- Clinical Centre of Reproductive Medicine, Lianyungang Maternal and Child Health Hospital, Lianyungang, Jiangsu, China
Background: Previous studies have investigated the relationship between nesfatin-1 level and polycystic ovary syndrome (PCOS). However, these studies have produced conflicting results. Thus, in this meta-analysis, we aimed to clarify the association between blood nesfatin-1 levels and PCOS, and the ability of nesfatin-1 as a biomarker in PCOS.
Methods: Meta-analysis was performed using STATA 12.0 software. We computed standard mean difference (SMD) and 95% confidence interval (CI) regarding the comparison of blood nesfatin-1 in patients with PCOS and controls.
Results: The present meta-analysis showed no significant difference in blood nesfatin-1 level between patients with PCOS and controls with a random effects model (SMD = 0.03; 95%CI: -0.71, 0.77; I2 = 97.1%, p value for Q test < 0.001). Subgroup analysis for different ethnicities reported no significant difference in blood nesfatin-1 level between patients with PCOS and controls in both Caucasian and Asian populations. Subgroup analysis for different sample types reported no significant difference in serum nesfatin-1 level between patients with PCOS and controls. Subgroup studies reported no significant difference in blood nesfatin-1 level between PCOS and controls in both obese and non-obese populations.
Conclusion: In conclusion, there is no significant relationship between blood nesfatin-1 levels and PCOS.
1 Introduction
Polycystic ovary syndrome (PCOS) is one of the most common female endocrine disorders without exact etiology currently, affecting approximately 6%-10% of women worldwide (1). PCOS patients are most characterized by sex hormone imbalance, with hallmark features of acne, hirsutism, infertility, irregular menstrual cycle, and polycystic appearing ovaries on ultrasound (2). The Rotterdam diagnostic criteria for PCOS are now internationally endorsed and are based on two of three features: oligo- or anovulation, hyperandrogenism (clinical or biochemical), and polycystic ovaries (3). Additionally, the evidence indicates that PCOS is associated with several endocrine and metabolic disorders, including insulin resistance, and dyslipidemia (4, 5). A recent narrative review proposed that the levels of nesfatin-1, myonectin, omentin, and neudesin were decreased in PCOS patients, while the levels of the other considered agents (e.g., preptin, gremlin-1, neuregulin-4, xenopsin-related peptide, xenin-25, and galectin-3) were increased (6).
Nesfatin-1 is widely expressed in both the central nervous system and peripheral tissue with the role of regulating metabolism, appetite, gut motility, and feeding behavior (7, 8). As a multifunctional biomolecule, nesfatin-1 plays an important role in the diagnosis and treatment of many diseases, including coronary artery disease (9), multiple sclerosis (10), type 2 diabetes mellitus (11). Studies have shown that nesfatin-1 is related to the inhibition of lipid-related diseases, because it can reduce fat accumulation and increase lipid decomposition in the lipid metabolism (12).
As a newly discovered cytokine in 2006, previous studies have investigated the relationship between nesfatin-1 level and PCOS. However, these studies have produced conflicting results. Some studies revealed higher levels of nesfatin-1 in patients with PCOS relative to healthy controls, while others reported opposite findings. Thus, in this meta-analysis, we aimed to clarify the association between blood nesfatin-1 levels and PCOS, and the ability of nesfatin-1 as biomarker in PCOS.
2 Methods
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) guidelines (13) and Meta-analyses of Observational Studies in Epidemiology (MOOSE) guidelines (14).
2.1 Literature search
Two reviewers (MW and JT) independently searched these databases (PubMed, Web of Science, EMBASE, Medline and Google Scholar) from the inception of the databases to June 30, 2023. We only included studies written in English. The search terms were (“nesfatin-1” OR “nesfatin” OR “markers” OR “biomarkers”) AND (“polycystic ovary syndrome” OR “PCOS”). Articles were discussed and decided by the three authors (MW, JT and QZ) after the appearance of inconsistent selections.
2.2 Study selection
Inclusion criteria: 1) study investigated blood nesfatin-1; 2) study investigated PCOS; 3) study written in English; 4) studies used control group. The control group had no clinical or biochemical evidence of PCOS.
Exclusion criteria: 1) reviews, meta-analysis and case reports; 2) letters book chapters, animal studies and published abstracts; 3) study which did not provide sufficient information about blood nesfatin-1 level in PCOS.
2.3 Data extraction
Two reviewers screened titles and abstracts of all articles. We extracted these data from included articles: first author, publication year, country, sample size, mean age, body mass index (BMI), blood nesfatin-1 concentrations, sample type and detection method.
2.4 Statistical analysis
Meta-analysis was performed using STATA 12.0 software. We computed standard mean difference (SMD) and 95% confidence interval (CI) regarding the comparison of blood nesfatin-1 in patients with PCOS and controls. Heterogeneity across studies was explored with I2 and Q test. A random effects model was used for I2 ≥ 50% and p value for Q test ≤ 0.05. A fixed-effects model was used for I2 < 50% and p value for Q test > 0.05. Meta-regression analysis was adopted to investigate the source of heterogeneity. Subgroup studies for different ethnicities and different sample types were conducted to investigate the source of the heterogeneity. Obesity in adults was defined by the World Health Organization (WHO) (15) as BMI > 30kg/m² for obese. Subgroup studies depending on the presence and absence of obesity was conducted to investigate the source of the heterogeneity. Sensitivity analysis was adopted to evaluate the stabilization of meta-analysis. Begg’s test was adopted to evaluate publication bias.
3 Results
3.1 Characteristics of included studies
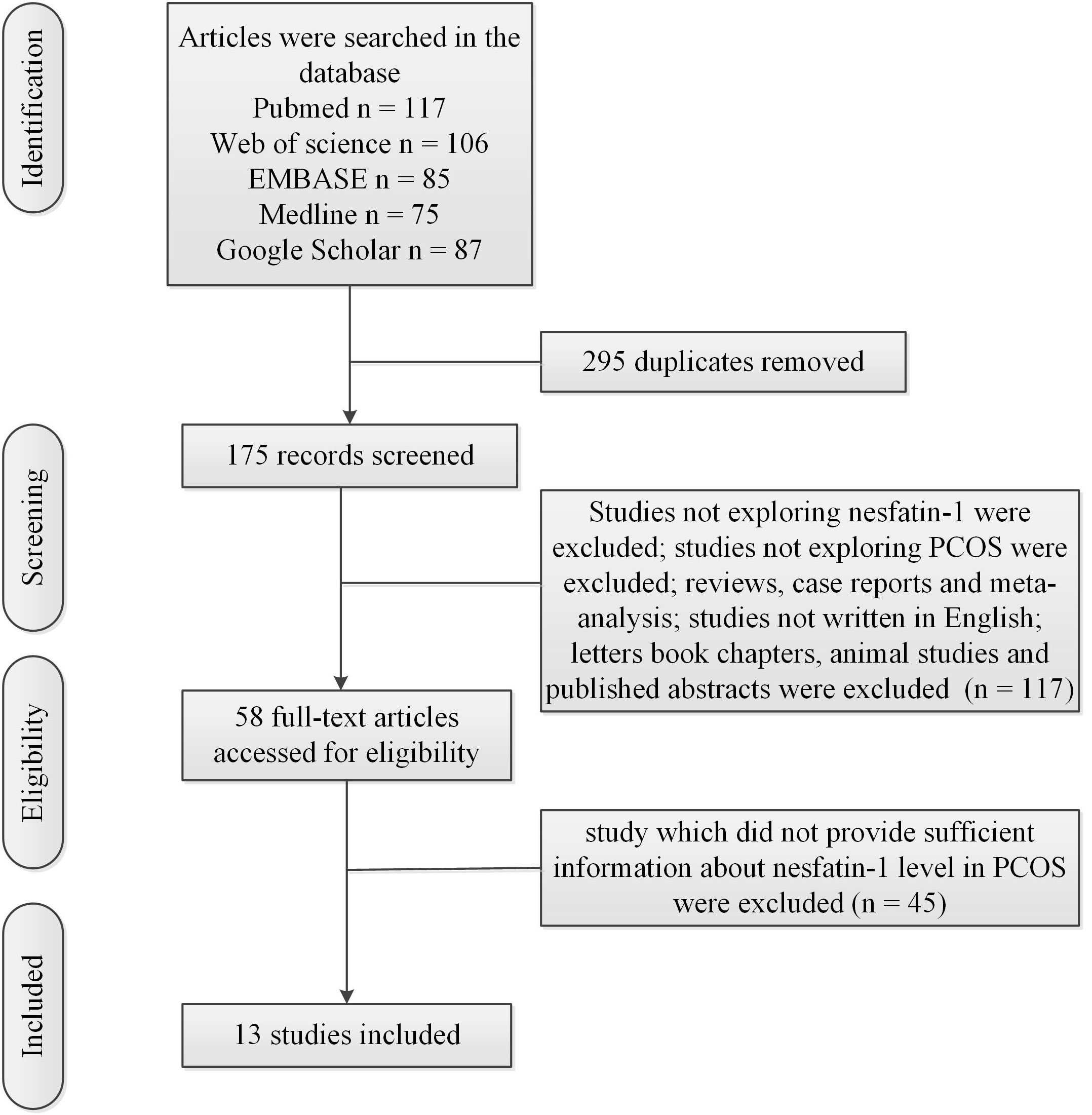
Figure 1 showed the flow chart of the literature search and selection process. Table 1 showed characteristics of included studies. Mean value and standard deviation (SD) of blood nesfatin-1 in patients with PCOS and controls were collected from 13 studies (16–28) (patients with PCOS: n = 757, controls: n = 569).
3.2 Meta-analysis results
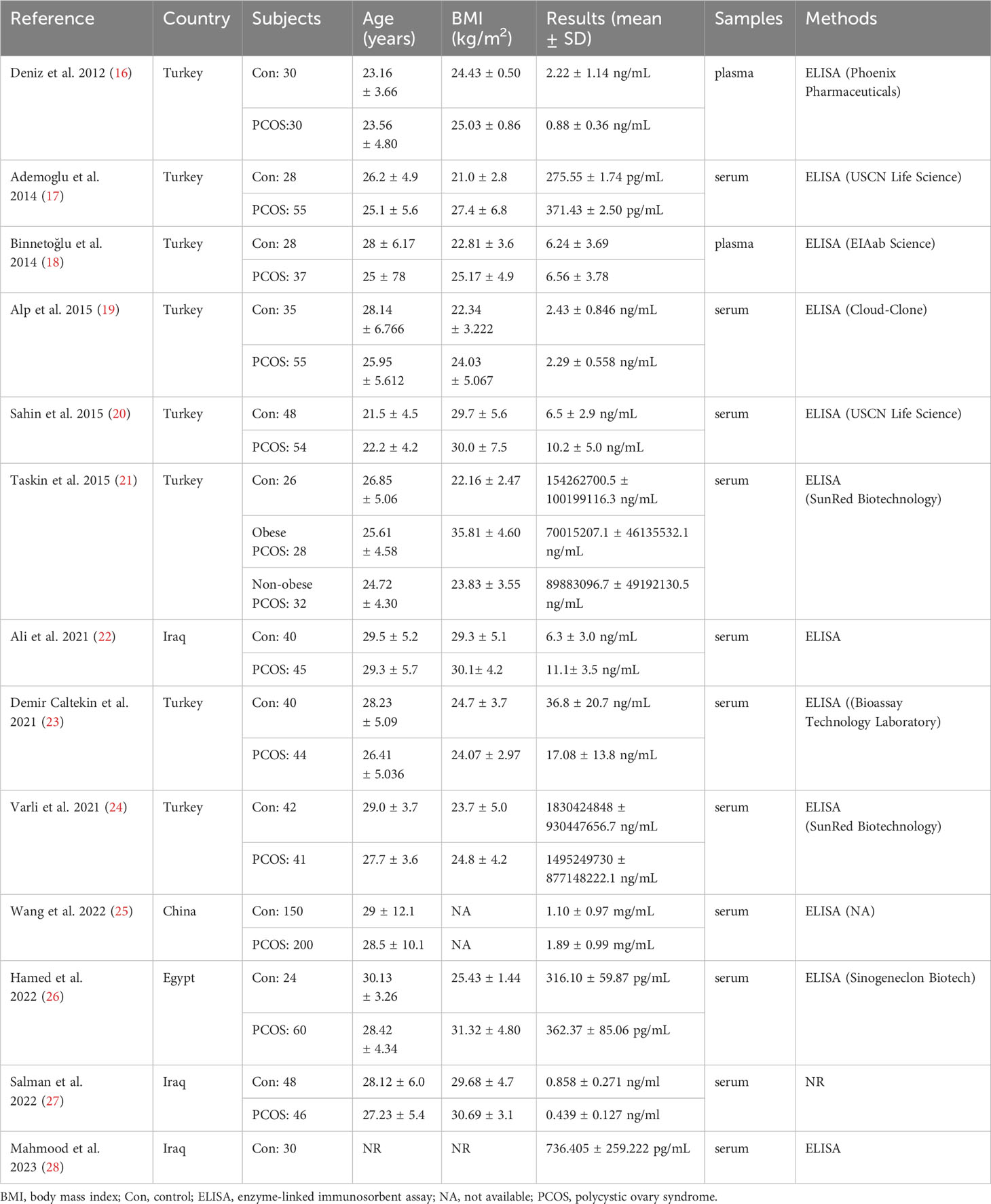
The present meta-analysis showed no significant difference in blood nesfatin-1 level between patients with PCOS and controls with a random effects model (SMD = 0.03; 95%CI: -0.71, 0.77; I2 = 97.1%, p value for Q test < 0.001; Figure 2). Meta-regression analysis showed that publication year and age were not responsible for heterogeneity across studies (publication year: p value = 0.369; age: p value = 0.632). Subgroup analysis for different ethnicities reported no significant difference in blood nesfatin-1 level between patients with PCOS and controls in both Caucasian and Asian populations (Caucasian: SMD = 0.30; 95%CI: -0.68, 1.28; Asian: SMD = -0.41; 95%CI: -2.03, 1.21; Figure 3). Subgroup analysis for different sample types reported no significant difference in serum nesfatin-1 level between patients with PCOS and controls (SMD = 0.20; 95%CI: -0.63, 1.03; Figure 4). Subgroup studies depending on presence and absence of obesity reported no significant difference in blood nesfatin-1 level between PCOS and controls in both obese and non-obese populations (Figure 5). Sensitivity analysis reported no changes in the direction of effect when any one study was excluded (Figure 6). Begg’s test and funnel plots showed no significant risk of publication bias (Begg’s test: p = 0.125; Figure 7).
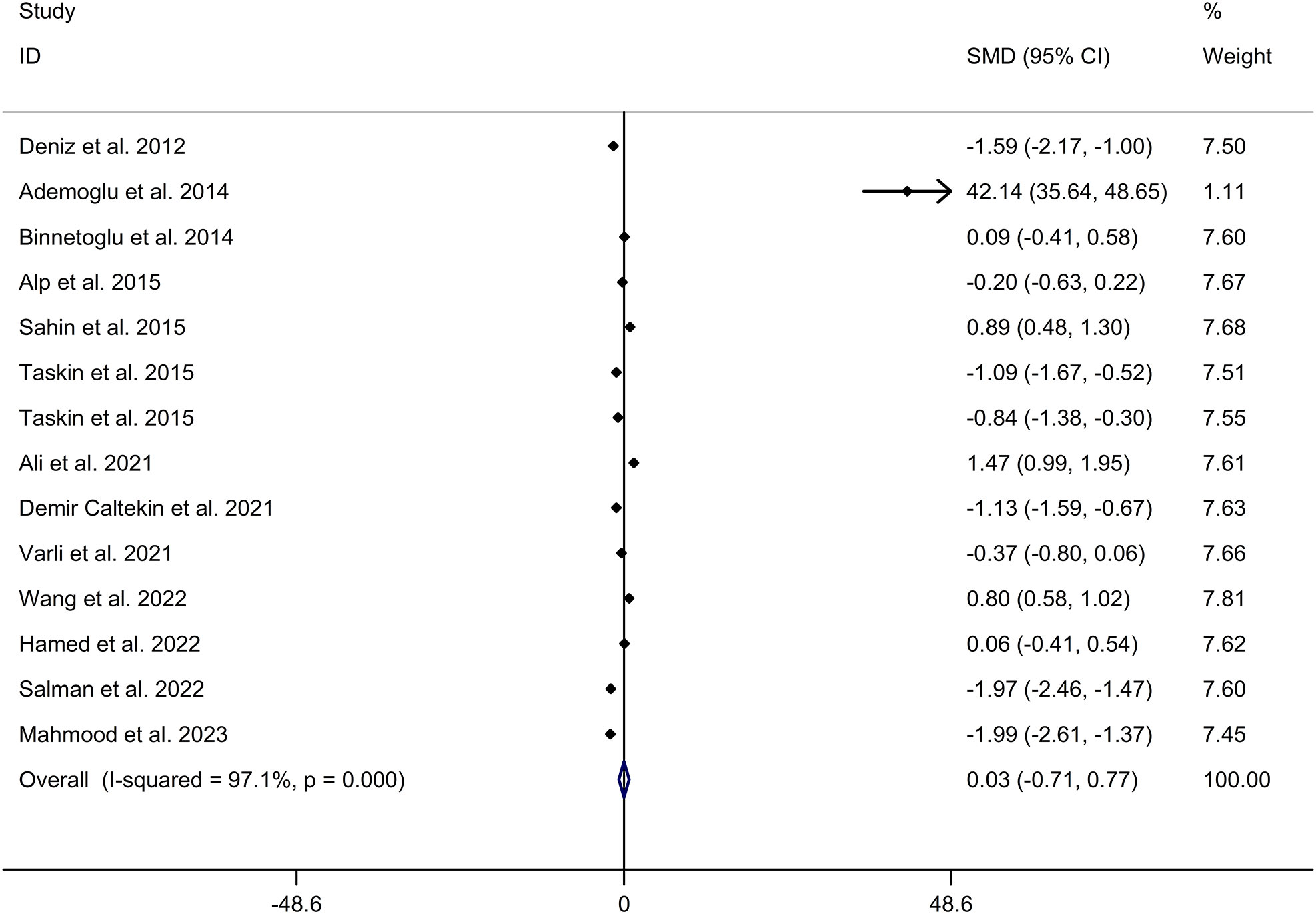
Figure 2 Forest plot for comparison in blood nesfatin-1 level between patients with PCOS and controls. CI, confidence interval; PCOS, polycystic ovary syndrome; SMD, standard mean difference.
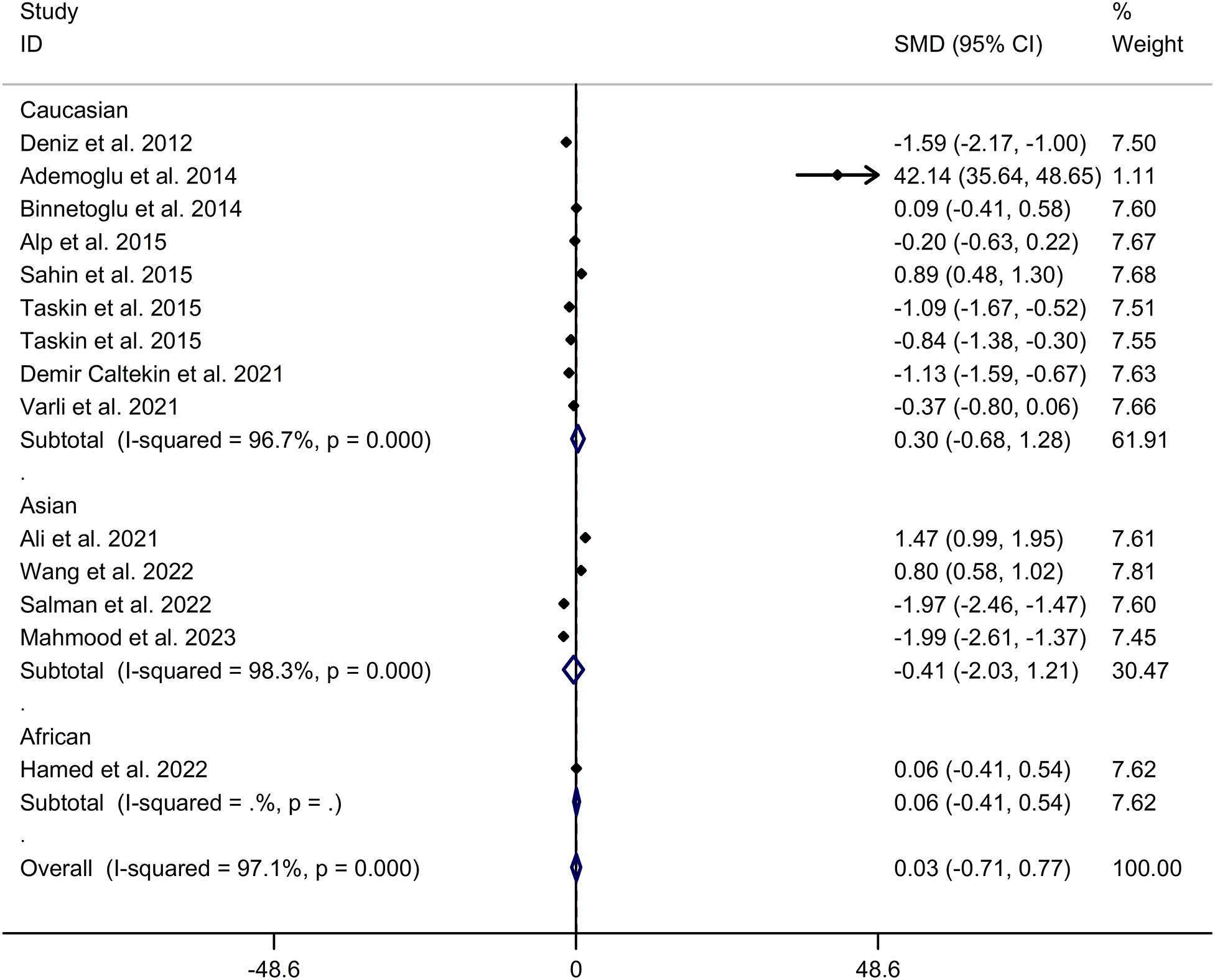
Figure 3 Subgroup analysis for comparison in blood nesfatin-1 level between patients with PCOS and controls with different ethnicities. CI, confidence interval; PCOS, polycystic ovary syndrome; SMD, standard mean difference.
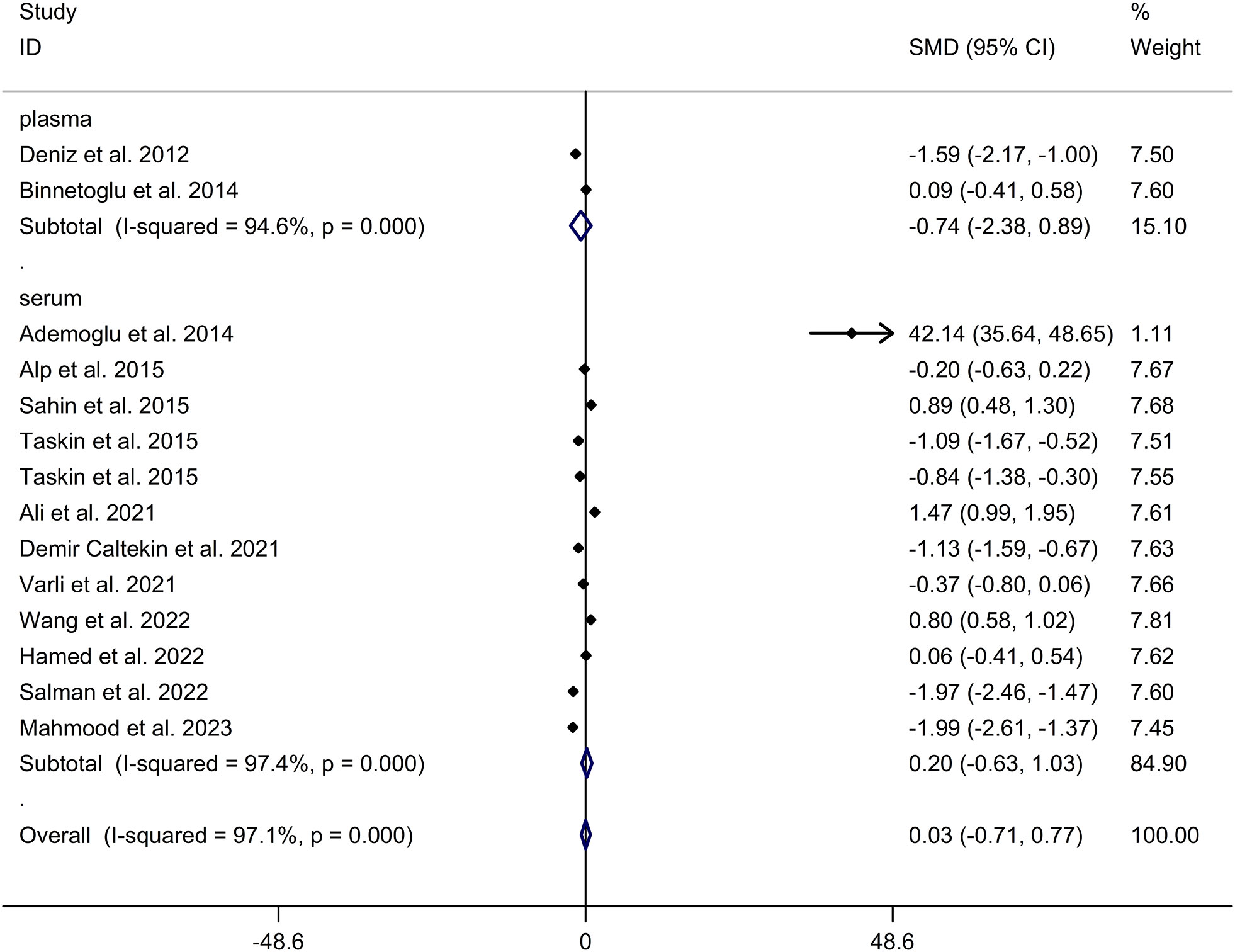
Figure 4 Subgroup analysis for comparison in nesfatin-1 level detected by different sample types between patients with PCOS and controls. CI, confidence interval; PCOS, polycystic ovary syndrome; SMD, standard mean difference.
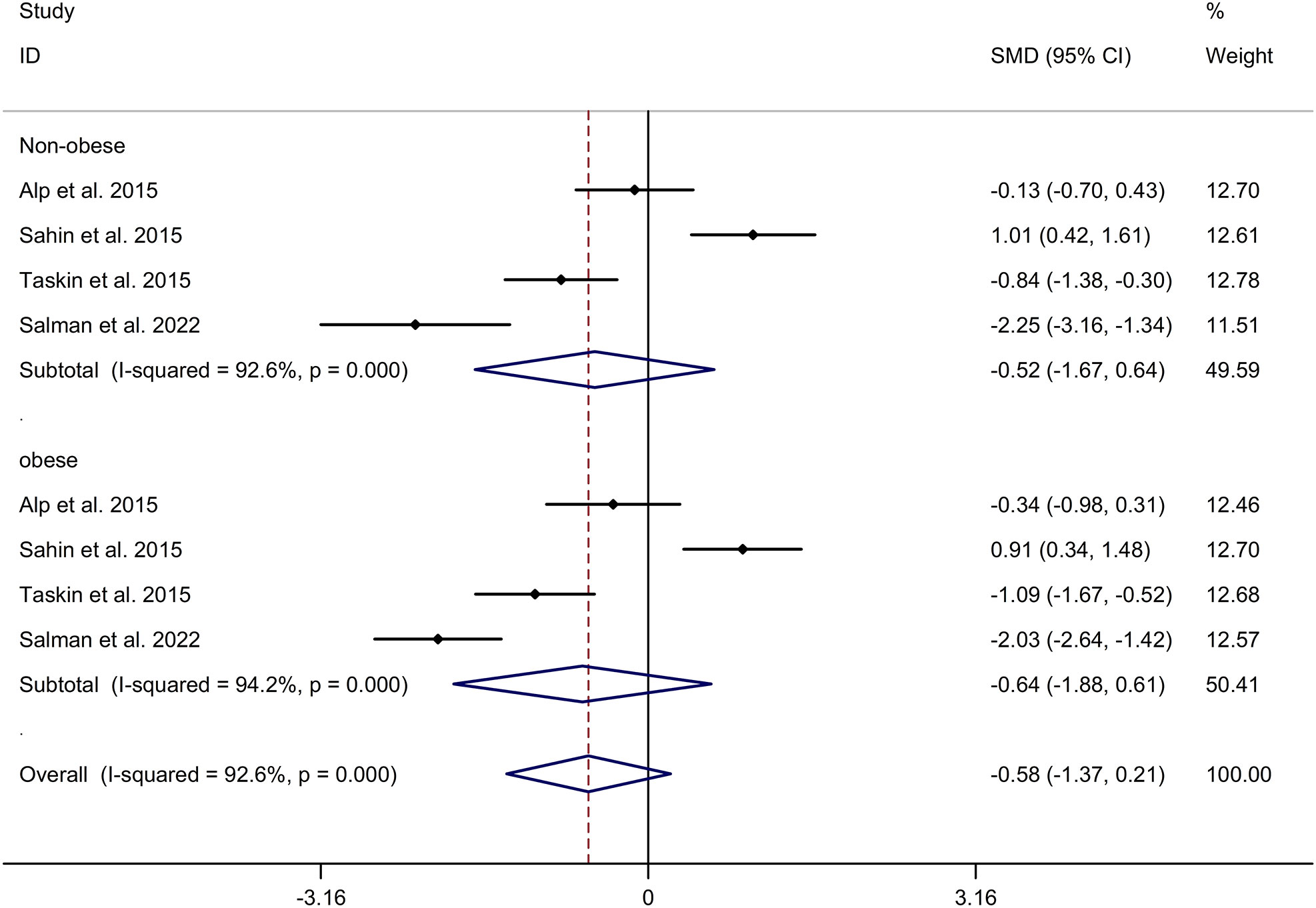
Figure 5 Subgroup analysis depending on presence and absence of obesity for comparison in nesfatin-1 level between patients with PCOS and controls. CI, confidence interval; PCOS, polycystic ovary syndrome; SMD, standard mean difference.
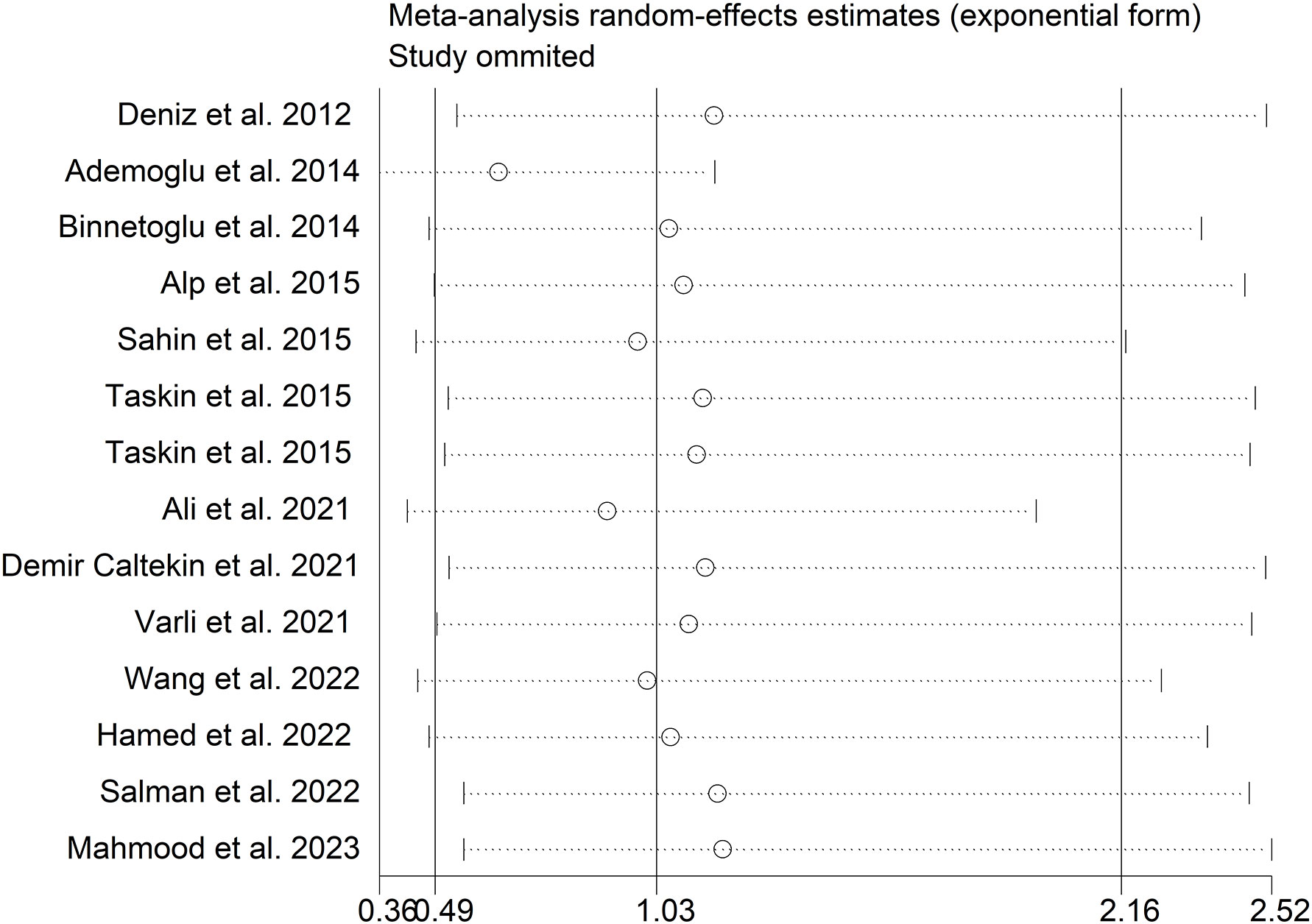
Figure 6 Sensitivity analysis for comparison in blood nesfatin-1 level between patients with PCOS and controls. PCOS, polycystic ovary syndrome.
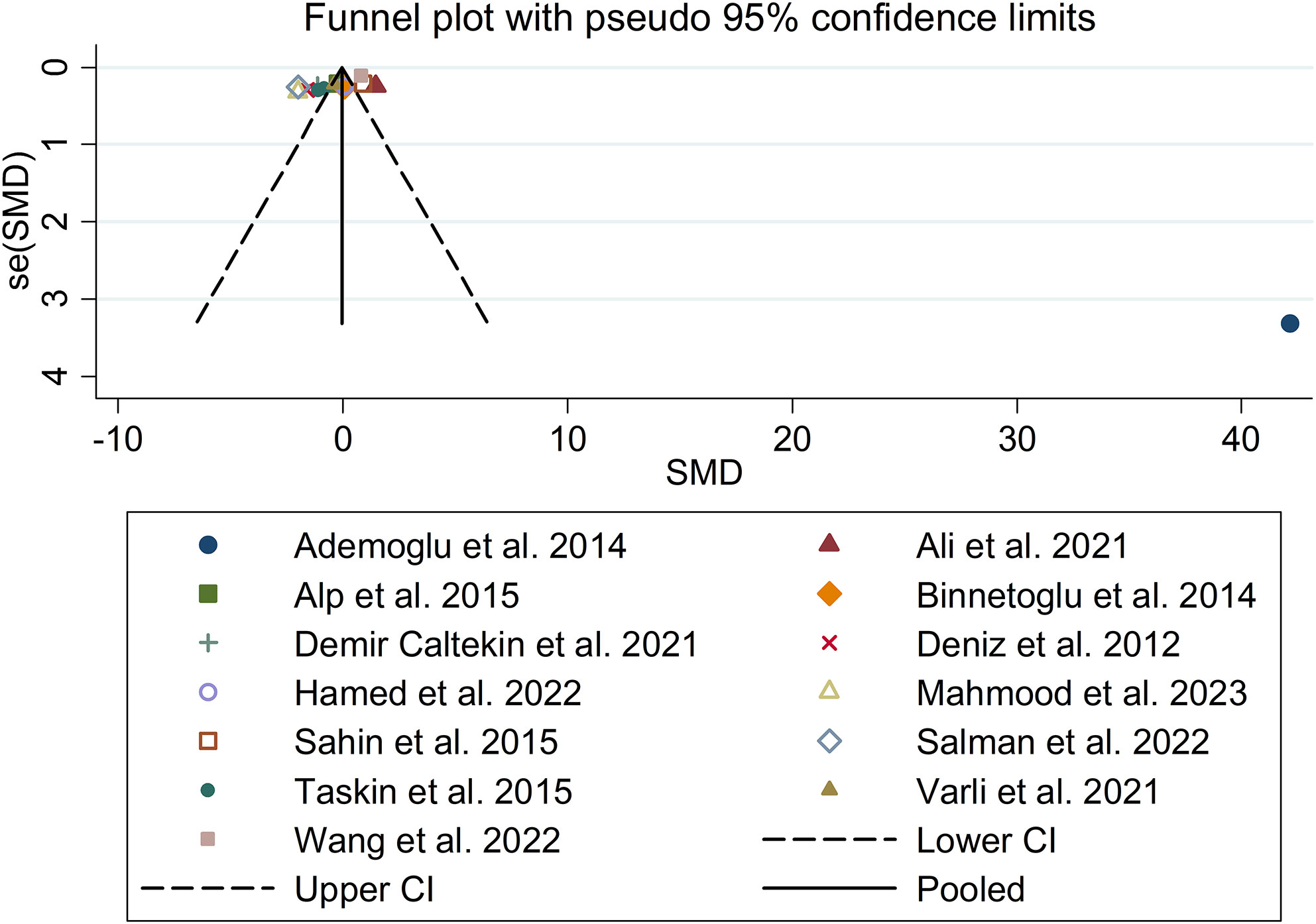
Figure 7 Funnel plot for comparison in blood nesfatin-1 level between patients with PCOS and controls. PCOS, polycystic ovary syndrome.
4 Discussion
Our literature search yielded a comprehensive selection of 13 studies involving a substantial cohort of approximately 1300 participants, which enabled us to obtain more precise and potentially more accurate estimates of standardized mean differences (SMD) compared to the individual primary studies. Additionally, this extensive pool of data provided us with the opportunity to explore the potential factors contributing to any observed heterogeneity among these studies. Our literature search yielded a comprehensive selection of 13 studies involving a substantial cohort of approximately 1300 participants, which enabled us to obtain more precise and potentially more accurate estimates of SMD compared to the individual primary studies. Additionally, this extensive pool of data provided us with the opportunity to explore the potential factors contributing to any observed heterogeneity among these studies (SMD = 0.03; 95%CI: -0.71, 0.77). Furthermore, subgroup analysis revealed no differences in nesfatin-1 levels between Caucasian and Asian populations suffering from PCOS (Caucasian: SMD = 0.30; 95%CI: -0.68, 1.28; Asian: SMD = -0.41; 95%CI: -2.03, 1.21). PCOS cases exhibit a variable phenotypic spectrum, and previous studies have suggested that nesfatin-1 has effects on obesity (29, 30). Therefore, nesfatin-1 levels in PCOS may vary depending on the presence or absence of obesity. However, our present study reported no significant difference in blood nesfatin-1 levels between PCOS and controls in both obese and non-obese populations. Salman et al. (27) reported a sensitivity 93.5%, specificity of 79% and accuracy of 86.2% for serum nesfatin -1 level as predictor of PCOS using receiver operating characteristic (ROC) curve analysis. More studies were essential to explore the change of blood nesfatin-1 levels in PCOS.
Nesfatin-1, a peptide derived from the precursor nucleobindin2 (NUCB2), plays a significant role in regulating feeding behavior and energy metabolism (31). The etiology of PCOS involves multiple aspects, including ovarian and adrenal hyperandrogenism, neuro-endocrine and hypothalamic-pituitary dysfunction, disorders of peripheral insulin resistance, and overweight or obesity (32, 33). Many studies have suggested that nesfatin-1 has a direct influence on obesity, including food intake, glucose metabolism, weight loss, and cardiac functions (22, 34). A study by Algual et al. reported lower serum nesfatin-1 levels in individuals with metabolic syndrome compared to the control group (35). However, other studies have shown that serum nesfatin-1 concentrations were significantly lower in obese subjects compared to non-obese subjects (36, 37). These inconsistent results may contribute to the lack of significant difference in blood nesfatin-1 levels between PCOS subjects and controls. It has been observed to have an anorexic effect by reducing meal frequency and increasing the time interval between meals (19). In a study involving PCOS model rats, it was found that nesfatin-1 serum levels decreased significantly compared to the normal control group (38). These results were consistent with the analysis of ovarian nesfatin-1 mRNA and protein levels using RT-PCR and western blot techniques (38). Additionally, the study revealed a positive correlation between nesfatin-1 and follicle-stimulating hormone (FSH), estradiol (E2), and progesterone (P) (38). This suggests that the decrease in nesfatin-1 levels in PCOS may disrupt follicular cell development through the inhibition of FSH in folliculogenesis (38). For PCOS patients, elevated serum nesfatin-1 concentrations were directly associated with serum levels of prolactin (26). This may be attributed to the co-localization of nesfatin-1 and prolactin-releasing peptide producing neurons in adrenal medullary A1 and A2 catecholamine cell groups, as well as the co-expression of nesfatin-1 and prolactin-releasing peptide (39, 40). However, it is worth noting that some studies have reported the opposite findings. A previous study demonstrated that intravenous injection of nesfatin-1 significantly decreased blood sugar in hyperglycemic db/db mice, indicating that nesfatin-1 has hypoglycemic effects by accelerating insulin secretion through increased calcium ion influx via L-type channels in mouse pancreas islet beta-cells (41, 42). Caltekin et al. also revealed lower nesfatin-1 levels in PCOS patients compared to healthy individuals, suggesting that PCOS shares similarities with diabetes and gestational diabetes mellitus (GDM) due to weight and insulin resistance (23). A separate Japanese study provided evidence supporting a relationship between nesfatin-1 and the insulin receptor (43). However, further research is necessary to elucidate the precise mechanisms underlying the association between nesfatin-1 and PCOS.
In the current meta-analysis, several limitations should be acknowledged. Firstly, the number of included studies was limited, comprising only 13 studies, and most of these studies had small sample sizes. Secondly, the study solely focused on articles published in the English language, potentially introducing bias. This exclusion of non-English literature may have restricted the generalizability of the findings. Thirdly, complete access to detailed data sets, including potential confounders such as BMI, fasting blood glucose, insulin levels, homeostasis model assessment-insulin resistance (HOMA-IR) index, luteinizing hormone (LH), follicle stimulating hormone (FSH), smoking, and physical activity, was not available. These confounders may have influenced the results.
From this meta-analysis, it is concluded that there is no significant relationship between blood nesfatin-1 levels and PCOS. However, the precise role of nesfatin-1 in the pathogenesis of PCOS remains poorly understood. Consequently, further examination of our findings necessitates additional prospective evidence-like clinical studies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
MW: Data curation, Formal Analysis, Investigation, Writing – original draft. JT: Formal Analysis, Investigation, Methodology, Project administration, Writing – original draft. QZ: Investigation, Methodology, Writing – review & editing. HT: Formal Analysis, Investigation, Methodology, Writing – review & editing. LT: Investigation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Natural Science Foundation of Jiangsu Province for Youth (No. BK20210140) and the Youth Science and Technology Project of Lianyungang Health Commission (No. QN202009).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1275753/full#supplementary-material
References
1. Gomez JMD, VanHise K, Stachenfeld N, Chan JL, Merz NB, Shufelt C. Subclinical cardiovascular disease and polycystic ovary syndrome. Fertil Steril (2022) 117(5):912–23. doi: 10.1016/j.fertnstert.2022.02.028
2. Wolf WM, Wattick RA, Kinkade ON, Olfert MD. Geographical prevalence of polycystic ovary syndrome as determined by region and race/ethnicity. Int J Environ Res Public Health (2018) 15(11):2589. doi: 10.3390/ijerph15112589
3. ESHRE TR, Group A-SPCW. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril (2004) 81(1):19–25. doi: 10.1016/j.fertnstert.2003.10.004
4. Witchel SF, Teede HJ, Peña AS. Curtailing PCOS. Pediatr Res (2020) 87(2):353–61. doi: 10.1038/s41390-019-0615-1
5. Dapas M, Dunaif A. Deconstructing a syndrome: genomic insights into PCOS causal mechanisms and classification. Endocr Rev (2022) 43(6):927–65. doi: 10.1210/endrev/bnac001
6. Kruszewska J, Laudy-Wiaderny H, Kunicki M. Review of novel potential insulin resistance biomarkers in PCOS patients-the debate is still open. Int J Environ Res Public Health (2022) 19(4):2099. doi: 10.3390/ijerph19042099
7. Szeliga A, Podfigurna A, Meczekalski B. Nesfatin-1 as a potential marker for functional hypothalamic amenorrhea. Gynecol Endocrinol (2022) 38(11):992–6. doi: 10.1080/09513590.2022.2126455
8. Farhangi MA, Dehghan P, Tajmiri S, Abbasi MM. The effects of Nigella sativa on thyroid function, serum Vascular Endothelial Growth Factor (VEGF) - 1, Nesfatin-1 and anthropometric features in patients with Hashimoto’s thyroiditis: a randomized controlled trial. BMC Complement Altern Med (2016) 16(1):471. doi: 10.1186/s12906-016-1432-2
9. Kadoglou NPE, Korakas E, Lampropoulos S, Maratou E, Kassimis G, Patsourakos N, et al. Plasma nesfatin-1 and DDP-4 levels in patients with coronary artery disease: Kozani study. Cardiovasc Diabetol (2021) 20(1):166. doi: 10.1186/s12933-021-01355-x
10. Altas M, Uca AU, Akdag T, Odabas FO, Tokgoz OS. Serum levels of irisin and nesfatin-1 in multiple sclerosis. Arq Neuropsiquiatr (2022) 80(2):161–7. doi: 10.1590/0004-282x-anp-2020-0520
11. Zhai T, Li SZ, Fan XT, Tian Z, Lu XQ, Dong J. Circulating nesfatin-1 levels and type 2 diabetes: A systematic review and meta-analysis. J Diabetes Res (2017) 2017:7687098. doi: 10.1155/2017/7687098
12. Luo JJ, Wen FJ, Qiu D, Wang SZ. Nesfatin-1 in lipid metabolism and lipid-related diseases. Clin Chim Acta (2021) 522:23–30. doi: 10.1016/j.cca.2021.08.005
13. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj (2021) 372:n71. doi: 10.1136/bmj.n71
14. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama (2000) 283(15):2008–12. doi: 10.1001/jama.283.15.2008
15. Rahman M, Berenson AB. Accuracy of current body mass index obesity classification for white, black, and Hispanic reproductive-age women. Obstet Gynecol (2010) 115(5):982–8. doi: 10.1097/AOG.0b013e3181da9423
16. Deniz R, Gurates B, Aydin S, Celik H, Sahin I, Baykus Y, et al. Nesfatin-1 and other hormone alterations in polycystic ovary syndrome. Endocrine (2012) 42(3):694–9. doi: 10.1007/s12020-012-9638-7
17. Ademoglu EN, Gorar S, Carlıoglu A, Yazıcı H, Dellal FD, Berberoglu Z, et al. Plasma nesfatin-1 levels are increased in patients with polycystic ovary syndrome. J Endocrinol Invest (2014) 37(8):715–9. doi: 10.1007/s40618-014-0089-2
18. Binnetoğlu E, Erbağ G, Gencer M, Turkön H, Aşik M, Güneş F, et al. Plasma levels of nesfatin-1 in patients with polycystic ovary syndrome. Acta Med Mediterranea (2014) 30(1):201–4.
19. Alp E, Görmüş U, Güdücü N, Bozkurt S. Nesfatin-1 levels and metabolic markers in polycystic ovary syndrome. Gynecol Endocrinol (2015) 31(7):543–7. doi: 10.3109/09513590.2015.1024219
20. Sahin FK, Sahin SB, Ural UM, Cure MC, Senturk S, Tekin YB, et al. Nesfatin-1 and Vitamin D levels may be associated with systolic and diastolic blood pressure values and hearth rate in polycystic ovary syndrome. Bosn J Basic Med Sci (2015) 15(3):57–63. doi: 10.17305/bjbms.2015.432
21. Taskin MI, Eser B, Adali E, Kara H, Cuce C, Hismiogulları AA. NUCB2 gene polymorphism and its relationship with nesfatin-1 levels in polycystic ovary syndrome. Gynecol Endocrinol (2016) 32(1):46–50. doi: 10.3109/09513590.2015.1081682
22. Ali EA, AL-Jedda WA J, AL-Khateeb SM, AL-Samarriae AY. The Association between Serum Nesfatin-1 level and BMI in Iraqi patients with Polycystic Ovary Syndrome (PCOS). Indian J Forensic Med Toxicol (2021) 15(2). doi: 10.37506/ijfmt.v15i2.14549
23. Demir Çaltekin M, Caniklioğlu A, Eris Yalçın S, Aydoğan Kırmızı D, Baser E, Yalvaç ES. DLK1 and Nesfatin-1 levels and the relationship with metabolic parameters in polycystic ovary syndrome: Prospective, controlled study. Turk J Obstet Gynecol (2021) 18(2):124–30. doi: 10.4274/tjod.galenos.2021.39024
24. Varlı B, Şükür YE, Özmen B, Ergüder B, Sönmezer M, Berker B, et al. Anorexigenic peptide (leptin, obestatin, nesfatin-1) levels and their impact on assisted reproductive technology treatment outcomes in patients with polycystic ovary syndrome. Clin Exp Reprod Med (2021) 48(4):368–73. doi: 10.5653/cerm.2021.04420
25. Wang Y, Ma X, Luo J, Wang X, Han L. Expression of serum PSA, nesfatin-1, and AMH in patients with polycystic ovary syndrome. Cell Mol Biol (Noisy-le-grand) (2022) 67(5):57–63. doi: 10.14715/cmb/2021.67.5.8
26. Hamed EA, Sayyed HG, Abbas AM, Gaber MMA, Aleem H. Nesfatin-1, dopamine, and NADPH levels in infertile women with polycystic ovary syndrome: is there a relationship between their levels and metabolic and hormonal variables. J Reprod Infertil (2022) 23(3):160–8. doi: 10.18502/jri.v23i3.10006
27. Salman SS, Athab SS. Serum nesfatin-1 level in polycystic ovarian syndrome and non polysystic ovarian syndrome women. Himalayan J Appl Med Sci Res (2022) 3(3). doi: 10.47310/Hjamsr.2022.v03i03.008
28. Mahmood NS, Al-Sammarai RRH, Al-Fanar RNJ. Evaluation of the role of nesfatin-1 and myonectin as A diagnostic marker for polycystic ovary syndrome and also for treatment response with metformin. Egyptian Acad J Biol Sci C Physiol Mol Biol (2023) 15(1):577–84. doi: 10.21608/eajbsc.2023.305089
29. Stengel A, Mori M, Taché Y. The role of nesfatin-1 in the regulation of food intake and body weight: recent developments and future endeavors. Obes Rev (2013) 14(11):859–70. doi: 10.1111/obr.12063
30. Schalla MA, Unniappan S, Lambrecht NWG, Mori M, Taché Y, Stengel A. NUCB2/nesfatin-1 - Inhibitory effects on food intake, body weight and metabolism. Peptides (2020) 128:170308. doi: 10.1016/j.peptides.2020.170308
31. Bani Mohammad M, Majdi Seghinsara A. Polycystic ovary syndrome (PCOS), diagnostic criteria, and AMH. Asian Pac J Cancer Prev (2017) 18(1):17–21. doi: 10.22034/APJCP.2017.18.1.17
32. Pirotta S, Joham A, Grieger JA, Tay CT, Bahri-Khomami M, Lujan M, et al. Obesity and the risk of infertility, gestational diabetes, and type 2 diabetes in polycystic ovary syndrome. Semin Reprod Med (2020) 38(6):342–51. doi: 10.1055/s-0041-1726866
33. Glueck CJ, Goldenberg N. Characteristics of obesity in polycystic ovary syndrome: Etiology, treatment, and genetics. Metabolism (2019) 92:108–20. doi: 10.1016/j.metabol.2018.11.002
34. Tekin T, Cicek B, Konyaligil N. Regulatory peptide nesfatin-1 and its relationship with metabolic syndrome. Eurasian J Med (2019) 51(3):280–4. doi: 10.5152/eurasianjmed.2019.18420
35. Algul S, Ozkan Y, Ozcelik O. Serum nesfatin-1 levels in patients with different glucose tolerance levels. Physiol Res (2016) 65(6):979–85. doi: 10.33549/physiolres.933186
36. Başar O, Akbal E, Köklü S, Koçak E, Tuna Y, Ekiz F, et al. A novel appetite peptide, nesfatin-1 in patients with non-alcoholic fatty liver disease. Scand J Clin Lab Invest (2012) 72(6):479–83. doi: 10.3109/00365513.2012.699097
37. Mirakhor Samani S, Ghasemi H, Rezaei Bookani K, Shokouhi B. Serum nesfatin-1 level in healthy subjects with weight-related abnormalities and newly diagnosed patients with type 2 diabetes mellitus; A case-control study. Acta Endocrinol (Buchar) (2019) 5(1):69–73. doi: 10.4183/aeb.2019.69
38. Xu Y, Zhang H, Li Q, Lao K, Wang Y. The role of nesfatin-1 expression in letrozole-induced polycystic ovaries in the rat. Gynecol Endocrinol (2017) 33(6):438–41. doi: 10.1080/09513590.2017.1290068
39. Könczöl K, Bodnár I, Zelena D, Pintér O, Papp RS, Palkovits M, et al. Nesfatin-1/NUCB2 may participate in the activation of the hypothalamic-pituitary-adrenal axis in rats. Neurochem Int (2010) 57(3):189–97. doi: 10.1016/j.neuint.2010.04.012
40. Könczöl K, Pintér O, Ferenczi S, Varga J, Kovács K, Palkovits M, et al. Nesfatin-1 exerts long-term effect on food intake and body temperature. Int J Obes (Lond) (2012) 36(12):1514–21. doi: 10.1038/ijo.2012.2
41. Su Y, Zhang J, Tang Y, Bi F, Liu JN. The novel function of nesfatin-1: anti-hyperglycemia. Biochem Biophys Res Commun (2010) 391(1):1039–42. doi: 10.1016/j.bbrc.2009.12.014
42. Ayada C, Toru Ü, Korkut Y. Nesfatin-1 and its effects on different systems. Hippokratia (2015) 19(1):4–10.
Keywords: meta-analysis, nesfatin-1, polycystic ovary syndrome, serum, systematic review
Citation: Wang M, Tong J, Zhu Q, Tang H and Tang L (2024) Blood nesfatin-1 levels in patients with polycystic ovary syndrome: a systematic review and meta-analysis. Front. Endocrinol. 14:1275753. doi: 10.3389/fendo.2023.1275753
Received: 10 August 2023; Accepted: 28 December 2023;
Published: 24 January 2024.
Edited by:
Thozhukat Sathyapalan, Hull York Medical School, United KingdomReviewed by:
Esra Hatipoglu, University of Health Sciences, TürkiyeMichał Kunicki, Medical University of Warsaw, Poland
Manvendra Pratap Singh, HYDERABAD, India
Copyright © 2024 Wang, Tong, Zhu, Tang and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lisha Tang, SDg4MzkyXzIzakBvdXRsb29rLmNvbQ==; Huaiyun Tang, c3RldWlpZTIxQDIxY24uY29t
†These authors have contributed equally to this work
 Mei Wang
Mei Wang Lisha Tang
Lisha Tang