- 1Country Renal Research Institution of Beijing University of Chinese Medicine, Key Laboratory of Chinese Internal Medicine of Ministry of Education and Beijing, Dongzhimen Hospital Affiliated to Beijing University of Chinese Medicine, Beijing, China
- 2Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 3Institute of Basic Research in Clinical Medicine, China Academy of Chinese Medical Sciences, Beijing, China
Diabetic nephropathy (DN) is a complication of diabetes mellitus (DM) and the main cause of excess mortality in patients with type 2 DM. The pathogenesis and progression of DN are closely associated with disorders of glucose and lipid metabolism. As a member of the sirtuin family, SIRT6 has deacetylation, defatty-acylation, and adenosine diphosphate-ribosylation enzyme activities as well as anti-aging and anticancer activities. SIRT6 plays an important role in glucose and lipid metabolism and signaling, especially in DN. SIRT6 improves glucose and lipid metabolism by controlling glycolysis and gluconeogenesis, affecting insulin secretion and transmission and regulating lipid decomposition, transport, and synthesis. Targeting SIRT6 may provide a new therapeutic strategy for DN by improving glucose and lipid metabolism. This review elaborates on the important role of SIRT6 in glucose and lipid metabolism, discusses the potential of SIRT6 as a therapeutic target to improve glucose and lipid metabolism and alleviate DN occurrence and progression of DN, and describes the prospects for future research.
1 Introduction
Diabetic nephropathy (DN) is the main microvascular complication of diabetes mellitus (DM) (1). Approximately 30–40% of patients with DM will develop DN, the main cause of end-stage renal disease (2). DN is the main cause of mortality in patients with type 2 DM (T2DM) (3). The all-cause mortality of patients with DM and DN is approximately 30 times that of those without DN, and the vast majority of patients with DN die of cardiovascular disease before end-stage renal disease (4). Multiple risk factors accelerate DN progression, including hypertension, hyperglycemia, obesity, insulin resistance, atherosclerotic dyslipidemia, and familial aggregation (5–9). DN pathogenesis is complex and includes glucose metabolism disorders, changes in fatty acid metabolism, oxidative stress, changes in energy utilization, and mitochondrial dysfunction, which can lead to endothelial dysfunction, glomerular sclerosis, inflammatory cell recruitment, renal tubular fibrosis, and other pathological changes (10, 11). Dyslipidemia and renal ectopic lipid accumulation are associated with kidney disease (especially DN) (12). Almost all renal cell types, from mesangial cells (MCs) to podocytes and proximal tubular epithelial cells (PTECs), can deposit lipids (13). Therefore, glucose and lipid metabolism disorders are important causes of DN onset and progression.
High blood glucose levels and excessive carbohydrate intake can produce toxic effects on cells and tissues through hyperglycemia and carbon stress (14). Hyperglycemia stress including reduction of stress, polyol pathway (15–19), hexosamine pathway (20, 21), protein kinase C (PKC) activation pathway (22, 23), advanced glycation end-product pathway (24–26) and oxidative stress (27–29). Excessive uptake of nutrients (including glucose and lipids) causes carbon overload in cells, resulting in accumulation of a large number of reactive acyl metabolites (including malonyl-coa, succinyl-coa, and acetyl-coa) and ultimately leading to protein modification and dysfunction (30, 31), including through protein acetylation (30, 32) and succinylation (30, 33). Long-term exposure to high concentrations of lipids and lipid derivatives can produce lipotoxicity to cells (34). Long-term elevation of free fatty acid (FFA) levels destroys glucose homeostasis, and exposure to high glucose (HG) causes synergistic glucolipotoxicity (35). Lipotoxicity in DM can aggravate glucotoxicity-induced mitochondrial damage (36). Enhanced fatty acid synthesis and inhibition of fatty acid oxidation are the main causes of renal lipid accumulation (37). Renal lipid deposition induces cell damage by activating oxidative stress, inflammation, fibrosis, and apoptosis pathways (38). Aging is not only a risk factor for the occurrence and development of kidney disease (39), but also leads to adipose tissue dysfunction (40) and decreased glucose tolerance (41), which lead to glucose and lipid metabolism disorders. Therefore, changes in carbohydrate and lipid metabolism as well as kidney aging are associated with the development of chronic kidney disease (42, 43).
Sirtuins, as a diverse group of histone deacetylases, that are core participants in anti-aging effects and metabolism (44) and can play an anti-aging role in DN (45, 46). SIRT6 is an important regulator of glucose and lipid metabolism (Figure 1) (47–49). It is also involved in anti-aging (45), NAD+ metabolism (50), inflammation (51, 52), autophagy (53, 54) and oxidative stress (55, 56). SIRT6 deacetylase activity prevents the transcription of genes involved in renal fibrosis (57). SIRT6 is a key regulator of DN progression. SIRT6 expression is downregulated in DN kidney tissues (58), and podocyte-specific SIRT6 deletion aggravates podocyte injury and proteinuria in mice with DN (58). In addition, SIRT6 deficiency is associated with mitochondrial and podocyte apoptosis (59, 60).
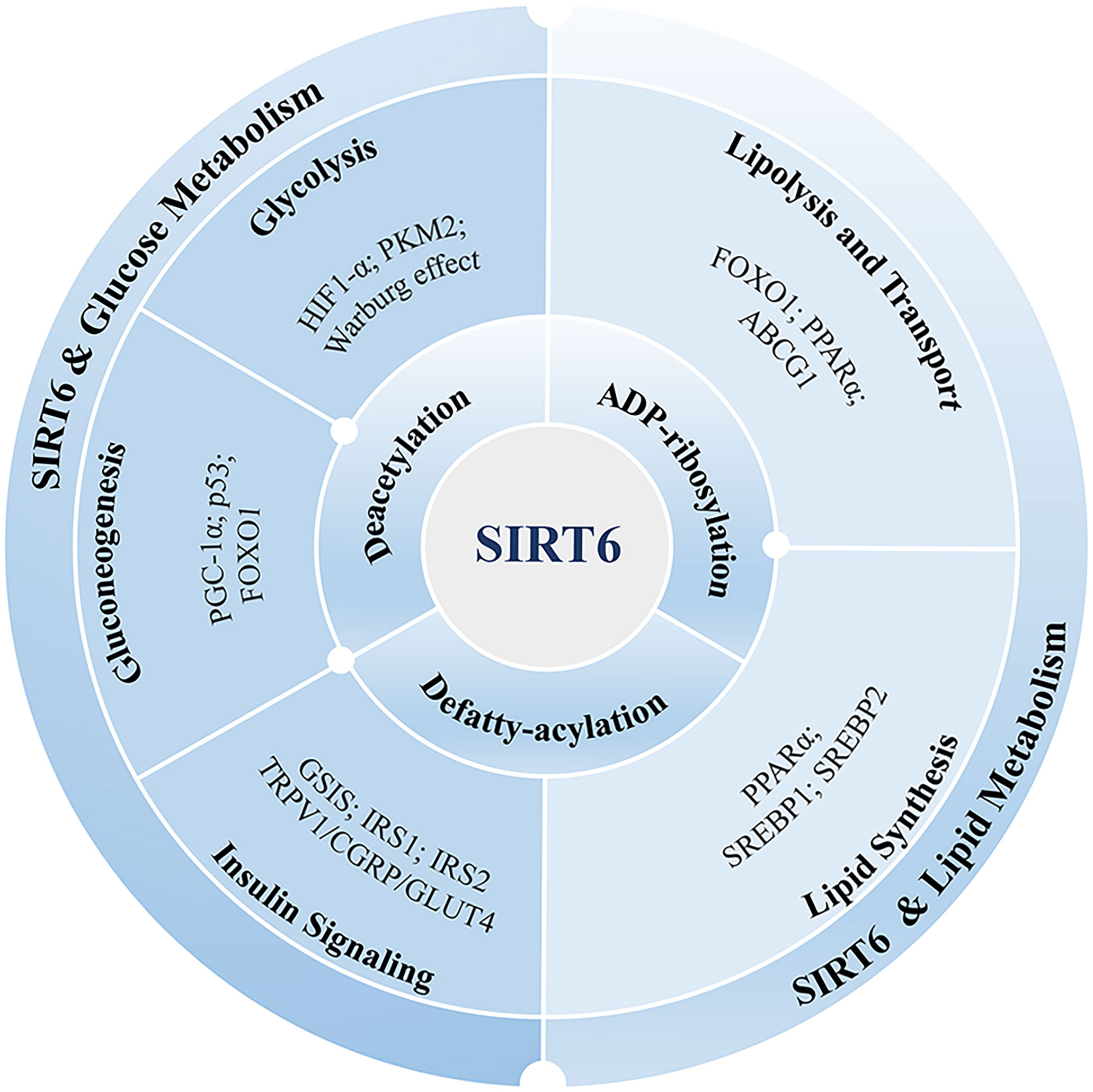
Figure 1 SIRT6’s function in the metabolism of lipids and glucose. SIRT6 exerts its influence on lipid and glucose metabolism through various enzymatic activities, including deacetylation, defatty-acylation, and ADP-ribosylation. These activities enable SIRT6 to modulate metabolic pathways in multiple ways. Glucose metabolism encompasses important processes such as glycolysis, gluconeogenesis, and insulin signaling. SIRT6 is implicated in these processes, and its involvement is associated with several proteins, including HIF-1α, PKM2, PGC-1α, FOXO1, p53, and GLUT4. These proteins play a role in mediating the effects of SIRT6 on glucose metabolism. Lipid metabolism involves the lipolysis, transport, and synthesis of lipids. SIRT6 is involved in regulating lipid metabolism through interactions with various proteins, including FOXO1, PPARα, ABCG1, and SREBP. These proteins collectively contribute to the control of lipid metabolism. By understanding the impact of SIRT6 on these metabolic pathways and its interactions with specific proteins, we can gain valuable insights into its potential as a therapeutic target for managing metabolic disorders.
In this review, we systematically elaborate on the targeting of SIRT6 to regulate glucose and lipid metabolism to delay DN progression and on the feasibility of utilizing SIRT6 in DN treatment.
2 Localization, structure, and enzymatic activity of SIRT6
2.1 Localization and structure
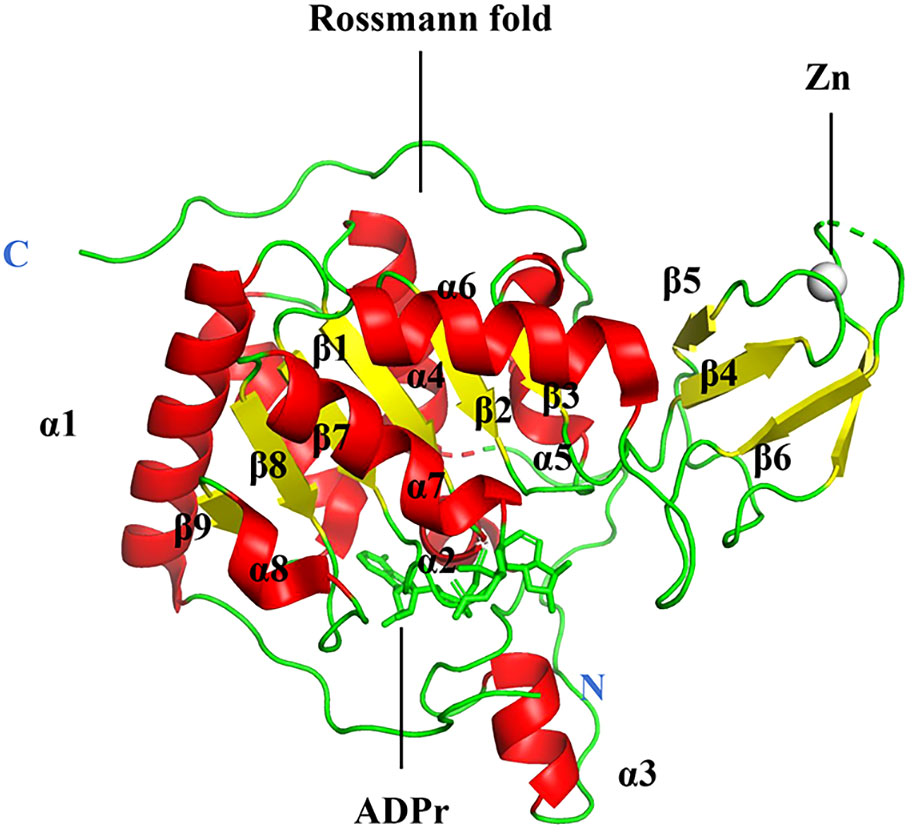
The sirtuin family includes seven proteins (SIRT1–SIRT7), of which SIRT6 is a member of class IV (61). SIRT6 is primarily localized in the nucleus (62). The human SIRT6 gene contains eight exons, with exon 4 being the shortest at 60 bases and exon 8 the longest at 838 bases (63). The gene is located on chromosome 19p13.3. A protein of 355 amino acids with a projected molecular weight of 39.1 kDa and an isoelectric point of 9.12 is encoded by the human SIRT6 mRNA (63). Most tissues produce SIRT6, and research has shown that its gene is mostly expressed in the embryonic heart, kidney, and brain (64). Eight α-sheets and nine β-strands make up the two globular domains found in SIRT6: a large Rossmann fold for NAD+ binding (residues 25–128 and 191–266) and a smaller zinc-binding domain (residues 129–190). The parallel β-sheets of six strands (β1, β2, β3, β7, β8, and β9) that make up the large Rossmann fold domain are surrounded by two helices (α6 and α7) on one side and four on the other (α1, α4, α5, and α8). The smaller domain, which consists of three antiparallel β-sheets (sheets β4, β5, and β6), is created by two extension loops of the large domain (linking loops β3 and α6) (Figure 2) (65). Despite lacking acetylated substrates, SIRT6 has a structurally strong single helix that allows it to bind NAD (65). Deacetylation, defatty-acylation, and ADP-ribosylation are three unique enzymatic activities that SIRT6 has shown (66).
2.2 Deacetylation
NAD+-dependent histone deacetylases are the most distinctive features of SIRT6. There are multiple SIRT6-targeted deacetylation sites on histone H3, including at H3K9, H3K18, and H3K27 (67). Histone H3 lysine 9 (H3K9Ac) is the first specific deacetylation substrate that regulates telomere chromatin (68). Lysine 56 in the histone H3 globular nucleus (H3K56Ac) is the second substrate (69) and is involved in DNA repair (70). SIRT6 promotes H3K18 deacetylation in paracentric heterochromatin (71). Further research has shown that the role of SIRT6 as a protein deacetylase extends beyond the scope of histones. C-terminal-binding protein interacting protein (CtIP) was the first discovered non-histone substrate and promotes DNA end resection and homologous recombination (72) to maintain genomic stability. When SIRT6 is activated by ribosomes or fatty acids, its deacetylation activity is significantly enhanced (73, 74).
2.3 Defatty-acylation
Fatty acylation of lysine is a novel mechanism that regulates protein secretion (75). Palmitoylation affects cellular protein dynamics and differential regulation (76). Myristoylation affects plasma targeting, subcellular tracking, and protein localization (77). Enzymatic and structural studies have shown that SIRT6 preferentially hydrolyzes long-chain fatty acyl groups (myristoyl and palmitoyl) (73, 78). SIRT6 knockdown increases lysine fatty acylation of the RAS-related protein R-Ras2 (79). SIRT6 regulates the lipid acylation level of K19 and K20 and affects the secretion of tumor necrosis factor α (TNFα) (78). In addition, SIRT6 can remove the fatty acylation of H3K9, H3K18, and H3K27 in fatty-acylated nucleosomes; however, the physiological function of this reaction requires further study (67).
2.4 ADP-ribosylation
ADP-ribosylation is a post-translational modification (80) involved in glucose and lipid metabolism (81), DNA repair (82) and cell proliferation (83). SIRT6 is an ADP-ribosyltransferase (62). SIRT6 mono-ADP-ribosylation of KDM2A can locally increase H3K36me2 at DNA damage sites, thereby inhibiting transcription and promoting repair (84). In response to oxidative stress, SIRT6 ribosylates K521 and activates poly (ADP-ribose) polymerase 1 to promote double-strand break repair (85). SIRT6 inhibits long interspersed element 1 retrotransposons by ribosylating KRAB domain-associated protein 1 (86).
2.5 Regulation of SIRT6 enzyme activity
Deficiency of SIRT6 SUMOylation specifically reduces H3K56 deacetylation (87). Compared to patients without DM, SIRT6 DNA methylation levels in patients with DM are lower and are negatively correlated with blood glucose levels, suggesting that epigenetic mechanisms regulate SIRT6 expression (88). Oxidative stress inhibited SIRT6 expression in a mouse model of DM embryopathy (89). SIRT6 expression was inhibited by 2,3-dimethoxy-1,4-naphthoquinone (89) in vitro. p53 directly activates SIRT6 expression (90, 91). Under normal growth conditions, p53 positively regulates SIRT6 protein levels; however, under nutrient-limited conditions, p53 has no relationship with SIRT6 stability (92). Ubiquitination is a common post-translational modification that regulates target protein stability (93). Ubiquitin-specific peptidase 10 (USP10) inhibits SIRT6 ubiquitination and degradation, reducing liver fat deposition, insulin resistance, and inflammation (94). The ubiquitin ligase CHIP (carboxyl terminus of HSP70-interacting protein) ubiquitinates SIRT6 at K170 (95).
3 SIRT6 regulation in glycolipid metabolism
3.1 SIRT6 and glucose metabolism
3.1.1 SIRT6 and blood glucose
Cys144 of SIRT6 is a functional redox-sensitive site that regulates glucose metabolism in monocytes (96), including inhibition of glucose transporters and glycolytic enzyme expression (97, 98). SIRT6 inhibitors increase expression of glucose transporters and glycolytic enzymes, reducing blood glucose levels (99). Sirt6-deficient mice exhibit lethal hypoglycemia in early life (100). SIRT6 deficiency did not affect intestinal glucose absorption or renal glucose secretion in mice (98). The kidney regulates glucose homeostasis through gluconeogenesis, glucose uptake from circulation, and glucose reabsorption from glomerular filtrate (101). In DM, the kidneys increase blood glucose by increasing glucose reabsorption in the prourine and upregulating gluconeogenesis in the proximal tubules (PTs) (102). The glucose transporter (GLUT) and sodium-glucose co-transporter (SGLT) are both expressed in renal tissues (103). SIRT6 deletion enhances the membrane association between GLUT1 and GLUT4, thereby enhancing glucose uptake (104). In cell-specific SIRT6 KO mice, SIRT6-mediated forkhead box protein O1 (FOXO1) deacetylation leads to nuclear export and restoration of pancreatic duodenal homeobox 1 (Pdx1) expression. It may also promote glucose-stimulated insulin secretion (GSIS) and upregulate GLUT2 expression (105).
3.1.2 SIRT6 and glycolysis
Glycolysis, a key energy production process in almost all mammalian cells, converts glucose into pyruvate. Under aerobic conditions, it enter the mitochondria (106). When cells are deprived of nutrients or under hypoxia, they undergo anaerobic respiration and convert pyruvate to lactate (107–109). Hyperglycemic toxicity can be reduced by increasing glycolysis. The elevation of enzymes involved in the metabolism of free glucose and its metabolites in glomerular cells is related to the maintenance of renal function in T2DM (110). Anaerobic glycolysis and glucose fermentation into lactate are the main metabolic pathways in podocytes. Under physiological conditions, podocytes do not rely on mitochondrial energy sources, but metabolize glucose to lactate to meet energy demands, similar to the Warburg effect (111). In DN, regulating glucose metabolism, reducing the levels of glucotoxic products, and improving mitochondrial function can protect the kidneys (112). After 8 days of hyperglycemia intervention in renal tubular cells, the downregulation of respiratory parameters persisted and glycolysis increased to compensate (113).
Hypoxia-inducible factor-1α (HIF-1α) regulates glycolytic gene expression. HIF-1 activates glycolytic genes such as pyruvate dehydrogenase kinase (PDK), which is key to hypoxic metabolism adaptations by increasing the conversion rate of glucose to pyruvate and lactate (114). SIRT6 negatively regulates HIF-1α to regulate glycolysis. The two SIRT6 Cys residues Cys18 and HIF-1α (Cys800) form a reversible disulfide bond, thereby inhibiting the transcriptional activity of HIF-1α (115). In a cross-sectional study of patients with T2DM (313 cases), patients with pre-DM (102 cases), and healthy volunteers (100 cases), SIRT6 was elevated in patients with different severities of DM and microalbuminuria with increased TNFα, HIF1-α, and urinary protein biomarkers (116). Thus, HIF1-α is a target for SIRT6 intervention in glycolysis. In SIRT6-deficient cells, HIF-1α protein synthesis and stability are increased, leading to the overexpression of HIF-1α target genes involved in glycolysis, such as those coding for lactate dehydrogenase, triose phosphate isomerase, aldolase, and the rate-limiting glycolytic enzyme phosphofructokinase (98). Under normal nutritional conditions, SIRT6 acts as a histone deacetylase to inhibit the expression of glycolytic genes and maintain an appropriate flux of glucose into the tricarboxylic acid cycle (98). Under nutritional stress, SIRT6 inactivation can activate HIF-1α and recruit p300. Acetylation of H3K9 at the promoter increases the expression of a variety of metabolic genes, resulting in increased glycolysis and decreased mitochondrial respiration (98). In mice specifically overexpressing pyruvate kinase M2 (PKM2) in podocytes, PKM2 protects mitochondrial function in all glomerular cells by activating and inducing the HIF-1α/VEGF pathway, resisting hyperglycemic toxicity, and slowing down DN progression (117). PKM2 activation protects podocytes from glucose-induced injury by increasing glucose metabolic flux, inhibiting the production of toxic glucose metabolites, and inducing mitochondrial biogenesis to restore mitochondrial function (118). SIRT6 deacetylates PKM2, leading to its nuclear export. Therefore, the interaction of SIRT6 with PKM2 and HIF-1α can be further investigated to provide strategies for the treatment of altered podocyte metabolism in DN.
3.1.3 SIRT6 and gluconeogenesis
Gluconeogenesis is an important metabolic process that provides energy to the body, particularly during fasting and physical activities. Systemic SIRT6 overexpression improves the utilization of two major gluconeogenic precursors (glycerol and lactate), blocking age-dependent deterioration of euglycemia and gluconeogenic capacity, indicating that organs other than the liver are critical for SIRT6-mediated gluconeogenesis activation (50). PTs are the second most important gluconeogenic tissue after the liver (119). In DM, both the liver and kidneys increase gluconeogenesis; however, the relative increase in glucose production in the kidneys is much stronger than that in the liver (102). The most important renal gluconeoprecursors are lactate, glutamine, and glycerol (101).
During gluconeogenesis, SIRT6 is regulated by the peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α), FOXO1, and other targets. PGC-1α is a key mediator of gluconeogenic gene transcription, and this function depends on its acetylation status (120, 121). Metabolomics suggests that the characteristics of mitochondrial dysfunction in DN are related to decreased expression of the PGC-1α gene, which is evidence of the global impairment of mitochondrial biogenesis (122, 123). SIRT6 binds the histone acetyltransferase general control of nucleotide synthesis 5 (GCN5) at K549 to deacetylate it, changing protein phosphorylation to activate GCN5. This in turn suppresses hepatic gluconeogenesis by increasing PGC-1α acetylation (120). p53 downregulates the rate-limiting enzymes of gluconeogenesis (phosphoenolpyruvate carboxykinase 1 and glucose-6-phosphatase) and activates SIRT6 expression. SIRT6 deacetylates FOXO1 and exports it to the cytoplasm to regulate gluconeogenesis (90). SIRT6 also regulates FOXO1 nuclear translocation, affecting renal glucose reabsorption and gluconeogenesis in type 1 DM (124).
3.1.4 SIRT6 and insulin signaling
Insulin is the only hormone in the body that lowers blood glucose levels, and it is secreted by pancreatic β-cells. The kidney plays a major role in insulin degradation, removing 6–8 U of insulin daily via two major pathways (125). The GSIS of pancreatic β-cell SIRT6-knockout mice decreased by approximately 50%, suggesting that SIRT6 activation may improve insulin secretion in DM (126). SIRT6 deficiency also leads to abnormal upregulation of thioredoxin-interacting protein in islet β-cells, thereby inhibiting insulin secretion (127). SIRT6 overexpression can reduce palmitate (PA)-induced lipotoxicity, improve pancreatic β-cell viability, and increase GSIS (128). SIRT6 also regulates GSIS via mitochondrial glucose oxidation, plasma membrane depolarization, and calcium dynamics (126). Furthermore, SIRT6 inhibits multiple upstream molecules, such as insulin receptor, insulin receptor substrate 1, and insulin receptor substrate 2. Additionally, SIRT6 negatively regulates AKT phosphorylation (104).
Insulin resistance (IR) is a factor that promotes DN progression (129). SIRT6 overexpression activates transient receptor potential vallinoid 1 (TRPV1)/calcitonin gene-related peptide (CGRP) signaling and regulates GLUT expression at the protein and mRNA levels, which are involved in the TRPV1-CGRP-GLUT4 signaling axis, thereby increasing glucose intake and reducing IR in mice fed high-fat diets (HFDs) and 3T3-L1 adipocytes (130). Therefore, SIRT6 not only affects insulin secretion and sensitivity, but also serves as a potential target for the treatment of IR.
3.2 SIRT6 and lipid metabolism
3.2.1 SIRT6 and adipocytes
Adipose tissue is mainly composed of adipocytes, interstitial fibroblasts, and progenitor cells, which form energy storage organelles in the form of triglycerides packaged into lipid droplets (LDs). Adipose tissue also plays an important role in regulating systemic metabolic homeostasis (131–134). Depending on adipocyte type, fat can be classified as white, brown, or beige. White adipocytes have unilocular LDs mainly responsible for energy storage (131). Brown fat cells are rich in mitochondria that consume energy to produce heat (135). Mice with SIRT6-deficient adipose tissue have shown elevated blood glucose levels and severe IR (136). Obesity, hyperglycemia, and other factors can reduce SIRT6 expression. SIRT6 expression was observed to have decreased in the adipose tissue of db/db mice in a model of T2DM (120). SIRT6 expression in the abdominal adipose tissue of patients with obesity and pre-DM is lower than that in healthy patients, while nuclear transcription factor-κB (NF-κB), peroxisome proliferator-activated receptor γ (PPARγ), and sterol regulatory element-binding protein 1 (SREBP-1) expression levels increase, suggesting their involvement in the inflammatory pathway (137). SIRT6 expression in subcutaneous adipose tissue increases significantly after weight loss (138). Low temperature can induce SIRT6 to interact with the PGC-1α promoter and promote phospho-activating transcription factor 2 (p-ATF2) binding, thereby activating thermogenic genes and promoting fat thermogenesis (136). SIRT6 also inhibits preadipocyte differentiation by activating the adenosine monophosphate-activated protein kinase-α (AMPKα) pathway (139). Adipose tissue can also function as a secretory organ for leptin and adiponectin (140, 141). SIRT6 deficiency impairs leptin-induced signal transduction (142). Increased adiponectin can reduce proteinuria, glomerular hypertrophy, and inflammatory responses in the renal tissue (143).
3.2.2 SIRT6, lipolysis, and transport
SIRT6 overexpression significantly reduces blood triglycerides in mice (144). FOXO1 is involved in lipid metabolism, promotes lipolysis, and inhibits adipocyte differentiation. Acetylation and deacetylation are the most important regulatory mechanisms affecting FOXO1 expression and activity (145). SIRT6 is a FOXO1 deacetylase that drives lipid catabolism, and its activity is enhanced by the loss of mTOR complex 2 (mTORC2) (146). mTORC2 promotes glucose uptake and adipogenesis in adipocytes, and counteracts the inflammatory response of macrophages (147). It also regulates lipid metabolism in brown adipocytes via the SIRT6-FOXO1 pathway (146). However, the lack of SIRT6 can increase FOXO1 acetylation, promote FOXO1 nuclear export, and reduce the positive regulation of adipose triglyceride lipase, a key enzyme in fat mobilization (148). PPARα, one of the PPAR isoforms, is a key transcription factor involved in hepatic oxidation. PPARα activates PDK4 to inhibit the oxidation of pyruvate produced by glycolysis and increase the production of lactate and alanine, thereby indirectly promoting lipid oxidation in the liver (149). SIRT6 can bind PPARα and its response elements in the promoter region to activate gene transcription and promote lipid β-oxidation (150). Lipoproteins include phospholipids, free cholesterol, and apolipoproteins (151). Disorders of cholesterol metabolism are also associated with lipotoxicity and lipid accumulation in DM (152). SIRT6 affects cholesterol efflux in podocytes by regulating the expression of ATP-binding cassette transporter G1 (ABCG1) expression. SIRT6 deficiency exacerbates Ang II-induced cholesterol accumulation and podocyte injury SIRT6 is a potential target for renin-angiotensin system-related podocyte injury (153).
3.2.3 SIRT6 and lipid synthesis
SREBP is a lipogenic transcription factor regulated by cholesterol, insulin, and glucose. PPARα can inhibit the SREBP-mediated synthesis of cholesterol and triglycerides (154, 155). SREBP1 regulates adipogenesis by activating the genes involved in fatty acid and triglyceride biosynthesis, whereas SREBP2 activates the genes involved in cholesterol synthesis (156). SREBP1 overexpression in the kidneys induces glomerulosclerosis (157). SIRT6 can bind to the promoter regions of SREBP1c and SREBP2 and repress transcription by deacetylating histone H3K56 in the promoter. FOXO3 recruits SIRT6 to the SREBP-2 gene promoter, and SIRT6 deacetylates H3K9AC and H3K56AC to reduce low-density lipoprotein (LDL) cholesterol (158). SIRT6 also inhibits SREBP1c by increasing the adenosine monophosphate (AMP)/ATP ratio and stimulating AMPK phosphorylation (159). miRNAs are key regulators of lipid synthesis, fatty acid oxidation, and lipoprotein formation and secretion (160). However, miR33a and miR33b from the SREBP2 and SREBP1 introns can inhibit SIRT6 expression (159, 161). SIRT6 inhibits lipid deposition by activating the AMPKα pathway (139). Ectopic lipid deposition (ELD) is associated with DN progression (12). SIRT6 improves lipid accumulation via FOXO1 and PPARγ (162). Therefore, SIRT6 can affect the lipogenic transcription factors SREBP1 and SREBP2 through a variety of mechanisms. Further studies are needed to determine whether SIRT6 alleviates renal ELD.
4 Effect of glucose and lipid metabolism on DN
Disorders of glucose and lipid metabolism are closely related to the occurrence and progression of DN (Figure 3). Glucotoxicity and lipotoxicity can affect a variety of intrinsic renal cells in DN, causing structural and functional changes in the glomeruli and tubules.
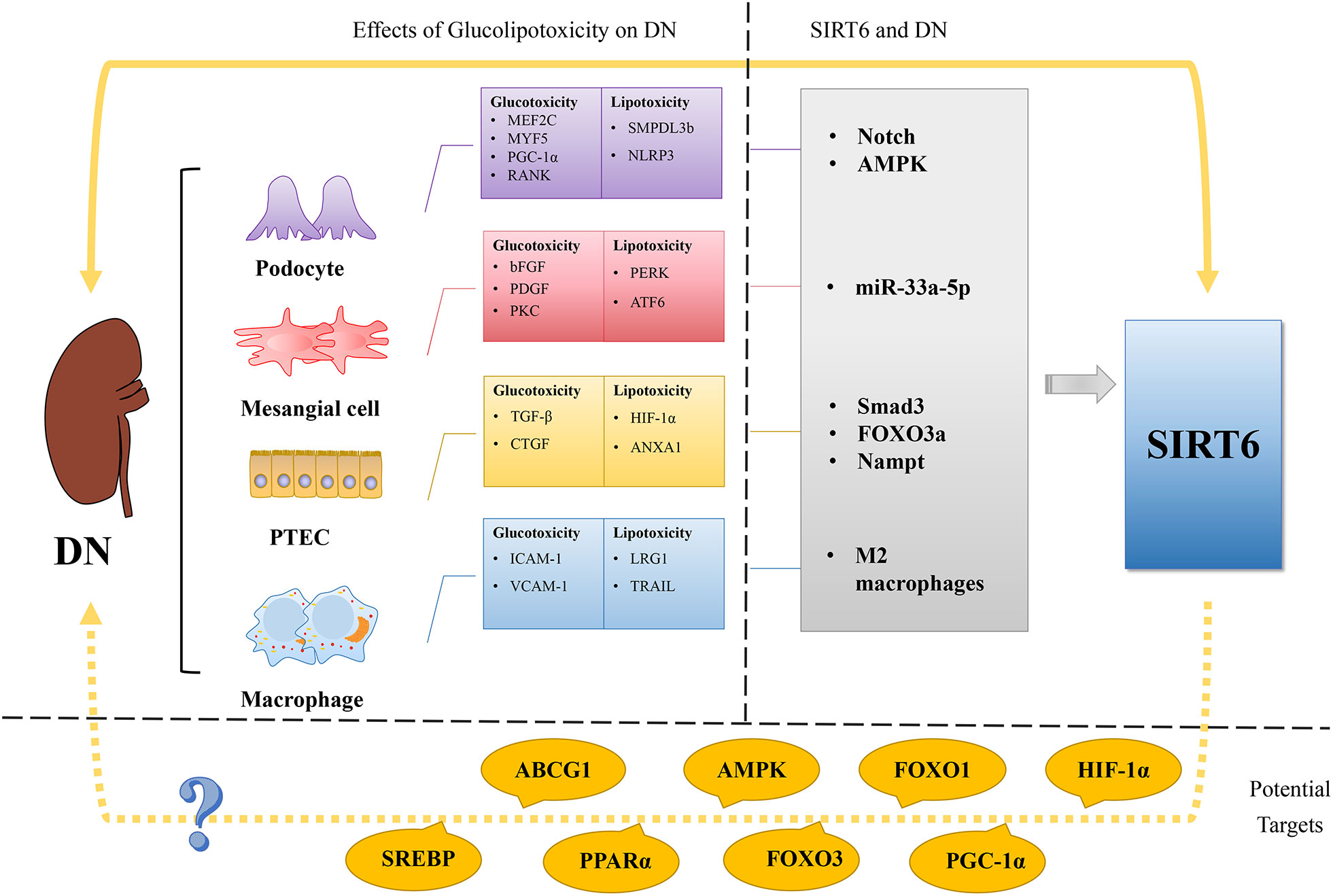
Figure 3 Regulation of glucose and lipid metabolism in DN and SIRT6’s possible role as a treatment target for DN. The imbalance of lipid and glucose metabolism is a crucial etiological component in the development of DN, a microvascular complication of DM. In podocyte glucotoxicity, MEF2C, MYF5, PGC-1α, and RANK are involved, whereas in podocyte lipotoxicity, SMPDL3b and NLRP3 are. The glucotoxicity of mesangial cells is mediated by bFGF, PDGF, and PKC, whereas the lipotoxicity of mesangial cells is mediated by PERK and ATF6. TGF-β and CTGF support glucotoxicity in PTCs, whereas HIF-1α and ANXA1 support lipotoxicity. In contrast to lipotoxicity, which is mediated by LRG1 and TRAIL, macrophage glucotoxicity is mediated by ICAM-1 and VCAM-1. Recent studies show that SIRT6 plays a role in the activation of the Notch pathway, AMPK, miR-33a-5p, Smad3, FOXO3a, Nampt, and M2 macrophages in DN. Potential targets for treating DN using SIRT6 are suggested, including HIF-1α, PGC-1α, FOXO1, FOXO3, AMPK, PPARα, ABCG1, and SREBP, given the involvement of SIRT6 in glucose and lipid metabolism. By targeting SIRT6 and its associated pathways, there is potential to modulate glucose and lipid metabolism and mitigate the development and progression of DN. Further research and investigation are warranted to explore the therapeutic implications of targeting SIRT6 in DN treatment.
4.1 Glucotoxicity and DN
DN is a microvascular disease in which vascular endothelial cells are unable to downregulate glucose transport in response to high glucose levels, resulting in a large flow of intracellular glucose that triggers the production of pathogenic mediators (163). Hyperglycemia is considered a key initiating factor in DN-related renal injury. Excess glucose flux generates reactive oxygen species via several pathways (164). Mesangial expansion and podocyte loss are important early features of DN, and tubulointerstitial injury and fibrosis are key to the progression of DN to renal failure (11).
Podocyte structure and dysfunction are the core factors in DN pathogenesis. Hyperglycemia can induce podocytopathy, which is characterized by cell hypertrophy, foot process loss, and podocyte depletion (165, 166). Expression levels of myocyte-specific enhancer factor 2C (MEF2C), myogenic factor 5 (MYF5), and PGC-1α are decreased in renal tissues of patients with DN. This suggests that hyperglycemia reshapes energy metabolism in human podocytes (167). SIRT6 promotes the expression of PGC-1α (168). Receptor activator of NF-κB (RANK) is induced in DM and promotes glomerular oxidative stress as well as the secretion of pro-inflammatory cytokines, leading to podocyte injury and mediating the occurrence of DN (169). SIRT6 attenuates NF-κB signaling via deacetylation of H3K9 on chromatin (170).
Increased glucose load in PTs in the early stage of DM leads to maladaptive hypertrophy, hyperplasia of cortical tubules (171), and, at the same time, upregulated glucose transport (172), which promotes glucose reabsorption. In PTECs, SGLT2 and SGLT1 actively reabsorb glucose and passively return it to the blood via GLUT2 (173). These conditions can activate tubule-glomerular feedback, leading to increased intraglomerular pressure and ultrafiltration (174, 175). SIRT6 regulates the expression of GLUT2 during glucose reabsorption and gluconeogenesis (124). Renal tubular epithelial-to-mesenchymal transition (EMT) and tubulointerstitial fibrosis are important pathological features of DN (176, 177) and represent the “final common pathway” of associated renal function loss (178). Increased extracellular matrix (ECM) deposition in the kidney can be regulated by transforming growth factor-beta (TGF-β), connective tissue growth factor (CTGF), and other profibrotic mediators (179). Human PTECs and cortical fibroblasts exposed to HG show altered cell growth and collagen synthesis independent of hemodynamics and glomerular or vascular pathology (180). SIRT6 attenuates TGF-β-induced fibrosis in renal tubular cells by blocking β-catenin expression (57, 181).
MCs proliferate in the early stages of DN and are closely related to basic fibroblast growth factor (bFGF) and platelet-derived growth factor (PDGF) (182). Studies shows that SIRT6 regulate the expression of PDGF (183). PKC activation by glucose increases the permeability of endothelial cells to albumin, stimulates the synthesis of matrix proteins in MCs, and changes the function and structure of DM glomeruli (184). PKC also phosphorylates SIRT6 to mediate fatty acid β-oxidation (185).
Macrophages are the main immune cells, and activation of resident and infiltrating macrophages in DN can promote inflammation and fibrosis of the glomeruli and tubulointerstitium (186). HG induces high expression of intracellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) in vascular endothelial cells, which promotes the recruitment of renal macrophages in DN (187). Deacetylation of MRTF-A by SIRT6 leads to nuclear expulsion, thereby inhibiting the binding of MRTF-A to the ICAM-1 promoter and subsequently inhibiting the transcription of ICAM-1 (188). SIRT6 inhibits monocyte adhesion through downregulation of endothelial VCAM-1 expression (189).
4.2 Lipotoxicity and DN
Renal lipotoxicity, caused by lipid metabolism disorders, is involved in DN progression and renal dysfunction. Lipid metabolism disorders are significantly correlated with inflammation, podocyte dysfunction, fibrosis, and estimated glomerular filtration rate (eGFR), while lipid deposition is related to disorders of lipid metabolism genes (152). DM often coexists with obesity and leads to renal lipid accumulation (190). The degree of renal lipid deposition is related to renal function in DN (152, 191). Cholesterol accumulation in podocytes is associated with glomerulosclerosis progression (192). In DN, accumulation of lipids exceeding LD storage damages podocytes and renal tubular cells (193). Compared to healthy patients, patients with obesity have increased phospholipid accumulation, larger lysosomes, and impaired autophagic flux in the kidney (194). HFDs induce autophagolysosome dysfunction in mice accompanied by impaired autophagy, increased hypertrophy, lipid peroxidation and aging markers in the S2 segment of PTECs, sparse peritubular capillaries with localized interstitial fibrosis, and glomerular hypertrophy with mesangial expansion (195).
The expression of sphingomyelinase-like phosphodiesterase 3b (SMPDL3b) is increased in DN podocytes, and SMPDL3b promotes degradation of ceramide-1-phosphate (C1P) to ceramides and sphingolipids, which causes the insulin receptor to shift from the caveolin-1-rich domain in a C1P-dependent manner, leading to impaired AKT phosphorylation and podocyte injury (196, 197). Inhibition of nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) inflammasome activation inhibits lipid accumulation and improves podocyte injury (198). SIRT6 is involved in the NLRP3-mediated cell pyroptosis (199).
Healthy PTECs are rich in mitochondria and mainly depend on fatty acid beta-oxidation (FAO) for energy. They activate PGC-1α transcription through multiple signaling pathways, including the mTOR and AMPK pathways. The balance between mitochondrial dynamics and energetics maintains mitochondrial homeostasis (119). A shift from fatty acid utilization to glycolysis and lipid accumulation is a metabolic change characteristic of PTs in the development of DN and progression of renal fibrosis and is associated with increased HIF-1α expression (200). LDs are energy storage cellular organelles closely related to mitochondria (201). A single phospholipid bilayer can isolate neutral lipids from the cytoplasm and protect cells from FFA toxicity (202). Lipophagy occurs when LDs are isolated by autophagosomes and fuse with lysosomes to form autolysosomes, which are subsequently degraded by lysosomal hydrolases within the autolysosomes. This hydrolysis produces FFAs, which are recycled back into the cytoplasm for mitochondrial oxidation (203, 204). Lipophagy deficiency plays a key role in the development of ELD and lipid-related renal injury in DN (205). Overexpression of SIRT6 enhances autophagy (206). Annexin A1 (ANXA1) may improve mitochondrial FAO in PTECs through the AMPK/PPARα/CPT1b signaling pathway, thereby reducing intracellular lipid accumulation and improving lipotoxicity-mediated, DN-related tubular damage (191). The AMPK-SIRT6 pathway is involved in aging-related lipid deposition due to metabolic disorders (207).
MCs are susceptible to lipotoxicity, and lipotoxicity-induced MC apoptosis is related to decreased renal function (208, 209). Lipotoxicity is mediated by protein kinase R-like endoplasmic reticulum kinase (PERK) and activating transcription factor 6 (ATF6) signaling pathway-induced apoptosis in MCs (210). It is found that upregulation of SIRT6 expression inhibited the expression of p-PERK and ATF6 (211, 212).
Macrophages infiltration around apoptotic tubular epithelial cells induced by lipotoxicity has been observed in DN and is associated with leucine-rich α-2-glycoprotein 1 (LRG1) and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) (213).
5 SIRT6 and DN
SIRT6 expression was significantly decreased in HG-stimulated podocytes in a concentration- and time-dependent manner (47, 58). SIRT6 mRNA levels correlate positively with eGFR and negatively with proteinuria in renal biopsies of patients with podocyte disease (58). In streptozotocin (STZ)- and adriamycin (ADR)-treated mice and in db/db mice, SIRT6 expression in the kidneys decreased (58). The reduced expression of SIRT6 in podocytes suggests that SIRT6 reduction is an important cause of podocyte injury under various pathological conditions (58). SIRT6 inhibits the Notch pathway in HG to increase autophagic flux, reduce pro-inflammatory mediators, improve actin cytoskeleton disorders, and attenuate podocyte apoptosis to protect podocytes (58). SIRT6 activates AMPK and inhibits HG-induced mitochondrial dysfunction and podocyte apoptosis (47).
SIRT6 promoted Smad3 deacetylation and inhibits Smad3 nuclear accumulation to alleviate DN kidney injury in HG-induced HK-2 cells and in db/db mice. FOXO3a binds to the SIRT6 promoter and enhances its expression to prevent EMT and renal tubular injury in DN and can mediate SIRT6/Smad3 signaling to treat DN (214). Albuminuria decreases nicotinamide phosphoribosyltransferase (Nampt) expression in the PTs of STZ-induced diabetic mice, ultimately leading to matrix metalloproteinase (MMP) inactivation and reduced fibrous tissue disintegration by increasing H3K9 acetylation and decreasing SIRT6 expression in the tissue inhibitor of metal protease 1 (TIMP-1) promoter. Therefore, ECM remodeling linked to DN fibrosis can be efficiently controlled by the Nampt–SIRT6 axis within PTs (215).
In HG-induced rat MCs and STZ-induced DM mice, circ-ITCH regulated SIRT6 expression through miR-33a-5p to reduce inflammation and fibrosis (216).
In addition, SIRT6 protected podocytes from injury in a simulated DN microenvironment by activating M2 macrophages (217). Although there have been few reports on the relationship between SIRT6 and glomerular endothelial cells, SIRT6 has been shown to protect endothelial cells and exert anti-atherosclerotic effects. SIRT6 attenuates the endothelial dysfunction induced by cholesterol crystals by activating nuclear erythroid 2-related factor 2 (Nrf2) (218). SIRT6 deacetylates and reduces the expression of tumor necrosis factor ligand superfamily member 4 (TNFSF4) to maintain endothelial cell function and mitigate atherosclerosis (189).
6 Potential therapies targeting SIRT6 in DN
Recently, SIRT6 has been shown to play therapeutic roles in various diseases. Small molecules and compounds that regulate SIRT6 include MDL-811 (219) in ischemic brain injury, UBCS039 (220, 221) in cancer and liver injury, and anthocyanins in osteoarthritis (222). Because SIRT6 can inhibit glycolysis, it is considered part of a potential new generation of anticancer treatment targets (223).
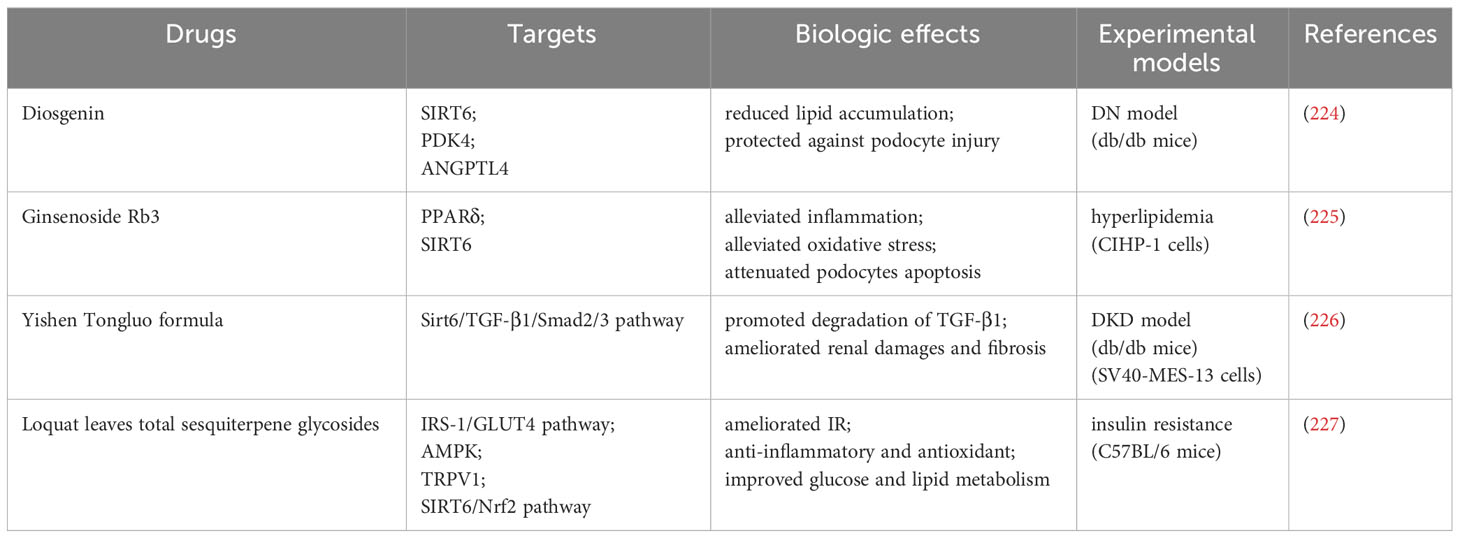
Drugs targeting SIRT6 also play important roles in the treatment of DN (Table 1). Diosgenin can reduce lipid accumulation by regulating SIRT6 in early DN while affecting PDK4 and angiopoietin-like-4 (ANGPTL4) to protect podocytes and reduce damage (224). After ginsenoside Rb3 treatment of palmitic acid-induced podocytes (CIHP-1 cells), PPARδ and SIRT6 expression increased in a dose-dependent manner and reduced inflammation and oxidative stress, thereby reducing podocyte apoptosis (225). Yishen Tongluo formula (YSTLF) treatment of db/db mice improved renal injury and fibrosis by positively regulating SIRT6 expression, inhibiting the TGF-β1/Smad2/3 signaling pathway and promoting TGF-β1 degradation (226). IR is closely associated with DN (228). Total sesquiterpene glycosides in loquat leaves can promote the SIRT6/Nrf2 signaling pathway to improve IR (227).
7 Conclusions and perspectives
Accumulating evidence suggests that SIRT6 plays a key role in DN treatment. This review describes the structure and enzymatic activity of SIRT6 and summarizes its important role in glucose and lipid metabolism. Additionally, we described the regulation of glucose and lipid metabolic pathways, including glycolysis, gluconeogenesis, lipolysis, and lipid synthesis, achievable by targeting SIRT6 to affect the progression of DN. Several compounds act as SIRT6 agonists and play potential roles in the treatment of DN. We have focused on the role of the sirtuin family in kidney diseases, especially in DN (46, 162, 229). However, the role of SIRT6 in regulating glucose and lipid metabolism remains unclear. As a potentially underappreciated and understudied target in DN, many challenges remain in the study of SIRT6. Most studies on SIRT6 have been preclinical, and the focus should be shifted to clinical applications, as well as to the efficacy, safety, and stability of targeted drugs. In conclusion, further exploration of the properties of SIRT6 is of potential value, and targeting SIRT6 has important clinical implications for the treatment of DN.
Author contributions
YW and JG conceived and designed the study. YW and TL wrote the manuscript. YW and TL designed the figures and edited the manuscript. JG and YC revised the paper. WL and JG supervised the writing. All authors have read and approved the final version of manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Natural Science Foundation of China (82104820).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kosiborod M, Gomes MB, Nicolucci A, Pocock S, Rathmann W, Shestakova MV, et al. Vascular complications in patients with type 2 diabetes: prevalence and associated factors in 38 countries (the discover study program). Cardiovasc Diabetol (2018) 17(1):150. doi: 10.1186/s12933-018-0787-8
2. Umanath K, Lewis JB. Update on diabetic nephropathy: core curriculum 2018. Am J Kidney Dis (2018) 71(6):884–95. doi: 10.1053/j.ajkd.2017.10.026
3. Tancredi M, Rosengren A, Svensson AM, Kosiborod M, Pivodic A, Gudbjornsdottir S, et al. Excess mortality among persons with type 2 diabetes. N Engl J Med (2015) 373(18):1720–32. doi: 10.1056/NEJMoa1504347
4. Sagoo MK, Gnudi L. Diabetic nephropathy: an overview. Methods Mol Biol (2020) 2067:3–7. doi: 10.1007/978-1-4939-9841-8_1
5. Reidy K, Kang HM, Hostetter T, Susztak K. Molecular mechanisms of diabetic kidney disease. J Clin Invest (2014) 124(6):2333–40. doi: 10.1172/JCI72271
6. Lytvyn Y, Bjornstad P, van Raalte DH, Heerspink HL, Cherney DZI. The new biology of diabetic kidney disease-mechanisms and therapeutic implications. Endocr Rev (2020) 41(2):202–31. doi: 10.1210/endrev/bnz010
7. Russo G, Piscitelli P, Giandalia A, Viazzi F, Pontremoli R, Fioretto P, et al. Atherogenic dyslipidemia and diabetic nephropathy. J Nephrol (2020) 33(5):1001–8. doi: 10.1007/s40620-020-00739-8
8. Gurley SB, Ghosh S, Johnson SA, Azushima K, Sakban RB, George SE, et al. Inflammation and immunity pathways regulate genetic susceptibility to diabetic nephropathy. Diabetes (2018) 67(10):2096–106. doi: 10.2337/db17-1323
9. Kato M, Natarajan R. Epigenetics and epigenomics in diabetic kidney disease and metabolic memory. Nat Rev Nephrol (2019) 15(6):327–45. doi: 10.1038/s41581-019-0135-6
10. Thomas MC, Brownlee M, Susztak K, Sharma K, Jandeleit-Dahm KA, Zoungas S, et al. Diabetic kidney disease. Nat Rev Dis Primers (2015) 1:15018. doi: 10.1038/nrdp.2015.18
11. Vallon V, Komers R. Pathophysiology of the diabetic kidney. Compr Physiol (2011) 1(3):1175–232. doi: 10.1002/cphy.c100049
12. Opazo-Rios L, Mas S, Marin-Royo G, Mezzano S, Gomez-Guerrero C, Moreno JA, et al. Lipotoxicity and diabetic nephropathy: novel mechanistic insights and therapeutic opportunities. Int J Mol Sci (2020) 21(7):2632. doi: 10.3390/ijms21072632
13. Gai Z, Wang T, Visentin M, Kullak-Ublick GA, Fu X, Wang Z. Lipid accumulation and chronic kidney disease. Nutrients (2019) 11(4):722. doi: 10.3390/nu11040722
14. Luo X, Wu J, Jing S, Yan LJ. Hyperglycemic stress and carbon stress in diabetic glucotoxicity. Aging Dis (2016) 7(1):90–110. doi: 10.14336/AD.2015.0702
15. Yan LJ. Pathogenesis of chronic hyperglycemia: from reductive stress to oxidative stress. J Diabetes Res (2014) 2014:137919. doi: 10.1155/2014/137919
16. Lipinski B. Evidence in support of a concept of reductive stress. Br J Nutr (2002) 87(1):93–4. doi: 10.1079/BJN2001435
17. Teodoro JS, Rolo AP, Palmeira CM. The nad ratio redox paradox: why does too much reductive power cause oxidative stress? Toxicol Mech Methods (2013) 23(5):297–302. doi: 10.3109/15376516.2012.759305
18. Valadi H, Valadi A, Ansell R, Gustafsson L, Adler L, Norbeck J, et al. Nadh-reductive stress in saccharomyces cerevisiae induces the expression of the minor isoform of glyceraldehyde-3-phosphate dehydrogenase (Tdh1). Curr Genet (2004) 45(2):90–5. doi: 10.1007/s00294-003-0469-1
19. Tilton RG. Diabetic vascular dysfunction: links to glucose-induced reductive stress and vegf. Microsc Res Tech (2002) 57(5):390–407. doi: 10.1002/jemt.10092
20. Hardiville S, Hart GW. Nutrient regulation of signaling, transcription, and cell physiology by O-Glcnacylation. Cell Metab (2014) 20(2):208–13. doi: 10.1016/j.cmet.2014.07.014
21. Issad T, Masson E, Pagesy P. O-Glcnac modification, insulin signaling and diabetic complications. Diabetes Metab (2010) 36(6 Pt 1):423–35. doi: 10.1016/j.diabet.2010.09.001
22. Xia L, Wang H, Munk S, Frecker H, Goldberg HJ, Fantus IG, et al. Reactive oxygen species, Pkc-beta1, and Pkc-zeta mediate high-glucose-induced vascular endothelial growth factor expression in mesangial cells. Am J Physiol Endocrinol Metab (2007) 293(5):E1280–8. doi: 10.1152/ajpendo.00223.2007
23. Bey EA, Xu B, Bhattacharjee A, Oldfield CM, Zhao X, Li Q, et al. Protein kinase C delta is required for P47phox phosphorylation and translocation in activated human monocytes. J Immunol (2004) 173(9):5730–8. doi: 10.4049/jimmunol.173.9.5730
24. Queisser MA, Yao D, Geisler S, Hammes HP, Lochnit G, Schleicher ED, et al. Hyperglycemia impairs proteasome function by methylglyoxal. Diabetes (2010) 59(3):670–8. doi: 10.2337/db08-1565
25. Thornalley PJ, Langborg A, Minhas HS. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J (1999) 344(Pt 1):109–16. doi: 10.1042/bj3440109
26. Maessen DE, Stehouwer CD, Schalkwijk CG. The role of methylglyoxal and the glyoxalase system in diabetes and other age-related diseases. Clin Sci (Lond) (2015) 128(12):839–61. doi: 10.1042/CS20140683
27. Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res (2010) 107(9):1058–70. doi: 10.1161/CIRCRESAHA.110.223545
28. Tiganis T. Reactive oxygen species and insulin resistance: the good, the bad and the ugly. Trends Pharmacol Sci (2011) 32(2):82–9. doi: 10.1016/j.tips.2010.11.006
29. Wolff SP. Diabetes mellitus and free radicals. Free radicals, transition metals and oxidative stress in the aetiology of diabetes mellitus and complications. Br Med Bull (1993) 49(3):642–52. doi: 10.1093/oxfordjournals.bmb.a072637
30. Trub AG, Hirschey MD. Reactive acyl-coa species modify proteins and induce carbon stress. Trends Biochem Sci (2018) 43(5):369–79. doi: 10.1016/j.tibs.2018.02.002
31. Zheng H, Wu J, Jin Z, Yan LJ. Protein modifications as manifestations of hyperglycemic glucotoxicity in diabetes and its complications. Biochem Insights (2016) 9:1–9. doi: 10.4137/BCI.S36141
32. Wagner GR, Hirschey MD. Nonenzymatic protein acylation as a carbon stress regulated by sirtuin deacylases. Mol Cell (2014) 54(1):5–16. doi: 10.1016/j.molcel.2014.03.027
33. Frizzell N, Thomas SA, Carson JA, Baynes JW. Mitochondrial stress causes increased succination of proteins in adipocytes in response to glucotoxicity. Biochem J (2012) 445(2):247–54. doi: 10.1042/BJ20112142
34. Mota M, Banini BA, Cazanave SC, Sanyal AJ. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism (2016) 65(8):1049–61. doi: 10.1016/j.metabol.2016.02.014
35. Lytrivi M, Castell AL, Poitout V, Cnop M. Recent insights into mechanisms of beta-cell lipo- and glucolipotoxicity in type 2 diabetes. J Mol Biol (2020) 432(5):1514–34. doi: 10.1016/j.jmb.2019.09.016
36. Kumar B, Kowluru A, Kowluru RA. Lipotoxicity augments glucotoxicity-induced mitochondrial damage in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci (2015) 56(5):2985–92. doi: 10.1167/iovs.15-16466
37. Thongnak L, Pongchaidecha A, Lungkaphin A. Renal lipid metabolism and lipotoxicity in diabetes. Am J Med Sci (2020) 359(2):84–99. doi: 10.1016/j.amjms.2019.11.004
38. Izquierdo-Lahuerta A, Martinez-Garcia C, Medina-Gomez G. Lipotoxicity as a trigger factor of renal disease. J Nephrol (2016) 29(5):603–10. doi: 10.1007/s40620-016-0278-5
39. Ogura Y, Kitada M, Koya D. Sirtuins and renal oxidative stress. Antioxidants (Basel) (2021) 10(8):1198. doi: 10.3390/antiox10081198
40. Stout MB, Justice JN, Nicklas BJ, Kirkland JL. Physiological aging: links among adipose tissue dysfunction, diabetes, and frailty. Physiol (Bethesda) (2017) 32(1):9–19. doi: 10.1152/physiol.00012.2016
41. Preuss HG. Effects of glucose/insulin perturbations on aging and chronic disorders of aging: the evidence. J Am Coll Nutr (1997) 16(5):397–403. doi: 10.1080/07315724.1997.10718704
42. Braun F, Rinschen MM, Bartels V, Frommolt P, Habermann B, Hoeijmakers JH, et al. Altered lipid metabolism in the aging kidney identified by three layered omic analysis. Aging (Albany NY) (2016) 8(3):441–57. doi: 10.18632/aging.100900
43. Chen Y, Kanwar YS, Chen X, Zhan M. Aging and diabetic kidney disease: emerging pathogenetic mechanisms and clinical implications. Curr Med Chem (2023). doi: 10.2174/0929867330666230621112215
44. Canto C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, et al. The Nad(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab (2012) 15(6):838–47. doi: 10.1016/j.cmet.2012.04.022
45. Akter R, Afrose A, Rahman MR, Chowdhury R, Nirzhor SSR, Khan RI, et al. A comprehensive analysis into the therapeutic application of natural products as Sirt6 modulators in Alzheimer's disease, aging, cancer, inflammation, and diabetes. Int J Mol Sci (2021) 22(8):4180. doi: 10.3390/ijms22084180
46. Guo J, Zheng HJ, Zhang W, Lou W, Xia C, Han XT, et al. Accelerated kidney aging in diabetes mellitus. Oxid Med Cell Longev (2020) 2020:1234059. doi: 10.1155/2020/1234059
47. Fan Y, Yang Q, Yang Y, Gao Z, Ma Y, Zhang L, et al. Sirt6 suppresses high glucose-induced mitochondrial dysfunction and apoptosis in podocytes through Ampk activation. Int J Biol Sci (2019) 15(3):701–13. doi: 10.7150/ijbs.29323
48. Hou T, Tian Y, Cao Z, Zhang J, Feng T, Tao W, et al. Cytoplasmic Sirt6-mediated acsl5 deacetylation impedes nonalcoholic fatty liver disease by facilitating hepatic fatty acid oxidation. Mol Cell (2022) 82(21):4099–115 e9. doi: 10.1016/j.molcel.2022.09.018
49. Zhao Y, Jia X, Yang X, Bai X, Lu Y, Zhu L, et al. Deacetylation of caveolin-1 by Sirt6 induces autophagy and retards high glucose-stimulated Ldl transcytosis and atherosclerosis formation. Metabolism (2022) 131:155162. doi: 10.1016/j.metabol.2022.155162
50. Roichman A, Elhanati S, Aon MA, Abramovich I, Di Francesco A, Shahar Y, et al. Restoration of energy homeostasis by Sirt6 extends healthy lifespan. Nat Commun (2021) 12(1):3208. doi: 10.1038/s41467-021-23545-7
51. He Y, Yang G, Sun L, Gao H, Yao F, Jin Z, et al. Sirt6 inhibits inflammatory response through regulation of Nrf2 in vascular endothelial cells. Int Immunopharmacol (2021) 99:107926. doi: 10.1016/j.intimp.2021.107926
52. He Y, Yang G, Yao F, Xian Y, Wang G, Chen L, et al. Sitagliptin inhibits vascular inflammation via the Sirt6-dependent signaling pathway. Int Immunopharmacol (2019) 75:105805. doi: 10.1016/j.intimp.2019.105805
53. Shao J, Yang X, Liu T, Zhang T, Xie QR, Xia W. Autophagy induction by Sirt6 is involved in oxidative stress-induced neuronal damage. Protein Cell (2016) 7(4):281–90. doi: 10.1007/s13238-016-0257-6
54. Lu J, Sun D, Liu Z, Li M, Hong H, Liu C, et al. Sirt6 suppresses isoproterenol-induced cardiac hypertrophy through activation of autophagy. Transl Res (2016) 172:96–112 e6. doi: 10.1016/j.trsl.2016.03.002
55. Zhou Y, Fan X, Jiao T, Li W, Chen P, Jiang Y, et al. Sirt6 as a key event linking P53 and Nrf2 counteracts apap-induced hepatotoxicity through inhibiting oxidative stress and promoting hepatocyte proliferation. Acta Pharm Sin B (2021) 11(1):89–99. doi: 10.1016/j.apsb.2020.06.016
56. Kim HG, Huang M, Xin Y, Zhang Y, Zhang X, Wang G, et al. The epigenetic regulator Sirt6 protects the liver from alcohol-induced tissue injury by reducing oxidative stress in mice. J Hepatol (2019) 71(5):960–9. doi: 10.1016/j.jhep.2019.06.019
57. Cai J, Liu Z, Huang X, Shu S, Hu X, Zheng M, et al. The deacetylase sirtuin 6 protects against kidney fibrosis by epigenetically blocking beta-catenin target gene expression. Kidney Int (2020) 97(1):106–18. doi: 10.1016/j.kint.2019.08.028
58. Liu M, Liang K, Zhen J, Zhou M, Wang X, Wang Z, et al. Sirt6 deficiency exacerbates podocyte injury and proteinuria through targeting notch signaling. Nat Commun (2017) 8(1):413. doi: 10.1038/s41467-017-00498-4
59. Chen Z, Liang W, Hu J, Zhu Z, Feng J, Ma Y, et al. Sirt6 deficiency contributes to mitochondrial fission and oxidative damage in podocytes via Rock1-drp1 signalling pathway. Cell Prolif (2022) 55(10):e13296. doi: 10.1111/cpr.13296
60. Fan Y, Cheng J, Yang Q, Feng J, Hu J, Ren Z, et al. Sirt6-mediated Nrf2/Ho-1 activation alleviates angiotensin ii-induced DNA dsbs and apoptosis in podocytes. Food Funct (2021) 12(17):7867–82. doi: 10.1039/d0fo03467c
61. Frye RA. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun (2000) 273(2):793–8. doi: 10.1006/bbrc.2000.3000
62. Liszt G, Ford E, Kurtev M, Guarente L. Mouse sir2 homolog Sirt6 is a nuclear adp-ribosyltransferase. J Biol Chem (2005) 280(22):21313–20. doi: 10.1074/jbc.M413296200
63. Mahlknecht U, Ho AD, Voelter-Mahlknecht S. Chromosomal organization and fluorescence in situ hybridization of the human sirtuin 6 gene. Int J Oncol (2006) 28(2):447–56. doi: 10.3892/ijo.28.2.447
64. Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human sirt proteins. Mol Biol Cell (2005) 16(10):4623–35. doi: 10.1091/mbc.e05-01-0033
65. Pan PW, Feldman JL, Devries MK, Dong A, Edwards AM, Denu JM. Structure and biochemical functions of Sirt6. J Biol Chem (2011) 286(16):14575–87. doi: 10.1074/jbc.M111.218990
66. Kugel S, Mostoslavsky R. Chromatin and beyond: the multitasking roles for Sirt6. Trends Biochem Sci (2014) 39(2):72–81. doi: 10.1016/j.tibs.2013.12.002
67. Wang WW, Zeng Y, Wu B, Deiters A, Liu WR. A chemical biology approach to reveal Sirt6-targeted histone H3 sites in nucleosomes. ACS Chem Biol (2016) 11(7):1973–81. doi: 10.1021/acschembio.6b00243
68. Michishita E, McCord RA, Berber E, Kioi M, Padilla-Nash H, Damian M, et al. Sirt6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature (2008) 452(7186):492–6. doi: 10.1038/nature06736
69. Michishita E, McCord RA, Boxer LD, Barber MF, Hong T, Gozani O, et al. Cell cycle-dependent deacetylation of telomeric histone H3 lysine K56 by human Sirt6. Cell Cycle (2009) 8(16):2664–6. doi: 10.4161/cc.8.16.9367
70. Toiber D, Erdel F, Bouazoune K, Silberman DM, Zhong L, Mulligan P, et al. Sirt6 recruits Snf2h to DNA break sites, preventing genomic instability through chromatin remodeling. Mol Cell (2013) 51(4):454–68. doi: 10.1016/j.molcel.2013.06.018
71. Tasselli L, Xi Y, Zheng W, Tennen RI, Odrowaz Z, Simeoni F, et al. Sirt6 deacetylates H3k18ac at pericentric chromatin to prevent mitotic errors and cellular senescence. Nat Struct Mol Biol (2016) 23(5):434–40. doi: 10.1038/nsmb.3202
72. Kaidi A, Weinert BT, Choudhary C, Jackson SP. Retracted: human Sirt6 promotes DNA end resection through ctip deacetylation. Science (2010) 329(5997):1348–53. doi: 10.1126/science.1192049
73. Feldman JL, Baeza J, Denu JM. Activation of the protein deacetylase Sirt6 by long-chain fatty acids and widespread deacylation by mammalian sirtuins. J Biol Chem (2013) 288(43):31350–6. doi: 10.1074/jbc.C113.511261
74. Gil R, Barth S, Kanfi Y, Cohen HY. Sirt6 exhibits nucleosome-dependent deacetylase activity. Nucleic Acids Res (2013) 41(18):8537–45. doi: 10.1093/nar/gkt642
75. Zhang X, Khan S, Jiang H, Antonyak MA, Chen X, Spiegelman NA, et al. Identifying the functional contribution of the defatty-acylase activity of Sirt6. Nat Chem Biol (2016) 12(8):614–20. doi: 10.1038/nchembio.2106
76. Cassinelli S, Vinola-Renart C, Benavente-Garcia A, Navarro-Perez M, Capera J, Felipe A. Palmitoylation of voltage-gated ion channels. Int J Mol Sci (2022) 23(16):9357. doi: 10.3390/ijms23169357
77. Yuan M, Song ZH, Ying MD, Zhu H, He QJ, Yang B, et al. N-myristoylation: from cell biology to translational medicine. Acta Pharmacol Sin (2020) 41(8):1005–15. doi: 10.1038/s41401-020-0388-4
78. Jiang H, Khan S, Wang Y, Charron G, He B, Sebastian C, et al. Sirt6 regulates tnf-alpha secretion through hydrolysis of long-chain fatty acyl lysine. Nature (2013) 496(7443):110–3. doi: 10.1038/nature12038
79. Zhang X, Spiegelman NA, Nelson OD, Jing H, Lin H. Sirt6 regulates ras-related protein R-ras2 by lysine defatty-acylation. Elife (2017) 6:e25158. doi: 10.7554/eLife.25158
80. Luscher B, Butepage M, Eckei L, Krieg S, Verheugd P, Shilton BH. Adp-ribosylation, a multifaceted posttranslational modification involved in the control of cell physiology in health and disease. Chem Rev (2018) 118(3):1092–136. doi: 10.1021/acs.chemrev.7b00122
81. Hopp AK, Gruter P, Hottiger MO. Regulation of glucose metabolism by Nad(+) and adp-ribosylation. Cells (2019) 8(8):890. doi: 10.3390/cells8080890
82. Groslambert J, Prokhorova E, Ahel I. Adp-ribosylation of DNA and rna. DNA Repair (Amst) (2021) 105:103144. doi: 10.1016/j.dnarep.2021.103144
83. Ling F, Tang Y, Li M, Li QS, Li X, Yang L, et al. Mono-Adp-Ribosylation of Histone 3 at Arginine-117 Promotes Proliferation through Its Interaction with P300. Oncotarget (2017) 8(42):72773–87. doi: 10.18632/oncotarget.20347
84. Rezazadeh S, Yang D, Biashad SA, Firsanov D, Takasugi M, Gilbert M, et al. Sirt6 mono-adp ribosylates kdm2a to locally increase H3k36me2 at DNA damage sites to inhibit transcription and promote repair. Aging (Albany NY) (2020) 12(12):11165–84. doi: 10.18632/aging.103567
85. Mao Z, Hine C, Tian X, Van Meter M, Au M, Vaidya A, et al. Sirt6 promotes DNA repair under stress by activating parp1. Science (2011) 332(6036):1443–6. doi: 10.1126/science.1202723
86. Van Meter M, Kashyap M, Rezazadeh S, Geneva AJ, Morello TD, Seluanov A, et al. Sirt6 represses line1 retrotransposons by ribosylating kap1 but this repression fails with stress and age. Nat Commun (2014) 5:5011. doi: 10.1038/ncomms6011
87. Cai J, Zuo Y, Wang T, Cao Y, Cai R, Chen FL, et al. A crucial role of sumoylation in modulating Sirt6 deacetylation of H3 at lysine 56 and its tumor suppressive activity. Oncogene (2016) 35(37):4949–56. doi: 10.1038/onc.2016.24
88. Scisciola L, Rizzo MR, Marfella R, Cataldo V, Fontanella RA, Boccalone E, et al. New insight in molecular mechanisms regulating Sirt6 expression in diabetes: hyperglycaemia effects on Sirt6 DNA methylation. J Cell Physiol (2021) 236(6):4604–13. doi: 10.1002/jcp.30185
89. Yu J, Wu Y, Yang P. High glucose-induced oxidative stress represses sirtuin deacetylase expression and increases histone acetylation leading to neural tube defects. J Neurochem (2016) 137(3):371–83. doi: 10.1111/jnc.13587
90. Zhang P, Tu B, Wang H, Cao Z, Tang M, Zhang C, et al. Tumor suppressor P53 cooperates with Sirt6 to regulate gluconeogenesis by promoting foxo1 nuclear exclusion. Proc Natl Acad Sci USA. (2014) 111(29):10684–9. doi: 10.1073/pnas.1411026111
91. Jung ES, Choi H, Song H, Hwang YJ, Kim A, Ryu H, et al. P53-dependent Sirt6 expression protects abeta42-induced DNA damage. Sci Rep (2016) 6:25628. doi: 10.1038/srep25628
92. Kanfi Y, Shalman R, Peshti V, Pilosof SN, Gozlan YM, Pearson KJ, et al. Regulation of Sirt6 protein levels by nutrient availability. FEBS Lett (2008) 582(5):543–8. doi: 10.1016/j.febslet.2008.01.019
93. D'Arcy P, Wang X, Linder S. Deubiquitinase inhibition as a cancer therapeutic strategy. Pharmacol Ther (2015) 147:32–54. doi: 10.1016/j.pharmthera.2014.11.002
94. Luo P, Qin C, Zhu L, Fang C, Zhang Y, Zhang H, et al. Ubiquitin-specific peptidase 10 (Usp10) inhibits hepatic steatosis, insulin resistance, and inflammation through Sirt6. Hepatology (2018) 68(5):1786–803. doi: 10.1002/hep.30062
95. Ronnebaum SM, Wu Y, McDonough H, Patterson C. The ubiquitin ligase chip prevents Sirt6 degradation through noncanonical ubiquitination. Mol Cell Biol (2013) 33(22):4461–72. doi: 10.1128/MCB.00480-13
96. Long D, Wu H, Tsang AW, Poole LB, Yoza BK, Wang X, et al. The oxidative state of cysteine thiol 144 regulates the Sirt6 glucose homeostat. Sci Rep (2017) 7(1):11005. doi: 10.1038/s41598-017-11388-6
97. Huang L, Sun H, Song F, Cao Z, Jiang X, Zhang L, et al. Sirt6 overexpression inhibits cementogenesis by suppressing glucose transporter 1. J Cell Physiol (2019) 234(4):4005–14. doi: 10.1002/jcp.27213
98. Zhong L, D'Urso A, Toiber D, Sebastian C, Henry RE, Vadysirisack DD, et al. The histone deacetylase Sirt6 regulates glucose homeostasis via hif1alpha. Cell (2010) 140(2):280–93. doi: 10.1016/j.cell.2009.12.041
99. Sociali G, Magnone M, Ravera S, Damonte P, Vigliarolo T, Von Holtey M, et al. Pharmacological Sirt6 inhibition improves glucose tolerance in a type 2 diabetes mouse model. FASEB J (2017) 31(7):3138–49. doi: 10.1096/fj.201601294R
100. Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, et al. Genomic instability and aging-like phenotype in the absence of mammalian Sirt6. Cell (2006) 124(2):315–29. doi: 10.1016/j.cell.2005.11.044
101. Mitrakou A. Kidney: its impact on glucose homeostasis and hormonal regulation. Diabetes Res Clin Pract (2011) 93 Suppl 1:S66–72. doi: 10.1016/S0168-8227(11)70016-X
102. Ansermet C, Centeno G, Bignon Y, Ortiz D, Pradervand S, Garcia A, et al. Dysfunction of the circadian clock in the kidney tubule leads to enhanced kidney gluconeogenesis and exacerbated hyperglycemia in diabetes. Kidney Int (2022) 101(3):563–73. doi: 10.1016/j.kint.2021.11.016
103. Sedzikowska A, Szablewski L. Human glucose transporters in renal glucose homeostasis. Int J Mol Sci (2021) 22(24):13522. doi: 10.3390/ijms222413522
104. Xiao C, Kim HS, Lahusen T, Wang RH, Xu X, Gavrilova O, et al. Sirt6 deficiency results in severe hypoglycemia by enhancing both basal and insulin-stimulated glucose uptake in mice. J Biol Chem (2010) 285(47):36776–84. doi: 10.1074/jbc.M110.168039
105. Song MY, Wang J, Ka SO, Bae EJ, Park BH. Insulin secretion impairment in Sirt6 knockout pancreatic beta cells is mediated by suppression of the foxo1-pdx1-glut2 pathway. Sci Rep (2016) 6:30321. doi: 10.1038/srep30321
106. Yuan Z, Zeng Y, Tian Y, Wang S, Hong B, Yang M. Sirt6 serves as a polyhedron in glycolytic metabolism and ageing-related diseases. Exp Gerontol (2022) 162:111765. doi: 10.1016/j.exger.2022.111765
107. Aragones J, Fraisl P, Baes M, Carmeliet P. Oxygen sensors at the crossroad of metabolism. Cell Metab (2009) 9(1):11–22. doi: 10.1016/j.cmet.2008.10.001
108. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the warburg effect: the metabolic requirements of cell proliferation. Science (2009) 324(5930):1029–33. doi: 10.1126/science.1160809
109. Li J, Wang T, Xia J, Yao W, Huang F. Enzymatic and nonenzymatic protein acetylations control glycolysis process in liver diseases. FASEB J (2019) 33(11):11640–54. doi: 10.1096/fj.201901175R
110. Gordin D, Shah H, Shinjo T, St-Louis R, Qi W, Park K, et al. Characterization of glycolytic enzymes and pyruvate kinase M2 in type 1 and 2 diabetic nephropathy. Diabetes Care (2019) 42(7):1263–73. doi: 10.2337/dc18-2585
111. Brinkkoetter PT, Bork T, Salou S, Liang W, Mizi A, Ozel C, et al. Anaerobic glycolysis maintains the glomerular filtration barrier independent of mitochondrial metabolism and dynamics. Cell Rep (2019) 27(5):1551–66 e5. doi: 10.1016/j.celrep.2019.04.012
112. Luo L, Luo J, Cai Y, Fu M, Li W, Shi L, et al. Inulin-type fructans change the gut microbiota and prevent the development of diabetic nephropathy. Pharmacol Res (2022) 183:106367. doi: 10.1016/j.phrs.2022.106367
113. Czajka A, Malik AN. Hyperglycemia induced damage to mitochondrial respiration in renal mesangial and tubular cells: implications for diabetic nephropathy. Redox Biol (2016) 10:100–7. doi: 10.1016/j.redox.2016.09.007
114. Kim JW, Tchernyshyov I, Semenza GL, Dang CV. Hif-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metab (2006) 3(3):177–85. doi: 10.1016/j.cmet.2006.02.002
115. Yang J, Gupta V, Carroll KS, Liebler DC. Site-specific mapping and quantification of protein S-sulphenylation in cells. Nat Commun (2014) 5:4776. doi: 10.1038/ncomms5776
116. Bian C, Gao J, Wang Y, Li J, Luan Z, Lu H, et al. Association of Sirt6 circulating levels with urinary and glycometabolic markers in pre-diabetes and diabetes. Acta Diabetol (2021) 58(11):1551–62. doi: 10.1007/s00592-021-01759-x
117. Fu J, Shinjo T, Li Q, St-Louis R, Park K, Yu MG, et al. Regeneration of glomerular metabolism and function by podocyte pyruvate kinase M2 in diabetic nephropathy. JCI Insight (2022) 7(5):e155260. doi: 10.1172/jci.insight.155260
118. Qi W, Keenan HA, Li Q, Ishikado A, Kannt A, Sadowski T, et al. Pyruvate kinase M2 activation may protect against the progression of diabetic glomerular pathology and mitochondrial dysfunction. Nat Med (2017) 23(6):753–62. doi: 10.1038/nm.4328
119. Bhargava P, Schnellmann RG. Mitochondrial energetics in the kidney. Nat Rev Nephrol (2017) 13(10):629–46. doi: 10.1038/nrneph.2017.107
120. Dominy JE Jr., Lee Y, Jedrychowski MP, Chim H, Jurczak MJ, Camporez JP, et al. The deacetylase Sirt6 activates the acetyltransferase Gcn5 and suppresses hepatic gluconeogenesis. Mol Cell (2012) 48(6):900–13. doi: 10.1016/j.molcel.2012.09.030
121. Soyal S, Krempler F, Oberkofler H, Patsch W. Pgc-1alpha: A potent transcriptional cofactor involved in the pathogenesis of type 2 diabetes. Diabetologia (2006) 49(7):1477–88. doi: 10.1007/s00125-006-0268-6
122. Sharma K, Karl B, Mathew AV, Gangoiti JA, Wassel CL, Saito R, et al. Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J Am Soc Nephrol (2013) 24(11):1901–12. doi: 10.1681/ASN.2013020126
123. Li L, Wang C, Yang H, Liu S, Lu Y, Fu P, et al. Metabolomics reveal mitochondrial and fatty acid metabolism disorders that contribute to the development of Dkd in T2dm patients. Mol Biosyst (2017) 13(11):2392–400. doi: 10.1039/c7mb00167c
124. Bian C, Zhang R, Wang Y, Li J, Song Y, Guo D, et al. Sirtuin 6 affects glucose reabsorption and gluconeogenesis in type 1 diabetes via foxo1. Mol Cell Endocrinol (2022) 547:111597. doi: 10.1016/j.mce.2022.111597
125. Pina AF, Borges DO, Meneses MJ, Branco P, Birne R, Vilasi A, et al. Insulin: trigger and target of renal functions. Front Cell Dev Biol (2020) 8:519. doi: 10.3389/fcell.2020.00519
126. Xiong X, Wang G, Tao R, Wu P, Kono T, Li K, et al. Sirtuin 6 regulates glucose-stimulated insulin secretion in mouse pancreatic beta cells. Diabetologia (2016) 59(1):151–60. doi: 10.1007/s00125-015-3778-2
127. Qin K, Zhang N, Zhang Z, Nipper M, Zhu Z, Leighton J, et al. Sirt6-mediated transcriptional suppression of Txnip is critical for pancreatic beta cell function and survival in mice. Diabetologia (2018) 61(4):906–18. doi: 10.1007/s00125-017-4542-6
128. Xiong X, Sun X, Wang Q, Qian X, Zhang Y, Pan X, et al. Sirt6 protects against palmitate-induced pancreatic beta-cell dysfunction and apoptosis. J Endocrinol (2016) 231(2):159–65. doi: 10.1530/JOE-16-0317
129. Svensson M, Eriksson JW. Insulin resistance in diabetic nephropathy–cause or consequence? Diabetes Metab Res Rev (2006) 22(5):401–10. doi: 10.1002/dmrr.648
130. Tang W, Fan Y. Sirt6 as a potential target for treating insulin resistance. Life Sci (2019) 231:116558. doi: 10.1016/j.lfs.2019.116558
131. Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell (2014) 156(1-2):20–44. doi: 10.1016/j.cell.2013.12.012
132. Farmer SR. Transcriptional control of adipocyte formation. Cell Metab (2006) 4(4):263–73. doi: 10.1016/j.cmet.2006.07.001
133. Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol (2006) 7(12):885–96. doi: 10.1038/nrm2066
134. Ghaben AL, Scherer PE. Adipogenesis and metabolic health. Nat Rev Mol Cell Biol (2019) 20(4):242–58. doi: 10.1038/s41580-018-0093-z
135. Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med (2013) 19(10):1252–63. doi: 10.1038/nm.3361
136. Yao L, Cui X, Chen Q, Yang X, Fang F, Zhang J, et al. Cold-inducible Sirt6 regulates thermogenesis of brown and beige fat. Cell Rep (2017) 20(3):641–54. doi: 10.1016/j.celrep.2017.06.069
137. D'Onofrio N, Pieretti G, Ciccarelli F, Gambardella A, Passariello N, Rizzo MR, et al. Abdominal fat Sirt6 expression and its relationship with inflammatory and metabolic pathways in pre-diabetic overweight patients. Int J Mol Sci (2019) 20(5):1153. doi: 10.3390/ijms20051153
138. Moschen AR, Wieser V, Gerner RR, Bichler A, Enrich B, Moser P, et al. Adipose tissue and liver expression of sirt1, 3, and 6 increase after extensive weight loss in morbid obesity. J Hepatol (2013) 59(6):1315–22. doi: 10.1016/j.jhep.2013.07.027
139. Hong J, Mei C, Abbas Raza SH, Khan R, Cheng G, Zan L. Sirt6 cooperates with sirt5 to regulate bovine preadipocyte differentiation and lipid metabolism via the ampkalpha signaling pathway. Arch Biochem Biophys (2020) 681:108260. doi: 10.1016/j.abb.2020.108260
140. Ahima RS, Flier JS. Leptin. Annu Rev Physiol (2000) 62:413–37. doi: 10.1146/annurev.physiol.62.1.413
141. Straub LG, Scherer PE. Metabolic messengers: adiponectin. Nat Metab (2019) 1(3):334–9. doi: 10.1038/s42255-019-0041-z
142. Tang Q, Gao Y, Liu Q, Yang X, Wu T, Huang C, et al. Sirt6 in pro-opiomelanocortin neurons controls energy metabolism by modulating leptin signaling. Mol Metab (2020) 37:100994. doi: 10.1016/j.molmet.2020.100994
143. Zha D, Wu X, Gao P. Adiponectin and its receptors in diabetic kidney disease: molecular mechanisms and clinical potential. Endocrinology (2017) 158(7):2022–34. doi: 10.1210/en.2016-1765
144. Kanfi Y, Peshti V, Gil R, Naiman S, Nahum L, Levin E, et al. Sirt6 protects against pathological damage caused by diet-induced obesity. Aging Cell (2010) 9(2):162–73. doi: 10.1111/j.1474-9726.2009.00544.x
145. Li Y, Ma Z, Jiang S, Hu W, Li T, Di S, et al. A global perspective on foxo1 in lipid metabolism and lipid-related diseases. Prog Lipid Res (2017) 66:42–9. doi: 10.1016/j.plipres.2017.04.002
146. Jung SM, Hung CM, Hildebrand SR, Sanchez-Gurmaches J, Martinez-Pastor B, Gengatharan JM, et al. Non-canonical mtorc2 signaling regulates brown adipocyte lipid catabolism through Sirt6-foxo1. Mol Cell (2019) 75(4):807–22 e8. doi: 10.1016/j.molcel.2019.07.023
147. Festuccia WT. Regulation of adipocyte and macrophage functions by mtorc1 and 2 in metabolic diseases. Mol Nutr Food Res (2021) 65(1):e1900768. doi: 10.1002/mnfr.201900768
148. Kuang J, Zhang Y, Liu Q, Shen J, Pu S, Cheng S, et al. Fat-specific Sirt6 ablation sensitizes mice to high-fat diet-induced obesity and insulin resistance by inhibiting lipolysis. Diabetes (2017) 66(5):1159–71. doi: 10.2337/db16-1225
149. Peeters A, Baes M. Role of pparalpha in hepatic carbohydrate metabolism. PPAR Res (2010) 2010:572405. doi: 10.1155/2010/572405
150. Naiman S, Huynh FK, Gil R, Glick Y, Shahar Y, Touitou N, et al. Sirt6 promotes hepatic beta-oxidation via activation of pparalpha. Cell Rep (2019) 29(12):4127–43 e8. doi: 10.1016/j.celrep.2019.11.067
151. Feingold KR. Lipid and lipoprotein metabolism. Endocrinol Metab Clin North Am (2022) 51(3):437–58. doi: 10.1016/j.ecl.2022.02.008
152. Herman-Edelstein M, Scherzer P, Tobar A, Levi M, Gafter U. Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J Lipid Res (2014) 55(3):561–72. doi: 10.1194/jlr.P040501
153. Yang Q, Hu J, Yang Y, Chen Z, Feng J, Zhu Z, et al. Sirt6 deficiency aggravates angiotensin ii-induced cholesterol accumulation and injury in podocytes. Theranostics (2020) 10(16):7465–79. doi: 10.7150/thno.45003
154. Konig B, Koch A, Spielmann J, Hilgenfeld C, Stangl GI, Eder K. Activation of pparalpha lowers synthesis and concentration of cholesterol by reduction of nuclear srebp-2. Biochem Pharmacol (2007) 73(4):574–85. doi: 10.1016/j.bcp.2006.10.027
155. Konig B, Koch A, Spielmann J, Hilgenfeld C, Hirche F, Stangl GI, et al. Activation of pparalpha and ppargamma reduces triacylglycerol synthesis in rat hepatoma cells by reduction of nuclear srebp-1. Eur J Pharmacol (2009) 605(1-3):23–30. doi: 10.1016/j.ejphar.2009.01.009
156. Horton JD, Goldstein JL, Brown MS. Srebps: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest (2002) 109(9):1125–31. doi: 10.1172/JCI15593
157. Ishigaki N, Yamamoto T, Shimizu Y, Kobayashi K, Yatoh S, Sone H, et al. Involvement of glomerular Srebp-1c in diabetic nephropathy. Biochem Biophys Res Commun (2007) 364(3):502–8. doi: 10.1016/j.bbrc.2007.10.038
158. Tao R, Xiong X, DePinho RA, Deng CX, Dong XC. Hepatic srebp-2 and cholesterol biosynthesis are regulated by foxo3 and Sirt6. J Lipid Res (2013) 54(10):2745–53. doi: 10.1194/jlr.M039339
159. Elhanati S, Kanfi Y, Varvak A, Roichman A, Carmel-Gross I, Barth S, et al. Multiple regulatory layers of srebp1/2 by Sirt6. Cell Rep (2013) 4(5):905–12. doi: 10.1016/j.celrep.2013.08.006
160. Yang Z, Cappello T, Wang L. Emerging role of micrornas in lipid metabolism. Acta Pharm Sin B (2015) 5(2):145–50. doi: 10.1016/j.apsb.2015.01.002
161. Davalos A, Goedeke L, Smibert P, Ramirez CM, Warrier NP, Andreo U, et al. Mir-33a/B contribute to the regulation of fatty acid metabolism and insulin signaling. Proc Natl Acad Sci USA. (2011) 108(22):9232–7. doi: 10.1073/pnas.1102281108
162. Liu T, Yang L, Mao H, Ma F, Wang Y, Li S, et al. Sirtuins as novel pharmacological targets in podocyte injury and related glomerular diseases. BioMed Pharmacother (2022) 155:113620. doi: 10.1016/j.biopha.2022.113620
163. Kaiser N, Sasson S, Feener EP, Boukobza-Vardi N, Higashi S, Moller DE, et al. Differential regulation of glucose transport and transporters by glucose in vascular endothelial and smooth muscle cells. Diabetes (1993) 42(1):80–9. doi: 10.2337/diab.42.1.80
164. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature (2001) 414(6865):813–20. doi: 10.1038/414813a
165. Lin JS, Susztak K. Podocytes: the weakest link in diabetic kidney disease? Curr Diabetes Rep (2016) 16(5):45. doi: 10.1007/s11892-016-0735-5
166. Nakamichi R, Hayashi K, Itoh H. Effects of high glucose and lipotoxicity on diabetic podocytes. Nutrients (2021) 13(1):241. doi: 10.3390/nu13010241
167. Imasawa T, Obre E, Bellance N, Lavie J, Imasawa T, Rigothier C, et al. High glucose repatterns human podocyte energy metabolism during differentiation and diabetic nephropathy. FASEB J (2017) 31(1):294–307. doi: 10.1096/fj.201600293R
168. Gao S, Yang Q, Liu Z, Kong W, Chen J, Li X, et al. Metformin alleviates hfd-induced oxidative stress in hepatocyte via activating Sirt6/pgc-1alpha/endog signaling. Clin Sci (Lond) (2022) 136(22):1711–30. doi: 10.1042/CS20220242
169. Ke G, Chen X, Liao R, Xu L, Zhang L, Zhang H, et al. Receptor activator of nf-kappab mediates podocyte injury in diabetic nephropathy. Kidney Int (2021) 100(2):377–90. doi: 10.1016/j.kint.2021.04.036
170. Kawahara TL, Michishita E, Adler AS, Damian M, Berber E, Lin M, et al. Sirt6 links histone H3 lysine 9 deacetylation to nf-kappab-dependent gene expression and organismal life span. Cell (2009) 136(1):62–74. doi: 10.1016/j.cell.2008.10.052
171. Osterby R, Gundersen HJ. Glomerular size and structure in diabetes mellitus. I. Early abnormalities. Diabetologia (1975) 11(3):225–9. doi: 10.1007/BF00422326
172. Rahmoune H, Thompson PW, Ward JM, Smith CD, Hong G, Brown J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes (2005) 54(12):3427–34. doi: 10.2337/diabetes.54.12.3427
173. Vallon V, Thomson SC. The tubular hypothesis of nephron filtration and diabetic kidney disease. Nat Rev Nephrol (2020) 16(6):317–36. doi: 10.1038/s41581-020-0256-y
174. Vallon V, Thomson SC. Renal function in diabetic disease models: the tubular system in the pathophysiology of the diabetic kidney. Annu Rev Physiol (2012) 74:351–75. doi: 10.1146/annurev-physiol-020911-153333
175. Vallon V, Rose M, Gerasimova M, Satriano J, Platt KA, Koepsell H, et al. Knockout of Na-glucose transporter Sglt2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol Renal Physiol (2013) 304(2):F156–67. doi: 10.1152/ajprenal.00409.2012
176. Peng W, Zhou X, Xu T, Mao Y, Zhang X, Liu H, et al. Bmp-7 ameliorates partial epithelial-mesenchymal transition by restoring snon protein level via Smad1/5 pathway in diabetic kidney disease. Cell Death Dis (2022) 13(3):254. doi: 10.1038/s41419-022-04529-x
177. Yan R, Wang Y, Shi M, Xiao Y, Liu L, Liu L, et al. Regulation of Pten/Akt/Fak pathways by ppargamma impacts on fibrosis in diabetic nephropathy. J Cell Biochem (2019) 120(5):6998–7014. doi: 10.1002/jcb.27937
178. Bohle A, Mackensen-Haen S, von Gise H, Grund KE, Wehrmann M, Batz C, et al. The consequences of tubulo-interstitial changes for renal function in glomerulopathies. A morphometric and cytological analysis. Pathol Res Pract (1990) 186(1):135–44. doi: 10.1016/S0344-0338(11)81021-6
179. Wang B, Herman-Edelstein M, Koh P, Burns W, Jandeleit-Dahm K, Watson A, et al. E-cadherin expression is regulated by Mir-192/215 by a mechanism that is independent of the profibrotic effects of transforming growth factor-beta. Diabetes (2010) 59(7):1794–802. doi: 10.2337/db09-1736
180. Jones SC, Saunders HJ, Pollock CA. High glucose increases growth and collagen synthesis in cultured human tubulointerstitial cells. Diabetes Med (1999) 16(11):932–8. doi: 10.1046/j.1464-5491.1999.00174.x
181. Cai J, Wang T, Zhou Y, Tang C, Liu Y, Dong Z. Phosphorylation by Gsk-3beta increases the stability of Sirt6 to alleviate Tgf-beta-induced fibrotic response in renal tubular cells. Life Sci (2022) 308:120914. doi: 10.1016/j.lfs.2022.120914
182. Young BA, Johnson RJ, Alpers CE, Eng E, Gordon K, Floege J, et al. Cellular events in the evolution of experimental diabetic nephropathy. Kidney Int (1995) 47(3):935–44. doi: 10.1038/ki.1995.139
183. Giacconi R, Chiodi L, Boccoli G, Costarelli L, Piacenza F, Provinciali M, et al. Reduced levels of plasma selenium are associated with increased inflammation and cardiovascular disease in an italian elderly population. Exp Gerontol (2021) 145:111219. doi: 10.1016/j.exger.2020.111219
184. Derubertis FR, Craven PA. Activation of protein kinase C in glomerular cells in diabetes. Mechanisms and potential links to the pathogenesis of diabetic glomerulopathy. Diabetes (1994) 43(1):1–8. doi: 10.2337/diab.43.1.1
185. Gao T, Li M, Mu G, Hou T, Zhu WG, Yang Y. Pkczeta phosphorylates Sirt6 to mediate fatty acid beta-oxidation in colon cancer cells. Neoplasia (2019) 21(1):61–73. doi: 10.1016/j.neo.2018.11.008
186. Tang PM, Nikolic-Paterson DJ, Lan HY. Macrophages: versatile players in renal inflammation and fibrosis. Nat Rev Nephrol (2019) 15(3):144–58. doi: 10.1038/s41581-019-0110-2
187. Li HD, You YK, Shao BY, Wu WF, Wang YF, Guo JB, et al. Roles and crosstalks of macrophages in diabetic nephropathy. Front Immunol (2022) 13:1015142. doi: 10.3389/fimmu.2022.1015142
188. Huang S, Shao T, Liu H, Wang Q, Li T, Zhao Q. Sirt6 mediates Mrtf-a deacetylation in vascular endothelial cells to antagonize Oxldl-induced Icam-1 transcription. Cell Death Discovery (2022) 8(1):96. doi: 10.1038/s41420-022-00903-y
189. Xu S, Yin M, Koroleva M, Mastrangelo MA, Zhang W, Bai P, et al. Sirt6 protects against endothelial dysfunction and atherosclerosis in mice. Aging (Albany NY) (2016) 8(5):1064–82. doi: 10.18632/aging.100975
190. Katsiki N, Anagnostis P, Kotsa K, Goulis DG, Mikhailidis DP. Obesity, metabolic syndrome and the risk of microvascular complications in patients with diabetes mellitus. Curr Pharm Des (2019) 25(18):2051–9. doi: 10.2174/1381612825666190708192134
191. Wu L, Liu C, Chang DY, Zhan R, Zhao M, Man Lam S, et al. The attenuation of diabetic nephropathy by annexin A1 via regulation of lipid metabolism through the Ampk/Pparalpha/Cpt1b Pathway. Diabetes (2021) 70(10):2192–203. doi: 10.2337/db21-0050
192. Jiang T, Wang Z, Proctor G, Moskowitz S, Liebman SE, Rogers T, et al. Diet-induced obesity in C57bl/6j mice causes increased renal lipid accumulation and glomerulosclerosis via a sterol regulatory element-binding protein-1c-dependent pathway. J Biol Chem (2005) 280(37):32317–25. doi: 10.1074/jbc.M500801200
193. Schelling JR. The contribution of lipotoxicity to diabetic kidney disease. Cells (2022) 11(20):3236. doi: 10.3390/cells11203236
194. Yamamoto T, Takabatake Y, Takahashi A, Kimura T, Namba T, Matsuda J, et al. High-fat diet-induced lysosomal dysfunction and impaired autophagic flux contribute to lipotoxicity in the kidney. J Am Soc Nephrol (2017) 28(5):1534–51. doi: 10.1681/ASN.2016070731
195. Kuwahara S, Hosojima M, Kaneko R, Aoki H, Nakano D, Sasagawa T, et al. Megalin-mediated tubuloglomerular alterations in high-fat diet-induced kidney disease. J Am Soc Nephrol (2016) 27(7):1996–2008. doi: 10.1681/ASN.2015020190
196. Yoo TH, Pedigo CE, Guzman J, Correa-Medina M, Wei C, Villarreal R, et al. Sphingomyelinase-like phosphodiesterase 3b expression levels determine podocyte injury phenotypes in glomerular disease. J Am Soc Nephrol (2015) 26(1):133–47. doi: 10.1681/ASN.2013111213
197. Mitrofanova A, Mallela SK, Ducasa GM, Yoo TH, Rosenfeld-Gur E, Zelnik ID, et al. Smpdl3b modulates insulin receptor signaling in diabetic kidney disease. Nat Commun (2019) 10(1):2692. doi: 10.1038/s41467-019-10584-4
198. Wu M, Yang Z, Zhang C, Shi Y, Han W, Song S, et al. Inhibition of Nlrp3 inflammasome ameliorates podocyte damage by suppressing lipid accumulation in diabetic nephropathy. Metabolism (2021) 118:154748. doi: 10.1016/j.metabol.2021.154748
199. He J, Deng Y, Ren L, Jin Z, Yang J, Yao F, et al. Isoliquiritigenin from licorice flavonoids attenuates Nlrp3-mediated pyroptosis by Sirt6 in vascular endothelial cells. J Ethnopharmacol (2023) 303:115952. doi: 10.1016/j.jep.2022.115952
200. Cai T, Ke Q, Fang Y, Wen P, Chen H, Yuan Q, et al. Sodium-glucose cotransporter 2 inhibition suppresses hif-1alpha-mediated metabolic switch from lipid oxidation to glycolysis in kidney tubule cells of diabetic mice. Cell Death Dis (2020) 11(5):390. doi: 10.1038/s41419-020-2544-7
201. Yang M, Luo S, Yang J, Chen W, He L, Liu D, et al. Lipid droplet - mitochondria coupling: A novel lipid metabolism regulatory hub in diabetic nephropathy. Front Endocrinol (Lausanne) (2022) 13:1017387. doi: 10.3389/fendo.2022.1017387
202. Wilfling F, Haas JT, Walther TC, Farese RV Jr. Lipid droplet biogenesis. Curr Opin Cell Biol (2014) 29:39–45. doi: 10.1016/j.ceb.2014.03.008
203. Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, et al. Autophagy regulates lipid metabolism. Nature (2009) 458(7242):1131–5. doi: 10.1038/nature07976
204. Jaishy B, Abel ED. Lipids, lysosomes, and autophagy. J Lipid Res (2016) 57(9):1619–35. doi: 10.1194/jlr.R067520
205. Han Y, Xiong S, Zhao H, Yang S, Yang M, Zhu X, et al. Lipophagy deficiency exacerbates ectopic lipid accumulation and tubular cells injury in diabetic nephropathy. Cell Death Dis (2021) 12(11):1031. doi: 10.1038/s41419-021-04326-y
206. Liu H, Wang S, Gong L, Shen Y, Xu F, Wang Y, et al. Sirt6 ameliorates Lps-induced apoptosis and tight junction injury in ards through the Erk1/2 pathway and autophagy. Int J Med Sci (2023) 20(5):581–94. doi: 10.7150/ijms.80920
207. Wang Z, Liang Y, Zhang L, Zhang N, Liu Q, Wang Z. Phosphodiesterase 4 inhibitor activates Ampk-Sirt6 pathway to prevent aging-related adipose deposition induced by metabolic disorder. Aging (Albany NY) (2018) 10(9):2394–406. doi: 10.18632/aging.101559
208. Tanaka Y, Kume S, Araki S, Isshiki K, Chin-Kanasaki M, Sakaguchi M, et al. Fenofibrate, a pparalpha agonist, has renoprotective effects in mice by enhancing renal lipolysis. Kidney Int (2011) 79(8):871–82. doi: 10.1038/ki.2010.530
209. Mishra R, Simonson MS. Saturated free fatty acids and apoptosis in microvascular mesangial cells: palmitate activates pro-apoptotic signaling involving caspase 9 and mitochondrial release of endonuclease G. Cardiovasc Diabetol (2005) 4:2. doi: 10.1186/1475-2840-4-2
210. Park MJ, Han HJ, Kim DI. Lipotoxicity-induced Prmt1 exacerbates mesangial cell apoptosis via endoplasmic reticulum stress. Int J Mol Sci (2017) 18(7):1421. doi: 10.3390/ijms18071421
211. Tan HY, Wan C, Wu GL, Qiao LJ, Cai YF, Wang Q, et al. Taohong siwu decoction ameliorates cognitive dysfunction through Sirt6/Er stress pathway in alzheimer's disease. J Ethnopharmacol (2023) 314:116580. doi: 10.1016/j.jep.2023.116580
212. Peng D, Xia Q, Guan L, Li HY, Qiao LJ, Chen YB, et al. Carnosine improves cognitive impairment through promoting Sirt6 expression and inhibiting endoplasmic reticulum stress in a diabetic encephalopathy model. Rejuvenation Res (2022) 25(2):79–88. doi: 10.1089/rej.2022.0002
213. Jiang WJ, Xu CT, Du CL, Dong JH, Xu SB, Hu BF, et al. Tubular epithelial cell-to-macrophage communication forms a negative feedback loop via extracellular vesicle transfer to promote renal inflammation and apoptosis in diabetic nephropathy. Theranostics (2022) 12(1):324–39. doi: 10.7150/thno.63735
214. Wang X, Ji T, Li X, Qu X, Bai S. Foxo3a protects against kidney injury in type ii diabetic nephropathy by promoting Sirt6 expression and inhibiting smad3 acetylation. Oxid Med Cell Longev (2021) 2021:5565761. doi: 10.1155/2021/5565761
215. Muraoka H, Hasegawa K, Sakamaki Y, Minakuchi H, Kawaguchi T, Yasuda I, et al. Role of Nampt-Sirt6 axis in renal proximal tubules in extracellular matrix deposition in diabetic nephropathy. Cell Rep (2019) 27(1):199–212 e5. doi: 10.1016/j.celrep.2019.03.024
216. Liu J, Duan P, Xu C, Xu D, Liu Y, Jiang J. Circrna circ-itch improves renal inflammation and fibrosis in streptozotocin-induced diabetic mice by regulating the Mir-33a-5p/Sirt6 axis. Inflammation Res (2021) 70(7):835–46. doi: 10.1007/s00011-021-01485-8
217. Ji L, Chen Y, Wang H, Zhang W, He L, Wu J, et al. Overexpression of Sirt6 promotes M2 macrophage transformation, alleviating renal injury in diabetic nephropathy. Int J Oncol (2019) 55(1):103–15. doi: 10.3892/ijo.2019.4800
218. Jin Z, Xiao Y, Yao F, Wang B, Zheng Z, Gao H, et al. Sirt6 inhibits cholesterol crystal-induced vascular endothelial dysfunction via Nrf2 activation. Exp Cell Res (2020) 387(1):111744. doi: 10.1016/j.yexcr.2019.111744
219. He T, Shang J, Gao C, Guan X, Chen Y, Zhu L, et al. A novel Sirt6 activator ameliorates neuroinflammation and ischemic brain injury via Ezh2/Foxc1 axis. Acta Pharm Sin B (2021) 11(3):708–26. doi: 10.1016/j.apsb.2020.11.002
220. Iachettini S, Trisciuoglio D, Rotili D, Lucidi A, Salvati E, Zizza P, et al. Pharmacological activation of Sirt6 triggers lethal autophagy in human cancer cells. Cell Death Dis (2018) 9(10):996. doi: 10.1038/s41419-018-1065-0
221. Jiao F, Zhang Z, Hu H, Zhang Y, Xiong Y. Sirt6 activator Ubcs039 inhibits thioacetamide-induced hepatic injury in vitro and in vivo. Front Pharmacol (2022) 13:837544. doi: 10.3389/fphar.2022.837544
222. Jiang C, Sun ZM, Hu JN, Jin Y, Guo Q, Xu JJ, et al. Cyanidin ameliorates the progression of osteoarthritis via the Sirt6/nf-kappab axis in vitro and in vivo. Food Funct (2019) 10(9):5873–85. doi: 10.1039/c9fo00742c
223. Fiorentino F, Carafa V, Favale G, Altucci L, Mai A, Rotili D. The two-faced role of Sirt6 in cancer. Cancers (Basel) (2021) 13(5):1156. doi: 10.3390/cancers13051156
224. Wang Z, Wu Q, Wang H, Gao Y, Nie K, Tang Y, et al. Diosgenin Protects against Podocyte Injury in Early Phase of Diabetic Nephropathy through Regulating Sirt6. Phytomedicine (2022) 104:154276. doi: 10.1016/j.phymed.2022.154276
225. Oh H, Cho W, Park SY, Abd El-Aty AM, Jeong JH, Jung TW. Ginsenoside Rb3 ameliorates podocyte injury under hyperlipidemic conditions via ppardelta- or Sirt6-mediated suppression of inflammation and oxidative stress. J Ginseng Res (2023) 47(3):400–7. doi: 10.1016/j.jgr.2022.11.006
226. Zhang X, Zhao L, Xiang S, Sun Y, Wang P, Chen JJ, et al. Yishen tongluo formula alleviates diabetic kidney disease through regulating Sirt6/Tgf-Beta1/Smad2/3 pathway and promoting degradation of Tgf-Beta1. J Ethnopharmacol (2023) 307:116243. doi: 10.1016/j.jep.2023.116243
227. Wu R, Jian T, Ding X, Lv H, Meng X, Ren B, et al. Total sesquiterpene glycosides from loquat leaves ameliorate Hfd-induced insulin resistance by modulating Irs-1/Glut4, Trpv1, and Sirt6/Nrf2 signaling pathways. Oxid Med Cell Longev (2021) 2021:4706410. doi: 10.1155/2021/4706410
228. Karalliedde J, Gnudi L. Diabetes mellitus, a complex and heterogeneous disease, and the role of insulin resistance as a determinant of diabetic kidney disease. Nephrol Dial Transplant (2016) 31(2):206–13. doi: 10.1093/ndt/gfu405
Keywords: diabetic nephropathy, SIRT6, glucose metabolism, lipid metabolism, treatment
Citation: Wang Y, Liu T, Cai Y, Liu W and Guo J (2023) SIRT6’s function in controlling the metabolism of lipids and glucose in diabetic nephropathy. Front. Endocrinol. 14:1244705. doi: 10.3389/fendo.2023.1244705
Received: 23 June 2023; Accepted: 21 September 2023;
Published: 09 October 2023.
Edited by:
Yao-Wu Liu, Xuzhou Medical University, ChinaReviewed by:
Peng Gao, Army Medical University, ChinaZhi Wang, Huazhong University of Science and Technology, China
Copyright © 2023 Wang, Liu, Cai, Liu and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weijing Liu, bGl1d2VpamluZy0xOTc3QGhvdG1haWwuY29t; Jing Guo, NTEyNDkxNDY3QHFxLmNvbQ==
 Ying Wang
Ying Wang Tongtong Liu
Tongtong Liu Yuzi Cai
Yuzi Cai Weijing Liu
Weijing Liu Jing Guo
Jing Guo