- 1Department of Biochemistry, Purdue University, West Lafayette, IN, United States
- 2Center for Fundamental and Applied Microbiomics, Biodesign Institute, Arizona State University, Tempe, AZ, United States
Climate changes cause altering rainfall patterns resulting in an increase in drought occurrences globally. These events are disrupting plants and agricultural productivity. To evade droughts, plants try to adapt and modify in the best capacities possible. The plants have adapted by structurally modifying roots, stems, and leaves, as well as modifying functions. Lately, the association of microbial communities with plants has also been proven to be an important factor in aiding resilience. The fungal representatives of the microbial community also help safeguard the plants against drought. We discuss how these fungi associate with plants and contribute to evading drought stress. We specifically focus on Arbuscular mycorrhizal fungi (AMF) mediated mechanisms involving antioxidant defenses, phytohormone mediations, osmotic adjustments, proline expressions, fungal water absorption and transport, morphological modifications, and photosynthesis. We believe understanding the mechanisms would help us to optimize the use of fungi in agricultural practices. That way we could better prepare the plants for the anticipated future drought events.
1 Introduction
Climate change is an immediate and global concern, as we anticipate more frequent drought events in the future (Leemans and Eickhout, 2004). Plants are expected to be directly affected by these drought events, impacting agricultural productivity (Backhaus et al., 2014; Feller and Vaseva, 2014; Mukherjee et al., 2018). Plants employ various strategies to cope with drought stress, enabling them to either evade stress or enhance their ability to tolerate drought. Plants can increase diffusive resistance, enhance water uptake by forming extensive root systems, and reduce transpiration loss, among others (Farooq et al., 2012). One of the other mechanisms enables plants to endure water-limited environments by sustaining a higher water status. Steadily, the knowledge about the involvement of the soil microbial communities aiding plants’ resilience is populating (Gamalero and Glick, 2011; Meena et al., 2017; Sarkar et al., 2022a, Sarkar et al., 2022b; Feng et al., 2023). Plants modify their microbiomes in response to various stressors, seeking assistance to cope with these challenges (Song and Haney, 2021). The role of yeast in environmental remediation is also well-known (Sarkar et al., 2019; Mukherjee et al., 2020; Ghosh et al., 2023; Rana et al., 2023; Vaksmaa et al., 2023). However, there is a dearth of understanding regarding the involvement of the fungal counterpart, particularly in terms of the mechanisms. Comprehending the role of soil fungi in bolstering plant resilience during drought conditions continues to be a formidable task, given the intricate nature of their composition and function (Emmett et al., 2021). Arbuscular mycorrhizal fungi (AMF) contribute to the resilience and adaptation of plants by withstanding environmental constraints, especially drought (Genre et al., 2020; Boczoń et al., 2021; Song and Haney, 2021; Abdalla et al., 2023; Cosme, 2023). AMF establishes a symbiotic association with around 80% of terrestrial plant species (Zobel and Öpik, 2014) enhancing plant tissue hydration and physiology during periods of drought stress (Ruiz-Lozano et al., 2012). It significantly affects plant growth, retention of water, mineral nutrition, as well as defense against abiotic stresses (Zhao et al., 2015). AMF plays a crucial role as a biological tool in enhancing plant resilience to drought alongside promoting phenotypic plasticity through the establishment of mutualistic associations with the host plant species. It is now acknowledged that a combination of physical, nutritional, physiological, and cellular processes results in AM symbiosis’s contribution to plants’ ability to withstand drought (Zobel and Öpik, 2014). This mini review delves into the role of AMF in helping plants cope with drought stress, both in model plants and agriculturally important species, using a range of mechanisms involving antioxidant defenses, phytohormone mediations, osmotic adjustments, proline expressions, fungal water absorption and transport, morphological modifications, and photosynthesis (Figure 1). Understanding these mechanisms would be beneficial for agricultural productivity under anticipated future drought conditions.
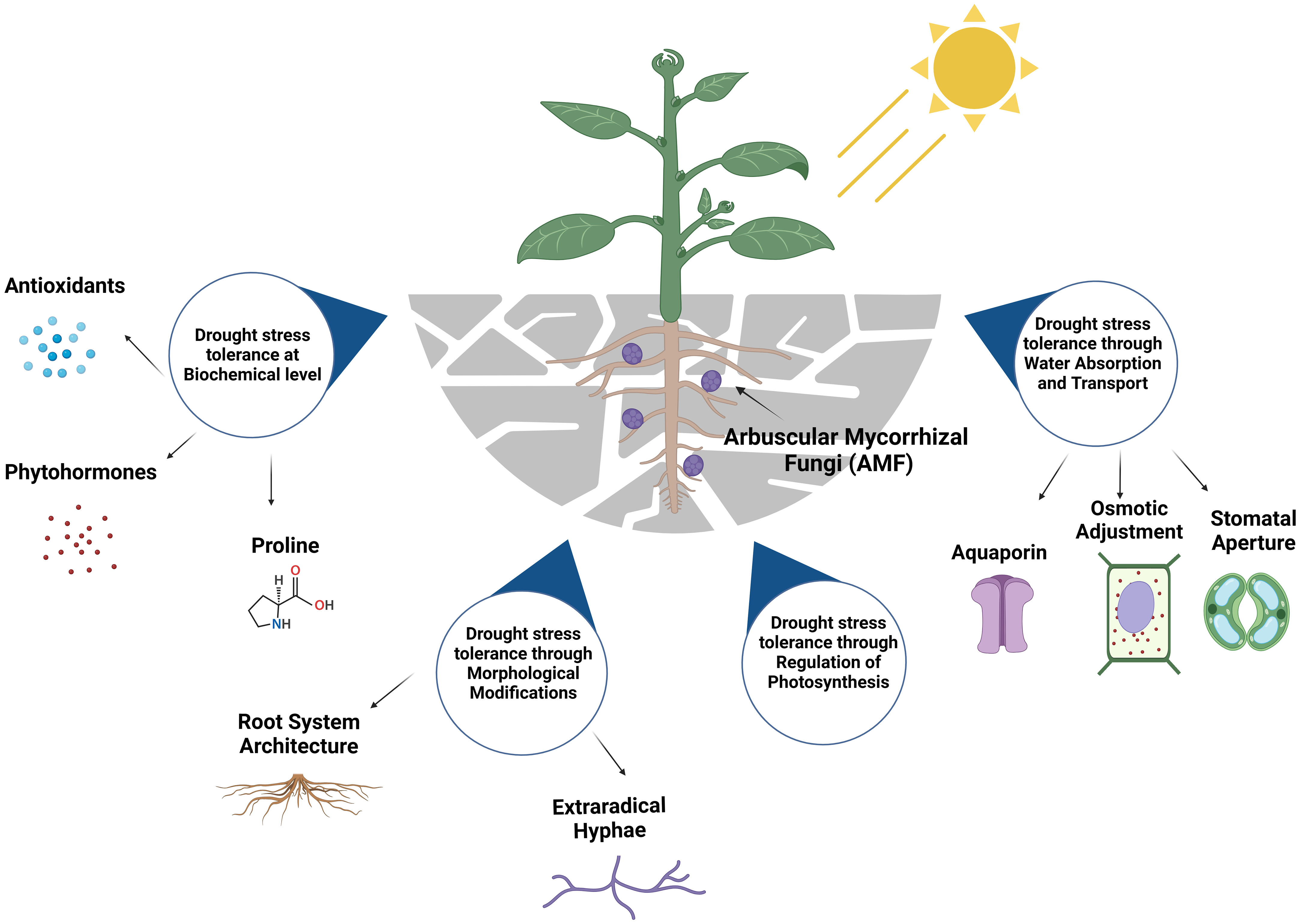
Figure 1 Key mechanisms for Arbuscular Mycorrhizal Fungi (AMF) to induce drought stress tolerance qualities into plants. The figure was created with BioRender.com.
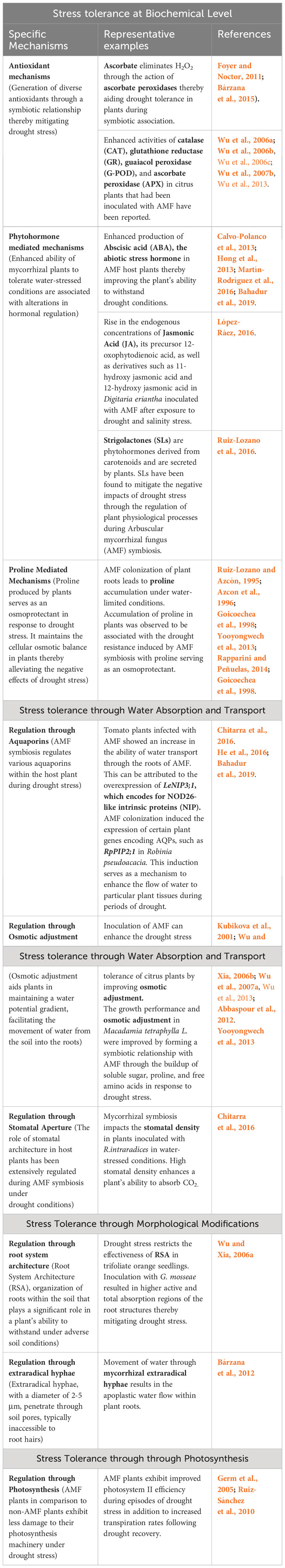
2 Drought stress tolerance at biochemical level
2.1 Antioxidant defense mechanisms
Oxidative damage and drought stress are closely intertwined. Plants undergo an elevation in reactive oxygen species (ROS) like superoxide anion free radical (O2–), hydrogen peroxide (H2O2), and hydroxyl radical (OH·) among others, as well as their buildup caused by drought stress (Tiwari et al., 2017; Hasanuzzaman et al., 2021; Li et al., 2022). Drought stress can lead to an overabundance of reactive oxygen species (ROS), which can trigger an “oxidative burst” and oxidative damage in plants (Li et al., 2022). This eventually results in the structural damage of essential biomolecules leading to membrane damage and subsequent cell death in plants (Fobert and Després, 2005; Rhoads et al., 2006; Gill and Tuteja, 2010; Demidchik, 2015; Bahadur et al., 2019). AMF’s generation of ROS is a well-studied phenomenon (Fester & Hause, 2005; Zou et al., 2021) and is essential to the process of fungal colonization. The early colonization of roots by AM fungus is largely dependent on the formation of hydrogen peroxide in the cortical cells containing mycorrhiza. Nevertheless, this production is quickly eliminated by enzymes like superoxide dismutase (SOD), catalase (CAT), and carotenoids (Kapoor and Singh, 2017; Zou et al., 2021). An optimal ROS level is crucial for molecular signaling in plant growth, development, adaptation, and response to different abiotic and biotic stresses (Liu and He, 2016; Mittler, 2017; Li et al., 2022). Thus, maintaining a balance between ROS generation and ROS scavenging in stressful environments is crucial for the survival of plants (Li et al., 2022). AMF mitigates oxidative damage and enhances drought tolerance through two distinct strategies. The first strategy entails the absorption of water using hyphae followed by its subsequent transfer to the host. This process enhances the water content and reduces the production of ROS (Huang et al., 2017; Bahadur et al., 2019). The second strategy involves an increase in the generation of diverse antioxidants through a symbiotic relationship (Abbaspour et al., 2012; Bahmani et al., 2018; Bahadur et al., 2019). Heat shock transcription factors (Hsfs) play a crucial role in signal transduction and gene response to stress (Si et al., 2021; Ma et al., 2022). In addition, certain members of the Hsfs, including SPL7, HsfA1b, HsfA4a and HsfA8, play a role in maintaining the balance of reactive oxygen species (ROS) during drought stressed conditions (Hoang et al., 2019). Furthermore, it is worth noting that Hsfs possess the ability to detect ROS in plant cells. These Hsfs play a crucial role in regulating the oxidative burst during times of stress (Miller et al., 2008). AMF has the potential to activate antioxidant defense systems and enhance Hsfs transcription levels, thereby mitigating the oxidative damage induced by drought stress. Diversispora spurca enhances the expression of JrHsf03, JrHsf22, and JrHsf24 in drought stressed Juglans regia (walnut), helping to alleviate the effects of drought stress (Ma et al., 2022). Ascorbate plays a crucial role in eliminating H2O2 through the action of ascorbate peroxidases that utilize ascorbate as an electron donor (Foyer and Noctor, 2011; Bárzana et al., 2015). A recent study highlighted the ascorbate buildup during drought. This process scavenges H2O2, as its concentration decreases significantly compared to well-watered conditions. Results also indicate that the activities of catalase (CAT), glutathione reductase (GR), guaiacol peroxidase (G-POD), and ascorbate peroxidase (APX) had a more positive impact on drought recovery in citrus plants that had been inoculated with AMF compared to non-AMF inoculated plants (Wu et al., 2006a, Wu et al., 2006b; Wu et al., 2006c; Wu et al., 2007b, Wu et al., 2013) (Table 1). In a related study on the inoculation of Diversispora spurca in Juglans regia, it was found that mycorrhizal plants exhibited increased peroxidase, catalase, and superoxide dismutase activities compared to non-mycorrhizal plants during periods of drought (Ma et al., 2022). The production of reactive oxygen species (ROS) is caused by respiratory burst oxidase homologs (Rbohs), a NADPH oxidase that also regulates a wide variety of biological processes related to biotic and abiotic stressors, including plant responses to drought (Sagi and Fluhr, 2006; Chapman et al., 2019; Tarawneh et al., 2020; Li et al., 2022; Zhang et al., 2022). AMF significantly reduced the expression of several Rbohs genes in drought stressed Bombax ceiba seedlings (Ma et al., 2022). In seedlings, Rbohs were slightly upregulated by AMF for well-water (WW) treatment (Ma et al., 2022). Additional studies have indicated that the symbiotic relationship between AMF and plants results in higher transcription levels of enzymatic antioxidants and components involved in the biosynthesis of ascorbate and glutathione (Marulanda et al., 2007; Abbaspour et al., 2012; Bahadur et al., 2019). This suggests a sophisticated transcriptional regulation of the antioxidant system. Additional investigations are needed to investigate the notable interplay between fungal symbiosis with plants and antioxidant systems.
2.2 Phytohormone-mediated mechanisms
The enhanced ability of mycorrhizal plants to tolerate water-stressed conditions is associated with alterations in hormonal regulation, specifically in Abscisic acid (ABA) signaling (López-Ráez, 2016) (Table 1). ABA has been shown to play a crucial role in arbuscular development during AMF symbiosis (Herrera-Medina et al., 2007; Martín-Rodríguez et al., 2011; López-Ráez, 2016). Several investigations have yielded valuable insights into the mechanisms behind the enhanced production of ABA in AMF host plants. This increased ABA production plays a crucial role in improving the plant’s ability to withstand drought conditions (Calvo-Polanco et al., 2013; Hong et al., 2013; Martín-Rodríguez et al., 2016; Bahadur et al., 2019). The fungus regulates ABA content in host roots during drought (Estrada-Luna and Davies, 2003; Aroca et al., 2008; López-Ráez, 2016). A study by (Calvo-Polanco et al., 2013) found that mycorrhizal plants exposed to severe stress showed a significant rise in ABA levels, also known as the ‘abiotic stress hormone’. This rise is associated with priming, which enhances the plant’s ability to tolerate stress. ABA is an essential factor for the successful establishment and functioning of AMF symbiosis. It plays a crucial role in regulating arbuscular development (Herrera-Medina et al., 2007; Martín-Rodríguez et al., 2011; Pozo et al., 2015; López-Ráez, 2016). Researchers also observed a concurrent upregulation of two plant genes, D-myo-inositol-3-phosphate synthase, and 14-3-3-like protein GF14, involved in ABA signaling transduction, indicating their involvement in the synergistic effects of the symbiotic partners to improve the plant’s resistance to drought (Li et al., 2016; Bahadur et al., 2019).
Jasmonic acid (JA) and its derivatives, known as jasmonates, are believed to play a crucial role in AMF symbiosis (López-Ráez, 2016) (Table 1). Reports suggests that there is an observed rise in the endogenous concentrations of JA, its precursor 12-oxophytodienoic acid, as well as derivatives such as 11-hydroxy jasmonic acid and 12-hydroxy jasmonic acid in Digitaria eriantha plants inoculated with AMF after exposure to drought and salinity stress (López-Ráez, 2016; Pedranzani et al., 2016)). Previous research by (Sánchez-Romera et al., 2016) has demonstrated that AMF symbiosis along with the application of exogenous methyl jasmonate can mitigate the negative impact of drought on root hydraulic conductivity within common bean plants (López-Ráez, 2016). It has been suggested that the observed protection might be linked to a decrease in salicylic acid (SA) amounts due to a negative interaction between JA and SA (Pieterse et al., 2009; López-Ráez, 2016; Sánchez-Romera et al., 2016).
Strigolactones (SLs) are phytohormones derived from carotenoids and are secreted by plants. During the pre-contact phase, labile signaling molecules are released to attract AMF and help them identify a nearby host. AMF induces oxidative metabolism upon detecting SLs, leading to enhanced hyphal branching and growth. This promotes physical contact with the roots of a host plant, ultimately driving symbiotic association (Kretzschmar et al., 2012; Mori et al., 2016; Pandey et al., 2016; Bahadur et al., 2019) (Table 1). Rhizophagus irregularis has been found to stimulate the biosynthesis of SLs within lettuce and tomato plants during periods of drought, suggesting that AMF symbiosis promotes SL production (Ruiz-Lozano et al., 2016). The study demonstrated that the expression of the SlCCD7 gene, which is responsible for the production of SLs in tomatoes, was significantly increased during drought stress in the host roots (Ruiz-Lozano et al., 2016). ABA and SLs share a common biosynthetic origin as apocarotenoids and are classified as “stress hormones.” In mycorrhizal plants under stress conditions, a positive correlation between ABA-SLs was also observed (Aroca et al., 2013; López-Ráez, 2016; Ruiz-Lozano et al., 2016). The interplay between AM symbiosis and strigolactones have been found to mitigate the adverse impacts of drought through their regulation of plant physiological processes.
2.3 Proline mediated mechanisms
Proline serves as an osmoprotectant, which is produced by plants as a response to stress caused by drought. Maintaining cellular osmotic balance is beneficial in alleviating the negative effects of drought stress (Koyro et al., 2012; Bahadur et al., 2019) (Table 1). Additional evidence supports the significant role of proline in osmoregulation and the scavenging of free radicals (Yooyongwech et al., 2013). Moreover, it serves as a molecular chaperone, aiding in the stabilization of subcellular structures thereby safeguarding plant cells from the detrimental impacts of drought stress (de Carvalho et al., 2013; Yooyongwech et al., 2013). Studies pointed out that AMF colonization of plant roots leads to proline accumulation under water-limited conditions (Ruiz-Lozano and Azcón, 1995; Azcon et al., 1996; Goicoechea et al., 1998; Yooyongwech et al., 2013; Rapparini and Peñuelas, 2014). The increased buildup of proline observed in these experiments was found to be associated with the drought resistance induced by AMF symbiosis, with proline serving as an osmoprotectant. The colonization of Medicago sativa L. roots by AMF leads to the accumulation of proline in both roots and under water stress conditions (Goicoechea et al., 1998). During the symbiosis of AMF in tomato plants (Solanum lycopersicum), the level of proline concentrations showed an extensive rise in response to water stress (WS) (Chitarra et al., 2016).
3 Drought stress tolerance through water absorption and transport
3.1 Regulation through aquaporins
Aquaporins (AQPs) are a group of integral membrane proteins that have an essential role in facilitating the transportation of water across cell membranes (Chitarra et al., 2016). AMF symbiosis regulates various aquaporins within the host plant, which include those from different subfamilies (Table 1). The mechanism of AQP gene regulation is influenced by watering conditions as well as the extent of drought stress. Certain aquaporins can transport both water and other physiologically important molecules, thereby contributing to the performance of the host plant (Bárzana et al., 2014). During drought stress, the upregulation of two AQP genes, GintAQPF1 and GintAQPF2, was observed in the extraradical mycelia of R. irregularis and mycorrhizal roots. This finding suggests that AMF plays a direct role in enhancing the resilience of plants to water deprivation (Ying-Ning et al., 2013; Li et al., 2013a). In a separate study, tomato plants infected with AMF showed an increase in the ability of water transport through the roots of AMF. This increase was found to be associated with the overexpression of a gene called LeNIP3;1, which encodes for NOD26-like intrinsic proteins (NIP) (Chitarra et al., 2016). AMF colonization induced the expression of certain plant genes encoding AQPs, such as RpPIP2;1 in Robinia pseudoacacia (He et al., 2016; Bahadur et al., 2019). This induction may serve as a mechanism to enhance the flow of water to particular plant tissues, which is crucial for the survival of the host plant during periods of drought-related stress. In contrast, the expression of the GintAQP1 gene in lettuce roots was found to be downregulated under conditions of water deficit, despite the improvement in root AMF (Aroca et al., 2007). In their study (Bárzana et al., 2014), offered new insights into the regulation of aquaporins in maize plants during drought stress, specifically in the context of AMF symbiosis. They found that under short-term drought-stressed conditions, AMF plants showed higher sap flow rate (Jv) and osmotic root hydraulic conductance (Lo) values compared to non-AMF plants. This can be attributed to the fact that the expression levels of several PIP proteins (ZmPIP1;1, ZmPIP1;2, ZmPIP1;3, ZmPIP1;4, ZmPIP1;6, ZmPIP2;2, and ZmPIP2;4) remained high or even increased. During prolonged periods of drought, the availability of soil water resources is reduced significantly, resulting in a decrease in both Jv and Lo values in AMF plants. In that scenario, AMF has been observed to downregulate several PIP genes, including ZmPIP1;1, ZmPIP1;3, ZmPIP1;4, ZmPIP2;2, and ZmPIP2;4, in both well-watered and sustained drought situations. This observed downregulation might serve as an approach to prevent water loss (Porcel et al., 2006). AM fungal aquaporins may also contribute to drought tolerance during the event of AMF symbiosis (Aroca et al., 2009; Li et al., 2013a, Li et al., 2013b). AMF aquaporins are known to play a role in facilitating water movement in both the extraradical mycelium and periarbuscular membrane (Li et al., 2013a). The increased Lo values observed in AMF plants during short-term drought stress and the elevated hydrostatic root hydraulic conductance (Lh) values observed during prolonged drought can be attributed to the functioning of fungal aquaporins (Bárzana et al., 2014).
3.2 Regulation through osmotic adjustment
Osmotic adjustment (OA) is considered an effective way to promote drought tolerance in plants (Wu et al., 2013). OA aids plants in maintaining a water potential gradient, facilitating the movement of water from the soil into the roots (Yooyongwech et al., 2013; Zhang et al., 2016; Bahadur et al., 2019) (Table 1). It involves the reduction of osmotic potential by accumulating low molecular weight solutes when exposed to stress (Martı̀nez et al., 2004; Wu et al., 2013). Organic (proline, glycinbetain, aspartic acid, protein, and sugars) and inorganic solutes (K+, Ca2+, and Mg2+) function as osmoprotectants, aiding in water absorption and stabilizing macromolecular frameworks and subcellular membranes during dehydration stress (Gomes et al., 2010; Wu et al., 2013). The growth performance and osmotic adjustment in Macadamia tetraphylla L. were improved by forming a symbiotic relationship with AMF. This improvement was achieved through a buildup of various compounds, including soluble sugar, proline, and free amino acids, in response to drought conditions (Yooyongwech et al., 2013). Multiple studies have shown that the inoculation of AMF can enhance the drought stress tolerance of citrus plants by improving osmotic adjustment (OA) (Kubikova et al., 2001; Wu and Xia, 2006b; Wu et al., 2007a; Abbaspour et al., 2012; Wu et al., 2013).
3.3 Regulation through stomatal aperture
The role of stomatal architecture has been extensively studied during AMF symbiosis in response to the water-stressed condition in Solanum lycopersicum (tomato plants) (Chitarra et al., 2016). The study quantified the stomatal density in mature leaves of AMF and control NS plants. The findings showed that mycorrhizal symbiosis has an impact on stomatal density, particularly in plants inoculated with R. intraradices. The density of stomatal cells in this condition was approximately double compared to that of the control and plants inoculated with F. mosseae. A high stomatal density enhances a plant’s ability to absorb CO2 (Chitarra et al., 2016) (Table 1). The study quantified LeEPFL9 transcripts, which have an effect in regulating stomatal development, alongside the genes encoding EPF1 and EPF2 that act as antagonists of LeEPFL9 thereby negatively regulating stomatal development. In tomato leaves undergoing development, the expression of these genes was observed to be significant only when AMF symbiosis was present. The steady-state levels of LeEPFL9 transcripts showed a strong positive correlation in accordance with the higher stomatal density observed in plants colonized by R. intraradices (Chitarra et al., 2016).
4 Drought stress tolerance through morphological modifications
4.1 Regulation through root system architecture
Root system architecture (RSA) refers to the organization of roots within the soil particularly playing a significant role in a plant’s ability to withstand adverse soil conditions (de Dorlodot et al., 2007; Wu et al., 2013). AMF colonization can cause RSA modifications to host plants, which are influenced by factors such as plant and fungal species or genotypes, in addition to both water and nutrient availability (Wu et al., 2012; Chatzistathis et al., 2013; Wu et al., 2013; Ying-Ning et al., 2013) (Table 1). One report suggests that drought stress greatly restricted the effectiveness of RSA in trifoliate orange seedlings. However, inoculation with G. mosseae successfully mitigated this limitation and resulted in higher active and total absorption regions of the root structures. This effect was observed in seedlings grown under different soil water content levels (20%, 16%, and 12%) contrasted to those that were not inoculated with AMF (Wu and Xia, 2006a). Studies by (Orfanoudakis et al., 2010) suggest that the combined inoculation of AMF and Frankia resulted in a bigger spike in root branching in plants, specifically Alnus glutinosa. The alterations in RSA caused by AMF can be attributed to multiple factors such as an altered balance of cytokinin to gibberellin, improved nutritional condition in AMF plants, and the tightly controlled metabolism of endogenous polyamines (Berta et al., 1993; Wu et al., 2012; Chatzistathis et al., 2013; Wu et al., 2013).
4.2 Regulation through extraradical hyphae
In dry soil conditions as opposed to wet soil conditions, the hyphal water transfer may play a greater role. The movement of water through mycorrhizal hyphae plays a role in the apoplastic water flow within roots (Bárzana et al., 2012) (Table 1). Extraradical hyphae, with a diameter of 2-5 μm, penetrate through soil pores that are typically inaccessible to root hairs (Gianinazzi et al., 1994; Khan, 2003). K+ is essential for water movement by mycorrhizal hyphae. The presence of additional K+ simply enhanced root hydraulic conductivity in AMF plants, compared to non-AMF plants, irrespective of water conditions (El-Mesbahi et al., 2012). The mycorrhizal association is crucial in aiding the absorption of mineral nutrients, particularly those that have limited movement within the soil, like phosphorus (P), zinc (Zn), and copper (Cu) (Srivastava et al., 2002; Smith and Smith, 2011).
5 Regulation through photosynthesis
The symbiotic relationship between AMF and Oryza sativa (Rice) plants improved the efficiency of photosynthesis by more than 40% during stress conditions (Ruiz-Sánchez et al., 2010). AMF plants demonstrated improved photosystem II efficiency under drought stress in addition to increased transpiration rates following drought recovery (Table 1). Reports already suggest that AMF plants exhibit higher photosynthetic efficiency indicating less damage to their photosynthesis machinery under drought stress (Germ et al., 2005; Ruiz-Sánchez et al., 2010). The two combined effects probably contributed to the improved plant growth of AMF plants through improved CO2 fixation during and after periods of drought stress (Ruiz-Sánchez et al., 2010). Furthermore, the improved efficiency of photosystem II together with increased transpiration in AMF plants may have resulted in reduced photorespiration and subsequently reduced levels of ROS in these plants (Cadenas, 1989).
6 Conclusions
Current and future drought events are a serious cause of concern. As a scientific community, we must be prepared to mitigate drought events through natural and organic efforts. We anticipate heavy losses to plants and agricultural productivity due to the disturbances. AMF helps plants withstand environmental constraints, particularly drought, thereby enhancing their resilience. We discussed how AMF could protect plants at biochemical level through antioxidant defense mechanisms, phytohormone and proline-mediated mechanisms. AMF also aids in drought stress tolerance through water absorption and transport using aquaporins, making osmotic adjustments, and also through photosynthesis. Moreover, morphological modifications in plants and AMF can also contribute to the drought stress tolerance. We believe this knowledge would help fathom the ways fungal interaction with plants is useful in tolerating extreme situations.
Author contributions
SD: Conceptualization, Investigation, Software, Visualization, Writing – original draft, Writing – review & editing. SS: Conceptualization, Investigation, Resources, Software, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to acknowledge the Department of Biochemistry, Purdue University, USA, and the Center for Fundamental and Applied Microbiomics, Biodesign Institute, Arizona State University. The authors are thankful to Maria Zea Rojas, Associate Researcher, Department of Biochemistry, Purdue University for her assistance with the figure. Figure 1 was created with BioRender (www.biorender.com).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbaspour H., Saeidi-Sar S., Afshari H., Abdel-Wahhab M. A. (2012). Tolerance of Mycorrhiza infected Pistachio (Pistacia vera L.) seedling to drought stress under glasshouse conditions. J. Plant Physiol. 169, 704–709. doi: 10.1016/j.jplph.2012.01.014
Abdalla M., Bitterlich M., Jansa J., Püschel D., Ahmed M. A. (2023). The role of arbuscular mycorrhizal symbiosis in improving plant water status under drought. J. Exp. Bot. 74, 4808–4824. doi: 10.1093/jxb/erad249
Aroca R., Bago A., Sutka M., Paz J. A., Cano C., Amodeo G., et al. (2009). Expression analysis of the first arbuscular mycorrhizal fungi aquaporin described reveals concerted gene expression between salt-stressed and nonstressed mycelium. Mol. Plant-Microbe Interactions® 22, 1169–1178. doi: 10.1094/MPMI-22-9-1169
Aroca R., del Mar Alguacil M., Vernieri P., Ruiz-Lozano J. M. (2008). Plant responses to drought stress and exogenous ABA application are modulated differently by mycorrhization in tomato and an ABA-deficient mutant (Sitiens). Microbial Ecol. 56, 704–719. doi: 10.1007/s00248-008-9390-y
Aroca R., Porcel R., Ruiz-Lozano J. M. (2007). How does arbuscular mycorrhizal symbiosis regulate root hydraulic properties and plasma membrane aquaporins in Phaseolus vulgaris under drought, cold or salinity stresses? New Phytol. 173, 808–816. doi: 10.1111/j.1469-8137.2006.01961.x
Aroca R., Ruiz-Lozano J. M., Zamarreño Á.M., Paz J. A., García-Mina J. M., Pozo M. J., et al. (2013). Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J. Plant Physiol. 170, 47–55. doi: 10.1016/j.jplph.2012.08.020
Azcon R., Gomez M., Tobar R. (1996). ). Physiological and nutritional responses by Lactuca Sativa L. @ to nitrogen sources and mycorrhizal fungi under drought conditions. Biol. Fertility Soils 22, 156–161. doi: 10.1007/BF00384448
Backhaus S., Kreyling J., Grant K., Beierkuhnlein C., Walter J., Jentsch A. (2014). Recurrent mild drought events increase resistance toward extreme drought stress. Ecosystems 17, 1068–1081. doi: 10.1007/s10021-014-9781-5
Bahadur A., Batool A., Nasir F., Jiang S., Mingsen Q., Zhang Q., et al. (2019). Mechanistic insights into arbuscular mycorrhizal fungi-mediated drought stress tolerance in plants. Int. J. Mol. Sci. 20, 4199. doi: 10.3390/ijms20174199
Bahmani M., Naghdi R., Kartoolinejad D. (2018). Milkweed seedlings tolerance against water stress: Comparison of inoculations with Rhizophagus irregularis and Pseudomonas putida. Environ. Technol. Innovation 10, 111–121. doi: 10.1016/j.eti.2018.01.001
Bárzana G., Aroca R., Bienert G. P., Chaumont F., Ruiz-Lozano J. M. (2014). New insights into the regulation of aquaporins by the arbuscular mycorrhizal symbiosis in maize plants under drought stress and possible implications for plant performance. Mol. Plant-Microbe Interactions® 27, 349–363. doi: 10.1094/MPMI-09-13-0268-R
Bárzana G., Aroca R., Paz J. A., Chaumont F., Martinez-Ballesta M. C., Carvajal M., et al. (2012). Arbuscular mycorrhizal symbiosis increases relative apoplastic water flow in roots of the host plant under both well-watered and drought stress conditions. Ann. Bot. 109, 1009–1017. doi: 10.1093/aob/mcs007
Bárzana G., Aroca R., Ruiz-Lozano J. M. (2015). Localized and non-localized effects of arbuscular mycorrhizal symbiosis on accumulation of osmolytes and aquaporins and on antioxidant systems in maize plants subjected to total or partial root drying. Plant Cell Environ. 38, 1613–1627. doi: 10.1111/pce.12507
Berta G., Fusconi A., Trotta A. (1993). VA mycorrhizal infection and the morphology and function of root systems. Environ. Exp. Bot. 33, 159–173. doi: 10.1016/0098-8472(93)90063-L
Boczoń A., Hilszczańska D., Wrzosek M., Szczepkowski A., Sierota Z. (2021). Drought in the forest breaks plant–fungi interactions. Eur. J. For. Res. 140, 1301–1321. doi: 10.1007/s10342-021-01409-5
Cadenas E. (1989). Biochemistry of oxygen toxicity. Annu. Rev. Biochem. 58, 79–110. doi: 10.1146/annurev.bi.58.070189.000455
Calvo-Polanco M., Sánchez-Romera B., Aroca R. (2013). Arbuscular mycorrhizal fungi and the tolerance of plants to drought and salinity. Soil Biol. 37, 271–288. doi: 10.1007/978-3-642-39317-4_14
Chapman J. M., Muhlemann J. K., Gayomba S. R., Muday G. K. (2019). RBOH-dependent ROS synthesis and ROS scavenging by plant specialized metabolites to modulate plant development and stress responses. Chem. Res. Toxicol. 32, 370–396. doi: 10.1021/acs.chemrestox.9b00028
Chatzistathis T., Orfanoudakis M., Alifragis D., Therios I. (2013). Colonization of Greek olive cultivars’ root system by arbuscular mycorrhiza fungus: Root morphology, growth, and mineral nutrition of olive plants. Scientia Agricola 70, 185–194. doi: 10.1590/S0103-90162013000300007
Chitarra W., Pagliarani C., Maserti B., Lumini E., Siciliano I., Cascone P., et al. (2016). Insights on the impact of arbuscular mycorrhizal symbiosis on tomato tolerance to water stress. Plant Physiol. 171 (2), 00307.2016. doi: 10.1104/pp.16.00307
Cosme M. (2023). Mycorrhizas drive the evolution of plant adaptation to drought. Commun. Biol. 6, 346. doi: 10.1038/s42003-023-04722-4
de Carvalho K., de Campos M. K. F., Domingues D. S., Pereira L. F. P., Vieira L. G. E. (2013). The accumulation of endogenous proline induces changes in gene expression of several antioxidant enzymes in leaves of transgenic Swingle citrumelo. Mol. Biol. Rep. 40, 3269–3279. doi: 10.1007/s11033-012-2402-5
de Dorlodot S., Forster B., Pagès L., Price A., Tuberosa R., Draye X. (2007). Root system architecture: opportunities and constraints for genetic improvement of crops. Trends Plant Sci. 12, 474–481. doi: 10.1016/j.tplants.2007.08.012
Demidchik V. (2015). Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environmental and Experimental Botany 109, 212–228. doi: 10.1016/j.envexpbot.2014.06.021
El-Mesbahi M. N., Azcón R., Ruiz-Lozano J. M., Aroca R. (2012). Plant potassium content modifies the effects of arbuscular mycorrhizal symbiosis on root hydraulic properties in maize plants. Mycorrhiza 22, 555–564. doi: 10.1007/s00572-012-0433-3
Emmett B. D., Lévesque-Tremblay V., Harrison M. J. (2021). Conserved and reproducible bacterial communities associate with extraradical hyphae of arbuscular mycorrhizal fungi. ISME J. 15, 2276–2288. doi: 10.1038/s41396-021-00920-2
Estrada-Luna A. A., Davies F. T. (2003). Arbuscular mycorrhizal fungi influence water relations, gas exchange, abscisic acid and growth of micropropagated Chile ancho pepper (Capsicum annuum) plantlets during acclimatization and post-acclimatization. J. Plant Physiol. 160, 1073–1083. doi: 10.1078/0176-1617-00989
Farooq M., Hussain M., Wahid A., Siddique K. H. M. (2012). “Drought stress in plants: An overview,” in Plant Responses to Drought Stress (Springer, Berlin Heidelberg), 1–33. doi: 10.1007/978-3-642-32653-0_1
Feller U., Vaseva I. I. (2014). Extreme climatic events: impacts of drought and high temperature on physiological processes in agronomically important plants. Front. Environ. Sci. 2. doi: 10.3389/fenvs.2014.00039
Feng Q., Cao S., Liao S., Wassie M., Sun X., Chen L., et al. (2023). Fusarium equiseti-inoculation altered rhizosphere soil microbial community, potentially driving perennial ryegrass growth and salt tolerance. Sci. Total Environ. 871, 162153. doi: 10.1016/j.scitotenv.2023.162153
Fester T., Hause G. (2005). Accumulation of reactive oxygen species in arbuscular mycorrhizal roots. Mycorrhiza 15, 373–379. doi: 10.1007/s00572-005-0363-4
Fobert P. R., Després C. (2005). Redox control of systemic acquired resistance. Curr. Opin. Plant Biol. 8, 378–382. doi: 10.1016/j.pbi.2005.05.003
Foyer C. H., Noctor G. (2011). Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 155, 2–18. doi: 10.1104/pp.110.167569
Gamalero E., Glick B. R. (2011). “Mechanisms used by plant growth-promoting bacteria,” in Bacteria in Agrobiology: Plant Nutrient Management (Springer, Berlin Heidelberg), 17–46. doi: 10.1007/978-3-642-21061-7_2
Genre A., Lanfranco L., Perotto S., Bonfante P. (2020). Unique and common traits in mycorrhizal symbioses. Nat. Rev. Microbiol. 18, 649–660. doi: 10.1038/s41579-020-0402-3
Germ M., Kreft I., Osvald J. (2005). Influence of UV-B exclusion and selenium treatment on photochemical efficiency of photosystem II, yield and respiratory potential in pumpkins (Cucurbita pepo L.). Plant Physiol. Biochem. 43, 445–448. doi: 10.1016/j.plaphy.2005.03.004
Ghosh S., Rusyn I., Dmytruk O. V., Dmytruk K. V., Onyeaka H., Gryzenhout M., et al. (2023). Filamentous fungi for sustainable remediation of pharmaceutical compounds, heavy metal and oil hydrocarbons. Front. Bioengineering Biotechnol. 11. doi: 10.3389/fbioe.2023.1106973
Gianinazzi S., Schiiepp H., Sanchez-Diaz M., Honrubial M. (1994). “Water Relations And Alleviation Of Drought Stress In Mycorrhizal Plants,” in Impact of arbuscular mycorrhizas on sustainable agriculture and natural ecosystems (Basel: Springerlink, Birkhäuser).
Gill S. S., Tuteja N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48, 909–930. doi: 10.1016/j.plaphy.2010.08.016
Goicoechea N., Szalai G., Antolín M. C., Sánchez-Díaz M., Paldi E. (1998). Influence of arbuscular mycorrhizae and Rhizobium on free polyamines and proline levels in water-stressed alfalfa. J. Plant Physiol. 153, 706–711. doi: 10.1016/S0176-1617(98)80224-1
Gomes F. P., Oliva M. A., Mielke M. S., Almeida A.-A. F., Aquino L. A. (2010). Osmotic adjustment, proline accumulation and cell membrane stability in leaves of Cocos nucifera submitted to drought stress. Scientia Hortic. 126, 379–384. doi: 10.1016/j.scienta.2010.07.036
Hasanuzzaman M., Md. R. H., Masud A. A. C., Rahman K., Nowroz F., Rahman M., et al. (2021). Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int. J. Mol. Sci. 22, 9326. doi: 10.3390/ijms22179326
He D., Xiang X., He J.-S., Wang C., Cao G., Adams J., et al. (2016). Composition of the soil fungal community is more sensitive to phosphorus than nitrogen addition in the alpine meadow on the Qinghai-Tibetan Plateau. Biol. Fertility Soils 52, 1059–1072. doi: 10.1007/s00374-016-1142-4
Herrera-Medina M. J., Steinkellner S., Vierheilig H., Ocampo Bote J. A., García Garrido J. M. (2007). Abscisic acid determines arbuscule development and functionality in the tomato arbuscular mycorrhiza. New Phytol. 175, 554–564. doi: 10.1111/j.1469-8137.2007.02107.x
Hoang T. V., Vo K. T. X., Rahman M. M., Choi S.-H., Jeon J.-S. (2019). Heat stress transcription factor OsSPL7 plays a critical role in reactive oxygen species balance and stress responses in rice. Plant Sci. 289, 110273. doi: 10.1016/j.plantsci.2019.110273
Hong J. H., Seah S. W., Xu J. (2013). The root of ABA action in environmental stress response. Plant Cell Rep. 32, 971–983. doi: 10.1007/s00299-013-1439-9
Huang Y.-M., Zou Y.-N., Wu Q.-S. (2017). Alleviation of drought stress by mycorrhizas is related to increased root H2O2 efflux in trifoliate orange. Sci. Rep. 7, 42335. doi: 10.1038/srep42335
Kapoor R., Singh N. (2017). “Arbuscular mycorrhiza and reactive oxygen species,” in Arbuscular Mycorrhizas And Stress Tolerance Of Plants (Springer, Singapore), 225–243. doi: 10.1007/978-981-10-4115-0_10
Khan S. A. (2003). Interaction of vesicular arbuscular mycorrhizae, hormones and drought in soybeans.
Koyro H.-W., Ahmad P., Geissler N. (2012). “Abiotic stress responses in plants: an overview,” in Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change (Springer, New York), 1–28. doi: 10.1007/978-1-4614-0815-4_1
Kretzschmar T., Kohlen W., Sasse J., Borghi L., Schlegel M., Bachelier J. B., et al. (2012). A petunia ABC protein controls strigolactone-dependent symbiotic signalling and branching. Nature 483, 341–344. doi: 10.1038/nature10873
Kubikova E., Jennifer L. M., Bonnie H. O., Michael D. M., Augé M. R. (2001). Mycorrhizal impact on osmotic adjustment in Ocimum basilicum during a lethal drying episode. J. Plant Physiol. 158, 1227–1230. doi: 10.1078/0176-1617-00441
Leemans R., Eickhout B. (2004). Another reason for concern: regional and global impacts on ecosystems for different levels of climate change. Global Environ. Change 14, 219–228. doi: 10.1016/j.gloenvcha.2004.04.009
Li T., Hu Y.-J., Hao Z.-P., Li H., Chen B.-D. (2013b). Aquaporin genes GintAQPF1 and GintAQPF2 from Glomus intraradices contribute to plant drought tolerance. Plant Signaling Behav. 8, e24030. doi: 10.4161/psb.24030
Li T., Hu Y., Hao Z., Li H., Wang Y., Chen B. (2013a). First cloning and characterization of two functional aquaporin genes from an arbuscular mycorrhizal fungus Glomus intraradices. New Phytol. 197, 617–630. doi: 10.1111/nph.12011
Li T., Sun Y., Ruan Y., Xu L., Hu Y., Hao Z., et al. (2016). Potential role of D-myo-inositol-3-phosphate synthase and 14-3-3 genes in the crosstalk between Zea mays and Rhizophagus intraradices under drought stress. Mycorrhiza 26, 879–893. doi: 10.1007/s00572-016-0723-2
Li Z., Zhang Y., Liu C., Gao Y., Han L., Chu H. (2022). Arbuscular mycorrhizal fungi contribute to reactive oxygen species homeostasis of Bombax ceiba L. under drought stress. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.991781
Liu Y., He C. (2016). Regulation of plant reactive oxygen species (ROS) in stress responses: learning from AtRBOHD. Plant Cell Rep. 35, 995–1007. doi: 10.1007/s00299-016-1950-x
López-Ráez J. A. (2016). How drought and salinity affect arbuscular mycorrhizal symbiosis and strigolactone biosynthesis? Planta 243, 1375–1385. doi: 10.1007/s00425-015-2435-9
Ma W.-Y., Qin Q.-Y., Zou Y.-N., Kuča K., Giri B., Wu Q.-S., et al. (2022). Arbuscular mycorrhiza induces low oxidative burst in drought-stressed walnut through activating antioxidant defense systems and heat shock transcription factor expression. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.1089420
Martín-Rodríguez J. A., Huertas R., Ho-Plágaro T., Ocampo J. A., Turečková V., Tarkowská D., et al. (2016). Gibberellin–abscisic acid balances during arbuscular mycorrhiza formation in tomato. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.01273
Martín-Rodríguez J.Á., León-Morcillo R., Vierheilig H., Ocampo J. A., Ludwig-Müller J., García-Garrido J. M. (2011). Ethylene-dependent/ethylene-independent ABA regulation of tomato plants colonized by arbuscular mycorrhiza fungi. New Phytol. 190, 193–205. doi: 10.1111/j.1469-8137.2010.03610.x
Martı̀nez J. P., Lutts S., Schanck A., Bajji M., Kinet J.-M. (2004). Is osmotic adjustment required for water stress resistance in the Mediterranean shrub Atriplex halimus L? J. Plant Physiol. 161, 1041–1051. doi: 10.1016/j.jplph.2003.12.009
Marulanda A., Porcel R., Barea J. M., Azcón R. (2007). Drought tolerance and antioxidant activities in lavender plants colonized by native drought-tolerant or drought-sensitive glomus species. Microbial Ecol. 54, 543–552. doi: 10.1007/s00248-007-9237-y
Meena K. K., Sorty A. M., Bitla U. M., Choudhary K., Gupta P., Pareek A., et al. (2017). Abiotic stress responses and microbe-mediated mitigation in plants: The omics strategies. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.00172
Miller G., Shulaev V., Mittler R. (2008). Reactive oxygen signaling and abiotic stress. Physiologia Plantarum 133, 481–489. doi: 10.1111/j.1399-3054.2008.01090.x
Mori N., Nishiuma K., Sugiyama T., Hayashi H., Akiyama K. (2016). Carlactone-type strigolactones and their synthetic analogues as inducers of hyphal branching in arbuscular mycorrhizal fungi. Phytochemistry 130, 90–98. doi: 10.1016/j.phytochem.2016.05.012
Mukherjee S., Mishra A., Trenberth K. E. (2018). Climate change and drought: A perspective on drought indices. Curr. Climate Change Rep. 4, 145–163. doi: 10.1007/s40641-018-0098-x
Mukherjee A., Sarkar S., Parvin R., Bera D., Roy U., Gachhui R. (2020). Remarkably high Pb2+ binding capacity of a novel, regenerable bioremediator Papiliotrema laurentii RY1: Functional in both alkaline and neutral environments. Ecotoxicology Environ. Saf. 195, 110439. doi: 10.1016/j.ecoenv.2020.110439
Orfanoudakis M., Wheeler C. T., Hooker J. E. (2010). Both the arbuscular mycorrhizal fungus Gigaspora rosea and Frankia increase root system branching and reduce root hair frequency in Alnus glutinosa. Mycorrhiza 20, 117–126. doi: 10.1007/s00572-009-0271-0
Pandey A., Sharma M., Pandey G. K. (2016). Corrigendum: Emerging roles of strigolactones in plant responses to stress and development. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.00860
Pedranzani H., Rodríguez-Rivera M., Gutiérrez M., Porcel R., Hause B., Ruiz-Lozano J. M. (2016). Arbuscular mycorrhizal symbiosis regulates physiology and performance of Digitaria eriantha plants subjected to abiotic stresses by modulating antioxidant and jasmonate levels. Mycorrhiza 26, 141–152. doi: 10.1007/s00572-015-0653-4
Pieterse C. M. J., Leon-Reyes A., van der Ent S., Van Wees S. C. M. (2009). Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 5, 308–316. doi: 10.1038/nchembio.164
Porcel R., Aroca R., Azcón R., Ruiz-Lozano J. M. (2006). PIP Aquaporin Gene Expression in Arbuscular Mycorrhizal Glycine max and Lactuca sativa Plants in Relation to Drought Stress Tolerance. Plant Mol. Biol. 60, 389–404. doi: 10.1007/s11103-005-4210-y
Pozo M. J., López-Ráez J. A., Azcón-Aguilar C., García-Garrido J. M. (2015). Phytohormones as integrators of environmental signals in the regulation of mycorrhizal symbioses. New Phytol. 205, 1431–1436. doi: 10.1111/nph.13252
Rana S., Handa S., Aggarwal Y., Puri S., Chatterjee M. (2023). Role of Candida in the bioremediation of pollutants: A review. Lett. Appl. Microbiol. 76. doi: 10.1093/lambio/ovad103
Rapparini F., Peñuelas J. (2014). “Mycorrhizal fungi to alleviate drought stress on plant growth,” in Use of Microbes For The Alleviation Of Soil Stresses, vol. 1. (Springer, New York), 21–42. doi: 10.1007/978-1-4614-9466-9_2
Rhoads D. M., Umbach A. L., Subbaiah C. C., Siedow J. N. (2006). Mitochondrial reactive oxygen species. Contribution to oxidative stress and interorganellar signaling. Plant Physiol. 141, 357–366. doi: 10.1104/pp.106.079129
Ruiz-Lozano J. M., Aroca R., Zamarreño Á.M., Molina S., Andreo-Jiménez B., Porcel R., et al. (2016). Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ. 39, 441–452. doi: 10.1111/pce.12631
Ruiz-Lozano J. M., Azcón R. (1995). Hyphal contribution to water uptake in mycorrhizal plants as affected by the fungal species and water status. Physiologia Plantarum 95, 472–478. doi: 10.1111/j.1399-3054.1995.tb00865.x
Ruiz-Lozano J., Porcel R., Bárzana G., Azcón R., Aroca R. (2012). “Contribution of arbuscular mycorrhizal symbiosis to plant drought tolerance: State of the art,” in Plant Responses To Drought Stress (Springer, Berlin Heidelberg), 335–362. doi: 10.1007/978-3-642-32653-0_13
Ruiz-Sánchez M., Aroca R., Muñoz Y., Polón R., Ruiz-Lozano J. M. (2010). The arbuscular mycorrhizal symbiosis enhances the photosynthetic efficiency and the antioxidative response of rice plants subjected to drought stress. J. Plant Physiol. 167, 862–869. doi: 10.1016/j.jplph.2010.01.018
Sagi M., Fluhr R. (2006). Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 141, 336–340. doi: 10.1104/pp.106.078089
Sánchez-Romera B., Ruiz-Lozano J. M., Zamarreño Á.M., García-Mina J. M., Aroca R. (2016). Arbuscular mycorrhizal symbiosis and methyl jasmonate avoid the inhibition of root hydraulic conductivity caused by drought. Mycorrhiza 26, 111–122. doi: 10.1007/s00572-015-0650-7
Sarkar S., Kamke A., Ward K., Hartung E., Ran Q., Feehan B., et al. (2022a). Pseudomonas cultivated from Andropogon gerardii rhizosphere show functional potential for promoting plant host growth and drought resilience. BMC Genomics 23, 784. doi: 10.1186/s12864-022-09019-0
Sarkar S., Kamke A., Ward K., Rudick A. K., Baer S. G., Ran Q., et al. (2022b). Bacterial but Not Fungal Rhizosphere Community Composition Differ among Perennial Grass Ecotypes under Abiotic Environmental Stress. Microbiol. Spectr. 10. doi: 10.1128/spectrum.02391-21
Sarkar S., Mukherjee A., Parvin R., Das S., Roy U., Ghosh S., et al. (2019). Removal of Pb (II), As (III), and Cr (VI) by nitrogen-starved Papiliotrema laurentii strain RY1. J. Basic Microbiol. 59, 1016–1030. doi: 10.1002/jobm.201900222
Si W., Liang Q., Chen L., Song F., Chen Y., Jiang H. (2021). Ectopic overexpression of maize heat stress transcription factor zmHsf05 confers drought tolerance in transgenic rice. Genes 12, 1568. doi: 10.3390/genes12101568
Smith S. E., Smith F. A. (2011). Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 62, 227–250. doi: 10.1146/annurev-arplant-042110-103846
Song Y., Haney C. H. (2021). Drought dampens microbiome development. Nat. Plants 7, 994–995. doi: 10.1038/s41477-021-00977-z
Srivastava A. K., Singh S., Marathe R. A. (2002). Organic citrus: Soil fertility and plant nutrition. J. Sustain. Agric. 19, 5–29. doi: 10.1300/J064v19n03_03
Tarawneh R. A., Alqudah A. M., Nagel M., Börner A. (2020). Genome-wide association mapping reveals putative candidate genes for drought tolerance in barley. Environ. Exp. Bot. 180, 104237. doi: 10.1016/j.envexpbot.2020.104237
Tiwari S., Tiwari S., Singh M., Singh A., Prasad S. M. (2017). “Generation mechanisms of reactive oxygen species in the Plant cell,” in Reactive Oxygen Species In Plants (Hoboken, New Jersey, US: Wiley), 1–22. doi: 10.1002/9781119324928.ch1
Vaksmaa A., Guerrero-Cruz S., Ghosh P., Zeghal E., Hernando-Morales V., Niemann H. (2023). Role of fungi in bioremediation of emerging pollutants. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1070905
Wu Q.-S., He X.-H., Zou Y.-N., Liu C.-Y., Xiao J., Li Y. (2012). Arbuscular mycorrhizas alter root system architecture of Citrus tangerine through regulating metabolism of endogenous polyamines. Plant Growth Regul. 68, 27–35. doi: 10.1007/s10725-012-9690-6
Wu Q.-S., Srivastava A. K., Zou Y.-N. (2013). AMF-induced tolerance to drought stress in citrus: A review. Scientia Hortic. 164, 77–87. doi: 10.1016/j.scienta.2013.09.010
Wu Q. S., Wang Y. S., Xia R. X. (2006a). Comparison of Arbuscular Mycorrhizal Fungi for drought resistance of trifoliate orange ( Poncirus trifoliata L. Raf. ) seedlings. Acta Hortic. Sin. 33, 613–616.
Wu Q., Xia R. (2006a). Effects of arbuscular mycorrhizal fungi on leaf solutes and root absorption areas of trifoliate orange seedlings under water stress conditions. Front. Forestry China 1, 312–317. doi: 10.1007/s11461-006-0035-3
Wu Q.-S., Xia R.-X. (2006b). Arbuscular mycorrhizal fungi influence growth, osmotic adjustment and photosynthesis of citrus under well-watered and water stress conditions. J. Plant Physiol. 163, 417–425. doi: 10.1016/j.jplph.2005.04.024
Wu Q.-S., Xia R.-X., Zou Y.-N. (2006c). Reactive oxygen metabolism in mycorrhizal and non-mycorrhizal citrus (Poncirus trifoliata) seedlings subjected to water stress. J. Plant Physiol. 163, 1101–1110. doi: 10.1016/j.jplph.2005.09.001
Wu Q.-S., Xia R.-X., Zou Y.-N., Wang G.-Y. (2007a). Osmotic solute responses of mycorrhizal citrus (Poncirus trifoliata) seedlings to drought stress. Acta Physiologiae Plantarum 29, 543–549. doi: 10.1007/s11738-007-0065-y
Wu Q. S., Zou Y. N., Xia R. X. (2006b). Effects of water stress and arbuscular mycorrhizal fungi on reactive oxygen metabolism and antioxidant production by citrus (Citrus tangerine) roots. Eur. J. Soil Biol. 42, 166–172. doi: 10.1016/j.ejsobi.2005.12.006
Wu Q.-S., Zou Y.-N., Xia R.-X., Wang M.-Y. (2007b). Five Glomus species affect water relations of Citrus tangerine during drought stress. Botanical Stud. 48, 147–154.
Ying-Ning Z., Wu Q.-S., Li Y., Zou Y.-N. (2013). Effects of Diversispora spurca Inoculation on Growth, Root System Architecture and Chlorophyll Contents of Four Citrus Genotypes INTERNATIONAL JOURNAL OF AGRICULTURE & BIOLOGY Effects of Diversispora spurca Inoculation on Growth, Root System Architecture and Chlorophyll Contents of Four Citrus Genotypes. Int. J. Agric. Biol. 15, 342–346.
Yooyongwech S., Phaukinsang N., Cha-um S., Supaibulwatana K. (2013). Arbuscular mycorrhiza improved growth performance in Macadamia tetraphylla L. grown under water deficit stress involves soluble sugar and proline accumulation. Plant Growth Regul. 69, 285–293. doi: 10.1007/s10725-012-9771-6
Zhang B., Chang S. X., Anyia A. O. (2016). Mycorrhizal inoculation and nitrogen fertilization affect the physiology and growth of spring wheat under two contrasting water regimes. Plant Soil 398, 47–57. doi: 10.1007/s11104-015-2635-x
Zhang Y., Zhang Y., Luo L., Lu C., Kong W., Cheng L., et al. (2022). Genome wide identification of respiratory burst oxidase homolog (Rboh) genes in citrus sinensis and functional analysis of csRbohD in cold tolerance. Int. J. Mol. Sci. 23, 648. doi: 10.3390/ijms23020648
Zhao R., Guo W., Bi N., Guo J., Wang L., Zhao J., et al. (2015). Arbuscular mycorrhizal fungi affect the growth, nutrient uptake and water status of maize ( Zea mays L.) grown in two types of coal mine spoils under drought stress. Appl. Soil Ecol. 88, 41–49. doi: 10.1016/j.apsoil.2014.11.016
Zobel M., Öpik M. (2014). Plant and arbuscular mycorrhizal fungal (AMF) communities – which drives which? J. Vegetation Sci. 25, 1133–1140. doi: 10.1111/jvs.12191
Keywords: plants, AMF, drought, symbiosis, mechanism
Citation: Das S and Sarkar S (2024) Arbuscular mycorrhizal fungal contribution towards plant resilience to drought conditions. Front. Fungal Biol. 5:1355999. doi: 10.3389/ffunb.2024.1355999
Received: 14 December 2023; Accepted: 05 February 2024;
Published: 16 February 2024.
Edited by:
Shekhar Jain, Mandsaur University, IndiaReviewed by:
Matteo Chialva, University of Turin, ItalyCopyright © 2024 Das and Sarkar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Subhadeep Das, subhadeepdas207@gmail.com; Soumyadev Sarkar, sarkar.soumyadev@gmail.com
 Subhadeep Das
Subhadeep Das