
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Fungal Biol. , 21 December 2023
Sec. Fungi-Plant Interactions
Volume 4 - 2023 | https://doi.org/10.3389/ffunb.2023.1276287
Brazil has a long history of using biological control and has the largest program in sugarcane agriculture to which a biocontrol program has been applied. This achievement is at least partly due to the utilization of the entomopathogenic fungus Metarhizium. This well-known fungal genus exhibits pathogenicity against a broad range of arthropod hosts and has been used globally as a biocontrol agent. This fungus is also a root symbiont, and in this capacity, it is a plant growth promoter. However, this feature (i.e., as a plant symbiont) has yet to be fully explored and implemented in Brazil, although the number of reports demonstrating Metarhizium’s utility as a plant bioinoculant is increasing. The Brazilian bioproduct industry targets agricultural pests, and is limited to two Metarhizium species represented by four fungal isolates as active ingredients. Entomopathogenic fungi have also been successful in controlling arthropods of public health concern, as shown in their control of mosquitoes, which are vectors of diseases. The isolation of new indigenous Metarhizium isolates from a variety of substrates such as soil, insects, and plants shows the wide genetic diversity within this fungal genus. In this review, we emphasize the significance of Metarhizium spp. for the biological control of insects in Brazil. We also suggest that the experience and success of biological control with fungi in Brazil is an important resource for developing integrated pest management and sustainable strategies for pest control worldwide. Moreover, the future implementation prospects of species of Metarhizium being used as bioinoculants and possible new advances in the utility of this fungus are discussed.
Metarhizium is a genus of entomopathogenic fungi in the family Clavicipitaceae, order Hypocreales. These fungi play multiple roles, as endophytes, saprobes, and pathogens of insects (Stone and Bidochka, 2020). Phylogenetic analysis showed that Metarhizium and Pochonia chlamydosporia form a monophyletic clade that evolved from the plant root symbionts Claviceps and Epichloë approximately 300 million years ago (MYA), and then diverged with pathogenic ability against nematodes and insects approximately 180 MYA (Sheng et al., 2022). In addition to this, there have been more recent studies carried out on entomopathogenic fungi as endophytes. Vega (2018) highlighted entomopathogenic fungal–plants interactions to integrate aspects of endophytism with insect pathogenesis in an applied sense. However, there is limited research on the effects of fungus-inoculated plants on arthropod pests in Brazil.
Based on the insect host range, Metarhizium species have been classified as generalists with broad host ranges and specialists with narrow host ranges (Gao et al., 2011; St Leger and Wang, 2020). For example, Metarhizium acridum was classified as a specialist pathogen restricted to Orthoptera (Wang et al., 2016), and generalists such as Metarhizium anisopliae infect a wide spectrum of insect hosts in the orders Lepidoptera, Coleoptera, Hemiptera, and Orthoptera (Balachander et al., 2009).
Both the generalist and specialist Metarhizium insect pathogens retain their ancestral ability to colonize plant roots (Moonjely and Bidochka, 2019). As plant symbionts, Metarhizium can improve plant growth (Ahmad et al., 2020; Hu et al., 2023), resist plant pathogens (Sasan and Bidochka, 2013; Gupta et al., 2022), and ameliorate salt stress (Chaudhary et al., 2023). As a bioremediator, Metarhizium can alleviate heavy metal pollution of mercury in soil and water (Wu et al., 2022) and enhance the cadmium efflux capacity of plants (Jiang et al., 2022).
With recent developments in the application of Metarhizium as a biocontrol agent, this review will focus on the utility and potential prospects of Metarhizium as a mycoinsecticide and plant bioinoculant in Brazil.
There is accumulating knowledge of the diversity and abundance of indigenous Brazilian strains (Mesquita et al., 2020; Couceiro et al., 2022; Diniz et al., 2021). According to Luz et al. (2019), M. robertsii, Metarhizium humberi, and M. anisopliae sensu stricto (s. str.) are abundant in Brazilian soils. The Metarhizium spp. diversity was explored using the nuclear intergenic region MzIGS3 (Kepler and Rehner, 2013) collected from several Brazilian ecological biomes (Amazon, Caatinga, Cerrado, Atlantic Forest, and Pampa) in the dry and humid seasons (Riguetti Zanardo Botelho et al., 2019). This study showed that Metarhizium spp. occurrence is correlated with Brazilian biomes, that is, M. robertsii was the only species identified in the Pampas biome, while the taxonomically uncharacterized “Metarhizium sp. indet. 3” was identified mostly in the Caatinga biome. Currently, M. humberi (referred to as Metarhizium sp. indet. 1 in the study) is the most diverse haplotype, and, interestingly, the haplotypes identified from the Cerrado biome soils were entirely different from those identified from soils in the Amazon biome. The haplotype diversity of M. humberi has also been noted in previous studies (Rocha et al., 2013; Lopes et al., 2014; Rezende et al., 2015). According to Riguetti Zanardo Botelho et al. (2019), the Amazon biome was the only one where all Metarhizium spp. were identified, which is not unexpected as it holds great ecological diversity. These authors confirmed a great abundance of M. robertsii in soils, which is in agreement with Iwanicki et al. (2019). However, for M. anisopliae, it was suggested that in Brazil, the occurrence of this species was strongly correlated with arthropod hosts (Riguetti Zanardo Botelho et al., 2019; Rezende et al., 2015). The highest occurrence of M. anisopliae was detected by Rezende et al. (2015) in a diverse group of environments, that is, in soils from different biomes and insects.
The diversity of Metarhizium spp. identified in agricultural and non-agricultural habitats has revealed the predominance of M. anisopliae sensu lato (Mani 2 subclade) in sugarcane fields, while M. humberi (Metarhizium sp. indet. 1) was predominantly found in the undisturbed soils of native plant communities (Rezende et al., 2015). Moreover, regarding the natural occurrence of Metarhizium spp. in Brazilian soils, M. brunneum and M. pingshaense were detected in a strawberry field previously treated with two different Metarhizium spp. (Castro et al., 2016). Within these four species, the authors identified two additional M. anisopliae haplotypes, five M. robertsii haplotypes, and one each of Metarhizium brunneum and Metarhizium pingshaense.
The genetic and biochemical basis of the ability of Metarhizium to penetrate the insect cuticle is well known (Wang et al., 2016; Beys-da-Silva et al., 2020; Hong et al., 2023). After the conidium attaches to the insect cuticle, a germ tube is formed and terminates in an appressoria. From this structure, a penetration peg is formed, and through mechanical and enzymatic action (i.e., secreted proteases, chitinases, and lipases) (Zimmermann, 2007), the cuticle is breached, and the fungus reaches the arthropod hemolymph. Once inside the nutrient-rich hemocoel, the fungus grows and forms hyphal bodies termed blastospores. Blastospores can evade insect immune responses by producing a collagenous coat (Wang and St. Leger, 2006)) and producing an array of toxins and secondary metabolites that leads to arthropod death (Zimmermann, 2007).
Mycoinsecticides based on M. anisopliae s. str. in Brazil target the following insects: the spittlebugs Mahanarva fimbriolata, Deois flavopicta, and Zulia entreriana (Mascarin et al., 2019), while two products based on Metarhizium rileyi target the fall armyworm Spodotera frugiperda (Agrofit, 2023). However, Metarhizium spp. reportedly infect a broader range of insects in Brazil. Examples of the studies reporting the diversity of Metarhizium spp. in terms of their infecting a variety of insects are found in Table 1. For instance, the generalist M. anisopliae has been used to control arthropods important for public health such as Aedes aegypti larvae (Oliveira Barbosa Bitencourt et al., 2021; Gomes et al., 2023) and the Chagas disease vector Triatoma infestans (Rangel et al., 2020). More recently, less common Metarhizium spp. have been shown to infect other arthropod hosts. For example, Metarhizium marquandii demonstrated virulence against the termite Nasutitermes sp. (Diniz et al., 2021), and Metarhizium braziliense infected the corn leafhopper Dalbulus maidis (Hemiptera: Cicadellidae) naturally in maize crops (Souza et al., 2021). Furthermore, Metarhizium spp. infections in ticks have been reported, both in the field and in semi-field conditions, demonstrating biocontrol results for Rhipicephalus microplus (Camargo et al., 2016; Marciano et al., 2021; Carneiro et al., 2022) and Rhipicephalus sanguineus (Reis et al., 2008) in Brazil.
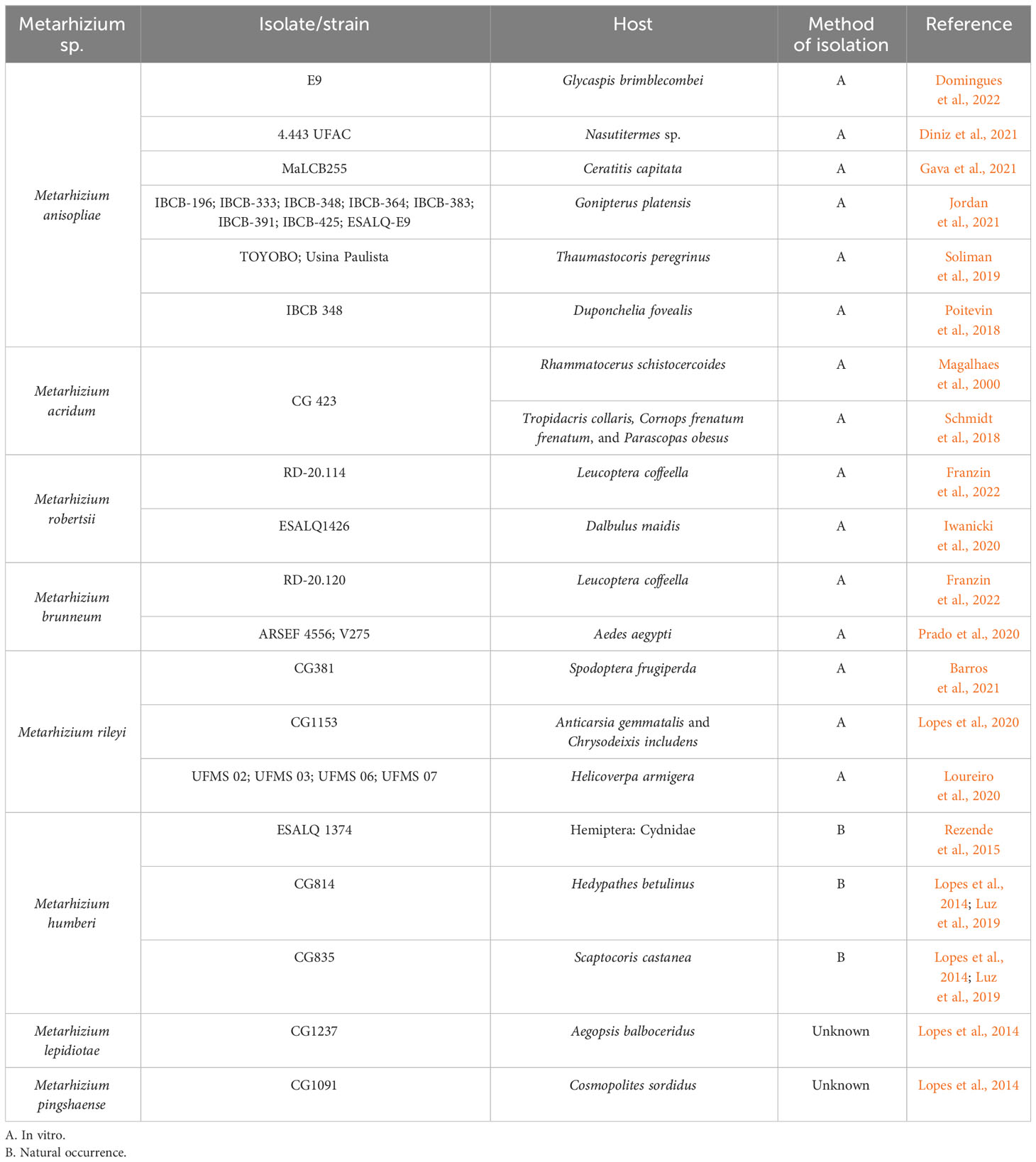
Table 1 Diversity of Metarhizium spp. and strains infecting different species of arthropods in Brazilian territory.
Metarhizium spp. are ecologically soil-borne fungi (Jaronski, 2007), and many have been demonstrated to be rhizosphere competent (Hu and St. Leger, 2002; Hu and Bidochka, 2021a), and these features can be exploited in biocontrol efforts (Bamisile et al., 2023). For example, in a sugarcane fields, an indigenous M. anisopliae strain—ESALQ 1604—persisted for up to 60 days after a soil drench application (Iwanicki et al., 2019). In a semi-field experiment, a native strain of M. anisopliae LCM S04 was shown to persist for up to 5 months post inoculation in soil in switchgrass pots (Mesquita et al., 2020). Additionally, in soil in which strawberry crops were grown, Metarhizium persistence was detected up to 1 year post treatment (Castro et al., 2016). According to Iwanicki et al. (2019), M. brunneum shows greater association with the rhizosphere than with bulk soil. In the same study, in addition to the spittlebugs that were infected with M. brunneum ESALQ 1604, endemic strains of M. anisopliae were found to infect up to 50% of the spittlebugs collected in the field. In Brazil, there is still limited information on the association of Metarhizium spp. with plant roots. It has been recovered from roots of strawberry (Canassa et al., 2020), sugarcane (Iwanicki et al., 2019), tomato (Siqueira et al., 2020), coffee (Franzin et al., 2022), grass (Marciano et al., 2021), peanut (Vinha et al., 2023), and soybean (Holz et al., 2023) (Figure 1). The recognition, connection, and relevance of these studies are shown in Supplementary Figure S1. Although not common, Metarhizium spp. were isolated in Goiás state from aquatic habitats (i.e., small- to medium-sized water bodies and lakes and rivers), where A. aegypti larvae were found (Rocha et al., 2022). The aquatic environment is suggested to be important for conidial recycling, as mosquito egg rafts are found on the surface of water bodies and mycosed mosquito larvae float on the surface of water bodies.
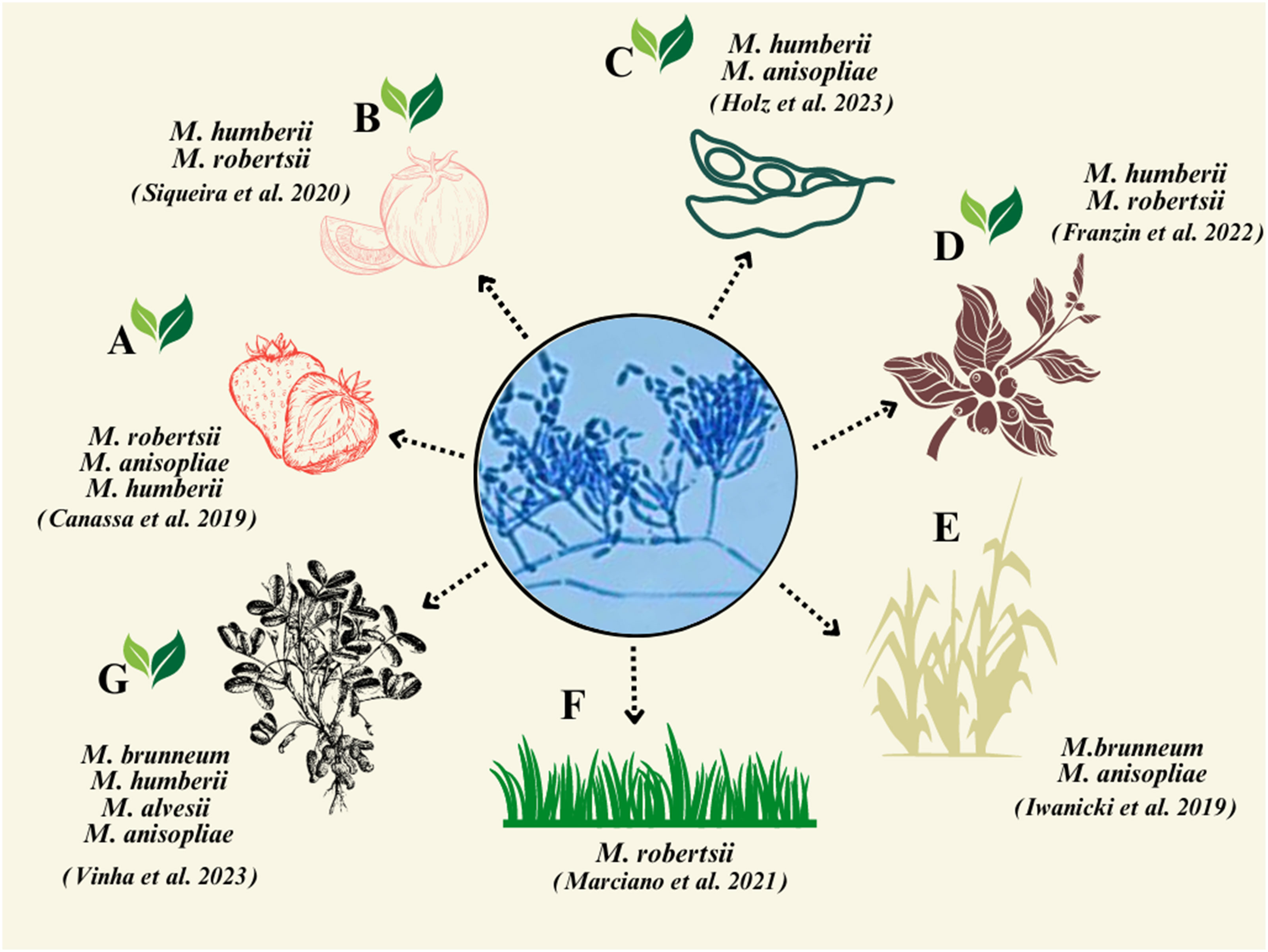
Figure 1 Representation of plant species studied for the isolation of Metarhizium spp. demonstrating the corresponding fungal species isolated from agricultural plants in Brazil. (A) Strawberry; (B) tomato; (C) soybean; (D) coffee; (E) sugarcane; (F) switchgrass; and (G) peanut. The green leaves indicate dicotyledons plants.
One of the first reports of entomopathogenic fungi (probably of Metarhizium) killing crop insect pests in Brazil was done by Pestana (1923), who described sugarcane spittlebugs and their muscardine disease in Minas Gerais State (southeast Brazil). Because of the increasing occurrence of the sugarcane leaf spittlebug Mahanarva posticata in the northeastern states of Brazil in the 1960s and 1970s (Marques and Vilas Boas, 1978), along with reports of natural epizootics of the green muscardine disease in insects caused by Metarhizium across the country (Alves, 1998a), Metarhizium became a key subject in research and extension projects of several Brazilian government institutions (Li et al., 2010). An individual who was particularly instrumental in developing fungal biocontrol in Brazil was Dr. Donald W. Roberts (in memoriam), who received several Brazilian awards for his efforts and whose work is considered crucial to the success of the biological control narrative in Brazil. He was engaged in several projects in the country, especially at Embrapa Arroz e Feijão in Goiás state, where the work began, and supervised Brazilian students and researchers for many years. In recognition of his contributions to biocontrol efforts and to fundamental research, M. robertsii was named after him.
According to the Food and Agriculture Organization of the United Nations (FAO), Brazil is one of the world’s largest producers of agricultural and livestock commodities, including rice, barley, corn, soy, wheat, and beef (FAOSTAT, 2021). Brazil’s boom in agriculture production is claimed to have started with the “Green Revolution” (Nehring, 2022). Although using fertilizers and pesticides represents a major part of the crop production landscape, insecticide/acaricide resistance and pesticide residue are consequential environmental and health risks in Brazil and worldwide (Deguine et al., 2021.; Valentim et al., 2023). These concerns can be traced back many decades when the Brazilian government started seeking sustainable and safer alternatives for arthropod pest control, including the use of entomopathogenic fungi.
Since the mid 1900s, Metarhizium has been mass produced in Brazil, first using 1-L glass bottles that were later replaced by autoclavable plastic bags (Aquino et al., 1977; Alves, 1988b). Public and private research institutions have been working on developing more efficient and low-cost methods capable of large-scale, economical production of these fungi (Mascarin et al., 2015; Mascarin et al., 2019). In addition to low production costs, several factors are involved in the high acceptance of the use of entomopathogenic fungi for insect pest control in Brazil, including (i) effectiveness (Iwanicki et al., 2019), (ii) standard registration protocol, and (iii) on-farm production (on-farm production is defined as the production of beneficial microorganisms by growers exclusively for their own use) (Faria et al., 2023). Both solid-state (i.e., production of aerial conidia) and submerged liquid fermentations (i.e., production of hyphal bodies and/or blastospores) have been reported by Brazilian farmers. However, solid-state production is the most widely practiced form of fungal production (Faria et al., 2023). Only fungal-based products registered with the ANVISA that are manufactured or imported by companies authorized and licensed by the government may be commercialized in Brazil. Despite this, some of the current issues with mycoinsecticides in the country rely on the illegal production and distribution of non-registered products (Mascarin et al., 2019). These products usually do not undergo quality control during production, or shelf-life tests before distribution, resulting in low credibility. The reports of this condition have been addressed by Li et al. (2010). These authors also highlighted the program of pasture spittlebug control by M. anisopliae where the control rate was not satisfactory. Although most Metarhizium-based products are registered to control agriculture insect pests, some of these mycoinsecticides have been successfully tested against ticks under laboratory and field conditions (Camargo et al., 2014; Camargo et al., 2016; Nogueira et al., 2020). In addition to the existing commercial Metarhizium products, a wide variety of other Metarhizium fungal isolates have been tested against ticks in Brazil (Quinelato et al., 2012; Alves et al., 2017; Bernardo et al., 2018; Jones et al., 2021).
The use of native isolates of Metarhizium for research and technological development in Brazil is now regulated by the new biodiversity law established in 2015 (Law 13, 123). This law considers any microorganism isolated in the country as part of Brazilian genetic heritage, including Metarhizium spp. isolates (da Silva and de Oliveira, 2018). According to the law, researchers need to register their access to Metarhizium species in an online system (National System for the Management of Genetic Heritage and Associated Traditional Knowledge—SisGen) before disseminating results, shipment, and application for intellectual propriety. Some authors claim that there are positive aspects of this law (these pertain to its protection of Brazilian biodiversity), whereas others have expressed concern about research bureaucratization and barriers to basic research and international collaboration (da Silva and de Oliveira, 2018; Alves et al., 2018).
Metarhizium spp. are reported as plant growth promoters, root colonizers, and endophytes (Garcia et al., 2011; Wyrebek et al., 2011; Hu and Bidochka, 2021a), and have the ability to protect plants from phytopathogenic fungi and can affect insect pest feeding and oviposition behavior in inoculated plants (Sasan and Bidochka, 2013; Canassa et al., 2020; Hao et al., 2021). Plant recognition of Metarhizium spp. as a beneficial symbiont may occur through the downregulation of plant defense mechanisms (Hu and Bidochka, 2021b) and decreases in plant oxidative responses, for example, soybean under salinity stress (Khan et al., 2012). However, studies of plant association with Metarhizium are more recent than the long-term studies of these entomopathogenic fungi in insect pest control programs. In Brazil, recent publications have started to analyze the diversity of native Brazilian strains in association with soil and plants and assess the potential effects on plant health and growth. While the development of these fungi as plant bioinoculants is still in its early stages, such research efforts are essential to study the feasibility and future use of Metarhizium spp. for plant growth promotion.
Seed treatment and direct soil drenching are usually successful in establishing fungi as rhizoplane colonizers and as endophytes. In the coffee plant (Coffea arabica), a study by Franzin et al. (2022) found that a soil drench with conidial suspensions promoted plant growth and provided protection against the coffee leaf miner (Leucoptera coffeella) using the Brazilian isolates M. robertsii (RD-20.114) and M. brunneum (RD-20.120) (Franzin et al., 2022). The application of M. robertsii significantly increased the coffee leaf area and suppressed foliar damage by the coffee leaf miner. This study reported that female insects that emerged from the plants inoculated with M. robertsii produced half the number of eggs produced by those from control plants. The inoculation method was successful in establishing both species in the root area for up to 43 days, although this study did not differentiate between rhizoplane soil or plant tissue when assessing colonization. Soil inoculation with Brazilian isolates of M. robertsii and M. humberi also promoted growth in tomato (Solanum lycopersicum L., ‘Micro Tom’ variety), with significant effects reported for M. robertsii ESALQ 1635 (Siqueira et al., 2020). After 30 days, the plants inoculated with M. robertsii showed a significant increase in traits such as height, root length, root weight, and overall biomass compared with controls, as well as a larger number of flowers and increased fruit weight. Both species were retrieved from rhizoplane soils and, interestingly, were also found to colonize the plant endophytically in all tissues, although a higher level of colonization was observed in the roots. This has also been observed in some studies that reisolated Metarhizium spp. from aboveground tissues following plant inoculations, although usually at lower levels than in the root region (Garcia et al., 2011; Jaber and Enkerli, 2016; Ahmad et al., 2020). Siqueira et al. (2020) also analyzed these strains of Metarhizium for certain biochemical traits and observed that the levels of phosphorus solubilization and plant hormone indole-3-acetic acid (IAA) production were comparable to those observed in a commercial strain of Trichoderma harzianum. T. harzianum is a well-known plant growth promoter that is widely used in Brazil, mostly as a biological control agent for its antagonistic interactions with soil-dwelling phytopathogenic fungi and nematodes (Nascimento et al., 2022).
In addition to Trichoderma spp., Metarhizium spp. have also been reported for their antagonistic performance against other fungi. Holz et al. (2023) recently described the ability of two Brazilian Metarhizium isolates to protect host plants from the fungal pathogen, Phakopsora pachyrhizi, the causal agent of Asian soybean rust. Soil drench applications of the M. robertsii Brazilian strain MHBR-03 later resulted in a significant decrease in rust disease symptoms in soybean following the foliar spray application of P. pachyrhizi spores on plants. Interestingly, foliar applications of Metarhizium cell-free culture filtrates also showed a degree of protection against the symptoms of rust in vivo and affected P. pachyrhizi development in vitro, which is an indication that metabolites produced by M. robertsii and released into the aqueous media could be responsible for rust inhibition, either directly or indirectly by activating plant defense mechanisms. Although further investigation was not performed to elucidate this particular finding, entomopathogenic fungi are known for their production of secondary metabolites that can potentially inhibit phytopathogens (Lozano-Tovar et al., 2017; Wei et al., 2022).
In addition to soil drench, seed treatments have been reported as an effective method for the application of Metarhizium spp. as plant bioinoculants. Bean (Phaseolus vulgaris) seed inoculations with a Brazilian strain of M. robertsii (ESALQ 1622) resulted in increased plant growth, root area, and aerial weight in treated plants (Canassa et al., 2019). It was possible to reisolate M. robertsii only from root rhizoplane with low levels of endophytic colonization. M. robertsii is a well-known plant rhizoplane colonizer and endophyte (Liao et al., 2014; Behie et al., 2015; Barelli et al., 2018). This fungus also promoted indirect protection against the spider mite (Tetranychus urticae), a primary pest mite commonly found in beans and other crops, which had a lower rate of population growth in inoculated plants (Canassa et al., 2019). The same species of mite was also suppressed in strawberry plants (Fragaria × ananassa) following root inoculation with Metarhizium spp., with lower oviposition rates by female mites (Canassa et al., 2020). The authors described plant improvements associated with fungal inoculations, such as increased fruit yield and overall plant growth, with effects that varied among different species and isolates. Once more, a comparison with commercial strains showed that the plant growth-promoting abilities of several Metarhizium isolates were comparable to those of T. harzianum, Bacillus subtilis, and Bacillus licheniformis, which are active ingredients in commercial agricultural products.
A novel application strategy using seed treatments to establish Metarhizium as a plant bioinoculant was investigated by Lira et al. (2020), who selected three Brazilian isolates for their ability to produce microsclerotia, and used this propagule for corn plant colonization. Microsclerotia are resistant fungal structures which, given their hardiness and ability to withstand desiccation, have been studied as potential active ingredients in microbial bioinoculants in a variety of fungal species (Kobori et al., 2015; Huarte-Bonnet et al., 2019; Marciano et al., 2021; Rodrigues et al., 2021). Seed coating using microsclerotia granules with Brazilian isolates of M. humberi, M. anisopliae, and M. robertsii influenced plant traits, such as root length, plant dry weight (Lira et al., 2020), and mortality of the fall armyworm (S. frugiperda) larvae when feeding on treated plants. However, fungal inoculation did not affect the mortality of the leafhopper, D. maidis, and this could be due to the differences in the feeding behaviors of the two insects. Leafhoppers are Hemiptera, with sucking mouth parts, while armyworm larvae are Lepidoptera, with chewing mouth parts. This study highlighted the potential of microsclerotia not only in biopesticide formulations, but also in seed treatments aiming to establish fungal colonization in host plants.
As more studies investigate Metarhizium spp. with a focus on their relationship with host plants, the potential of these fungi beyond their use as entomopathogens is being revealed. Different species and strains of Metarhizium interact differently with plant hosts, which shows the importance of strain selection for specific objectives when developing novel biological control tools. The majority of Metarhizium-based products commercially available in Brazil are used specifically as topical sprays against insect pests, meaning that they may not be optimal candidates as plant bioinoculants; however, this assertion is currently underexplored.
With six distinct biomes and a vast land area, Brazil has a huge variety of naturally occurring strains of Metarhizium both in natural and agricultural areas (Rocha et al., 2013; Riguetti Zanardo Botelho et al., 2019; Couceiro et al., 2022), many of which have been isolated from soils and in association with plants, and which could therefore be explored for their potential as plant growth promoters. The results described earlier in this section show the high levels of genetic diversity within M. robertsii, M. humberi, and M. anisopliae, and exemplify how this genetic variability could be explored by Brazilian biopesticide producers for the development of Metarhizium as a plant bioinoculant.
The registration of biological products in Brazil is regulated by the Brazilian Health and Surveillance Agency (ANVISA) under resolution RDC 55/2010 (Brazil Official Union Diary). According to the Brazilian Ministry of Agriculture, Livestock and Supply, there are 91 registered products based on Metarhizium spp. (alone or with other biological agents) (Agrofit, 2023). Most of these products have M. anisopliae as their active ingredient, that is, M. anisopliae IBCB 425 has 87 registered products, M. rileyi CCT7771 has two products, and M. anisopliae IBCB 348 and M. anisopliae have one product each. These products are essentially directed to the control of spittlebug species, as previously mentioned. The use of Metarhizium against sugarcane insect pests in Brazil is considered one of the most successful biological control programs in the world, with millions of hectares treated annually (Parra, 2014; Iwanicki et al., 2019; Mascarin et al., 2019). According to the Brazilian Ministry of Agriculture, Livestock and Supply there are 3,293 registered pesticides. Of these, 593 are based on biological organisms (i.e., microbiological insecticide/acaricide/bactericide/fungicide/nematicide or other biological control agents), which constitute approximately 18% of the pesticide market. Mycoinsecticides and mycoacaricides based on Metarhizium and Beauveria spp. represent approximately 6% of the pesticide market (198 products). Although this review focused on Metarhizium spp., Beauveria bassiana products constitute up to 101 registered pesticide products according to government data (Agrofit, 2023). Commercial products in Brazil must rely on formulation techniques and consider environmental conditions that may impair fungal biology, such as temperature, UV radiation, and humidity (Acheampong et al., 2020a; Acheampong et al., 2020b). Although the microbial control business in Brazil is continuously increasing, available products in the market are mainly based on wettable powder formulations and the addition of oil as an adjuvant (Faria and Wraight, 2007; Mascarin et al., 2019). Unfortunately, industry places little emphasis on shelf life and technologies that improve insect pathogenicity and delivery. There is a perceived lack of investment and interest in formulation research to boost efficacy, although the relevance of this improvement has already been reported (Vemmer and Patel, 2013; Iwanicki et al., 2021; Marciano et al., 2021; Meirelles et al., 2023). Nonetheless, this is not peculiar to Brazil, especially due to the time required to approve a novel formulation. Biocontrol companies worldwide follow similar patterns. In addition to this, more recently, a report on the product ATTRACAP® (BIOCARE GmbH, Germany) described its efficacy against wireworms (Coleoptera: Elateridae) in an “attract-and-kill” strategy (Gvozdenac et al., 2022). This granular formulation is a M. brunneum-based bead constituted with alginate (polymer), starch (nutrient), and Saccharomyces cerevisiae (CO2 source) (Working Group Patel, 2023).
The majority of the mass production of Metarhizium is done using solid substrate fermentation with cereal grains and rice, with the aim of producing high yields of aerial conidia (Jaronski, 2013; Mascarin et al., 2019; Jaronski, 2022; Rangel et al., 2023). In addition to this, liquid culture fermentation yielding blastospores and hyphal bodies has been studied, as it has a better cost-to-benefit ratio and faster production (Mascarin et al., 2015; Mascarin et al., 2019). The biggest concern around the use of blastospores is their suggested low tolerance to abiotic factors. However, Bernardo et al. (2020) have compared conidia and blastospores of Metarhizium spp. and Beauveria bassiana with respect to susceptibility to UV-B and heat stress. Their study showed that blastospores of B. bassiana CG 307 exhibited higher tolerance to heat than conidia, while M. robertsii and M. anisopliae blastospores and conidia were equally tolerant to UV-B.
Brazil has a well-established agricultural market and is an international leader in insect biocontrol, particularly with regard to sugarcane. However, there is a paucity of information on the use of Metarhizium as a plant growth promoter. Given Brazil’s geography and biome diversity, there is an abundance of and diversity within Brazilian isolates of Metarhizium that is currently underexplored. The Brazilian government has astutely protected this diversity, which could also serve as a potential export resource and could benefit the agricultural market in neighboring countries in South America. In this study, we highlighted Brazilian products commercially available based on Metarhizium that rely mostly on only four Metarhizium isolates among the 91 registered products. This approach, however, underrepresents the variety of species and underexplored genetic diversity found in Brazil. Farmers and bioproduct business owners could better assess and potentially exploit the diversity of Metarhizium not only as insect pathogens but also as plant bioinoculants. Moreover, the widespread use of biological control agents and bioinoculants for both pest control and plant improvement could benefit Brazil’s agroindustry. According to Guida et al. (2018), Brazil has been the number one user of agrochemicals globally since 2008. The application of bioproducts could support and diversify agroindustry in Brazil as well as affording benefits to human health and a sustainable environment.
EM: Writing – original draft, Writing – review & editing. SH: Writing – original draft. TL: Writing – original draft. PG: Writing – original draft, Writing – review & editing. MB: Conceptualization, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The National Council for Scientific and Technological Development(CNPq) of Brazil provided a postdoctoral scholarship for EM under the call CNPq 16/2020.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffunb.2023.1276287/full#supplementary-material
Supplementary Figure 1 | Map of citations per paper according to the studies presented in Table 1 and Figure 1. Each circle correlates to one paper. The grouped circles represent the connection of papers by similarity. The number of citations and importance of papers are demonstrated by the size of the circle and the color demonstrated in the legend.
Acheampong M. A., Coombes C. A., Moore S. D., Hill M. P. (2020a). Temperature tolerance and humidity requirements of select entomopathogenic fungal isolates for future use in citrus IPM programmes. J. Invertebr Pathol. 174, 107436. doi: 10.1016/j.jip.2020.107436
Acheampong M. A., Hill M. P., Moore S. D., Coombes C. A. (2020b). UV sensitivity of Beauveria bassiana and Metarhizium anisopliae isolates under investigation as potential biological control agents in South African citrus orchards. Fungal Biol. 124, 304–310. doi: 10.1016/j.funbio.2019.08.009
Agrofit (2023) Agrofit - sistema de agrotóxicos fitossanitários - ministério da agricultura, pecuária e abasteciment, 2023. (Accessed 8 Jun 2023).
Ahmad I., Del Mar Jiménez-Gasco M., Luthe D. S., Barbercheck M. E. (2020). Systemic colonization by Metarhizium robertsii enhances cover crop growth. J. Fungi 6, 64. doi: 10.3390/jof6020064
Alves F. M., Bernardo C. C., Paixão F. R. S., Barreto L. P., Luz C., Humber R. A., et al. (2017). Heat-stressed Metarhizium anisopliae: viability (in vitro) and virulence (in vivo) assessments against the tick Rhipicephalus sanguineus. Parasitol. Res. 116, 111–121. doi: 10.1007/s00436-016-5267-z
Alves R. J. V., Weksler M., Oliveira J. A., Buckup P. A., Pombal J. P., Santana H. R. G., et al. (2018). Brazilian legislation on genetic heritage harms biodiversity convention goals and threatens basic biology research and education. Acad. Bras. Cienc 90, 1279–1284. doi: 10.1590/0001-3765201820180460
Alves S. B. (1988a). “Patologia e controle microbiano: vantagens e desvantagens,” in Controle microbiano de insetos. Ed. Alves S. B. (Piracicaba: FEALQ), 21–37.
Alves S. B. (1988b). “Fungos entomopatogênicos,” in Controle microbiano de insetos. Ed. Alves S. B. (Piracicaba: FEALQ), 289–381.
Aquino M. L. N., Vital A. F., Cavalcanti V. L. B., Nascimento M. G. (1977). “Cultura de metarhizium anisopliae (Metsch.) sorokin em sacos de polipropileno,” in Boletim técnico CODECAP, vol. 5, 7–11.
Balachander M., Remadevi O. K., Sasidharan T. O., Sapna Bai N. (2009). Infectivity of Metarhizium anisopliae (Deuteromycotina: Hyphomycetes) isolates to the arboreal termite Odontotermes sp. (Isoptera: Termitidae). Int. J. Trop. Insect Sci. 29, 202–207. doi: 10.1017/S1742758409990294
Bamisile B. S., Afolabi O. G., Siddiqui J. A., Xu Y. (2023). Endophytic insect pathogenic fungi-host plant-herbivore mutualism: elucidating the mechanisms involved in the tripartite interactions. World J. Microbiol. Biotechnol. 39, 326. doi: 10.1007/s11274-023-03780-4
Barelli L., Moreira C. C., Bidochka M. J. (2018). Initial stages of endophytic colonization by Metarhizium involves Rhizoplane colonization. Microbiol. (United Kingdom) 164, 1531–1540. doi: 10.1099/mic.0.000729
Barros S. K. A., de Almeida E. G., Ferreira F. T. R., Barreto M. R., Lopes R. B., Pitta R. M. (2021). Field efficacy of Metarhizium rileyi applications against Spodoptera frugiperda (Lepidoptera: Noctuidae) in maize. Neotropl Entomol 50, 976–988. doi: 10.1007/s13744-021-00903-0
Behie S. W., Jones S. J., Bidochka M. J. (2015). Plant tissue localization of the endophytic insect pathogenic fungi Metarhizium and Beauveria. Fungal Ecol. 13, 112–119. doi: 10.1016/j.funeco.2014.08.001
Bernardo C. C., Barreto L. P., C. de S. R., Luz C., Arruda W., Fernandes É.K.K. (2018). Conidia and blastospores of Metarhizium spp. and Beauveria bassiana s.l.: Their development during the infection process and virulence against the tick Rhipicephalus microplus. Ticks Tick Borne Dis. 9, 1334–1342. doi: 10.1016/j.ttbdis.2018.06.001
Bernardo C., das C., Pereira-Junior R. A., Luz C., Mascarin G. M., Kamp Fernandes É.K. (2020). Differential susceptibility of blastospores and aerial conidia of entomopathogenic fungi to heat and UV-B stresses. Fungal Biol. 124, 714–722. doi: 10.1016/j.funbio.2020.04.003
Beys-da-Silva W. O., Rosa R. L., Berger M., Coutinho-Rodrigues C. J., Vainstein M. H., Schrank A., et al. (2020). Updating the application of Metarhizium anisopliae to control cattle tick Rhipicephalus microplus (Acari: Ixodidae). Exp. Parasitol. 208, 107812. doi: 10.1016/j.exppara.2019.107812
Camargo M. G., Marciano A. F., Sá F. A., Perinotto W. M. S., Quinelato S., Gôlo P. S., et al. (2014). Commercial formulation of Metarhizium anisopliae for the control of Rhipicephalus microplus in a pen study. Vet. Parasitol. 205, 271–276. doi: 10.1016/j.vetpar.2014.07.011
Camargo M. G., Nogueira M. R. S., Marciano A. F., Perinotto W. M. S., Coutinho-Rodrigues C. J. B., Scott F. B., et al. (2016). Metarhizium anisopliae for controlling Rhipicephalus microplus ticks under field conditions. Vet. Parasitol. 223, 38–42. doi: 10.1016/j.vetpar.2016.04.014
Canassa F., Esteca F. C. N., Moral R. A., Meyling N. V., Klingen I., Delalibera I. (2020). Root inoculation of strawberry with the entomopathogenic fungi Metarhizium robertsii and Beauveria bassiana reduces incidence of the twospotted spider mite and selected insect pests and plant diseases in the field. J. Pest Sci. (2004) 93, 261–274. doi: 10.1007/s10340-019-01147-z
Canassa F., Tall S., Moral R. A., Lara I. A. R., Delalibera I., Meyling N. V. (2019). Effects of bean seed treatment by the entomopathogenic fungi Metarhizium robertsii and Beauveria bassiana on plant growth, spider mite populations and behavior of predatory mites. Biol. Control 132, 199–208. doi: 10.1016/j.biocontrol.2019.02.003
Carneiro A., da S., Mesquita E., Meirelles L. N., Bittencourt V. R. E. P., Golo P. S. (2022). Compatibility of different Metarhizium spp. propagules with synthetic acaricides for controlling Rhipicephalus microplus. Rev. Bras. Parasitol. Vet. 31, e018221. doi: 10.1590/S1984-29612022018
Castro T., Mayerhofer J., Enkerli J., Eilenberg J., Meyling N. V., Moral R., et al. (2016). Persistence of Brazilian isolates of the entomopathogenic fungi Metarhizium anisopliae and M. robertsii in strawberry crop soil after soil drench application. Agric. Ecosyst. Environ. 233, 361–369. doi: 10.1016/j.agee.2016.09.031
Chaudhary P. J., Patel H. K., Mehta P. V., Patel N. B., Sonth B., Dave A., et al. (2023). Plant Growth-Promoting Potential of Entomopathogenic Fungus Metarhizium pinghaense AAUBC-M26 under Elevated Salt Stress in Tomato. Agronomy 13, 1577. doi: 10.3390/agronomy13061577
Couceiro J., da C., De Fine Licht H. H., Delalibera I., Meyling N. V. (2022). Comparative gene expression and genomics reflect geographical divergence in the plant symbiotic and entomopathogenic fungal genus Metarhizium. Fungal Ecol. 60, 101190. doi: 10.1016/j.funeco.2022.101190
da Silva M., de Oliveira D. R. (2018). The new Brazilian legislation on access to the biodiversity (Law 13,123/15 and Decree 8772/16). Braz. J. Microbiol. 49, 1–4. doi: 10.1016/j.bjm.2017.12.001
Deguine J.-P., Aubertot J.-N., Flor R. J., Lescourret F., Wyckhuys K. A. G., Ratnadass A. (2021). Integrated pest management: good intentions, hard realities. A review. Agron. Sustain Dev. 41, 38. doi: 10.1007/s13593-021-00689-w/Published
Diniz F. V., Gleison G. R. Q. M., Atilon A. V., de A., Leila L. P. P., Clarice C. M. C. (2021). Native amazonian fungi to control termites Nasutitermes sp. (BLATTODEA: TERMITIDAE). Acta Biolo Colomb 27, 36–43. doi: 10.15446/abc.v27n1.86848
Domingues M. M., Dos Santos P. L., Gêa B. C. C., Carvalho V. R., Oliveira F. N., Soliman E. P. (2022). Entomopathogenic fungi, isolated from soils and Bemisia tabaci (Hemiptera: Aleyrodidae) adults, to manage the eucalyptus red gum lerp psyllid Glycaspis brimblecombei (Hemiptera: Aphalaridae). J. Econ Entomol 115, 1886–1893. doi: 10.1093/jee/toac165
FAOSTAT Statistical Database. (2021). Food and agriculture organization of the united nations, rome. Available at: http://www.fao.org/faostat (Accessed 11 June 2023).
Faria M., Mascarin G. M., Butt T., Lopes R. B. (2023). On-farm production of microbial entomopathogens for use in agriculture: Brazil as a case Study. Neotrop Entomol 52, 122–133. doi: 10.1007/s13744-023-01033-5
Faria M. R., Wraight S. P. (2007). Mycoinsecticides and Mycoacaricides: A comprehensive list with worldwide coverage and international classification of formulation types. Biol. Control 43, 237–256. doi: 10.1016/j.biocontrol.2007.08.001
Franzin M. L., Moreira C. C., da Silva L. N. P., Martins E. F., Fadini M. A. M., Pallini A., et al. (2022). Metarhizium associated with coffee seedling roots: positive effects on plant growth and protection against Leucoptera coffeella. Agric. (Switzerland) 12, 2030. doi: 10.3390/agriculture12122030
Gao Q., Jin K., Ying S. H., Zhang Y., Xiao G., Shang Y., et al. (2011). Genome sequencing and comparative transcriptomics of the model entomopathogenic fungi Metarhizium anisopliae and M. acridum. PloS Genet. 7, e1001264. doi: 10.1371/journal.pgen.1001264
Garcia J., Posadas J. B., Perticari A., García J., Elena J., Beatriz P., et al. (2011). Metarhizium anisopliae (Metschnikoff) Sorokin promotes growth and has endophytic activity in tomato plants. Adv. Biol. Res. (Rennes) 5, 22–27.
Gava C. A. T., da Silva J. C., Simões W. L., Paranhos B. A. J. (2021). Impact of soil texture on conidia movement and residual effect of entomopathogenic fungi applied through irrigation to control fruit-fly pupae in mango orchards. Biol. Control 163, 104559. doi: 10.1016/j.biocontrol.2021.104559
Gomes S. A., Carolino A. T., Teodoro T. B. P., Silva G. A., Bitencourt R. D. O. B., Silva C. P., et al. (2023). The potential of Metarhizium anisopliae blastospores to control Aedes aEgypti larvae in the field. J. Fungi 9, 759. doi: 10.3390/jof9070759
Guida Y. S., Meire R. O., Torres J. P. M., Malm O. (2018). Air contamination by legacy and current-use pesticides in brazilian mountains: an overview of national regulations by monitoring pollutant presence in pristine areas. Environ. pollut. 242, 19–30. doi: 10.1016/j.envpol.2018.06.061
Gupta R., Keppanan R., Leibman-Markus M., Rav-David D., Elad Y., Ment D., et al. (2022). The entomopathogenic fungi Metarhizium brunneum and Beauveria bassiana promote systemic immunity and confer resistance to a broad range of pests and pathogens in tomato. Phytopathology 112, 784–793. doi: 10.1094/PHYTO-08-21-0343-R
Gvozdenac S., Milovac Ž., Vidal S., Crvenković Z. L., Papuga I.Š., Franeta F. (2022). Comparison of chemical and biological wireworm control options in Serbian sunflower fields and a proposition for a refined wireworm damage assessment. Agronomy 12, 758. doi: 10.3390/agronomy12040758
Hao Q., Albaghdady D. M. D., Xiao Y., Xiao X., Mo C., Tian T., et al. (2021). Endophytic Metarhizium anisopliae is a potential biocontrol agent against wheat Fusarium head blight caused by Fusarium graminearum. J. Plant Pathol. 103, 875–885. doi: 10.1007/s42161-021-00866-6
Holz S., D’Alessandro C. P., Maximo H. J., Nascimento de Souza P. H., Raruang Y., Demétrio C. G. B., et al. (2023). The potential of using Metarhizium anisopliae and Metarhizium humberi to control the Asian soybean rust caused by Phakopsora pachyrhizi. Biocontrol Sci. Technol. 33, 366–382. doi: 10.1080/09583157.2023.2191299
Hong S., Shang J., Sun Y., Tang G., Wang C. (2023). Fungal infection of insects: molecular insights and prospects. Trends Microbiol. doi: 10.1016/j.tim.2023.09.005
Hu S., Bidochka M. J. (2021a). Root colonization by endophytic insect-pathogenic fungi. J. Appl. Microbiol. 130, 570–581. doi: 10.1111/jam.14503
Hu S., Bidochka M. J. (2021b). Abscisic acid implicated in differential plant responses of Phaseolus vulgaris during endophytic colonization by Metarhizium and pathogenic colonization by Fusarium. Sci. Rep. 11, 11327. doi: 10.1038/s41598-021-90232-4
Hu S., Mojahid M. S., Bidochka M. J. (2023). Root colonization of industrial hemp (Cannabis sativa L.) by the endophytic fungi Metarhizium and Pochonia improves growth. Ind. Crops Prod 198, 116716. doi: 10.1016/j.indcrop.2023.116716
Hu G., St. Leger R. J. (2002). Field studies using a recombinant mycoinsecticide (Metarhizium anisopliae) reveal that it is rhizosphere competent. Appl. Environ. Microbiol. 68, 6383–6387. doi: 10.1128/AEM.68.12.6383-6387.2002
Huarte-Bonnet C., Paixão F. R. S., Mascarin G. M., Santana M., Fernandes É.K.K., Pedrini N. (2019). The entomopathogenic fungus Beauveria bassiana produces microsclerotia-like pellets mediated by oxidative stress and peroxisome biogenesis. Environ. Microbiol. Rep. 11, 518–524. doi: 10.1111/1758-2229.12742
Iwanicki N. S. A., Mascarin G. M., Moreno S. G., Eilenberg J., Delalibera I. (2021). Development of novel spray-dried and air-dried formulations of Metarhizium robertsii blastospores and their virulence against Dalbulus maidis. Appl. Microbiol. Biotechnol. 105, 7913–7933. doi: 10.1007/s00253-021-11576-5
Iwanicki N. S. A., Mascarin G. M., Moreno S. G., Eilenberg J., Delalibera Junior I. (2020). Growth kinetic and nitrogen source optimization for liquid culture fermentation of Metarhizium robertsii blastospores and bioefficacy against the corn leafhopper Dalbulus maidis. World J. Microbiol. Biotechnol. 36, 1–13. doi: 10.1007/s11274-020-02844-z
Iwanicki N. S., Pereira A. A., Botelho A. B. R. Z., Rezende J. M., Moral R., de A., et al. (2019). Monitoring of the field application of Metarhizium anisopliae in Brazil revealed high molecular diversity of Metarhizium spp in insects, soil and sugarcane roots. Sci. Rep. 9, 4443. doi: 10.1038/s41598-019-38594-8
Jaber L. R., Enkerli J. (2016). Effect of seed treatment duration on growth and colonization of Vicia faba by endophytic Beauveria bassiana and Metarhizium brunneum. Biol. Control 103, 187–195. doi: 10.1016/j.biocontrol.2016.09.008
Jaronski S. T. (2007). “Soil ecology of the entomopathogenic ascomycetes: a critical examination of what we (think) we know,” in Use of entomopathogenic fungi in biological pest management. Eds. Ekesi S., Maniania N. K. (Kerala, India: Research Signpost), 91–144.
Jaronski S. T. (2013). “Mass production of entomopathogenic fungi: state of the art,” in Mass production of beneficial organisms: invertebrates and entomopathogens (Amsterdam, Netherlands: Elsevier Inc), 357–413.
Jaronski S. T. (2022). “Mass production of entomopathogenic fungi—state of the art,” in Mass production of beneficial organisms: invertebrates and entomopathogens (Amsterdam, Netherlands: Elsevier), 317–357.
Jiang X., Dai J., Zhang X., Wu H., Tong J. H., Shi J., et al. (2022). Enhanced Cd efflux capacity and physiological stress resistance: The beneficial modulations of Metarhizium robertsii on plants under cadmium stress. J. Hazard Mater 437, 129429. doi: 10.1016/j.jhazmat.2022.129429
Jones G. A., de Souza Perinotto W. M., Camargo M. G., Golo P. S., Bittencourt V. R. E. P. (2021). Selection of Metarhizium spp. Brazilian isolates to control Rhipicephalus microplus ticks: in vitro virulence tests and conidiogenesis. Rev. Bras. Med. Vet. 43, e002020. doi: 10.29374/2527-2179.BJVM002020
Jordan C., Dos Santos P. L., Oliveira L. R. D. S., Domingues M. M., Gêa B. C. C., Ribeiro M. F. (2021). Entomopathogenic fungi as the microbial frontline against the alien Eucalyptus pest Gonipterus platensis in Brazil. Sci. Rep. 11, 1–13. doi: 10.1038/s41598-021-86638-9
Kepler R. M., Rehner S. A. (2013). Genome-assisted development of nuclear intergenic sequence markers for entomopathogenic fungi of the Metarhizium anisopliae species complex. Mol. Ecol. Resour 13, 210–217. doi: 10.1111/1755-0998.12058
Khan A. L., Hamayun M., Khan S. A., Kang S. M., Shinwari Z. K., Kamran M., et al. (2012). Pure culture of Metarhizium anisopliae LHL07 reprograms soybean to higher growth and mitigates salt stress. World J. Microbiol. Biotechnol. 28, 1483–1494. doi: 10.1007/s11274-011-0950-9
Kobori N. N., Mascarin G. M., Jackson M. A., Schisler D. A. (2015). Liquid culture production of microsclerotia and submerged conidia by Trichoderma harzianum active against damping-off disease caused by Rhizoctonia solani. Fungal Biol. 119, 179–190. doi: 10.1016/j.funbio.2014.12.005
Liao X., O’Brien T. R., Fang W., St. Leger R. J. (2014). The plant beneficial effects of Metarhizium species correlate with their association with roots. Appl. Microbiol. Biotechnol. 98, 7089–7096. doi: 10.1007/s00253-014-5788-2
Lira A. C., Mascarin G. M., Delalibera Júnior Í. (2020). Microsclerotia production of Metarhizium spp. for dual role as plant biostimulant and control of Spodoptera frugiperda through corn seed coating. Fungal Biol. 124, 689–699. doi: 10.1016/j.funbio.2020.03.011
Li Z., Alves S. B., Roberts D. W., Fan M., Delalibera I. Jr., Tang J., et al. (2010). Biological control of insects in brazil and china: history, current programs and reasons for their successes using entomopathogenic fungi. Biocontrol Sci. Techn. 20, 117–136. doi: 10.1080/09583150903431665
Lopes R. B., Faria M., Souza D. A., Bloch C., Silva L. P., Humber R. A. (2014). MALDI-TOF mass spectrometry applied to identifying species of insect-pathogenic fungi from the Metarhizium anisopliae complex. Mycologia 106, 865–878. doi: 10.3852/13-401
Lopes R. B., Sosa-Gómez D. R., Oliveira C. M., Sanches M. M., de Souza D. A., Benito N. P., et al. (2020). Efficacy of an oil-based formulation combining Metarhizium rileyi and nucleopolyhedroviruses against lepidopteran pests of soybean. J. Appl. Entomol 144, 678–689. doi: 10.1111/jen.12787
Loureiro E. S., de Souza Tosta R. A., Dias P. M., Pessoa L. G. A., de Oliveira Neto F. M., Devoz G. L. R., et al. (2020). Performance of Metarhizium rileyi applied on Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae). Rev. Agric. Neotrop 7, 60–65. doi: 10.32404/rean.v7i1.4208
Lozano-Tovar M. D., Garrido-Jurado I., Quesada-Moraga E., Raya-Ortega M. C., Trapero-Casas A. (2017). Metarhizium brunneum and Beauveria bassiana release secondary metabolites with antagonistic activity against Verticillium dahliae and Phytophthora megasperma olive pathogens. Crop Prot. 100, 186–195. doi: 10.1016/j.cropro.2017.06.026
Luz C., Rocha L. F. N., Montalva C., Souza D. A., Botelho A. B. R. Z., Lopes R. B., et al. (2019). Metarhizium humberi sp. nov. (Hypocreales: Clavicipitaceae), a new member of the PARB clade in the Metarhizium anisopliae complex from Latin America. J. Invertebr Pathol. 166, 107216. doi: 10.1016/j.jip.2019.107216
Magalhaes B. P., Lecoq M., Faria M. D., Schmidt F. G. V., Guerra W. D. (2000). Field trial with the entomopathogenic fungus Metarhizium anisopliae var. acridum against bands of the grasshopper Rhammatocerus schistocercoides in Brazil. Biocontrol Sci. Technol. 10, 427–441. doi: 10.1080/09583150050115016
Marciano A. F., Mascarin G. M., Franco R. F. F., Golo P. S., Jaronski S. T., Fernandes É.K.K., et al. (2021). Innovative granular formulation of Metarhizium robertsii microsclerotia and blastospores for cattle tick control. Sci. Rep. 11, 4972. doi: 10.1038/s41598-021-84142-8
Marques E. J., Vilas Boas A. M. (1978). Avaliação de danos de mahanarva posticata (STAL, 1855) (HOM., CERCOPIDAE) em cana-de-açucar. Anais da Sociedade Entomológica do Bras. 72, 99–104.
Mascarin G. M., Jackson M. A., Kobori N. N., Behle R. W., Delalibera Júnior Í. (2015). Liquid culture fermentation for rapid production of desiccation tolerant blastospores of Beauveria bassiana and Isaria fumosorosea strains. J. Invertebr Pathol. 127, 11–20. doi: 10.1016/j.jip.2014.12.001
Mascarin G. M., Lopes R. B., Delalibera Í., Fernandes É.K.K., Luz C., Faria M. (2019). Current status and perspectives of fungal entomopathogens used for microbial control of arthropod pests in Brazil. J. Invertebr Pathol. 165, 46–53. doi: 10.1016/j.jip.2018.01.001
Meirelles L. N., Mesquita E., Corrêa T. A., Bitencourt R., de O. B., Oliveira J. L., et al. (2023). Encapsulation of entomopathogenic fungal conidia: evaluation of stability and control potential of Rhipicephalus microplus. Ticks Tick Borne Dis. 14, 102184. doi: 10.1016/j.ttbdis.2023.102184
Mesquita E., Marciano A. F., Corval A. R. C., Fiorotti J., Corrêa T. A., Quinelato S., et al. (2020). Efficacy of a native isolate of the entomopathogenic fungus Metarhizium anisopliae against larval tick outbreaks under semifield conditions. BioControl 65, 353–362. doi: 10.1007/s10526-020-10006-1
Moonjely S., Bidochka M. J. (2019). Generalist and specialist Metarhizium insect pathogens retain ancestral ability to colonize plant roots. Fungal Ecol. 41, 209–217. doi: 10.1016/j.funeco.2019.06.004
Nascimento V. C., Rodrigues-Santos K. C., Carvalho-Alencar K. L., Castro M. B., Kruger R. H., Lopes F. A. C. (2022). Trichoderma: biological control efficiency and perspectives for the Brazilian Midwest states and Tocantins. Braz. J. Biol. 82, e260161. doi: 10.1590/1519-6984.260161
Nehring R. (2022). The Brazilian green revolution. Polit Geogr. 95, 102574. doi: 10.1016/j.polgeo.2021.102574
Nogueira M. R., dos S., Camargo M. G., Rodrigues C. J. B. C., Marciano A. F., Quinelato S., et al. (2020). In vitro efficacy of two commercial products of Metarhizium anisopliae s.L. for controlling the cattle tick Rhipicephalus microplus. Rev. Bras. Parasitol. Vet. 29, 1–8. doi: 10.1590/S1984-29612020035
Oliveira Barbosa Bitencourt R., Reis dos Santos Mallet J., Mesquita E., Silva Gôlo P., Fiorotti J., Rita Elias Pinheiro Bittencourt V., et al. (2021). Larvicidal activity, route of interaction and ultrastructural changes in Aedes aEgypti exposed to entomopathogenic fungi. Acta Trop. 213, 105732. doi: 10.1016/j.actatropica.2020.105732
Parra J. R. P. (2014). Biological control in Brazil: An overview. Sci. Agric. 71, 420–429. doi: 10.1590/0103-9016-2014-0167
Pestana A. C. (1923). Dois cercopídeos parasitas da cana de açúcar (Ministério da Agricultura Indústria e Comércio, Campos), 17.
Poitevin C. G., Porsani M. V., Poltronieri A. S., Zawadneak M. A. C., Pimentel I. C. (2018). Fungi isolated from insects in strawberry crops act as potential biological control agents of Duponchelia fovealis (Lepidoptera: Crambidae). Appl. entomol zool 53, 323–331. doi: 10.1007/s13355-018-0561-0
Prado R., Macedo-Salles P. A., Duprat R. C., Baptista A. R., Feder D., Lima J. B. P., et al. (2020). Action of Metarhizium brunneum (Hypocreales: Clavicipitaceae) against organophosphate-and pyrethroid-resistant Aedes aEgypti (Diptera: Culicidae) and the synergistic effects of phenylthiourea. J. Med. Entomol 57, 454–462. doi: 10.1093/jme/tjz161
Quinelato S., Golo P. S., Perinotto W. M. S., Sá F. A., Camargo M. G., Angelo I. C., et al. (2012). Virulence potential of Metarhizium anisopliae s.l. isolates on Rhipicephalus (Boophilus) microplus larvae. Vet. Parasitol. 190, 556–565. doi: 10.1016/j.vetpar.2012.06.028
Rangel D. E., Acheampong M. A., Bignayan H. G., Golez H. G., Roberts D. W. (2023). Conidial mass production of entomopathogenic fungi and tolerance of their mass-produced conidia to UV-B radiation and heat. Fungal Biol. 127, 1524–1533. doi: 10.1016/j.funbio.2023.07.001
Rangel D. E. N., Piedrabuena A. E., Roitman I., Messias C. L. (2020). Laboratory and field studies for the control of Chagas disease vectors using the fungus Metarhizium anisopliae. Arch. Insect Biochem. Physiol. 105, e21745. doi: 10.1002/arch.21745
Reis R. C. S., Fernandes É.K.K., Bittencourt V. R. E. P. (2008). “Fungal formulations to control Rhipicephalus sanguineus engorged females,” in Annals of the New York Academy of Sciences (New York, USA: Blackwell Publishing Inc), 239–241.
Rezende J. M., Zanardo A. B. R., da Silva Lopes M., Delalibera I., Rehner S. A. (2015). Phylogenetic diversity of Brazilian Metarhizium associated with sugarcane agriculture. BioControl 60, 495–505. doi: 10.1007/s10526-015-9656-5
Riguetti Zanardo Botelho A. B., Alves-Pereira A., Colonhez Prado R., Zucchi M. I., Delalibera Júnior I. (2019). Metarhizium species in soil from Brazilian biomes: a study of diversity, distribution, and association with natural and agricultural environments. Fungal Ecol. 41, 289–300. doi: 10.1016/j.funeco.2019.07.004
Rocha L. F. N., Inglis P. W., Humber R. A., Kipnis A., Luz C. (2013). Occurrence of Metarhizium spp. in Central Brazilian soils. J. Basic Microbiol. 53, 251–259. doi: 10.1002/jobm.201100482
Rocha L. F. N., Rodrigues J., Martinez J. M., Pereira T. C. D., Neto J. R. C., Montalva C., et al. (2022). Occurrence of entomopathogenic hypocrealean fungi in mosquitoes and their larval habitats in Central Brazil, and activity against Aedes aEgypti. J. Invertebr Pathol. 194, 107803. doi: 10.1016/j.jip.2022.107803
Rodrigues J., Lopes Catão A. M., Soares A., Santos D., Regina F., Paixão S., et al. (2021). Relative humidity impacts development and activity against Aedes aEgypti adults by granular formulations of Metarhizium humberi microsclerotia. Appl. Microbiol. Biotechnol. 105, 2725–2736. doi: 10.1007/s00253-021-11157-6/Published
Sasan R. K., Bidochka M. J. (2013). Antagonism of the endophytic insect pathogenic fungus Metarhizium robertsii against the bean plant pathogen Fusarium solani f. sp. phaseoli. Can. J. Plant Pathol. 35, 288–293. doi: 10.1080/07060661.2013.823114
Schmidt F. G. V., de Jesus Conceição P., Benito N. P., Lopes R. B. (2018). Susceptibility of three orthopteran species to infection by Metarhizium acridum (Hypocreales: Clavicipitaceae). Int. J. Trop. Insect Sci. 38, 117–121. doi: 10.1017/S1742758417000352
Sheng H., McNamara P. J., St. Leger R. J. (2022). Metarhizium: an opportunistic middleman for multitrophic lifestyles. Curr. Opin. Microbiol. 69, 102176. doi: 10.1016/j.mib.2022.102176
Siqueira A. C. O., Mascarin G. M., Gonçalves C. R. N. C. B., Marcon J., Quecine M. C., Figueira A., et al. (2020). Multi-Trait biochemical features of Metarhizium Species and their activities that stimulate the growth of tomato plants. Front. Sustain Food Syst. 4. doi: 10.3389/fsufs.2020.00137
Soliman E. P., Wilcken C. F., Firmino A. C., Pogetto M. H. F. A. D., Barbosa L. R., Zanuncio J. C. (2019). Susceptibility of Thaumastocoris peregrinus (Hemiptera: Thaumastocoridae), a Eucalyptus pest, to entomopathogenic fungi. Sci. Agric. 76, 255–260. doi: 10.1590/1678-992x-2017-0043
Souza D. A., de Oliveira C. M., Tamai M. A., Faria M., Lopes R. B. (2021). First report on the natural occurrence of entomopathogenic fungi in populations of the leafhopper Dalbulus maidis (Hemiptera: Cicadellidae): Pathogen identifications and their incidence in maize crops. Fungal Biol. 125, 980–988. doi: 10.1016/j.funbio.2021.08.004
St Leger R. J., Wang J. B. (2020). Metarhizium: Jack of all trades, master of many: Sex and host switching in a fungus. Open Biol. 10, 200307. doi: 10.1098/rsob.200307
Stone L. B. L., Bidochka M. J. (2020). The multifunctional lifestyles of Metarhizium: evolution and applications. Appl. Microbiol. Biotechnol. 104, 9935–9945. doi: 10.1007/s00253-020-10968-3/Published
Valentim B. M. J. M., Fagundes T. R., Ferreira O. M., Micheletti L. P., Oliveira B. G. E., Souza C. M. (2023). Monitoring residues of pesticides in food in Brazil: A multiscale analysis of the main contaminants, dietary cancer risk estimative and mechanisms associated. Front. Public Health 11. doi: 10.3389/fpubh.2023.1130893
Vega F. E. (2018). The use of fungal entomopathogens as endophytes in biological control: a review. Mycologia 110, 4–30. doi: 10.1080/00275514.2017.1418578
Vemmer M., Patel A. V. (2013). Review of encapsulation methods suitable for microbial biological control agents. Biol. Control 67, 380–389. doi: 10.1016/j.biocontrol.2013.09.003
Vinha F. B., Rojas L. A. C., Ramos Sales C., Monteiro Lima N. S., Do Nascimento J., De Carvalho L. A. L., et al. (2023). Negative effects on the development of Chrysodeixis includens and Spodoptera cosmioides fed by peanut plants inoculated with entomopathogenic fungi. Front. Fungal Biol. 3. doi: 10.3389/ffunb.2022.968528
Wang C., St. Leger R. J. (2006). A collagenous protective coat enables Metarhizium anisopliae to evade insect immune responses. Proc. Natl. Acad. Sci. 103, 6647–6652. doi: 10.1073/pnas.0601951103
Wang J. B., St. Leger R. J., Wang C. (2016). Advances in genomics of entomopathogenic fungi. Adv. Genet. 94, 67–105. doi: 10.1016/bs.adgen.2016.01.002
Wei J., Zhou X., Dong M., Yang L., Zhao C., Lu R., et al. (2022). Metabolites and novel compounds with anti-microbial or antiaging activities from Cordyceps fumosorosea. AMB Express 12, 1–14. doi: 10.1186/s13568-022-01379-w
Working Group Patel (2023) ATTRACAP. Available at: https://workinggrouppatel.wordpress.com/attracap/ (Accessed October 05, 2023).
Wu C., Tang D., Dai J., Tang X., Bao Y., Ning J., et al. (2022). Bioremediation of mercury-polluted soil and water by the plant symbiotic fungus Metarhizium robertsii. Proc. Natl. Acad. Sci. 119, e2214513119. doi: 10.1073/pnas.2214513119
Wyrebek M., Huber C., Sasan R. K., Bidochka M. J. (2011). Three sympatrically occurring species of Metarhizium show plant rhizosphere specificity. Microbiol. (N Y) 157, 2904–2911. doi: 10.1099/mic.0.051102-0
Keywords: entomopathogenic fungi, endophytes, native isolates, rhizosphere-competence, integrated pest management (IPM)
Citation: Mesquita E, Hu S, Lima TB, Golo PS and Bidochka MJ (2023) Utilization of Metarhizium as an insect biocontrol agent and a plant bioinoculant with special reference to Brazil. Front. Fungal Biol. 4:1276287. doi: 10.3389/ffunb.2023.1276287
Received: 11 August 2023; Accepted: 30 November 2023;
Published: 21 December 2023.
Edited by:
Chandra Nayak, University of Mysore, IndiaReviewed by:
Mavis Agyeiwaa Acheampong, University of Ghana, GhanaCopyright © 2023 Mesquita, Hu, Lima, Golo and Bidochka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael J. Bidochka, bWJpZG9jaGthQGJyb2NrdS5jYQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.