- 1Department of Pediatrics, Incheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea
- 2Department of Pediatrics, Eunpyeong St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea
- 3Department of Pediatrics, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea
- 4Department of Pediatrics, St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea
- 5Department of Pediatrics, Bucheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea
- 6Department of Pediatrics, Yeouido St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea
Background: Irisin is an adipomyokine secreted by muscle and adipose cells, and it plays a role in glucose, fat, and bone metabolism. This study aimed to determine the correlation of serum irisin levels with anthropometric, metabolic, and bone parameters in obese children and adolescents.
Methods: This single-center study included 103 Korean children and adolescents: 54 (52.4%) obese participants with a body mass index (BMI) ≥95th percentile and 49 (47.6%) healthy controls with BMI within the 15th to 85th percentile. Various parameters were measured, including fasting blood glucose, fasting insulin, homeostasis model assessment of insulin resistance (HOMA-IR), triglyceride and glucose (TyG) index, lipid profile, alkaline phosphatase (ALP), osteocalcin, and 25(OH)-Vitamin D levels. Bone mineral density (BMD) was measured using dual-energy X-ray absorptiometry (DEXA) in 33 healthy subjects.
Results: Serum irisin was significantly higher in the obese group than in the control group (mean 18.1 ± 3.5 vs. 16.2 ± 2.0 ng/mL; p = 0.001). Serum irisin level was positively correlated with chronological age (r = 0.28; p = 0.004), height SDS (r = 0.24; p = 0.02), BMI SDS (r = 0.37; p < 0. 001), fasting glucose (r = 0.27; p = 0.007), fasting insulin (r = 0.23; p = 0.03), HOMA-IR (r = 0.21; p = 0.04), osteocalcin (r = 0.27; p = 0.006) and negatively correlated with HDL cholesterol (r = -0.29; p = 0.005). All these correlations were evident in obese subjects but not in healthy subjects. ALP and 25(OH)-Vitamin D were unrelated to irisin levels. Among 33 healthy subjects, total body-less head (TBLH) BMD Z-score was positively correlated with serum irisin (r = 0.39; p = 0.03), osteocalcin (r = 0.40; p = 0.02), fasting insulin (r = 0.39; p = 0.04), and HOMA-IR (r = 0.38; p = 0.047).
Conclusion: This study demonstrated an association between irisin levels and glucose, lipid, and bone parameters in children and adolescents. Our findings suggest that irisin has a potential role in metabolic disorders and bone health in obese children and adolescents.
Introduction
Muscle cells produce and release cytokines and chemokines, collectively known as myokines, during physical activity. Representative myokines include myostatin, brain-derived neurotrophic factor (BDNF), interleukin-6, fibroblast growth factor-21 (FGF-21), monocyte chemotactic protein 1 (MCP1), insulin-like growth factor-1 (IGF-1), and irisin (1). Irisin was first identified as a myokine secreted by muscle in response to exercise (2), later determined to be also secreted by white adipose tissue (3). Irisin is cleaved from its precursor fibronectin type III domain-containing protein 5 (FNDC5) in skeletal muscle cells and is responsible for white fat browning, promoting thermogenesis and energy expenditure (4).
Irisin facilitates glucose uptake by skeletal muscles and improves glucose and lipid profile levels in obesity and type 2 diabetes patients, making it an important factor in metabolic regulation (5). Circulating irisin was significantly lower in individuals with T2DM than in non-diabetic controls (6). In non-diabetic patients, circulating irisin levels correlate positively with fasting glucose, fasting insulin, and homeostatic model assessment (HOMA) (6, 7). Irisin has recently received much attention in the treatment of obesity, which is one of the biggest public health problems worldwide. Animal studies have shown downregulation of FNDC5 in both skeletal muscle and adipose tissue due to obesity (8, 9), while others have shown no significant association between obesity and FNDC5/irisin (10, 11). In humans, some studies showed a positive correlation between serum irisin levels and body mass index (BMI) (6, 7), but others found negative (12) or no association (13).
Recent evidence suggests that irisin also plays an important role in bone metabolism and health (14). In vitro experiments, irisin promotes osteoblast proliferation and differentiation via activating mitogen-activated protein (MAP) kinase signaling pathways (15). Colaianni et al. found that recombinant irisin increased cortical bone content and prevented bone loss in mice (16). Cortical and trabecular bone mineral density (BMD), bone volume fraction loss, and fractal dimension are preserved in irisin-treated mice subjected to musculoskeletal unloading (17). In humans, irisin levels positively correlated with BMD and strength in soccer players (18).
Despite these concerns, limited studies have addressed the relationship between irisin and obesity and metabolic parameters in children and adolescents (19–22). Obesity is a growing health threat in children and adolescents, and irisin has therapeutic potential. Furthermore, scarce data exist on the relationship between irisin and bone status in this population (23, 24). Therefore, our study aimed to investigate the relationship of serum irisin levels with anthropometric, metabolic, and bone parameters in Korean children and adolescents.
Materials and methods
Subjects
This study was conducted with 103 children and adolescent patients who visited the outpatient Department of Pediatrics at Incheon St. Mary’s Hospital from March 2020 to August 2021. Based on Korean children and adolescent growth charts in 2017, the standard deviation score (SDS) of height, weight, and BMI was calculated for gender and age (25). Obesity was defined as a BMI greater than or equal to the 95th percentile. The normal weight was defined as a BMI between the 15th and 84th percentile of the same age and gender. Exclusion criteria included children with chronic disease (liver disease, renal disease), genetic disorders (Turner syndrome, Prader-Willi syndrome, Congenital adrenal hypoplasia), and endocrine disorders (thyroid disease, Cushing disease, Type 2 diabetes mellitus). Written informed consent was obtained from all participants and their parents. The study was approved by the Institutional Review Board of Incheon St. Mary’s Hospital (IRB number: OC19OESI0063, OC21OESI0119).
Anthropometry measurements
Height was recorded to the nearest 0.1 cm using a Harpenden Stadiometer, and weight was recorded to the nearest 0.1 kg using an electronic scale. Weight, height, and BMI were not evenly distributed among the different age groups, and the standard deviation scores (SDS) were based on the reference data published by the 2017 Korean National Growth Charts (25).
The pubertal stage was assessed by the pediatric endocrinologist according to Marshall and Tanner standards (26). The pubertal stage was defined as testicular volume ≥4 mL in boys and breast development in girls. Tanner stage 1 was defined as prepuberty, Tanner stage 2–3 as early puberty, and Tanner stage 4–5 as late puberty.
Laboratory evaluations
Venous blood samples were obtained in the morning after a 10-hour overnight fast. Serum samples were analyzed for glucose, insulin, total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), alkaline phosphatase (ALP), 25(OH)-Vitamin D, osteocalcin, and irisin. Dyslipidemia was defined as TC ≥200 mg/dL, LDL-C ≥ 130 mg/dL, HDL-C ≤ 45 mg/dL, and TG ≥150 mg/dL (or a combination thereof) according to the Third Report of the National Cholesterol Education Program (27) and the American Diabetes Association (28). Insulin resistance was estimated from fasting glucose and insulin serum levels using the homeostasis model assessment of insulin resistance (HOMA-IR): fasting insulin (µU/mL)×fasting glucose (mmol/L)/22.5. Serum osteocalcin was measured using an electrochemiluminescence immunoassay (ECLIA) and an Elecsys autoanalyzer (Roche Diagnostics GmbH, Mannheim, Germany). Serum irisin levels were measured using an ELISA assay (Phoenix Pharmaceuticals, Burlingame, CA). The intra- and inter-assay coefficients of variation (CV) were 4–8% and 8–12%, respectively.
Dual-energy X-ray absorptiometry
BMD was measured in the total body-less head region (TBLH) using a Lunar model DXA (Lunar Prodigy Advance; GE Healthcare Lunar Corporation, Madison, WI, USA). BMD SDS (TBLH Z-score) refers to the standard value of BMD in Korean children and adolescents (29). Due to a limited research budget, BMD was measured only in healthy subjects but not in obese subjects. We have selected healthy rather than obese subjects for BMD measurement to exclude the confounding effects of metabolic parameters associated with obesity.
Statistical analysis
All statistical analyses were conducted using SPSS for Windows version 20.0 (SPSS Inc., Chicago, IL, USA). Chi-square tests or Fisher’s exact tests were employed for categorical variables between two groups, as appropriate. The Student’s t-test and Mann-Whitney U test were utilized for continuous variables with normal and non-normal distributions, respectively. The normality of distribution was assessed using the Shapiro-Wilk test. The correlation between continuous variables was examined using Pearson’s correlation coefficient analysis.
Multiple linear regression was used to estimate the independent association between irisin levels and metabolic parameters. All p-values < 0.05 were considered statistically significant.
Results
Clinical characteristics of obese and normal weight groups
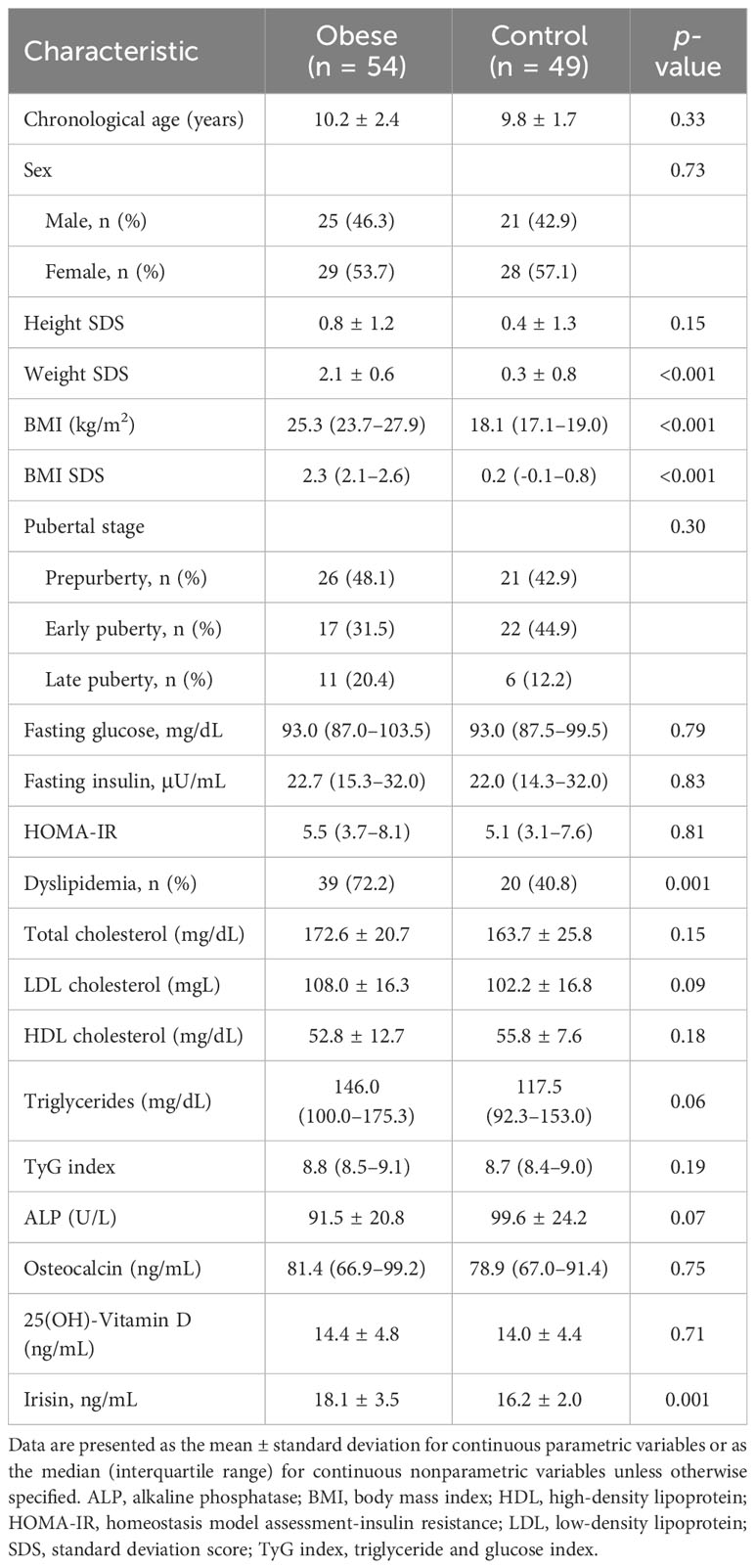
Of all subjects, 54 (52.4%) were obese and 49 (47.6%) were normal weight. Table 1 shows the clinical and biochemical characteristics in the obese and normal weight groups. The mean age of the obese and normal weight groups was 10.2 ± 2.4 years and 9.8 ± 1.7 years, respectively. Median BMI was 25.3 kg/m2 in the obese group and 18.1 kg/m2 in the normal weight group (p < 0.001). Dyslipidemia was more common in the obese group than in the normal weight group (72.2% vs. 40.8%; p = 0.001).
Correlation of serum irisin with patient characteristics
Serum irisin level was significantly higher in boys than in girls (median 17.5 vs. 15.8; p = 0.003) and in the obese group than in the control group (mean 18.1 ± 3.5 vs. 16.2 ± 2.0 ng/mL; p = 0.001). Serum irisin levels were not significantly different between prepubertal and early pubertal children. However, serum irisin levels were significantly higher in late puberty than early puberty (median 19.6 vs. 16.7; p = 0.02) in boys, but this difference did not reach a significant level in girls (median 18.5 vs. 15.4; p = 0.08) (Figure 1). The subjects with dyslipidemia had significantly higher serum irisin levels than those without (mean 17.8 ± 3.3 vs. 16.4 ± 2.4 ng/mL; p = 0.01).
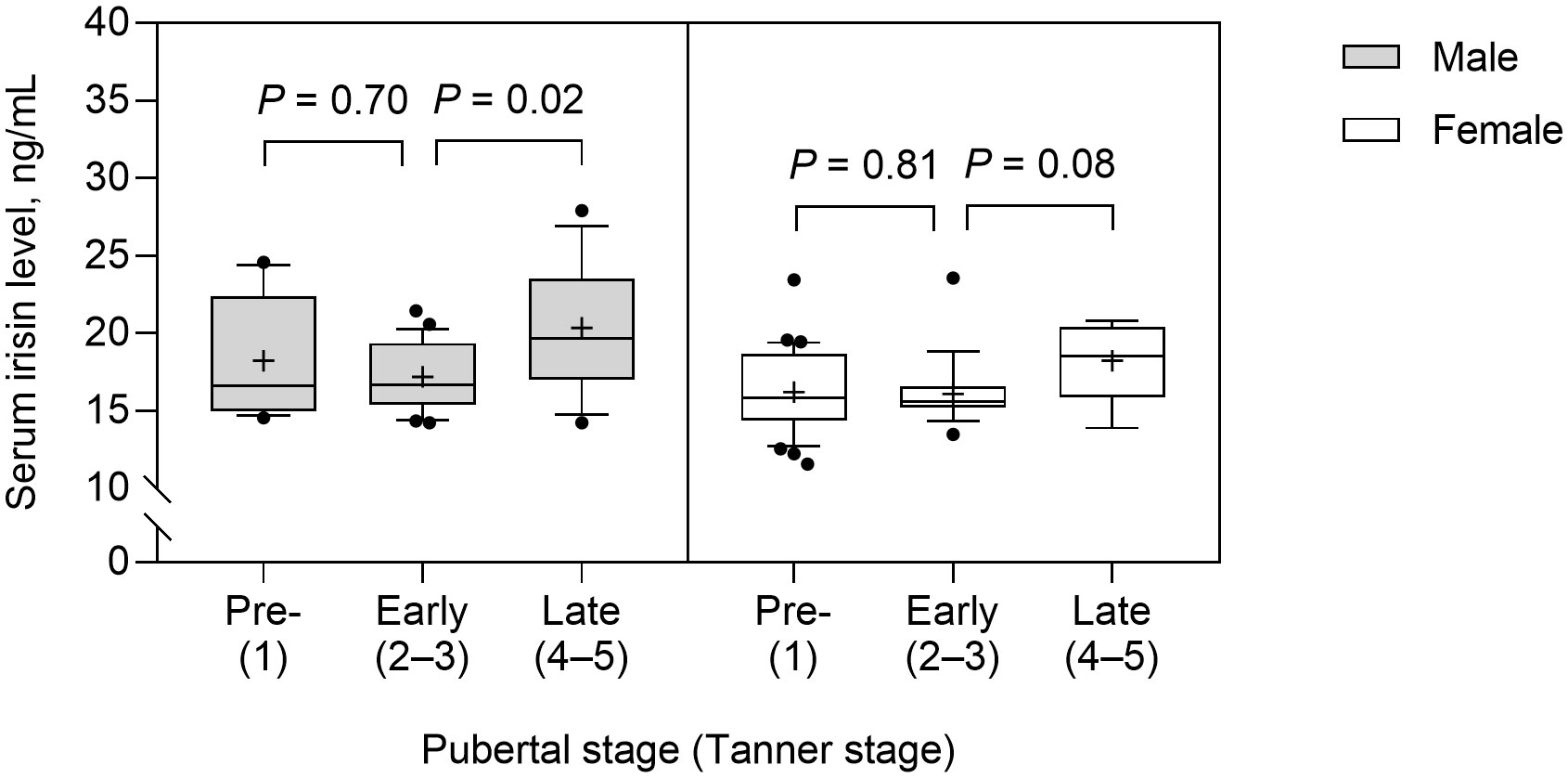
Figure 1 Serum irisin levels according to the pubertal stage in male and female subjects. The box and whisker plots indicate the median (horizontal line in the box), 25th percentile (bottom line of the box), 75th percentile (top line of the box), and 5th and 95th percentiles (whiskers). The black cross and dots indicate the mean and outliers, respectively.
Correlation of serum irisin with anthropometric and metabolic parameters
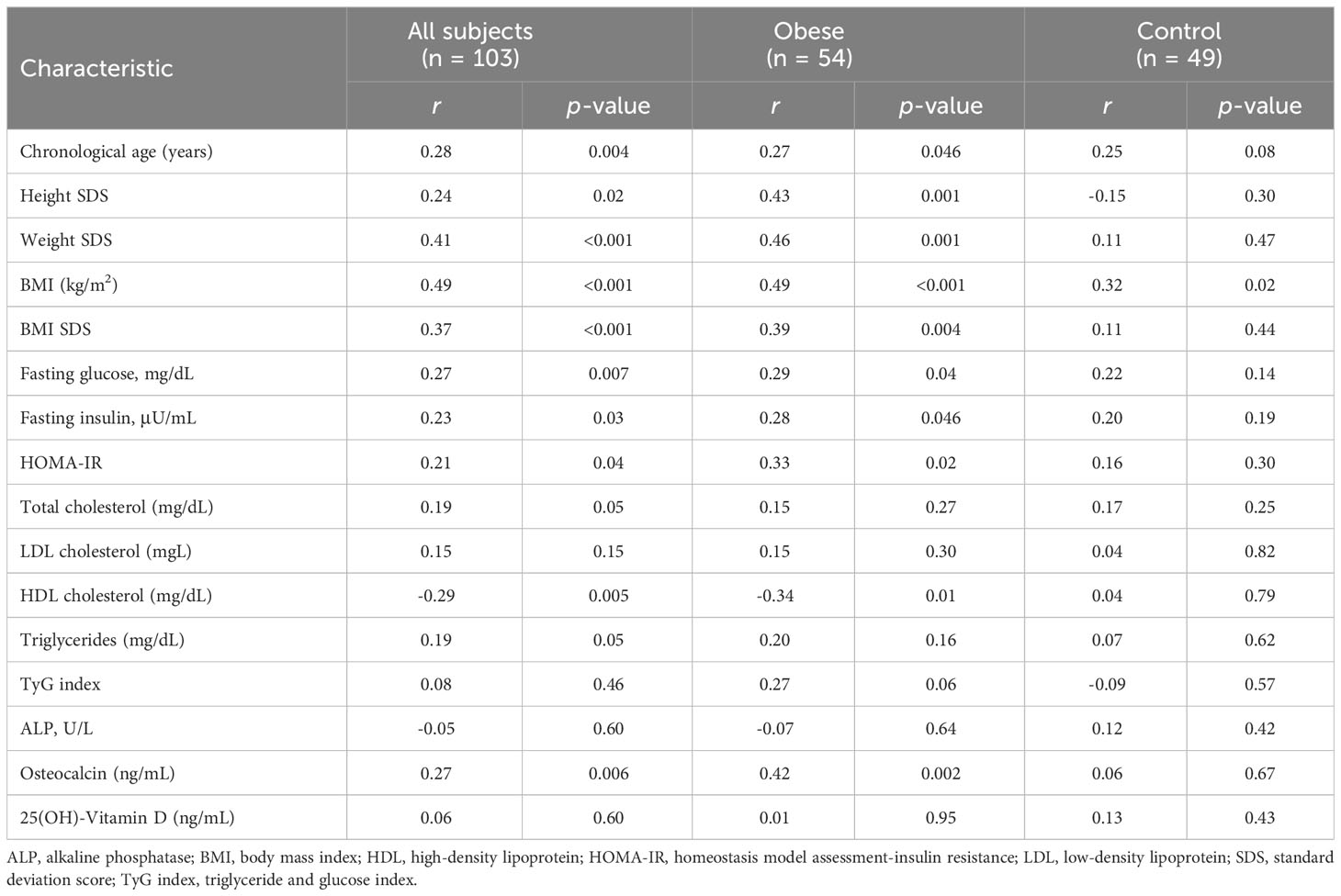
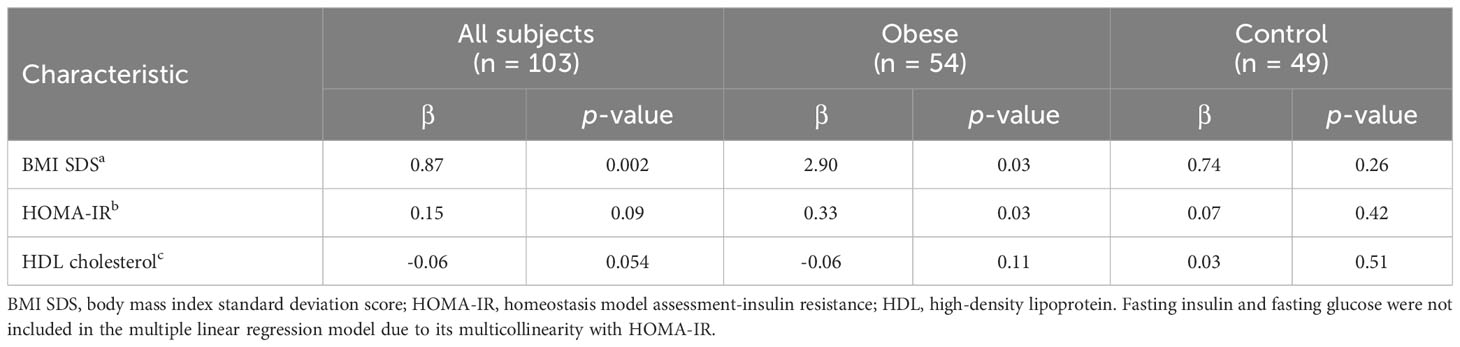
Table 2 shows the correlation between serum irisin and anthropometric and metabolic parameters. Serum irisin level was positively correlated with chronological age (r = 0.28; p = 0.004), height SDS (r = 0.24; p = 0.02), weight SDS (r = 0.41; p < 0.001), BMI SDS (r = 0.37; p < 0. 001), fasting glucose (r = 0.27; p = 0.007), and fasting insulin (r = 0.23; p = 0.03), HOMA-IR (r = 0.21; p = 0.04). Serum irisin level negatively correlated with HDL cholesterol (r = -0.29; p = 0.005). All these correlations were evident in obese subjects but not in healthy subjects. Table 3 shows multiple linear regression analysis between serum irisin levels and metabolic parameters. Multiple linear regression analysis indicated that BMI SDS was independently associated with serum irisin (β = 0.87; p = 0.002) in all subjects. In the obese group, BMI SDS (β = 2.90; p = 0.03) and HOMA-IR (β = 0.33; p = 0.03) were independently associated with serum irisin levels. In the healthy group, no factors were associated with serum irisin levels.
Correlation of serum irisin with bone parameters and BMD
The correlation of serum irisin with bone parameters is shown in Table 2. Serum irisin level was positively associated with osteocalcin (r = 0.27; p = 0.006). This association was evident in obese subjects (r = 0.42; p = 0.002) but not in healthy subjects (r = 0.06; p = 0.67). Serum irisin levels were not associated with ALP (r = -0.05; p = 0.60) and 25(OH)-Vitamin D (r = 0.06; p = 0.60).
Of 49 healthy subjects, 33 (67.3%) agreed to the BMD measurement. There were no significant differences in parameters except for weight SDS (p = 0.04) and LDL cholesterol (p = 0.03) between subjects who received BMD measurement and those who did not (Table 4). Table 5 shows the correlation of TBLH BMD Z-score with anthropometric, metabolic, and bone parameters. The TBLH BMD Z-score was positively correlated with fasting insulin (r = 0.39; p = 0.04), HOMA-IR (r = 0.38; p = 0.047), osteocalcin (r = 0.40; p = 0.02), and serum irisin (r = 0.39; p = 0.03). ALP and 25(OH)-Vitamin D was not associated with TBLH BMD Z-score.
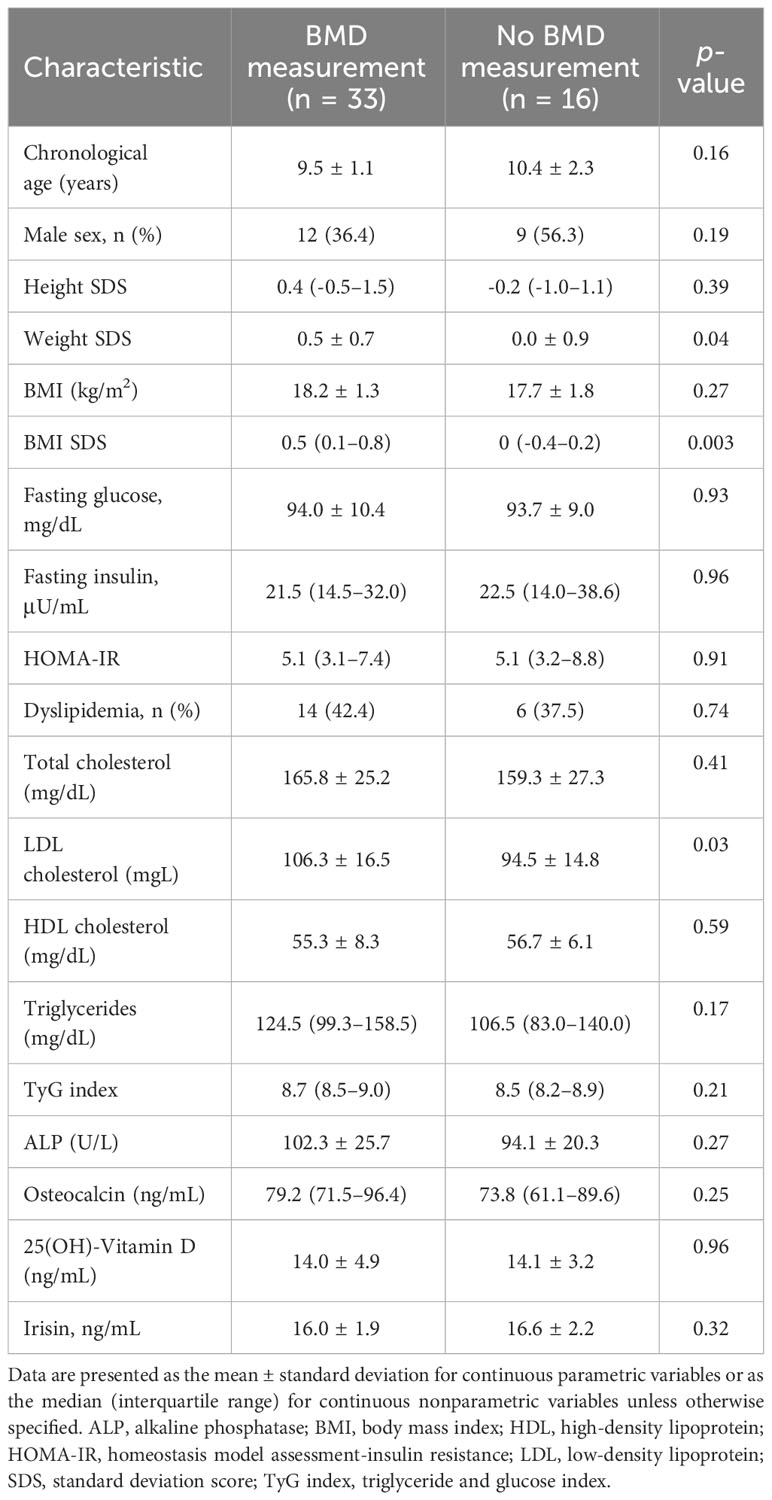
Table 4 Comparison between healthy subjects who underwent bone mineral density measurement and those who did not.
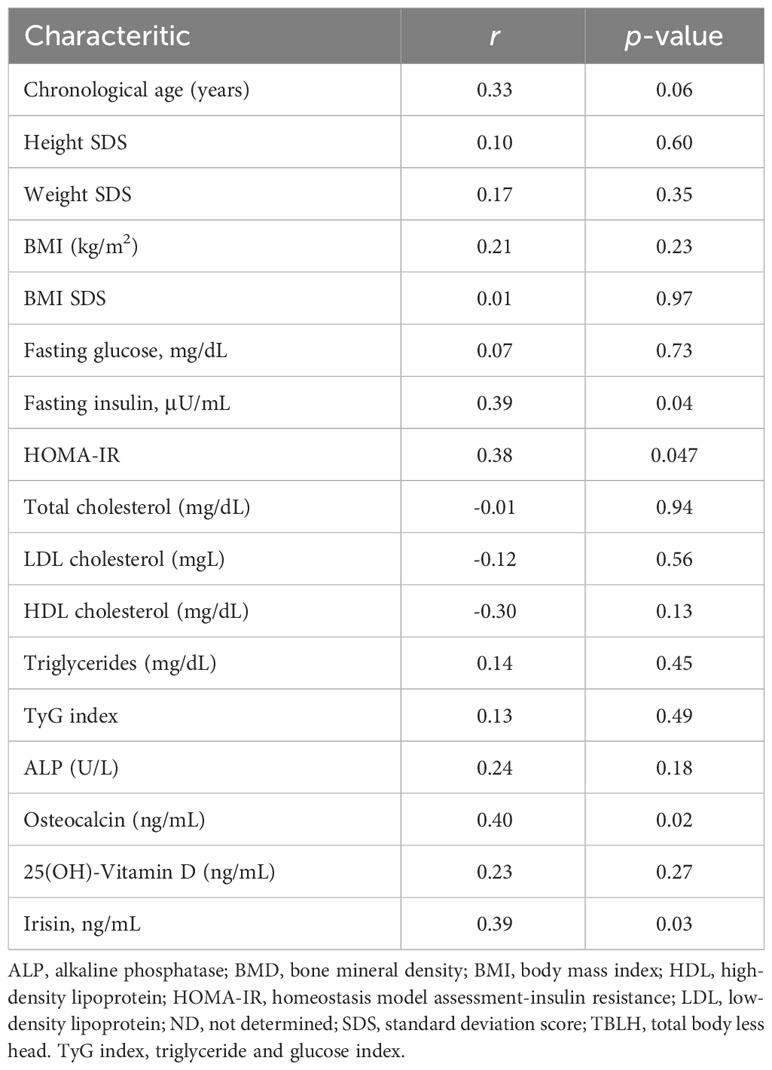
Table 5 Correlation of TBLH BMD Z-score with anthropometric and metabolic parameters, bone turnover markers, and serum irisin.
Discussion
This study explores the correlation of serum irisin levels with anthropometric, metabolic, and bone parameters in Korean children and adolescents. The results indicate that irisin levels were positively correlated with age, BMI, fasting glucose, HOMA-IR, and osteocalcin and negatively correlated with osteocalcin. All these associations were evident in obese subjects but not in healthy subjects. In healthy subjects, a positive correlation was found between serum and BMD.
Consistent with the previous results (19–22), we found that serum irisin levels were higher in obese healthy children and adolescents than in healthy subjects and were positively associated with BMI. Although this finding may be expected given the increase in muscle mass (30), increased secretion from adipose tissue is another possible source of increased circulating irisin in obesity. A molecular animal-based study confirmed the contribution of adipose tissue to circulating irisin levels (31). In rats, secretion of irisin was higher from white adipose tissues of diet-induced obese compared to lean controls (31). A study including 145 female adults reported that both fat mass and fat-free mass positively correlated with irisin levels (32). In adolescents, increased body fat mass rather than BMI was an independent factor for increased irisin levels (21). The results of previous studies and ours suggest that increased irisin may compensate for increasing body mass, especially body fat mass in obese individuals, to maintain the balance of energy storage and expenditure. It is also suggested that increased irisin in obesity may be to overcome insulin and irisin resistance (7), similar to well-documented leptin resistance in obesity (33). Although we have not collected the data on physical activity, the association between irisin and BMI in children and adolescents should be cautiously interpreted according to physical activity. One study showed that adolescents who engaged in regular physical activity had higher levels of irisin than sedentary adolescents (21). Another study found significant interactions between circulating irisin concentrations and physical activity on BMI in children (34).
We found a positive correlation between irisin and glucose and HOMA-IR in children and adolescents. In a large study of 618 Korean adolescents, circulating serum irisin positively correlated with glucose, insulin, and HOMAR-IR (21). Elevated serum irisin was associated with increased risk odds of having obesity (OR = 2.2) and metabolic syndrome (OR = 2.0) (21). However, different results were reported in children of different ethnicities and ages. In a cohort study of 153 Saudi children, circulating irisin negatively correlated with HOMA-IR (35). In prepubertal children, circulating negatively correlated with fasting glucose, and children with metabolic syndrome exhibited lower irisin concentrations than those without (36). Notably, the significant association between irisin and HOMA-IR was not observed after controlling for BMI in the previous study (21, 35) and ours. In the pediatric population, serum irisin may be more likely to be strongly associated with body mass and composition than metabolic disease.
In this study, serum irisin levels were negatively correlated with HDL cholesterol and significantly higher in subjects with dyslipidemia. In children, serum irisin negatively correlated with HDL cholesterol (22, 37) and positively correlated with LDL cholesterol (22) and triglyceride (22). One adult study evaluated the association between irisin and lipoprotein subparticles (38). In that study, serum irisin was positively correlated with HDL cholesterol, primarily large HDL particles. Literature suggests a potential role of irisin in cardiac function and cardiovascular diseases. In rats, more irisin is produced in the cardiac muscle than in the skeletal muscle, and serum irisin increased after exercise, higher in younger than older rats (39). In humans, the dysfunction of irisin has been shown to be involved in cardiovascular diseases such as hypertension, coronary artery disease, and myocardial infarction (40). In children, irisin levels were significantly correlated with systolic, diastolic blood pressure, and circulating endothelial progenitor cell levels (37, 41).
We found serum irisin levels positively correlated with osteocalcin in children and adolescents. This finding was consistent with a recent study evaluating 34 healthy children (24). ALP, another osteoblast differentiation marker, did not correlate with serum irisin in the previous study (24) and ours. We found no significant association between irisin and 25(OH)-Vitamin D in children and adolescents. A negative association between irisin and 25(OH)-Vitamin D was reported in children with type 1 diabetes mellitus (42) and Charcot-Marie-Tooth disease (43). In contrast, another study showed that vitamin D supplementation in patients with hypovitaminosis associated with primary hyperparathyroidism increased serum irisin levels (44). Based on these findings, disease type may influence the relationship between irisin and vitamin D. In this study, BMD positively correlated with serum irisin but did not correlate with ALP. Our result was consistent with two recent studies in healthy children of 6–8 years (23) and those of 7–13 years (24). Multivariate regression indicated that irisin was the more powerful determinant of bone mineral status than bone ALP (24). In post-menopausal women, it is negatively associated with vertebral fragility fractures (45, 46). In mice, exercise increased the expression of FNDC5/irisin and osteocalcin in bone tissues (47). Overall, these results suggest that encouraging children to exercise may preserve their bone health, and this could also apply to pediatric pathologic conditions at high risk of poor bone health (48).
Irisin plays an important role in activating the hypothalamic-pituitary-gonadal axis (HPG axis) and in reproductive development. When comparing prepubertal and pubertal children, irisin was higher in pubertal children (37), but other studies have found no difference in irisin levels (21). In this study, there was no significant difference in irisin levels between prepubertal and early pubertal subjects, but irisin levels were higher in late puberty relative to early puberty in boys. Irisin can vary with the ratio of body fat to muscle mass, which varies with sex and pubertal stage, and this should be taken into account when examining changes in irisin levels during puberty (49).
This study has several limitations. First, this is a single-hospital study with a small number of subjects. Thus, this study had limited power to confirm the independent association between irisin and other parameters in obese and healthy subgroups. Second, due to a limited research budget, we have measured BMD in only healthy subjects. Third, body fat mass, muscle mass, diet, exercise, and weight change status were unknown, and the effect of these factors on irisin level remains unclear.
In conclusion, serum irisin levels were associated with BMI, glucose, lipid, and bone parameters in Korean adolescents and children. Our observation is evident in obese subjects but not in healthy subjects. We suggest that irisin is important in regulating glucose, lipid, and bone metabolism in children and adolescents, especially in obese subjects. We found a positive correlation between irisin and BMD in healthy subjects. The potential role of irisin should be confirmed in further studies evaluating the impact of exercise and lifestyle change in obesity on circulating irisin levels, metabolic parameters, and bone health.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The study was approved by the Institutional Review Board of Incheon St. Mary’s Hospital (IRB number: OC19OESI0063, OC21OESI0119). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
S-HK: Data curation, Formal Analysis, Funding acquisition, Writing – original draft, Writing – review & editing, Conceptualization, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization. SEK: Data curation, Investigation, Writing – review & editing. SK: Methodology, Writing – review & editing, Data curation. MA: Investigation, Writing – review & editing, Methodology. WC: Formal Analysis, Writing – review & editing. KC: Data curation, Writing – review & editing. MJ: Funding acquisition, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Clinical Trials Center of Incheon St. Mary’s Hospital, The Catholic University of Korea.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol (2012) 8(8):457–65. doi: 10.1038/nrendo.2012.49
2. Hecksteden A, Wegmann M, Steffen A, Kraushaar J, Morsch A, Ruppenthal S, et al. Irisin and exercise training in humans - results from a randomized controlled training trial. BMC Med (2013) 11:235. doi: 10.1186/1741-7015-11-235
3. Moreno-Navarrete JM, Ortega F, Serrano M, Guerra E, Pardo G, Tinahones F, et al. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J Clin Endocrinol Metab (2013) 98(4):E769–78. doi: 10.1210/jc.2012-2749
4. Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature (2012) 481(7382):463–8. doi: 10.1038/nature10777
5. Perakakis N, Triantafyllou GA, Fernández-Real JM, Huh JY, Park KH, Seufert J, et al. Physiology and role of irisin in glucose homeostasis. Nat Rev Endocrinol (2017) 13(6):324–37. doi: 10.1038/nrendo.2016.221
6. Liu JJ, Wong MD, Toy WC, Tan CS, Liu S, Ng XW, et al. Lower circulating irisin is associated with type 2 diabetes mellitus. J Diabetes Complications (2013) 27(4):365–9. doi: 10.1016/j.jdiacomp.2013.03.002
7. Park KH, Zaichenko L, Brinkoetter M, Thakkar B, Sahin-Efe A, Joung KE, et al. Circulating irisin in relation to insulin resistance and the metabolic syndrome. J Clin Endocrinol Metab (2013) 98(12):4899–907. doi: 10.1210/jc.2013-2373
8. Yang Z, Chen X, Chen Y, Zhao Q. Decreased irisin secretion contributes to muscle insulin resistance in high-fat diet mice. Int J Clin Exp Pathol (2015) 8(6):6490–7.
9. Morton TL, Galior K, McGrath C, Wu X, Uzer G, Uzer GB, et al. Exercise increases and browns muscle lipid in high-fat diet-fed mice. Front Endocrinol (Lausanne) (2016) 7:80. doi: 10.3389/fendo.2016.00080
10. Pekkala S, Wiklund PK, Hulmi JJ, Ahtiainen JP, Horttanainen M, Pöllänen E, et al. Are skeletal muscle FNDC5 gene expression and irisin release regulated by exercise and related to health? J Physiol (2013) 591(21):5393–400. doi: 10.1113/jphysiol.2013.263707
11. Roberts MD, Bayless DS, Company JM, Jenkins NT, Padilla J, Childs TE, et al. Elevated skeletal muscle irisin precursor FNDC5 mRNA in obese OLETF rats. Metabolism (2013) 62(8):1052–6. doi: 10.1016/j.metabol.2013.02.002
12. Hou N, Han F, Sun X. The relationship between circulating irisin levels and endothelial function in lean and obese subjects. Clin Endocrinol (Oxf) (2015) 83(3):339–43. doi: 10.1111/cen.12658
13. Gouni-Berthold I, Berthold HK, Huh JY, Berman R, Spenrath N, Krone W, et al. Effects of lipid-lowering drugs on irisin in human subjects in vivo and in human skeletal muscle cells ex vivo. PloS One (2013) 8(9):e72858. doi: 10.1371/journal.pone.0072858
14. Zhao R, Chen Y, Wang D, Zhang C, Song H, Ni G. Role of irisin in bone diseases. Front Endocrinol (Lausanne) (2023) 14:1212892. doi: 10.3389/fendo.2023.1212892
15. Qiao X, Nie Y, Ma Y, Chen Y, Cheng R, Yin W, et al. Irisin promotes osteoblast proliferation and differentiation via activating the MAP kinase signaling pathways. Sci Rep (2016) 6:18732. doi: 10.1038/srep18732
16. Colaianni G, Cuscito C, Mongelli T, Pignataro P, Buccoliero C, Liu P, et al. The myokine irisin increases cortical bone mass. Proc Natl Acad Sci U.S.A. (2015) 112(39):12157–62. doi: 10.1073/pnas.1516622112
17. Colaianni G, Mongelli T, Cuscito C, Pignataro P, Lippo L, Spiro G, et al. Irisin prevents and restores bone loss and muscle atrophy in hind-limb suspended mice. Sci Rep (2017) 7(1):2811. doi: 10.1038/s41598-017-02557-8
18. Colaianni G, Notarnicola A, Sanesi L, Brunetti G, Lippo L, Celi M, et al. Irisin levels correlate with bone mineral density in soccer players. J Biol Regul Homeost Agents (2017) 31(4 suppl 1):21–8.
19. Nigro E, Scudiero O, Ludovica Monaco M, Polito R, Schettino P, Grandone A, et al. Adiponectin profile and Irisin expression in Italian obese children: Association with insulin-resistance. Cytokine (2017) 94:8–13. doi: 10.1016/j.cyto.2016.12.018
20. Palacios-González B, Vadillo-Ortega F, Polo-Oteyza E, Sánchez T, Ancira-Moreno M, Romero-Hidalgo S, et al. Irisin levels before and after physical activity among school-age children with different BMI: a direct relation with leptin. Obes (Silver Spring) (2015) 23(4):729–32. doi: 10.1002/oby.21029
21. Jang HB, Kim HJ, Kang JH, Park SI, Park KH, Lee HJ. Association of circulating irisin levels with metabolic and metabolite profiles of Korean adolescents. Metabolism (2017) 73:100–8. doi: 10.1016/j.metabol.2017.05.007
22. Catli G, Kume T, Tuhan HU, Anik A, Calan OG, Bober E, et al. Relation of serum irisin level with metabolic and antropometric parameters in obese children. J Diabetes Complications (2016) 30(8):1560–5. doi: 10.1016/j.jdiacomp.2016.07.019
23. Soininen S, Sidoroff V, Lindi V, Mahonen A, Kröger L, Kröger H, et al. Body fat mass, lean body mass and associated biomarkers as determinants of bone mineral density in children 6-8years of age - The Physical Activity and Nutrition in Children (PANIC) study. Bone (2018) 108:106–14. doi: 10.1016/j.bone.2018.01.003
24. Colaianni G, Faienza MF, Sanesi L, Brunetti G, Pignataro P, Lippo L, et al. Irisin serum levels are positively correlated with bone mineral status in a population of healthy children. Pediatr Res (2019) 85(4):484–8. doi: 10.1038/s41390-019-0278-y
25. Kim JH, Yun S, Hwang SS, Shim JO, Chae HW, Lee YJ, et al. The 2017 Korean National Growth Charts for children and adolescents: development, improvement, and prospects. Korean J Pediatr (2018) 61(5):135–49. doi: 10.3345/kjp.2018.61.5.135
26. Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child (1969) 44(235):291–303. doi: 10.1136/adc.44.235.291
27. Expert Panel on Detection E. Treatment of high blood cholesterol in A. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA (2001) 285(19):2486–97. doi: 10.1001/jama.285.19.2486
28. American Diabetes Association. Management of dyslipidemia in children and adolescents with diabetes. Diabetes Care (2003) 26(7):2194–7. doi: 10.2337/diacare.26.7.2194
29. Lim JS, Hwang JS, Lee JA, Kim DH, Park KD, Cheon GJ, et al. Bone mineral density according to age, bone age, and pubertal stages in korean children and adolescents. J Clin Densitom (2010) 13(1):68–76. doi: 10.1016/j.jocd.2009.09.006
30. Huh JY, Panagiotou G, Mougios V, Brinkoetter M, Vamvini MT, Schneider BE, et al. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism (2012) 61(12):1725–38. doi: 10.1016/j.metabol.2012.09.002
31. Roca-Rivada A, Castelao C, Senin LL, Landrove MO, Baltar J, Belén Crujeiras A, et al. FNDC5/irisin is not only a myokine but also an adipokine. PloS One (2013) 8(4):e60563. doi: 10.1371/journal.pone.0060563
32. Pardo M, Crujeiras AB, Amil M, Aguera Z, Jiménez-Murcia S, Baños R, et al. Association of irisin with fat mass, resting energy expenditure, and daily activity in conditions of extreme body mass index. Int J Endocrinol (2014) 2014:857270. doi: 10.1155/2014/857270
33. Moon HS, Dalamaga M, Kim SY, Polyzos SA, Hamnvik OP, Magkos F, et al. Leptin’s role in lipodystrophic and nonlipodystrophic insulin-resistant and diabetic individuals. Endocr Rev (2013) 34(3):377–412. doi: 10.1210/er.2012-1053
34. Cai L, Tan M, Tan W, Zeng X, Wan N, Wong SH, et al. Associations of circulating irisin concentrations with cardiometabolic risk factors among children vary by physical activity or sedentary time levels. Front Endocrinol (Lausanne) (2019) 10:549. doi: 10.3389/fendo.2019.00549
35. Al-Daghri NM, Alkharfy KM, Rahman S, Amer OE, Vinodson B, Sabico S, et al. Irisin as a predictor of glucose metabolism in children: sexually dimorphic effects. Eur J Clin Invest (2014) 44(2):119–24. doi: 10.1111/eci.12196
36. Shim YS, Kang MJ, Yang S, Hwang IT. Irisin is a biomarker for metabolic syndrome in prepubertal children. Endocr J (2018) 65(1):23–31. doi: 10.1507/endocrj.EJ17-0260
37. Reinehr T, Elfers C, Lass N, Roth CL. Irisin and its relation to insulin resistance and puberty in obese children: a longitudinal analysis. J Clin Endocrinol Metab (2015) 100(5):2123–30. doi: 10.1210/jc.2015-1208
38. Panagiotou G, Mu L, Na B, Mukamal KJ, Mantzoros CS. Circulating irisin, omentin-1, and lipoprotein subparticles in adults at higher cardiovascular risk. Metabolism (2014) 63(10):1265–71. doi: 10.1016/j.metabol.2014.06.001
39. Aydin S, Kuloglu T, Aydin S, Eren MN, Celik A, Yilmaz M, et al. Cardiac, skeletal muscle and serum irisin responses to with or without water exercise in young and old male rats: cardiac muscle produces more irisin than skeletal muscle. Peptides (2014) 52:68–73. doi: 10.1016/j.peptides.2013.11.024
40. Fu J, Li F, Tang Y, Cai L, Zeng C, Yang Y, et al. The emerging role of irisin in cardiovascular diseases. J Am Heart Assoc (2021) 10(20):e022453. doi: 10.1161/jaha.121.022453
41. De Meneck F, Victorino de Souza L, Oliveira V, do Franco MC. High irisin levels in overweight/obese children and its positive correlation with metabolic profile, blood pressure, and endothelial progenitor cells. Nutrition Metab Cardiovasc Dis (2018) 28(7):756–64. doi: 10.1016/j.numecd.2018.04.009
42. Faienza MF, Brunetti G, Sanesi L, Colaianni G, Celi M, Piacente L, et al. High irisin levels are associated with better glycemic control and bone health in children with Type 1 diabetes. Diabetes Res Clin Pract (2018) 141:10–7. doi: 10.1016/j.diabres.2018.03.046
43. Colaianni G, Oranger A, Dicarlo M, Lovero R, Storlino G, Pignataro P, et al. Irisin serum levels and skeletal muscle assessment in a cohort of charcot-marie-tooth patients. Front Endocrinol (Lausanne) (2022) 13:886243. doi: 10.3389/fendo.2022.886243
44. Sanesi L, Dicarlo M, Pignataro P, Zerlotin R, Pugliese F, Columbu C, et al. Vitamin D increases irisin serum levels and the expression of its precursor in skeletal muscle. Int J Mol Sci (2023) 24(4):4129. doi: 10.3390/ijms24044129
45. Anastasilakis AD, Polyzos SA, Makras P, Gkiomisi A, Bisbinas I, Katsarou A, et al. Circulating irisin is associated with osteoporotic fractures in postmenopausal women with low bone mass but is not affected by either teriparatide or denosumab treatment for 3 months. Osteoporos Int (2014) 25(5):1633–42. doi: 10.1007/s00198-014-2673-x
46. Palermo A, Strollo R, Maddaloni E, Tuccinardi D, D’Onofrio L, Briganti SI, et al. Irisin is associated with osteoporotic fractures independently of bone mineral density, body composition or daily physical activity. Clin Endocrinol (Oxf) (2015) 82(4):615–9. doi: 10.1111/cen.12672
47. Zhang J, Valverde P, Zhu X, Murray D, Wu Y, Yu L, et al. Exercise-induced irisin in bone and systemic irisin administration reveal new regulatory mechanisms of bone metabolism. Bone Res (2017) 5:16056. doi: 10.1038/boneres.2016.56
48. Colaianni G, Sanesi L, Storlino G, Brunetti G, Colucci S, Grano M. Irisin and bone: from preclinical studies to the evaluation of its circulating levels in different populations of human subjects. Cells (2019) 8(5):451. doi: 10.3390/cells8050451
Keywords: irisin, obesity, insulin resistance, bone mineral density, children
Citation: Kim S-H, Kim SE, Kim S, Ahn MB, Cho WK, Cho KS and Jung MH (2024) The association of serum irisin with anthropometric, metabolic, and bone parameters in obese children and adolescents. Front. Endocrinol. 14:1326851. doi: 10.3389/fendo.2023.1326851
Received: 24 October 2023; Accepted: 27 December 2023;
Published: 25 January 2024.
Edited by:
Dénes Molnár, University of Pécs, HungaryReviewed by:
Maria Felicia Faienza, University of Bari Aldo Moro, ItalyGiuseppe Petito, University of Campania Luigi Vanvitelli, Italy
Copyright © 2024 Kim, Kim, Kim, Ahn, Cho, Cho and Jung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Ho Jung, am1ocGVAY2F0aG9saWMuYWMua3I=
 Shin-Hee Kim
Shin-Hee Kim Sung Eun Kim1
Sung Eun Kim1 Moon Bae Ahn
Moon Bae Ahn Won Kyoung Cho
Won Kyoung Cho