
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Genet., 03 March 2017
Sec. Evolutionary and Population Genetics
Volume 8 - 2017 | https://doi.org/10.3389/fgene.2017.00025
This article is part of the Research TopicEvolutionary Feedbacks Between Population Biology and Genome ArchitectureView all 11 articles
In animals, interactions among gene products of mitochondrial and nuclear genomes (mitonuclear interactions) are of profound fitness, evolutionary, and ecological significance. Most fundamentally, the oxidative phosphorylation (OXPHOS) complexes responsible for cellular bioenergetics are formed by the direct interactions of 13 mitochondrial-encoded and ∼80 nuclear-encoded protein subunits in most animals. It is expected that organisms will develop genomic architecture that facilitates co-adaptation of these mitonuclear interactions and enhances biochemical efficiency of OXPHOS complexes. In this perspective, we present principles and approaches to understanding the co-evolution of these interactions, with a novel focus on how genomic architecture might facilitate it. We advocate that recent interdisciplinary advances assist in the consolidation of links between genotype and phenotype. For example, advances in genomics allow us to unravel signatures of selection in mitochondrial and nuclear OXPHOS genes at population-relevant scales, while newly published complete atomic-resolution structures of the OXPHOS machinery enable more robust predictions of how these genes interact epistatically and co-evolutionarily. We use three case studies to show how integrative approaches have improved the understanding of mitonuclear interactions in OXPHOS, namely those driving high-altitude adaptation in bar-headed geese, allopatric population divergence in Tigriopus californicus copepods, and the genome architecture of nuclear genes coding for mitochondrial functions in the eastern yellow robin.
Rapid improvements in genomics hold much promise in advancing one of the most important but demanding tasks in evolutionary biology: establishing genotype-to-phenotype links for features of organisms that are important in adaptation and speciation (Savolainen et al., 2013; Seehausen et al., 2014). The main challenge is that fitness-conferring characteristics in complex organisms are typically quantitative traits, controlled by many loci with small individual effect sizes (Mackay et al., 2009). This is compounded by the astronomical numbers of both meaningful gene interactions and spurious correlations that arise from population structure and history. Accordingly, adaptation can be implicated in species evolution only when disentangled from population history (Hoban et al., 2016). Identifying genotype-to-phenotype links of complex traits can be made more tractable by focussing on genomic variation expected to bestow major fitness differences based on prior knowledge. If such predictions are consistent with population genomic analyses, this will increase confidence that the candidate genes and mechanisms are true positives worthy of the demanding empirical investigations in wild populations needed to test them (Cheviron et al., 2014; Gompert et al., 2014; Egan et al., 2015).
An excellent opportunity to study the interplay between population biology and genome architecture is presented by interactions between mitochondrial proteins encoded by genes of the mitochondrial and nuclear genomes. Such mitonuclear interactions are required for fundamental physiological processes such as cellular respiration (Bar-Yaacov et al., 2012) and thus influence processes at multiple levels of biological organization: cellular function, organismal fitness, and ecosystem processes (Dowling et al., 2008; Wolff et al., 2014; Latorre-Pellicer et al., 2016). Moreover, these interactions are so central to evolutionary and ecological processes, including adaptation and speciation, that the term ‘mitonuclear ecology’ was recently proposed for their study (Hill, 2015, 2016).
While we have an incomplete understanding of most mitonuclear interactions (Pagliarini et al., 2008), we have a rich knowledge of a fundamental subset of them: those that form the core protein complexes responsible for oxidative phosphorylation (OXPHOS) (Rand et al., 2004; Gershoni et al., 2009). This essential system is responsible for the availability of nearly all cellular energy in eukaryotes, and thus through metabolic, trophic and thermal biology, at some level underpins virtually all eukaryotic ecological and evolutionary phenomena (Rand et al., 2004). These interactions are amenable to experimental investigation through interdisciplinary approaches. Essential for fitness, tractably complex, and relatively well-understood, these interactions thus represent strong study systems for understanding the evolution of adaptive traits. In this article, we present principles and case studies of investigations of the mitonuclear co-evolution of OXPHOS complexes in wildlife. We suggest an integrated experimental approach to this key issue in evolutionary biology, including a novel perspective on the role of genomic architecture in optimizing mitonuclear interactions.
Oxidative phosphorylation depends on the interaction of protein complexes in the inner mitochondrial membrane. In most animals, the four core complexes mediating OXPHOS are encoded by the 13 protein-encoding mitochondrial genes and an estimated 80 nuclear-encoded genes (Nicholls and Ferguson, 2013). The respirasome (comprising complexes I, III, and IV) uses the energy liberated during electron transfer from NADH to O2 to drive proton-translocation to the intermembrane space and thus establish a proton gradient across the inner mitochondrial membrane (Figure 1) (Gu et al., 2016; Letts et al., 2016; Wu et al., 2016). The ATP synthase (complex V) uses the proton-motive force thus generated to chemiosmotically drive ATP synthesis (Allegretti et al., 2015; Hahn et al., 2016). Nuclear-encoded proteins also serve as electron carriers (e.g., cytochrome c), alternative electron inputs (e.g., complex II), and assembly factors (e.g., NDUFC1, SURF1) throughout the OXPHOS system (Mashkevich et al., 1997; Nicholls and Ferguson, 2013; Stroud et al., 2016).
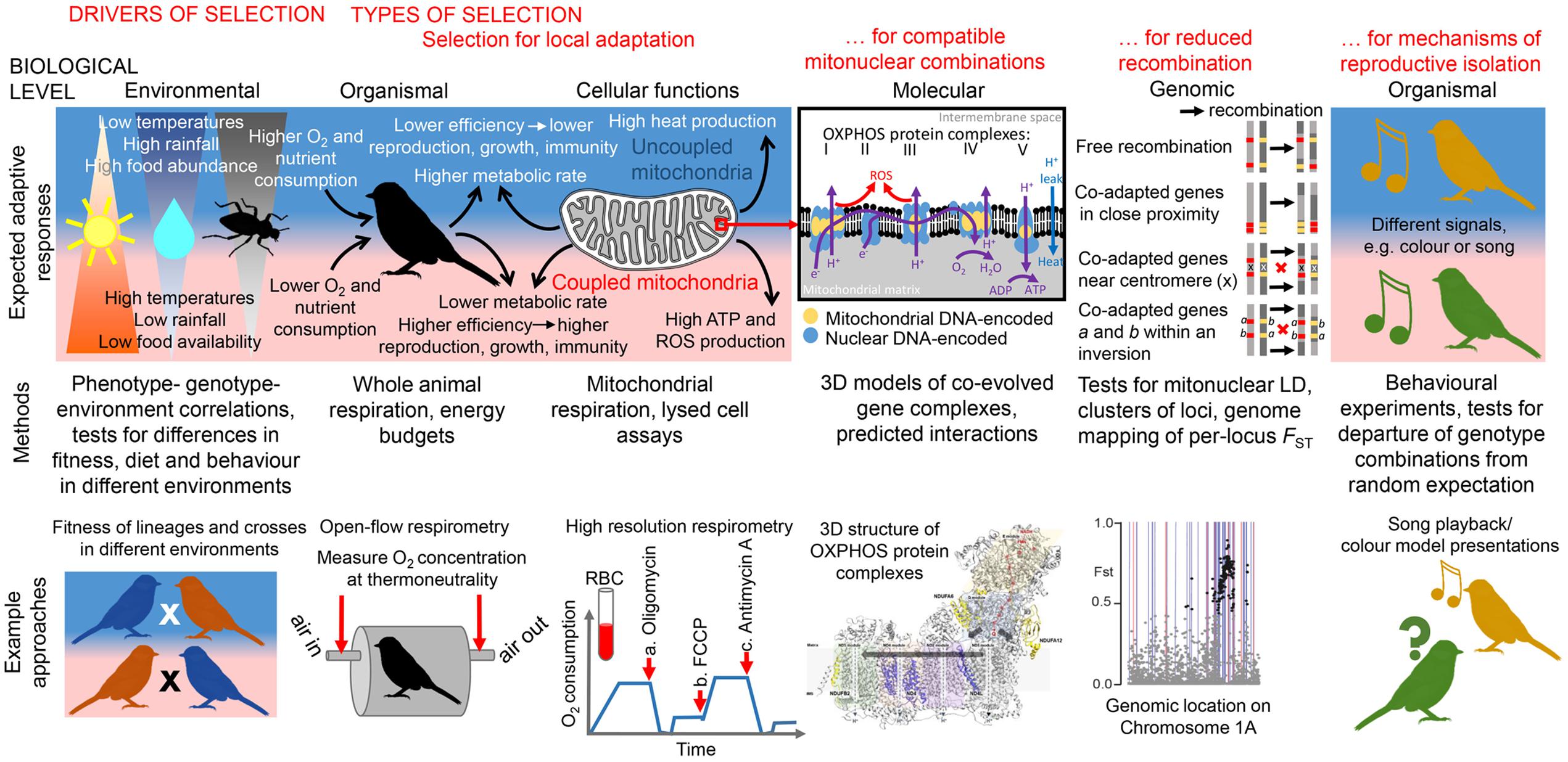
FIGURE 1. Overview of expected responses to selective pressures related to thermal metabolism at various levels of biological organization and integrative approaches to studying the mitonuclear interactions they modulate. Environmental level: Significant differences in temperature and precipitation can drive differences in food abundance and selection for local adaptation. Significant correlations between phenotype, genotype, and environment, after controlling for confounding factors (e.g., genetic drift), can suggest the presence of local adaptation. Fitness/metabolic performance of organisms with diverged mitolineages measured in different environments can indicate the presence of local metabolic adaptation, whereas fitness/metabolic performance of several generations of crosses can show whether mitonuclear incompatibilities have evolved between lineages. Organismal level: Heat produced from less-coupled mitochondria may be adaptive in colder environments for endothermic organisms (Pörtner et al., 1998); individuals with less-coupled metabolism are expected to produce fewer ATP molecules (leading to lower amount of energy available for growth, immune function, or reproduction) and fewer ROS (leading to lower oxidative stress and greater longevity) per unit of O2/nutrients consumed (Stier et al., 2014a,b). Higher O2/nutrient consumption could be expected to compensate for metabolic inefficiency. O2 consumption at thermoneutrality can be measured with an open-flow respirometry system (Lighton, 2008) after some acclimation time in a metabolic chamber; this can be used to calculate an organism’s resting metabolism, expected to be lower in organisms adapted to warmer environments (White et al., 2007). Cellular function level: The level of mitochondrial coupling between substrate oxidation and ATP production determines the amount of ATP and heat (through proton leak) produced per unit of O2/substrate consumed. A low level of coupling resulting in high heat production might be adaptive in cold climates. Mitochondrial respiration in birds, fish, amphibians and reptiles can be measured non-destructively from red blood cells (RBC) instead of liver or muscle tissues; O2 consumption (blue line) can be measured at a baseline for comparison with responses to the additions of (a) the ATP synthase inhibitor oligomycin (measures residual O2 consumption during proton leakage), (b) the mitochondrial uncoupler FCCP (measures maximal uncoupled O2 consumption), and (c) the inhibitor of mitochondrial respiration antimycin A (measures non-mitochondrial oxygen consumption) (Stier et al., 2016). Molecular level: Complex I (NADH dehydrogenase), complex II (succinate dehydrogenase), complex III (bc1 complex) and complex IV (cytochrome c oxidase) transport electrons (e-) from NADH, succinate and FAD-linked substrates (not shown) to create a proton-motive force (H+ gradient). Complex V (ATP synthase) uses energy released by backflow of protons to create ATP from ADP. Proteins of complexes I, III, IV, and V are encoded by both nuclear and mitochondrial genomes, leading to strong selection for compatible functional allele combinations. Mapping genes with amino acid candidates for positive selection onto 3D models of OXPHOS complexes enables better understanding of mitonuclear interactions. Here, the 3D structure of OXPHOS complex I (Fiedorczuk et al., 2016) is shown, with mitochondrially encoded subunits ND4 and ND4L, found to contain positively selected amino acids in two eastern yellow robin lineages (Morales et al., 2016a), highlighted in purple. Genomic level: Selection for co-transmission of co-adapted nuclear-encoded mitochondrial allele combinations with mitochondrial DNA lineages can drive the evolution of genomic architecture that suppresses recombination between co-adapted genes; examples of such mechanisms include close proximity of co-adapted genes and location of co-adapted genes near a centromere or within an inversion. Mapping single-nuclear-locus FST between populations fixed for alternative mitochondrial lineages to a reference genome can help detect clusters of loci co-inherited with mtDNA. Here, FSTs between two eastern yellow robin lineages mapped to chromosome 1A (dots; black dots- top 1% outliers) show the presence of the mtDNA-linked cluster of loci (Morales et al., 2016a); background lines show the location of genes with predicted mitochondrial functions (red lines- OXPHOS genes), for which this genomic region was enriched. Organismal level (reproductive isolation and incompatibilities): Selection against incompatible mitonuclear combinations [postzygotic reproductive isolation can drive evolution of prezygotic reproductive isolation and result in speciation (Sloan et al., 2016)]. For example, organisms can advertize their mitonuclear genotypes through differences in color or vocalization (Hill and Johnson, 2013; Hill, 2016). Behavioral experiments involving model presentations can elucidate whether individuals mate assortatively according to their mitonuclear genotype, implying late stages of speciation.
Intimate associations between mitochondrial- and nuclear-encoded subunits are required for the electron transport chain of the respirasome and ATP synthase activity for efficient mitochondrial ‘coupling,’ i.e., the ratio of ATP synthesis per unit substrate and O2 consumed (Lowell and Spiegelman, 2000). The intimacy of such interactions is particularly reflected by OXPHOS complex I. This enzyme couples electron transfer through its nuclear-encoded hydrophilic arm to proton translocation through its four mitochondrially encoded proton pumps. This depends on long-range conformational changes mediated through protein–protein interactions of the mitochondrially encoded subunits with core and supernumerary nuclear-encoded subunits (Fiedorczuk et al., 2016; Zhu et al., 2016). Genetic studies have demonstrated that assembly of this complex depends strictly on 39 of its 45 subunits (Stroud et al., 2016), and even single amino acid substitutions can alter coupling efficiency (Mimaki et al., 2012; Gershoni et al., 2014). In addition to serving as the primary electron input in the respiratory chain (Nicholls and Ferguson, 2013), OXPHOS complex I is also the main site of cellular reactive oxygen species (ROS) production (Murphy, 2009).
The central physiological importance of OXPHOS means that mitonuclear compatibilities are required for optimal fitness. Even minor biochemical inefficiencies can have major fitness consequences for an organism by modulating their energetic efficiency and oxidative stress levels. There are therefore strong selective pressures to maintain optimal mitonuclear interactions in the OXPHOS system (Rand et al., 2004; Dowling et al., 2008; Burton et al., 2013).
There are multiple lines of evidence that OXPHOS is important for local adaptation. Experimental approaches with model organisms have allowed researchers to test an impressive array of mitonuclear combinations and assess their functional effects under a wide set of conditions (Dowling et al., 2007; Arnqvist et al., 2010; Paliwal et al., 2014; Ma et al., 2016; Mossman et al., 2016). These efforts have revealed that mismatched mitonuclear interactions (gene–gene interactions) can have profound consequences, such as reduced metabolic performance, fecundity, and lifespan. When mitonuclear combinations are assessed in multiple environments (e.g., diet, temperature or hypoxia), interaction effects are commonly context dependent (gene–gene-environment interactions; Koevoets et al., 2012; Hoekstra et al., 2013).
In the wild, OXPHOS traits have been correlated with a wide range of environmental pressures, including heat stress (Morales et al., 2016a,b), cold stress (Cheviron et al., 2014; Stier et al., 2014a,b), nutrient limitation (da Fonseca et al., 2008), and hypoxia (da Fonseca et al., 2008; Scott et al., 2011). Consistently, there is evidence for positive selection and climate-linked differences in the sequences and expression of OXPHOS genes in a range of animal species with wide biogeographic ranges (Mishmar et al., 2003; Ruiz-Pesini et al., 2004; Toews and Brelsford, 2012; Garvin et al., 2015; Morales et al., 2016a). Accordingly, genes encoding the OXPHOS machinery are frequently candidates for positive selection. This likely reflects the high levels of mitochondrial DNA variation within and among populations combined with the selection pressures for optimally adapted phenotypes (Gershoni et al., 2009; Bar-Yaacov et al., 2012).
As mechanistic understanding of OXPHOS activity continues to improve, it should be increasingly possible to make more specific predictions of what kinds of protein-level changes should be adaptive. For example, it is hypothesized that the coupling efficiencies of OXPHOS complexes are closely linked to adaptive thermal biology. OXPHOS generates chemical energy and heat in proportions that depend on the coupling efficiency of the respirasome and ATP synthase (Lowell and Spiegelman, 2000). It is proposed that the heat produced from less-coupled mitochondria may be particularly beneficial for adaptation of endothermic organisms to colder environments. In contrast, heat production may be deleterious in warm environments, necessitate higher nutritional intake, and is associated with high oxidative stress due to increased ROS production (Figure 1) (Pörtner et al., 1998; Brand, 2000; Somero, 2002; Fangue et al., 2009; Stier et al., 2014a,b). It is important to account for variation in such predictions among organisms and environments. For example, contrary to the expectations for endotherms, cold adaptation in fishes is linked to higher mitochondrial densities in muscle (White et al., 2011), and so the associated high energy demands for synthesis and maintenance of mitochondria may favor genotypes with high coupling efficiency (Pavlova et al., 2017).
Mitonuclear co-evolution should be enforced under strong selection given the complex interactions and essential functions mediated by OXPHOS (Burton et al., 2013). Challenges to positive co-evolution include the fast mutation rate of the mitochondrial genome (due to its proximity to ROS production, high rate of replication and lack of efficient repair mechanisms), typically maternal inheritance, and lack of recombination, which generate a mutation load that the nuclear genome must counter by compensatory mutation (Rand et al., 2004; Lynch et al., 2006; Osada and Akashi, 2012; Havird et al., 2015; Havird and Sloan, 2016). In addition, mitonuclear co-evolution can be disrupted by mechanisms that generate genetic variation or promote gene flow. In particular, substantial gene flow and recombination in nuclear DNA will tend to break up optimally functioning allele combinations of co-adapted genes in each sexual generation (Rand et al., 2004; Burton and Barreto, 2012; Burton et al., 2013). Accordingly, we propose that nuclear genomic architecture should tend to evolve to suppress recombination and prevent the segregation of genome regions that mediate epistatic functions of nuclear-encoded mitochondrial genes.
With improvements in techniques to explore genome structure, examples are building of how genomic architecture can drive evolutionary adaptation, for example the ‘supergene’ of 125 genes associated with differences in male mating strategies in birds (Küpper et al., 2016). Natural selection can locate co-adapted loci in genome areas of low recombination (Schwander et al., 2014; Thompson and Jiggins, 2014) or promote genomic clustering of synergistically adaptive alleles (Yeaman, 2013) so that they can be co-inherited and/or co-regulated. The three-dimensional organization of the genome can dictate how and which loci should be subject to genome changes that will favor their co-location (Lanctôt et al., 2007; Wijchers and de Laat, 2011). Reduced recombination among co-adapted genes (increasing their co-inheritance) can occur through the evolution of recombination modifiers or chromosomal re-arrangements, such as transposition of a gene to a location close to a co-adapted gene, movement of co-adapted genes toward a centromere or into a region within an inversion between diverged lineages (Figure 1) (Rieseberg, 2001; Butlin, 2005; Kirkpatrick and Barton, 2006; Yeaman and Whitlock, 2011; Yeaman, 2013; Ortiz-Barrientos et al., 2016).
To date, mitonuclear genomic architecture (encompassing mitochondrial-nuclear and nuclear-nuclear) remains relatively underexplored, except in the context of biased co-transmission of mitochondrial and nuclear-encoded mitochondrial genes on sex chromosomes. Because mtDNA, due to its maternal inheritance, accumulates mutations that are deleterious in males (mother’s curse), selection to restore fitness in males that drives compensatory evolution of nuclear-encoded mitochondrial genes could be expected to prevent concentrations of such genes on female-linked chromosomes (Havird and Sloan, 2016). Results for different taxa variously support overrepresentation, underrepresentation, or unbiased distribution of nuclear-encoded mitochondrial genes on X and Z chromosomes with respect to autosomes, supporting multiple theories of the distribution of nuclear-encoded mitochondrial genes: co-adaptation, sexual conflict and sexual selection (Drown et al., 2012; Hill and Johnson, 2013; Dean et al., 2014, 2015; Hill, 2014; Rogell et al., 2014). More recently, we uncovered an autosomal genomic island of divergence associated with mitonuclear interactions in a passerine (Figure 1) (Morales et al., 2016a). This genomic island of divergence is implicated in maintaining deep mitochondrial divergence between two parapatric lineages connected by nuclear gene flow. Observations of the fluidity of positioning of nuclear-encoded mitochondrial genes in some systems (but see Dean et al., 2015) raise questions about the role of genome organization in rates of co-evolution between mitochondria and nuclear genes (Hill, 2014). We contend that exploration of genomic architecture may be crucial for understanding mitonuclear co-evolution, and also vice versa given the crucial roles and considerable number of genes concerned with mitochondrial function (Pagliarini et al., 2008).
While signals of selection have frequently been identified in OXPHOS-encoded genes, few studies have examined changes in function due to observed substitutions; thus empirical demonstration of local adaptation is limited (Burton et al., 2013; Levin et al., 2014). However, it is possible to develop relatively strong genotype-to-phenotype links by bridging population genetic studies with biochemical and physiological approaches developed for studying OXPHOS. In the five decades since its discovery by Mitchell (1961), a wealth of physiological, biochemical, and structural studies have developed a rich understanding of oxidative phosphorylation (Nicholls and Ferguson, 2013), and much of the knowledge and methodology can be translated to wild populations.
Here we suggest a flexible framework that draws on recent technical advances in multiple fields for testing the significance of mitonuclear interactions. First, candidate interacting loci can be identified by improved methods for inferring loci under selection. Second, these candidates can then be examined through structural mapping and modeling to develop hypotheses about biochemical interactions relevant to the species biology in question. Third, these hypotheses can then be tested by measuring phenotypic responses at different scales, notably whole cell, whole animal, and fitness in the wild. Finally, experimental approaches could be used to test for reproductive isolation between differently adapted lineages (Figure 1). It is particularly desirable to compare multiple species for repeated signals of selection in the same genomic regions: common signals of selection between lineages in the context of their geographic arrangement relative to selection pressures provide strong evidence of adaptation (Garvin et al., 2014). Adopting this proposed framework should increase comparability among studies.
Detection of natural selection is one of the most contentious and active fields in evolutionary biology. Here we highlight, in the context of mitonuclear co-evolution, the more general issues that are explored in depth elsewhere (Nielsen, 2005; Haasl and Payseur, 2016; Manel et al., 2016; Stephan, 2016).
The power to detect candidate loci that evolve under natural selection rests on the molecular tools available for a given system: reduced representation genomic scans (SNPs), sequence-based genomic scans (candidate genes, exome-sequencing, or RNA-sequencing), whole genome re-sequencing, and/or physical linkage maps (Manel et al., 2016; Stephan, 2016). The key limitation to detecting natural selection in the wild is that several ecological and evolutionary processes can leave a similar signature to selection and lead to a high rate of false positives. Confounding factors include demographic processes (e.g., population size change and structure), background selection, and heterogeneous mutation and recombination rates. Given that mitochondrial and nuclear genomes can have largely independent evolutionary histories (e.g., different introgression patterns and mutation load), knowledge of the demographic history of the study system is especially useful to interpret patterns of mitonuclear co-evolution (Bar-Yaacov et al., 2015; Morales et al., 2016a,b; Pereira et al., 2016; Sloan et al., 2016). Given that mitonuclear co-evolution is likely to respond to environmental variation (Burton et al., 2013), approaches to detecting selection that rely on gene-environment associations could be particularly useful to identify candidate loci under selection (Rellstab et al., 2015; Forester et al., 2016).
A common starting point in the search for signatures of selection in mitonuclear co-evolution is sequencing full mitochondrial genomes. A family of methods proven to be especially useful in the context of mitogenome evolution are codon-based approaches, which rely on the estimation of the non-synonymous to synonymous ratio (ω = dN/dS) [HyPhy and Datamonkey (Pond and Frost, 2005); PAML (Yang, 2007)]. There are multiple examples in the literature of how complementary codon-based approaches have been combined to discriminate positive and relaxed purifying selection in mitogenome-encoded OXPHOS components (da Fonseca et al., 2008; Garvin et al., 2011; Morales et al., 2015; Wertheim et al., 2015; Pavlova et al., 2017). However, these types of methods have important limitations: they require data across multiple species, or sequences that are reasonably diverged, and only allow selection inference within coding regions (Gonçalves da Silva, 2017). It is important to consider this last limitation since mitonuclear incompatibilities have been mapped to non-coding regulatory genes, non-coding sequences such as transfer RNAs and the mitochondrial control region (Montooth et al., 2009; Meiklejohn et al., 2013; Rollins et al., 2016; Jhuang et al., 2017).
A natural follow-up is to look for evidence of natural selection in nuclear-encoded mitochondrial genes and signals of mitonuclear co-evolutionary adaptation (Mishmar et al., 2006; Gagnaire et al., 2012; Bar-Yaacov et al., 2015; Pereira et al., 2016). For example, such approaches have identified supernumerary and assembly factors of OXPHOS complex I implicated in local adaptation (Garvin et al., 2016; Morales et al., 2016a).
Recent advances in understanding structure-function relationships in oxidative phosphorylation enable better prediction of how genetic substitutions affect mitochondrial function. Largely as a result of recent advances in cryo-electron microscopy, complete atomic-resolution structures of all components in the mammalian electron transport chain are now available, including the mitochondrially co-encoded complex I (Figure 1) (Fiedorczuk et al., 2016; Zhu et al., 2016), complex III (Iwata et al., 1998), complex IV (Tsukihara et al., 1996), and the respirasome supercomplex (Gu et al., 2016; Letts et al., 2016; Wu et al., 2016). In addition, near-complete structures of yeast ATP synthase are also available (Allegretti et al., 2015; Hahn et al., 2016).
With these newly available protein structures, it is now possible to map the locations of subunits and amino acids predicted to be under selection using protein visualization software (Pettersen et al., 2004) and to develop homology models using public servers (Källberg et al., 2012; Kelley et al., 2015). Such approaches have been used to predict the mechanistic effects of amino acid substitutions observed in OXPHOS subunits across diverse species (Scott et al., 2011; Finch et al., 2014; Caballero et al., 2015; Zhang and Broughton, 2015; Campana et al., 2016; Morales et al., 2016a). This approach was first highlighted by Garvin et al. (2011) who detected an amino acid under positive selection in the Pacific salmon in an unusual region of OXPHOS complex I: the piston-like horizontal helix (helix HL) of ND5. Meta-analysis has since suggested that this helix is the most common region of positive selection in the mitogenomes of diverse animal species (Garvin et al., 2014). While the function of the helix HL remains unresolved, it is hypothesized to influence coupling by propagating conformational changes from proximal to distal proton pumps; hence fine-tuning its properties may have adaptive consequences for heat and energy production (Torres-Bacete et al., 2011; Sazanov, 2014).
In animal systems, it is challenging to validate experimentally that certain amino acid substitutions affect mitochondrial function. Due to their membrane localisation, multi-subunit cofactor-bound composition, and complex assembly pathways, OXPHOS complexes are incompatible with recombinant protein expression and can rarely be purified natively (Nicholls and Ferguson, 2013). However, a suite of physiological techniques enable us to measure the activities, kinetics, and efficiencies of OXPHOS complexes. For example, classical respirometry techniques enable measurement of the rates of substrate oxidation or oxygen consumption by whole cells or purified mitochondria; it is possible to calculate mitochondrial coupling efficiencies and to probe the activities of specific protein complexes by systematically comparing basal respiration rates with those in the presence of specific agonists, inhibitors, and uncouplers (Figure 1) (Nicholls and Ferguson, 2013; Stier et al., 2013, 2016; Toews et al., 2014). Well-established assays using lysed cells also enable measurement of potentially relevant parameters such as the expression levels, protein content, and kinetic parameters of individual OXPHOS complexes (Scott et al., 2011).
Another development that enhances the ability to measure biologically relevant mitochondrial function is the recent discovery that non-mammalian animals harbor functional mitochondria in their erythrocytes. This presents options for non-destructive sampling of wild populations (Stier et al., 2013, 2015, 2016). These cellular measurements of mitochondrial respiration can be complemented with whole-organism measurements of basal and maximal metabolic rates; while rarely adopted in mitonuclear ecology, such approaches may have value for understanding relationships between nutritional intake and energy expenditure (White et al., 2007, 2011; Halsey and White, 2010).
Taking an approach amenable to experimental manipulations, laboratory-based crossings have also been used to assess the effects of intraspecific and interspecific mitonuclear compatibilities using individuals sampled from wild populations from different environments. As elaborated in a case study below, there are several examples of how crosses have been combined with measurements of enzymatic, cellular, or organismal performance to consolidate genotype-to-phenotype links (Edmands and Burton, 1999; Willett and Burton, 2001; Arnqvist et al., 2010; Chang et al., 2015; Dordevic et al., 2016). In the few study systems where cell lines can be established, experimental cellular approaches can provide valuable functional insights in study systems (Blier et al., 2006), for example through the construction of mitonuclear hybrid cell systems (cybrids) that allow testing of the effects of mitogenome variation on fitness in a constant nuclear background (e.g., Barrientos et al., 1998; Moreno-Loshuertos et al., 2006; Dingley et al., 2014). Within these kinds of manipulative approaches, as well as more broadly, rapid advances in molecular genomics, including the increasing tractability of RNA sequencing, are facilitating investigations of the roles, mechanisms and evolutionary genomics of genes of interest in adaptation and divergence of wild species (Harrisson et al., 2014; Havird and Sloan, 2016; Latorre-Pellicer et al., 2016).
The level of resolution that can be reached in examining genomic architecture depends on the genomic resources available. Full resolution requires assembled genomes and physical linkage maps, rarely available for wild organisms. However, powerful approximations can be made by mapping genetic variants of interest (e.g., candidate genes or loci under selection) on to a reference annotated genome of the same species or a close relative with known conserved synteny. Mapped variation provides the presumed order, position and identity of loci of interest (e.g., nuclear-encoded mitochondrial genes).
Linkage (gametic) disequilibrium (LD) is the non-random association of alleles at different loci within individuals. These correlations can arise through genes being near each other on a chromosome, via population subdivision, and driven by epistatic selection. Accordingly, LD can arise between markers on different nuclear chromosomes, and between the mitochondrial and nuclear genomes (Sloan et al., 2015). Linkage disequilibrium has proven a powerful tool for studying the genomic architecture of population divergence, local adaptation, and reproductive isolation (Nosil et al., 2009; Servedio, 2009; Smadja and Butlin, 2011). Natural selection can favor the evolution of high LD when multiple loci that influence a trait experience the same divergent selection (Nielsen, 2005). Strong LD between nuclear-encoded mitochondrial alleles could signal co-adapted genes responding to the same selective drivers, which may or may not be maintained by genomic architecture favoring reduced levels of recombination. Significant clustering of nuclear genes encoding mitochondrial or chloroplast proteins in Arabidopsis has been demonstrated (Alexeyenko et al., 2006). Similar analysis for animal taxa has rarely been performed, not least because fully assembled genomes are unavailable for many of the organisms for which mitonuclear co-evolution might be relevant, but some significant mitochondrial-nuclear LD is present in humans (Sloan et al., 2015). As genomic resources for non-model organisms expand, we expect to see more studies of mitonuclear genomic architecture (e.g., genomic re-arrangements of nuclear-encoded mitochondrial genes), as is increasingly the case for other traits linked to reproductive isolation (Lowry and Willis, 2010; Jones et al., 2012; Egan et al., 2015).
Even without a reference genome, LD can be estimated by analyzing LD clustering with LDna (Kemppainen et al., 2015). This tool finds clusters of loci with similarly high levels of LD independently of their position in the genome. This is a valuable first step to studying the genomic architecture of organisms without genomic resources. It is important to note that high LD can also emerge through processes unrelated to selection, such as population history and structure, which should be accounted for (Mangin et al., 2012; Goicoechea et al., 2015). Demographic factors, however, should impact many loci across the genome, so significant excesses of high LD among nuclear genes with mitochondrial functions is indicative of non-neutral processes (Morales et al., 2016a). Comparative genomic approaches are also recommended to investigate whether re-arranged or ancestral genomic architectures in closely related taxa are more or less prone to evolution of mitonuclear interactions, and whether nuclear-encoded mitochondrial gene re-arrangements can be favored to reduce recombination between locally co-adapted alleles and can promote adaptive genetic divergence (Faria et al., 2011; Yeaman, 2013).
The strength of oxidative phosphorylation as an evolutionary study system has been highlighted by several in-depth studies on specific organisms. Each of these studies integrated different combinations of the techniques described above to address different questions about the role of OXPHOS in evolutionary and ecological processes.
One of the richest examples of the role of mitochondrial evolution in local adaptation comes from studies of the bar-headed goose Anser indicus. During its well-documented migrations over the Himalayas, this bird sustains high metabolic rates as a result of a multitude of physiological adaptations (Bishop et al., 2015). Comparative physiological studies show that adaptations at multiple levels of organization, from protein activity to organ morphology, enable this species to enhance O2 supply and modulate O2 usage compared to low-altitude geese species (Scott et al., 2009, 2011).
Among the potentially adaptive differences observed is a difference in the substrate kinetics of OXPHOS complex IV in bar-headed geese compared to other species. Cardiac muscle measurements show that this enzyme has a comparatively high affinity but low activity for its nuclear-encoded substrate cytochrome c. Scott et al. (2011) proposed that this may be adaptively relevant by enabling the mitochondrion to maintain redox balance in response to limitations and fluctuations in their O2 supply during their extreme flights. The authors inferred the genotypic basis of these changes by comparing the sequences of the mtDNA and nuclear-encoded mitochondrial genes from complex IV subunits between low- and high-altitude species. This revealed several non-synonymous substitutions in bar-headed geese, including a unique W116R substitution in COX3, as well as subtle differences in the expression levels of the complex (Scott et al., 2011).
Despite these strong phenotype-to-genotype links, it remains to be determined how these substitutions affect the biochemistry of the complex. Homology modeling suggests that the W116R mutation disrupts inter-subunit interactions in complex IV, but it is unclear if and how this affects the binding of cytochrome c (Scott et al., 2011). These gaps reflect the major challenges associated with purifying this enzyme complex for kinetic or structural characterisation. A striking contrast is provided by studies on why the O2-affinity of hemoglobin is so high in bar-headed geese; the relative ease of purifying this protein from erythrocytes has facilitated structural studies showing that a single amino acid substitution markedly shifts the cooperative behavior of the hemoglobin tetramer and in turn modulates O2 affinity (Zhang et al., 1996; Liang et al., 2001).
These studies of the bar-headed goose offer a strong example of integrated research leading to an understanding of the mechanistic links between genes affecting mitochondrial function and ecophysiological phenotype. While there are multiple classes of gene that might be expected to contribute to the adaptive phenotype (Scott et al., 2011), there is no particular expectation that strong genome architecture is required to promote the co-evolution of these: the species does not interbreed with another, and there are no apparent differently adapted lineages within the bar-headed goose, so gene flow should not disrupt co-adapted combinations.
Through extensive studies, Burton and colleagues have demonstrated that mitonuclear discordance has contributed to allopatric population divergence of Tigriopus californicus copepods. There is extraordinary genetic differentiation between populations of this intertidal copepod across geographic barriers in the Californian coast, with mtDNA divergence exceeding 18% between Santa Cruz and San Diego populations (Burton and Lee, 1994; Burton, 1998). Elegantly designed aquarium experiments revealed that F1 hybrids from the T. californicus populations are viable, but subsequent generations exhibit a range of fitness defects and reduced ATP production rates (Burton and Lee, 1994; Ellison and Burton, 2006, 2008). Consistent with a mitochondrial origin, maternal but not paternal backcrossing can restore fitness of progeny (Ellison and Burton, 2008).
Targeted sequencing revealed that there are high levels of divergence in genes encoding key determinants of the mitochondrial electron transport chain. While most of these substitutions appear to be neutral, ω-based approaches provided strong evidence for positive selection for substitutions in mitochondrially encoded complex IV and its nuclear-encoded substrate cytochrome c (Rawson et al., 2000; Willett and Burton, 2004). Interpopulation crossing experiments substantiated this by showing that mitonuclear compatibility was required for optimal complex IV activity (Edmands and Burton, 1999) and that the mitotype modulated segregation ratios of cytochrome c (Willett and Burton, 2001). The authors went further by validating these predictions using biochemical approaches. Intrapopulation pairs of complex IV and cytochrome c consistently showed up to fourfold higher activity than did interpopulation pairs (Rawson and Burton, 2002). Moreover, recombinantly produced cytochrome c variants (reflecting different nuclear backgrounds) interacted differentially with complex IV in tissue homogenates (reflecting different mitochondrial backgrounds) (Harrison and Burton, 2006). This work proved that single substitutions are sufficient to cause mitonuclear incompatibilities in wild populations.
Despite these accomplishments, the evolutionary processes and pressures that result in allopatric population divergence remain under investigation. Pereira et al. (2016) recently approximated the contribution of genetic drift and natural selection in T. californicus divergence by comparing whole-transcriptome sequences of allopatric populations at different stages of divergence (Pereira et al., 2016). They found that the pattern of shared polymorphism could be partially explained by genetic drift, as lower effective population sizes led to less shared polymorphism between populations, and higher mutation load. However, natural selection possibly drives accelerated evolution of some genes, including nuclear-encoded mitochondrial ones. The authors predict that genomic architecture should regulate the efficiency of selection and the impact of drift, for example by modulating recombination rates, but this prediction was not tested.
Our studies on the population structure of eastern yellow robin Eopsaltria australis have emphasized the importance of studying genomic architecture (Morales et al., 2016a). This songbird is one of multiple animals that maintains functional mitonuclear interactions despite discordance between its mitochondrial and nuclear genomes (Toews and Brelsford, 2012). Population genetic data have shown that the major axis of nuclear DNA differentiation runs north-south through the species range in Eastern Australia, whereas mitochondrial DNA has diverged into two mitolineages in the perpendicular coastal-inland direction (Pavlova et al., 2013; Morales et al., 2016a). Coalescent analyses suggest that the two genomes initially differentiated together in a north–south direction during the early Pleistocene, but their evolutionary history became separated due to two mitochondrial introgression events in the mid-to-late Pleistocene (Pavlova et al., 2013; Morales et al., 2016b). The two mitolineages show sharp climate-correlated differences in their distributions. This suggests that the mitochondrial introgression and resulting divergence were driven by natural selection (Morales et al., 2016b).
There is evidence of positive selection for non-conservative amino acid differences in the proton pumps ND4 and ND4L of OXPHOS complex I between the mitolineages of the eastern yellow robin. These polymorphisms are predicted to cause differences in electrostatic subunit-subunit interactions and in turn influence coupling efficiencies of the complex, though this remains to be validated experimentally (Morales et al., 2015). Comparison of fixation indexes in the nuclear genomes between eastern yellow robin populations across their biogeographic range revealed the existence of two genomic islands of divergence against a background of low differentiation (Morales et al., 2016a). One of these islands, located on autosome 1A (Figure 1), is statistically overrepresented with nuclear-encoded genes with predicted mitochondrial functions; among them are three complex I supernumerary subunits proposed to be functionally linked to the mitochondrially encoded ND4 and ND4L genes. Moreover, markers within the genomic island of divergence exhibit very strongly elevated LD, suggesting genome architecture that promotes reduced recombination between nuclear-encoded genes with mitochondrial functions. Further research will disentangle whether mitonuclear co-evolution promoted the evolution of this particular genomic architecture or pre-existing genomic architecture enabled this tight mitonuclear co-evolution.
In this perspective, we have summarized some of the wealth of information of the adaptive consequences of mitochondrial-nuclear interactions, with particular focus on OXPHOS functions. We make the case that the powerful fitness consequences of mitonuclear gene interactions are likely to select for optimized genome architecture that will hold together effective combinations of nuclear-encoded mitochondrial gene in the face of gene flow. Demonstrating the phenotypic consequences of genome variation in wildlife species is challenging: we suggest a workflow that utilizes advances in detection of candidates of selection, biochemical understanding of OXPHOS, phenotyping and studying genome organization. The number of studies demonstrating major evolutionary impacts of mitonuclear interactions in wildlife is currently limited, but we anticipate they will be found to be common under the application of the strong emerging methods of investigation such as we present here. Comparative genomic approaches will be important for deriving the deepest insights into mitonuclear evolution and genome architecture, and accordingly, we encourage the application of consistent methodologies.
All authors listed have made substantial, direct and intellectual contribution to the work, and approved it for publication.
Our work on eastern yellow robin has not yet been funded by a large grant. Partial funding was provided by the Australian Research Council Linkage Grant (LP0776322). HM was supported by the Holsworth Wildlife Research Endowment and Stuart Leslie Bird Research Award from BirdLife Australia, a Monash Graduate Scholarship (MGS), a Monash Faculty of Science Dean’s International Postgraduate Research Scholarship and a Monash Postgraduate Publication Award. CG was supported by Australian Research Council DECRA Fellowship DE170100310.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Scott Edwards and Tariq Ezaz for the invitation to write this perspective. We are grateful to the many researchers with whom we have discussions on topics covered here, including Antoine Stier, Ricardo Pereira, Geoff Hill, Damian Dowling, Jonci Wolff, and our research group members including Stephanie Falk and Lynna Kvistad. The comments of Dan Mishmar and Alan Brelsford helped improve the manuscript.
Alexeyenko, A., Millar, A. H., Whelan, J., and Sonnhammer, E. L. L. (2006). Chromosomal clustering of nuclear genes encoding mitochondrial and chloroplast proteins in Arabidopsis. Trends Genet. 22, 589–593. doi: 10.1016/j.tig.2006.09.002
Allegretti, M., Klusch, N., Mills, D. J., Vonck, J., Kühlbrandt, W., and Davies, K. M. (2015). Horizontal membrane-intrinsic alpha-helices in the stator a-subunit of an F-type ATP synthase. Nature 521, 237–240. doi: 10.1038/nature14185
Arnqvist, G., Dowling, D. K., Eady, P., Gay, L., Tregenza, T., Tuda, M., et al. (2010). Genetic architecture of metabolic rate: environment specific epistasis between mitochondrial and nuclear genes in an insect. Evolution 64, 3354–3363. doi: 10.1111/j.1558-5646.2010.01135.x
Barrientos, A., Kenyon, L., and Moraes, C. T. (1998). Human xenomitochondrial cybrids cellular models of mitochondrial complex I deficiency. J. Biol. Chem. 273, 14210–14217. doi: 10.1074/jbc.273.23.14210
Bar-Yaacov, D., Blumberg, A., and Mishmar, D. (2012). Mitochondrial-nuclear co-evolution and its effects on OXPHOS activity and regulation. Biochim. Biophys. Acta 1819, 1107–1111. doi: 10.1016/j.bbagrm.2011.10.008
Bar-Yaacov, D., Hadjivasiliou, Z., Levin, L., Barshad, G., Zarivach, R., Bouskila, A., et al. (2015). Mitochondrial involvement in vertebrate speciation? The case of mito-nuclear genetic divergence in chameleons. Genome Biol. Evol. 7, 3322–3336. doi: 10.1093/gbe/evv226
Bishop, C. M., Spivey, R. J., Hawkes, L. A., Batbayar, N., Chua, B., Frappell, P. B., et al. (2015). The roller coaster flight strategy of bar-headed geese conserves energy during Himalayan migrations. Science 347, 250–254. doi: 10.1126/science.1258732
Blier, P. U., Breton, S., Desrosiers, V., and Lemieux, H. (2006). Functional conservatism in mitochondrial evolution: insight from hybridization of arctic and brook charrs. J. Exp. Zool. Part B Mol. Dev. Evol. 306, 425–432. doi: 10.1002/jez.b.21089
Brand, M. D. (2000). Uncoupling to survive? The role of mitochondrial inefficiency in ageing. Exp. Gerontol. 35, 811–820. doi: 10.1016/S0531-5565(00)00135-2
Burton, R. S. (1998). Intraspecific phylogeography across the Point Conception biogeographic boundary. Evolution 52, 734–745. doi: 10.2307/2411268
Burton, R. S., and Barreto, F. S. (2012). A disproportionate role for mtDNA in Dobzhansky–Muller incompatibilities? Mol. Ecol. 21, 4942–4957. doi: 10.1111/mec.12006
Burton, R. S., and Lee, B. N. (1994). Nuclear and mitochondrial gene genealogies and allozyme polymorphism across a major phylogeographic break in the copepod Tigriopus californicus. Proc. Natl. Acad. Sci. U.S.A. 91, 5197–5201. doi: 10.1073/pnas.91.11.5197
Burton, R. S., Pereira, R. J., and Barreto, F. S. (2013). Cytonuclear genomic interactions and hybrid breakdown. Annu. Rev. Ecol. Evol. Syst. 44, 281–302. doi: 10.1146/annurev-ecolsys-110512-135758
Butlin, R. K. (2005). Recombination and speciation. Mol. Ecol. 14, 2621–2635. doi: 10.1111/j.1365-294X.2005.02617.x
Caballero, S., Duchêne, S., Garavito, M. F., Slikas, B., and Baker, C. S. (2015). Initial evidence for adaptive selection on the NADH Subunit two of freshwater dolphins by analyses of mitochondrial genomes. PLoS ONE 10:e0123543. doi: 10.1371/journal.pone.0123543
Campana, M. G., Parker, L. D., Hawkins, M. T. R., Young, H. S., Helgen, K. M., Gunther, M. S., et al. (2016). Genome sequence, population history, and pelage genetics of the endangered African wild dog (Lycaon pictus). BMC Genomics 17, 1013. doi: 10.1186/s12864-016-3368-9
Chang, C.-C., Rodriguez, J., and Ross, J. (2015). Mitochondrial-nuclear epistasis impacts fitness and mitochondrial physiology of interpopulation Caenorhabditis briggsae hybrids. G3 (Bethesda). 6, 209–219. doi: 10.1534/g3.115.022970
Cheviron, Z. A., Connaty, A. D., McClelland, G. B., and Storz, J. F. (2014). Functional genomics of adaptation to hypoxic cold-stress in high-altitude deer mice: transcriptomic plasticity and thermogenic performance. Evolution 68, 48–62. doi: 10.1111/evo.12257
da Fonseca, R. R., Johnson, W. E., O’Brien, S. J., Ramos, M. J., and Antunes, A. (2008). The adaptive evolution of the mammalian mitochondrial genome. BMC Genomics 9, 1. doi: 10.1186/1471-2164-9-119
Dean, R., Zimmer, F., and Mank, J. E. (2014). The potential role of sexual conflict and sexual selection in shaping the genomic distribution of mito-nuclear genes. Genome Biol. Evol. 6, 1096–1104. doi: 10.1093/gbe/evu063
Dean, R., Zimmer, F., and Mank, J. E. (2015). Deficit of mitonuclear genes on the human X chromosome predates sex chromosome formation. Genome Biol. Evol. 7, 636–641. doi: 10.1093/gbe/evv017
Dingley, S. D., Polyak, E., Ostrovsky, J., Srinivasan, S., Lee, I., Rosenfeld, A. B., et al. (2014). Mitochondrial DNA variant in COX1 subunit significantly alters energy metabolism of geographically divergent wild isolates in Caenorhabditis elegans. J. Mol. Biol. 426, 2199–2216. doi: 10.1016/j.jmb.2014.02.009
Dordevic, M., Stojkovic, B., Savkovic, U., Immonen, E., Tucic, N., Lazarevic, J., et al. (2016). Sex-specific mitonuclear epistasis and the evolution of mitochondrial bioenergetics, ageing, and life history in seed beetles. Evolution 71, 274–288. doi: 10.1111/evo.13109
Dowling, D. K., Friberg, U., Hailer, F., and Arnqvist, G. (2007). Intergenomic epistasis for fitness: within-population interactions between cytoplasmic and nuclear genes in Drosophila melanogaster. Genetics 175, 235–244. doi: 10.1534/genetics.105.052050
Dowling, D. K., Friberg, U., and Lindell, J. (2008). Evolutionary implications of non-neutral mitochondrial genetic variation. Trends Ecol. Evol. 23, 546–554. doi: 10.1016/j.tree.2008.05.011
Drown, D. M., Preuss, K. M., and Wade, M. J. (2012). Evidence of a paucity of genes that interact with the mitochondrion on the X in mammals. Genome Biol. Evol. 4, 875–880. doi: 10.1093/gbe/evs064
Edmands, S., and Burton, R. S. (1999). Cytochrome c oxidase activity in interpopulation hybrids of a marine copepod: a test for nuclear-nuclear or nuclear-cytoplasmic coadaptation. Evolution 53, 1972–1978. doi: 10.2307/2640456
Egan, S. P., Ragland, G. J., Assour, L., Powell, T. H. Q., Hood, G. R., Emrich, S., et al. (2015). Experimental evidence of genome-wide impact of ecological selection during early stages of speciation-with-gene-flow. Ecol. Lett. 18, 817–825. doi: 10.1111/ele.12460
Ellison, C. K., and Burton, R. S. (2006). Disruption of mitochondrial function in interpopulation hybrids of Tigriopus californicus. Evolution (N. Y). 60, 1382–1391.
Ellison, C. K., and Burton, R. S. (2008). Interpopulation hybrid breakdown maps to the mitochondrial genome. Evolution (N. Y). 62, 631–638. doi: 10.1111/j.1558-5646.2007.00305.x
Fangue, N. A., Richards, J. G., and Schulte, P. M. (2009). Do mitochondrial properties explain intraspecific variation in thermal tolerance? J. Exp. Biol. 212, 514–522. doi: 10.1242/jeb.024034
Faria, R., Neto, S., Noor, M. A. F., and Navarro, A. (2011). “Role of natural selection in chromosomal speciation,” in Encyclopedia of Life Sciences (ELS) (Chichester: John Wiley & Sons Ltd.). doi: 10.1002/9780470015902.a0022850
Fiedorczuk, K., Letts, J. A., Degliesposti, G., Kaszuba, K., Skehel, M., and Sazanov, L. A. (2016). Atomic structure of the entire mammalian mitochondrial complex I. Nature 538, 406–410. doi: 10.1038/nature19794
Finch, T. M., Zhao, N., Korkin, D., Frederick, K. H., and Eggert, L. S. (2014). Evidence of positive selection in mitochondrial complexes I and V of the African elephant. PLoS ONE 9:e92587. doi: 10.1371/journal.pone.0092587
Forester, B. R., Jones, M. R., Joost, S., Landguth, E. L., and Lasky, J. R. (2016). Detecting spatial genetic signatures of local adaptation in heterogeneous landscapes. Mol. Ecol. 25, 104–120. doi: 10.1111/mec.13476
Gagnaire, P.-A., Normandeau, E., and Bernatchez, L. (2012). Comparative genomics reveals adaptive protein evolution and a possible cytonuclear incompatibility between European and American eels. Mol. Biol. Evol. 29, 2909–2919. doi: 10.1093/molbev/mss076
Garvin, M. R., Bielawski, J. P., and Gharrett, A. J. (2011). Positive Darwinian selection in the piston that powers proton pumps in complex I of the mitochondria of Pacific salmon. PLoS ONE 6:e24127. doi: 10.1371/journal.pone.0024127
Garvin, M. R., Bielawski, J. P., Sazanov, L. A., and Gharrett, A. J. (2014). Review and meta-analysis of natural selection in mitochondrial complex I in metazoans. J. Zool. Syst. Evol. Res. 53, 1–17. doi: 10.1111/jzs.12079
Garvin, M. R., Templin, W. D., Gharrett, A. J., DeCovich, N., Kondzela, C. M., Guyon, J. R., et al. (2016). Potentially adaptive mitochondrial haplotypes as a tool to identify divergent nuclear loci. Methods Ecol. Evol. doi: 10.1111/2041-210X.12698
Garvin, M. R., Thorgaard, G. H., and Narum, S. R. (2015). Differential expression of genes that control respiration contribute to thermal adaptation in redband trout (Oncorhynchus mykiss gairdneri). Genome Biol. Evol. 7, 1404–1414. doi: 10.1093/gbe/evv078
Gershoni, M., Levin, L., Ovadia, O., Toiw, Y., Shani, N., Dadon, S., et al. (2014). Disrupting mitochondrial-nuclear coevolution affects OXPHOS Complex I integrity and impacts human health. Genome Biol. Evol. 6, 2665–2680. doi: 10.1093/gbe/evu208
Gershoni, M., Templeton, A. R., and Mishmar, D. (2009). Mitochondrial bioenergetics as a major motive force of speciation. Bioessays 31, 642–650. doi: 10.1002/bies.200800139
Goicoechea, P. G., Herrán, A., Durand, J., Bodénès, C., Plomion, C., and Kremer, A. (2015). A linkage disequilibrium perspective on the genetic mosaic of speciation in two hybridizing Mediterranean white oaks. Heredity (Edinb). 114, 373–386. doi: 10.1038/hdy.2014.113
Gompert, Z., Comeault, A. A., Farkas, T. E., Feder, J. L., Parchman, T. L., Buerkle, C. A., et al. (2014). Experimental evidence for ecological selection on genome variation in the wild. Ecol. Lett. 17, 369–379. doi: 10.1111/ele.12238
Gonçalves da Silva, A. G. (2017). “Measuring natural selection,” in Bioinformatics: Data, Sequence Analysis, and Evolution, ed. J. M. Keith (New York, NY: Springer), 315–347.
Gu, J., Wu, M., Guo, R., Yan, K., Lei, J., Gao, N., et al. (2016). The architecture of the mammalian respirasome. Nature 537, 639–643. doi: 10.1038/nature19359
Haasl, R. J., and Payseur, B. A. (2016). Fifteen years of genomewide scans for selection: trends, lessons and unaddressed genetic sources of complication. Mol. Ecol. 25, 5–23. doi: 10.1111/mec.13339
Hahn, A., Parey, K., Bublitz, M., Mills, D. J., Zickermann, V., Vonck, J., et al. (2016). Structure of a complete ATP synthase dimer reveals the molecular basis of inner mitochondrial membrane morphology. Mol. Cell 63, 445–456. doi: 10.1016/j.molcel.2016.05.037
Halsey, L. G., and White, C. R. (2010). Measuring energetics and behaviour using accelerometry in cane toads Bufo marinus. PLoS ONE 5:e10170. doi: 10.1371/journal.pone.0010170
Harrison, J. S., and Burton, R. S. (2006). Tracing hybrid incompatibilities to single amino acid substitutions. Mol. Biol. Evol. 23, 559–564. doi: 10.1093/molbev/msj058
Harrisson, K. A., Pavlova, A., Telonis-Scott, M., and Sunnucks, P. (2014). Using genomics to characterize evolutionary potential for conservation of wild populations. Evol. Appl. 7, 1008–1025. doi: 10.1111/eva.12149
Havird, J. C., Hall, M. D., and Dowling, D. K. (2015). The evolution of sex: a new hypothesis based on mitochondrial mutational erosion. Bioessays 37, 951–958. doi: 10.1002/bies.201500057
Havird, J. C., and Sloan, D. B. (2016). The roles of mutation, selection, and expression in determining relative rates of evolution in mitochondrial vs. nuclear genomes. Mol. Biol. Evol. 33, 3042–3053. doi: 10.1093/molbev/msw185
Hill, G. E. (2014). Sex linkage of nuclear-encoded mitochondrial genes. Heredity (Edinb). 112, 469–470. doi: 10.1038/hdy.2013.125
Hill, G. E. (2016). Mitonuclear coevolution as the genesis of speciation and the mitochondrial DNA barcode gap. Ecol. Evol. 6, 5831–5842. doi: 10.1002/ece3.2338
Hill, G. E., and Johnson, J. D. (2013). The mitonuclear compatibility hypothesis of sexual selection. Proc. Biol. Sci. 280:20131314. doi: 10.1098/rspb.2013.1314
Hoban, S., Kelley, J. L., Lotterhos, K. E., Antolin, M. F., Bradburd, G., Lowry, D. B., et al. (2016). Finding the genomic basis of local adaptation: pitfalls, practical solutions, and future directions. Am. Nat. 188, 379–397. doi: 10.1086/688018
Hoekstra, L. A., Siddiq, M. A., and Montooth, K. L. (2013). Pleiotropic effects of a mitochondrial–nuclear incompatibility depend upon the accelerating effect of temperature in Drosophila. Genetics 195, 1129–1139. doi: 10.1534/genetics.113.154914
Iwata, S., Lee, J. W., Okada, K., Lee, J. K., Iwata, M., Rasmussen, B., et al. (1998). Complete structure of the 11-subunit bovine mitochondrial cytochrome bc1 complex. Science 281, 64–71. doi: 10.1126/science.281.5373.64
Jhuang, H., Lee, H., and Leu, J. (2017). Mitochondrial–nuclear co-evolution leads to hybrid incompatibility through pentatricopeptide repeat proteins. EMBO Rep. 18, 87–101. doi: 10.15252/embr.201643311
Jones, F. C., Grabherr, M. G., Chan, Y. F., Russell, P., Mauceli, E., Johnson, J., et al. (2012). The genomic basis of adaptive evolution in threespine sticklebacks. Nature 484, 55–61. doi: 10.1038/nature10944
Källberg, M., Wang, H., Wang, S., Peng, J., Wang, Z., Lu, H., et al. (2012). Template-based protein structure modeling using the RaptorX web server. Nat. Protoc. 7, 1511–1522. doi: 10.1038/nprot.2012.085
Kelley, L. A., Mezulis, S., Yates, C. M., Wass, M. N., and Sternberg, M. J. E. (2015). The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 10, 845–858. doi: 10.1038/nprot.2015.053
Kemppainen, P., Knight, C. G., Sarma, D. K., Hlaing, T., Prakash, A., Maung, M., et al. (2015). Linkage disequilibrium network analysis (LDna) gives a global view of chromosomal inversions, local adaptation and geographic structure. Mol. Ecol. Resour. 15, 1031–1045. doi: 10.1111/1755-0998.12369
Kirkpatrick, M., and Barton, N. (2006). Chromosome inversions, local adaptation and speciation. Genetics 173, 419–434. doi: 10.1534/genetics.105.047985
Koevoets, T., Van de Zande, L., and Beukeboom, L. W. (2012). Temperature stress increases hybrid incompatibilities in the parasitic wasp genus Nasonia. J. Evol. Biol. 25, 304–316. doi: 10.1111/j.1420-9101.2011.02424.x
Küpper, C., Stocks, M., Risse, J. E., dos Remedios, N., Farrell, L. L., Mcrae, S. B., et al. (2016). A supergene determines highly divergent male reproductive morphs in the ruff. Nat. Genet. 48, 79–83. doi: 10.1038/ng.3443
Lanctôt, C., Cheutin, T., Cremer, M., Cavalli, G., and Cremer, T. (2007). Dynamic genome architecture in the nuclear space: regulation of gene expression in three dimensions. Nat. Rev. Genet. 8, 104–115. doi: 10.1038/nrg2041
Latorre-Pellicer, A., Moreno-Loshuertos, R., Lechuga-Vieco, A. V., Sánchez-Cabo, F., Torroja, C., Acín-Pérez, R., et al. (2016). Mitochondrial and nuclear DNA matching shapes metabolism and healthy ageing. Nature 535, 561–565. doi: 10.1038/nature18618
Letts, J. A., Fiedorczuk, K., and Sazanov, L. A. (2016). The architecture of respiratory supercomplexes. Nature 537, 644–648. doi: 10.1038/nature19774
Levin, L., Blumberg, A., Barshad, G., and Mishmar, D. (2014). Mito-nuclear co-evolution: the positive and negative sides of functional ancient mutations. Front. Genet. 5:448. doi: 10.3389/fgene.2014.00448
Liang, Y., Hua, Z., Liang, X., Xu, Q., and Lu, G. (2001). The crystal structure of bar-headed goose hemoglobin in deoxy form: the allosteric mechanism of a hemoglobin species with high oxygen affinity. J. Mol. Biol. 313, 123–137. doi: 10.1006/jmbi.2001.5028
Lighton, J. R. B. (2008). Measuring Metabolic Rates: A Manual for Scientists. Oxford: Oxford University Press. doi: 10.1093/acprof:oso/9780195310610.001.0001
Lowell, B. B., and Spiegelman, B. M. (2000). Towards a molecular understanding of adaptive thermogenesis. Nature 404, 652–660.
Lowry, D. B., and Willis, J. H. (2010). A widespread chromosomal inversion polymorphism contributes to a major life-history transition, local adaptation, and reproductive isolation. PLoS Biol. 8:e1000500. doi: 10.1371/journal.pbio.1000500
Lynch, M., Koskella, B., and Schaack, S. (2006). Mutation pressure and the evolution of organelle genomic architecture. Science 311, 1727–1730. doi: 10.1126/science.1118884
Ma, H., Gutierrez, N. M., Morey, R., Van Dyken, C., Kang, E., Hayama, T., et al. (2016). Incompatibility between nuclear and mitochondrial genomes contributes to an interspecies reproductive barrier. Cell Metab. 24, 283–294. doi: 10.1016/j.cmet.2016.06.012
Mackay, T. F. C., Stone, E. A., and Ayroles, J. F. (2009). The genetics of quantitative traits: challenges and prospects. Nat. Rev. Genet. 10, 565–577. doi: 10.1038/nrg2612
Manel, S., Perrier, C., Pratlong, M., Abi-Rached, L., Paganini, J., Pontarotti, P., et al. (2016). Genomic resources and their influence on the detection of the signal of positive selection in genome scans. Mol. Ecol. 25, 170–184. doi: 10.1111/mec.13468
Mangin, B., Siberchicot, A., Nicolas, S., Doligez, A., This, P., and Cierco-Ayrolles, C. (2012). Novel measures of linkage disequilibrium that correct the bias due to population structure and relatedness. Heredity (Edinb). 108, 285–291. doi: 10.1038/hdy.2011.73
Mashkevich, G., Repetto, B., Glerum, D. M., Jin, C., and Tzagoloff, A. (1997). SHY1, the yeast homolog of the mammalian SURF-1 gene, encodes a mitochondrial protein required for respiration. J. Biol. Chem. 272, 14356–14364. doi: 10.1074/jbc.272.22.14356
Meiklejohn, C. D., Holmbeck, M. A., Siddiq, M. A., Abt, D. N., Rand, D. M., and Montooth, K. L. (2013). An incompatibility between a mitochondrial tRNA and its nuclear-encoded tRNA synthetase compromises development and fitness in Drosophila. PLoS Genet. 9:e1003238. doi: 10.1371/journal.pgen.1003238
Mimaki, M., Wang, X., McKenzie, M., Thorburn, D. R., and Ryan, M. T. (2012). Understanding mitochondrial complex I assembly in health and disease. Biochim. Biophys. Acta 1817, 851–862. doi: 10.1016/j.bbabio.2011.08.010
Mishmar, D., Ruiz-Pesini, E., Golik, P., Macaulay, V., Clark, A. G., Hosseini, S., et al. (2003). Natural selection shaped regional mtDNA variation in humans. Proc. Natl. Acad. Sci. U.S.A. 100, 171–176. doi: 10.1073/pnas.0136972100
Mishmar, D., Ruiz-Pesini, E., Mondragon-Palomino, M., Procaccio, V., Gaut, B., and Wallace, D. C. (2006). Adaptive selection of mitochondrial complex I subunits during primate radiation. Gene 378, 11–18. doi: 10.1016/j.gene.2006.03.015
Mitchell, P. (1961). Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 191, 144–148. doi: 10.1038/191144a0
Montooth, K. L., Abt, D. N., Hofmann, J. W., and Rand, D. M. (2009). Comparative genomics of Drosophila mtDNA: novel features of conservation and change across functional domains and lineages. J. Mol. Evol. 69, 94–114. doi: 10.1007/s00239-009-9255-0
Morales, H. E., Pavlova, A., Amos, N., Major, R., Bragg, J., Kilian, A., et al. (2016a). Mitochondrial-nuclear interactions maintain a deep mitochondrial split in the face of nuclear gene flow. bioRxiv 095596. doi: 10.1101/095596
Morales, H. E., Pavlova, A., Joseph, L., and Sunnucks, P. (2015). Positive and purifying selection in mitochondrial genomes of a bird with mitonuclear discordance. Mol. Ecol. 24, 2820–2837. doi: 10.1111/mec.13203
Morales, H. E., Sunnucks, P., Joseph, L., and Pavlova, A. (2016b). Perpendicular axes of incipient speciation generated by mitochondrial introgression. bioRxiv 072942. doi: 10.1101/072942
Moreno-Loshuertos, R., Acín-Pérez, R., Fernández-Silva, P., Movilla, N., Pérez-Martos, A., de Cordoba, S. R., et al. (2006). Differences in reactive oxygen species production explain the phenotypes associated with common mouse mitochondrial DNA variants. Nat. Genet. 38, 1261–1268. doi: 10.1038/ng1897
Mossman, J. A., Biancani, L. M., Zhu, C.-T., and Rand, D. M. (2016). Mitonuclear epistasis for development time and its modification by diet in Drosophila. Genetics 203, 463–484. doi: 10.1534/genetics.116.187286
Murphy, M. P. (2009). How mitochondria produce reactive oxygen species. Biochem. J. 417, 1–13. doi: 10.1042/BJ20081386
Nielsen, R. (2005). Molecular signatures of natural selection. Annu. Rev. Genet. 39, 197–218. doi: 10.1146/annurev.genet.39.073003.112420
Nosil, P., Funk, D. J., and Ortiz-Barrientos, D. (2009). Divergent selection and heterogeneous genomic divergence. Mol. Ecol. 18, 375–402. doi: 10.1111/j.1365-294X.2008.03946.x
Ortiz-Barrientos, D., Engelstädter, J., and Rieseberg, L. H. (2016). Recombination rate evolution and the origin of species. Trends Ecol. Evol. 31, 226–236. doi: 10.1016/j.tree.2015.12.016
Osada, N., and Akashi, H. (2012). Mitochondrial–nuclear interactions and accelerated compensatory evolution: evidence from the primate cytochrome c oxidase complex. Mol. Biol. Evol. 29, 337–346. doi: 10.1093/molbev/msr211
Pagliarini, D. J., Calvo, S. E., Chang, B., Sheth, S. A., Vafai, S. B., Ong, S.-E., et al. (2008). A mitochondrial protein compendium elucidates complex I disease biology. Cell 134, 112–123. doi: 10.1016/j.cell.2008.06.016
Paliwal, S., Fiumera, A. C., and Fiumera, H. L. (2014). Mitochondrial-nuclear epistasis contributes to phenotypic variation and coadaptation in natural isolates of Saccharomyces cerevisiae. Genetics 198, 1251–1265. doi: 10.1534/genetics.114.168575
Pavlova, A., Amos, J. N., Joseph, L., Loynes, K., Austin, J. J., Keogh, J. S., et al. (2013). Perched at the mito-nuclear crossroads: divergent mitochondrial lineages correlate with environment in the face of ongoing nuclear gene flow in an australian bird. Evolution (N. Y). 67, 3412–3428. doi: 10.1111/evo.12107
Pavlova, A., Gan, H. M., Lee, Y. P., Austin, C. M., Gilligan, D. M., Lintermans, M., et al. (2017). Purifying selection and genetic drift shaped Pleistocene evolution of the mitochondrial genome in an endangered Australian freshwater fish. Heredity (Edinb) doi: 10.1038/hdy.2016.120 [Epub ahead of print].
Pereira, R. J., Barreto, F. S., Pierce, N. T., Carneiro, M., and Burton, R. S. (2016). Transcriptome-wide patterns of divergence during allopatric evolution. Mol. Ecol. 25, 1478–1493. doi: 10.1111/mec.13579
Pettersen, E. F., Goddard, T. D., Huang, C. C., Couch, G. S., Greenblatt, D. M., Meng, E. C., et al. (2004). UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612. doi: 10.1002/jcc.20084
Pond, S. L. K., and Frost, S. D. W. (2005). Datamonkey: rapid detection of selective pressure on individual sites of codon alignments. Bioinformatics 21, 2531–2533. doi: 10.1093/bioinformatics/bti320
Pörtner, H.-O., Hardewig, I., Sartoris, F. J., and Van Dijk, P. L. M. (1998). Energetic aspects of cold adaptation: critical temperatures in metabolic, ionic and acid-base regulation. Cold Ocean Physiol. 66, 88–120. doi: 10.1017/CBO9780511661723.005
Rand, D. M., Haney, R. A., and Fry, A. J. (2004). Cytonuclear coevolution: the genomics of cooperation. Trends Ecol. Evol. 19, 645–653. doi: 10.1016/j.tree.2004.10.003
Rawson, P. D., Brazeau, D. A., and Burton, R. S. (2000). Isolation and characterization of cytochrome c from the marine copepod Tigriopus californicus. Gene 248, 15–22. doi: 10.1016/S0378-1119(00)00145-1
Rawson, P. D., and Burton, R. S. (2002). Functional coadaptation between cytochrome c and cytochrome c oxidase within allopatric populations of a marine copepod. Proc. Natl. Acad. Sci. U.S.A. 99, 12955–12958. doi: 10.1073/pnas.202335899
Rellstab, C., Gugerli, F., Eckert, A. J., Hancock, A. M., and Holderegger, R. (2015). A practical guide to environmental association analysis in landscape genomics. Mol. Ecol. 24, 4348–4370. doi: 10.1111/mec.13322
Rieseberg, L. H. (2001). Chromosomal rearrangements and speciation. Trends Ecol. Evol. 16, 351–358. doi: 10.1016/S0169-5347(01)02187-5
Rogell, B., Dean, R., Lemos, B., and Dowling, D. K. (2014). Mito-nuclear interactions as drivers of gene movement on and off the X-chromosome. BMC Genomics 15, 1. doi: 10.1186/1471-2164-15-330
Rollins, L. A., Woolnough, A. P., Fanson, B. G., Cummins, M. L., Crowley, T. M., Wilton, A. N., et al. (2016). Selection on mitochondrial variants occurs between and within individuals in an expanding invasion. Mol. Biol. Evol. 33, 995–1007. doi: 10.1093/molbev/msv343
Ruiz-Pesini, E., Mishmar, D., Brandon, M., Procaccio, V., and Wallace, D. C. (2004). Effects of purifying and adaptive selection on regional variation in human mtDNA. Science 303, 223–226. doi: 10.1126/science.1088434
Savolainen, O., Lascoux, M., and Merilä, J. (2013). Ecological genomics of local adaptation. Nat. Rev. Genet. 14, 807–820. doi: 10.1038/nrg3522
Sazanov, L. A. (2014). The mechanism of coupling between electron transfer and proton translocation in respiratory complex I. J. Bioenerg. Biomembr. 46, 247–253. doi: 10.1007/s10863-014-9554-z
Schwander, T., Libbrecht, R., and Keller, L. (2014). Supergenes and complex phenotypes. Curr. Biol. 24, R288–R294. doi: 10.1016/j.cub.2014.01.056
Scott, G. R., Richards, J. G., and Milsom, W. K. (2009). Control of respiration in flight muscle from the high-altitude bar-headed goose and low-altitude birds. Am. J. Physiol. Integr. Comp. Physiol. 297, R1066–R1074. doi: 10.1152/ajpregu.00241.2009
Scott, G. R., Schulte, P. M., Egginton, S., Scott, A. L. M., Richards, J. G., and Milsom, W. K. (2011). Molecular evolution of cytochrome c oxidase underlies high-altitude adaptation in the bar-headed goose. Mol. Biol. Evol. 28, 351–363. doi: 10.1093/molbev/msq205
Seehausen, O., Butlin, R. K., Keller, I., Wagner, C. E., Boughman, J. W., Hohenlohe, P. A., et al. (2014). Genomics and the origin of species. Nat. Rev. Genet. 15, 176–192. doi: 10.1038/nrg3644
Servedio, M. R. (2009). The role of linkage disequilibrium in the evolution of premating isolation. Heredity (Edinb). 102, 51–56. doi: 10.1038/hdy.2008.98
Sloan, D. B., Fields, P. D., and Havird, J. C. (2015). Mitonuclear linkage disequilibrium in human populations. Proc. Biol. Sci. 282:20151704. doi: 10.1098/rspb.2015.1704
Sloan, D. B., Havird, J. C., and Sharbrough, J. (2016). The on-again, off-again relationship between mitochondrial genomes and species boundaries. Mol. Ecol doi: 10.1111/mec.13959 [Epub ahead of print].
Smadja, C. M., and Butlin, R. K. (2011). A framework for comparing processes of speciation in the presence of gene flow. Mol. Ecol. 20, 5123–5140. doi: 10.1111/j.1365-294X.2011.05350.x
Somero, G. N. (2002). Thermal physiology and vertical zonation of intertidal animals: optima, limits, and costs of living. Integr. Comp. Biol. 42, 780–789. doi: 10.1093/icb/42.4.780
Stephan, W. (2016). Signatures of positive selection: from selective sweeps at individual loci to subtle allele frequency changes in polygenic adaptation. Mol. Ecol. 25, 79–88. doi: 10.1111/mec.13288
Stier, A., Bize, P., Roussel, D., Schull, Q., Massemin, S., and Criscuolo, F. (2014a). Mitochondrial uncoupling as a regulator of life-history trajectories in birds: an experimental study in the zebra finch. J. Exp. Biol. 217, 3579–3589. doi: 10.1242/jeb.103945
Stier, A., Bize, P., Schull, Q., Zoll, J., Singh, F., Geny, B., et al. (2013). Avian erythrocytes have functional mitochondria, opening novel perspectives for birds as animal models in the study of ageing. Front. Zool. 10:33. doi: 10.1186/1742-9994-10-33
Stier, A., Massemin, S., and Criscuolo, F. (2014b). Chronic mitochondrial uncoupling treatment prevents acute cold-induced oxidative stress in birds. J. Comp. Physiol. B 184, 1021–1029. doi: 10.1007/s00360-014-0856-6
Stier, A., Reichert, S., Criscuolo, F., and Bize, P. (2015). Red blood cells open promising avenues for longitudinal studies of ageing in laboratory, non-model and wild animals. Exp. Gerontol. 71, 118–134. doi: 10.1016/j.exger.2015.09.001
Stier, A., Romestaing, C., Schull, Q., Lefol, E., Robin, J. P., Roussel, D., et al. (2016). How to measure mitochondrial function in birds using red blood cells: a case study in the king penguin and perspectives in ecology and evolution. Methods Ecol. Evol. doi: 10.1111/2041-210X.12724
Stroud, D. A., Surgenor, E. E., Formosa, L. E., Reljic, B., Frazier, A. E., Dibley, M. G., et al. (2016). Accessory subunits are integral for assembly and function of human mitochondrial complex I. Nature 538, 123–126. doi: 10.1038/nature19754
Thompson, M. J., and Jiggins, C. D. (2014). Supergenes and their role in evolution. Heredity (Edinb). 113, 1–8. doi: 10.1038/hdy.2014.20
Toews, D. P. L., and Brelsford, A. (2012). The biogeography of mitochondrial and nuclear discordance in animals. Mol. Ecol. 21, 3907–3930. doi: 10.1111/j.1365-294X.2012.05664.x
Toews, D. P. L., Mandic, M., Richards, J. G., and Irwin, D. E. (2014). Migration, mitochondria, and the yellow-rumped warbler. Evolution 68, 241–255. doi: 10.1111/evo.12260
Torres-Bacete, J., Sinha, P. K., Matsuno-Yagi, A., and Yagi, T. (2011). Structural contribution of C-terminal segments of NuoL (ND5) and NuoM (ND4) subunits of complex I from Escherichia coli. J. Biol. Chem. 286, 34007–34014. doi: 10.1074/jbc.M111.260968
Tsukihara, T., Aoyama, H., Yamashita, E., and Tomizaki, T. (1996). The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 angstrom. Science 272, 1136. doi: 10.1126/science.272.5265.1136
Wertheim, J. O., Murrell, B., Smith, M. D., Pond, S. L. K., and Scheffler, K. (2015). RELAX: detecting relaxed selection in a phylogenetic framework. Mol. Biol. Evol. 32, 820–832. doi: 10.1093/molbev/msu400
White, C. R., Alton, L. A., and Frappell, P. B. (2011). Metabolic cold adaptation in fishes occurs at the level of whole animal, mitochondria and enzyme. Proc. Biol. Sci. 279, 1740–1747. doi: 10.1098/rspb.2011.2060
White, C. R., Blackburn, T. M., Martin, G. R., and Butler, P. J. (2007). Basal metabolic rate of birds is associated with habitat temperature and precipitation, not primary productivity. Proc. Biol. Sci. 274, 287–293. doi: 10.1098/rspb.2006.3727
Wijchers, P. J., and de Laat, W. (2011). Genome organization influences partner selection for chromosomal rearrangements. Trends Genet. 27, 63–71. doi: 10.1016/j.tig.2010.11.001
Willett, C. S., and Burton, R. S. (2001). Viability of cytochrome c genotypes depends on cytoplasmic backgrounds in Tigriopus californicus. Evolution (N. Y). 55, 1592–1599. doi: 10.1111/j.0014-3820.2001.tb00678.x
Willett, C. S., and Burton, R. S. (2004). Evolution of interacting proteins in the mitochondrial electron transport system in a marine copepod. Mol. Biol. Evol. 21, 443–453. doi: 10.1093/molbev/msh031
Wolff, J. N., Ladoukakis, E. D., Enríquez, J. A., and Dowling, D. K. (2014). Mitonuclear interactions: evolutionary consequences over multiple biological scales. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369:20130443. doi: 10.1098/rstb.2013.0443
Wu, M., Gu, J., Guo, R., Huang, Y., and Yang, M. (2016). Structure of mammalian respiratory supercomplex I1III2IV1. Cell 167, 1598–1609. doi: 10.1016/j.cell.2016.11.012
Yang, Z. (2007). PAML 4: phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 24, 1586–1591. doi: 10.1093/molbev/msm088
Yeaman, S. (2013). Genomic rearrangements and the evolution of clusters of locally adaptive loci. Proc. Natl. Acad. Sci. U.S.A. 110, E1743–E1751. doi: 10.1073/pnas.1219381110
Yeaman, S., and Whitlock, M. C. (2011). The genetic architecture of adaptation under migration–selection balance. Evolution (N. Y). 65, 1897–1911. doi: 10.1111/j.1558-5646.2011.01269.x
Zhang, F., and Broughton, R. E. (2015). Heterogeneous natural selection on oxidative phosphorylation genes among fishes with extreme high and low aerobic performance. BMC Evol. Biol. 15, 173. doi: 10.1186/s12862-015-0453-7
Zhang, J., Hua, Z., Tame, J. R. H., Lu, G., Zhang, R., and Gu, X. (1996). The crystal structure of a high oxygen affinity species of haemoglobin (bar-headed goose haemoglobin in the oxy form). J. Mol. Biol. 255, 484–493. doi: 10.1006/jmbi.1996.0040
Keywords: mitochondrial, nuclear, mitonuclear, oxidative phosphorylation, OXPHOS, co-evolution, genome architecture
Citation: Sunnucks P, Morales HE, Lamb AM, Pavlova A and Greening C (2017) Integrative Approaches for Studying Mitochondrial and Nuclear Genome Co-evolution in Oxidative Phosphorylation. Front. Genet. 8:25. doi: 10.3389/fgene.2017.00025
Received: 13 January 2017; Accepted: 17 February 2017;
Published: 03 March 2017.
Edited by:
Tariq Ezaz, University of Canberra, AustraliaReviewed by:
Dan Mishmar, Ben-Gurion University of the Negev, IsraelCopyright © 2017 Sunnucks, Morales, Lamb, Pavlova and Greening. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paul Sunnucks, paul.sunnucks@monash.edu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.