- 1Department of Oncology, Yueyang Central Hospital, Yueyang, China
- 2Department of Oncology, Third Xiangya Hospital, Central South University, Changsha, China
- 3Department of Oncology, Yueyang People’s Hospital, Yueyang, China
- 4Department of Hepatobiliary Surgery, Yueyang Central Hospital, Yueyang, China
Background: Anlotinib may boost the efficacy of pancreatic cancer (PC) treatment if timely added to the GS regimen (Gemcitabine, Tegafur-gimeracil-oteracil potassium); however, no data has been published. This study evaluated the safety and efficacy of anlotinib in combination with the GS regimen(hereafter referred to as the A+GS regimen) in the first-line treatment of patients with unresectable or metastatic PC.
Methods: Patients with unresectable or metastatic PC treated at Yueyang Central Hospital and Yueyang People’s Hospital between October 2018 and June 2022 were enrolled in this retrospective real-world investigation. Treatment efficacy was evaluated based on the overall survival (OS), progression-free survival (PFS), disease control rate (DCR), and objective response rate (ORR), while the treatment safety was assessed by the frequency of major adverse events (AEs).
Results: Seventy-one patients were included in this study, 41 in the GS group and 30 in the A+GS group. The A+GS group had a longer mPFS than the GS group (12.0 months (95% CI, 6.0–18.0) and 6.0 months (95% CI, 3.0–8.1)), respectively (P = 0.005). mOS was longer in the GS+A group) when compared with the GS group (17.0 months (95%CI, 14.0–20.0) and 10.0 months (95% CI, 7.5–12.5)), respectively (P = 0.018). The GS+A group had higher ORR (50.0% vs 26.8%, P = 0.045) and DCR (83.3% vs 58.5%, P = 0.026). Furthermore, there were no grade 4-5 AEs and no treatment-related deaths, and no discernible increase in AEs in the GS+A group when compared with the GS group.
Conclusion: The A+GS regimen therapy holds great promise in managing treatment-naive advanced PC, except that future prospective studies with larger sample sizes and multiple centers are required to determine its efficacy and safety.
Introduction
Pancreatic cancer(PC) is one of the most prevalent diseases of the digestive system across the globe, with late diagnosis, rapid progression, and a poor prognosis (1, 2). With a five-year survival rate of 10%, PC is currently the fourth leading cause of cancer-related deaths (3, 4). A majority of PC patients show middle or advanced stage when they first consult a doctor owing to its insidious onset, rapid progression, and lack of typical clinical symptoms in the early stage; less than 20% of this patient group have a chance of receiving radical resection (5). Patients with inoperable metastatic PC have a 6-month median overall survival (mOS) (6). Although systemic chemotherapy remains the principal treatment for advanced PC patients to improve the quality of life and lengthen survival time (7), its overall effectiveness remains inadequate. At present, systemic chemotherapy has been found to improve median survival by 2–4 months, and it is associated with considerable toxicity (8).
Radiotherapy and immunotherapy have only made a modest improvement over the past few years in treating advanced PC patients (9, 10). Nonetheless, chemotherapy remains the first option for most advanced PC patients. Adjuvant chemotherapy with Gemcitabine (GEM) has been shown to considerably prolong disease-free survival and overall survival (OS) of PC patients (11). A novel generation of oral compound preparation from the 5-FU family, Tegafur-gimeracil-oteracil potassium (S-1) has proved in clinical tests to be effective as GEM in treating metastatic or locally advanced PC (12, 13). The 2018 edition of the guidelines of the Chinese Society of Clinical Oncology (CSCO) for Pancreatic Cancer indicates that GEM combined with an S-1 chemotherapy regimen (GS regimen) is one of the first-line treatments for advanced PC (14).
Anlotinib, as a novel oral small-molecule multi-target tyrosine kinase inhibitor, has been demonstrated to inhibit tumor growth and exert anti-tumor angiogenesis effects, effectively inhibiting PDGFR, C-KIT, FGFR, VEGFR, and other kinases that are critical to cancer progression (15, 16). In recent years, the Chinese National Medical Products Administration (NMPA) approved anlotinib for the treatment of small cell lung cancer(SCLC) (17), advanced non-small cell lung cancer (NSCLC) (16, 18), thyroid cancer (19), soft tissue sarcoma (20), and esophageal cancer (21). Many clinical trials in liver cancer, gastric cancer, colorectal cancer, kidney cancer, breast cancer, endometrial cancer, ovarian cancer, NK/T cell lymphoma, Ewing’s sarcoma, diffuse large B cell lymphoma, and other cancers are currently underway.
Considering these factors, we hypothesized that adding anlotinib to the GS regimen may improve treatment efficacy for PC, which has not previously been documented. In this view, the present work evaluated the safety and efficacy of anlotinib combined with the GS regimen (hence referred to as the A+GS regimen) in the first-line treatment of patients with metastatic or unresectable PC to better understand the therapy choices for advanced PC.
Materials and methods
Patient selection
This retrospective real-world study used data from patients with unresectable or metastatic PC who received GS regimen alone or in combination with anlotinib at Yueyang Central Hospital and Yueyang People’s Hospital between October 2018 and June 2022. The inclusion criteria were as follows: (1) PC diagnosis was made based on pathological examination; (2) Eastern Cooperative Oncology Group (ECOG) performance status score ≤2; (3) age ≥18 years; (4) tumor lesions are unresectable or patients are unwilling to undergo surgery for various reasons, including a small number of postoperative recurrence; (5) no other systemic therapy received before first-line chemotherapy, or neoadjuvant or adjuvant chemotherapy received with one regimen but relapsed more than 6 months after the end of last chemotherapy; (6) no obvious contraindication in using chemotherapy and antiangiogenic drugs before treatment; (7) at least one measurable lesion according to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1); (8) projected survival time ≥3 months. The exclusion criteria were as follows: (1) Patients who received systemic treatment the last within 6 months.
Patients who received the A+GS regimen were classified in the A+GS group, whereas those who received the GS regimen were classified in the GS group. The treatment approaches were chosen with consent from patients. The study followed the Declaration of Helsinki, and because a retrospective study does not require ethics committee approval, formal informed consent from patients was waived.
Treatment
(1) GS group: Patients received sequential chemotherapy with GEM + S-1, with GEM doses of 800-1000mg/m2 ivdrip d1, d8, Q3W + S-1 40-60mg po bid, d1-d14, Q3W, lasting 4 to 6 cycles, followed by S-1 monotherapy maintenance therapy. (2) A+GS group: Patients received a three-drug combination of GEM + S-1+ anlotinib, the dosage of GEM + S-1 was similar to the GS group, the anlotinib(Chia Tai Tianqing Company) dose was 8-12mg po qd, d1-d14, Q3W, administered orally before breakfast.
The dosage of oral S-1 for PC patients was calculated based on their body mass index (BMI), as follows (BMI<1.25 kg/m2: 40mg po bid d1-14 Q3W,1.25kg/m2<BMI<1.5 kg/m2: 50mg po bid d1-14 Q3W, BMI>1.5 kg/m2: 60mg po bid d1-14 Q3W), and the initial dose of anlotinib was 12mg po qd, d1-d14, Q3W. Patients were closely monitored for adverse events (AEs), and drug dose during treatment was adjusted if major AEs occurred. The dosage of anlotinib or chemotherapeutic drug was decreased appropriately if grade 3 AEs occurred, for the AEs of anlotinib, the first adjustment dosage was 10mg po qd, d1-d14, Q3W, and the second adjustment dosage was 8mg po qd, d1-d14, Q3W. Treatment was discontinued completely if 8mg was not tolerable, for the AEs of S-1 or GEM, the dose was reduced by 25% until the AEs improved to grade 0-1. Meanwhile, the current treatment was discontinued or replaced if grade 4-5 AEs occurred. In our investigation, we only recorded the incidence of grade ≥2 AEs because of the low incidence of grade 4-5 AEs. In addition, patients were allowed to receive local radiotherapy or interventional therapy. Follow-up information and clinical data were obtained using telephone or hospital records.
Follow-up and response evaluation
Patients were regularly monitored and evaluated every 1-2 months till death or the censoring date. The size of primary tumors was measured using magnetic resonance imaging (MRI), and/or computed tomography (CT) at baseline, and then every 2-3 months during the treatment. Treatment responses were classified as complete response (CR), partial response(PR), stable disease (SD), and progressive disease (PD) using the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1) standards. The toxicity was assessed using the Common Terminology Criteria for Adverse Events version 5.0 (CTCAE5.0).
Primary outcomes were overall survival (OS) and progression-free survival(PFS). Secondary outcomes were disease control rate(DCR=CR+PR+SD), objective response rate (ORR=CR+PR), and toxic side effects. OS was defined as the time interval from the start of treatment to the date of the last follow-up or death. PFS was defined as the period between the commencement of treatment and disease progression or follow-up termination if there was no relapse or death.
Statistical analysis
All statistical analyses were performed with SPSS 23.0 software. The Chi-square test was used to compare the clinical parameters of the two groups. The median PFS(mPFS) and mOS were estimated with the Kaplan-Meier method. Survival curves were generated using GraphPad Prism 8.0. and Cox proportional hazards regression analyses were used to identify prognostic factors influencing PFS and OS.
Result
Patient characteristics
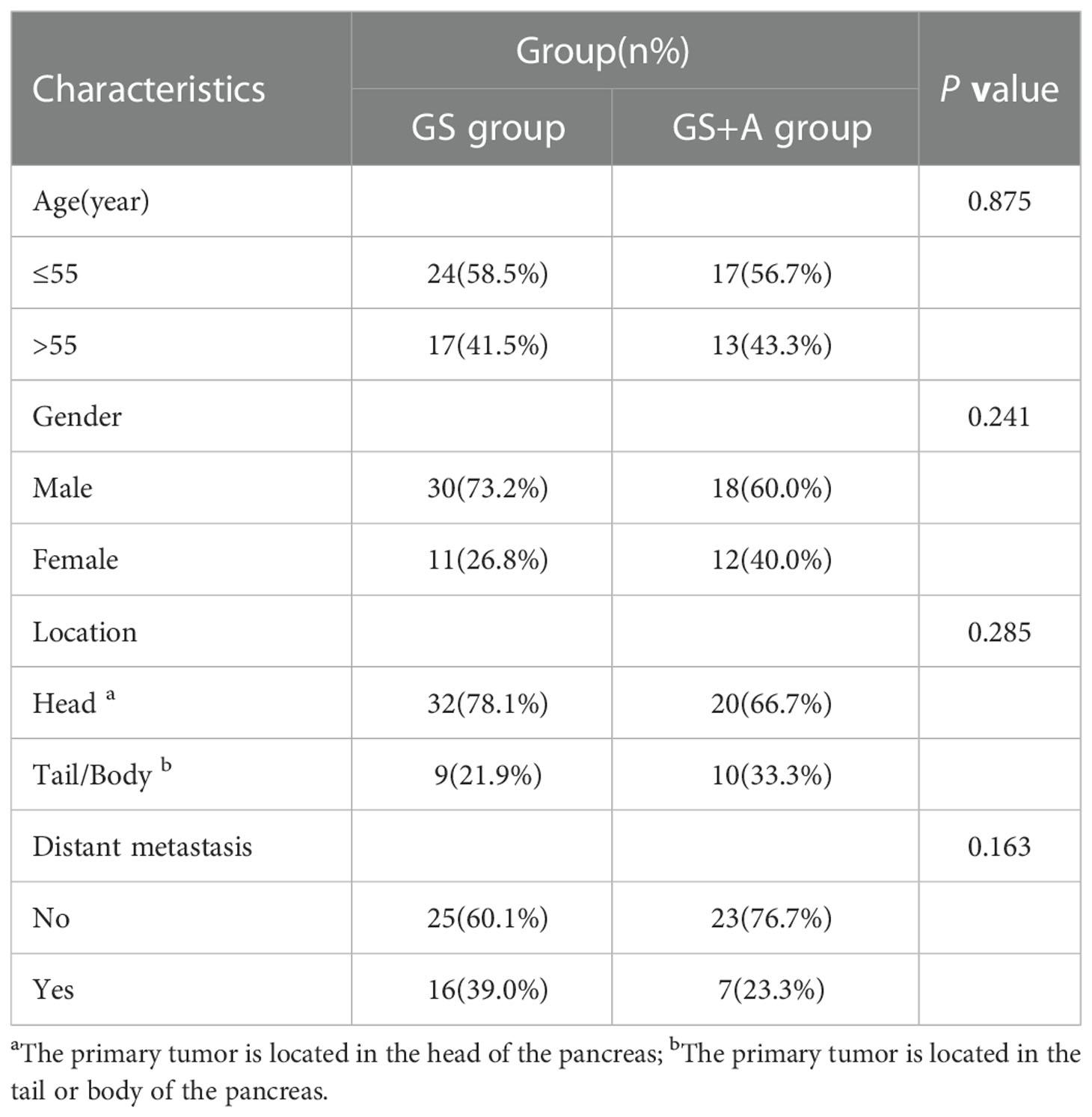
Seventy-one patients (48 males and 23 females) were enrolled in this study between October 2018 and June 2022; 41 subjects were classified in the GS group, while 30 subjects were classified in the GS+A group (Table 1). The primary lesion developed in the head of the pancreas in 52 of these patients, and in the body or tail of the pancreas in 19 patients. Twenty-three (32.4%) patients had distant metastases, and 3 patients had tumor recurrence following previous radical resection. Simple liver metastasis occurred in 3 patients, simple lung metastasis in 2 patients, simple retroperitoneal lymph node metastasis in 1 patient, simple peritoneal metastasis in 1 patient, and simultaneous multiple metastases in 16 patients. Among them, 41 patients could not undergo surgical treatment because the tumor encircled the abdominal trunk and superior mesenteric artery or locally invaded the duodenum or liver, and 7 patients with resectable tumors were not considered for surgical treatment for various reasons. Moreover, 4 patients received local radiotherapy following first-line systemic therapy. The two groups did not differ significantly in terms of age, gender, primary tumor site, and distant metastasis state.
Survival outcomes
There were no treatment-related deaths in either group during the follow-up and treatment period. At the last follow-up, 10(33.33%) and 18 (43.90%) patients in the A+GS group and the GS group, respectively, died. Figure 1 depicts the Kaplan–Meier curves for the two groups. The A+GS group showed a longer mPFS than the GS group (12.0 months (95% confidence interval (CI), 6.0-18.0) and 6.0 months (95% CI, 3.9-8.1)), respectively (P = 0.005; Figure 1A). Similarly, the A+GS group had a longer mOS compared with the GS group (17.0 months (95%CI, 14.0-20.0) and 10.0 months (95% CI, 7.5-12.5)), respectively (P = 0.018; Figure 1B). Furthermore, the A+GS group had a longer time for disease progression and better prognosis compared with the GS group.
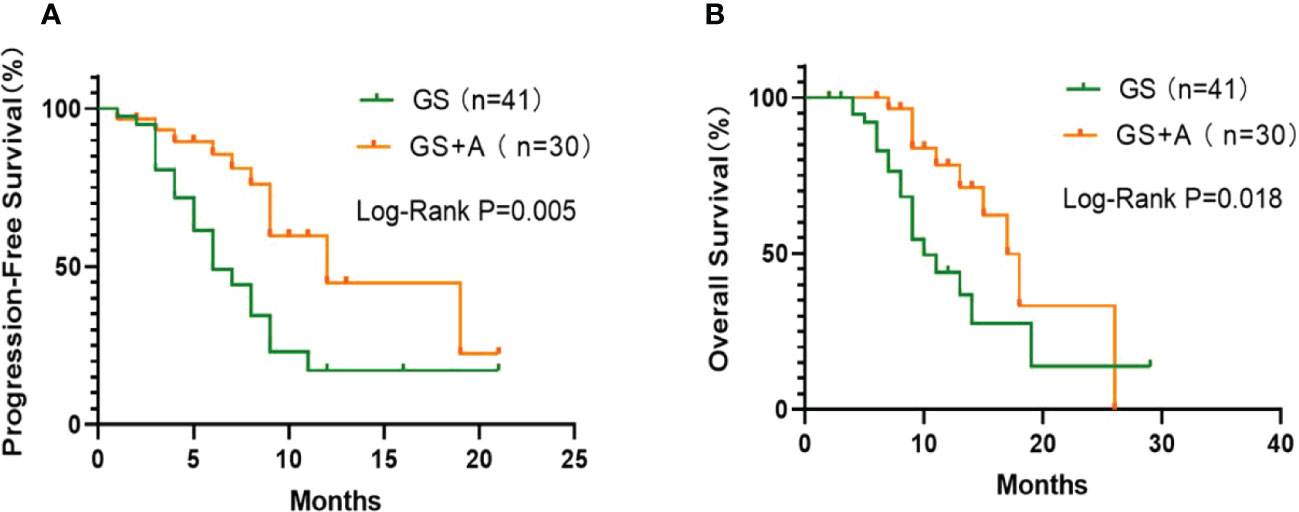
Figure 1 Kaplan-Meier plots: The GS+A group exhibited longer median progression‐free survival (mPFS; (A) and median overall survival (mOS; (B) than the GS.
The A+GS group had a longer mOS (P=0.012) and mPFS(P=0.006) than the GS group in the subgroup of PC patients with head tumors. However, there was no significant difference (P>0.05) in PFS and OS between the two groups in the subgroup analysis of PC patients with tumors in the tail or body, and different distant metastasis states. Supplementary Figure 1 depicts the comparison results and Kaplan–Meier curves for different subgroups.
Tumor response
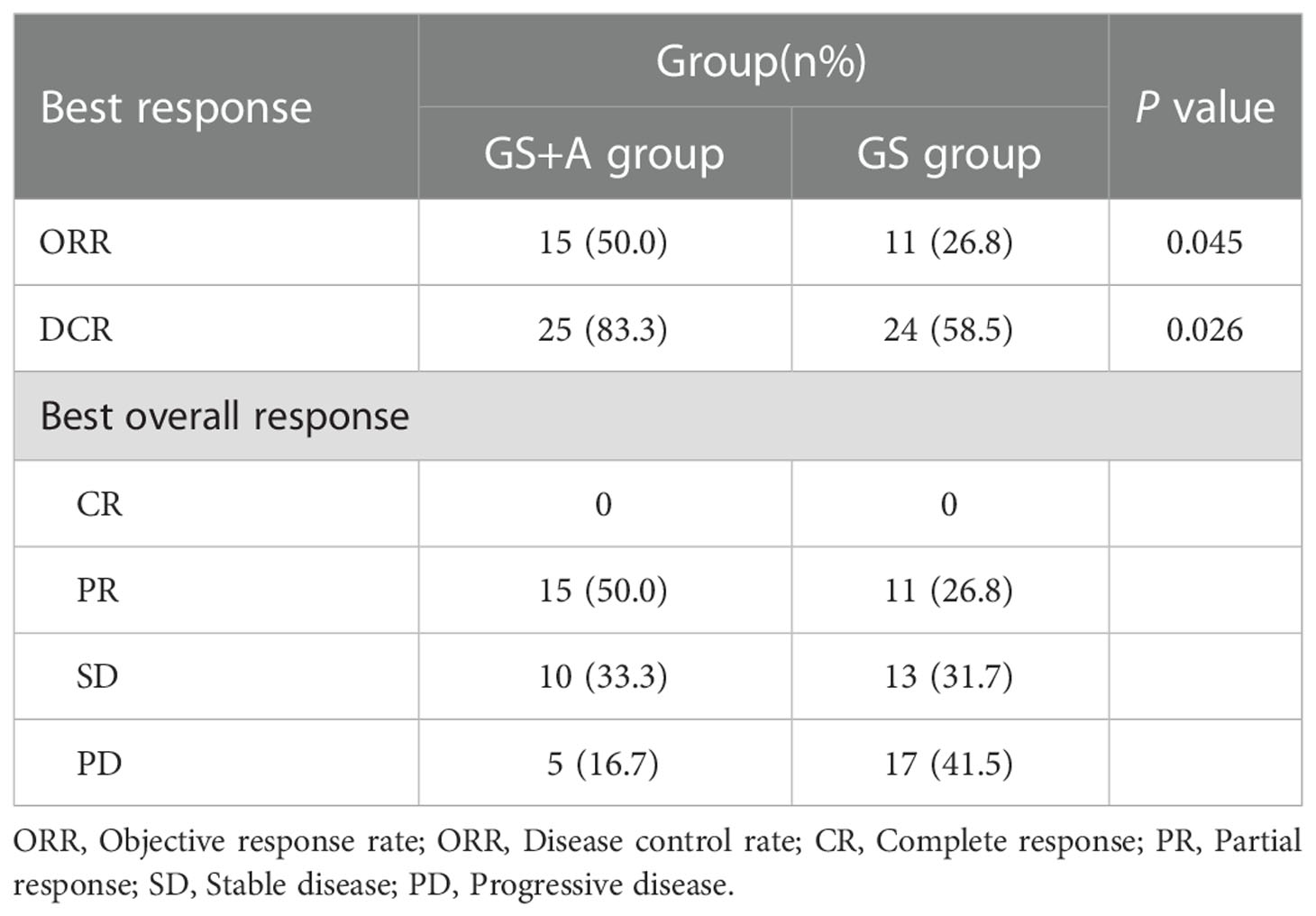
The GS+A group had 15 PR and 10 SD cases, while the GS group had 11 PR and 13 SD cases. The DCR and ORR of the A+GS group(83.3% and 50.0% respectively) were significantly higher than those of the GS group (58.5% and 26.8%, respectively) (Table 2). In addition, we selected a PC patient with multiple liver metastases who received the A+GS regimen as first-line treatment. Figure 2 depicts continuous changes in CT images before and after treatment.
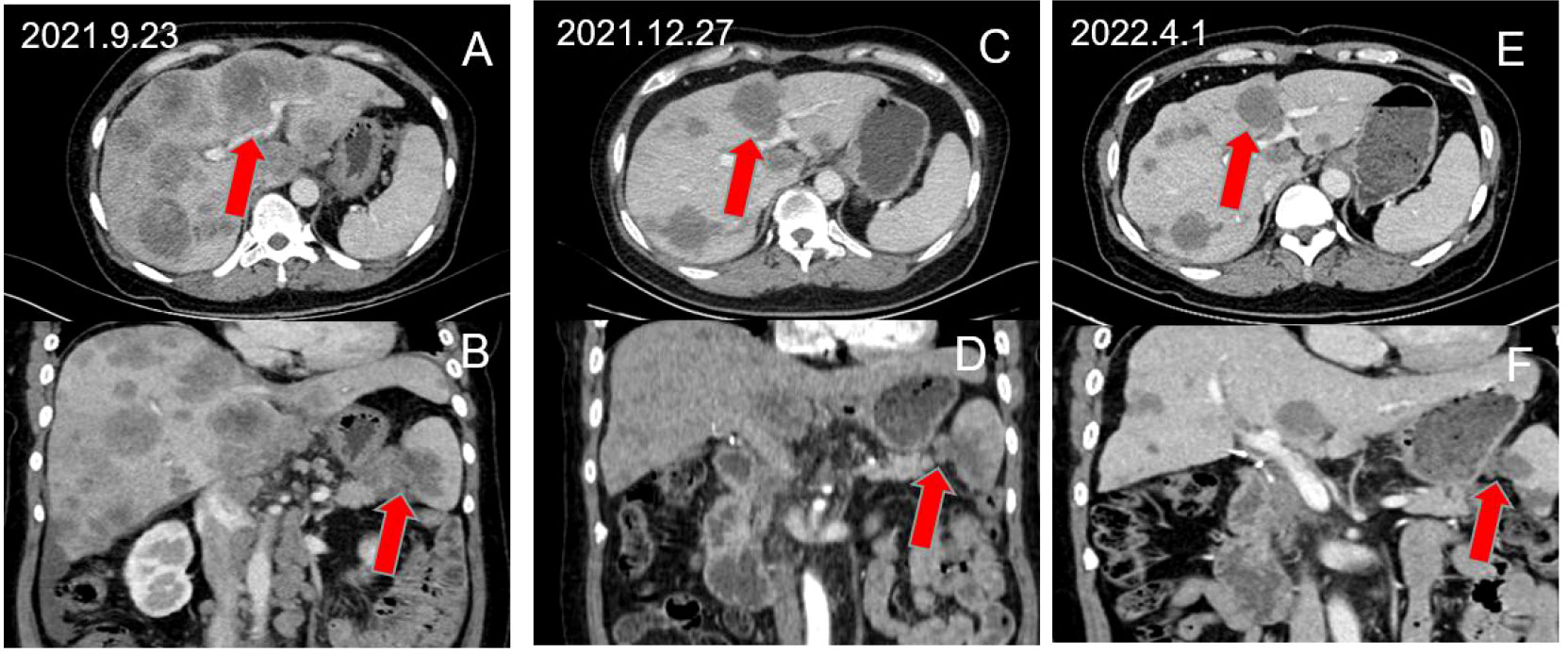
Figure 2 A 52-year-old female patient with advanced PC with multiple liver metastases achieved partial response after receiving A+GS regimen therapy. (A, B) show the pre-treatment baseline images, (C, D) show the images after 3 cycles of treatment, and (E, F) show the images after 6 cycles of treatment.
Adverse effects
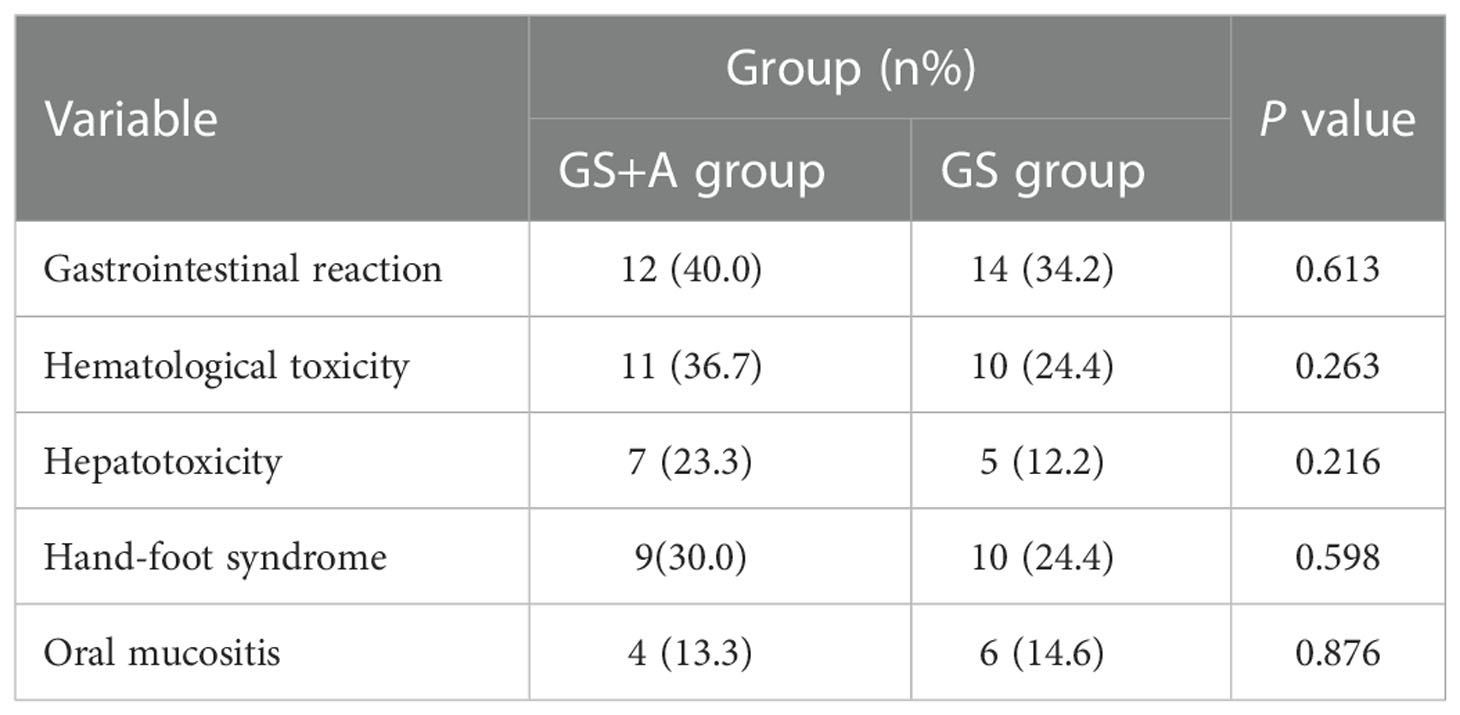
Analysis of the adverse effects in the two groups revealed that the addition of anlotinib did not increase the incidence of adverse effects(AEs) compared with the GS regimen alone (P>0.05 for all) (Table 3). The chemotherapy drug dosage was adjusted for four patients due to hematological toxicity. Anlotinib dosage was adjusted for 2 patients due to hepatotoxicity; notably, the AEs resolved after dose adjustment or symptomatic supportive treatment. No grade 4-5 AEs or treatment-related deaths were recorded.
Factors associated with OS and PFS
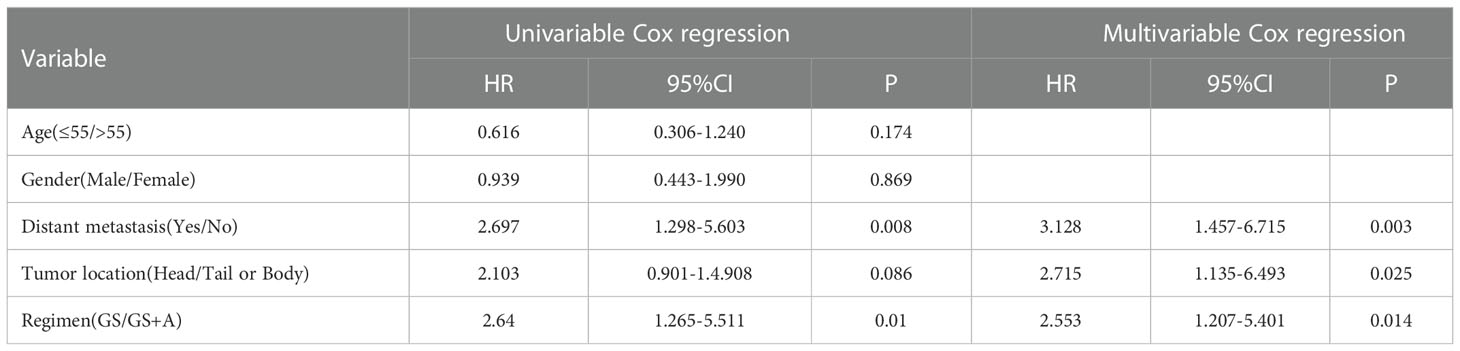
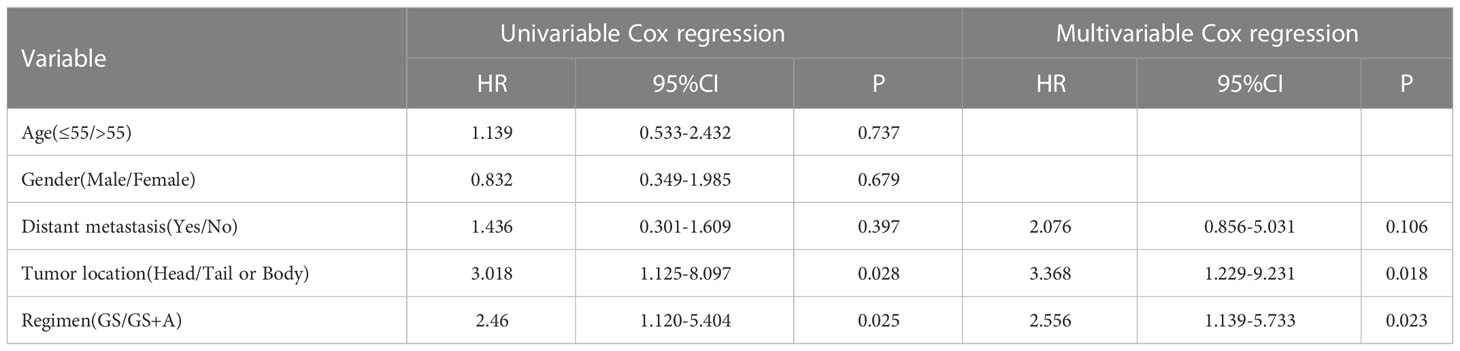
Prognostic indicators influencing PFS and OS were revealed with univariate and multivariate Cox regression analyses. Multivariate analysis demonstrated that distant metastasis states, tumor location, and treatment regimen were independent prognostic factors for PFS (Table 4), whereas tumor location and treatment regimen were independent prognostic factors for OS (Table 5).
Discussion
The NCCN and CSCO guidelines for pancreatic adenocarcinoma (14, 22), for unresectable locally advanced PC or patients with distant metastasis, recommend GS regimen, GA regimen (GEM, albumin-bound paclitaxel), GX regimen (GEM, Capecitabine), FOLFRINOX (oxaliplatin, fluorouracil, irinotecan, and leucovorin), among others, as the first-line treatment options. However, the overall treatment efficacy remains unsatisfactory. In this view, more relevant therapeutic modalities that can improve the prognosis of patients with advanced PC are urgently needed. To the best of our knowledge, this was the first study comparing A+GS regimen vs GS regimen alone in managing advanced PC.
The preliminary findings of this retrospective real-world investigation demonstrated that the A+GS group had better ORR and DCR, longer mOS, and mPFS than the GS group in the treatment of patients with advanced PC. Additionally, there was no discernible increase in AEs between the A+GS group and the GS group, indicating a satisfactory safety profile. These data demonstrate a novel therapeutic approach that may benefit patients with treatment-naive advanced PC in terms of survival and efficacy.
According to recent evidence, neither immunotherapy nor targeted therapy, two recent revolutions in cancer treatment, have produced statistically significant positive results in PC treatment (23). Immunotherapeutic interventions and targeted therapies are not currently the primary treatment options for PC (9, 24, 25), and the benefit of radiotherapy is also insufficient (26). Of note, GEM has been the first-line chemotherapy regimen for PC patients with the locally advanced or metastatic disease since 1997, but the mOS of PC patients treated with single-agent GEM was only 5.7 months (27). Several combinations of GEM with biological agents and cytotoxic agents have been investigated, but a majority have not significantly improved prognosis as compared with GEM alone (28–31). Although GEM is widely accepted as the first-line treatment option for advanced PC, overcoming GEM resistance remains a significant challenge for pancreatic cancer patients.
S-1 is an oral chemotherapeutic drug that is well-tolerated and simple to administer in clinical practice. S-1 outperformed GEM in terms of OS in the GEST Phase III clinical trial research, with an mOS of 8.8 months for GEM and 9.7 months for S-1 in the treatment of patients with locally advanced or metastatic PC (12). S-1 monotherapy and GS regimen have been listed as first-line chemotherapy options for unresectable locally advanced or metastatic PC in the 2018 CSCO guidelines (14).
EGFR overexpression was reported in approximately 30% to 89% of PC patients (32). GEM combined with erlotinib, an EGFR tyrosine kinase inhibitor, was found to be more effective than GEM alone in both mPFS and mOS in metastatic or locally advanced PC in phase III randomized controlled clinical study. However, the mOS was only extended by 0.33 months (6.24 months vs 5.91 months) and the mPFS was only extended by 0.20 months (3.75 months vs 3.55 months) (33). Furthermore, because the combination of GEM and erlotinib has limitations in prolonging PFS and OS in patients with metastatic or locally advanced PC compared to GEM monotherapy, it is necessary to investigate a novel small molecule tyrosine-kinase inhibitor drug in combination with chemotherapy for PC.
Anlotinib is a novel oral small-molecule tyrosine kinase inhibitor with multi-targets. Anlotinib, unlike other tyrosine kinase inhibitors such as sunitinib and sorafenib, can effectively inhibit multiple targets, among them FGFR, PDGFR, VEGFR, C-Kit, and other kinases (34). Since its introduction as a broad-spectrum anti-tumor-targeted drug, anlotinib has made significant progress in the treatment of cancers and has played a role in a wide range of malignancies (34–37). Zhang et al. discovered that anlotinib inhibited PC cell proliferation while inducing apoptosis (38). Yang et al. demonstrated that anlotinib killed PC cells both in vitro and in vivo (39). Moreover, several case reports show that anlotinib improved the prognosis of patients with advanced PC (40–42). A retrospective study of 33 patients with advanced PC (17 patients received anlotinib combined with GA regimen (GEM, albumin-bound paclitaxel) and 16 patients received GA regimen alone revealed that the anlotinib combination GA regimen group had significantly improved mOS (9.0months vs 6.0 months, P = 0.006) and mPFS(5.0months vs 2.7months, P = 0.022) when compared with the GA regimen group alone (43). In this view, anlotinib is expected to achieve a therapeutic advantage in treating metastatic and locally advanced PC.
The present investigation revealed that the A+GS group had a longer mOS and mPFS than the GS group in the subgroup of PC patients with tumors in the head. However, in the other subgroups, no significant difference in PFS and OS was reported between the two groups, which could be attributed to the smaller sample size. Furthermore, our multivariate analysis revealed that distant metastasis and tumor location are both independent risk factors for PFS and OS, respectively. Similarly, previous research found a link between distant metastasis and prognosis (5). Although there is controversy regarding whether cancer location is a prognostic factor in PC (44), we could not extensively explore this phenomenon due to the small sample size. Previous studies revealed that the adverse reactions of anlotinib are relatively mild, and the proportion of patients whose dose was reduced or discontinued due to adverse reactions was low than in group GS (45). In our investigation, the most common AEs in the two groups were in most cases mild and manageable, including hematological toxicity, hepatotoxicity, and gastrointestinal reactions. Furthermore, the incidence of AEs was not significantly different between the two groups, demonstrating that A+GS regimen therapy is clinically safe and feasible. Our findings strongly demonstrate that anlotinib improves the efficacy of the GS regimen. Although individual differences may influence patient prognosis, the short-term efficacy, based on PFS and tumor response rate, that the present work fully evaluated was not affected by subsequent treatment. These data could accurately represent the clinical efficacy of A+GS regimen therapy. As such, we will provide more data on efficacy and safety in the future.
While the present investigation is the first to report preliminary clinical results of anlotinib combined with GS regimen for advanced PC, providing evidence for future prospective clinical trials, a few limitations cannot be ignored. First, this was a retrospective real-world study conducted in China with small sample size and inevitable potential bias. Second, although this is the largest study reported so far, the number of PC patients in the A+GS regimen therapy group remained small. Future prospective multicenter randomized clinical trials are warranted to validate these findings.
Conclusions
The A+GS regimen therapy is of great promise in managing treatment-naive advanced PC. Our data provide a theoretical foundation for further investigation of the A+GS regimen therapy for advanced PC. The efficacy of the A+GS regimen therapy warrants further validation with more prospective studies with larger sample sizes and multiple centers.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
YS and DZ contributed to the idea and design. GZ performed the research and edited the manuscript. JH, SD, JW and CZ organized and data analyzed results. FW, SL, FF, ES, QZ, PL and MX contributed to the manuscript writing and revision. All authors approved the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Yueyang Science and Technology Department (D202305019011).
Acknowledgments
All authors are grateful for the contributions all parties made and the support they received in writing this paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1110624/full#supplementary-material
References
1. Zhang Y, Liu X, Wang Y, Lai S, Wang Z, Yang Y, et al. The m6A demethylase ALKBH5-mediated upregulation of DDIT4-AS1 maintains pancreatic cancer stemness and suppresses chemosensitivity by activating the mTOR pathway. Mol Cancer. (2022) 21(1):174. doi: 10.1186/s12943-022-01647-0
2. Ansari D, Tingstedt B, Andersson B, Holmquist F, Sturesson C, Williamsson C, et al. Pancreatic cancer: yesterday, today and tomorrow. Future Oncol (2016) 12(16):1929–46. doi: 10.2217/fon-2016-0010
3. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
4. Weibel P, Pavic M, Lombriser N, Gutknecht S, Weber M. Chemoradiotherapy after curative surgery for locally advanced pancreatic cancer: A 20-year single center experience. Surg Oncol (2021) 36:36–41. doi: 10.1016/j.suronc.2020.11.012
5. Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol (2016) 22(44):9694–705. doi: 10.3748/wjg.v22.i44.9694
6. Allendorf JD, Lauerman M, Bill A, DiGiorgi M, Goetz N, Vakiani E, et al. Neoadjuvant chemotherapy and radiation for patients with locally unresectable pancreatic adenocarcinoma: feasibility, efficacy, and survival. J Gastrointest Surg (2008) 12(1):91–100. doi: 10.1007/s11605-007-0296-7
7. Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet (2020) 395(10242):2008–20. doi: 10.1016/S0140-6736(20)30974-0
8. Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med (2013) 369(18):1691–703. doi: 10.1056/NEJMoa1304369
9. Eso Y, Seno H. Current status of treatment with immune checkpoint inhibitors for gastrointestinal, hepatobiliary, and pancreatic cancers. Therap Adv Gastroenterol (2020) 13:1756284820948773. doi: 10.1177/1756284820948773
10. Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. (2015) 15(7):409–25. doi: 10.1038/nrc3958
11. Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA (2013) 310(14):1473–81. doi: 10.1001/jama.2013.279201
12. Ueno H, Ioka T, Ikeda M, Ohkawa S, Yanagimoto H, Boku N, et al. Randomized phase III study of gemcitabine plus s-1, s-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in japan and taiwan: GEST study. J Clin Oncol (2013) 31(13):1640–8. doi: 10.1200/JCO.2012.43.3680
13. Uesaka K, Boku N, Fukutomi A, Okamura Y, Konishi M, Matsumoto I, et al. Adjuvant chemotherapy of s-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet (2016) 388(10041):248–57. doi: 10.1016/S0140-6736(16)30583-9
14. National Health Commission Of The People's Republic Of China. Chinese guidelines for diagnosis and treatment of pancreatic cancer 2018 (English version). Chin J Cancer Res (2019) 31(2):278–94. doi: 10.21147/j.issn.1000-9604.2019.02.03
15. Xie C, Wan X, Quan H, Zheng M, Fu L, Li Y, et al. Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor. Cancer Sci (2018) 109(4):1207–19. doi: 10.1111/cas.13536
16. Han B, Li K, Zhao Y, Li B, Cheng Y, Zhou J, et al. Anlotinib as a third-line therapy in patients with refractory advanced non-small-cell lung cancer: a multicentre, randomised phase II trial (ALTER0302). Br J Cancer. (2018) 118(5):654–61. doi: 10.1038/bjc.2017.478
17. Wu D, Nie J, Hu W, Dai L, Zhang J, Chen X, et al. A phase II study of anlotinib in 45 patients with relapsed small cell lung cancer. Int J Cancer. (2020) 147(12):3453–60. doi: 10.1002/ijc.33161
18. Zhou M, Chen X, Zhang H, Xia L, Tong X, Zou L, et al. China national medical products administration approval summary: anlotinib for the treatment of advanced non-small cell lung cancer after two lines of chemotherapy. Cancer Commun (Lond). (2019) 39(1):36. doi: 10.1186/s40880-019-0383-7
19. Sun Y, Du F, Gao M, Ji Q, Li Z, Zhang Y, et al. Anlotinib for the treatment of patients with locally advanced or metastatic medullary thyroid cancer. Thyroid (2018) 28(11):1455–61. doi: 10.1089/thy.2018.0022
20. Chi Y, Fang Z, Hong X, Yao Y, Sun P, Wang G, et al. Safety and efficacy of anlotinib, a multikinase angiogenesis inhibitor, in patients with refractory metastatic soft-tissue sarcoma. Clin Cancer Res (2018) 24(21):5233–8. doi: 10.1158/1078-0432
21. Shi J, Zhang Y, Wang J, Li J, Li Z. Anlotinib combined with chemoradiotherapy exhibits significant therapeutic efficacy in esophageal squamous cell carcinoma. Front Oncol (2020) 10:995. doi: 10.3389/fonc.2020.00995
22. Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, et al. Pancreatic adenocarcinoma, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2021) 19(4):439–57. doi: 10.6004/jnccn.2021.0017
23. Ducreux M, Seufferlein T, Van Laethem JL, Laurent-Puig P, Smolenschi C, Malka D, et al. Systemic treatment of pancreatic cancer revisited. Semin Oncol (2019) 46(1):28–38. doi: 10.1053/j.seminoncol.2018.12.003
24. Henriksen A, Dyhl-Polk A, Chen I, Nielsen D. Checkpoint inhibitors in pancreatic cancer. Cancer Treat Rev (2019) 78:17–30. doi: 10.1016/j.ctrv.2019.06.005
25. Macherla S, Laks S, Naqash AR, Bulumulle A, Zervos E, Muzaffar M. Emerging role of immune checkpoint blockade in pancreatic cancer. Int J Mol Sci (2018) 19(11):3505. doi: 10.3390/ijms19113505
26. Barcellini A, Peloso A, Pugliese L, Vitolo V, Cobianchi L. Locally advanced pancreatic ductal adenocarcinoma: Challenges and progress. Onco Targets Ther (2020) 13:12705–20. doi: 10.2147/OTT.S220971
27. Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol (1997) 15(6):2403–13. doi: 10.1200/JCO.1997.15.6.2403
28. Colucci G, Giuliani F, Gebbia V, Biglietto M, Rabitti P, Uomo G, et al. Gemcitabine alone or with cisplatin for the treatment of patients with locally advanced and/or metastatic pancreatic carcinoma: a prospective, randomized phase III study of the gruppo oncologia dell'Italia meridionale. Cancer (2002) 94(4):902–10. doi: 10.1002/cncr.10323
29. Rocha Lima CM, Green MR, Rotche R, Miller WH Jr, Jeffrey GM, Cisar LA, et al. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol (2004) 22(18):3776–83. doi: 10.1200/JCO.2004.12.082
30. Louvet C, Labianca R, Hammel P, Lledo G, Zampino MG, André T, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol (2005) 23(15):3509–16. doi: 10.1200/JCO.2005.06.023
31. Heinemann V, Quietzsch D, Gieseler F, Gonnermann M, Schönekäs H, Rost A, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol (2006) 24(24):3946–52. doi: 10.1200/JCO.2005.05.1490
32. Troiani T, Martinelli E, Capasso A, Morgillo F, Orditura M, De Vita F, et al. Targeting EGFR in pancreatic cancer treatment. Curr Drug Targets. (2012) 13(6):802–10. doi: 10.2174/138945012800564158
33. Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the national cancer institute of canada clinical trials group. J Clin Oncol (2007) 25(15):1960–6. doi: 10.1200/JCO.2006.07.9525
34. Shen G, Zheng F, Ren D, Du F, Dong Q, Wang Z, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol (2018) 11(1):120. doi: 10.1186/s13045-018-0664-7
35. Si X, Zhang L, Wang H, Zhang X, Wang M, Han B, et al. Quality of life results from a randomized, double-blinded, placebo-controlled, multi-center phase III trial of anlotinib in patients with advanced non-small cell lung cancer. Lung Cancer. (2018) 122:32–7. doi: 10.1016/j.lungcan.2018.05.013
36. Sun Y, Zhou A, Zhang W, Jiang Z, Chen B, Zhao J, et al. Anlotinib in the treatment of advanced hepatocellular carcinoma: an open-label phase II study (ALTER-0802 study). Hepatol Int (2021) 15(3):621–9. doi: 10.1007/s12072-021-10171-0
37. Chi Y, Shu Y, Ba Y, Bai Y, Qin B, Wang X, et al. Anlotinib monotherapy for refractory metastatic colorectal cancer: A double-blinded, placebo-controlled, randomized phase III trial (ALTER0703). Oncologist (2021) 26(10):e1693–703. doi: 10.1002/onco.13857
38. Zhang X, Liu Y, Zhang Z, Tan J, Zhang J, Ou H, et al. Multi-omics analysis of anlotinib in pancreatic cancer and development of an anlotinib-related prognostic signature. Front Cell Dev Biol (2021) 9:649265. doi: 10.3389/fcell.2021.649265
39. Yang L, Zhou X, Sun J, Lei Q, Wang Q, Pan D, et al. Reactive oxygen species mediate anlotinib-induced apoptosis via activation of endoplasmic reticulum stress in pancreatic cancer. Cell Death Dis (2020) 11(9):766. doi: 10.1038/s41419-020-02938-4
40. Li S, Niu M, Deng W, Li N, Wei C, Luo S. Anlotinib is effective in the treatment of advanced pancreatic cancer: a case report. Anticancer Drugs (2022) 33(1):e558–61. doi: 10.1097/CAD.0000000000001173
41. Luo D, Liao S, Li Q, Lin Y, Wei J, Li Y, et al. Case report: A case of locally advanced pancreatic cancer which achieved progression free for over 12 months by subsequent therapy with anlotinib hydrochloride plus tegafur-Gimeracil-Oteracil potassium (TS-1). Front Oncol (2022) 12:862600. doi: 10.3389/fonc.2022.862600
42. Wang Y, Wang B, Xiang L, Deng J, Xu B, He P, et al. Case report: Anlotinib combined with PD-1 inhibitor and sequential GA regimen or FOLFIRINOX chemotherapy in treatment of KRAS G12V mutated pancreatic ductal adenocarcinoma with liver metastasis: A case and literature review. Front Immunol (2022) 13:1016647. doi: 10.3389/fimmu.2022.1016647
43. Wu H, Huang N, Zhao C, Hu X, Da L, Huang W, et al. Anlotinib plus nab-paclitaxel/gemcitabine as first-line treatment prolongs survival in patients with unresectable or metastatic pancreatic adenocarcinoma: a retrospective cohort. Ann Transl Med (2022) 10(6):294. doi: 10.21037/atm-22-544
44. Lee M, Kwon W, Kim H, Byun Y, Han Y, Kang JS, et al. The role of location of tumor in the prognosis of the pancreatic cancer. Cancers (Basel). (2020) 12(8):2036. doi: 10.3390/cancers12082036
Keywords: pancreatic cancer, anlotinib, GS regimen, efficacy, safety, overall survival, progression-free survival
Citation: Zhan G, Hu J, Da S, Weng J, Zhou C, Wen F, Liu S, Fang F, Shen E, Zhou Q, Luo P, Xu M, Zhan D and Su Y (2023) A real-world study of anlotinib combined with GS regimen as first-line treatment for advanced pancreatic cancer. Front. Endocrinol. 14:1110624. doi: 10.3389/fendo.2023.1110624
Received: 29 November 2022; Accepted: 04 January 2023;
Published: 20 January 2023.
Edited by:
Ruiqin Han, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences, ChinaReviewed by:
Hongzhu Yu, First Affiliated Hospital of Anhui Medical University, ChinaLeilei Wu, Tongji University School of Medicine, China
Copyright © 2023 Zhan, Hu, Da, Weng, Zhou, Wen, Liu, Fang, Shen, Zhou, Luo, Xu, Zhan and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dahe Zhan, emhhbmRhaGVAMTYzLmNvbQ==; Yuqi Su, c3V5dXFpMTAwMDBAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Gouling Zhan
Gouling Zhan Jianbing Hu1
Jianbing Hu1