
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 19 June 2023
Sec. Obesity
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1188487
This article is part of the Research Topic Gut Microbiota, Obesity, and Associated Metabolic Disorders: Quo Vadis? View all 7 articles
A commentary has been posted on this article:
Commentary: Association between Helicobacter pylori infection and metabolic syndrome and its components
Background and aim: The association between Helicobacter pylori (H. pylori) infection and metabolic syndrome (MetS) has been studied previously; however, the results remain controversial, which could be partly due to the different criteria used for defining MetS. We adopted five MetS criteria to provide better understanding of the association between H. pylori infection and MetS.
Methods: Physical examination data of 100,708 subjects were obtained from January 2014 to December 2018. MetS was defined based on five criteria including: International Diabetes Federation (IDF), The Third Report of the National Cholesterol Education Program Expert Panel, Adult Treatment Panel III (ATP III), Joint Statement of International Multi-Societies (JIS), Chinese Diabetes Society (CDS), and the Guidelines for the Prevention and Treatment of Type 2 Diabetes in China (2017 edition)(CDS DM). Multivariate logistic regression analysis was performed to elucidate the association between H. pylori infection and MetS and its components.
Results: The prevalence of MetS defined assessed using IDF, ATP III, JIS, CDS and CDS DM criteria was 15.8%, 19.9%, 23.7%, 8.7% and 15.4%, respectively. In males, the prevalence of MetS assessed using the five criteria in H. pylori-positive group was higher than that in negative-group; however, in females, same results were obtained using the three international criteria. In males, the prevalence of all MetS components was found to be higher in the H. pylori-positive group than those in the negative group; however, in females, only the prevalence of dyslipidemia and waist circumferences exhibited significant differences. Multivariate logistic regression analysis revealed that H. pylori infection in males was positively correlated with MetS. Additionally, H. pylori infection was found to be positively correlated with the waist circumference in the general population, and with hypertension and hyperglycemia in males.
Conclusions: H. pylori infection was found to be positively associated with MetS in males in China.
Metabolic syndrome (MetS) is a major health issue in the contemporary society. It is reported that about 20-25% of adults worldwide suffer from MetS, which is a syndrome associated with the accumulation of multiple metabolic risk factors in individuals (1). Although MetS is an outcome of the interaction between multiple genetic and environmental risk factors, insulin resistance (IR) is considered as the key factor associated with MetS (2). The disorders of glucose, lipid, and protein metabolism are all associated with IR and eventually lead to obesity, cardiovascular disease, non-alcoholic fatty liver, and other related metabolic diseases (3). Therefore, Stern (4) proposed the “common soil” theory to explain the basic role of IR and effects of IR on multiple organs and systems, and evealing the factors that promote IR will help to understand the mechanism of MetS formation. In recent years, the accumulating evidence indicates that gastrointestinal microbes play a significant role in IR development (5). Moreover, previous studies have revealed that gut microbes are associated with MetS components such as diabetes, hypertension, and obesity (6–8), which would link gastrointestinal microbes to the development of MetS.
Helicobacter pylori (H. pylori), as an important and widely concerned gastrointestinal microbe, has a high infection rate worldwide. The infection rate of H. pylori is considerably high in developing countries, and up to 50% in China (9). In addition to the well-known association with chronic gastritis, gastric ulcer, and gastric cancer, growing attention has been paid to the extra-gastrointestinal effects of H. pylori infection, which has been found to impaired insulin signaling in hepatocytes, and increase the expression of a variety of inflammatory factors involved in IR development that has been related to MetS (10, 11). Based on this potential pathogenesis link mentioned above, researchers are interested in exploring the correlation between H. pylori infection and MetS and its components.
Some studies have attempted to describe the possible correlation between H. pylori infection and MetS, but the results are inconsistent and conflicting (12–15). Polyzos et al. indicated that the inconsistency of the results may be partly attributed to the different criteria used for defining MetS and different methods used for the detection of H. pylori infection (16). As known, different criteria with different parameters were developed to identify MetS, such as International Diabetes Federation (IDF) (17), Third Report of the National Cholesterol Education Program Expert Panel, Adult Treatment Panel III (ATP III) (18), Joint Statement of International Multi-Societies (JIS) (19), Chinese Diabetes Society (CDS) (20), Guidelines for the Prevention and Treatment of Type 2 Diabetes in China (2017 edition) (CDS DM) (21). Meanwhile, the methods for detecting H. pylori infection vary among different studies, including the specific antibody of H. pylori in serum and 13C urea breath test (UBT). When discussing the association between H. pylori infection and MetS, it is worth investigating whether applying different criteria for MetS would affect the results. In our study, H. pylori infection was determined using the UBT, which is highly recommended to conduct non-invasive screening of H. pylori, and MetS was defined using different international and Chinese criteria mentioned-above that are widely accepted. Using this strategy, we aimed to improve our understanding on the association between H. pylori infection and MetS and its components after excluding the influence of differing criteria.
This was a retrospective cross-sectional study conducted at the Health Management Center of Sichuan Provincial People’s Hospital (Chengdu, China) from January 2014 to December 2018. The subjects were categorized based on their sex. The differences in the prevalence of MetS and its components were compared between the H. pylori-positive and -negative groups. Multivariate logistic regression analysis was performed to elucidate the association between H. pylori infection and MetS and its components.
All subjects were asked to complete a medical history questionnaire, followed by physical examination (height, body weight, blood pressure, circumference of waist, hip, and neck), and laboratory examination (blood routine test, liver and kidney function, fasting plasma glucose [FPG], hemoglobin A1c [HbA1c], uric acid [UA], total cholesterol [TC], triglycerides [TG], low-density lipoprotein cholesterol [LDL-C], high-density lipoprotein cholesterol [HDL-C]), abdominal color Doppler ultrasound, chest imaging (X-ray or CT), and 13C urea breath tests (UBT).
Subjects were excluded if they exhibited the following: (a) history of gastrectomy or subtotal gastrectomy; (b) unable to perform 13C urea breath test due to pregnancy, lactation or other reasons; (c) immune system diseases, severe heart, liver and kidney dysfunction, and tumor patients; (d) history of anti-H. pylori therapy in the past three months.
Five criteria were used to define MetS, including IDF, ATP III, JIS, CDS, and CDS DM. In IDF, ATP III and JIS criteria, the standards applicable to Chinese were adopted, as shown in Table 1.
H. pylori infection was determined using the 13C urea breath tests (Beijing Boran Pharmaceutical Co., Ltd., Beijing, China). We followed a standardized procedure for the sample collection. All subjects fasted overnight for more than 8 h, maintained normal breath, inserted the straw into the bottom of one sample tube, and exhaled slowly into the sample tube through the straw for 4 to 5 s. Thereafter, they pulled the straw out, tightened the cap immediately, and this was considered as the 0 min sample. Then, the subjects took another bottle with urea 13C granules and 80 to 100 mL cold drinking water, rested for 30 min, and then collected the breath sample again. The two collected gas samples were tested for 13CO2, and δ‰ was used to represent the determination result. δ‰ = (isotopic abundance of 13C for the test sample ─ isotopic abundance of 13C for reference sample) × 1000/isotopic abundance 13C for reference sample. The detection value was defined as δ‰ measured at 30 min subtracted from that measured at 0 min. H. pylori infection was considered positive when the detection value was ≥ 4.0.
IBM SPSS (version 21.0; IBM Corp., NY, USA) was used to perform the statistical analysis. Continuous data are expressed as mean ± standard deviation (SD) for normally distributed data and median with 25th and 75th percentiles for non-normally distributed data. Categorical data are expressed as percentages. The significant differences were evaluated using either the Student’s t-test (for continuous variables) or the chi-square test (for categorical variables). Significant and independent predictors of MetS and its components were identified using the multivariable logistic regression analysis. The models were generated wherein the covariates included age, drinking, smoking (Model 1), or Model 1 plus ALT, AST, GGT, creatinine (Model 2), and modeling was performed using the backward stepwise selection methods of variables. The results are expressed as odds ratios (ORs) and 95% confidence intervals (CIs). Statistical significance was set at p < 0.05.
The data of 100,708 subjects (56,301 males and 44,407 females) were obtained in this study, with an average age of 44.76 ± 12.69 years, ranging between 18 to 95 years. The prevalence of MetS according to IDF, ATP III, JIS, CDS and CDS DM criteria was 15.8% (18.7% in males and 12.1% in females), 19.9% (24.3% in males and 14.3% in females), 23.7% (31.2% in males and 14.3% in females), 8.7% (12.0% in males and 4.4% in females), and 15.4% (22.5% in males and 6.4% in females), respectively. The overall infection rate of H. pylori was 39.6%, which was higher in males than in females (40.8% vs. 38.2%, p < 0.001). These results are presented in Table 2. The overlap of MetS defined using the five criteria was shown in Figure 1.
The subjects were stratified based on their age. Among males and females, the prevalence of H. pylori infection increased with age and reached the peak in the age group of 50-59 years; however, it somehow declined in individuals after 60 years of age. The prevalence of H. pylori infection in males was significantly higher than that in females, except in the age group of 40-49 years. Under the five criteria used for defining MetS, the males exhibited a peak prevalence of MetS in the age group of 50-59 years, which also decreased after 60 years of age; however, in females, it continued to increase with age. The prevalence of MetS in males was higher than that in females before 60 years old (IDF, ATP III and JIS criteria) or 70 years old (CDS and CDS DM criteria), and then lowered compared to females; the difference was statistically significant, as shown in Table 3.
In males, the prevalence of MetS defined using the five criteria in H. pylori-positive group was higher than that in the negative group (19.9% vs. 17.9% for IDF criteria; 25.8% vs. 23.3% for ATP III criteria; 32.8% vs. 30.1% for JIS criteria; 12.9% vs. 11.5% for CDS criteria; 23.9% vs. 21.6% for CDS DM criteria, all p < 0.001). In females, the prevalence of MetS defined using the three international criteria in the H. pylori-positive group was higher than that in the negative group (12.8% vs. 11.6% for IDF criteria, p < 0.001; 15.0% vs. 13.9% for ATP III and JIS criteria, p = 0.003), but no significant differences were observed upon using the two Chinese criteria (4.5% vs. 4.3% for CDS criteria, p = 0.180; 6.6% vs. 6.3% for CDS DM criteria, p = 0.343), as shown in Figure 2.
The prevalence of MetS components under different H. pylori status was also investigated. The results revealed that in males, the prevalence of different components defined using the five criteria of MetS in H. pylori-positive group was significantly higher than that in the negative group (all p < 0.05). However, in females, the prevalence of excess waist circumference, overweight and dyslipidemia (except the CDS criteria) was higher in the H. pylori-positive group than that in the negative group (all p < 0.05), but no significant differences were observed in the prevalence of hypertension and hyperglycemia (all p > 0.05), as shown in Figure 3.

Figure 3 The prevalence of components of MetS under different H. pylori status. Footnote: The letters in brackets of the abscissa item name represent different criteria of MetS: A. IDF criteria; B. ATP III criteria; C. JIS criteria; D. CDS criteria; E. CDS DM criteria.
Univariate logistic regression analysis was performed with MetS defined using the five criteria as the dependent variable. The results revealed that H. pylori infection is a potential risk factor associated with MetS defined using the five criteria in males and the three international criteria in females. After adjusting for age, smoking, and drinking, the OR for MetS remained significant in males but no longer in females except for the IDF criteria. Furthermore, upon adjusting for ALT, AST, GGT and creatinine, the OR for MetS remained significant in males, but H. pylori infection was no longer associated with the risk of MetS defined using all the five criteria in females, as shown in Table 4.
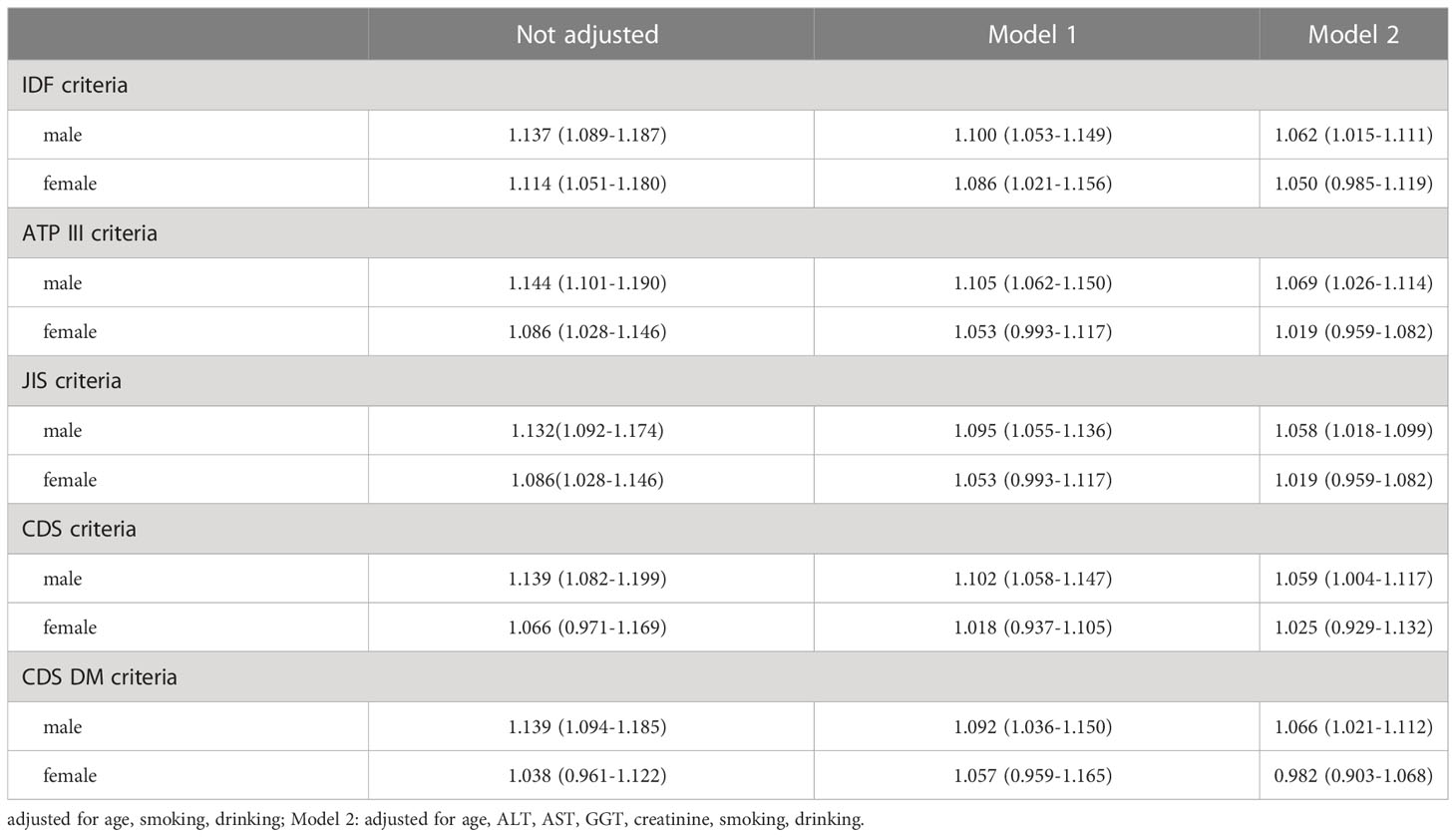
Table 4 Logistic regression analysis of the effect of H. pylori infection on MetS defined by different criteria [OR (95% CI)].
The association between H. pylori infection and the components of MetS was also investigated using the multivariate logistic regression analysis. After adjusting for age, smoking, drinking, ALT, AST, GGT and creatinine, the results revealed that using the five criteria of MetS, H. pylori infection was identified as a risk factor associated with excess waist circumference and BMI in both males and females. H. pylori infection was also identified as a risk factor associated with hypertension and hyperglycemia in males, but not in females. No association was identified between H. pylori infection and dyslipidemia in both males and females, as shown in Table 5.
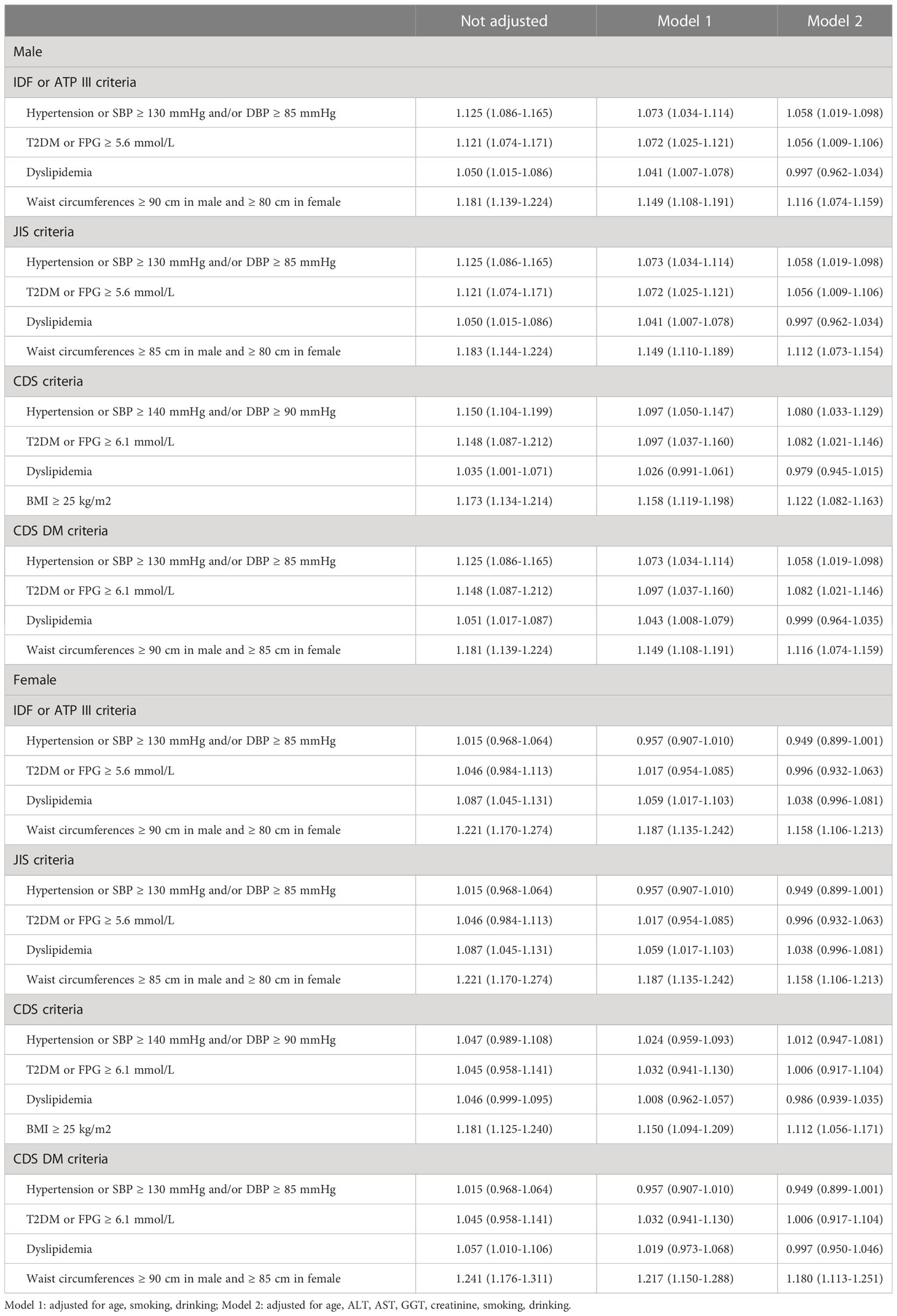
Table 5 Logistic regression analysis of the effect of H. pylori infection on the components of MetS defined by different criteria [OR (95% CI)].
H. pylori infection and MetS are two important public health issues in the contemporary society. Some researchers attempted to discussed the correlation between these two, but the results are inconsistent and conflicting. Gunji et al. investigated the data of 7,394 Japanese subjects and suggested that there was a significant and independent correlation between the positive rate of serum H. pylori antibody and MetS (12). Lim et al. conducted an international multicenter study including 15,195 Korean subjects, and suggested that H. pylori infection plays an independent role in the development of MetS in Korean people under 65 years of age (15). However, inconsistent results have also been reported. Takeoka et al. investigated 1,044 Japanese subjects, and found no significant association between H. pylori-seropositive status and the risk of MetS upon adjusting for age and sex (14). Naja et al. reported no association between H. pylori seropositivity and MetS or IR among 308 Lebanese adults (13). The inconsistency in the results was thought to be partly due to the different criteria used for defining MetS (16).
In our study, five MetS criteria were used for the analysis to avoid the impact of using different criteria on the results. We found that in males, the prevalence of MetS defined using the five criteria was higher in the H. pylori-positive group than in the negative group. In females, the prevalence of MetS defined using the three international criteria in H. pylori-positive group was higher than that in the negative group, but the differences were not significant using the two Chinese criteria. According to the multivariate regression analysis upon adjusting for age, smoking, drinking, ALT, AST, GGT and creatinine, we found that H. pylori positivity was still a risk factor associated with MetS defined using the five criteria in males.
However, the results based on gender differences in our study were different from those of previous similar studies, which suggest that H. pylori infection increased the risk of MetS in females (22, 23). We further analyzed the relationship between H. pylori infection and different MetS components. The results of the multivariate regression analysis revealed that H. pylori positivity was a risk factor associated with excess waist circumference and BMI in both males and females upon using the five criteria for defining MetS. Furthermore, H. pylori positivity was also identified as a risk factor associated with hypertension and hyperglycemia in males, but not in females. No correlation was identified between H. pylori positivity and dyslipidemia in either males or females.
As well known, IR forms the pathophysiological basis of MetS, and growing evidences have shown that H. pylori infection is associated with IR (24, 25). Some researchers thought that H. pylori infection caused IR through insulin dysfunction in the liver, which is an important target organ for insulin, via the c-Jun/microRNA203/suppressor of cytokine signaling 3 pathway (11). Additionally, the immune response to H. pylori infection has also been shown to increase the levels of some proinflammatory cytokines, such as IL-1, IL-6, and TNFa, which causes the phosphorylation of serine/threonine residues on the insulin receptor, disrupt the activation of the receptor, and eventually reduce insulin sensitivity (10, 26). Furthermore, leptin, ghrelin, fetuin A and monocyte chemoattractant protein-1 (MCP-1) are related to the IR process. During H. pylori infection, the intestinal hormones and protein molecules mentioned above exhibited the corresponding changes in their levels that contributed in the process of IR promotion (27–30). H. pylori infection also induced the production of reactive oxygen species (ROS) and platelet activation, which plays a critical role in IR development (16). Therefore, we hypothesized that H. pylori infection may participate in the development of MetS through IR promotion as the underlying mechanism. The improvement of waist circumference, fasting blood glucose, glycosylated hemoglobin, and high-density lipoprotein in MetS patients after radical treatment of H. pylori infection also supported its participation in the process of MetS development (31).
IR is also a mechanism underlying the development of hyperglycemia. However, it is worth noting that in our study, we found a correlation between hyperglycemia and H. pylori infection only in males. Previous studies have demonstrated gender differences in IR and suggested that females exhibited less insulin resistance (32, 33). Androgen itself appeared to contribute to IR development (34). This difference in IR between the males and females would be further reflected through the effect on serum glucose levels.
Similarly, there was a clear gender difference in the correlation between H. pylori infection and hypertension. Moreover, the prevalence of hypertension was different between males and females, and males more likely developed hypertension (35). As for the mechanisms that would link H. pylori infection to hypertension, it was thought to be related to the increase in the levels of many inflammatory factors induced upon H. pylori infection. These inflammatory factors are considered to play important roles in the pathogenesis of atherosclerosis and reduce the elasticity of blood vessels (36). IR itself has also been reported to increase the peripheral blood pressure (37). Animal experiments demonstrated the association of hyperinsulinemia and insulin resistance with hypertension in male only (38). Therefore, the correlation between IR and hypertension appears to be sex-dependent, and more attention should be paid to the improvement of hypertension and hyperglycemia in males upon H. pylori infection.
In our study, the results of waist circumference and BMI suggested a correlation between obesity and H. pylori infection in both males and females. This might be different from hypertension and hyperglycemia, which mainly occur as a result of IR, and the relationship between IR and obesity was more complex, for being a mutual cause and effect scenario (39). IR occurred as long as obesity was present, while hypertension and hyperglycemia required IR to reach a certain level. This difference in the cause-and-effect relationship might also be observed in case of various metabolic disorders caused by IR due to H. pylori infection.
Dyslipidemia is closely related to IR. Previous studies have suggested that H. pylori infection may cause dyslipidemia (40), and indicated that H. pylori infection was significantly and independently associated with dyslipidemia (41). However, conflicting results are also present (42). In our study, we did not find a correlation between H. pylori infection and dyslipidemia in both males and females. Whether there is a correlation between H. pylori infection and dyslipidemia remains to be elucidated yet.
The limitation of our study was that the subjects were from the health examination population rather than from random sampling of the community, which led to sample deviation, but this was somewhat compensated through the inclusion of relatively large number of subjects. Second, our study lacked socioeconomic data, which might have led to the exclusion of confounding factors. However, the prevalence of H. pylori was similar to that reported in a previous study (22), and the bias might not be significant. Moreover, this cross-sectional study might only reflect the association between H. pylori infection and MetS, rather than causality. The pathogenic mechanisms of H. pylori infection and MetS are diverse and complex. Moreover, MetS is considered as a chronic inflammatory or proinflammatory state, and cytokines induced upon H. pylori infection have also been observed in MetS (43). Even in the process of MetS caused due to H. pylori infection, MetS itself or its components could be correlated to the status of H. pylori infection through multiple mechanisms. This may lead to an interaction or even a vicious circle between them, thereby increasing the complexity of the result determination. Only through the study of specific biological mechanism, we may effectively elucidate the relationship between H. pylori infection and MetS and its components.
Conclusively, our study suggests that H. pylori infection is associated with MetS in males. Additionally, H. pylori infection was found to be correlated with MetS components (such as hyperglycemia, hypertension, obesity, etc.) in males. As a warning, it might be needed for males with H. pylori infection to pay greater attention to weight control and proper diet. However, these results need to be verified further through more clinical studies, especially cohort ones with high sample sizes. Authoritative and credible research conclusions may provide a new strategy for treating MetS in males.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics committees of the Sichuan Provincial People’s Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
YiL and YuL designed the study. YiL, WC and DL wrote the manuscript. YiL and PS performed the data collection and analysis. YuL assisted in data collection and document writing. All authors read and approved the manuscript.
This work was supported by Science & Technology Department of Sichuan Province (grant number 2020YFS0557).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Tanner RM, Brown TM, Muntner P. Epidemiology of obesity, the metabolic syndrome, and chronic kidney disease. Curr Hypertens Rep (2012) 14(2):152–9. doi: 10.1007/s11906-012-0254-y
2. Grundy SM, Hansen B, Smith SC Jr., Cleeman JI, Kahn RA, American Heart A, et al. Clinical management of metabolic syndrome: report of the American heart Association/National heart, lung, and blood Institute/American diabetes association conference on scientific issues related to management. Arterioscler Thromb Vasc Biol (2004) 24(2):e19–24. doi: 10.1161/01.ATV.0000112379.88385.67
3. Jornayvaz FR, Samuel VT, Shulman GI. The role of muscle insulin resistance in the pathogenesis of atherogenic dyslipidemia and nonalcoholic fatty liver disease associated with the metabolic syndrome. Annu Rev Nutr (2010) 30:273–90. doi: 10.1146/annurev.nutr.012809.104726
4. Stern MP. Diabetes and cardiovascular disease. the “common soil” hypothesis. Diabetes (1995) 44(4):369–74. doi: 10.2337/diab.44.4.369
5. Shen J, Obin MS, Zhao L. The gut microbiota, obesity and insulin resistance. Mol Aspects Med (2013) 34(1):39–58. doi: 10.1016/j.mam.2012.11.001
6. Gerard P. Gut microbiota and obesity. Cell Mol Life Sci (2016) 73(1):147–62. doi: 10.1007/s00018-015-2061-5
7. Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome (2017) 5(1):14. doi: 10.1186/s40168-016-0222-x
8. Wu H, Tremaroli V, Schmidt C, Lundqvist A, Olsson LM, Kramer M, et al. The gut microbiota in prediabetes and diabetes: a population-based cross-sectional study. Cell Metab (2020) 32(3):379–390.e373. doi: 10.1016/j.cmet.2020.06.011
9. Nagy P, Johansson S, Molloy-Bland M. Systematic review of time trends in the prevalence of helicobacter pylori infection in China and the USA. Gut Pathog (2016) 8:8. doi: 10.1186/s13099-016-0091-7
10. Polyzos SA, Kountouras J, Zavos C, Deretzi G. The association between helicobacter pylori infection and insulin resistance: a systematic review. Helicobacter (2011) 16(2):79–88. doi: 10.1111/j.1523-5378.2011.00822.x
11. Zhou X, Liu W, Gu M, Zhou H, Zhang G. Helicobacter pylori infection causes hepatic insulin resistance by the c-Jun/miR-203/SOCS3 signaling pathway. J Gastroenterol (2015) 50(10):1027–40. doi: 10.1007/s00535-015-1051-6
12. Gunji T, Matsuhashi N, Sato H, Fujibayashi K, Okumura M, Sasabe N, et al. Helicobacter pylori infection is significantly associated with metabolic syndrome in the Japanese population. Am J Gastroenterol (2008) 103(12):3005–10. doi: 10.1111/j.1572-0241.2008.02151.x
13. Naja F, Nasreddine L, Hwalla N, Moghames P, Shoaib H, Fatfat M, et al. Association of h. pylori infection with insulin resistance and metabolic syndrome among Lebanese adults. Helicobacter (2012) 17(6):444–51. doi: 10.1111/j.1523-5378.2012.00970.x
14. Takeoka A, Tayama J, Yamasaki H, Kobayashi M, Ogawa S, Saigo T, et al. Impact of helicobacter pylori immunoglobulin G levels and atrophic gastritis status on risk of metabolic syndrome. PloS One (2016) 11(11):e0166588. doi: 10.1371/journal.pone.0166588
15. Lim SH, Kim N, Kwon JW, Kim SE, Baik GH, Lee JY, et al. Positive association between helicobacter pylori infection and metabolic syndrome in a Korean population: a multicenter nationwide study. Dig Dis Sci (2019) 64(8):2219–30. doi: 10.1007/s10620-019-05544-3
16. Polyzos SA, Kountouras J. Novel advances in the association between helicobacter pylori infection, metabolic syndrome, and related morbidity. Helicobacter (2015) 20(6):405–9. doi: 10.1111/hel.12228
17. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome–a new world-wide definition. a consensus statement from the international diabetes federation. Diabetes Med (2006) 23(5):469–80. doi: 10.1111/j.1464-5491.2006.01858.x
18. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome. an American heart Association/National heart, lung, and blood institute scientific statement. executive summary. Cardiol Rev (2005) 13(6):322–7. doi: 10.1161/CIRCULATIONAHA.105.169404
19. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation (2009) 120(16):1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644
20. Metabolic syndrome study cooperation group of Chinese diabetes society. Chinese Diabetes society suggestions for defnition of metabolic syndrome. Chin J Diabetes (2004) 12:156–61. doi: 10.3321/j.issn:1006-6187.2004.03.002
21. Chinese diabetes society. The guidelines for the prevention and treatment of type 2 diabetes in china, (2017 Edition). Chin J Diabetes (2018) 10:4–67. doi: 10.3760/cma.j.issn.1674-5809.2018.01.003
22. Chen TP, Hung HF, Chen MK, Lai HH, Hsu WF, Huang KC, et al. Helicobacter pylori infection is positively associated with metabolic syndrome in Taiwanese adults: a cross-sectional study. Helicobacter (2015) 20(3):184–91. doi: 10.1111/hel.12190
23. Yu Y, Cai J, Song Z, Wang J, Wu L. Association of helicobacter pylori infection with metabolic syndrome in aged Chinese females. Exp Ther Med (2019) 17(6):4403–8. doi: 10.3892/etm.2019.7509
24. Aydemir S, Bayraktaroglu T, Sert M, Sokmen C, Atmaca H, Mungan G, et al. The effect of helicobacter pylori on insulin resistance. Dig Dis Sci (2005) 50(11):2090–3. doi: 10.1007/s10620-005-3012-z
25. Vafaeimanesh J, Bagherzadeh M, Mirzaei A, Parham M, Norouzinia M, Vafaee R. Effect of helicobacter pylori on metabolic syndrome parameters in diabetic patients. Gastroenterol Hepatol Bed Bench (2016) 9(Suppl1):S36–41. doi: 10.22037/GHFBB.V0I0.1020
26. Wellen KE. Inflammation, stress, and diabetes. J Clin Invest (2005) 115(5):1111–9. doi: 10.1172/jci200525102
27. Futagami S, Hiratsuka T, Tatsuguchi A, Suzuki K, Kusunoki M, Shinji Y, et al. Monocyte chemoattractant protein 1 (MCP-1) released from helicobacter pylori stimulated gastric epithelial cells induces cyclooxygenase 2 expression and activation in T cells. Gut (2003) 52(9):1257–64. doi: 10.1136/gut.52.9.1257
28. Salles N, Menard A, Georges A, Salzmann M, de Ledinghen V, de Mascarel A, et al. Effects of helicobacter pylori infection on gut appetite peptide (leptin, ghrelin) expression in elderly inpatients. J Gerontol A Biol Sci Med Sci (2006) 61(11):1144–50. doi: 10.1093/gerona/61.11.1144
29. Kawashima J, Ohno S, Sakurada T, Takabayashi H, Kudo M, Ro S, et al. Circulating acylated ghrelin level decreases in accordance with the extent of atrophic gastritis. J Gastroenterol (2009) 44(10):1046–54. doi: 10.1007/s00535-009-0120-0
30. Manolakis AC, Tiaka EK, Kapsoritakis AN, Georgoulias P, Tsiopoulos F, Valotassiou V, et al. Increased fetuin a levels in helicobacter pylori infection: a missing link between h. pylori and insulin resistance? Diabetologia (2011) 54(2):472–4. doi: 10.1007/s00125-010-1995-2
31. Mokhtare M, Mirfakhraee H, Arshad M, Samadani Fard SH, Bahardoust M, Movahed A, et al. The effects of helicobacter pylori eradication on modification of metabolic syndrome parameters in patients with functional dyspepsia. Diabetes Metab Syndr (2017) 11 Suppl 2:S1031–5. doi: 10.1016/j.dsx.2017.07.035
32. Nuutila P, Knuuti MJ, Maki M, Laine H, Ruotsalainen U, Teras M, et al. Gender and insulin sensitivity in the heart and in skeletal muscles. studies using positron emission tomography. Diabetes (1995) 44(1):31–6. doi: 10.2337/diab.44.1.31
33. Moran A, Jacobs DR Jr., Steinberger J, Steffen LM, Pankow JS, Hong CP, et al. Changes in insulin resistance and cardiovascular risk during adolescence: establishment of differential risk in males and females. Circulation (2008) 117(18):2361–8. doi: 10.1161/CIRCULATIONAHA.107.704569
34. Livingstone C, Collison M. Sex steroids and insulin resistance. Clin Sci (Lond) (2002) 102(2):151–66. doi: 10.1042/cs1020151
35. Colafella KMM, Denton KM. Sex-specific differences in hypertension and associated cardiovascular disease. Nat Rev Nephrol (2018) 14(3):185–201. doi: 10.1038/nrneph.2017.189
36. Ramji DP, Davies TS. Cytokines in atherosclerosis: key players in all stages of disease and promising therapeutic targets. Cytokine Growth Factor Rev (2015) 26(6):673–85. doi: 10.1016/j.cytogfr.2015.04.003
37. Kotsis V, Stabouli S, Papakatsika S, Rizos Z, Parati G. Mechanisms of obesity-induced hypertension. Hypertens Res (2010) 33(5):386–93. doi: 10.1038/hr.2010.9
38. Galipeau DM, Yao L, McNeill JH. Relationship among hyperinsulinemia, insulin resistance, and hypertension is dependent on sex. Am J Physiol Heart Circ Physiol (2002) 283(2):H562–567. doi: 10.1152/ajpheart.00238.2002
39. Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest (2000) 106(4):473–81. doi: 10.1172/JCI10842
40. Shin DW, Kwon HT, Kang JM, Park JH, Choi HC, Park MS, et al. Association between metabolic syndrome and helicobacter pylori infection diagnosed by histologic status and serological status. J Clin Gastroenterol (2012) 46(10):840–5. doi: 10.1097/MCG.0b013e3182522477
41. Kim TJ, Lee H, Kang M, Kim JE, Choi YH, Min YW, et al. Helicobacter pylori is associated with dyslipidemia but not with other risk factors of cardiovascular disease. Sci Rep (2016) 6:38015. doi: 10.1038/srep38015
42. Elizalde JI, Pique JM, Moreno V, Morillas JD, Elizalde I, Bujanda L, et al. Influence of helicobacter pylori infection and eradication on blood lipids and fibrinogen. Aliment Pharmacol Ther (2002) 16(3):577–86. doi: 10.1046/j.1365-2036.2002.01202.x
Keywords: metabolic syndrome, Helicobacter pylori, obesity, hypertension, hyperglycemia
Citation: Liu Y, Shuai P, Chen W, Liu Y and Li D (2023) Association between Helicobacter pylori infection and metabolic syndrome and its components. Front. Endocrinol. 14:1188487. doi: 10.3389/fendo.2023.1188487
Received: 17 March 2023; Accepted: 02 June 2023;
Published: 19 June 2023.
Edited by:
Yuhang Ma, Shanghai General Hospital, ChinaReviewed by:
Salvatore Vaccaro, IRCCS Local Health Authority of Reggio Emilia, ItalyCopyright © 2023 Liu, Shuai, Chen, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongyu Li, ZG9uZ3l1emhpeWluQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.