
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. For. Glob. Change , 09 December 2024
Sec. Forests and the Atmosphere
Volume 7 - 2024 | https://doi.org/10.3389/ffgc.2024.1489081
In recent decades, continued growth decline has been observed in various beech forest regions of Central and Western Europe, especially in the warmer lowlands, which is not necessarily linked to increased mortality. While earlier dendrochronological studies have shown that a deteriorating climatic water balance in the course of climate warming can drive negative growth trends, less is known about the effects of climatic extremes on tree growth, notably heat and rising atmospheric vapor pressure deficits (VPD). Through climate-growth analysis, we analyzed the influence of summer heat duration (frequency of hot days with Tmax > 30°C) and elevated VPD on the basal area increment (BAI) of dominant beech trees in 30 stands across a precipitation gradient in the northern German lowlands. Summer heat (especially in June) and elevated VPD are reducing BAI in a similar manner as does a deteriorated climatic water balance. While growing season length (GSL), derived from thermal thresholds of growth activity, has substantially increased since 1980, BAI has declined in the majority of stands, demonstrating a recent decoupling of tree productivity from GSL. We conclude that heat and elevated VPD most likely are important drivers of the recent beech growth decline in this region, while growing season length has lost its indicative value of beech productivity.
Climate warming is threatening the productivity and health of trees and forests in many regions on Earth (Allen et al., 2010; Anderegg et al., 2013). Three climatic factors are exposing trees to increasing climatic stress, (1) rising summer temperatures that are associated with more frequent and more severe heat extremes, (2) increasing atmospheric vapor pressure deficits (VPD), and (3) an increasing frequency and severity of droughts (Adams et al., 2017; Hammond et al., 2022). Central Europe with its intensive forest management on most of the forested area is particularly vulnerable to climate change-induced destabilization of forests, as (i) warming and the increase in heat exposure are proceeding faster than in other northern temperate regions (IPCC, 2021; Vautard et al., 2023), and (ii) the pool of native tree species and especially the number of valuable timber species is small (Leuschner and Ellenberg, 2017). This has prompted intensive research on the climate vulnerability and drought and heat response of the major tree species of this region in recent time, notably of European beech (Fagus sylvatica L.), Norway spruce (Picea abies Karst.), Scots pine (Pinus sylvestris L.), Sessile and Common oak [Quercus petraea (Matt.) LIebl. and Q. robur L.], as well as of introduced Douglas fir [Pseudotsuga menziesii (Mirb.)Franco] (Bose et al., 2020, 2021; Braun et al., 2021; Debel et al., 2021; Diers et al., 2024; Enderle et al., 2024; Gribbe et al., 2024; Leuschner et al., 2024; Martinez del Castillo et al., 2022; Obladen et al., 2021; Thom et al., 2023; Walthert et al., 2021; Weigel et al., 2023). This research has demonstrated that the majority of these species have suffered vitality declines and increased mortality in various Central European regions after recent severe hot droughts, as in 2003, 2015, and 2018/19 (Arend et al., 2022; George et al., 2022; Leuschner et al., 2023; Schuldt et al., 2020; Senf et al., 2020; Thonfeld et al., 2022). This has raised concern about the future perspective of the forestry sector and timber yield in Central Europe (Hanewinkel et al., 2013; Yousefpour and Hanewinkel, 2016), which was in various regions hit hard by the extreme 2018/19 hot drought (Möhring et al., 2021). The future health status and development of natural forests especially in Central Europe’s warmer regions are also questionable.
European beech is the most important tree species of Central Europe’s natural forest vegetation, which would dominate for example in Germany two third of the forest area in the absence of human impact (Suck et al., 2014). Physiological and dendrochronological research in the last two decades has demonstrated that the species is fairly drought sensitive (Gessler et al., 2007; Lendzion and Leuschner, 2008; Dorado-Liñán et al., 2017; Serra-Maluquer et al., 2019; Leuschner, 2020; Martinez del Castillo et al., 2022). In accordance, numerous studies have reported declining radial growth rates of beech in various Central and Western European regions in recent time that were related to climate warming, mostly at lower elevations (Jump et al., 2006; Piovesan et al., 2008; Lakatos and Molnár, 2009; Bontemps et al., 2010; Charru et al., 2010; Scharnweber et al., 2011; Kint et al., 2012; Härdtle et al., 2013; Zimmermann et al., 2015; Knutzen et al., 2017; Braun et al., 2021). For example, a study covering 30 mature beech forests in the lowlands of northern Germany found negative basal area increment (BAI) trends over the last 20–30 years in about 60 percent of studied trees, with negative BAI trends increasing toward stands with lower summer precipitation (Weigel et al., 2023). Premature foliage discoloration and leaf shedding, and crown damage and mortality have increased especially in the aftermath of the extreme 2018/19 hot drought, as was observed in Switzerland, southern Germany, and elsewhere (Braun et al., 2021; Frei et al., 2022; Klesse et al., 2022; Neycken et al., 2022). Stands in southern exposition, on shallow soils and at forest edges were generally hit hardest (Schuldt et al., 2020). On a forest patch scale, some beech forests suffered mortality rates up to 25% or even >80% of the stems (Frei et al., 2022; Wohlgemuth et al., 2020), but mortality rates were lower on the landscape scale (usually <5%; see review in Leuschner, 2024). While the direct causes of mortality remain unclear in the majority of cases, catastrophic hydraulic failure could in some studies be confirmed as the main driver (Schuldt et al., 2020; Arend et al., 2022).
Beech pursues a more anisohydric regulation of foliar water status, tolerating fairly large diurnal and seasonal leaf water potential drops, when water is scarce and evaporative demand is high (Leuschner et al., 2019). With P50 values of mature tree sun-canopy branches usually in the range of −2.8 to −3.8 MPa (Herbette et al., 2010; Schuldt et al., 2016; Weithmann et al., 2022), beech has a fairly embolism-resistant branch xylem, which faces catastrophic hydraulic failure only during rare extreme droughts (Dietrich and Kahmen, 2019; Leuschner, 2020; Walthert et al., 2021). This may happen especially on shallow soils with low water storage capacity, when local crown dieback has been observed that may have been caused by xylem cavitation (Schuldt et al., 2020; Frei et al., 2022; Henkel et al., 2022). However, with respect to the widespread growth declines observed in various central and western European beech stands during the last 20–40 years, it is unlikely that drought-induced embolism is the main cause, as the shift from positive (or stable) to negative growth trends occurred gradually and not abruptly (Knutzen et al., 2017; Scharnweber et al., 2011; Weigel et al., 2023). Among the factors that could have caused the vitality loss and growth decline are a rise in VPD (which might have reduced stomatal conductance and thus photosynthetic carbon gain), a continuous lowering of foliar and cambial water potentials due to a deteriorating climatic water balance (which could have reduced leaf and stem growth), negative heat effects on leaf metabolism, and carbon allocation shifts to more root growth at the expense of stem growth, triggered by reduced soil water availability in a drying climate (Leuschner et al., 2023). Much of this is speculative, as the causes of continued recent growth declines in beech (and other temperate tree species) are far less understood than those of sudden crown dieback and increased mortality (Arend et al., 2022; McDowell et al., 2022).
Physiological measurements suggest that heat may become an increasingly important factor impairing the vitality of temperate tree species in a warming climate (Ruehr et al., 2015; Teskey et al., 2015; Münchinger et al., 2023). In addition, much research has recently focused on the role of VPD for tree vitality and growth (Grossiord et al., 2020; Köcher et al., 2012; Lendzion and Leuschner, 2008; Novick et al., 2024). However, analyses of climate-growth relationships in the context of dendrochronological studies have mostly focused on temperature means, precipitation sums and the climatic water balance of summer months (Debel et al., 2021; Stolz et al., 2021; Weigel et al., 2023), but have rarely investigated effects of heat and elevated VPD on radial growth (Enderle et al., 2024). These omissions may hinder a full understanding of the drivers of the recent growth trend shifts that were observed in European beech and other Central European trees species.
Another factor that should influence annual ring width is the length of the growing season, as earlier spring greening in the course of climate warming may increase annual canopy carbon gain and forest productivity (Keenan et al., 2014; Ren et al., 2019). For example, a modeling study predicted an increase in deciduous forest productivity by 5.9 g C m−2 per day growing season length extension (Baldocchi and Wilson, 2001), which is supported by eddy covariance and remote sensing studies (Churkina et al., 2005; Griffis et al., 2003). Over longer times spans, extended growing seasons may have the potential to trigger changes in species composition, when phenological patterns are fundamentally altered. On the other hand, a longer growing season may increase transpiration rates, leaving less moisture in the soil with negative effects on productivity (Lian et al., 2020), or it can increase the trees’ vulnerability to pests and diseases (Thackeray et al., 2016; Walther et al., 2002). In the lowlands of northern Germany, recent dendrochronological findings suggest for various sites a negative rather than a positive relation between GSL and BAI, as growth trends have often turned negative, even though growing season length has been found to increase with climate warming (Menzel and Fabian, 1999; Menzel et al., 2006). A closer look on the relation between GSL and growth rate is thus needed.
Here, we analyze the influence of increasing heat, elevated VPD and extended growing season length on the basal area increment of beech in a sample of 30 stands in the lowlands of northern Germany. This region is characterized by a gradient from a moister temperate-oceanic to a drier temperate sub-continental climate, with precipitation decreasing from c. 850 to 500 mm yr.−1 over 300 km distance. An earlier study has analyzed recent growth trends and possible drivers of growth decline in this sample, but did not investigate heat, VPD and GSL effects (Weigel et al., 2023). We use a 66-yr record of monthly weather data from a dense net of stations in this region to analyze long-term trends in the number of hot days (days with >30°C maximum temperature), in the year’s maximum mean daily VPD, and in growing season length to investigate the influence of these heat- and warmth-related variables on BAI trends of beech populations that grow under high (>800 mm) to low precipitation (<600 mm). We tested the following hypotheses: (1) The recent warming has increased the frequency of hot days and of maximum VPD and has extended growing season length, but at different rates in oceanic and sub-continental climates. (2) As suggested by earlier research (Enderle et al., 2024), heat and maximum VPD are important drivers of beech basal area increment. (3) Increasing exposure to heat and elevated VPD can shift the relation between long-term BAI trend and GSL change from a positive to a negative relation.
The study was carried out in the lowlands of northern Germany on Pleistocene deposits between the Dutch border in the west and the Polish border in the east, covering an area of about 138,000 km2 in the range of c. 7 °E – 14 °E longitude and 51 °N – 55 °N latitude. At elevations of 19–159 m a.s.l., the region comprises with the federal states Lower Saxony, Schleswig-Holstein, Bremen, Hamburg, Saxony-Anhalt, Mecklenburg-Vorpommern, Berlin, and Brandenburg roughly 40% of the area of Germany. The study area is characterized by a marked climate gradient from the north-west to the south-east with a transition from a cool-temperate oceanic climate to a cool-temperate sub-continental climate (mean annual temperature 9.0–10.0°C) and a decrease in mean annual precipitation from ca. 900 mm at the North Sea coast to 500–550 mm at river Oder in the east (Deutscher Wetterdienst, 2023). Dystric to eutric Cambisols and Luvisols, and dystric Podzols developed in fluvio-glacial deposits or moraine till of the penultimate (Saalian) and last glaciation (Weichselian) are the dominant soil types. All sites were selected on deep sandy to sandy-loamy substrates without groundwater influence. The capacity of the soil (0–100 cm profile) for plant-available water was estimated from soil texture data determined for all stands (Supplementary Table S1).
Forests of European beech (Fagus sylvatica) cover an area of ca. 401,300 ha in the lowlands of northern Germany (Leuschner et al., 2023). For characterizing the beech forests by their precipitation regime, we subdivided the lowlands into four classes defined by mean growing season precipitation (MGSP, April–September): Wettest region: 418–448 mm, wet region: 364–417 mm, dry region: 329–358 mm, and driest region: 306–328 mm. Figure 1 shows the location of beech forests within these four MGSP-defined regions, marked by different colors. For a more detailed description of the physiography and stand structure of the 30 beech stands see Weigel et al. (2023).
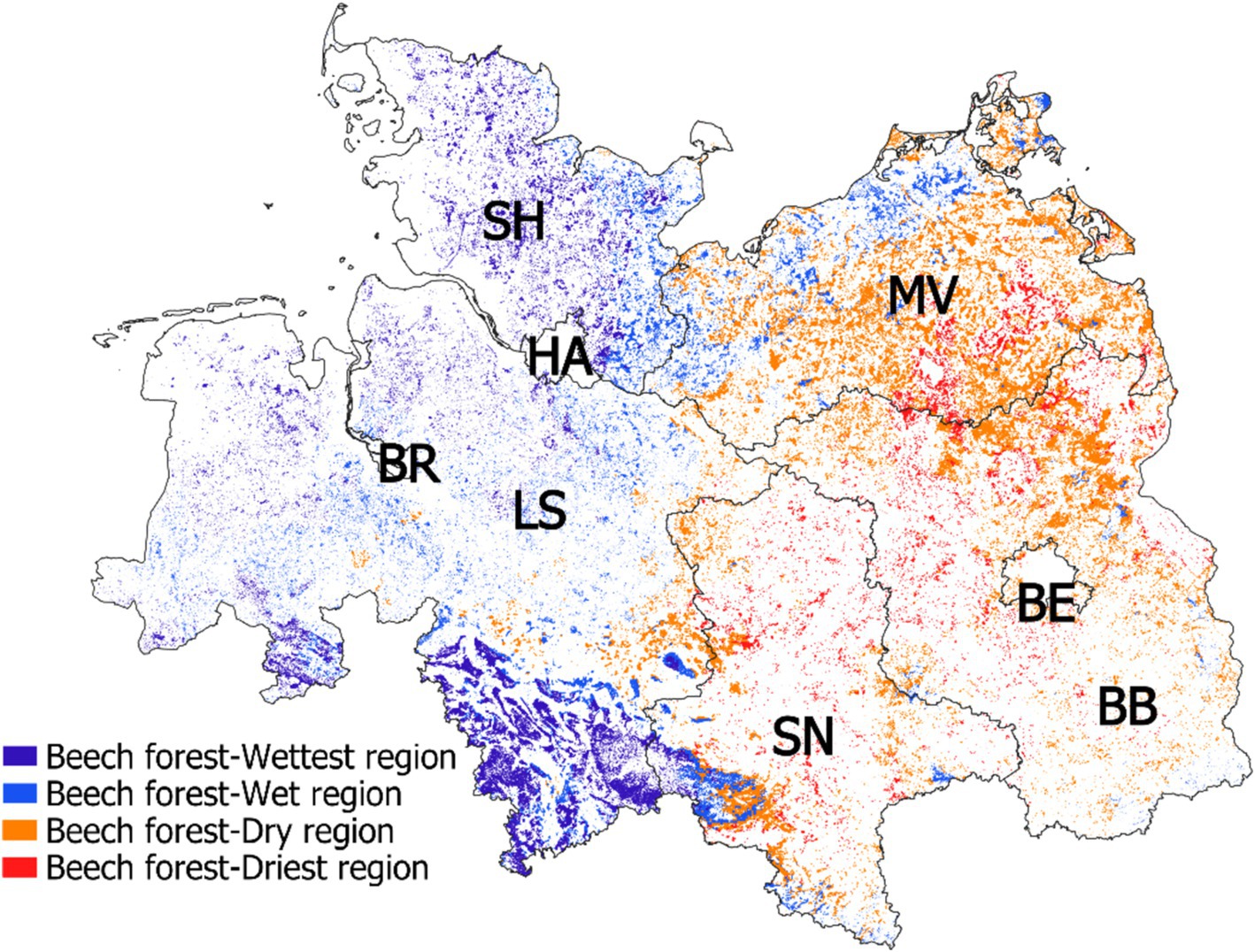
Figure 1. Distribution of beech forests in northern Germany with assignment to four classes of mean growing season precipitation (wettest: 418–448 mm, wet: 364–417 mm, dry: 329–358 mm; driest: 306–328 mm). Note the highly fragmented forest cover in the region. Federal states: BB, Brandenburg; BE, Berlin; BR, Bremen; LS, Lower Saxony; MV, Mecklenburg-Vorpommern; SN, Saxony-Anhalt; SH, Schleswig-Holstein. Distribution of beech forests after Blickensdörfer et al. (2022).
Thirty monospecific stands of mature beech were selected for dendrochronological study, about five stands each in the four MGSP classes, which were dispersed over the entire lowland region (Supplementary Table S1). Most trees were between 80 and 120 years old. All stands had a cohort-like stand structure with only beech present, and had a canopy closure >0.9. In each plot, 15 (co-)dominant beech trees of the upper canopy layer were sampled by extracting each one core at breast height (1.3 m) with a 5-mm increment borer (Haglöf, Langsele, Sweden). Measurements of tree-ring widths were carried out with an accuracy of 10 μm using a moveable Lintab 5 measuring table (Rinntech, Heidelberg, Germany) and the TSAP-Win software of Rinntech. All further tree-ring statistics were computed with the R package dplR (Bunn, 2008). Cross-dating of time series was done based on the coefficient of agreement (Gleichläufigkeit; > 0.65) (Eckstein and Bauch, 1969). All statistical analyses were performed in R version 4.3.2 (R Core Team, 2023).
In order to remove age trends, the tree-ring series were detrended with a 30-year smoothing spline and low-frequency cut-off set to 50%, using the function “detrend” of the package “dplR.” Subsequently, master chronologies were built for every population by calculating Tukey’s bi-weight robust mean of the standardized ring width index (RWI) series. Within-chronology growth coherence was quantified through the mean inter-series correlation (Rbar), and the expressed population signal (EPS; ≥0.85) (Wigley et al., 1984). The age of the trees was approximated by counting the number of rings from tree pith to bark (Supplementary Table S1). Basal area increment (BAI) was computed from the ring width series and measured DBH for each tree with the “bai.out” function of the “dplR” package.
We used monthly climate data of the last 70 years (1951–2020) that were provided in gridded form (spatial resolution of 1 km) for the northern German lowlands by the Climate Data Centre (CDC) of the German Weather Service (Deutscher Wetterdienst, 2023). We retrieved monthly values of precipitation sums, mean relative air humidity (RH) and mean air temperature, and mean daily maximum and minimum air temperatures for the 70-year period at the 30 beech forest sites by download from the CDC data base using the rdwd package in R (Boessenkool, 2023). Vapor pressure deficit (VPD) was computed from air temperature and RH using the Magnus formula (Tetens, 1930). Climatic water balance was expressed through the 3-months Standardized Precipitation-Evapotranspiration Index (SPEI-3) calculated on a monthly basis, using the SPEI package in R (Vicente-Serrano et al., 2010), to indicate long-term change in the climatic water balance and identify abnormally dry (SPEI <0) and wet (SPEI >0) months compared to the long-term average. Since hourly temperature data are not available for the 70-yr period, we approximated the frequency and severity of heat and VPD extremes through the analysis of long-term change in mean monthly maximum temperature, the number of hot days (Tmax > 30°C) per year, mean temperature of the year’s warmest day, and mean VPD of the year’s driest day. The 30°C threshold is best suited to reveal long-term heat trends in the 66-year observation period, as a higher threshold (e.g., 35°C) would have left too few hot days in the cooler first decades to analyze.
Long-term trends in climate variables were analyzed by regressing the different monthly parameters against calendar year. This was done separately for the periods 1951–1980 (before the recent warming) and 1981–2017 (the warming period), as well as for the entire (66-yr) period. Testing for significance was done with a Mann-Kendall trend test.
A climatic response analysis was carried out using the “treeclim” package in R (Zang and Biondi, 2015) to investigate the relationships between ring width index data and selected climatic variables in the 66-yr observation period (1951–2017), notably monthly mean temperature, mean monthly maximum and minimum temperatures, monthly VPD means and precipitation totals, and mean monthly SPEI (based on 3-month SPEI) for the current year (January – September) and the previous year (April – December). In addition, the relation between RWI and the number of hot days (Tmax > 30°C) in a year, the mean temperature of the year’s hottest day, and the mean VPD of the year’s driest day was analyzed. The strength of the correlation was expressed with Pearson’s r. Previous-year months were included in the analysis to account for carry-over effects of the last growing season on beech growth. A 1,000-fold bootstrapping procedure was used for significance testing of the RWI–climate relationships.
A widespread approach to model growing season length of beech and other Central European deciduous trees bases on the analysis of phenological data compiled by Menzel (1997). It uses an empirically determined critical temperature for beech growth activity and local daily temperature data for prediction. In accordance, growing season length was computed for the 30 sites from the daily temperature data of the CDC data base (Deutscher Wetterdienst, 2023) using the “vegperiod” package in R (Nuske, 2022), which applies temperature thresholds for bud burst and leaf fall. Based on the empirical data assembled by Menzel (1997), bud burst was assumed to happen, when, for the first time in the year, five consecutive days with daily temperature means >5°C occur. The end of the vegetation period was computed according to von Wilpert (1990), who set the threshold to the occurrence after mid-summer of five consecutive days with daily mean temperature < 5°C (Menzel, 1997). For the date of bud burst, an error margin of 4–8 days was assumed according to the authors. We attempted to compute the standard error of our calculated growing season extension by adding the model error (Menzel model) and the observation error to obtain the total error = √((observed error)^2 + (model error)^2).
Pearson correlation analysis was further employed to analyze relationships between different climate variables, between growing season length and BAI, and between growing season length and the number of hot days. A significance level of p < 0.05 was used throughout the paper.
For the earlier 1951–1980 period, the climate data do not show any significant trends in the studied thermal and hydrometeorological parameters for the northern German lowlands (Figure 2). Yet, mean summer temperature decreased slightly (but non-significantly) from 1951 to 1980 (Supplementary Figure S1). In contrast, mean annual (and summer) temperature has significantly increased by 0.3–0.4°C decade−1 since 1980 in all parts of the lowlands (Figure 2A). This is also valid for the mean temperature of the year’s hottest day as a proxy of heat extremes (increase by 0.4°C decade−1) (Figure 2C). A different picture emerges for the number of hot days (Tmax > 30°C) per year, which is a measure of heat duration: It rose much faster in the dry and driest regions of the lowlands (by 1.9–2.0 decade−1) than in the wet and wettest regions (by 0.3–0.7 decade−1) (Figure 2B). The three maps in Figure 3A demonstrate that the number of hot days has changed only little from 1951–1960 to 1981–1990, but there has been a marked increase since then especially in the states of Saxony-Anhalt and in the central and southern part of Brandenburg with warmest climate, while the North Sea and Baltic Sea coastal regions faced the smallest increase. The number of hot days is closely related to other thermal and hydrometeorological parameters, especially to the mean temperature of the warmest day in July (Tmax-Jul) (Supplementary Figure S2D), the mean VPD of the year’s driest day (Supplementary Figure S2B), and SPEI-Jul and SPEI-Aug (Supplementary Figures S2G,H).
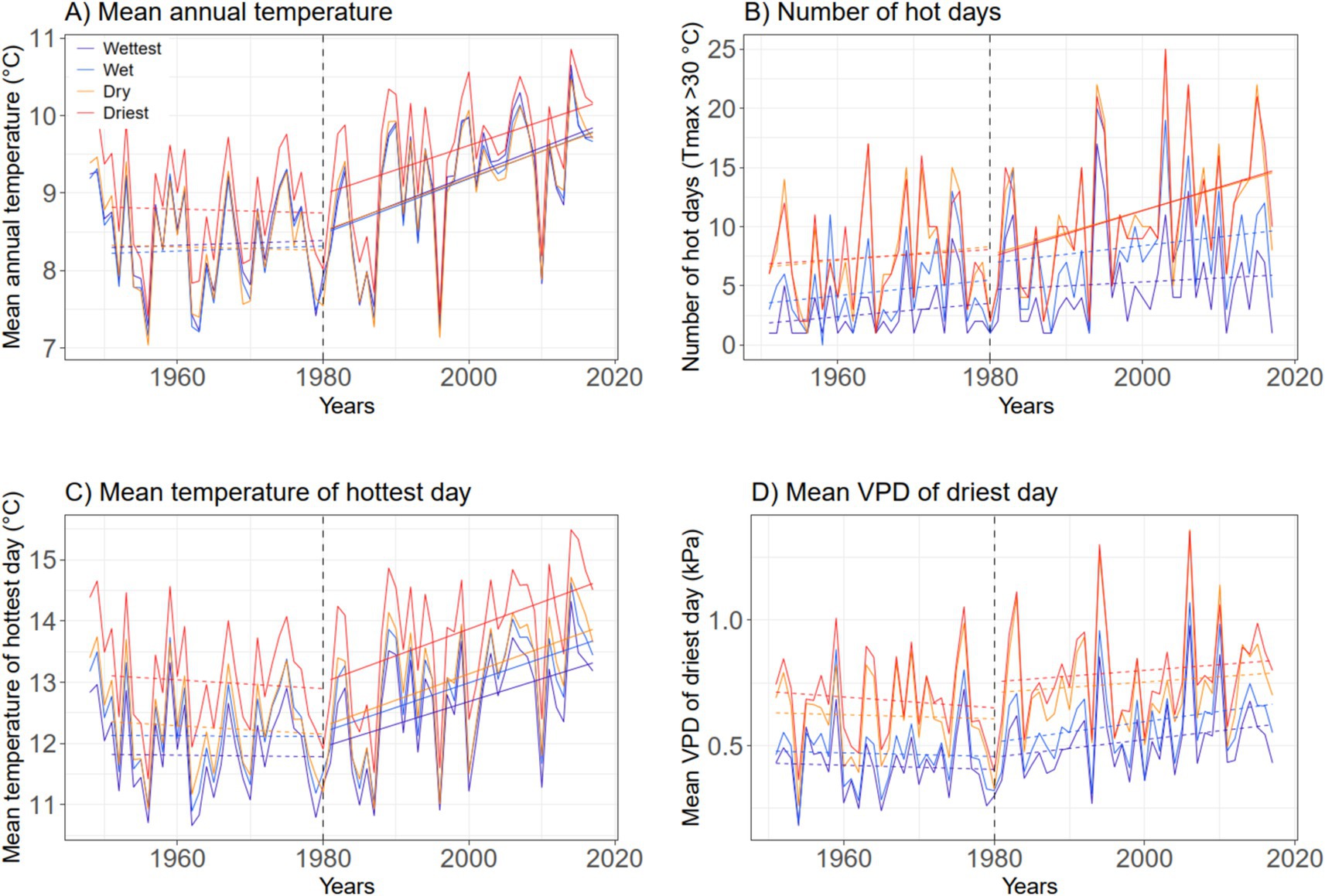
Figure 2. Change in mean annual temperature (A), the number of hot days per year (daily maximum temperature > 30°C) (B), mean temperature of the year’s hottest day (C), and mean VPD of the year’s driest day (D) in the 1951–2017 period in the northern German lowlands. Shown are the mean curves of all stations assigned to the four precipitation classes Wettest (mean growing season precipitation, April–September: 418–448 mm), Wet (364–417 mm), Dry (329–358 mm) and Driest (306–328 mm) with trend lines for the periods 1951–1980 and 1981–2017 (solid line: significant trend according to a Mann-Kendall test; dashed line: non-significant trend). The vertical dashed line (1980) separates the two periods.
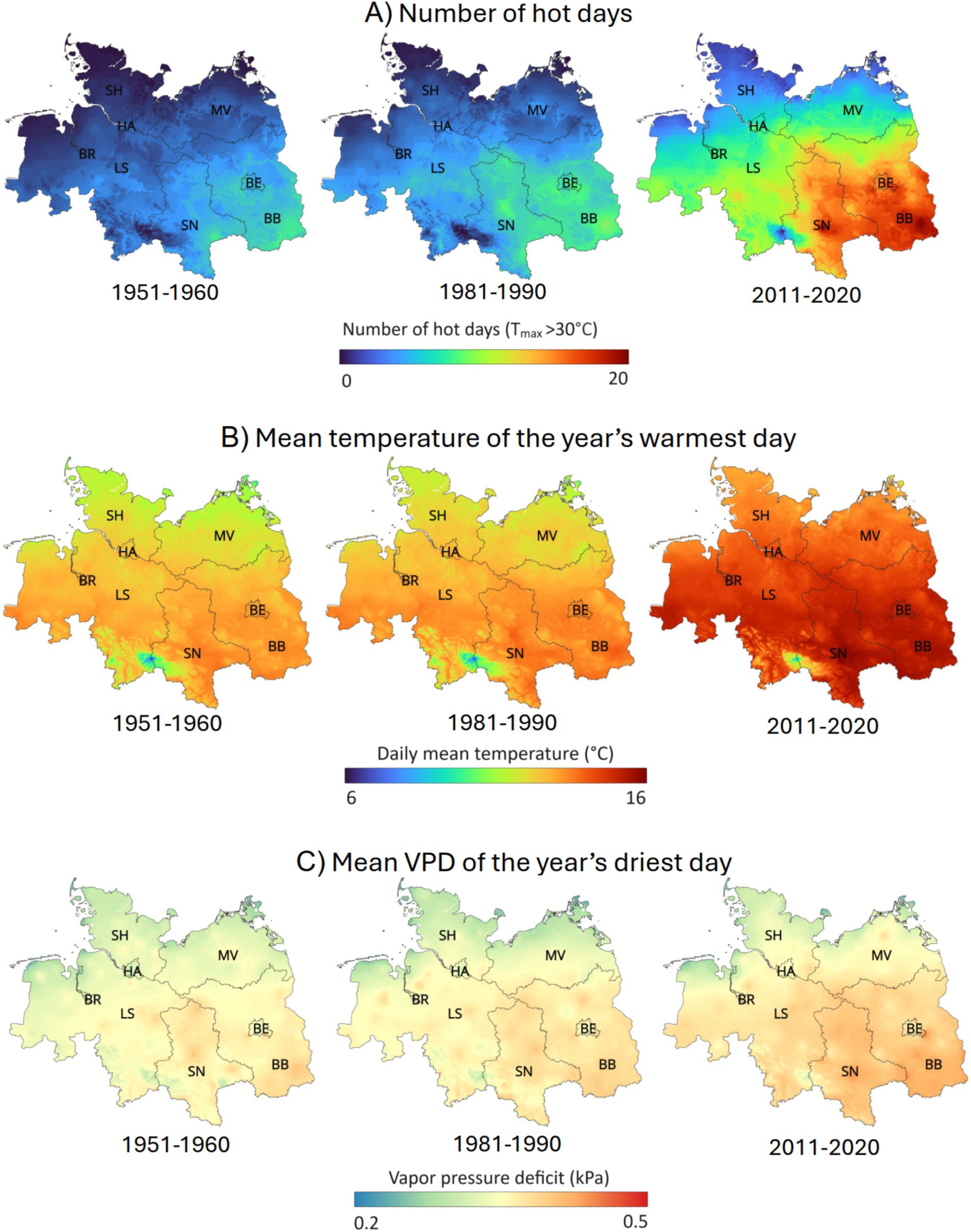
Figure 3. Spatial distribution of the number of hot days (Tmax > 30°C) (A), mean temperature of the year’s warmest day (B) and mean VPD of the year’s driest day (C) in the three periods 1951–1960, 1981–1990 and 2011–2020 (decadal means) in the northern German lowlands. BB, Brandenburg; BE, Berlin; BR, Bremen; HA, Hamburg; LS, Lower Saxony; MV, Mecklenburg-Vorpommern; SH, Schleswig-Holstein; SN, Saxony-Anhalt.
The mean VPD of the year’s driest day has increased in all regions by ca. 0.4 kPa decade−1, when the whole study period (1951–2017) is considered, but in the recent 1981–2017 period, there is only a tendency for an increase, which is not yet significant (Figure 2D). The maps in Figures 3B,C show that the temperature and VPD of the year’s warmest and driest days as indicators of heat and VPD extremes has increased since 1980 at a rather uniform rate across most of the lowlands, except for the direct coastal regions with slower change.
Growing season as derived from assumed temperature thresholds for bud burst and leaf fall is generally longer in the drier regions of the lowlands with a more continental climate (Supplementary Figure S3). It has decreased from 1951 to c. 1975 by 5–10 days especially at the drier sites, related to the (non-significant) decrease in mean summer temperatures in this period (see Supplementary Figure S2), but has rapidly increased by up to 10 days since then in all regions to reach a peak at around 2010 (Figure 4; Supplementary Figure S4). During the last decade (2010–2020), a slight decrease seems to materialize (Figure 4). From the 1951–1980 to the 1981–2020 period, mean growing season length has increased by on average 11 days in the wettest and the dry region, and by about 10 days in the wet region, and about 8 days in the driest region (Figure 5). At the site level, variation in growing season extension in this 35-year period was high (range: 6.5–12.0 days), even though all sites are located at similar elevation (70–140 m a.s.l.) (Supplementary Figure S5).

Figure 4. Change in growing season length in the four regions differing in mean growing season precipitation in the period 1951–2020. The vertical dashed line (1980) separates the two periods. Curve smoothing was done with a smoothing spline.
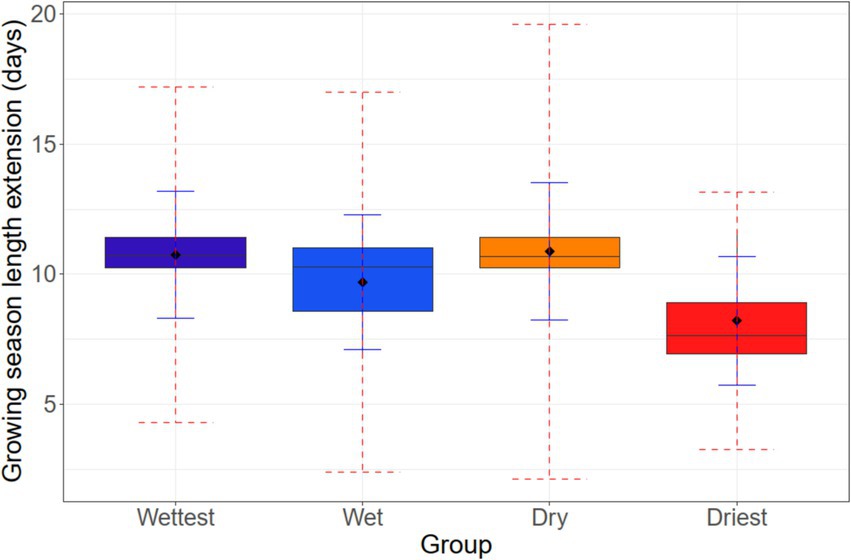
Figure 5. Growing season length extension from the 1951–1980 to the 1981–2020 period at the 30 locations in the northern German lowlands that were assigned to four growing-season precipitation classes (Wettest, Wet, Dry, and Driest). Given are the means of the four classes and the standard error of measurements (solid line) as well as the estimated total error, i.e., observed error plus model error (dashed line).
Of the tested thermal and hydrometeorological climate variables, SPEI of current June and July, and of previous July–September, had the most consistent influence on beech growth (Figure 6E), highlighting the growth-promoting effect of a positive climatic water balance in these summer months. Mean monthly VPD and monthly precipitation in current May and June and previous June/July had also a significant positive effect, but the signal was less consistent than for SPEI (Figure 6D). Interestingly, a negative effect of elevated June temperature (current year) and July–September temperatures (previous year) was more pronounced at the drier sites (Figures 6B,C). Elevated mean monthly minimum temperature had a positive effect in current May (most regions) and February (only wettest region) (Figure 6A), suggesting negative effects of spring and winter frost on growth.
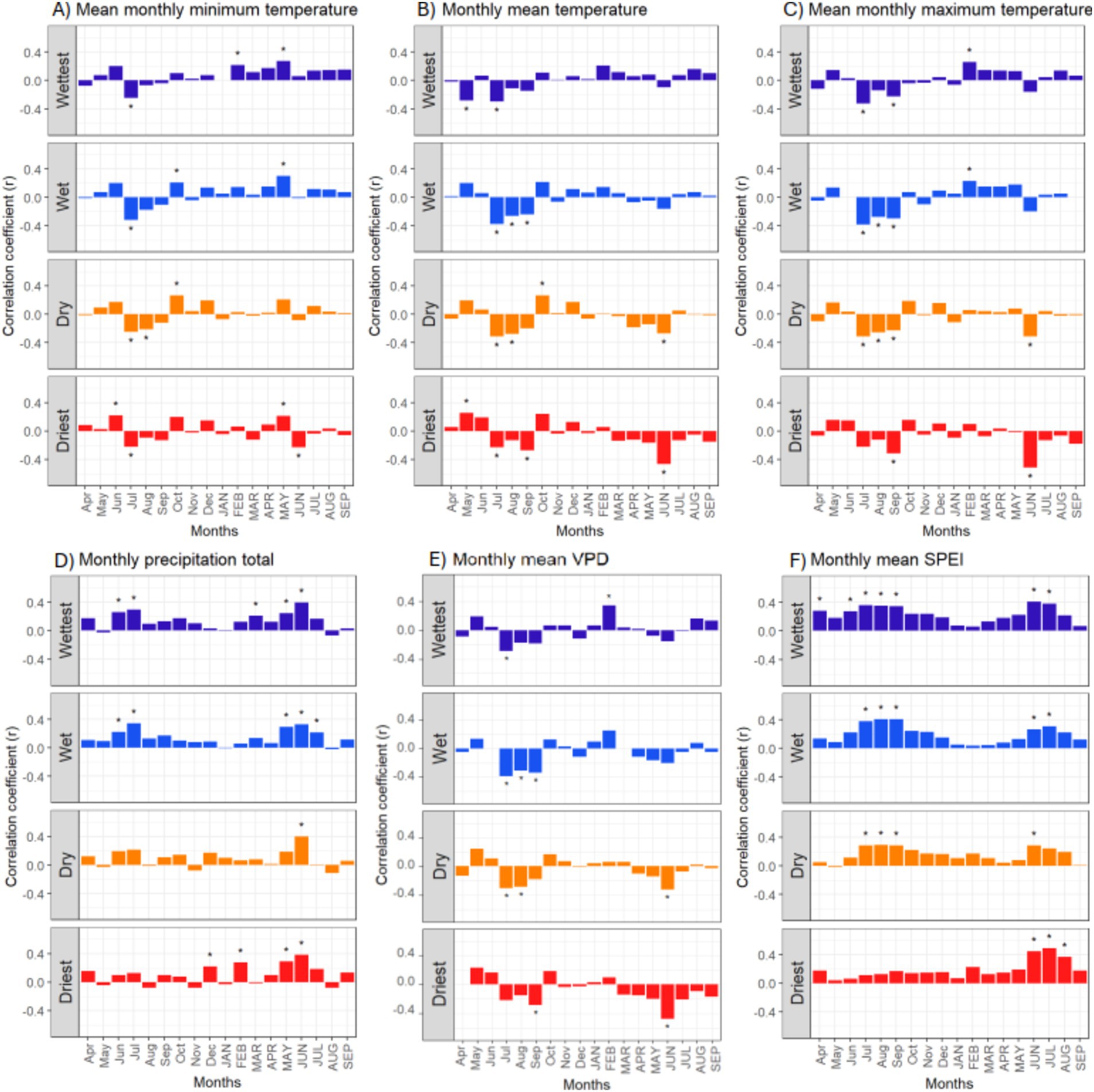
Figure 6. Climate-growth relationships for beech in the 1951–2017 period in the 30 stands that were assigned to four growing-season precipitation classes (Wettest, Wet, Dry, Driest) for the variables mean monthly minimum temperature (A), monthly mean temperature (B), mean of the monthly averaged daily maximum temperature (C), monthly precipitation total (D), monthly mean VPD (E), and monthly mean SPEI (F). Given are the Pearson correlation coefficients (r) for the relationships between ring width index (RWI) values and the monthly climate variables in the 18-month window from previous year’s April to current year’s September (small letters: previous year, capital letters: current year). Significant correlations (p < 0.05) are indicated by asterisks (*).
That extended heat periods are impeding growth is suggested by the negative correlation between growth and the number of hot days per year (significant for previous year’s influence in the wet, dry and driest regions; significant for current year’s influence in the driest region) (Figure 7A). The mean temperature of the year’s hottest day had a significant negative influence only in the driest region (Figure 7B). More influential was the VPD of the year’s driest day, which impacted growth negatively in all regions (previous year’s influence) (Figure 7C). While the correlation coefficients were generally higher for the monthly mean climate variables than for the annual heat and VPD extremes, the extremes displayed more clearly the contrasting behavior of the stands in the driest region.
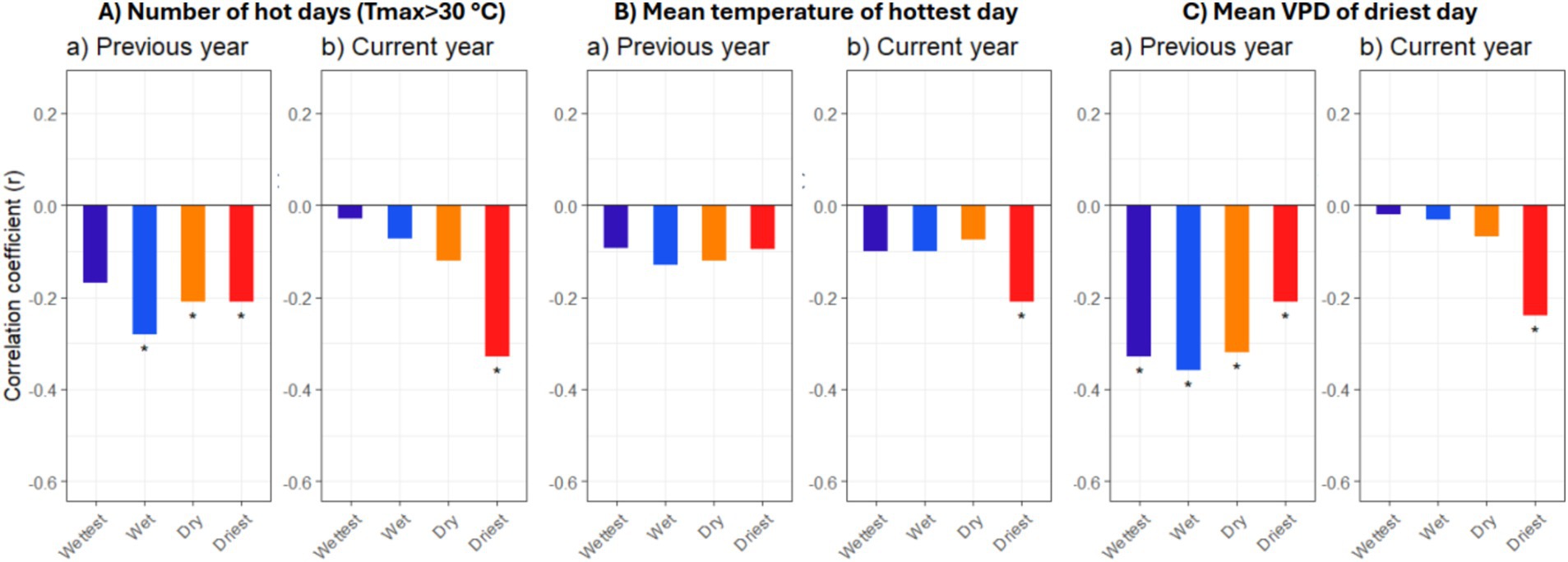
Figure 7. Climate-growth relationships for beech in the 1951–2017 period in the 30 stands that were assigned to four growing-season precipitation classes (Wettest, Wet, Dry, Driest) for the variables annual number of hot days (Tmax > 30°C) (A), mean temperature of the year’s hottest day (B), and mean VPD of the year’s driest day (C). Given are the Pearson correlation coefficients (r) for the relationships between ring width index (RWI) values and monthly climate variables either for the previous year (April–December) or current year (January–September) (averaged over the respective months). Significant correlations (p < 0.05) are indicated by asterisks (*).
Growing season length did not influence BAI in the wettest and wet regions, but showed a significant negative relation in the dry region, and a marginally significant one in the driest region (Supplementary Figure S6), indicating a growth decrease with an extension of growing season length. The significant positive relation between growing season length and the number of hot days in a year in the dry region (Supplementary Figure S7) suggests that heat is a main factor causing growth to decline with growing season extension. Interestingly, BAI was independent of growing season length, when the growing season varied between 130 and 150 days, but growth decreased when growing season length exceeded 150 days (Figure 8).
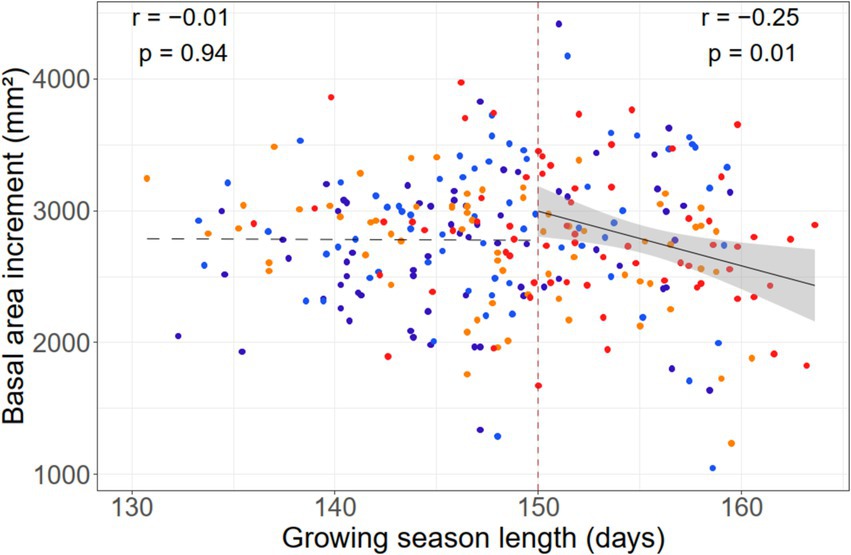
Figure 8. Dependence of the mean basal area increment of beech trees in the four growing-season precipitation classes (wettest: dark blue, wet: light blue, dry: orange, driest: red) on mean growing season length in the 1981–2017 period. While no relation appears for growing season lengths <150 days, a highly significant negative relation emerges for growing season lengths >150 days. For the latter case, the linear relationship with the 95% confidence interval is shown.
Since long-term hourly climate data, that would reflect climate extremes much better than daily means, were not available for the study region, we used the frequency of hot days with temperature maxima >30°C as a proxy for the occurrence of heat. Clearly, this measure does reflect the length of summer heat periods rather than the severity of heat events in a given year. Nevertheless, heat period length and heat intensity may often be correlated, and long-term trends in heat duration together with the mean temperature of the year’s hottest day can give physiologically relevant information on the growing exposure of the trees to heat during the last three to four decades. Since these data are available in the study region at high spatial resolution, we could also analyze regional differences in the long-term development of heat exposure. This situation is similar for VPD, where our daily mean values clearly miss the short-term vapor pressure deficit peaks that occur around noon and are most stressful to the plants. Again, by analyzing long-term trends in the mean VPD of the year’s driest day, we studied a proxy variable that likely correlates well with VPD maxima. Mean VPD of the year’s driest day increased since 1980 by about 0.18 kPa (12–20%) in our region; this increase was, however, not significant due to marked inter-annual fluctuation. This is similar to the global mean on the land surfaces, where average daily maximum VPD has increased in the 1980–2020 period by ca. 0.18 kPa, or by 0.021 hPa yr.−1 in the temperate zone (Novick et al., 2024). It is very likely that this increase has impacted the trees’ water status and growth rate (Köcher et al., 2012; Zweifel et al., 2021; Hammond et al., 2022).
The regional analysis of long-term thermal trends (Figure 3; Table 1) shows that the warming in the 1981–2020 period has proceeded at a similar rate in the wetter oceanic and the drier sub-continental regions of northern Germany. This is also valid for the mean temperature and mean VPD of the year’s hottest and driest day, suggesting in all sub-regions similar long-term trends for these climatic extremes. In contrast, the duration of heat periods as reflected in the number of hot days has increased faster in the drier (sub-continental) regions than in the wetter (more oceanic) regions, which could result from the establishment of more stable high pressure cells during heat episodes in the continental interior than near the coast. Atmospheric circulation patterns in summer in Western Europe are characterized by an increasing frequency of southerly inflows of air masses (Vautard et al., 2023). Moreover, weather conditions with an anticyclonic anomaly over the northern Atlantic (Labrador Sea and Greenland) and a cyclonic anomaly to the East of the British Isles, which drive calm and dry conditions over Western-Central Europe, have increased since the 1950s, favoring summertime heat waves especially in the more continental regions of Central Europe (Faranda et al., 2023). In our region, the more rapidly increasing heat exposure of the vegetation in the drier, more continental regions in comparison to the wetter, more oceanic regions is driven by two factors, (i) the on average higher temperatures with greater heat extremes in these regions, and (ii) the faster increase in the length of heat periods. Both have the potential to drive the trees of the drier regions faster toward their thermal limits.
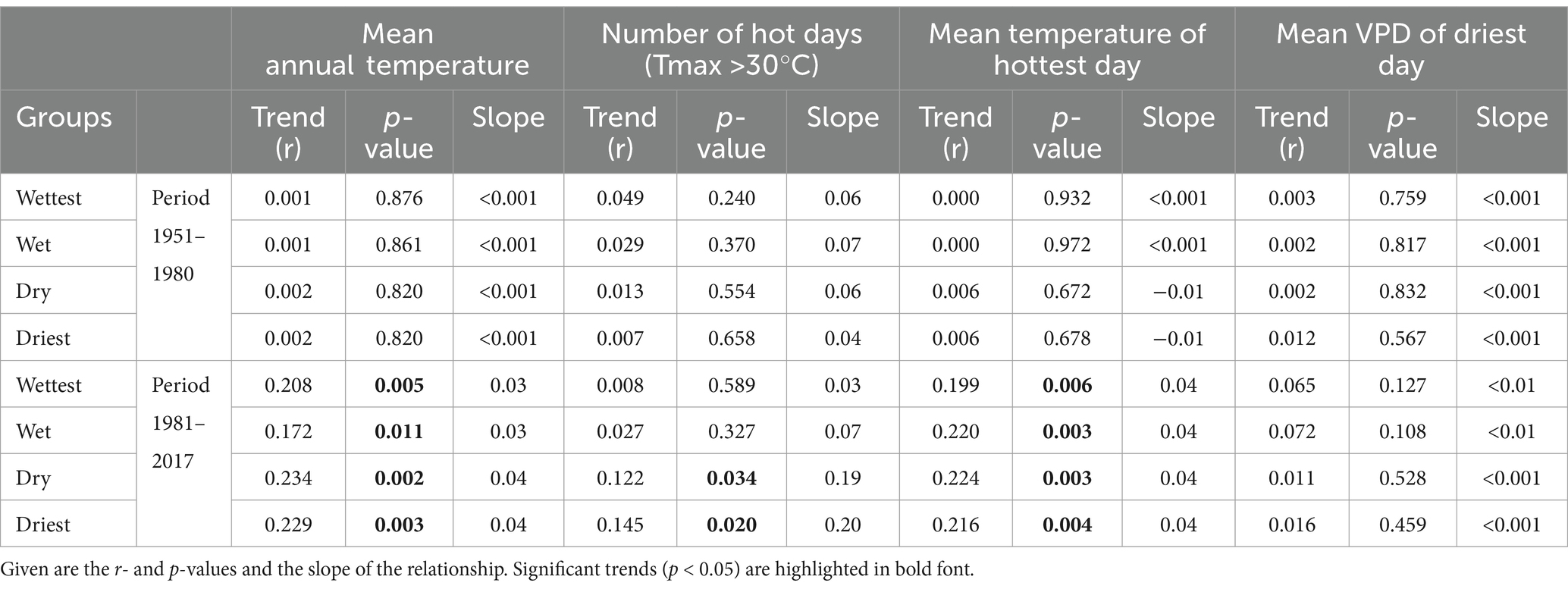
Table 1. Results of Mann-Kendall tests on trends in mean annual temperature, the number of hot days (Tmax > 30°C) per year, mean temperature of the year’s hottest day and mean VPD of the year’s driest day in the periods 1951–1980 and 1981–2017 in the northern German lowlands, analyzed separately for the wettest, wet, dry, and driest sites of the region.
It is increasingly recognized that heat and high VPD are exposing temperate trees to stress during hot drought episodes (Williams et al., 2013; Novick et al., 2024). Since these extremes typically last only for several hours of a day over a few days, their principal effect will be on leaf metabolism, while the longer-term impact on wood growth is less certain. Our study belongs to the few dendrochronological studies that have addressed the influence of heat and high VPD on radial growth. As higher temperatures are usually associated with higher atmospheric saturation deficits, both climatic factors are difficult to disentangle in dendrochronological studies, even though they are impacting plant metabolism in quite different ways. While drought is often acting in concert with heat, heat stress can independently harm plant metabolism (Adams et al., 2017; Kim and Portis, 2005). Particularly sensitive to high temperatures is the photosynthetic apparatus with photosystem II, where heat can negatively affect electron transport rate, Rubisco function, and thylakoid and cell membrane fluidity, increase photorespiration rate, and induce the production of reactive oxygen species (Salvucci and Crafts-Brandner, 2004; Teskey et al., 2015). The demonstrated increase in the number of hot days in the course of climate warming makes direct heat damage of adult and juvenile trees more likely (Williams et al., 2013).
That summer heat harms beech basal area increment in the study region, is suggested by the significant negative correlation between BAI and (i) mean maximum temperature in current June (only at the dry and driest sites; Figure 6C), (ii) mean temperature of the year’s hottest day (Figure 7B), and (iii) the number of hot days in the previous and current year (Figure 7A). The dendrochronological study of Enderle et al. (2024) in North-West and South-West German beech forests revealed a significant effect of the number of previous-year hot days on growth, but not of current-year hot days; this is confirmed in our study for the wettest, wet and dry regions, but not for the driest region. Here, the influence of current-year heat was clearly dominant over previous-year heat. A similar picture emerged for the influence of VPD extremes (mean VPD of the year’s driest day): in the driest region, current-year atmospheric drought was more important than previous-year VPD (Figure 7C). We speculate that the dominating negative heat and high-VPD effects in previous summer in all regions except for the driest sites are partly mediated through the stimulation of beech mast fruiting by these conditions, which reduces radial growth in the subsequent year (Hacket-Pain et al., 2015; Müller-Haubold et al., 2015). In the driest region, it is plausible that heat effects on current photosynthesis and growth are so strong that they are overlaying previous-year, mast fruiting-related effects of heat and VPD.
Fluorescence measurements on leaf discs suggest that the photosynthetic apparatus of beech is with T5 and T 50 values of 44.1 and 55.8°C, respectively (5 and 50% reduction of Fv/Fm, the ratio of variable to maximum fluorescence), somewhat more heat-sensitive than that of temperate light-demanding broad-leaf trees (Kunert and Hajek, 2022), but more heat-resistant than the needles of temperate conifers (Münchinger et al., 2023). From the correlation coefficients of the BAI − climate correlation analysis in our study, it appears that heat in current June can impact beech growth as severely as a reduction in the climatic water balance. This underpins that heat deserves more attention in the study of climate change effects on the health of beech and other temperate tree species.
Elevated VPD can negatively influence plant productivity through several causal pathways, among them lowered carbon gain due to reduced stomatal conductance and lowered leaf and cambial water potentials that reduce growth rate (Lendzion and Leuschner, 2008; Köcher et al., 2012; Grossiord et al., 2020; Novick et al., 2024). From the observation that beech growth was positively related to the climatic water balance (SPEI) in generally more summer months than it was to precipitation (Figures 6D,E), we conclude that VPD must act independently from precipitation on the water status and thus metabolism of beech, since SPEI is determined by both precipitation and VPD. Even though the long-term increase in the VPD of the year’s driest day was statistically weaker than was the increase of the heat-related variables, we assume from the largely different VPD influence on growth between the driest and the wetter regions (Figure 7C) that increasing VPD has the potential to impair beech growth. In accordance, air humidity manipulation experiments with beech saplings have demonstrated that increased VPD levels can reduce growth, independently of soil moisture availability (Lendzion and Leuschner, 2008).
Until recently, it was assumed that temperate forest productivity is primarily limited by low temperatures and low radiation (Nemani et al., 2003). In accordance, the substantial growth increase in Central European tree species during the last 100 years was largely attributed to rising temperatures and extended growing seasons (Spiecker et al., 2012; Pretzsch et al., 2014). Since growing season length is calculated with thermal parameters, rising temperatures are extending GSL, as the period with cold-limitation of growth shortens (Menzel et al., 2001; Linderholm, 2006). In contrast, climate cooling, as has happened in the study region in the 1951–1980 period, is associated with a GSL reduction. The slight GSL decrease calculated for the most recent decade in the study region relates to somewhat cooler spring and autumn temperatures in the years between 2008 and 2013 (as is indicated by Supplementary Figure S1). However, it is questionable whether growing season length can be deduced from temperature thresholds of growth onset and termination alone, especially in times of rapid climate aridification. Detailed monitoring of cambial activity in seven temperate tree species has shown that annual stem growth occurs only on 30–80% of the days within the growing season, when growth conditions are favorable (Etzold et al., 2022). Indeed, the radial growth of temperate trees occurs mainly at night, when VPD is lowest (Köcher et al., 2012; Zweifel et al., 2021) and it may cease in unfavorable periods during summer. This suggests that other factors than low temperatures are negatively impacting growth in much of the growing season. Among the most probable agents are soil and atmospheric drought as well as heat, all of which tend to increase in importance with climate warming. This must weaken the relation between GSL as defined by temperature thresholds and cumulative growth.
In fact, despite a marked warming since about 1980 in our region, our data do not show recent positive basal area increment trends of beech in the majority of stands (Supplementary Figure S8). Consequently, we did not find the anticipated positive relation between temperature-defined GSL and BAI. Rather, a negative GSL–BAI relation became visible in the full data set, when growing season length exceeded 150 days. A closer look revealed that this unexpected outcome was caused by growth decreases with growing season extension in the dry and driest regions, while no significant relationships existed in the wet and wettest regions. This is a clear hint that cambial activity has in recent decades indeed been constrained by additional factors than low temperature alone. Different GSL–BAI relationships in the wet and wettest regions as compared to the dry and driest regions support this finding. This discrepancy makes it likely that, in the drier, more continental climates, drought and/or heat have shifted the GSL–BAI relationship from a positive to a negative one in the recent past. Here, advancing climate warming does not only weaken low-temperature constraints on growth, but it apparently increasingly hampers growth despite an extended thermal growing season. This suggests that delimiting beech growing season length exclusively by means of thermal thresholds is not feasible in our region, but other growth controlling factors such as heat and drought have to be considered as well. The fact that GSL is in the dry region most tightly correlated with the number of hot days suggests that heat should be one of the additional growth-constraining factors, while drought is also plausible.
It should be mentioned that beech growth likely is influenced not only by climatic but by edaphic and demographic factors as well. While all stands were of relatively similar age and stocked on soils without groundwater influence, soil texture differed from sandy to loamy with related variation in soil water storage capacity. However, soil texture varied not systematically along the precipitation gradient and thus cannot explain the growth response pattern found between the wettest and driest sites.
The pronounced warming and drying of climate during the last 40 years has driven more than half of the dominant beech trees in the studied 30 northern German stands to negative growth trends. Yet, mortality rates have risen only slightly compared to the long-term mean, and only in the driest regions. Our climate-growth analysis suggests that the widespread growth decline likely is driven by heat and VPD extremes, besides the effect of a deteriorating climatic water balance in summer as a main cause. We therefore predict that further climatic warming will increase the stress exposure of these stands, at least in the upper canopy. Clearly, our dendroecological study has the shortcoming that the findings base on correlations, which do not allow firm conclusions on underlying mechanisms. Moreover, detecting the impact of climatic extremes on radial increment is likely complicated by the temporal mismatch between a short-term climatic trigger and a growth response that incorporates the influence of external and internal driving factors over a much longer time span. Future research should therefore combine dendrochronological studies with physiological research in mature trees and sapling experiments to deepen our understanding of the mechanisms through which heat and elevated VPD are reducing beech growth. This requires shifting some attention from the recent research focus on tree mortality to processes that drive long-term tree vitality decline, which may eventually lead to death as well. Further, systematic monitoring of canopy surface temperatures in different forest stands during heat episodes is needed to link weather station data to biologically meaningful temperature maxima and to assess the heat exposure of the foliage. Our study provides further evidence that growing season length, as computed from thermal thresholds, has lost its indicative value for tree and forest productivity in northern Germany.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
CL: Conceptualization, Funding acquisition, Project administration, Supervision, Validation, Writing – original draft. BB-E: Data curation, Formal analysis, Investigation, Software, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We thank two reviewers for their helpful comments on an early manuscript version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2024.1489081/full#supplementary-material
Adams, H. D., Zeppel, M. J., Anderegg, W. R., Hartmann, H., Landhäusser, S. M., Tissue, D. T., et al. (2017). A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat. Ecol. Evol. 1, 1285–1291. doi: 10.1038/s41559-017-0248-x
Allen, C. D., Macalady, A. K., Chenchouni, H., Bachelet, D., McDowell, N. G., Vennetier, M. T., et al. (2010). A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 259, 660–684. doi: 10.1016/j.foreco.2009.09.001
Anderegg, W. R. L., Kane, J. M., and Anderegg, L. D. L. (2013). Consequences of widespread tree mortality triggered by drought and temperature stress. Nat. Clim. Chang. 3, 30–36. doi: 10.1038/nclimate1635
Arend, M., Link, R. M., Zahnd, C., Hoch, G., Schuldt, B., and Kahmen, A. (2022). Lack of hydraulic recovery as a cause of post-drought foliage reduction and canopy decline in European beech. New Phytol. 234, 1195–1205. doi: 10.1111/nph.18065
Baldocchi, D. D., and Wilson, K. B. (2001). Modeling CO2 and water vapor exchange of a temperate broadleaved forest across hourly to decadal time scales. Ecol. Model. 142, 155–184. doi: 10.1016/S0304-3800(01)00287-3
Blickensdörfer, L., Oehmichen, K., Pflugmacher, D., Kleinschmit, B., and Hostert, P. (2022). Dominant tree species for Germany (2017/2018) : Open Agrar. [computer software]. Available at: https://atlas.thuenen.de/catalogue/#/dataset/100
Boessenkool, B. (2023). Rdwd: select and download climate data from “DWD” (German weather service) (version 1.8.0) [computer software]. Available at: https://cran.r-project.org/web/packages/rdwd/index.html (Accessed May 10, 2024).
Bontemps, J.-D., Hervé, J.-C., and Dhôte, J.-F. (2010). Dominant radial and height growth reveal comparable historical variations for common beech in North-Eastern France. For. Ecol. Manag. 259, 1455–1463. doi: 10.1016/j.foreco.2010.01.019
Bose, A. K., Gessler, A., Bolte, A., Bottero, A., Buras, A., Cailleret, M., et al. (2020). Growth and resilience responses of scots pine to extreme droughts across Europe depend on predrought growth conditions. Glob. Change Biol. 26, 4521–4537. doi: 10.1111/gcb.15153
Bose, A. K., Scherrer, D., Camarero, J. J., Ziche, D., Babst, F., Bigler, C., et al. (2021). Climate sensitivity and drought seasonality determine post-drought growth recovery of Quercus petraea and Quercus robur in Europe. Sci. Tot. Env. 784:147222. doi: 10.1016/j.scitotenv.2021.147222
Braun, S., Hopf, S.-E., Tresch, S., Remund, J., and Schindler, C. (2021). 37 years of Forest monitoring in Switzerland: drought effects on Fagus sylvatica. Front. For. Glob. Change 4:765782. doi: 10.3389/ffgc.2021.765782
Bunn, A. G. (2008). A dendrochronology program library in R (dplR). Dendrochronologia 26, 115–124. doi: 10.1016/j.dendro.2008.01.002
Charru, M., Seynave, I., Morneau, F., and Bontemps, J.-D. (2010). Recent changes in forest productivity: an analysis of national forest inventory data for common beech (Fagus sylvatica L.) in North-Eastern France. For. Ecol. Manag. 260, 864–874. doi: 10.1016/j.foreco.2010.06.005
Churkina, G., Schimel, D., Braswell, B. H., and Xiao, X. M. (2005). Spatial analysis of growing season length control over net ecosystem exchange. Glob. Change Biol. 11, 1777–1787. doi: 10.1111/j.1365-2486.2005.001012.x
Debel, A., Meier, W. J., and Bräuning, A. (2021). Climate signals for growth variations of F. sylvatica, P. abies, and P. sylvestris in Southeast Germany over the past 50 years. Forests 12:1433. doi: 10.3390/f12111433
Deutscher Wetterdienst. (2023). DWD climate data center (CDC). Available at: https://www.dwd.de/EN/ourservices/cdcftp/cdcftp.html (Accessed May 15, 2024).
Diers, M., Leuschner, C., Dulamsuren, C., Schulz, T. C., and Weigel, R. (2024). Increasing winter temperatures stimulate scots pine growth in the north German lowlands despite stationary sensitivity to summer drought. Ecosystems 27, 428–442. doi: 10.1007/s10021-023-00897-3
Dietrich, L., and Kahmen, A. (2019). Water relations of drought-stressed temperate trees benefit from short drought-intermitting rainfall events. Agric. For. Meteorol. 265, 70–77. doi: 10.1016/j.agrformet.2018.11.012
Dorado-Liñán, I., Akhmetzyanov, L., and Menzel, A. (2017). Climate threats on growth of rear-edge European beech peripheral populations in Spain. Int. J. Biometeorol. 61, 2097–2110. doi: 10.1007/s00484-017-1410-5
Eckstein, D., and Bauch, J. (1969). Beitrag zur Rationalisierung eines dendrochronologischen Verfahrens und zur Analyse seiner Aussagesicherheit. Forstwiss Centralbl. 88, 230–250. doi: 10.1007/BF02741777
Enderle, L., Gribbe, S., Muffler, L., Weigel, R., Hertel, D., and Leuschner, C. (2024). A warmer climate impairs the growth performance of Central Europe’s major timber species in lowland regions. Sci. Tot. Env. 941:173665. doi: 10.1016/j.scitotenv.2024.173665
Etzold, S., Sterck, F., Bose, A. K., Braun, S., Buchmann, N., Eugster, W., et al. (2022). Number of growth days and not length of the growth period determines radial stem growth of temperate trees. Ecol. Lett. 25, 427–439. doi: 10.1111/ele.13933
Faranda, D., Messori, G., Jezequel, A., and Yiou, P. (2023). Atmospheric circulation compounds anthropogenic warming and impacts of climate extremes in Europe. Proc. Natl. Acad. Sci. USA 120:e2214525120. doi: 10.1073/pnas.2214525120
Frei, E. R., Gossner, M. M., Vitasse, Y., Queloz, V., Dubach, V., Gessler, A., et al. (2022). European beech dieback after premature leaf senescence during the 2018 drought in northern Switzerland. Plant Biol. 24, 1132–1145. doi: 10.1111/plb.13467
George, J.-P., Bürkner, P.-C., Sanders, T. G., Neumann, M., Cammalleri, C., Vogt, J. V., et al. (2022). Long-term forest monitoring reveals constant mortality rise in European forests. Plant Biol. 24, 1108–1119. doi: 10.1111/plb.13469
Gessler, A., Keitel, C., Kreutzer, K., Matyssek, R., and Seiler, W. (2007). Potential risks for European beech (Fagus sylvatica L.) in a changing climate. Trees. 21, 1–11. Available at: https://link.springer.com/article/10.1007/s00468-006-0107-x
Gribbe, S., Enderle, L., Weigel, R., Hertel, D., Leuschner, C., and Muffler, L. (2024). Recent growth decline and shifts in climatic growth constraints suggest climate vulnerability of beech, Douglas fir, pine and oak in northern Germany. For. Ecol. Manag. 566:122022. doi: 10.1016/j.foreco.2024.122022
Griffis, T. J., Black, T. A., Morgenstern, K., Barr, A. G., Nesic, Z., Drewitt, G. B., et al. (2003). Ecophysiological controls on the carbon balances of three southern boreal forests. Agric. For. Meteorol. 117, 53–71. doi: 10.1016/S0168-1923(03)00023-6
Grossiord, C., Buckley, T. N., Cernusak, L. A., Novick, K. A., Poulter, B., Siegwolf, R. T. W., et al. (2020). Plant responses to rising vapor pressure deficit. New Phytol. 226, 1550–1566. doi: 10.1111/nph.16485
Hacket-Pain, A. J., Friend, A. D., Lageard, J. G., and Thomas, P. A. (2015). The influence of masting phenomenon on growth–climate relationships in trees: explaining the influence of previous summers’ climate on ring width. Tree Physiol. 35, 319–330. doi: 10.1093/treephys/tpv007
Hammond, W. M., Williams, A. P., Abatzoglou, J. T., Adams, H. D., Klein, T., López, R., et al. (2022). Global field observations of tree die-off reveal hotter-drought fingerprint for Earth’s forests. Nat. Commun. 13:1761. doi: 10.1038/s41467-022-29289-2
Hanewinkel, M., Cullmann, D. A., Schelhaas, M.-J., Nabuurs, G.-J., and Zimmermann, N. E. (2013). Climate change may cause severe loss in the economic value of European forest land. Nat. Clim. Chang. 3, 203–207. doi: 10.1038/nclimate1687
Härdtle, W., Niemeyer, T., Assmann, T., Baiboks, S., Fichtner, A., Friedrich, U., et al. (2013). Long-term trends in tree-ring width and isotope signatures (δ 13 C, δ 15 N) of Fagus sylvatica L. on soils with contrasting water supply. Ecosystems 16, 1413–1428. doi: 10.1007/s10021-013-9692-x
Henkel, A., Hese, S., and Thiel, C. (2022). Erhöhte Buchenmortalität im Nationalpark Hainich? AFZ Der Wald 3, 26–29.
Herbette, S., Wortemann, R., Awad, H., Huc, R., Cochard, H., and Barigah, T. S. (2010). Insights into xylem vulnerability to cavitation in Fagus sylvatica L.: phenotypic and environmental sources of variability. Tree Physiol. 30, 1448–1455. doi: 10.1093/treephys/tpq079
IPCC (2021). “Climate change 2021: the physical science basis” in Contribution of working group I to the sixth assessment report of the intergovernmental panel on climate change. (Cambridge, UK: Cambridge University Press).
Jump, A. S., Hunt, J. M., and Peñuelas, J. (2006). Rapid climate change-related growth decline at the southern range edge of Fagus sylvatica. Glob. Change Biol. 12, 2163–2174. doi: 10.1111/j.1365-2486.2006.01250.x
Keenan, T. F., Gray, J., Friedl, M. A., Toomey, M., Bohrer, G., Hollinger, D. Y., et al. (2014). Net carbon uptake has increased through warming-induced changes in temperate forest phenology. Nat. Clim. Chang. 4, 598–604. doi: 10.1038/nclimate2253
Kim, K., and Portis, A. R. Jr. (2005). Temperature dependence of photosynthesis in Arabidopsis plants with modifications in rubisco activase and membrane fluidity. Plant Cell Physiol. 46, 522–530. doi: 10.1093/pcp/pci052
Kint, V., Aertsen, W., Campioli, M., Vansteenkiste, D., Delcloo, A., and Muys, B. (2012). Radial growth change of temperate tree species in response to altered regional climate and air quality in the period 1901–2008. Clim. Chang. 115, 343–363. doi: 10.1007/s10584-012-0465-x
Klesse, S., Wohlgemuth, T., Meusburger, K., Vitasse, Y., von Arx, G., Lévesque, M., et al. (2022). Long-term soil water limitation and previous tree vigor drive local variability of drought-induced crown dieback in Fagus sylvatica. Sci. Tot. Env. 851:157926. doi: 10.1016/j.scitotenv.2022.157926
Knutzen, F., Dulamsuren, C., Meier, I. C., and Leuschner, C. (2017). Recent climate warming-related growth decline impairs European beech in the center of its distribution range. Ecosystems 20, 1494–1511. doi: 10.1007/s10021-017-0128-x
Köcher, P., Horna, V., and Leuschner, C. (2012). Environmental control of daily stem growth patterns in five temperate broad-leaved tree species. Tree Physiol. 32, 1021–1032. doi: 10.1093/treephys/tps049
Kunert, N., and Hajek, P. (2022). Shade-tolerant temperate broad-leaved trees are more sensitive to thermal stress than light-demanding species during a moderate heatwave. Trees For. People 9:100282. doi: 10.1016/j.tfp.2022.100282
Lakatos, F., and Molnár, M. (2009). Mass mortality of beech (Fagus sylvatica L.) in south-West Hungary. Acta Silv. Lign. Hung. 5, 75–82. doi: 10.37045/aslh-2009-0006
Lendzion, J., and Leuschner, C. (2008). Growth of European beech (Fagus sylvatica L.) saplings is limited by elevated atmospheric vapour pressure deficits. For. Ecol. Manag. 256, 648–655. doi: 10.1016/j.foreco.2008.05.008
Leuschner, C. (2020). Drought response of European beech (Fagus sylvatica L.)—a review. Perspect. Plant Ecol. Evol. Syst. 47:125576. doi: 10.1016/j.ppees.2020.125576
Leuschner, C. (2024). Trockenstress- und Hitzeempfindlichkeit wichtiger Baumarten - Vitalität von Norddeutschlands Wäldern im Klimawandel. Ber Reinh-Tüxen-Ges. 32, 133–156.
Leuschner, C., and Ellenberg, H. (2017). Ecology of central European forests: vegetation ecology of Central Europe. Cham: Springer International Publishing.
Leuschner, C., Fuchs, S., Wedde, P., Rüther, E., and Schuldt, B. (2024). A multi-criteria drought resistance assessment of temperate Acer, Carpinus, Fraxinus, Quercus, and Tilia species. Perspect. Plant Ecol. Evol. Syst. 62:125777. doi: 10.1016/j.ppees.2023.125777
Leuschner, C., Wedde, P., and Lübbe, T. (2019). The relation between pressure–volume curve traits and stomatal regulation of water potential in five temperate broadleaf tree species. Ann. For. Sci. 76, 1–14. doi: 10.1007/s13595-019-0838-7
Leuschner, C., Weithmann, G., Bat-Enerel, B., and Weigel, R. (2023). The future of European beech in northern Germany—climate change vulnerability and adaptation potential. Forests 14:1448. doi: 10.3390/f14071448
Lian, X., Piao, S., Li, L. Z., Li, Y., Huntingford, C., Ciais, P., et al. (2020). Summer soil drying exacerbated by earlier spring greening of northern vegetation. Sci. Adv. 6:eaax0255. doi: 10.1126/sciadv.aax0255
Linderholm, H. W. (2006). Growing season changes in the last century. Agric. For. Meteorol. 137, 1–14. doi: 10.1016/j.agrformet.2006.03.006
Martinez del Castillo, E., Zang, C. S., Buras, A., Hacket-Pain, A. J., Esper, J., Serrano-Notivoli, R., et al. (2022). Climate-change-driven growth decline of European beech forests. Commun. Biol. 5:163. doi: 10.1038/s42003-022-03107-3
McDowell, N. G., Sapes, G., Pivovaroff, A., Adams, H. D., Allen, C. D., Anderegg, W. R., et al. (2022). Mechanisms of woody-plant mortality under rising drought, CO2 and vapour pressure deficit. Nat. Rev. Earth Environ. 3, 294–308. doi: 10.1038/s43017-022-00272-1
Menzel, A. (1997). Phänologie von Waldbäumen unter sich ändernden Klimabedingungen: Auswertung der Beobachtungen in den internationalen phänologischen Gärten und Möglichkeiten der Modellierung von Phänodaten. München: Lehrstuhl für Bioklimatologie und Immissionsforschung der Univ Available at: https://books.google.de/books?id=bxvCNQAACAAJ (Accessed June 12, 2024).
Menzel, A., Estrella, N., and Fabian, P. (2001). Spatial and temporal variability of the phenological seasons in Germany from 1951 to 1996. Glob. Change Biol. 7, 657–666. doi: 10.1111/j.1365-2486.2001.00430.x
Menzel, A., and Fabian, P. (1999). Growing season extended in Europe. Nature 397:659. doi: 10.1038/17709
Menzel, A., Sparks, T., Estrella, N., Koch, E., Aasa, A., Ahas, R., et al. (2006). European phenological response to climate change matches the warming pattern. Glob. Change Biol. 12, 1969–1976. doi: 10.1111/j.1365-2486.2006.01193.x
Möhring, B., Bitter, A., Bub, G., Dieter, M., Dög, M., and Hanewinkel, M. (2021). Schadenssumme insgesamt 12,7 Mrd. Euro: Abschätzung der ökonomischen Schäden der Extremwetterereignisse der Jahre 2018 bis 2020 in der Forstwirtschaft. Holz-Zentralbl 147, 155–158. Available at: https://literatur.thuenen.de/digbib_extern/dn063403.pdf
Müller-Haubold, H., Hertel, D., and Leuschner, C. (2015). Climatic drivers of mast fruiting in European beech and resulting C and N allocation shifts. Ecosystems 18, 1083–1100. doi: 10.1007/s10021-015-9885-6
Münchinger, I. K., Hajek, P., Akdogan, B., Caicoya, A. T., and Kunert, N. (2023). Leaf thermal tolerance and sensitivity of temperate tree species are correlated with leaf physiological and functional drought resistance traits. J. Forestry Res. 34, 63–76. doi: 10.1007/s11676-022-01594-y
Nemani, R. R., Keeling, C. D., Hashimoto, H., Jolly, W. M., Piper, S. C., Tucker, C. J., et al. (2003). Climate-driven increases in global terrestrial net primary production from 1982 to 1999. Science 300, 1560–1563. doi: 10.1126/science.1082750
Neycken, A., Scheggia, M., Bigler, C., and Lévesque, M. (2022). Long-term growth decline precedes sudden crown dieback of European beech. Agric. For. Meteorol. 324:109103. doi: 10.1016/j.agrformet.2022.109103
Novick, K. A., Ficklin, D. L., Grossiord, C., Konings, A. G., Martinez-Vilalta, J., Sadok, W., et al. (2024). The impacts of rising vapour pressure deficit in natural and managed ecosystems. Plant Cell Environ. 47, 3561–3589. doi: 10.1111/pce.14846
Nuske, R. S. (2022). Vegperiod: determining vegetation periods based on temperature criteria. R Package Version 1.0.0. Available at: https://rnuske.github.io/vegperiod/reference/vergperiod.html
Obladen, N., Dechering, P., Skiadaresis, G., Tegel, W., Keßler, J., Höllerl, S., et al. (2021). Tree mortality of European beech and Norway spruce induced by 2018-2019 hot droughts in Central Germany. Agric. For. Meteorol. 307:108482. doi: 10.1016/j.agrformet.2021.108482
Piovesan, G., Biondi, F., Filippo, A. D., Alessandrini, A., and Maugeri, M. (2008). Drought-driven growth reduction in old beech (Fagus sylvatica L.) forests of the central Apennines, Italy. Glob. Change Biol. 14, 1265–1281. doi: 10.1111/j.1365-2486.2008.01570.x
Pretzsch, H., Biber, P., Schütze, G., Uhl, E., and Rötzer, T. (2014). Forest stand growth dynamics in Central Europe have accelerated since 1870. Nat. Commun. 5:4967. doi: 10.1038/ncomms5967
R Core Team. (2023). R: a language and environment for statistical computing. R Foundation for Statistical Computing. Available at: https://www.R-project.org/ (Accessed May 10, 2024)
Ren, P., Ziaco, E., Rossi, S., Biondi, F., Prislan, P., and Liang, E. (2019). Growth rate rather than growing season length determines wood biomass in dry environments. Agric. For. Meteorol. 271, 46–53. doi: 10.1016/j.agrformet.2019.02.031
Ruehr, N., Gast, A., Weber, C., Daub, B., and Arneth, A. (2015). Water availability as dominant control of heat stress responses in two contrasting tree species. Tree Physiol. 36, tpv102–tpv178. doi: 10.1093/treephys/tpv102
Salvucci, M. E., and Crafts-Brandner, S. J. (2004). Relationship between the heat tolerance of photosynthesis and the thermal stability of rubisco activase in plants from contrasting thermal environments. Plant Physiol. 134, 1460–1470. doi: 10.1104/pp.103.038323
Scharnweber, T., Manthey, M., Criegee, C., Bauwe, A., Schröder, C., and Wilmking, M. (2011). Climate-growth relationships of beech and oak along a precipitation gradient in northeastern Germany. For. Ecol. Manag. 262, 947–961. doi: 10.1016/j.foreco.2011.05.026
Schuldt, B., Buras, A., Arend, M., Vitasse, Y., Beierkuhnlein, C., Damm, A., et al. (2020). A first assessment of the impact of the extreme 2018 summer drought on central european forests. Basic Appl. Ecol. 45, 86–103. Available at: https://www.sciencedirect.com/science/article/pii/S1439179120300414
Schuldt, B., Knutzen, F., Delzon, S., Jansen, S., Müller-Haubold, H., Burlett, R., et al. (2016). How adaptable is the hydraulic system of European beech in the face of climate change-related precipitation reduction? New Phytol. 210, 443–458. doi: 10.1111/nph.13798
Senf, C., Buras, A., Zang, C. S., Rammig, A., and Seidl, R. (2020). Excess forest mortality is consistently linked to drought across Europe. Nat. Commun. 11:6200. doi: 10.1038/s41467-020-19924-1
Serra-Maluquer, X., Gazol, A., Sangüesa-Barreda, G., Sánchez-Salguero, R., Rozas, V., Colangelo, M., et al. (2019). Geographically structured growth decline of rear-edge Iberian Fagus sylvatica forests after the 1980s shift toward a warmer climate. Ecosystems 22, 1325–1337. doi: 10.1007/s10021-019-00339-z
Spiecker, H., Mielikäinen, K., Köhl, M., and Skovsgaard, J. P. (2012). Growth trends in European forests: studies from 12 countries. Berlin: Springer Science & Business Media.
Stolz, J., van der Maaten, E., Kalanke, H., Martin, J., Wilmking, M., and van der Maaten-Theunissen, M. (2021). Increasing climate sensitivity of beech and pine is not mediated by adaptation and soil characteristics along a precipitation gradient in northeastern Germany. Dendrochronologia 67:125834. doi: 10.1016/j.dendro.2021.125834
Suck, R., Bushart, M., Hofmann, G., and Schröder, L. (2014). Karte der Potentiellen Natürlichen Vegetation Deutschlands: 3. Band. BfN-Skripten 377. Bonn: Bundesamt für Naturschutz.
Teskey, R., Wertin, T., Bauweraerts, I., Ameye, M., McGuire, M. A., and Steppe, K. (2015). Responses of tree species to heat waves and extreme heat events. Plant Cell Environ. 38, 1699–1712. doi: 10.1111/pce.12417
Thackeray, S. J., Henrys, P. A., Hemming, D., Bell, J. R., Botham, M. S., Burthe, S., et al. (2016). Phenological sensitivity to climate across taxa and trophic levels. Nature 535, 241–245. doi: 10.1038/nature18608
Thom, D., Buras, A., Heym, M., Klemmt, H.-J., and Wauer, A. (2023). Varying growth response of central European tree species to the extraordinary drought period of 2018–2020. Agric. For. Meteorol. 338:109506. doi: 10.1016/j.agrformet.2023.109506
Thonfeld, F., Gessner, U., Holzwarth, S., Kriese, J., Da Ponte, E., Huth, J., et al. (2022). A first assessment of canopy cover loss in Germany’s forests after the 2018–2020 drought years. Remote Sens. 14:562. doi: 10.3390/rs14030562
Vautard, R., Cattiaux, J., Happé, T., Singh, J., Bonnet, R., Cassou, C., et al. (2023). Heat extremes in Western Europe increasing faster than simulated due to atmospheric circulation trends. Nat. Commun. 14:6803. doi: 10.1038/s41467-023-42143-3
Vicente-Serrano, S. M., Begueria, S., and Lopez-Moreno, J. I. (2010). A multi-scalar drought index sensitive to global warming: the standardized precipitation evapotranspiration index–SPEI. J. Clim. 23, 1696–1718. doi: 10.1175/2009JCLI2909.1
von Wilpert, K. (1990). Die Jahrringstruktur von Fichten in Abhängigkeit vom Bodenwasserhaushalt auf Pseudogley und Parabraunerde: Ein Methodenkonzept zur Erfassung standortsspezifischer Wasserstreßdisposition. Freiburg: Freiburger Bodenkundliche Abhandlungen.
Walther, O. G. R., Post, E., Convey, P., Menzel, A., Parmesan, C., Beebee, T. J. C., et al. (2002). Ecological responses to recent climate change. Nature 416, 389–395. doi: 10.1038/416389a
Walthert, L., Ganthaler, A., Mayr, S., Saurer, M., Waldner, P., Walser, M., et al. (2021). From the comfort zone to crown dieback: sequence of physiological stress thresholds in mature European beech trees across progressive drought. Sci. Tot. Environ. 753:141792. doi: 10.1016/j.scitotenv.2020.141792
Weigel, R., Bat-Enerel, B., Dulamsuren, C., Muffler, L., Weithmann, G., and Leuschner, C. (2023). Summer drought exposure, stand structure, and soil properties jointly control the growth of European beech along a steep precipitation gradient in northern Germany. Glob. Change Biol. 29, 763–779. doi: 10.1111/gcb.16506
Weithmann, G., Paligi, S. S., Schuldt, B., and Leuschner, C. (2022). Branch xylem vascular adjustments in European beech in response to decreasing water availability across a precipitation gradient. Tree Physiol. 42, 2224–2238. doi: 10.1093/treephys/tpac080
Wigley, T. M., Briffa, K. R., and Jones, P. D. (1984). On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J. Appl. Meteorol. Climatol. 23, 201–213. doi: 10.1175/1520-0450(1984)023<0201:OTAVOC>2.0.CO;2
Williams, A. P., Allen, C. D., Macalady, A. K., Griffin, D., Woodhouse, C. A., Meko, D. M., et al. (2013). Temperature as a potent driver of regional forest drought stress and tree mortality. Nat. Clim. Change 3, 292–297. doi: 10.1038/nclimate1693
Wohlgemuth, T., Kistler, M., Aymon, C., Hagedorn, F., Gessler, A., Gossner, M. M., et al. (2020). Früher Laubfall der Buche während der Sommertrockenheit 2018: Resistenz oder Schwächesymptom? Schweiz Zeitschr Forstwes 171, 257–269. doi: 10.3188/szf.2020.0257
Yousefpour, R., and Hanewinkel, M. (2016). Climate change and decision-making under uncertainty. Curr. For. Rep. 2, 143–149. doi: 10.1007/s40725-016-0035-y
Zang, C., and Biondi, F. (2015). Treeclim: an R package for the numerical calibration of proxy-climate relationships. Ecography 38, 431–436. doi: 10.1111/ecog.01335
Zimmermann, J., Hauck, M., Dulamsuren, C., and Leuschner, C. (2015). Climate warming-related growth decline affects Fagus sylvatica, but not other broad-leaved tree species in central European mixed forests. Ecosystems 18, 560–572. doi: 10.1007/s10021-015-9849-x
Keywords: dendrochronology, climate-growth analysis, growing season length, growth decline, number of hot days, vapor pressure deficit
Citation: Leuschner C and Bat-Enerel B (2024) Effects of heat, elevated vapor pressure deficits and growing season length on growth trends of European beech. Front. For. Glob. Change. 7:1489081. doi: 10.3389/ffgc.2024.1489081
Received: 31 August 2024; Accepted: 04 November 2024;
Published: 09 December 2024.
Edited by:
Tobias Rütting, University of Gothenburg, SwedenReviewed by:
Jesús Julio Camarero, Spanish National Research Council (CSIC), SpainCopyright © 2024 Leuschner and Bat-Enerel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christoph Leuschner, Y2xldXNjaEBnd2RnLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.