- 1Institute of Health Sciences, China Medical University, Shenyang, Liaoning, China
- 2School of Health and Life Sciences, University of Health and Rehabilitation Sciences, Qingdao, Shandong, China
- 3School of Public Health, China Medical University, Shenyang, Liaoning, China
Aim: Confirm and compare the degree of associations of non-traditional lipid profiles and metabolic syndrome (MetS) in Chinese adolescents, determine the lipid parameter with better predictive potential, and investigate their discriminatory power on MetS.
Methods: Medical measurements, including anthropometric measurements and biochemical blood tests, were undergone among a total sample of 1112 adolescents (564 boys and 548 girls) aged from 13 to 18 years. Univariate and multivariate logistic regression analyses were applied for assessing the relationships between the levels of traditional/non-traditional lipid profiles and MetS. We performed Receiver Operating Characteristic (ROC) analyses to mensurate the effectiveness of lipid accumulation product (LAP) on the diagnosis of MetS. Meanwhile, areas under the ROC curve and the cut-off values were calculated for MetS and its components.
Results: Univariate analysis showed that all our lipid profiles were closely associated with MetS (P< 0.05). LAP index showed the closest association with MetS than the other lipid profiles. Additionally, ROC analyses indicated that the LAP index showed sufficient capabilities to identify adolescents with MetS and its components.
Conclusion: The LAP index is a simple and efficient tool to identify individuals with MetS in Chinese adolescents.
1 Introduction
Metabolic syndrome (MetS), also known as syndrome X, is a complicated multifactorial disease with insulin resistance (IR) as the central feature, characterized by central obesity, abnormal glucose regulation, hypertension, and dyslipidemia (1, 2). The upstream MetS imply a prophetic potential enabling the subsequent diseases to be alerted. Previous studies have elucidated that MetS is associated with a higher risk of type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD), the significant causes of human morbidity and mortality (3, 4). Although it is not an absolute risk indicator, MetS have been proved to predispose adolescents to have twice or more the risk of CVD and T2DM in adulthood as adolescents without MetS (5, 6). The risk over a lifetime undoubtedly will be even higher. With the increment in childhood obesity, the prevalence of MetS in juveniles in the United States stepped-up from 4.3% in 1994 to 9.8% in 2016 (7, 8). In China, the percentage was 4.1% among minors aged 10 to 16 from 7 provinces in 2012 (9). Foreseeably, the rocketing prevalence of MetS may pose a threat to public health severely. Thus, further exploration should focus on identifying the MetS population as early as possible in adolescence.
Dysregulation of lipid homeostasis is now well accepted as a common characteristic of many diseases, especially metabolic diseases, and the alterations of lipid profiles may precede the occurrence of diseases (10, 11). Studies have confirmed that traditional lipid profiles are associated with MetS, and exhibit predictive abilities to some extent (12). Some innovative reports suggested that compared with traditional ones, the calculated non-traditional lipid profiles are better predictors for MetS (13–15), which may result from the fact that changes in newly calculated lipid parameters integrate the occurrence of multiple traditional lipid abnormalities. Additionally, the non-traditional lipid profiles do not require a high expenditure of time and expense, are more convenient and affordable detecting tools. These studies provide the possibility for early and expediently clinical diagnosing of MetS. But despite multifarious calculated non-traditional lipid profiles being studied, the most suitable parameters for clinical application have not been determined.
Hereby, this study aims to estimate the associations between non-traditional lipid profiles and MetS, and find the one with the most potential. To investigate the discriminating power of promising parameter for MetS, and provide the optimal cut-off value for reference.
2 Materials and methods
2.1 Study population
The study was carried out in two middle schools in Shenyang from August 2018 to March 2019. Students with restricted physical activity or (and) secondary obesity brought on by genetic diseases, congenital heart disease, endocrine abnormalities, etc. were excluded. After applying exclusion criteria, 1238 students were enrolled in our study. Afterward, participants whose ages were not specified (n = 58), or (and) did not participate in anthropometry (n = 65), or (and) were unable to obtain reports of biochemical blood tests (n = 1), or (and) duplicate data (n = 2) were excluded from our study. Data of the remaining 1112 individuals were used for further measurements.
2.2 Physical examinations
Physical examinations were performed following the standards of World Health Organization (16). Height of the subjects was measured to one decimal place (centimeter) by portable rangefinder (SECA 213, Hamburg, Germany), and weight was measured to kilograms with a body fat analysis meter (TANITA DC430MA, Tokyo, Japan). Neck girths, waistlines, hiplines, and thigh girths were measured manually with a soft ruler, respectively.
2.3 Blood sample collection and assessments
Intravenous blood samples were collected and preserved after 12 hours fasting using blood collection tubes containing EDTA (Becton Dickinson and Co., UK). The collected samples were directly centrifuged at 4°C for 10 min at a speed of 3000 rpm and all EDTA-plasma was aliquot and kept at -80°C until tested simultaneously. The plasma content of triglyceride (TG), cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), apolipoprotein A1 (apoA1) and B (apoB), fasting plasma glucose (FPG), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), γ-glutamyl transferase (GGT), and total bilirubin (BILT) were detected by an automatic biochemistry analyzer (ARCHITECT c1600, Japan).
2.4 Definition
MetS were diagnosed based on the International Diabetes Federation consensus report (17), combined with the items involved in this physical examination. In our study, we conducted an on-campus survey of 13 to 18-year-olds. Blood pressure was not measured, thus MetS were identified when any two of the following three factors are attached to central obesity (1): fasting plasma glucose (FPG) level above 5.6 mmol/L or known T2DM; (2) TG level of more than 150 mg/dL; (3) HDL-C level less than 40 mg/dL in boys of all ages and girls under 16, and less than 50 mg/dL for girls older than or equal to 16 years of age. Central obesity is defined as a child under 16 years of age with a waist circumference at or above the 90th percentile of children of the same age and sex, and a waist circumference greater than or equal to 90 cm and 80 cm for male and female, respectively, after 16 years old.
2.5 Statistical analysis
The non-traditional lipid parameters evaluated in the present study included TG/HDL-C, TC/HDL-C, LDL-C/HDL-C, non-HDL-C, atherogenic (ATH) index, lipoprotein combine index (LCI), apoB/apoA1, HDL-C/apoA1, LDL-C/apoB, lipid accumulation product (LAP), and visceral adiposity index (VAI). Continuous data were displayed with the median and interquartile range (upper quartile and lower quartile). Since the data distribution in this study is non-normal according to the Kolmogorov-Smirnov test (P< 0.05), we adopted the Mann-Whitney U test to compare participants’ general characteristics with and without MetS. Pearson chi-square was utilized to analyze the association between categorical variables. To eliminate the deviation caused by dimension and thus contribute to the comparisons of the associations between lipid profiles and MetS, each variable was standardized by the zero-mean normalization before logistic regression analyses. Univariate and multivariate logistic regression after adjusting for confounders were used to evaluate the associations between lipid profiles and the risk of MetS, and the adjusted odds ratio (OR) was presented with 95% confidence interval (95% CI). Additionally, we computed the area under the receiver operating characteristic (ROC) curve (AUC) for parameters with stable relevance to evaluate their resolving abilities in the detection of MetS and also determined their optimum cut-off values by the Yoden index. Statistical analyses were all conducted by SPSS 23.0 (IBM Corp). A P value< 0.05 was regarded statistically. The equations for ATH index, LCI, LAP and VAI are shown below:
2.6 Ethic approval
The ethics committee of the China Medical University approved this study, and each participant had informed consent (Ethics Approval No. [2020] 077). All methods were carried out under the Declaration of Helsinki and dependent guidelines.
3 Results
3.1 Basic characteristics
The prevalence of MetS among the subjects was 3.15%, and the prevalence by sex was 2.92% in boys and 3.4% in girls. According to the Pearson chi-square test, there was no significant difference in prevalence between boys and girls (P > 0.05). Most of the tested lipid profiles are higher in subjects diagnosed with MetS while HDL-C and apoA1 are lower. In girls, no group-related differences were observed in age, height, and the levels of ALP and BILT (P< 0.05). The same is true in boys with the addition of FPG. There are no sex-related differences in thigh girth, body mass index (BMI), FPG, TG, and LCI values among subjects (Supplemental Table 1).
3.2 Univariate and multiple logistic regression analyses
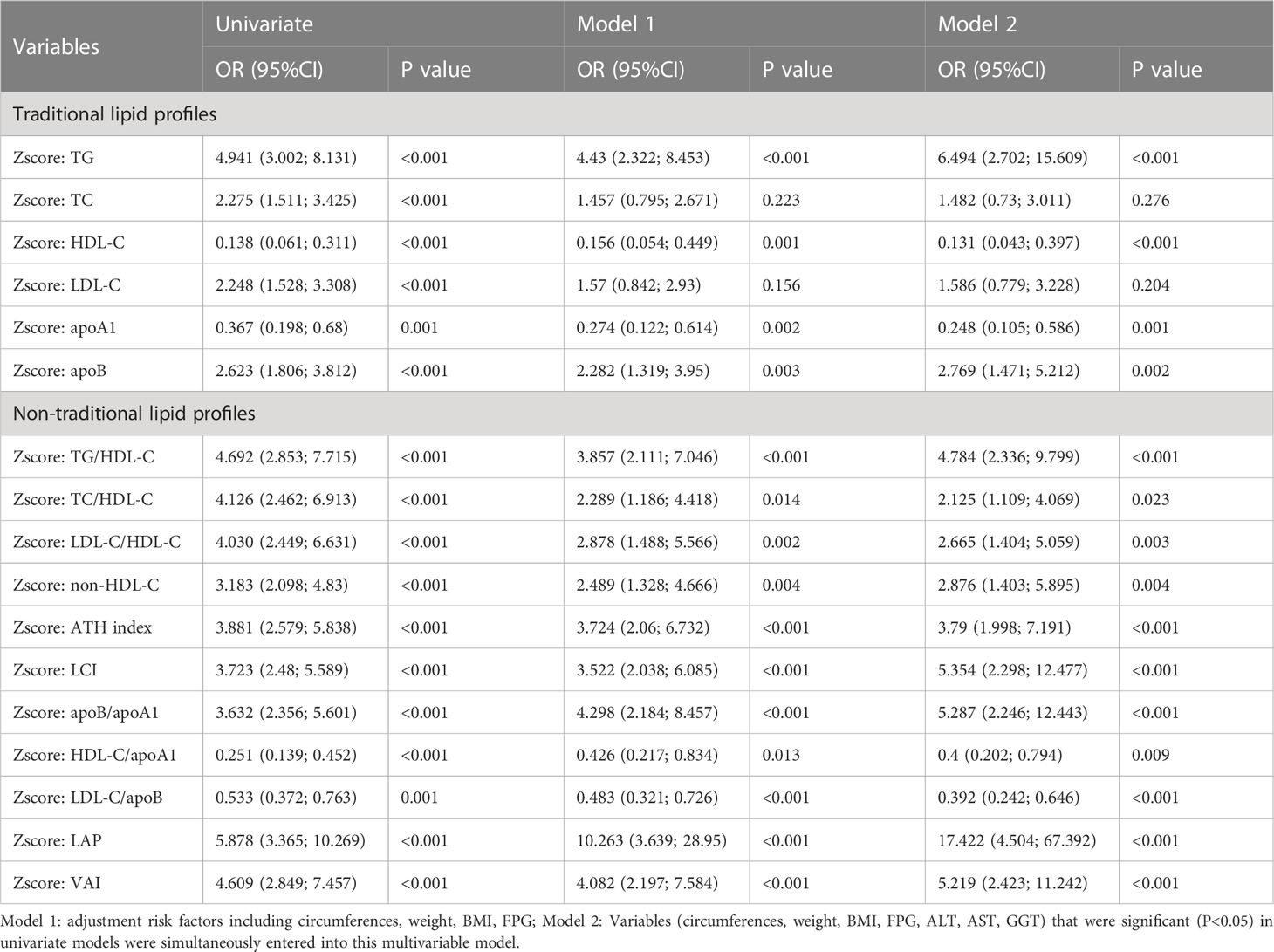
Univariate model was run for each predictive variable, using MetS as its outcome. Among male subjects, as illustrated in Table 1, from the univariate logistic regression analyses we found all lipid parameters are significantly associated with the risk of MetS. Notably, the association between TG and MetS is rivaled that between non-traditional lipid profiles and MetS. LAP (OR: 5.878, 95%CI: 3.365-10.269, P< 0.001) and HDL-C (OR: 0.138, 95%CI: 0.061-0.311, P< 0.001) were respectively the most influential negative and positive factor. Multivariate logistic regression was carried out to suppress the interference from other confounding factors. Common health metrics that are significant in univariate logistic regression (P< 0.1), including bodily circumferences, weight, BMI, and FPG, were synchronously put into model 1 with each lipid parameter. Furthermore, we incorporated conventional liver function indexes, factors with sufficient association strength with MetS (ALT, AST, and GGT) were qualified to be combined with lipid parameters to build model 2. After we threw all the steps together, LAP (OR: 17.422, 95%CI: 4.504-67.392, P< 0.001) was still the most significant negative factor, and HDL-C (OR: 0.131, 95%CI: 0.043-0.397, P< 0.001) still has the greatest positive association with MetS. The ORs of TC, LDL-C, and apoA1/HDL-C were no longer statistically significant, while the ORs of LCI, apoB/apoA1, apoB/LDL-C, and VAI increased.
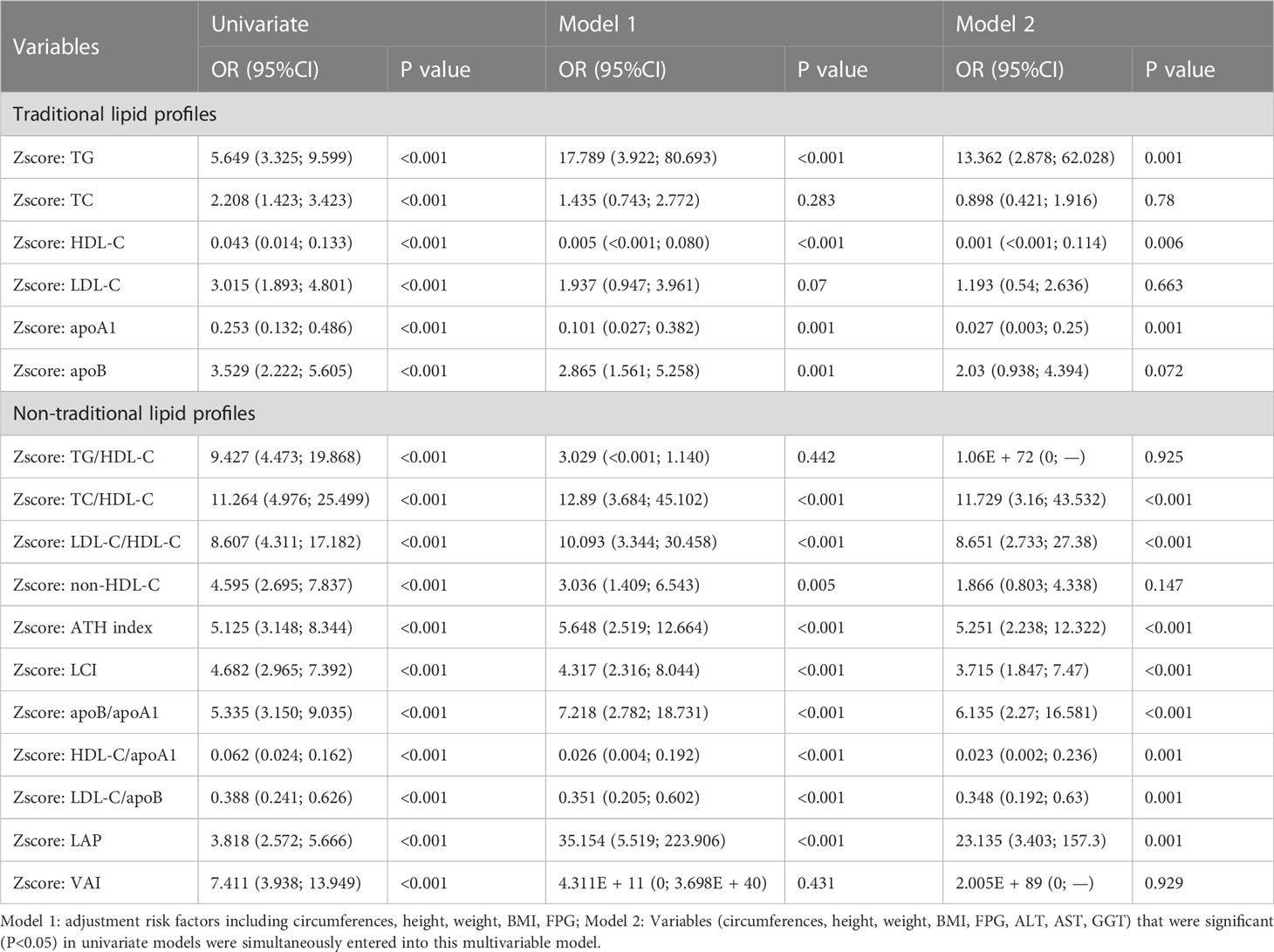
Similar methods were used for female subjects (Table 2). In univariate logistic regression analysis, both traditional and non-traditional lipid profiles showed significant associations with MetS, among which the HDL-C (OR: 0.043, 95%CI: 0.014-0.133, P< 0.001) and LAP (OR: 23.135, 95%CI: 3.403-157.3, P = 0.001) is the most significant positive and negative factor, respectively. Bodily circumferences, height, weight, BMI, and FPG were incorporated to build model 1. Whereafter, the ORs of TC, TG/HDL-C, and VAI were not statistically significant anymore (P > 0.05). In model 2, liver functions including ALT, AST, and GGT were added as confounding factors. The associations between LDL-C, apoB, non-HDL-C, and MetS were no longer statistically significant factors (P > 0.05). Among the remaining indicators, associations between TG, HDL-C, apoA1, apoB/apoA1, HDL-C/apoA1, LAP, and MetS enhanced obviously. The OR values of TC/HDL-C, LDL-C/HDL-C, ATH index, and LDL-C/apoB were relatively stable, while the OR value of LCI decreased.
We found that the associations between MetS and negative factors in female subjects were generally higher than that in males. LAP was the most closely associated negative factor with MetS in each sex.
3.3 Diagnostic value of LAP in detecting MetS
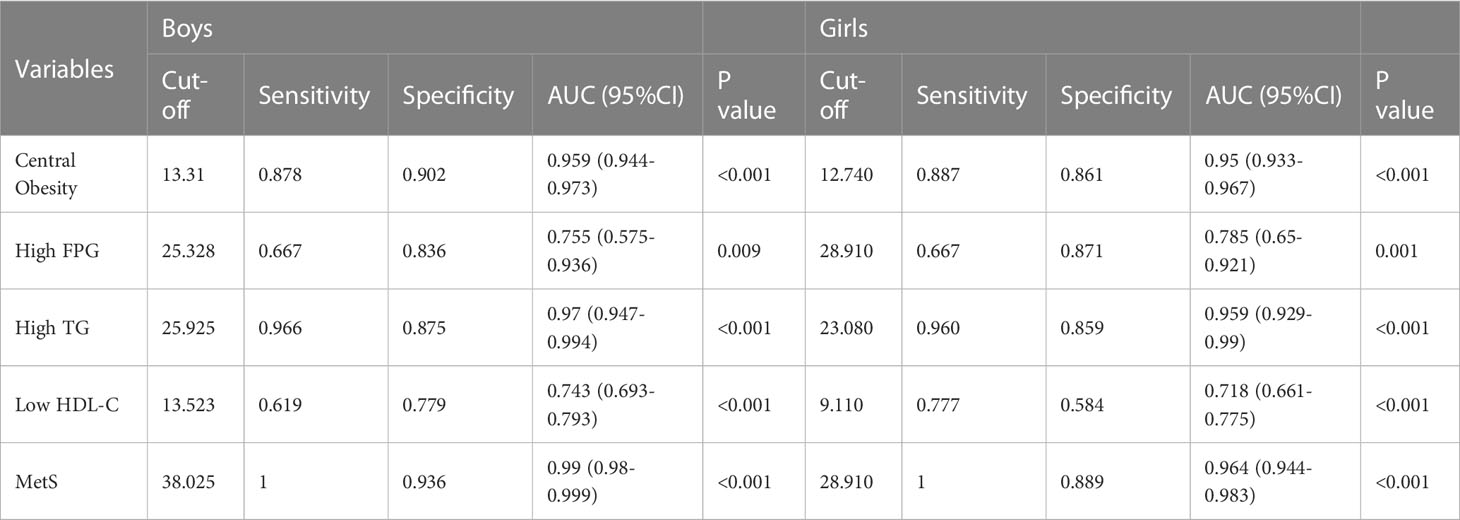
To evaluate the predictive value of LAP, the most remarkable parameter in logistic regression analyses, we drew the receiver operating characteristic curves (ROC) of MetS and its components based on LAP and calculated the areas under the curve (AUC). As shown in the ROC analyses (Table 3), the LAP ratio occupies outstanding prediction performance for MetS (girls: AUC = 0.964, boys: AUC = 0.99). Except for MetS, LAP was also an excellent predictor for high TG (girls: AUC = 0.959, boys: AUC = 0.97), central obesity (girls: AUC = 0.95, boys: AUC = 0.959) and effectual predictor for low HDL-C (girls: AUC = 0.743, boys: AUC = 0.718), high FPG (girls: AUC = 0.785, boys: AUC = 0.755). The cut-off values in detecting MetS are 38.025 and 28.91 in boys and girls, respectively. Our results suggested that LAP can identify each component of MetS and exhibited sufficient capacity to distinguish the MetS patients in the Chinese adolescent population.
4 Discussion
4.1 Summary
In the current study, we confirmed that the LAP levels increased in adolescents with MetS compared with normal subjects. Among the 11 common non-traditional lipid profiles we selected, LAP showed the strongest association with MetS. In agreement with previous studies, LAP has been confirmed to identify MetS independently and exhibited sufficient capacity to identity the components of MetS (18–22). Current research suggests that MetS develops after an accumulation of abdominal fat, and dyslipidemia precedes the occurrence of MetS. Therefore, LAP may have an ability to predict MetS in the early stages (23).
In addition, we reported the associations between lipid profiles and MetS after adjusting for significant common health metrics and liver function indexes. Although the non-traditional lipid profiles generally displayed a superior association with MetS, a couple of traditional lipid profiles have preeminent performances. Moreover, association with MetS was found to be reduced for some calculated parameters after the combination of these potent individual lipids. One of the possible reasons is the low prevalence in adolescent subjects resulting in the over-fitting of the model. This may reflect the non-traditional lipid profiles that integrate more than one lipid parameter are more reliable MetS predictors. TG and HDL-C, two components of MetS, are the most influential among all lipid profiles in subjects regardless of sex. Yet, there is still controversy about whether traditional lipid profiles can be used as MetS predictors and it is biased to regard them as powerful MetS predictors (24–26).
4.2 Strengths and limitations
Our study had a sufficient sample size, a balanced sex ratio, and kept as many eligible participants as possible. There are also several limitations to our study. Firstly, the MetS patients are few and may not be sufficient to represent the overall prevalence level in adolescents. Secondly, our cross-sectional study did not evaluate the long-term relationship between MetS and lipid profile levels, and prospective follow-up studies are needed. Thirdly, the diagnostic criteria of MetS have not been clearly defined yet, and the criteria we use may be different from other reports. Fourthly, there may be MetS patients who have not been diagnosed because we did not take blood pressure measurements. Besides, the prevalence of hypertension varies among MetS patients with different ages and sexes. Excluding hypertension from the components of MetS may affect the future design of specific treatments for different populations (27). Lastly, only Chinese adolescents were involved in our study and their ethnicity was not collected. Besides, the study sites are confined to Shenyang.
4.3 Comparison with existing literature
So far, only a few data concerning the association of LAP on MetS risk in Chinese, especially among adolescents. Cut-off values of non-traditional lipid profiles to identify MetS patients are rarely reported, and the majority of studies available only involved a specific population. Our study extends the current findings by proposing the cut-off values of LAP to distinguish individuals with MetS in Chinese adolescents. Our result differs from the data for Chinese elders (28), Indian PCOS women (29), and Iran adults (30), but close to a large community-based and multi-ethnic population study in China (31). These demonstrated that a single cut-off value is not adequate for a diverse population due to various ethnicity and age.
4.4 Implications for research and practice
Although the development of MetS is not entirely understood, existing studies believe that IR derived from central obesity is the most critical pathogenesis of MetS. Dyslipidemia leads to the accumulation of lipids and their metabolites, which impair the function of B cell, induce the occurrence of IR, then facilitate the development of MetS components, and finally leads to the occurrence of MetS (32). The LAP index has been considered a surrogate indicator of central obesity (33) and IR (34–37) formerly, which also showed an apparent association with MetS risk in our study. On one aspect, the calculation formula of the LAP index contains TG, which makes it possible to reflect dyslipidemia and MetS directly. On the other hand, LAP is generally regarded as an indicator in measuring visceral fat, while central obesity is another marker of ectopic fat accumulation (38) as a component of MetS. Accordingly, the role of LAP in metabolic diseases has been gradually gaining attention in late years. Researchers found that LAP level was positively correlated with an increased risk of long-term cardiovascular disease in populations with or without pre-existing CVD (39–41). In several large population-based studies, LAP was considered a powerful risk predictor for incident diabetes and capable of being used as a diagnostic tool (42–44). Some studies have also indicated that LAP is significantly associated with hypertension, but most of the current studies are concentrated in East Asia, and the data on different ethnic populations are temporarily lacking (45, 46).
An overview of the patient’s condition is needed to diagnose MetS, which is complicated in clinical practice. Nonetheless, levels of most lipid profiles are measured routinely and the non-traditional lipid ratios can be calculated easily. The strengths of this study are that we enumerated most of the non-traditional lipid profiles that are now extensively studied and made comparisons to help ascertain the most reliable tool for physicians to identify patients with MetS.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the ethics committee of the China Medical University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
Z-YC, LL: research design, data collection, data analysis, manuscript writing. Z-YC, LL, X-XZ, Y-CZ: blood biochemical measurements, data collection. YL: project development, data collection. YL, Y-NM, D-LW: data interpretation, manuscript revise. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the 111 project [grant number D21008] and the National Natural Science Foundation of China [grant number 72004233].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1179990/full#supplementary-material
References
1. Lemieux I, Després JP. Metabolic syndrome: past, present and future. Nutrients (2020) 12(11):3501. doi: 10.3390/nu12113501
2. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation (2009) 120(16):1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644
3. Aboonabi A, Meyer RR, Singh I. The association between metabolic syndrome components and the development of atherosclerosis. J Hum Hypertens (2019) 33(12):844–55. doi: 10.1038/s41371-019-0273-0
4. Lee MK, Han K, Kim MK, Koh ES, Kim ES, Nam GE, et al. Changes in metabolic syndrome and its components and the risk of type 2 diabetes: a nationwide cohort study. Sci Rep (2020) 10(1):2313. doi: 10.1038/s41598-020-59203-z
5. Zhu Y, Zheng H, Zou Z, Jing J, Ma Y, Wang H, et al. Metabolic syndrome and related factors in Chinese children and adolescents: analysis from a Chinese national study. J Atheroscler Thromb (2020) 27(6):534–44. doi: 10.5551/jat.50591
6. Asghari G, Hasheminia M, Heidari A, Mirmiran P, Guity K, Shahrzad MK, et al. Adolescent metabolic syndrome and its components associations with incidence of type 2 diabetes in early adulthood: Tehran lipid and glucose study. Diabetol Metab Syndr (2021) 13(1):1. doi: 10.1186/s13098-020-00608-1
7. Zhang Y, Hu J, Li Z, Li T, Chen M, Wu L, et al. A novel indicator of lipid accumulation product associated with metabolic syndrome in Chinese children and adolescents. Diabetes Metab Syndr Obes (2019) 12:2075–83. doi: 10.2147/DMSO.S221786
8. Lee AM, Gurka MJ, DeBoer MD. Trends in metabolic syndrome severity and lifestyle factors among adolescents. Pediatrics (2016) 137(3):e20153177. doi: 10.1542/peds.2015-3177
9. Wang ZH, Zou ZY, Wang S, Dong YH, Yang ZG, Yang ZP, et al. Analysis of the epidemiological characteristics of metabolic syndrome among 10-16 adolescents in 7 provinces in China, 2012. Zhonghua Yu Fang Yi Xue Za Zhi (2017) 51(4):295–9. doi: 10.3760/cma.j.issn.0253-9624.2017.04.004
10. Markgraf DF, Al-Hasani H, Lehr S. Lipidomics-reshaping the analysis and perception of type 2 diabetes. Int J Mol Sci (2016) 17(11):1841. doi: 10.3390/ijms17111841
11. Knebel B, Strassburger K, Szendroedi J, Kotzka J, Scheer M, Nowotny B, et al. Specific metabolic profiles and their relationship to insulin resistance in recent-onset type 1 and type 2 diabetes. J Clin Endocrinol Metab (2016) 101(5):2130–40. doi: 10.1210/jc.2015-4133
12. Datta Banik S, Pacheco-Pantoja E, Lugo R, Gómez-de-Regil L, Chim Aké R, Méndez González RM, et al. Evaluation of anthropometric indices and lipid parameters to predict metabolic syndrome among adults in Mexico. Diabetes Metab Syndr Obes (2021) 14:691–701. doi: 10.2147/DMSO.S281894
13. Chu SY, Jung JH, Park MJ, Kim SH. Risk assessment of metabolic syndrome in adolescents using the triglyceride/high-density lipoprotein cholesterol ratio and the total cholesterol/high-density lipoprotein cholesterol ratio. Ann Pediatr Endocrinol Metab (2019) 24(1):41–8. doi: 10.6065/apem.2019.24.1.41
14. Jung CH, Hwang JY, Yu JH, Shin MS, Bae SJ, Park JY, et al. The value of apolipoprotein B/A1 ratio in the diagnosis of metabolic syndrome in a Korean population. Clin Endocrinol (Oxf) (2012) 77(5):699–706. doi: 10.1111/j.1365-2265.2012.04329.x
15. Shin HG, Kim YK, Kim YH, Jung YH, Kang HC. The relationship between the triglyceride to high-density lipoprotein cholesterol ratio and metabolic syndrome. Korean J Fam Med (2017) 38(6):352–7. doi: 10.4082/kjfm.2017.38.6.352
16. World Health Organ Tech Rep Ser. Physical status: the use and interpretation of anthropometry. report of a WHO expert committee (Geneva, Switzerland: World Health Organization) (1995) 854:1–452.
17. Zimmet P, Alberti KG, Kaufman F, Tajima N, Silink M, Arslanian S, et al. The metabolic syndrome in children and adolescents - an IDF consensus report. Pediatr Diabetes (2007) 8(5):299–306. doi: 10.1111/j.1399-5448.2007.00271.x
18. Karatas S, Beysel S. Visceral adiposity index, Triglyceride/High-density lipoprotein ratio, and lipid accumulation product index to discriminate metabolic syndrome among adult type 1 diabetes patients. Metab Syndr Relat Disord (2021) 19(9):507–12. doi: 10.1089/met.2021.0047
19. Ray L, Ravichandran K, Nanda SK. Comparison of lipid accumulation product index with body mass index and waist circumference as a predictor of metabolic syndrome in Indian population. Metab Syndr Relat Disord (2018) 16(5):240–5. doi: 10.1089/met.2017.0119
20. Nascimento-Ferreira MV, Rendo-Urteaga T, Vilanova-Campelo RC, Carvalho HB, da Paz Oliveira G, Paes Landim MB, et al. The lipid accumulation product is a powerful tool to predict metabolic syndrome in undiagnosed Brazilian adults. Clin Nutr (2017) 36(6):1693–700. doi: 10.1016/j.clnu.2016.12.020
21. Biyik Z, Guney I. Lipid accumulation product and visceral adiposity ındex: two new indices to predict metabolic syndrome in chronic kidney disease. Eur Rev Med Pharmacol Sci (2019) 23(5):2167–73. doi: 10.26355/eurrev_201903_17262
22. Zhang X, Hong F, Liu L, Nie F, Du L, Guan H, et al. Lipid accumulation product is a reliable indicator for identifying metabolic syndrome: the China multi-ethnic cohort (CMEC) study. Qjm (2022) 115(3):140–7. doi: 10.1093/qjmed/hcaa325
23. Fernández-Verdejo R, Galgani JE. Exploring the sequential accumulation of metabolic syndrome components in adults. Sci Rep (2022) 12(1):15925. doi: 10.1038/s41598-022-19510-z
24. Gu Z, Zhu P, Wang Q, He H, Xu J, Zhang L, et al. Obesity and lipid-related parameters for predicting metabolic syndrome in Chinese elderly population. Lipids Health Dis (2018) 17(1):289. doi: 10.1186/s12944-018-0927-x
25. Naghshband Z, Kumar L, Mandappa S, Niranjana Murthy AS, Malini SS. Visceral adiposity index and lipid accumulation product as diagnostic markers of metabolic syndrome in south indians with polycystic ovary syndrome. J Hum Reprod Sci (2021) 14(3):234–43. doi: 10.4103/jhrs.jhrs_12_21
26. Motamed N, Razmjou S, Hemmasi G, Maadi M, Zamani F. Lipid accumulation product and metabolic syndrome: a population-based study in northern Iran, amol. J Endocrinol Invest (2016) 39(4):375–82. doi: 10.1007/s40618-015-0369-5
27. Song P, Zhang Y, Yu J, Zha M, Zhu Y, Rahimi K, et al. Global prevalence of hypertension in children: a systematic review and meta-analysis. JAMA Pediatr (2019) 173(12):1154–63. doi: 10.1001/jamapediatrics.2019.3310
28. Guo SX, Zhang XH, Zhang JY, He J, Yan YZ, Ma JL, et al. Visceral adiposity and anthropometric indicators as screening tools of metabolic syndrome among low income rural adults in xinjiang. Sci Rep (2016) 6:36091. doi: 10.1038/srep36091
29. Wang S. Association between serum low-density lipoprotein cholesterol and metabolic syndrome in a working population. Lipids Health Dis (2021) 20(1):73. doi: 10.1186/s12944-021-01500-1
30. Hajian-Tilaki K, Heidari B, Hajian-Tilaki A, Firouzjahi A, Bakhtiari A. Does the low-density lipoprotein cholesterol play a key role in predicting metabolic syndrome in the Iranian adult population? Caspian J Intern Med (2017) 8(4):289–95. doi: 10.22088/cjim.8.4.289
31. Janghorbani M, Amini M. Low-density lipoprotein cholesterol and metabolic syndrome in an Iranian high-risk population. Diabetes Metab Syndr (2015) 9(2):91–7. doi: 10.1016/j.dsx.2014.07.003
32. McCracken E, Monaghan M, Sreenivasan S. Pathophysiology of the metabolic syndrome. Clin Dermatol (2018) 36(1):14–20. doi: 10.1016/j.clindermatol.2017.09.004
33. Ahn N, Baumeister SE, Amann U, Rathmann W, Peters A, Huth C, et al. Visceral adiposity index (VAI), lipid accumulation product (LAP), and product of triglycerides and glucose (TyG) to discriminate prediabetes and diabetes. Sci Rep (2019) 9(1):9693. doi: 10.1038/s41598-019-46187-8
34. Mazidi M, Kengne AP, Katsiki N, Mikhailidis DP, Banach M. Lipid accumulation product and triglycerides/glucose index are useful predictors of insulin resistance. J Diabetes Complications (2018) 32(3):266–70. doi: 10.1016/j.jdiacomp.2017.10.007
35. Xia C, Li R, Zhang S, Gong L, Ren W, Wang Z, et al. Lipid accumulation product is a powerful index for recognizing insulin resistance in non-diabetic individuals. Eur J Clin Nutr (2012) 66(9):1035–8. doi: 10.1038/ejcn.2012.83
36. Lee J, Kim B, Kim W, Ahn C, Choi HY, Kim JG, et al. Lipid indices as simple and clinically useful surrogate markers for insulin resistance in the U. S population Sci Rep (2021) 11(1):2366. doi: 10.1038/s41598-021-82053-2
37. Anoop SS, Dasgupta R, Rebekah G, Jose A, Inbakumari MP, Finney G, et al. Lipid accumulation product (LAP) as a potential index to predict risk of insulin resistance in young, non-obese Asian Indian males from southern India: observations from hyperinsulinemic-euglycemic clamp studies. BMJ Open Diabetes Res Care (2021) 9(1):e002414. doi: 10.1136/bmjdrc-2021-002414
38. Smith U. Abdominal obesity: a marker of ectopic fat accumulation. J Clin Invest (2015) 125(5):1790–2. doi: 10.1172/JCI81507
39. Kyrou I, Panagiotakos DB, Kouli GM, Georgousopoulou E, Chrysohoou C, Tsigos C, et al. Lipid accumulation product in relation to 10-year cardiovascular disease incidence in Caucasian adults: the ATTICA study. Atherosclerosis (2018) 279:10–6. doi: 10.1016/j.atherosclerosis.2018.10.015
40. Maturana MA, Moreira RM, Spritzer PM. Lipid accumulation product (LAP) is related to androgenicity and cardiovascular risk factors in postmenopausal women. Maturitas (2011) 70(4):395–9. doi: 10.1016/j.maturitas.2011.09.012
41. Ioachimescu AG, Brennan DM, Hoar BM, Hoogwerf BJ. The lipid accumulation product and all-cause mortality in patients at high cardiovascular risk: a PreCIS database study. Obes (Silver Spring) (2010) 18(9):1836–44. doi: 10.1038/oby.2009.453
42. Tian T, Pei H, Chen Z, Hailili G, Wang S, Sun Y, et al. Comparison of lipid accumulation product and body mass index as indicators of diabetes diagnosis among 215,651 Chinese adults. PeerJ (2020) 8:e8483. doi: 10.7717/peerj.8483
43. Brahimaj A, Rivadeneira F, Muka T, Sijbrands EJG, Franco OH, Dehghan A, et al. Novel metabolic indices and incident type 2 diabetes among women and men: the Rotterdam study. Diabetologia (2019) 62(9):1581–90. doi: 10.1007/s00125-019-4921-2
44. Chen J, Sun H, Qiu S, Tao H, Yu J, Sun Z. Lipid accumulation product combined with urine glucose excretion improves the efficiency of diabetes screening in Chinese adults. Front Endocrinol (Lausanne) (2021) 12:691849. doi: 10.3389/fendo.2021.691849
45. Song J, Zhao Y, Nie S, Chen X, Wu X, Mi J. The effect of lipid accumulation product and its interaction with other factors on hypertension risk in Chinese han population: a cross-sectional study. PloS One (2018) 13(6):e0198105. doi: 10.1371/journal.pone.0198105
Keywords: lipid, lipid accumulation product, adolescent, metabolic syndrome, obesity
Citation: Chen Z-y, Liu L, Zhuang X-x, Zhang Y-c, Ma Y-n, Liu Y and Wen D-l (2023) Lipid accumulation product is a better predictor of metabolic syndrome in Chinese adolescents: a cross-sectional study. Front. Endocrinol. 14:1179990. doi: 10.3389/fendo.2023.1179990
Received: 05 March 2023; Accepted: 09 June 2023;
Published: 23 June 2023.
Edited by:
Hao Mei, University of Mississippi Medical Center School of Dentistry, United StatesReviewed by:
Yunxi Zhang, University of Mississippi Medical Center, United StatesAngelica Amato, University of Brasilia, Brazil
Copyright © 2023 Chen, Liu, Zhuang, Zhang, Ma, Liu and Wen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Liu, eWxpdTA1NjhAY211LmVkdS5jbg==; De-liang Wen, ZGx3ZW5AY211LmVkdS5jbg==
† These authors contributed equally to this work and share first authorship
 Zi-yi Chen
Zi-yi Chen Lei Liu2†
Lei Liu2† Ya-nan Ma
Ya-nan Ma Yang Liu
Yang Liu De-liang Wen
De-liang Wen