- Center for Reproductive Medicine, Zhongda Hospital, Southeast University, Nanjing, Jiangsu, China
Introduction: It is little known whether hyperlipidemia alone has adverse effects on the outcome of in vitro fertilization (IVF) in patients with polycystic ovarian syndrome (PCOS).
Methods: The PCOS patients with body mass index (BMI) < 30 kg/m2 were performed IVF or intracytoplasmic sperm injection treatment, including 208 fresh cycles and 127 frozen embryo transfer (FET) cycles. All the patients were divided into hyperlipidemia and control groups, and embryo quality and pregnancy outcomes between the two groups were compared.
Results: In the fresh cycles, total gonadotropin dosage in the control group was significantly lower than that in the hyperlipidemia group, and serum estradiol levels on trigger day were reversed (P < 0.05). The embryo fragment score was positively correlated with serum low-density lipoprotein level (r = 0.06, P < 0.05) and negatively with serum high-density lipoprotein (HDL) and lipoprotein A levels (r = -0.489 and -0.085, P < 0.01). Logistic regression analysis found that HDL was beneficial for clinical pregnancy (OR = 0.355, 95% CI: 0.135-0.938, P < 0.05). In the FET cycles, there were no differences in pulse index, systolic/diastolic ratio and serum estradiol and progesterone levels between the two groups, but resistance index in the hyperlipidemia group was significantly higher than that in the control group (P < 0.05).
Conclusion: Hyperlipidemia may increase the dosage of gonadotropin and have adverse effect on the embryo quality, endometrial receptivity, and clinical outcomes of lean PCOS patients. It is recommended that the non-obese patients with hyperlipidemia and PCOS perform lipid-lowering treatment before undergoing embryo transfer.
1 Introduction
In women of reproductive age, polycystic ovarian syndrome (PCOS) is the most prevalent endocrine condition and is characterized by hyperandrogenism, recurrent anovulation, and polycystic ovaries. A portion of PCOS is frequently linked to metabolic abnormalities such as obesity, insulin resistance (IR), poor glucose tolerance, lipid metabolic diseases, and others. Among these features obesity associated with IR is a major trigger of these disorders. Numerous researches in recent years have revealed that IR and obesity are associated with particular reproductive health issues, such as reduced clinical pregnancy rates in assisted reproductive technology (ART) cycles (1). However, there are few reports on the effects of dyslipidemia, especially in non-obese patients with PCOS, on the outcomes of assisted reproduction. It has been demonstrated that dyslipidemia in PCOS patients may exist without obesity (2), but it can occasionally exacerbate metabolic abnormalities such as obesity, IR, and others. About 70% of obese patients with PCOS have lipid abnormalities, and about 20% of lean PCOS individuals have impaired lipid metabolic disorders (3, 4). Some studies have proved that hyperlipidemia affected pregnancy outcomes of ART, such as implantation rate, clinical pregnancy rate, and miscarriage rate (5), which might be a result of alterations in the microenvironment of oocytes and endometrium brought on by lipids. Our study aimed to determine whether dyslipidemia affected the embryo quality and pregnancy outcome of non-obese PCOS patients undergoing in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI), which might indirectly reflect the oocyte quality and endometrial receptivity.
The ART cycle includes fresh embryo transfer and frozen embryo transfer (FET) cycles. Considering the many differences between fresh and FET cycles, such as the higher dosage of gonadotropin (Gn), different ovarian stimulation protocols, higher estrogen levels on the human chorionic gonadotropin (HCG) trigger day, and unfrozen embryos in fresh cycles, this study investigated the fresh and FET cycles separately and compared the effects of dyslipidemia on their pregnancy outcomes. Meanwhile, considering that lipids are the substrate of steroid hormones and that abnormal lipid metabolism may have an impact on endogenous hormones, this study only selected the hormone replacement therapy (HRT) protocol for the FET cycle, which may minimize the impact of confounding factors on the results by supplementing exogenous hormones as the endometrial preparation protocol.
2 Materials and methods
2.1 Patients
The clinical data of patients undergoing conventional IVF or ICSI treatment in Center for Reproductive Medicine of Zhongda Hospital during October 2017 and June 2021 were analyzed retrospectively. The implementation of IVF or ICSI was approved by the Reproductive Medicine Ethics Committee of Zhongda Hospital affiliated to Southeast University (Reproduction No. 2015-1), and all patients signed the informed consent. The inclusion criteria of the patients included: (1) females aged < 35 years old, (2) body mass index (BMI) < 30 kg/m2, and (3) patients diagnosed with PCOS. The diagnostic criteria for PCOS were based on the unified standards formulated by the Rotterdam International Conference in 2003 (6). The patients with any two of the following three conditions were diagnosed with PCOS: (1) infrequent ovulation or anovulation, (2) hyperandrogenism or clinical manifestations of high blood androgen, and (3) ultrasound findings of polycystic ovaries in one or two ovaries, 12 or more follicles with a diameter of 2-9 mm, and/or ovarian volume ≥ 10 ml after exclusion of other etiologies such as congenital adrenal hyperplasia, androgen-secreting tumors, Cushing syndrome, 21-hydroxylase-deficient nonclassic adrenal hyperplasia, androgenic/anabolic drug use or abuse. Exclusion criteria included: (1) women with endocrine or metabolic diseases such as thyroid dysfunction, hyperprolactinemia, type 2 diabetes mellitus, and cardiovascular disease, (2) oocyte donation cycles, and (3) chromosome abnormality or other genetic mutations. All the patients undergoing IVF or ICSI treatments were divided into dyslipidemia (hyperlipidemia) and normal (control) groups. The diagnostic criteria of dyslipidemia were based on the 2016 guidelines for the prevention and treatment of dyslipidemia in Chinese adults (7). Dyslipidemia was defined as having at least one of the following indexes: total cholesterol (TC) ≥ 5.18 mmol/L or ≥ 200 mg/dL, low-density lipoprotein cholesterol (LDL-C) ≥ 3.37 mmol/L or ≥ 130 mg/dL, high-density lipoprotein cholesterol (HDL-C) < 1.04 mmol/L or < 40 mg/dL, and triglyceride (TG) ≥ 1.7 mmol/L or ≥ 150.62 mg/dL. The patients underwent fresh embryo transfer or frozen embryo transfer (FET) for the first time were recruited for the study. A total of 208 fresh cycles and 127 FET cycles were included. The study conforms to the WMA Declaration of Helsinki.
2.2 Stimulation protocols for fresh cycles
Gonadotropin-releasing hormone (GnRH) antagonist and long GnRH agonist (GnRH-a) protocols were applied for controlled ovarian stimulation (COS). The initial gonadotropin dose was determined according to the patient’s age, body weight, number of antral follicles, etc. Stimulation was monitored using estradiol concentrations, together with ultrasound measurements of follicle numbers and diameters. When at least 2 follicles were 18 mm or 3 follicles were 17 mm in diameter, 0.2 mg GnRH-a or 5000-10000 U human chorionic gonadotropin (HCG) were injected for triggering. Oocyte retrieval was performed 36 hours after HCG was injected through the transvaginal route with an ultrasound guidance. Embryo transplantation was performed 3 days after ovum pick-up (OPU) on the basis of exact situations such as estradiol concentration and endometrial thickness. One to two embryos with best quality were transferred into the uterus, and then the patients were given routine corpus luteum support. The serum HCG levels of the patients were detected 14 days after embryo transfer, and serum HCG level > 50 U/L was regarded as biochemical pregnancy. B ultrasound examination was done 4 weeks after embryo transfer, and clinical pregnancy was considered if the gestational sac was found.
2.3 Endometrial preparation protocols for the FET cycle
All patients in the FET cycle used HRT for endometrial preparation. First, 4 mg of oral estradiol valerate (Progynova; Bayer, Germany) was administered per day from day 2–4 of the menstrual cycle. The patients were evaluated by transvaginal ultrasound 7 days later to adjust the dosage of estradiol based on the endometrial thickness. A supplementation of vaginal estradiol was added if the endometrial thickness continued to be unsatisfactory. When the endometrial thickness reached 8 mm, and serum progesterone level was below 1.5 ng/mL, intramuscular administration of 40 mg progesterone (Zhejiang Xianju Pharmaceutical Co., Ltd, Taizhou, Zhejiang, China) or vaginal supplementation with 90 mg progesterone (8% Crinone, Merck-Serono, Germany) was added. The parameters of resistance index (RI), pulse index (PI) and systolic/diastolic ratio (S/D) were measured on the day of progesterone administration using Voluson S8 color doppler ultrasonic diagnostic apparatus (GE Ultrasound Korea, Ltd.). One to two embryos with good quality were transferred into the uterus, and then the patients were given routine corpus luteum support. The serum HCG levels of the patients were detected 14 days after embryo transfer. If the serum HCG level was higher than 50 U/L, the patient was regarded as a biochemical pregnancy. Clinical pregnancy was confirmed by ultrasound, and one or two gestational sacs were visible approximately 4 weeks after embryo transfer.
2.4 Assessment of embryo quality
Embryo quality was evaluated on day 3 by assessing the embryo cell number and embryo fragment score (EFS). EFS was graded on the basis of the percentage of fragmentation as follows: < 5% of fragmentation, score 4; 5%–10% of fragmentation, score 3; 11%–25% of fragmentation, score 2; 26%–50% of fragmentation, score 1; and ≥ 51% of fragmentation, score 0 (8). Good quality embryos were defined as having over 6 cells with relatively equal sized blastomeres and less than 25% of fragmentation (EFS 2-4).
2.5 Laboratory analysis
Commercially available kits for the determinations of serum follicle-stimulating hormone (FSH), luteinizing hormone (LH), testosterone (T), estradiol (E2), progesterone, anti-Müllerian hormone (AMH) and insulin were purchased from Abbott Laboratories, Inc. USA, and serum FSH, LH, T, E2, progesterone, AMH and insulin levels were determined by chemiluminescence assay using an automated Abbott Architect i1000 system (Abbott Laboratories, Inc., USA). Commercially available kits for the determinations of TG, TC, LDL-C, HDL-C, and lipoprotein A were purchased from Shanghai Zhicheng Biotechnology Co., Ltd., China. Calibration and quality control products were purchased from Randox Laboratories Ltd., Northern Ireland, United Kingdom. The determinations of serum lipids were carried out using a Beckman Coulter AU5800 automatic biochemistry analyzer (Beckman Coulter, Inc., USA).
2.6 Statistical analysis
Statistical analysis was performed with SPSS 22.0 (SPSS Inc., Chicago, IL, USA). The measurement data were first performed by one-sample nonparametric tests (Kolmogorov-Smirnov test) to determine whether they were normal distribution. The data conforming to the normal distribution were expressed as mean ± standard deviation (x ± s), and those conforming to the non-normal distribution were expressed as median [P25, P75]. The comparisons between the control and hyperlipidemia groups were analyzed by independent samples t-test for normal distribution data or Mann-Whitney U test for non-normal distribution data. The count data were presented as percentages, and the comparisons between the control and hyperlipidemia groups were analyzed by the χ2 test. The standard errors (SE) for percentages in two samples were calculated using the following formulas (9): the observed percentage (p) in the combined samples = (n1p1 + n2p2)/(n1 + n2), and SE = [p(100-p)(1/n1 + 1/n2)]1/2. Among them, n1 and n2 were the sample size of two groups, respectively, and p1 and p2 were the percentage of two groups, respectively. The Spearman rank correlation coefficients between EFS and lipids levels were calculated to evaluate the correlations of lipids levels with embryo quality. The multiple logistic regression analysis was used to analyze the impacts of blood lipids on the clinical outcomes of non-obese PCOS patients. They all performed the 2-sided test. P ≤ 0.05 was considered to be statistically significant.
3 Results
3.1 Comparisons of basic clinical information between the control and hyperlipidemia groups in the non-obese PCOS patients undergoing fresh cycle transplantation
The basic clinical information of the PCOS patients undergoing fresh cycle transplantation was presented in Table 1. There were no statistical differences in the age, body mass index (BMI), number of antral follicles (AFC), fasting blood-glucose (FBG), insulin, FSH, LH, testosterone, and AMH (P > 0.05) except serum lipid levels such as TG, TC, HDL, LDL, and lipoprotein A (P < 0.05) between hyperlipidemia and control groups.
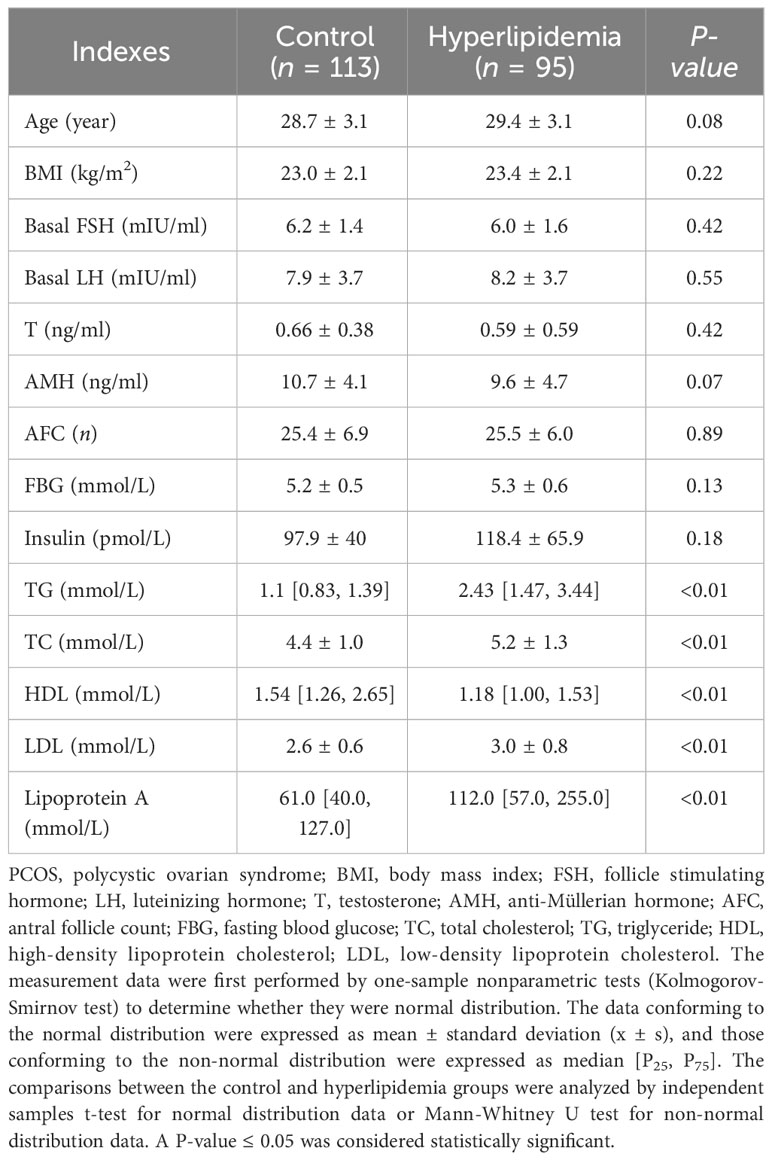
Table 1 Comparisons of basic clinical information between the control and hyperlipidemia groups in the non-obese PCOS patients undergoing fresh cycle transplantation.
3.2 Comparisons of COS protocols and clinical outcomes of assisted pregnancy between the control and hyperlipidemia groups in the non-obese PCOS patients undergoing fresh cycle transplantation
The COS protocols and clinical outcomes of the non-obese PCOS patients undergoing fresh cycle transplantation were presented in Table 2. There were no significant differences in the Gn usage time, endometrial thickness, progesterone level on trigger day, oocytes retrieved, normal fertilization rate, good quality embryos rate, implantation rate, clinical pregnancy rate, and miscarriage rate between hyperlipidemia and control groups. However, the total Gn dosage in the hyperlipidemia group was significantly higher than that in the control group, while the estradiol level on trigger day in the hyperlipidemia group was significantly lower than that in the control group.
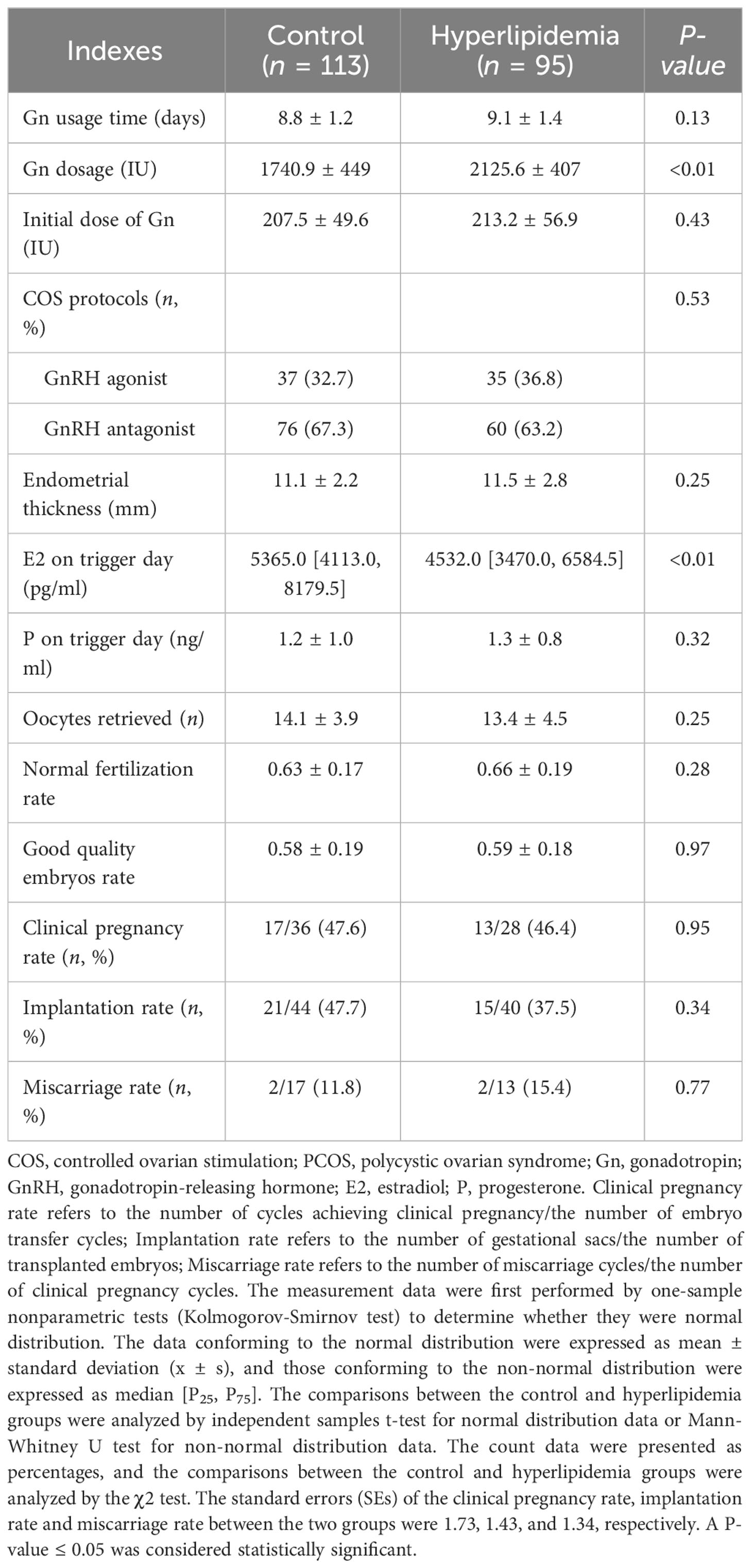
Table 2 Comparisons of COS protocols and clinical outcomes of assisted pregnancy between the control and hyperlipidemia groups in the non-obese PCOS patients undergoing fresh cycle transplantation.
3.3 Correlations of lipid levels with embryo quality in the non-obese PCOS patients undergoing fresh cycle transplantation
To further investigate the effects of lipid levels on embryo quality, we analyzed the correlations of lipid levels with EFS, and found that there were no significant correlations between EFS and TG or TC, but there were a positive correlation between EFS and LDL (r = 0.06, P = 0.015) and negative correlations between EFS and HDL or lipoprotein A (r = -0.489, P < 0.01; r = -0.085, P < 0.01) (Table 3).
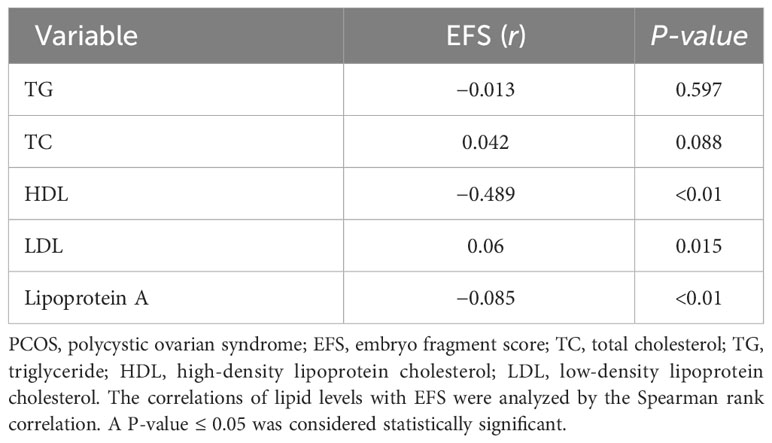
Table 3 Correlations of lipid levels with EFS in the non-obese PCOS patients undergoing fresh cycle transplantation (n = 208).
3.4 Multiple logistic regression analysis between blood lipids and the clinical outcomes of non-obese PCOS patients undergoing fresh cycle transplantation
The multiple logistic regression analysis between blood lipids and the clinical outcomes of non-obese PCOS patients undergoing fresh cycle transplantation was shown in Table 4. The results showed no any correlation between blood lipids and clinical outcomes, as shown by the crude odd ratios (ORs). However, after correcting the Gn dosage and estradiol on trigger day, HDL was shown to be a protective factor for clinical pregnancy (adjusted OR [AOR] = 0.355, 95% CI: 0.135-0.938, P = 0.037) and TC might be a potential risk factor (AOR = 4.072, 95% CI: 0.989-16.761, P = 0.052). It is indicated that HDL is beneficial for clinical pregnancy and TC may be harmful to clinical pregnancy.
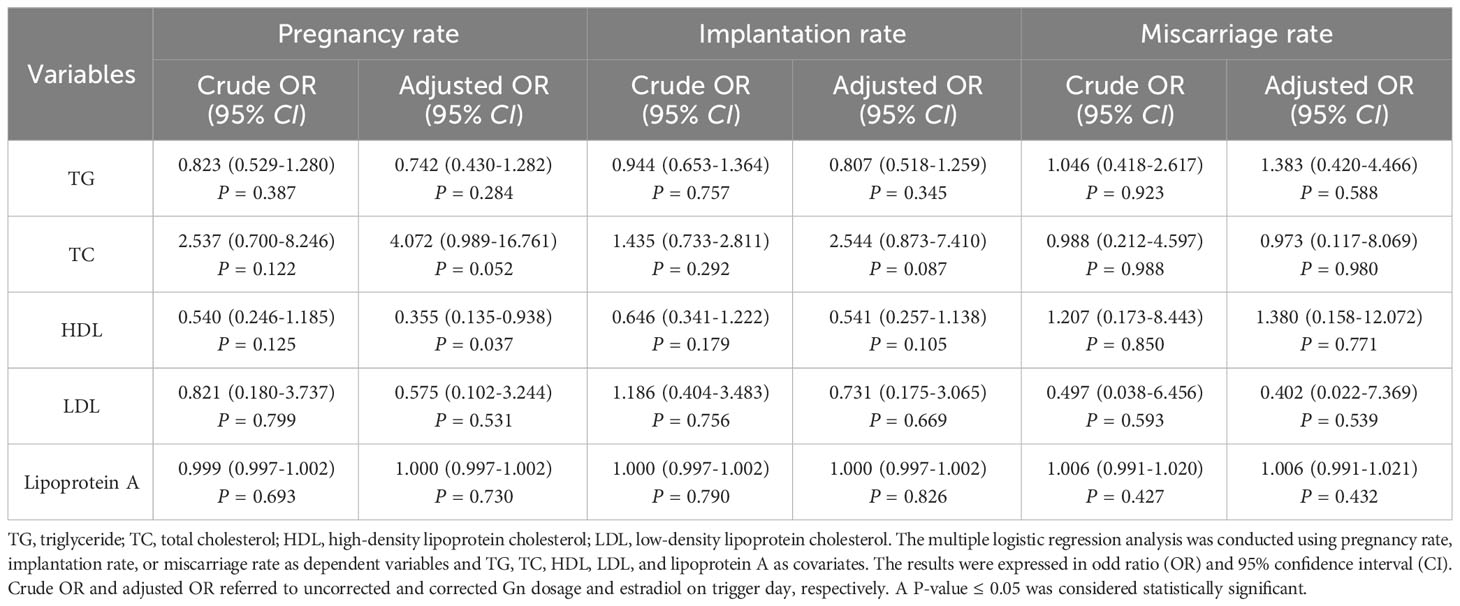
Table 4 Multiple logistic regression analysis between blood lipids and the clinical outcomes of non-obese PCOS patients undergoing fresh cycle transplantation.
3.5 Comparisons of basic clinical information and pregnancy outcomes between the control and hyperlipidemia groups in the non-obese PCOS patients undergoing FET
The basic clinical information and pregnancy outcomes of non-obese PCOS patients undergoing FET were shown in Table 5. Serum lipid levels, including TC, TG, LDL, and HDL, in the hyperlipidemia group were significantly higher than those in the control group (P < 0.01). However, there were no significant differences in the age, BMI, FBG, serum insulin level, serum P and E2 levels on the day of progesterone administration, PI, S/D, endometrial thickness, transferred embryos, implantation rate, clinical pregnancy rate, and miscarriage rate (all P > 0.05) except RI (P = 0.002) between the hyperlipidemia and control groups.
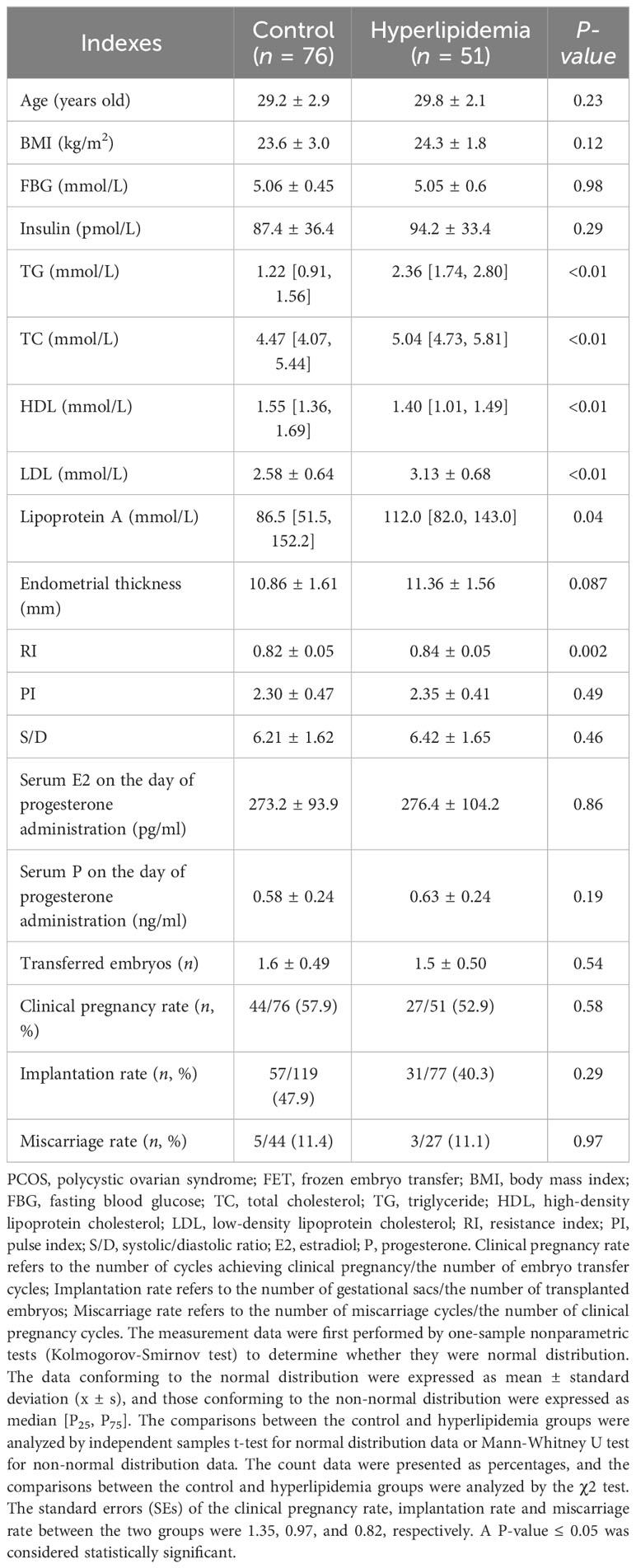
Table 5 Comparisons of basic clinical information and pregnancy outcomes between control and hyperlipidemia groups in the PCOS patients undergoing FET.
3.6 Multiple logistic regression analysis between blood lipids and the clinical outcomes of non-obese PCOS patients undergoing FET
The multiple logistic regression analysis between blood lipids and the clinical outcomes of non-obese PCOS patients undergoing FET was shown in Table 6. The results showed no any correlation between blood lipids and clinical outcomes, whether corrected or uncorrected for RI.
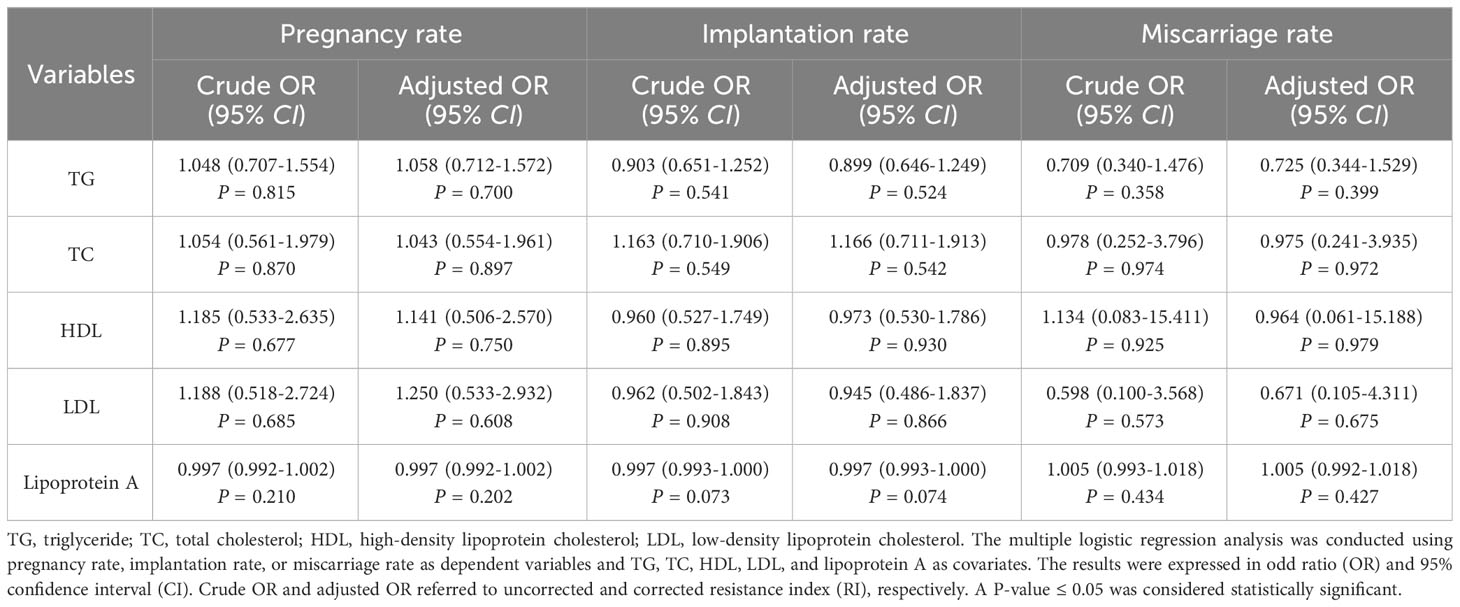
Table 6 Multiple logistic regression analysis between blood lipids and the clinical outcomes of non-obese PCOS patients undergoing FET.
4 Discussion
PCOS is the most common endocrine disorder in reproductive-aged women. Women with PCOS have increased risk of metabolic disorders such as IR, hyperandrogenism, obesity and hyperlipidemia. Researchers have confirmed the effects of obesity and IR on fertility. A compensatory hyperinsulinemic state caused by obesity and PCOS may disrupt endometrial homeostasis and result in insulin receptors decrease and defective decidualization (10). Hyperandrogenism could affect the window of implantation by decreasing the expression levels of HOXA10 and WT1 genes and influencing endometrial decidualization. Cui et al. (11) verified that the obese PCOS patients undergoing IVF/ICSI treatment had lower clinical pregnancy rate. Dyslipidemia often coexists with obesity and IR, and some studies have found that the incidence of hyperlipidemia in PCOS patients increased by 16.1% when compared with the general population (12). However, hyperlipidemia may exist without obesity in PCOS patients, and sometimes it can aggravate obesity, IR and a variety of metabolic abnormalities, which have serious effects on cardiovascular system and fertility (2). Therefore, we speculate that hyperlipidemia may affect the pregnancy outcome of ART. However, the relationship between serum lipids and the pregnancy outcomes of IVF/ICSI in PCOS patients is rarely reported.
The present study was to elucidate the characteristics of serum lipids and their effects on oocyte and endometrium in non-obese women with PCOS undergoing IVF/ICSI. The women chosen for the retrospective analysis had BMI below 30 kg/m2, and their hyperandrogenism were pretreated.
Our investigation found that the non-obese PCOS patients with hyperlipidemia required higher Gn dosage during ovulation induction, which was consistent with previous research results (13). However, the specific reasons are still unclear. Some researchers found that the serum level of sex hormone binding globulin in the patients with hyperlipidemia decreased, and that free testosterone levels increased, which would reduce the sensitivity of ovary to Gn (14). It was also found that tumor necrosis factor (TNF) was involved in the regulation of oocyte maturation and apoptosis, and that high TNFα levels might lead to the arrest of oocyte maturation and chromosome abnormality, and reduce the sensitivity of ovary to Gn (15). While, the increase of blood lipids especially triglyceride was positively correlated with TNFα (16).
An analysis of 1394 treatment cycles of 943 patients found that daily dose of Gn and total Gn dosage were negatively correlated with the number of oocytes, implantation rate, clinical pregnancy rate, and live-birth rate, which recommended that daily Gn dose is preferably less than 450 IU or total Gn dosage less than 3000 IU/cycle (17). Another study found that daily Gn dose exceeding 300 IU was significantly associated with a lower live birth rate (18). Excessive Gn exposure may lead to an unfavorable endometrium or a detrimental metabolic environment (19). It is speculated that increasing Gn dosage may cause high circulating progesterone level and a premature luteinization of the endometrium (20). It is also well established that the use of exogenous Gn affects the endometrium and can result in functional genomic disorder of the endometrium (21). Therefore, high Gn dosage in non-obese PCOS patients with hyperlipidemia may also affect endometrial receptivity.
In addition, numerous studies have demonstrated that the metabolic problems related to hyperlipidemia, such as hyperinsulinemia and aberrant adipokines, may affect embryonic development and endometrial receptivity (22). Some studies have demonstrated that hyperlipidemia has an adverse effect on embryo quality, and that the exact mechanisms need to be further verified. Some researches showed that hyperlipidemia could produce a large number of reactive oxygen species (ROS), and then destroy the morphology and integrity of endoplasmic reticulum of granulosa cells. All these changes would disturb the maturation of oocytes and reduce the blastocyst formation rate (8, 23, 24). Some animal experiments found that high fat diets might cause important defects and chromosome dislocations during meiosis of oocytes (25).
In our study, although there were no significant differences in the normal fertilization rate and good quality embryos rate between the control and hyperlipidemia groups, the embryo fragmentation score (EFS) was positively correlated with LDL and negatively with HDL and lipoprotein A (P < 0.05). Moreover, the multiple logistic regression analysis showed that HDL was beneficial for clinical pregnancy (AOR = 0.355, 95% CI: 0.135-0.938, P = 0.037) and TC may be a potential risk factor for clinical pregnancy (AOR = 4.072, 95% CI: 0.989-16.761, P = 0.052). Some studies also demonstrated that the levels of follicular fluid and plasma HDL were negatively correlated with embryo fragmentation (26). It was reported that transferring embryos with more fragments had lower implantation and pregnancy rates than transferring embryos with minimal fragments (27). Browne et al. (28) confirmed that HDL exhibited important cytoprotective effect on oocytes and granulosa cells around them. The most compelling evidence for the importance of HDL in mammalian oocyte development and competence was exemplified by studies of SR-BI KO mice (29). The SR-BI known as HDL receptor could facilitate the uptake of cholesterol for steroidogenesis. Oocyte and embryo morphology were disrupted in SR-BI KO female mice. However, it is unclear how HDL works to safeguard embryonic growth. Some researchers thought that it might be associated with antioxidation. The HDL particles include phospholipids, triglycerides and proteins such as PON1, which play an antioxidant role and would protect embryos from the damage of ROS (30). Theoretically, follicular fluid HDL is a filtration byproduct of human plasma. The plasma HDL level might partly reflect the HDL level in follicular fluid. However, the HDL particles in follicles contain less cholesterol and rich phospholipids relative to those in serum. Moreover, the HDL particles in follicles are heterogeneous in structure, and it is unclear which type of HDL is primarily related to embryo fragmentation. Kim et al. (31) found that higher concentrations of follicular fluid HDL subfractions in the large and medium-sized particles were associated with poorer embryo quality. Therefore, we speculate that HDL plays a protective role in oocyte and embryo development, but which kind of HDL is necessary still needs to be further explored.
Although we found a harmful effect of hyperlipidemia on embryos in fresh cycles, there were no differences in clinical pregnancy rate and miscarriage rate between hyperlipidemia and control groups. This may be attributed to the fact that the best embryos with few fragments in the first cycle were transplanted. Considering that serum E2 levels on trigger day in the control group were significantly higher than that in the hyperlipidemia group, and that serum E2 level was crucial for embryo implantation, we further investigated the FET outcomes of the two groups. All the patients undergone the FET for the first time received HRT. There were no significant differences in serum E2 levels and endometrial thickness on the day of progesterone administration. Meanwhile, all the transferred embryos were of good quality, similar to the fresh transfer cycle. The results showed that there were no significant differences in the implantation rate, pregnancy rate, and miscarriage rate between hyperlipidemia and control groups, but the resistance index (RI) was significantly different.
Uterine blood supply mainly comes from the uterine artery and its branches, including the arcuate artery supplying the myometrium and the spiral artery supplying the endometrium. During the embryo implantation stage, the blood supply to the uterus alters to ensure adequate blood supplementation. Kim et al. (32) used Doppler ultrasonography to measure endometrial and subendometrial vascularity on the day of HCG administration during IVF-ET, and found that endometrial and subendometrial vascularity in the pregnancy group was significantly higher than that in the control group. It was reported that a successful FET cycle was related to increased blood flow to the endometrium, which boosted endometrial receptivity (33). However, the results of this study showed that the difference of RI was not enough to lead to the difference of implantation rate. Subendometrial vascularity indexes such as vascularity index (VI) and vascularity flow index (VFI) are more accurate than uterus artery indexes including RI, PI and S/D. Some studies found that there were no differences in uterus artery indexes such as PI, RI and S/D scores between pregnancy and non-pregnancy groups (32, 34). In recent studies subendometrial vascularity indexes were more prone to be selected to detect endometrial receptivity. However, due to the significant technical requirements for measuring subendometrial blood vessels, we only measured the uterus artery indexes. This is one of the limitations of our study. This study is only conducted in one institute, which requires more data to confirm. Another limitation of this study is the limited sample size. Analyzing the FET cycle and fresh cycle separately may further reduce the testing power of the used samples. In the future, we will increase the sample size to further confirm the impact of hyperlipidemia on the clinical outcomes of ART in PCOS patients and explore its possible mechanisms.
5 Conclusions
In conclusion, our study found that the total Gn dosage in the hyperlipidemia group was significantly higher than that in the control group, and that the embryo fragment score was positively correlated with LDL and negatively with HDL and lipoprotein A. Moreover, HDL was beneficial for clinical pregnancy. In addition, in FET cycles, the resistance index in the hyperlipidemia group was significantly higher than that in the control group. It is indicated that hyperlipidemia may increase the dosage of gonadotropin and have adverse effect on the embryo quality, endometrial receptivity, and clinical outcomes of lean PCOS patients. It is recommended that the non-obese patients with hyperlipidemia and PCOS perform lipid-lowering treatment before undergoing embryo transfer.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The clinical data of patients undergoing conventional IVF or ICSI treatment in our hospital during October 2017 and June 2021 were analyzed retrospectively. The implenmentation of IVF or ICSI was approved by the Reproductive Medicine Ethics Committee of Zhongda Hospital affiliated to Southeast University (Reproduction No. 2015-1), and all patients signed the informed consent. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from primarily isolated as part of your previous study for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
FY: Data curation, Formal Analysis, Writing – original draft. J-CL: Conceptualization, Data curation, Writing – review & editing. TS: Data curation, Writing – original draft. Y-HJ: Data curation, Writing – original draft. Y-JL: Conceptualization, Resources, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
References
1. Gonzalez MB, Robker RL, Rose RD. Obesity and oocyte quality: Significant implications for ART and emerging mechanistic insights. Biol Reprod (2022) 106:338–50. doi: 10.1093/biolre/ioab228
2. Liu Q, Xie YJ, Qu LH, Zhang MX, Mo ZC. Dyslipidemia involvement in the development of polycystic ovary syndrome. Taiwan J Obstet Gynecol (2019) 58:447–53. doi: 10.1016/j.tjog.2019.05.003
3. Kim JJ, Choi YM. Dyslipidemia in women with polycystic ovary syndrome. Obstet Gynecol Sci (2013) 56:137–42. doi: 10.5468/ogs.2013.56.3.137
4. Li J, Xie LM, Song JL, Yau LF, Mi JN, Zhang CR, et al. Alterations of sphingolipid metabolism in different types of polycystic ovary syndrome. Sci Rep (2019) 9:3204. doi: 10.1038/s41598-019-38944-6
5. Liu T, Liu D, Song X, Qu J, Zheng X, Li J, et al. Lipid metabolism was associated with oocyte in vitro maturation in women with polycystic ovarian syndrome undergoing unstimulated natural cycle. Front Cell Dev Biol (2021) 9:719173. doi: 10.3389/fcell.2021.719173
6. Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod (2004) 19:41–7. doi: 10.1093/humrep/deh098
7. Hu DY. New guidelines and evidence for prevention and treatment of dyslipidemia and atherosclerotic cardiovascular disease in China. Chronic Dis Transl Med (2016) 44(10):826. doi: 10.1016/j.cdtm.2016.11.001
8. Niu Z, Lin N, Gu R, Sun Y, Feng Y. Associations between insulin resistance, free fatty acids, and oocyte quality in polycystic ovary syndrome during in vitro fertilization. J Clin Endocrinol Metab (2014) 99:E2269–76. doi: 10.1210/jc.2013-3942
9. Stuart A. Standard errors for percentages. J Roy Stat Soc C-App (1963) 12(2):87–101. doi: 10.2307/2985472
10. Schulte MM, Tsai JH, Moley KH. Obesity and PCOS: The effect of metabolic derangements on endometrial receptivity at the time of implantation. Reprod Sci (2015) 22:6–14. doi: 10.1177/1933719114561552
11. Cui N, Wang H, Wang W, Zhang J, Xu Y, Jiang L, et al. Impact of body mass index on outcomes of in vitro fertilization/intracytoplasmic sperm injection among polycystic ovarian syndrome patients. Cell Physiol Biochem (2016) 39:1723–34. doi: 10.1159/000447873
12. Ng NYH, Jiang G, Cheung LP, Zhang Y, Tam CHT, Luk AOY, et al. Progression of glucose intolerance and cardiometabolic risk factors over a decade in Chinese women with polycystic ovary syndrome: A case-control study. PloS Med (2019) 16:e1002953. doi: 10.1371/journal.pmed.1002953
13. Liu F, Jiang Q, Sun X, Huang Y, Zhang Z, Han T, et al. Lipid metabolic disorders and ovarian hyperstimulation syndrome: A retrospective analysis. Front Physiol (2020) 11:491892. doi: 10.3389/fphys.2020.491892
14. Fontana R, Torre SD. The deep correlation between energy metabolism and reproduction: A view on the effects of nutrition for women fertility. Nutrients (2016) 8:87. doi: 10.3390/nu8020087
15. Silva JRV, Lima FEO, Souza ALP, Silva AWB. Interleukin-1β and TNF-α systems in ovarian follicles and their roles during follicular development, oocyte maturation and ovulation. Zygote (2020) 28(4):270–7. doi: 10.1017/S0967199420000222
16. Niu Z, Ye Y, Xia L, Feng Y. Cytokine profile of follicular fluid and oocyte quality of PCOS patients with metabolic syndrome. J Reprod Med (2017) 26(5):409–14. doi: 10.3969/j.issn.1004-3845.2017.05.004
17. Friedler S, Meltzer S, Saar-Ryss B, Rabinson J, Lazer T, Liberty G. An upper limit of gonadotropin dose in patients undergoing ART should be advocated. Gynecol Endocrinol (2016) 32(12):965–9. doi: 10.1080/09513590.2016.1199018
18. Baker VL, Brown MB, Luke B, Smith GW, Ireland JJ. Gonadotropin dose is negatively correlated with live birth rate: analysis of more than 650,000 assisted reproductive technology cycles. Fertil Steril (2015) 104(5):1145–52. doi: 10.1016/j.fertnstert.2015.07.1151
19. Zhang JN, Wang J, Peng HM, Ma MY, Wang H, Zhao CC, et al. Effect of exogenous gonadotropin dosage on embryo aneuploidy rate and pregnancy outcome in patients of preimplantation genetic test. Zhonghua Fu Chan Ke Za Zhi (2020) 55(4):253–8. doi: 10.3760/cma.j.cn112141-20200309-00198
20. Gerber R, Fazzari M, Kappy M, Cohen A, Galperin S, Lieman H, et al. Differential impact of controlled ovarian hyperstimulation on live birth rate in fresh vs. frozen embryo transfer cycles: a SART CORS study. Fertil Steril (2020) 114(6):1225–31. doi: 10.1016/j.fertnstert.2020.06.021
21. Horcajadas JA, Mínguez P, Dopazo J, Esteban FJ, Domínguez F, Giudice LC, et al. Controlled ovarian stimulation induces a functional genomic delay of the endometrium with potential clinical implications. J Clin Endocrinol Metab (2008) 93(11):4500–10. doi: 10.1210/jc.2008-0588
22. Ye Q, Zeng X, Cai S, Qiao S, Zeng X. Mechanisms of lipid metabolism in uterine receptivity and embryo development. Trends Endocrinol Metab (2021) 32:1015–30. doi: 10.1016/j.tem.2021.09.002
23. Bradley J, Swann K. Mitochondria and lipid metabolism in mammalian oocytes and early embryos. Int J Dev Biol (2019) 63:93–103. doi: 10.1387/ijdb.180355ks
24. Mirabi P, Chaichi MJ, Esmaeilzadeh S, Ali Jorsaraei SG, Bijani A, Ehsani M, et al. The role of fatty acids on ICSI outcomes: a prospective cohort study. Lipids Health Dis (2017) 16:18. doi: 10.1186/s12944-016-0396-z
25. Grindler NM, Moley KH. Maternal obesity, infertility and mitochondrial dysfunction: potential mechanisms emerging from mouse model systems. Mol Hum Reprod (2013) 19:486–94. doi: 10.1093/molehr/gat026
26. Staessen C, Camus M, Bollen N, Devroey P, Van Steirteghem AC. The relationship between embryo quality and the occurrence of multiple pregnancies. Fertil Steril (1992) 57:626–30. doi: 10.1016/S0015-0282(16)54911-5
27. Ziebe S, Petersen K, Lindenberg S, Andersen AG, Gabrielsen A, Andersen AN. Embryo morphology or cleavage stage: how to select the best embryos for transfer after in-vitro fertilization. Hum Reprod (1997) 12:1545–9. doi: 10.1093/humrep/12.7.1545
28. Browne RW, Bloom MS, Shelly WB, Ocque AJ, Huddleston HG, Fujimoto VY. Follicular fluid high density lipoprotein-associated micronutrient levels are associated with embryo fragmentation during IVF. J Assist Reprod Genet (2009) 26:557–60. doi: 10.1007/s10815-009-9367-x
29. Yesilaltay A, Morales MG, Amigo L, Zanlungo S, Rigotti A, Karackattu SL, et al. Effects of hepatic expression of the high-density lipoprotein receptor SR-BI on lipoprotein metabolism and female fertility. Endocrinology (2006) 147:1577–88. doi: 10.1210/en.2005-1286
30. Maiga SF, Kalopissis AD, Chabert M. Apolipoprotein A-II is a key regulatory factor of HDL metabolism as appears from studies with transgenic animals and clinical outcomes. Biochimie (2014) 96:56–66. doi: 10.1016/j.biochi.2013.08.027
31. Kim K, Bloom MS, Browne RW, Bell EM, Yucel RM, Fujimoto VY. Associations between follicular fluid high density lipoprotein particle components embryo quality among in vitro fertilization patients. J Assist Reprod Genet (2017) 34:1–10. doi: 10.1007/s10815-016-0826-x
32. Kim A, Jung H, Choi WJ, Hong SN, Kim HY. Detection of endometrial and subendometrial vasculature on the day of embryo transfer and prediction of pregnancy during fresh in vitro fertilization cycles. Taiwan J Obstet Gynecol (2014) 53:360–5. doi: 10.1016/j.tjog.2013.05.007
33. Sardana D, Deepika K, Pranesh GT, Pranesh GT, Rao KA. Correlation of subendometrial endometrial blood flow assessment by two dimensional power Doppler with pregnancy outcome in frozen thawed embryo transfer cycles. J Hum Reprod Sci (2014) 7:130–5. doi: 10.4103/0974-1208.138872
Keywords: polycystic ovary syndrome, hyperlipidemia, in vitro fertilization, pregnancy outcome, non-obese
Citation: Yang F, Lu J-C, Shen T, Jin Y-H and Liang Y-J (2023) Effect of hyperlipidemia on the outcome of in vitro fertilization in non-obese patients with polycystic ovary syndrome. Front. Endocrinol. 14:1281794. doi: 10.3389/fendo.2023.1281794
Received: 23 August 2023; Accepted: 30 October 2023;
Published: 14 November 2023.
Edited by:
Alexandra E Butler, Royal College of Surgeons in Ireland, BahrainReviewed by:
Yujia Zhang, Centers for Disease Control and Prevention (CDC), United StatesShuo Yang, Peking University Third Hospital, China
Copyright © 2023 Yang, Lu, Shen, Jin and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin-Chun Lu, NDA2NjQ2MjI3QHFxLmNvbQ==; Yuan-Jiao Liang, eXVhbmppYW8xOTY1QDEyNi5jb20=
 Fang Yang
Fang Yang Jin-Chun Lu
Jin-Chun Lu