., Zhang, Z. H., and Zhang, Y. B. (2012). CIP2A with survivin protein expressions in human non-small-cell lung cancer correlates with prognosis. Med. Oncol. 29, 1643–1647. doi:10.1007/s12032-011-0053-3
Xu, P., Yao, J., He, J., Zhao, L., Wang, X., Li, Z., et al. (2016). CIP2A down regulation enhances the sensitivity of pancreatic cancer cells to gemcitabine. Oncotarget 7, 14831–14840. doi:10.18632/oncotarget.7447
Xue, Y., Wu, G., Wang, X., Zou, X., Zhang, G., Xiao, R., et al. (2013). CIP2A is a predictor of survival and a novel therapeutic target in bladder urothelial cell carcinoma. Med. Oncol. 30, 406. doi:10.1007/s12032-012-0406-6
Yang, X., Qu, K., Tao, J., Yin, G., Han, S., Liu, Q., et al. (2018). Inhibition of CIP2A attenuates tumor progression by inducing cell cycle arrest and promoting cellular senescence in hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 495, 1807–1814. doi:10.1016/j.bbrc.2017.11.124
Yang, X., Zhang, Y., Liu, H., and Lin, Z. (2016). Cancerous inhibitor of PP2A silencing inhibits proliferation and promotes apoptosis in human multiple myeloma cells. Biomed. Res. Int. 2016, 6864135. doi:10.1155/2016/6864135
Yi, F., Ni, W., Liu, W., Bai, J., and Li, W. (2013). Expression and biological role of CIP2A in human astrocytoma. Mol. Med. Rep. 7, 1376–1380. doi:10.3892/mmr.2013.1357
Yin, J., Chen, D., Luo, K., Lu, M., Gu, Y., Zeng, S., et al. (2019). Cip2a/miR-301a feedback loop promotes cell proliferation and invasion of triple-negative breast cancer. J. Cancer 10, 5964–5974. doi:10.7150/jca.35704
Yu, G., Liu, G., Dong, J., and Jin, Y. (2013). Clinical implications of CIP2A protein expression in breast cancer. Med. Oncol. 30, 524. doi:10.1007/s12032-013-0524-9
Yu, H. C., Chen, H. J., Chang, Y. L., Liu, C. Y., Shiau, C. W., Cheng, A. L., et al. (2013). Inhibition of CIP2A determines erlotinib-induced apoptosis in hepatocellular carcinoma. Biochem. Pharmacol. 85, 356–366. doi:10.1016/j.bcp.2012.11.009
Yu, H. C., Hung, M. H., Chen, Y. L., Chu, P. Y., Wang, C. Y., Chao, T. T., et al. (2014). Erlotinib derivative inhibits hepatocellular carcinoma by targeting CIP2A to reactivate protein phosphatase 2A. Cell Death Dis. 5, e1359. doi:10.1038/cddis.2014.325
Yu, N., Zhang, T., Zhao, D., Cao, Z., Du, J., and Zhang, Q. (2018). CIP2A is overexpressed in human endometrioid adenocarcinoma and regulates cell proliferation, invasion and apoptosis. Pathol. Res. Pract. 214, 233–239. doi:10.1016/j.prp.2017.11.011
Yu, X. J., Zhao, Q., Wang, X. B., Zhang, J. X., and Wang, X. B. (2016). Gambogenic acid induces proteasomal degradation of CIP2A and sensitizes hepatocellular carcinoma to anticancer agents. Oncol. Rep. 36, 3611–3618. doi:10.3892/or.2016.5188
Zhai, M., Cong, L., Han, Y., and Tu, G. (2014). CIP2A is overexpressed in osteosarcoma and regulates cell proliferation and invasion. Tumour Biol. 35, 1123–1128. doi:10.1007/s13277-013-1150-z
Zhang, H. F., Qin, J. J., Ren, P. F., Shi, J. X., Xia, J. F., Ye, H., et al. (2016). A panel of autoantibodies against multiple tumor-associated antigens in the immunodiagnosis of esophageal squamous cell cancer. Cancer Immunol. Immunother. 65, 1233–1242. doi:10.1007/s00262-016-1886-6
Zhang, J., Guo, X., Jin, B., and Zhu, Q. (2022). Editorial: Tumor-associated antigens and their autoantibodies, from discovering to clinical utilization. Front. Oncol. 12, 970623. doi:10.3389/fonc.2022.970623
Zhang, J. Y., and Tan, E. M. (2010). Autoantibodies to tumor-associated antigens as diagnostic biomarkers in hepatocellular carcinoma and other solid tumors. Expert Rev. Mol. Diagn 10, 321–328. doi:10.1586/erm.10.12
Zhang, J. Y., Wang, X., Peng, X. X., and Chan, E. K. (2002). Autoantibody responses in Chinese hepatocellular carcinoma. J. Clin. Immunol. 22, 98–105. doi:10.1023/a:1014483803483
Zhang, X., Xu, B., Sun, C., Wang, L., and Miao, X. (2015). Knockdown of CIP2A sensitizes ovarian cancer cells to cisplatin: An in vitro study. Int. J. Clin. Exp. Med. 8, 16941–16947.
Zhang, Y., Huang, P., Liu, X., Xiang, Y., Zhang, T., Wu, Y., et al. (2018). Polyphyllin I inhibits growth and invasion of cisplatin-resistant gastric cancer cells by partially inhibiting CIP2A/PP2A/Akt signaling axis. J. Pharmacol. Sci. 137, 305–312. doi:10.1016/j.jphs.2018.07.008
Zhang, Y., and Wang, X. (2020). Targeting the Wnt/β-catenin signaling pathway in cancer. J. Hematol. Oncol. 13, 165. doi:10.1186/s13045-020-00990-3
Zhao, Q., Zhao, M., Parris, A. B., Xing, Y., and Yang, X. (2016). Genistein targets the cancerous inhibitor of PP2A to induce growth inhibition and apoptosis in breast cancer cells. Int. J. Oncol. 49, 1203–1210. doi:10.3892/ijo.2016.3588
Zhao, S., Gao, X., Zang, S., Li, Y., Feng, X., and Yuan, X. (2017). MicroRNA-383-5p acts as a prognostic marker and inhibitor of cell proliferation in lung adenocarcinoma by cancerous inhibitor of protein phosphatase 2A. Oncol. Lett. 14, 3573–3579. doi:10.3892/ol.2017.6603
Zheng, Z., Qiao, Z., Chen, W., Gong, R., Wang, Y., Xu, L., et al. (2016). CIP2A regulates proliferation and apoptosis of multiple myeloma cells. Mol. Med. Rep. 14, 2705–2709. doi:10.3892/mmr.2016.5553
Keywords: CIP2A/p90, cancer, tumor-associated antigen (TAA), signaling pathways, biomarker, prognosis
Citation: Chen B, Hu H and Chen X (2023) From Basic Science to Clinical Practice: The Role of Cancerous Inhibitor of Protein Phosphatase 2A (CIP2A)/p90 in Cancer. Front. Genet. 14:1110656. doi: 10.3389/fgene.2023.1110656
Received: 29 November 2022; Accepted: 03 February 2023;
Published: 24 February 2023.
Edited by:
Bin Fang, the First Affiliated Hospital of Guangzhou University of Chinese Medicine, ChinaReviewed by:
Jianxian Lin, Fujian Medical University Union Hospital, ChinaCopyright © 2023 Chen, Hu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaobing Chen, emx5eWNoZW54YjA4MDdAenp1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.