- 1Department of Medicine, Division of Cardiology, Einstein Institute for Aging Research, Montefiore Health System, New York, NY, United States
- 2University of Naples “Federico II”, Naples, Italy
- 3ASL Avellino, Montefiore Health System, New York, NY, United States
- 4Department of Microbiology and Immunology, Montefiore Health System, New York, NY, United States
- 5Department of Molecular Pharmacology, Einstein-Sinai Diabetes Research Center (ES-DRC), Montefiore Health System, New York, NY, United States
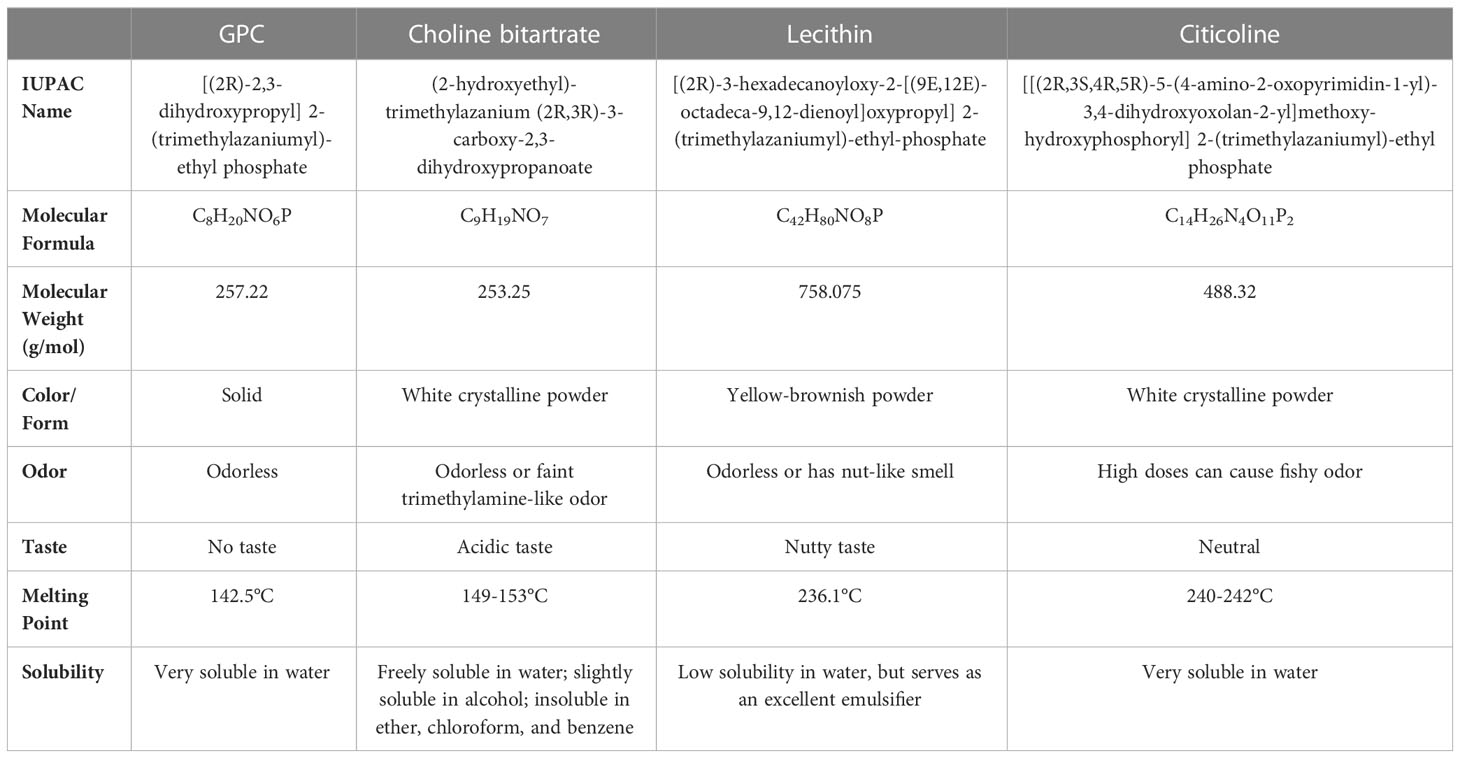
In this comprehensive review, we examine the main preclinical and clinical investigations assessing the effects of different forms of choline supplementation currently available, including choline alfoscerate (C8H20NO6P), also known as alpha-glycerophosphocholine (α-GPC, or GPC), choline bitartrate, lecithin, and citicoline, which are cholinergic compounds and precursors of acetylcholine. Extensively used as food supplements, they have been shown to represent an effective strategy for boosting memory and enhancing cognitive function.
Introduction
Choline is an important nutrient essential for proper functioning of liver, muscle, and brain (1–5). It is a main constituent of cell and organelle membranes and plays a vital role in numerous physiological processes including signal transduction, DNA and histone methylation, and nerve myelination (6, 7). Choline is a precursor of different metabolites including the neurotransmitter acetylcholine (ACh), the membrane phospholipids phosphatidylcholine (PC) and sphingomyelin, and the methyl donor betaine.
Choline can be obtained from the diet and via de novo biosynthesis from the methylation of phosphatidylethanolamine (PE) to PC (6, 8). The demand for choline increases particularly during pregnancy inasmuch as it is important for placental function, fetal growth, and brain development (7). Choline deficiency can cause serious medical conditions such as premature birth, cystic fibrosis, and hepato-steatosis. Therefore, a sufficient choline intake is necessary for growth and homeostasis.
The US Food and Drug Administration (FDA) identified choline as an essential nutrient in 1998. The National Academy of Medicine (NAM) of the USA and the European Food Safety Authority (EFSA) both specified adequate intake (AI) values for choline. Of course, age, sex, life conditions (pregnancy, breastfeeding), and genetic polymorphisms represent central factors in determining AI (9). In 2016, the European Food Safety Authority (EFSA) set an AI of 400 mg/day for all healthy adults. Similarly, the AIs for pregnant and lactating women are 480 mg/day for and 520 mg/day respectively. The US Institute of Medicine (IOM) has a slightly different choline AIs set for nonpregnant, pregnant, and lactating women: 425 mg/day, 450 mg/day, and 550 mg/day, respectively. Low AIs were set for infants of various ages: AI recommendations for infants 0–6 months are 125 mg choline/day whereas for infants 7–12 months are 150 mg choline/day. These AI values are set according to choline concentrations in human milk (160 mg/L) and estimated average volume of human milk intake (0.78 L/day) for a whole group of infants (aged 0–6 months) with a default body weight of 7 kg (approximately 18 mg/kg), and extrapolation for default body weight (aged 7-12 months) (10, 11). Plasma choline concentrations are three times higher in newborn infants than in their mothers as human milk is rich in choline (12–16).
Choline alfoscerate
In addition to choline intake from food, there are several forms of choline supplementation currently available (2). Choline alfoscerate (C8H20NO6P), also known as alpha-glycerophosphocholine (α-GPC, or GPC), is a cholinergic compound and ACh precursor extensively used as a food supplement. Its molecular weight is 257.22 g/mol. GPC is considered one of the most used sources of choline due to its high choline content (41% of choline by weight) and its ability to cross the blood-brain barrier. The content of choline and GPC in common foods is reported in Table 1.
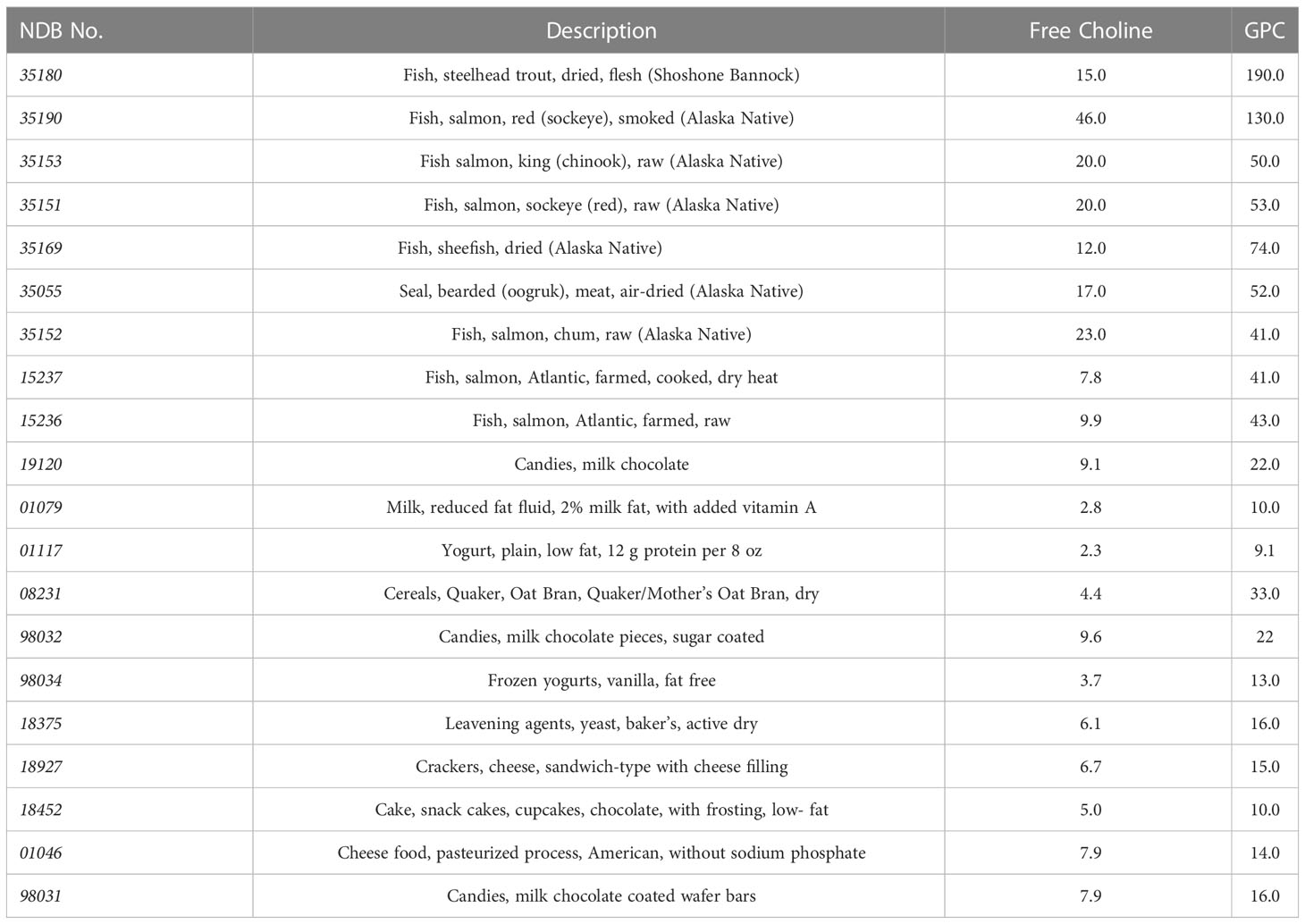
Table 1 Choline and GPC content in common foods (mg choline moiety/100 g of food) according to the US Department of Agriculture (USDA); the NDB (Nutrient DataBase) identifier is a five-digit numerical code used by the USDA for standard reference.
After oral administration, GPC can be readily metabolized to PC, the active form of choline that is able to increase the release of the neurotransmitter ACh (17, 18) and brain-derived neurotrophic factor (BDNF) (19, 20). GPC enhances memory and cognitive function and is well-known to be effective in the treatment of several neurodegenerative and vascular diseases such as Alzheimer’s disease and dementia (21–23). GPC has been shown to be more effective when combined with cholinesterase inhibitors (24, 25). Numerous studies have identified the favorable effects of GPC in the treatment of the sequelae of cerebrovascular accidents (26–28).
Nevertheless, GPC can be a friend or a foe depending on the doses and length of its administration. Uncovering a safe therapeutic window is essential to prevent adverse reactions.
Preclinical studies
GPC has been shown to exhibit a favorable action in experimental models of the aging brain as well as in a rat model of pilocarpine-induced seizure (29, 30), and to promote neuronal differentiation in a rat model of noise-restraint stress (29). In vitro assays performed in the SH-SY5Y human cell line have revealed that this cholinergic compound antagonizes neurotoxicity triggered by the fragment Aβ (25–35) of the Alzheimer’s amyloid β-peptide and attenuates the Aβ-induced phosphorylation of the Tau protein (31), by sustaining the expression level of synaptic vesicle proteins, such as synaptophysin (32–34). GPC was also shown to increase hippocampal neurogenesis, providing protection against seizure-induced neuronal death and cognitive impairment (26) and to antagonize scopolamine-induced amnesia enhancing hippocampal cholinergic transmission, suggesting that the behavioral effects of GPC could be related to its property to increase hippocampal synthesis and release of ACh (35–38).
Although GPC does not seem to be directly involved in the modulation of inflammatory responses (39), it has been shown to improve mitochondrial function and to reduce oxidative and nitrosative stress (40).
Chronic treatment of aged rats with GPC restored the number of muscarinic M1 receptors to levels found in the striatum and hippocampus from young animals (41). In young but not old rats, GPC significantly potentiated K+-stimulated intra-synaptosomal Ca2+ oscillations in purified synaptosomes derived from the hippocampus (17). Repeated injections of GPC significantly increased basal formation of [3H]inositol monophosphate in hippocampal, cortical, and striatal slices of male rats (42). Consistently, GPC potentiated receptor-stimulated phosphatidylinositol hydrolysis in cortical synapto-neurosomes (17).
In a model of acute cerebral ischemia in rats, GPC increased the tolerance of neurons to ischemic damage and slowed the execution of the cell death program (43). Consistent with these findings, in vitro assays in astroglial cell cultures have shown that GPC increases proliferation (44).
Clinical investigations
Cholinergic precursors have represented one of the first approaches attempting to relief cognitive impairment in dementia-related disorders. However, controlled clinical trials failed to show significant improvements with choline or PC, choline-containing phospholipids, alone or in association with cholinesterase inhibitors (tacrine plus choline, or physostigmine plus choline) (44, 45). Luckily, the lack of clinical benefits obtained with choline or lecithin are not shared by other phospholipids involved in choline biosynthetic pathways, including GPC and citicoline (cytidine 5′-diphosphocholine, also known as CDP-choline), which are able to increase ACh content and release (44, 46).
A study in male young adults demonstrated that the ingestion of 1000 mg GPC significantly increases plasma free choline levels (47). Numerous clinical reports suggest that GPC can improve memory and attention in patients with Alzheimer’s disease and dementia (26, 36, 48–54)
GPC advances physical and psychomotor performance in the context of muscle strength and conditioning (55–58). For instance, in a group of 13 college-aged male subjects, the administration of 600 mg GPC resulted in an increase of 98.8 N during an isometric mid-thigh pull assessment (55). Similarly, maximum velocity and maximum mechanical power were improved by the administration of 250 mg GPC (56) and nutritional supplements containing 300 mg or 150 mg GPC were shown to improve reaction time and vertical jump power (59), indicating the ergogenic properties of GPC.
The effects of GPC on cerebrovascular events remain controversial. Indeed, some investigators have conducted a multicenter clinical trial (daily intramuscular dose of 1000 mg for 28 days and oral dose of 800 mg during the following 5 months) that revealed the excellent tolerability and the therapeutic role of GPC on cognitive recovery of patients with acute stroke or transient ischemic attack (TIA) (18); on the other hand, a recent retrospective study has shown that GPC is associated with a higher 10-year incident stroke risk in a dose-response manner after adjusting for traditional cerebrovascular risk factors (60). A potential explanation for these different findings could be the diverse effects of GPC supplementation on the gut microbial community structure: in this sense, a recent preclinical report demonstrated that GPC can cause a shift in the murine microbiota, characterized by increased abundance of Bacteroides, Parabacteroides, and Ruminococcus, and decreased abundance of Lactobacillus, Akkermansia, and Roseburia (61).
Most recently, in a prospective study, GPC was suggested to enrich listening comprehension in older adults using hearing aids (62). Due to its action on the parasympathetic nervous system, GPC has also shown beneficial effects in patients with dry eye (keratoconjunctivitis sicca) and its combination with D-Panthenol accelerated and modulated the repair of the corneal innervation after cataract surgery (63–66).
Other forms of choline supplementation
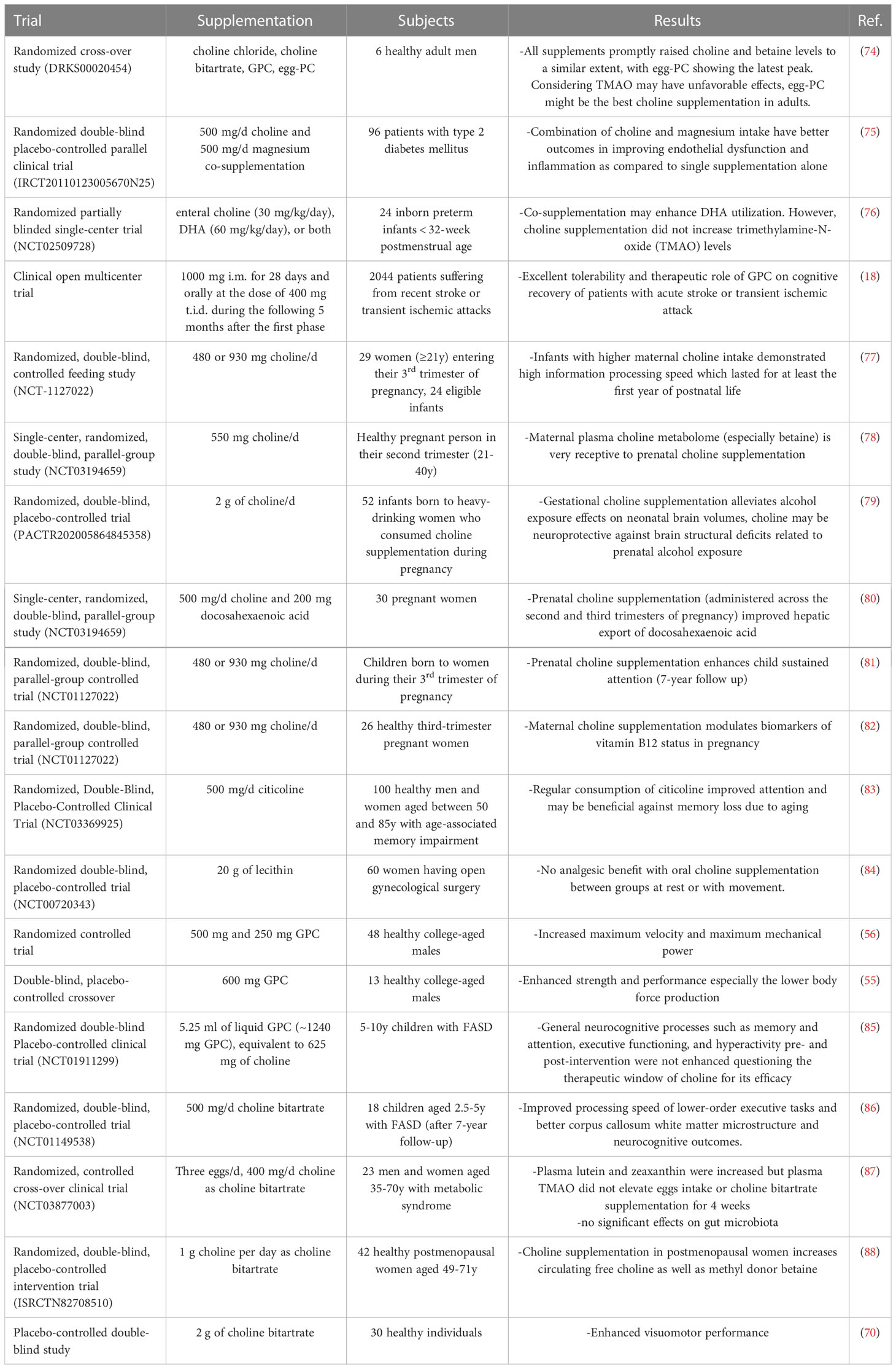
In addition to GPC, other supplements are available to ensure an adequate intake of choline (Table 2). One of the most used is choline bitartrate, which has shown favorable effects both in preclinical and clinical studies, especially in terms of improved cognitive function (67–73).
Importantly, a prospective randomized cross-over study was designed to compare four different choline supplements in terms of their impact on plasma concentration and kinetics of choline; participants received a single dose of 550 mg/d choline equivalent in the form of choline chloride, GPC, egg-PC, and choline bitartrate, in randomized sequence at least 1 week apart; the analysis of these revealed no difference in the area-under-curve of choline plasma concentrations after intake of the different supplements (74).
The main clinical trials assessing the effects of choline supplementation, in different formulations, are reported in Table 3.
Choline bitartrate
Choline bitartrate (C9H19NO7) is a white crystalline powder with no odor. Its molecular weight is 253.25 g/mol with 41.1% choline (104 g/mol choline in 253.25 g/mol choline bitartrate); 2 g of choline bitartrate administration provides 800 mg of choline action (70). It is freely soluble in water, slightly soluble in alcohol and insoluble in ether, chloroform, and benzene.
Choline bitartrate is widely used in dietary supplements. One of the main advantage of bitartrate is its lower hygroscopicity (89), a feature that in the last years has triggered an increase of its use. The methyl donor betaine, a choline derivative, has been shown to facilitate the cytosolic re-methylation of homocysteine to methionine in a reaction catalyzed by the enzyme betaine-homocysteine S-methyltransferase (BHMT). The same reaction is also catalyzed by the methionine synthase, which uses methyl-cobalamin as a co-factor and is a vitamin B12 dependent enzyme (82, 90). Preclinical studies have reported a choline-sparing effect of vitamin B12 supplementation (91–93) and patients deficient in vitamin B12 have lower blood concentrations of choline (94). These aspects provide a strong rationale for the preparation of formulations in which choline, especially choline bitartrate, is associated with vitamin B12.
Lecithin
Lecithin is a mixture of fats and can be obtained from food such as egg yolks (actually, the term lecithin derives from the Greek word λέκιθος, lekythos, which means ‘egg yolk’), soybeans, and nuts (95, 96). PC represents one of the main components of lecithin, albeit the two terms are sometimes used interchangeably. Lecithin is essential to cells in the human body. Since lecithin is converted into ACh, its consumption increases ACh concentrations in the brain (97). Several studies have been carried out showing the effects of consumption of lecithin on hypercholesterolemia and cardiovascular disorders (98, 99).
Citicoline
Citicoline is a brain chemical that occurs naturally in the cells, especially organs, of human and animals. It is a natural precursor of phospholipid synthesis, chiefly PC, and serves as a source of choline in the metabolic pathways for biosynthesis of ACh in the body (100). Citicoline enhances cerebral metabolism and has neuroprotective properties in animals and humans (101–103). Citicoline is effective in facilitating cognitive improvement in various conditions, including vascular and degenerative dementias, cerebrovascular diseases, amyotrophic lateral sclerosis, Alzheimer’s disease, and also Parkinson’s disease (104, 105); indeed, citicoline increases brain dopamine levels and may inhibit dopamine reuptake (104).
Choline supplementation and endothelial dysfunction
Endothelial cells play a crucial role in the exchange of choline and other nutrients between plasma and brain tissue (75, 106–108). Thus, choline must be incorporated into endothelial cells to be transported to the blood-brain barrier (109). Choline supplementation was shown to be effective against hypoxia-induced endothelial dysfunction by Zhang and co-workers, who demonstrated that choline enhanced rat aortic endothelial cell proliferation during hypoxia by secreting vascular endothelial growth factor (VEGF) (6). Moreover, choline supplementation activated the α7 non-neuronal nicotinic ACh receptors (nAChRs) and served as a key function in regulating blood vessels. Thus, choline can be protective against hypoxia-induced endothelial dysfunction (6, 110). Although the benefits of choline have been reported, the exact mechanisms in protecting endothelial function are yet to be fully defined.
Some investigators have reported that endothelial dysfunction is linked with various cardiovascular diseases (111, 112). Several studies have demonstrated the role of high choline intake and its metabolite trimethylamine N‐oxide (TMAO) in endothelial dysfunction and atherosclerosis (111, 113–117). Instead, phloretin, a flavonoid extracted from apple leaves, plays a protective role, and improves vascular endothelial dysfunction and liver injury (111).
Models of endothelial dysfunction like hypoxia or oxygen and glucose deprivation (OGD) were used to evaluate the effects of citicoline on human umbilical vein endothelial cells (HUVECs) and mouse brain microvascular endothelial cells (bEnd.3s) (105, 118–120). Citicoline attenuated the hypoxia/OGD-induced increase in endothelial permeability via upregulating the expression of tight junction proteins including zonula occludens-1, occludin, and claudin-5. Thus, citicoline could be an efficient therapeutic drug for targeting diseases characterized by endothelial barrier breakdown (105).
Choline supplementation and cardio-metabolic disorders
Choline plays a protective role in the heart and may be a promising candidate to improve doxorubicin-induced cardiotoxicity via vagal activity and Nrf2/HO-1 pathway (121). Moreover, choline exhibits protective effects against cardiovascular disorders, including arrhythmias, cardiac hypertrophy, and ischemia/reperfusion (I/R)-induced vascular injury by inhibiting the ROS-mediated Ca2+/calmodulin-dependent protein kinase II pathway (122–124). Citicoline acts as a myocardial protector from I/R injury via inhibiting mitochondrial permeability transition (125). Choline was also shown to ameliorate cardiovascular damage by slowing the progression of hypertension and enhancing cardiac function in spontaneously hypertensive rats (126).
Low amounts of choline can reduce cardiovascular risks and inflammatory markers as they have lowering effect on plasma homocysteine (127). In contrast, a choline‐ or carnitine‐rich diet was reported to promote atherosclerosis in mice as it increased the formation of TMAO produced by gut microbiota-related metabolite of choline (128). Similarly, other papers have reported the association of TMAO with an increased risk of cardiovascular disease and mortality (60, 113, 129–131). Dietary lecithin has shown favorable results with potential application in the treatment of dyslipidemia associated with metabolic disorders (132). Obesity is linked with several cardio-metabolic chronic diseases, such as non-alcoholic fatty acid liver disease (NAFLD), type-2 diabetes, and cardiovascular disease. Numerous studies have also investigated the beneficial effects of lecithin on obesity-related dyslipidemia (132–135). Lecithin-rich diets have hypocholesterolemic effects and display anti-atherogenic properties (136).
Intake of choline and betaine co-supplementation was not associated to cancer or cardiovascular disease; however, an adverse cardiovascular risk factor profile was linked with high choline and low betaine levels in plasma. Therefore, choline and betaine demonstrated opposite relationships with major components of metabolic syndrome (92). Choline and betaine supplementation has not been extensively studied in clinical trials for treating obesity and maintaining normal systemic metabolism. Notwithstanding, Sivanesan and co-workers revealed that choline and betaine administration is favorable for obese and insulin resistant Pcyt2+/- mice; they suggested that choline and betaine supplementations could be beneficial for the treatment of obesity and diabetes due to their participation in mitochondrial oxidative phosphorylation (137).
Choline supplementation and cognitive dysfunction
Environmental factors may contribute to the pathological progression of neurodegenerative diseases and epilepsy. Remarkably, dietary nutrients play an important part in facilitating mechanisms related to brain function (138). As mentioned above, ACh receptors orchestrate the immune response in the central nervous system, and their dysregulation plays a part in the pathogenesis of Alzheimer’s disease (139–144). In fact, Velasquez and collaborators demonstrated that a lifelong choline supplementation may have beneficial cognitive effects such as decreasing amyloid-β plaque load and improving spatial memory in the APP/PS1 mouse model of Alzheimer’s disease. Moreover, consumption of healthy diet throughout life may reduce Alzheimer’s disease pathology (139). In another paper, the same group reported that maternal choline supplementation has profound benefits in Alzheimer’s disease pathology by reducing brain homocysteine levels across multiple generations (145). Several studies have been carried out to investigate the impact of choline supplementation on cognitive functioning in the Ts65Dn mouse model of Down syndrome; for instance, perinatal choline supplementation was reported to enhance emotion regulation in Down syndrome (146). Other studies revealed that maternal choline supplementation improves spatial learning, increases adult hippocampal neurogenesis and basal forebrain cholinergic neurons (147, 148). Bottom and colleagues demonstrated that co-supplementation of choline protects against effects of prenatal ethanol exposure in fetal alcohol spectrum disorder (FASD) offspring (149). Increasing the intake of choline may also reduce spatial memory deficits due to the exposure of chemotherapeutic agents such as cyclophosphamide and doxorubicin in cancer patients (150).
Several researchers have tested the effects of high uptake of dietary choline in elderly patients suffering from impaired memory. A cross-sectional study conducted on ~2400 elderly patients demonstrated that choline intake, defined as the combination of dietary and supplement intake, correlates with cognitive performance (151). Choline supplements in the form of lecithin and choline chloride did not significantly improve memory performance in humans although some papers have reported positive outcomes in cognitive function of animal models (152–156). However, other choline supplements such as citicoline, choline bitartrate, and GPC appear to be very promising in the treatment of elderly patients suffering from dementia (49, 52, 54, 157, 158).
Conclusions
In summary, preclinical and clinical investigations have shown that GPC and other forms of choline supplementation have beneficial effects especially in terms of improved endothelial function and cognitive performance. Notwithstanding, further dedicated studies are warranted to compare the different effects of the currently available forms of choline supplementation.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
The Santulli’s Lab is supported in part by the National Institutes of Health (NIH): National Heart, Lung, and Blood Institute (NHLBI: R01-HL164772, R01-HL159062, R01-HL146691, T32-HL144456), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK: R01-DK123259, R01-DK033823), National Center for Advancing Translational Sciences (NCATS: UL1TR002556-06) to G.S., by the Diabetes Action Research and Education Foundation (to G.S.), and by the Monique Weill-Caulier and Irma T. Hirschl Trusts (to G.S.). U.K. is supported in part by a postdoctoral fellowship of the American Heart Association (AHA-23POST1026190). F.V. is supported in part by a postdoctoral fellowship of the American Heart Association (AHA-22POST995561).
Acknowledgments
The authors thank Dr. Wang for the valuable support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bernhard W, Poets CF, Franz AR. Choline and choline-related nutrients in regular and preterm infant growth. Eur J Nutr (2019) 58:931–45. doi: 10.1007/s00394-018-1834-7
2. Wiedeman AM, Barr SI, Green TJ, Xu Z, Innis SM, Kitts DD. Dietary choline intake: Current state of knowledge across the life cycle. Nutrients (2018) 10:1513. doi: 10.3390/nu10101513
3. Bernhard W, Lange R, Graepler-Mainka U, Engel C, Machann J, Hund V, et al. Choline supplementation in cystic fibrosis-the metabolic and clinical impact. Nutrients (2019) 11:356. doi: 10.3390/nu11030656
4. Wallace TC, Blusztajn JK, Caudill MA, Klatt KC, Natker E, Zeisel SH, et al. Choline: The underconsumed and underappreciated essential nutrient. Nutr Today (2018) 53:240–53. doi: 10.1097/NT.0000000000000302
5. Shim E, Park E. Choline intake and its dietary reference values in Korea and other countries: a review. Nutr Res Pract (2022) 16:S126–33. doi: 10.4162/nrp.2022.16.S1.S126
6. Zhang LC, Jin X, Huang Z, Yan ZN, Li PB, Duan RF, et al. Protective effects of choline against hypoxia-induced injuries of vessels and endothelial cells. Exp Ther Med (2017) 13:2316–24. doi: 10.3892/etm.2017.4276
7. Rees G, Brough L, Orsatti GM, Lodge A, Walker S. Do micronutrient and omega-3 fatty acid supplements affect human maternal immunity during pregnancy? a scoping review. Nutrients (2022) 14:367. doi: 10.3390/nu14020367
8. Blusztajn JK. Choline, a vital amine. Science (1998) 281:794–5. doi: 10.1126/science.281.5378.794
9. da Costa KA, Corbin KD, Niculescu MD, Galanko JA, Zeisel SH. Identification of new genetic polymorphisms that alter the dietary requirement for choline and vary in their distribution across ethnic and racial groups. FASEB J (2014) 28:2970–8. doi: 10.1096/fj.14-249557
10. Derbyshire E, Obeid R, Schon C. Habitual choline intakes across the childbearing years: A review. Nutrients (2021) 13:4390. doi: 10.3390/nu13124390
11. Mun JG, Legette LL, Ikonte CJ, Mitmesser SH. Choline and DHA in maternal and infant nutrition: Synergistic implications in brain and eye health. Nutrients (2019) 11:1125. doi: 10.3390/nu11051125
12. Wiedeman AM, Whitfield KC, March KM, Chen NN, Kroeun H, Sokhoing L, et al. Concentrations of water-soluble forms of choline in human milk from lactating women in Canada and Cambodia. Nutrients (2018) 10:381. doi: 10.3390/nu10030381
13. Friesen RW, Novak EM, Hasman D, Innis SM. Relationship of dimethylglycine, choline, and betaine with oxoproline in plasma of pregnant women and their newborn infants. J Nutr (2007) 137:2641–6. doi: 10.1093/jn/137.12.2641
14. Molloy AM, Mills JL, Cox C, Daly SF, Conley M, Brody LC, et al. Choline and homocysteine interrelations in umbilical cord and maternal plasma at delivery. Am J Clin Nutr (2005) 82:836–42. doi: 10.1093/ajcn/82.4.836
15. Holmes HC, Snodgrass GJ, Iles RA. Changes in the choline content of human breast milk in the first 3 weeks after birth. Eur J Pediatr (2000) 159:198–204. doi: 10.1007/s004310050050
16. Holmes-McNary MQ, Cheng WL, Mar MH, Fussell S, Zeisel SH. Choline and choline esters in human and rat milk and in infant formulas. Am J Clin Nutr (1996) 64:572–6. doi: 10.1093/ajcn/64.4.572
17. Schettini G, Ventra C, Florio T, Grimaldi M, Meucci O, Scorziello A, et al. Molecular mechanisms mediating the effects of l-alpha-glycerylphosphorylcholine, a new cognition-enhancing drug, on behavioral and biochemical parameters in young and aged rats. Pharmacol Biochem Behav (1992) 43:139–51. doi: 10.1016/0091-3057(92)90650-5
18. Barbagallo Sangiorgi G, Barbagallo M, Giordano M, Meli M, Panzarasa R. Alpha-glycerophosphocholine in the mental recovery of cerebral ischemic attacks. an Italian multicenter clinical trial. Ann N Y Acad Sci (1994) 717:253–69. doi: 10.1111/j.1749-6632.1994.tb12095.x
19. Burgess A, Aubert I. Polysialic acid limits choline acetyltransferase activity induced by brain-derived neurotrophic factor. J Neurochem (2006) 99:797–806. doi: 10.1111/j.1471-4159.2006.04110.x
20. Jamal M, Ito A, Tanaka N, Miki T, Takakura A, Suzuki S, et al. The role of apolipoprotein e and ethanol exposure in age-related changes in choline acetyltransferase and brain-derived neurotrophic factor expression in the mouse hippocampus. J Mol Neurosci (2018) 65:84–92. doi: 10.1007/s12031-018-1074-6
21. Kim J, Song Y, Lee SJ, Lee JE, Chung MY, Kim IH, et al. Enzymatic preparation of food-grade l-alpha-glycerylphosphorylcholine from soy phosphatidylcholine or fractionated soy lecithin. Biotechnol Prog (2020) 36:e2910. doi: 10.1002/btpr.2910
22. Oyeneye A, Shen J, Shim YY, Tse TJ, Reaney MJT. Production of alpha-glycerylphosphorylcholine and other compounds from wheat fermentation. ACS Omega (2020) 5:12486–94. doi: 10.1021/acsomega.0c01352
23. Catanesi M, d'Angelo M, Antonosante A, Castelli V, Alfonsetti M, Benedetti E, et al. Neuroprotective potential of choline alfoscerate against beta-amyloid injury: Involvement of neurotrophic signals. Cell Biol Int (2020) 44:1734–44. doi: 10.1002/cbin.11369
24. Tayebati SK, Di Tullio MA, Tomassoni D, Amenta F. Neuroprotective effect of treatment with galantamine and choline alphoscerate on brain microanatomy in spontaneously hypertensive rats. J Neurol Sci (2009) 283:187–94. doi: 10.1016/j.jns.2009.02.349
25. Kang M, Lee DB, Kwon S, Lee E, Kim WJ. Effectiveness of nootropics in combination with cholinesterase inhibitors on cognitive function in mild-to-Moderate dementia: A study using real-world data. J Clin Med (2022) 11:4661. doi: 10.3390/jcm11164661
26. Lee SH, Choi BY, Kim JH, Kho AR, Sohn M, Song HK, et al. Late treatment with choline alfoscerate (l-alpha glycerylphosphorylcholine, alpha-GPC) increases hippocampal neurogenesis and provides protection against seizure-induced neuronal death and cognitive impairment. Brain Res (2017) 1654:66–76. doi: 10.1016/j.brainres.2016.10.011
27. Roy P, Tomassoni D, Nittari G, Traini E, Amenta F. Effects of choline containing phospholipids on the neurovascular unit: A review. Front Cell Neurosci (2022) 16:988759. doi: 10.3389/fncel.2022.988759
28. Tayebati SK, Marucci G, Santinelli C, Buccioni M, Amenta F. Choline-containing phospholipids: Structure-activity relationships versus therapeutic applications. Curr Med Chem (2015) 22:4328–40. doi: 10.2174/0929867322666151029104152
29. Jeong Yu H, Lin Kim Y, Jung Kim M, Mee Park J, Young Park S, Nae Park S, et al. The effect of choline alphoscerate on non spatial memory and neuronal differentiation in a rat model of dual stress. Brain Res (2022) 1786:147900. doi: 10.1016/j.brainres.2022.147900
30. Narukawa M, Kamiyoshihara A, Izu H, Fujii T, Matsubara K, Misaka T. Efficacy of long-term feeding of alpha-glycerophosphocholine for aging-related phenomena in old mice. Gerontology (2020) 66:275–85. doi: 10.1159/000504962
31. Burgaletto C, Di Benedetto G, Munafo A, Bernardini R, Cantarella G. Beneficial effects of choline alphoscerate on amyloid-beta neurotoxicity in an In vitro model of alzheimer's disease. Curr Alzheimer Res (2021) 18:298–309. doi: 10.2174/1567205018666210608093658
32. Utz J, Berner J, Munoz LE, Oberstein TJ, Kornhuber J, Herrmann M, et al. Cerebrospinal fluid of patients with alzheimer's disease contains increased percentages of synaptophysin-bearing microvesicles. Front Aging Neurosci (2021) 13:682115. doi: 10.3389/fnagi.2021.682115
33. Yuki D, Sugiura Y, Zaima N, Akatsu H, Takei S, Yao I, et al. DHA-PC and PSD-95 decrease after loss of synaptophysin and before neuronal loss in patients with alzheimer's disease. Sci Rep (2014) 4:7130. doi: 10.1038/srep07130
34. Kshirsagar S, Sawant N, Morton H, Reddy AP, Reddy PH. Mitophagy enhancers against phosphorylated tau-induced mitochondrial and synaptic toxicities in Alzheimer disease. Pharmacol Res (2021) 174:105973. doi: 10.1016/j.phrs.2021.105973
35. Sigala S, Imperato A, Rizzonelli P, Casolini P, Missale C, Spano P. L-alpha-glycerylphosphorylcholine antagonizes scopolamine-induced amnesia and enhances hippocampal cholinergic transmission in the rat. Eur J Pharmacol (1992) 211:351–8. doi: 10.1016/0014-2999(92)90392-H
36. Drago F, Mauceri F, Nardo L, Valerio C, Lauria N, Rampello L, et al. Behavioral effects of l-alpha-glycerylphosphorylcholine: influence on cognitive mechanisms in the rat. Pharmacol Biochem Behav (1992) 41:445–8. doi: 10.1016/0091-3057(92)90124-X
37. Lopez CM, Govoni S, Battaini F, Bergamaschi S, Longoni A, Giaroni C, et al. Effect of a new cognition enhancer, alpha-glycerylphosphorylcholine, on scopolamine-induced amnesia and brain acetylcholine. Pharmacol Biochem Behav (1991) 39:835–40. doi: 10.1016/0091-3057(91)90040-9
38. Trabucchi M, Govoni S, Battaini F. Changes in the interaction between CNS cholinergic and dopaminergic neurons induced by l-alpha-glycerylphosphorylcholine, a cholinomimetic drug. Farmaco Sci (1986) 41:325–34.
39. Tayebati SK, Martinelli I, Moruzzi M, Amenta F, Tomassoni D. Choline and choline alphoscerate do not modulate inflammatory processes in the rat brain. Nutrients (2017) 9:1084. doi: 10.3390/nu9101084
40. Strifler G, Tuboly E, Gorbe A, Boros M, Pecz D, Hartmann P. Targeting mitochondrial dysfunction with l-alpha glycerylphosphorylcholine. PloS One (2016) 11:e0166682. doi: 10.1371/journal.pone.0166682
41. Muccioli G, Raso GM, Ghe C, Di Carlo R. Effect of l-alpha glycerylphosphorylcholine on muscarinic receptors and membrane microviscosity of aged rat brain. Prog Neuropsychopharmacol Biol Psychiatry (1996) 20:323–39. doi: 10.1016/0278-5846(95)00313-4
42. Aleppo G, Nicoletti F, Sortino MA, Casabona G, Scapagnini U, Canonico PL. Chronic l-alpha-glyceryl-phosphoryl-choline increases inositol phosphate formation in brain slices and neuronal cultures. Pharmacol Toxicol (1994) 74:95–100. doi: 10.1111/j.1600-0773.1994.tb01082.x
43. Onishchenko LS, Gaikova ON, Yanishevskii SN. Changes at the focus of experimental ischemic stroke treated with neuroprotective agents. Neurosci Behav Physiol (2008) 38:49–54. doi: 10.1007/s11055-008-0007-1
44. Bramanti V, Tomassoni D, Grasso S, Bronzi D, Napoli M, Campisi A, et al. Cholinergic precursors modulate the expression of heme oxigenase-1, p21 during astroglial cell proliferation and differentiation in culture. Neurochem Res (2012) 37:2795–804. doi: 10.1007/s11064-012-0873-3
45. Parnetti L, Mignini F, Tomassoni D, Traini E, Amenta F. Cholinergic precursors in the treatment of cognitive impairment of vascular origin: Ineffective approaches or need for re-evaluation? J Neurol Sci (2007) 257:264–9. doi: 10.1016/j.jns.2007.01.043
46. Amenta F, Tayebati SK. Pathways of acetylcholine synthesis, transport and release as targets for treatment of adult-onset cognitive dysfunction. Curr Med Chem (2008) 15:488–98. doi: 10.2174/092986708783503203
47. Kawamura T, Okubo T, Sato K, Fujita S, Goto K, Hamaoka T, et al. Glycerophosphocholine enhances growth hormone secretion and fat oxidation in young adults. Nutrition (2012) 28:1122–6. doi: 10.1016/j.nut.2012.02.011
48. Di Perri R, Coppola G, Ambrosio LA, Grasso A, Puca FM, Rizzo M. A multicentre trial to evaluate the efficacy and tolerability of alpha-glycerylphosphorylcholine versus cytosine diphosphocholine in patients with vascular dementia. J Int Med Res (1991) 19:330–41. doi: 10.1177/030006059101900406
49. De Jesus Moreno Moreno M. Cognitive improvement in mild to moderate alzheimer's dementia after treatment with the acetylcholine precursor choline alfoscerate: a multicenter, double-blind, randomized, placebo-controlled trial. Clin Ther (2003) 25:178–93. doi: 10.1016/S0149-2918(03)90023-3
50. Kolykhalov IV, Androsova LV, Gavrilova SI. [Clinical and immunological effects of choline alfoscerate in the treatment of amnestic type mild cognitive impairment]. Zh Nevrol Psikhiatr Im S S Korsakova (2022) 122:59–66. doi: 10.17116/jnevro202212211259
51. Gavrilova SI, Kolykhalov IV, Ponomareva EV, Fedorova YB, Selezneva ND. [Clinical efficacy and safety of choline alfoscerate in the treatment of late-onset cognitive impairment]. Zh Nevrol Psikhiatr Im S S Korsakova (2018) 118:45–53. doi: 10.17116/jnevro20181185145
52. Scapicchio PL. Revisiting choline alphoscerate profile: A new, perspective, role in dementia? Int J Neurosci (2013) 123:444–9. doi: 10.3109/00207454.2013.765870
53. Parnetti L, Abate G, Bartorelli L, Cucinotta D, Cuzzupoli M, Maggioni M, et al. Multicentre study of l-alpha-glyceryl-phosphorylcholine vs ST200 among patients with probable senile dementia of alzheimer's type. Drugs Aging (1993) 3:159–64. doi: 10.2165/00002512-199303020-00006
54. Parnetti L, Amenta F, Gallai V. Choline alphoscerate in cognitive decline and in acute cerebrovascular disease: An analysis of published clinical data. Mech Ageing Dev (2001) 122:2041–55. doi: 10.1016/S0047-6374(01)00312-8
55. Bellar D, LeBlanc NR, Campbell B. The effect of 6 days of alpha glycerylphosphorylcholine on isometric strength. J Int Soc Sports Nutr (2015) 12:42. doi: 10.1186/s12970-015-0103-x
56. Marcus L, Soileau J, Judge LW, Bellar D. Evaluation of the effects of two doses of alpha glycerylphosphorylcholine on physical and psychomotor performance. J Int Soc Sports Nutr (2017) 14:39. doi: 10.1186/s12970-017-0196-5
57. Barzanjeh SP, Pescatello LS, Figueroa A, Ahmadizad S. The effects of alpha-glycerylphosphorylcholine on heart rate variability and hemodynamic variables following sprint interval exercise in overweight and obese women. Nutrients (2022) 14:3970. doi: 10.3390/nu14193970
58. Tamura Y, Takata K, Matsubara K, Kataoka Y. Alpha-glycerylphosphorylcholine increases motivation in healthy volunteers: A single-blind, randomized, placebo-controlled human study. Nutrients (2021) 13:1091. doi: 10.3390/nu13062091
59. Hoffman JR, Ratamess NA, Gonzalez A, Beller NA, Hoffman MW, Olson M, et al. The effects of acute and prolonged CRAM supplementation on reaction time and subjective measures of focus and alertness in healthy college students. J Int Soc Sports Nutr (2010) 7:39. doi: 10.1186/1550-2783-7-39
60. Lee G, Choi S, Chang J, Choi D, Son JS, Kim K, et al. Association of l-alpha glycerylphosphorylcholine with subsequent stroke risk after 10 years. JAMA Netw Open (2021) 4:e2136008. doi: 10.1001/jamanetworkopen.2021.36008
61. Wang Z, Hazen J, Jia X, Org E, Zhao Y, Osborn LJ, et al. The nutritional supplement l-alpha glycerylphosphorylcholine promotes atherosclerosis. Int J Mol Sci (2021) 22. doi: 10.3390/ijms222413477
62. Na G, Kwak SH, Jang SH, Noh HE, Kim J, Yang S, et al. Supplementary effect of choline alfoscerate on speech recognition in patients with age-related hearing loss: A prospective study in 34 patients (57 ears). Front Aging Neurosci (2021) 13:684519. doi: 10.3389/fnagi.2021.684519
63. Choi JJ, Hwang JS, Shin YJ. Effect of oral choline alfoscerate on patients with keratoconjunctivitis sicca. Nutrients (2020) 12:1526. doi: 10.3390/nu12051526
64. Martone G, Balestrazzi A, Ciprandi G, Balestrazzi A. Alpha-glycerylphosphorylcholine and d-panthenol eye drops in patients undergoing cataract surgery. J Ophthalmol (2022) 2022:1951014. doi: 10.1155/2022/1951014
65. Greiner JV, Glonek T, Korb DR, Lindsay ME, Oliver PJ, Olson MCD. Corneal cryopreservation using glycerylphosphorylcholine-enriched medium. Cornea (2020) 39:370–5. doi: 10.1097/ICO.0000000000002214
66. Hwang JS, Shin YJ. Role of choline in ocular diseases. Int J Mol Sci (2021) 22. doi: 10.3390/ijms22094733
67. Hammoud R, Pannia E, Kubant R, Metherel A, Simonian R, Pausova Z, et al. High choline intake during pregnancy reduces characteristics of the metabolic syndrome in Male wistar rat offspring fed a high fat but not a normal fat post-weaning diet. Nutrients (2021) 13:1438. doi: 10.3390/nu13051438
68. Kitagawa E, Ota Y, Hasegawa M, Nakagawa T, Hayakawa T. Accumulation of liver lipids induced by vitamin B(6) deficiency was effectively ameliorated by choline and, to a lesser extent, betaine. J Nutr Sci Vitaminol (Tokyo) (2019) 65:94–101. doi: 10.3177/jnsv.65.94
69. Lyoo IK, Demopulos CM, Hirashima F, Ahn KH, Renshaw PF. Oral choline decreases brain purine levels in lithium-treated subjects with rapid-cycling bipolar disorder: a double-blind trial using proton and lithium magnetic resonance spectroscopy. Bipolar Disord (2003) 5:300–6. doi: 10.1034/j.1399-5618.2003.00041.x
70. Naber M, Hommel B, Colzato LS. Improved human visuomotor performance and pupil constriction after choline supplementation in a placebo-controlled double-blind study. Sci Rep (2015) 5:13188. doi: 10.1038/srep13188
71. Jacobson SW, Carter RC, Molteno CD, Stanton ME, Herbert JS, Lindinger NM, et al. Efficacy of maternal choline supplementation during pregnancy in mitigating adverse effects of prenatal alcohol exposure on growth and cognitive function: A randomized, double-blind, placebo-controlled clinical trial. Alcohol Clin Exp Res (2018) 42:1327–41. doi: 10.1111/acer.13769
72. Derbyshire E, Obeid R. Choline, neurological development and brain function: A systematic review focusing on the first 1000 days. Nutrients (2020) 12:1731. doi: 10.3390/nu12061731
73. Tabassum S, Haider S, Ahmad S, Madiha S, Parveen T. Chronic choline supplementation improves cognitive and motor performance via modulating oxidative and neurochemical status in rats. Pharmacol Biochem Behav (2017) 159:90–9. doi: 10.1016/j.pbb.2017.05.011
74. Bockmann KA, Franz AR, Minarski M, Shunova A, Maiwald CA, Schwarz J, et al. Differential metabolism of choline supplements in adult volunteers. Eur J Nutr (2022) 61:219–30. doi: 10.1007/s00394-021-02637-6
75. Rashvand S, Mobasseri M, Tarighat-Esfanjani A. The effects of choline and magnesium Co-supplementation on metabolic parameters, inflammation, and endothelial dysfunction in patients with type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled trial. J Am Coll Nutr (2019) 38:714–21. doi: 10.1080/07315724.2019.1599745
76. Bernhard W, Bockmann K, Maas C, Mathes M, Hovelmann J, Shunova A, et al. Combined choline and DHA supplementation: a randomized controlled trial. Eur J Nutr (2020) 59:729–39. doi: 10.1007/s00394-019-01940-7
77. Caudill MA, Strupp BJ, Muscalu L, Nevins JEH, Canfield RL. Maternal choline supplementation during the third trimester of pregnancy improves infant information processing speed: A randomized, double-blind, controlled feeding study. FASEB J (2018) 32:2172–80. doi: 10.1096/fj.201700692RR
78. Taesuwan S, McDougall MQ, Malysheva OV, Bender E, Nevins JEH, Devapatla S, et al. Choline metabolome response to prenatal choline supplementation across pregnancy: A randomized controlled trial. FASEB J (2021) 35:e22063. doi: 10.1096/fj.202101401RR
79. Warton FL, Molteno CD, Warton CMR, Wintermark P, Lindinger NM, Dodge NC, et al. Maternal choline supplementation mitigates alcohol exposure effects on neonatal brain volumes. Alcohol Clin Exp Res (2021) 45:1762–74. doi: 10.1111/acer.14672
80. Klatt KC, McDougall MQ, Malysheva OV, Taesuwan S, Loinard-Gonzalez AAP, Nevins JEH, et al. Prenatal choline supplementation improves biomarkers of maternal docosahexaenoic acid (DHA) status among pregnant participants consuming supplemental DHA: a randomized controlled trial. Am J Clin Nutr (2022) 116:820–32. doi: 10.1093/ajcn/nqac147
81. Bahnfleth CL, Strupp BJ, Caudill MA, Canfield RL. Prenatal choline supplementation improves child sustained attention: A 7-year follow-up of a randomized controlled feeding trial. FASEB J (2022) 36:e22054. doi: 10.1096/fj.202101217R
82. King JH, Kwan STC, Bae S, Klatt KC, Yan J, Malysheva OV, et al. Maternal choline supplementation alters vitamin b-12 status in human and murine pregnancy. J Nutr Biochem (2019) 72:108210. doi: 10.1016/j.jnutbio.2019.07.001
83. Nakazaki E, Mah E, Sanoshy K, Citrolo D, Watanabe F. Citicoline and memory function in healthy older adults: A randomized, double-blind, placebo-controlled clinical trial. J Nutr (2021) 151:2153–60. doi: 10.1093/jn/nxab119
84. Sidhu N, Davies S, Nadarajah A, Rivera J, Whittington R, Mercier RJ, et al. Oral choline supplementation for postoperative pain. Br J Anaesth (2013) 111:249–55. doi: 10.1093/bja/aet031
85. Nguyen TT, Risbud RD, Mattson SN, Chambers CD, Thomas JD. Randomized, double-blind, placebo-controlled clinical trial of choline supplementation in school-aged children with fetal alcohol spectrum disorders. Am J Clin Nutr (2016) 104:1683–92. doi: 10.3945/ajcn.116.142075
86. Gimbel BA, Anthony ME, Ernst AM, Roediger DJ, de Water E, Eckerle JK, et al. Long-term follow-up of a randomized controlled trial of choline for neurodevelopment in fetal alcohol spectrum disorder: corpus callosum white matter microstructure and neurocognitive outcomes. J Neurodev Disord (2022) 14:59. doi: 10.1186/s11689-022-09470-w
87. Thomas MS, DiBella M, Blesso CN, Malysheva O, Caudill M, Sholola M, et al. Comparison between egg intake versus choline supplementation on gut microbiota and plasma carotenoids in subjects with metabolic syndrome. Nutrients (2022) 14:1171. doi: 10.3390/nu14061179
88. Wallace JM, McCormack JM, McNulty H, Walsh PM, Robson PJ, Bonham MP, et al. Choline supplementation and measures of choline and betaine status: A randomised, controlled trial in postmenopausal women. Br J Nutr (2012) 108:1264–71. doi: 10.1017/S000711451100674X
89. Gangurde AB, Sav AK, Javeer SD, Moravkar KK, Pawar JN, Amin PD. Modified extrusion-spheronization as a technique of microencapsulation for stabilization of choline bitartrate using hydrogenated soya bean oil. Int J Pharm Investig (2015) 5:275–83. doi: 10.4103/2230-973X.167696
90. Sasaki H, Matsuzaki Y, Meguro K, Ikarashi Y, Maruyama Y, Yamaguchi S, et al. Vitamin B12 improves cognitive disturbance in rodents fed a choline-deficient diet. Pharmacol Biochem Behav (1992) 43:635–9. doi: 10.1016/0091-3057(92)90204-S
91. Schaefer AE, Salmon WD, Strength DR. Interrelationship of vitamin B12 and choline; effect on hemorrhagic kidney syndrome in the rat. Proc Soc Exp Biol Med (1949) 71:193–6. doi: 10.3181/00379727-71-17130
92. Ueland PM. Choline and betaine in health and disease. J Inherit Metab Dis (2011) 34:3–15. doi: 10.1007/s10545-010-9088-4
93. Schaefer AE, Salmon WD, Strength DR, Copeland DH. Interrelationship of folacin, vitamin B12 and choline; effect on hemorrhagic kidney syndrome in the rat and on growth of the chick. J Nutr (1950) 40:95–111. doi: 10.1093/jn/40.1.95
94. Wu BT, Innis SM, Mulder KA, Dyer RA, King DJ. Low plasma vitamin b-12 is associated with a lower pregnancy-associated rise in plasma free choline in Canadian pregnant women and lower postnatal growth rates in their male infants. Am J Clin Nutr (2013) 98:1209–17. doi: 10.3945/ajcn.113.060269
95. Zhu X, Wang Q, Leng Y, Chen F, Wu F, Mu G, et al. Lecithin alleviates protein flocculation and enhances fat digestion in a model of infant formula emulsion. Food Chem (2021) 346:128918. doi: 10.1016/j.foodchem.2020.128918
96. Higgins JP, Flicker L. Lecithin for dementia and cognitive impairment. Cochrane Database Syst Rev (2003), CD001015. doi: 10.1002/14651858.CD001015
97. Hirsch MJ, Wurtman RJ. Lecithin consumption increases acetylcholine concentrations in rat brain and adrenal gland. Science (1978) 202:223–5. doi: 10.1126/science.694529
98. Mourad AM, de Carvalho Pincinato E, Mazzola PG, Sabha M, Moriel P. Influence of soy lecithin administration on hypercholesterolemia. Cholesterol (2010) 2010:824813. doi: 10.1155/2010/824813
99. Rousset X, Vaisman B, Amar M, Sethi AA, Remaley AT. Lecithin: Cholesterol acyltransferase–from biochemistry to role in cardiovascular disease. Curr Opin Endocrinol Diabetes Obes (2009) 16:163–71. doi: 10.1097/MED.0b013e328329233b
100. Gareri P, Castagna A, Cotroneo AM, Putignano S, De Sarro G, Bruni AC. The role of citicoline in cognitive impairment: pharmacological characteristics, possible advantages, and doubts for an old drug with new perspectives. Clin Interv Aging (2015) 10:1421–9. doi: 10.2147/CIA.S87886
101. Synoradzki K, Grieb P. Citicoline: A superior form of choline? Nutrients (2019) 11:1569. doi: 10.3390/nu11071569
102. Jasielski P, Piedel F, Piwek M, Rocka A, Petit V, Rejdak K. Application of citicoline in neurological disorders: A systematic review. Nutrients (2020) 12:3113. doi: 10.3390/nu12103113
103. Marti-Carvajal AJ, Valli C, Marti-Amarista CE, Sola I, Marti-Fabregas J, Bonfill Cosp X. Citicoline for treating people with acute ischemic stroke. Cochrane Database Syst Rev (2020) 8:CD013066. doi: 10.1002/14651858.CD013066.pub2
104. Que DS, Jamora RDG. Citicoline as adjuvant therapy in parkinson's disease: A systematic review. Clin Ther (2021) 43:e19–31. doi: 10.1016/j.clinthera.2020.11.009
105. Ma X, Zhang H, Pan Q, Zhao Y, Chen J, Zhao B, et al. Hypoxia/Aglycemia-induced endothelial barrier dysfunction and tight junction protein downregulation can be ameliorated by citicoline. PloS One (2013) 8:e82604. doi: 10.1371/journal.pone.0082604
106. Bae CR, Zhang H, Kwon YG. The endothelial dysfunction blocker CU06-1004 ameliorates choline-deficient l-amino acid diet-induced non-alcoholic steatohepatitis in mice. PloS One (2020) 15:e0243497. doi: 10.1371/journal.pone.0243497
107. Dai X, Wang K, Fan J, Liu H, Fan X, Lin Q, et al. Nrf2 transcriptional upregulation of IDH2 to tune mitochondrial dynamics and rescue angiogenic function of diabetic EPCs. Redox Biol (2022) 56:102449. doi: 10.1016/j.redox.2022.102449
108. Ciccarelli M, Cipolletta E, Santulli G, Campanile A, Pumiglia K, Cervero P, et al. Endothelial beta2 adrenergic signaling to AKT: Role of gi and SRC. Cell Signal (2007) 19:1949–55. doi: 10.1016/j.cellsig.2007.05.007
109. Estrada C, Bready J, Berliner J, Cancilla PA. Choline uptake by cerebral capillary endothelial cells in culture. J Neurochem (1990) 54:1467–73. doi: 10.1111/j.1471-4159.1990.tb01193.x
110. Mehta AK, Arora N, Gaur SN, Singh BP. Choline supplementation reduces oxidative stress in mouse model of allergic airway disease. Eur J Clin Invest (2009) 39:934–41. doi: 10.1111/j.1365-2362.2009.02190.x
111. Ren D, Liu Y, Zhao Y, Yang X. Hepatotoxicity and endothelial dysfunction induced by high choline diet and the protective effects of phloretin in mice. Food Chem Toxicol (2016) 94:203–12. doi: 10.1016/j.fct.2016.06.004
112. Poredos P. Endothelial dysfunction and cardiovascular disease. Pathophysiol Haemost Thromb (2002) 32:274–7. doi: 10.1159/000073580
113. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature (2011) 472:57–63. doi: 10.1038/nature09922
114. Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med (2013) 368:1575–84. doi: 10.1056/NEJMoa1109400
115. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med (2013) 19:576–85. doi: 10.1038/nm.3145
116. Bennett BJ, de Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, et al. Trimethylamine-n-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab (2013) 17:49–60. doi: 10.1016/j.cmet.2012.12.011
117. Thomas MS, Fernandez ML. Trimethylamine n-oxide (TMAO), diet and cardiovascular disease. Curr Atheroscler Rep (2021) 23:12. doi: 10.1007/s11883-021-00910-x
118. Brown RC, Davis TP. Hypoxia/aglycemia alters expression of occludin and actin in brain endothelial cells. Biochem Biophys Res Commun (2005) 327:1114–23. doi: 10.1016/j.bbrc.2004.12.123
119. Koto T, Takubo K, Ishida S, Shinoda H, Inoue M, Tsubota K, et al. Hypoxia disrupts the barrier function of neural blood vessels through changes in the expression of claudin-5 in endothelial cells. Am J Pathol (2007) 170:1389–97. doi: 10.2353/ajpath.2007.060693
120. Zhu H, Wang Z, Xing Y, Gao Y, Ma T, Lou L, et al. Baicalin reduces the permeability of the blood-brain barrier during hypoxia in vitro by increasing the expression of tight junction proteins in brain microvascular endothelial cells. J Ethnopharmacol (2012) 141:714–20. doi: 10.1016/j.jep.2011.08.063
121. Guo F, Wang Y, Wang J, Liu Z, Lai Y, Zhou Z, et al. Choline protects the heart from doxorubicin-induced cardiotoxicity through vagal activation and Nrf2/HO-1 pathway. Oxid Med Cell Longev (2022) 2022:4740931. doi: 10.1155/2022/4740931
122. Xu M, Xue RQ, Lu Y, Yong SY, Wu Q, Cui YL, et al. Choline ameliorates cardiac hypertrophy by regulating metabolic remodelling and UPRmt through SIRT3-AMPK pathway. Cardiovasc Res (2019) 115:530–45. doi: 10.1093/cvr/cvy217
123. Lu XZ, Bi XY, He X, Zhao M, Xu M, Yu XJ, et al. Activation of M3 cholinoceptors attenuates vascular injury after ischaemia/reperfusion by inhibiting the Ca2+/calmodulin-dependent protein kinase II pathway. Br J Pharmacol (2015) 172:5619–33. doi: 10.1111/bph.13183
124. Yilmaz MS, Coskun C, Yalcin M, Savci V. CDP-choline prevents cardiac arrhythmias and lethality induced by short-term myocardial ischemia-reperfusion injury in the rat: Involvement of central muscarinic cholinergic mechanisms. Naunyn Schmiedebergs Arch Pharmacol (2008) 378:293–301. doi: 10.1007/s00210-008-0300-0
125. Hernandez-Esquivel L, Pavon N, Buelna-Chontal M, Gonzalez-Pacheco H, Belmont J, Chavez E. Citicoline (CDP-choline) protects myocardium from ischemia/reperfusion injury via inhibiting mitochondrial permeability transition. Life Sci (2014) 96:53–8. doi: 10.1016/j.lfs.2013.12.026
126. Liu L, Lu Y, Bi X, Xu M, Yu X, Xue R, et al. Choline ameliorates cardiovascular damage by improving vagal activity and inhibiting the inflammatory response in spontaneously hypertensive rats. Sci Rep (2017) 7:42553. doi: 10.1038/srep42553
127. Wortmann SB, Mayr JA. Choline-related-inherited metabolic diseases-a mini review. J Inherit Metab Dis (2019) 42:237–42. doi: 10.1002/jimd.12011
128. Yu ZL, Zhang LY, Jiang XM, Xue CH, Chi N, Zhang TT, et al. Effects of dietary choline, betaine, and l-carnitine on the generation of trimethylamine-n-oxide in healthy mice. J Food Sci (2020) 85:2207–15. doi: 10.1111/1750-3841.15186
129. Nam HS. Gut microbiota and ischemic stroke: The role of trimethylamine n-oxide. J Stroke (2019) 21:151–9. doi: 10.5853/jos.2019.00472
130. Haghikia A, Li XS, Liman TG, Bledau N, Schmidt D, Zimmermann F, et al. Gut microbiota-dependent trimethylamine n-oxide predicts risk of cardiovascular events in patients with stroke and is related to proinflammatory monocytes. Arterioscler Thromb Vasc Biol (2018) 38:2225–35. doi: 10.1161/ATVBAHA.118.311023
131. Heianza Y, Ma W, Manson JE, Rexrode KM, Qi L. Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: A systematic review and meta-analysis of prospective studies. J Am Heart Assoc (2017) 6:e004947. doi: 10.1161/JAHA.116.004947
132. Robert C, Couedelo L, Vaysse C, Michalski MC. Vegetable lecithins: A review of their compositional diversity, impact on lipid metabolism and potential in cardiometabolic disease prevention. Biochimie (2020) 169:121–32. doi: 10.1016/j.biochi.2019.11.017
133. Mastellone I, Polichetti E, Gres S, de la Maisonneuve C, Domingo N, Marin V, et al. Dietary soybean phosphatidylcholines lower lipidemia: Mechanisms at the levels of intestine, endothelial cell, and hepato-biliary axis. J Nutr Biochem (2000) 11:461–6. doi: 10.1016/S0955-2863(00)00115-7
134. Buang Y, Wang YM, Cha JY, Nagao K, Yanagita T. Dietary phosphatidylcholine alleviates fatty liver induced by orotic acid. Nutrition (2005) 21:867–73. doi: 10.1016/j.nut.2004.11.019
135. Lee HS, Nam Y, Chung YH, Kim HR, Park ES, Chung SJ, et al. Beneficial effects of phosphatidylcholine on high-fat diet-induced obesity, hyperlipidemia and fatty liver in mice. Life Sci (2014) 118:7–14. doi: 10.1016/j.lfs.2014.09.027
136. Wilson TA, Meservey CM, Nicolosi RJ. Soy lecithin reduces plasma lipoprotein cholesterol and early atherogenesis in hypercholesterolemic monkeys and hamsters: Beyond linoleate. Atherosclerosis (1998) 140:147–53. doi: 10.1016/S0021-9150(98)00132-4
137. Sivanesan S, Taylor A, Zhang J, Bakovic M. Betaine and choline improve lipid homeostasis in obesity by participation in mitochondrial oxidative demethylation. Front Nutr (2018) 5:61. doi: 10.3389/fnut.2018.00061
138. Gomez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci (2008) 9:568–78. doi: 10.1038/nrn2421
139. Velazquez R, Ferreira E, Knowles S, Fux C, Rodin A, Winslow W, et al. Lifelong choline supplementation ameliorates alzheimer's disease pathology and associated cognitive deficits by attenuating microglia activation. Aging Cell (2019) 18:e13037. doi: 10.1111/acel.13037
140. Winek K, Soreq H, Meisel A. Regulators of cholinergic signaling in disorders of the central nervous system. J Neurochem (2021) 158:1425–38. doi: 10.1111/jnc.15332
141. Takata K, Amamiya T, Mizoguchi H, Kawanishi S, Kuroda E, Kitamura R, et al. Alpha7 nicotinic acetylcholine receptor-specific agonist DMXBA (GTS-21) attenuates abeta accumulation through suppression of neuronal gamma-secretase activity and promotion of microglial amyloid-beta phagocytosis and ameliorates cognitive impairment in a mouse model of alzheimer's disease. Neurobiol Aging (2018) 62:197–209. doi: 10.1016/j.neurobiolaging.2017.10.021
142. Lehner KR, Silverman HA, Addorisio ME, Roy A, Al-Onaizi MA, Levine Y, et al. Forebrain cholinergic signaling regulates innate immune responses and inflammation. Front Immunol (2019) 10:585. doi: 10.3389/fimmu.2019.00585
143. Reale M, Costantini E. Cholinergic modulation of the immune system in neuroinflammatory diseases. Diseases (2021) 9:29. doi: 10.3390/diseases9020029
144. Neumann S, Shields NJ, Balle T, Chebib M, Clarkson AN. Innate immunity and inflammation post-stroke: An alpha7-nicotinic agonist perspective. Int J Mol Sci (2015) 16:29029–46. doi: 10.3390/ijms161226141
145. Velazquez R, Ferreira E, Winslow W, Dave N, Piras IS, Naymik M, et al. Maternal choline supplementation ameliorates alzheimer's disease pathology by reducing brain homocysteine levels across multiple generations. Mol Psychiatry (2020) 25:2620–9. doi: 10.1038/s41380-018-0322-z
146. Moon J, Chen M, Gandhy SU, Strawderman M, Levitsky DA, Maclean KN, et al. Perinatal choline supplementation improves cognitive functioning and emotion regulation in the Ts65Dn mouse model of down syndrome. Behav Neurosci (2010) 124:346–61. doi: 10.1037/a0019590
147. Velazquez R, Ash JA, Powers BE, Kelley CM, Strawderman M, Luscher ZI, et al. Maternal choline supplementation improves spatial learning and adult hippocampal neurogenesis in the Ts65Dn mouse model of down syndrome. Neurobiol Dis (2013) 58:92–101. doi: 10.1016/j.nbd.2013.04.016
148. Powers BE, Kelley CM, Velazquez R, Ash JA, Strawderman MS, Alldred MJ, et al. Maternal choline supplementation in a mouse model of down syndrome: Effects on attention and nucleus basalis/substantia innominata neuron morphology in adult offspring. Neuroscience (2017) 340:501–14. doi: 10.1016/j.neuroscience.2016.11.001
149. Bottom RT, Abbott CW 3rd, Huffman KJ. Rescue of ethanol-induced FASD-like phenotypes via prenatal co-administration of choline. Neuropharmacology (2020) 168:107990. doi: 10.1016/j.neuropharm.2020.107990
150. Johns BE, Ficken M, Engberg ME, Wecker L, Philpot RM. Increasing dietary choline attenuates spatial memory deficits resulting from exposure to the chemotherapeutic agents cyclophosphamide and doxorubicin. J Psychopharmacol (2021) 35:1300–9. doi: 10.1177/02698811211029752
151. Liu L, Qiao S, Zhuang L, Xu S, Chen L, Lai Q, et al. Choline intake correlates with cognitive performance among elder adults in the united states. Behav Neurol (2021) 2021:2962245. doi: 10.1155/2021/2962245
152. Bartus RT. On neurodegenerative diseases, models, and treatment strategies: Lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp Neurol (2000) 163:495–529. doi: 10.1006/exnr.2000.7397
153. Craig LA, Hong NS, McDonald RJ. Revisiting the cholinergic hypothesis in the development of alzheimer's disease. Neurosci Biobehav Rev (2011) 35:1397–409. doi: 10.1016/j.neubiorev.2011.03.001
154. Mohs RC, Davis KL, Tinklenberg JR, Hollister LE. Choline chloride effects on memory in the elderly. Neurobiol Aging (1980) 1:21–5. doi: 10.1016/0197-4580(80)90020-2
155. Thal LJ, Rosen W, Sharpless NS, Crystal H. Choline chloride fails to improve cognition of alzheimer's disease. Neurobiol Aging (1981) 2:205–8. doi: 10.1016/0197-4580(81)90022-1
156. Etienne P, Dastoor D, Gauthier S, Ludwick R, Collier B. Alzheimer Disease: Lack of effect of lecithin treatment for 3 months. Neurology (1981) 31:1552–4. doi: 10.1212/WNL.31.12.1552
157. Traini E, Bramanti V, Amenta F. Choline alphoscerate (alpha-glyceryl-phosphoryl-choline) an old choline- containing phospholipid with a still interesting profile as cognition enhancing agent. Curr Alzheimer Res (2013) 10:1070–9. doi: 10.2174/15672050113106660173
Keywords: choline, choline alfoscerate, choline bitartrate, choline supplementation, cognitive dysfunction, GPC, lecithin, supplements
Citation: Kansakar U, Trimarco V, Mone P, Varzideh F, Lombardi A and Santulli G (2023) Choline supplements: An update. Front. Endocrinol. 14:1148166. doi: 10.3389/fendo.2023.1148166
Received: 19 January 2023; Accepted: 07 February 2023;
Published: 07 March 2023.
Edited by:
Sergio Davinelli, University of Molise, ItalyReviewed by:
Paola Di Pietro, University of Salerno, ItalyMario Cioce, Campus Bio-Medico University, Italy
Alfonso Baldi, University of Campania Luigi Vanvitelli, Italy
Copyright © 2023 Kansakar, Trimarco, Mone, Varzideh, Lombardi and Santulli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Angela Lombardi, YW5nZWxhLmxvbWJhcmRpQGVpbnN0ZWlubWVkLm9yZw==
†These authors have contributed equally to this work
 Urna Kansakar
Urna Kansakar Valentina Trimarco2†
Valentina Trimarco2† Fahimeh Varzideh
Fahimeh Varzideh Angela Lombardi
Angela Lombardi