
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 24 March 2023
Sec. Pediatric Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1043370
This article is part of the Research Topic Childhood Diabetes in Low- and Middle-Income Countries: Progress, Challenges, and Actions Needed, volume II View all 6 articles
Introduction: In several of the Low and Middle Income countries , many patients with Type 1 diabetes (T1D) are most probably not diagnosed at all which may contribute to their low incidence. As an example of a country with low income and poor resources, we have chosen to study T1D in children/young people in Tanzania.
Methods: Analyses of casebooks and statistics at several Tanzanian hospitals treating young patients with insulin dependent diabetes, usually Type 1 diabetes, and collection of information from different organisations such a Tanzanian Diabetes Association, Life for a Child, Changing Diabetes in Children and World Diabetes Foundation.
Results: The incidence in several areas is low. However, a lot of data are often missing at studied clinics and therefore the incidence might be higher, and with increased awareness in recent years the number of patients has increased many-folds. Most patients present with typical symptoms and signs of T1D, and a high proportion with plausible ketoacidosis , although this proportion has decreased from about 90% to about 40% in recent decades. Many patients have poor blood glucose control, and complications often develop already after short diabetes duration. In recent years resources have increased, awareness has increased and diabetes clinics started where staff has got training.
Conclusions: There are problems with diabetes care in Tanzania but several facts give hope for the future.
Type 1 diabetes (T1D) is one of the most common chronic diseases in children. It is supposed to be caused by an autoimmune process, with underlying cause unknown but believed to be a combination of genetic susceptibility (1) and environmental factors, such as beta cell stress, early nutrition, increased hygiene, or viral infections (2). Type 1 diabetes can occur at any age but is much more frequent in children and young adults.
Those diagnosed with type 1 diabetes need daily injections of insulin to survive and keep their blood glucose within acceptable levels. To sustain daily insulin injections, frequent daily blood glucose monitoring, education, and support to the child/young adult and their family is essential to ensure that those with type 1 diabetes get a reasonable quality of life with enough good blood glucose balance to avoid acute complications and prevent or delay late complications.
Diabetes can be classified using genetics and occurrence of autoantibodies which are present in 85-90% of the cases of classical T1D in the western world (3), and to some extent, also via C-peptide (4). However, the initial classification of T1D is usually based on the clinical presentation and family history (5). T1D is often diagnosed after a short period of symptoms such as fatigue, polyuria, polydipsia, and weight loss and the majority of patients in the western world (80–90%) have no family history of T1D. In Tanzania, a typical representative of Low or Middle-Income Countries (LMIC), children with diabetes are mostly regarded as T1D unless they have signs typical for Type 2 diabetes (T2D) like obesity and/or acanthosis nigricans. Even though African people have similar genetic susceptibility for T1D, HLA-DR3/DR4, as people in Europe (6, 7), idiopathic T1D (8) is more common in populations with African origin with no or reduced signs of autoimmunity (9, 10). Furthermore, T1D may include subtypes, which so far are not classified (11).
T1D accounts for approximately 10% of all patients with diabetes in the world (12). According to IDF Diabetes Atlas, 2021 (13), globally 1,211,900 children and adolescents younger than 20 years were estimated to have T1D, and it was estimated that around 149,500 children and adolescents below the age of 20 years are diagnosed each year. Numbers of children and adolescents under 20 years of age with type 1 diabetes in IDF AFRICA region (51,000) have more than doubled since 2019, probably mainly because of better diagnosis and availability of new data and the annual incidence is supposedto be ca19,000.
The incidence of Type 1 diabetes varies around the world. Scandinavia has the highest incidence (Finland ca 60 and Sweden ca 45 (14, 15)). There is an increasing knowledge about the incidence of T1D in Sub-Saharan Africa (13, 16, 17) and the incidence seems to increase (18).
In several LMIC, many patients with diabetes are most probably not diagnosed at all, which may contribute to the low incidence in these countries. A study 1993 on juvenile diabetes in Dar es Salaam, Tanzania, estimated the annual incidence to be 1.5/100,000 (19), while IDF 2013 estimated the incidence in children aged 0–14 years in Tanzania to be 0.9/100,000 per year (20). We did a study in recent years and found an annual incidence of 1.8–1.9/100,000 children, with an incidence peak at 10–14 years (21). However, a lot of data were missing at the studied clinics and therefore the incidence might be higher.
The great majority of the patients in our study from Tanzania presented with typical signs and symptoms of T1D, and we estimated 83.7% to have presented with plausible ketoacidosis (DKA) (21). Furthermore, the frequency of misdiagnosis and subsequent deaths from DKA is not known but could be substantial. This should be compared with a much lower incidence of DKA at diagnosis in high-income countries (22).
Both in the health care and community an increased awareness of diabetes is needed. This is true in all countries, but especially so in LMIC. Efforts are done in Tanzania to improve the situation.
Tanzania Diabetes Association (TDA) was formed in 1985 as non-profit, non-Governmental organization (NGO) to unite the efforts of different people and stakeholders who were concerned with the care for people with diabetes in Tanzania. The purpose was not only to improve access to care for people living with diabetes in the country but also to put in strategies and initiatives to prevent and control diabetes.
TDA in collaboration with well-wishers, organizations, and institutions from within and outside Tanzania, and Government of Tanzania through the Ministry of Health (MoH) the President’s Office Regional Administration and Local Government (PORALG) has been able to establish network of diabetes/other non-communicable diseases (NCDs) clinics all over the country in tertiary and secondary public health facilities (Zonal, Regional, and District Hospitals) to improve access to care and quality of life of children, adolescents, and adults living with diabetes. At present, TDA is implementing further expansion into primary care facilities (Health Centers) all over the country.
Since year 2005, TDA with support from funders such as Life for a Child Project (LFAC), Changing Diabetes in Children (CDiC) project and the World Diabetes Foundation (WDF) has continued to identify and enrol children and adolescents with type 1 diabetes at 38 clinics within the public-sector all-over Tanzania, including Zanzibar and are specific for improving type 1 diabetes services (Figure 1). Additional funding has been obtained recently to establish Type 1 diabetes clinics in the remaining regional hospitals in the country and also in lower-level hospitals/Health Centers where the number of children attending the clinic are more than 10.
Thus, formally very much has been done, and progress has been seen, as shown by the increase in total number of children with diabetes (Figure 2) Still our own recent study (21), with investigations done both at national and regional centres, showed serious shortcomings. The great majority of patients lacked registered information on basal facts. One explanation to this may be that children with T1D did not come to their follow-ups at their diabetes clinic, another reason that HbA1c was not measured, or otherwise, that data were never registered. Only sporadic blood glucose values could be found, often registered as fasting blood glucose, although it was not clear if they were fasting. There were no registration or comments on home blood glucose values, which might have been used when HbA1c values were lacking. As a rule, even the most basal clinical information was lacking. A lot of missing data might, to some extent, be a reflection of limited resources, but even more an insufficient awareness and low priority among health care professionals.
In a majority of patients, HbA1c was never registered, but for those, we did find information the patients had overall poor blood glucose control, with calculated mean HbA1c 11.1% (98 mmol/mol) and most patients had HbA1c above 12.5% (113 mmol/mol). Others studies have got similar results (23). Even though progress is made in many countries, our findings from Tanzania are in agreement with those seen in other studies from Africa (24).
A poor blood glucose balance among children in Tanzania probably reflects the limitations of diabetes care, including lack of insulin supply (25, 26). When insulin is available, children in LMIC often get conventional insulin treatment in an inconsistent manner in contrast to developed countries where intensive diabetes treatment is offered. But lack of knowledge and motivation with inappropriate insulin doses also play an important role, poor monitoring practices missed doses of insulin, sometimes due to work place and school based stigma. Insulin supply with the program have improved but it is the “last mile” which is a challenge, i.e., frequent blood glucose testing, utilization of syringes, stigma at home/schools and insulin storage. Very hard work is necessary to improve awareness of diabetes both in the general population and in health care and this work is ongoing. The clinics in Tanzania are nowadays supplied on regular basis with insulin and other commodities such as glucometers, strips, lancets, and educational materials. Currently about 4000 youth living to type 1 diabetes have been registered with these clinics and they have access to these supplies at no cost. Thus, there is good grounds to improve care. Furthermore, health care workers from different facilities in the country have been trained in early diagnosis and management and are subjected to regular retraining. This is to ensure that they are updated on the latest science, knowledge, and skills required for the management of type 1 diabetes and they provide the highest quality of care to those in need. More than 350 healthcare workers have been trained on management of type 1 diabetes and additional numbers will be trained as TDA in partnership with MoH and PORALG, rolls out the National Type 1 diabetes and National NCD Program all over the country.
Even when patients have got their access to insulin unfortunately structured management programs are often limited resulting in poor metabolic control, which we know causes both acute complications such as diabetes ketoacidosis, hypoglycemia, and chronic complications affecting the eyes, kidneys, nerves, and cardiovascular system. Complications are common in patients with T1D in LMIC and appear often after a rather short duration of the disease (27–33) in the worst case also increased mortality (23, 34, 35). Despite all the different challenges, the number of children diagnosed with DKA has been decreasing from about 90% to current 38% (Figure 3). But the true burden of type 1 diabetes in most LMIC is unknown. When we did a retrospective study analyzing medical recordings from 2010 – 2016 or 604 children and young adults with T1D who were recruited from five hospitals with pediatric diabetes clinics we had great difficulties to find information (28). The results showed a high prevalence of complications already after short duration of T1D, associated with poor metabolic control. Only 42.2% of the patients had registered HbA1c values, and among them, 36% had HbA1c >12.5%. There was high prevalence of retinopathy (21.5%) and neuropathy (29.4%) in spite of short mean duration of diabetes (6.2 ± 4.1 years). But as most data were missing the picture is unclear. HbA1C, plasma glucose, and complications were documented in less than half of the patient files. Thus, our results had to be interpreted with great caution, which is probably the same with other studies from LMIC.
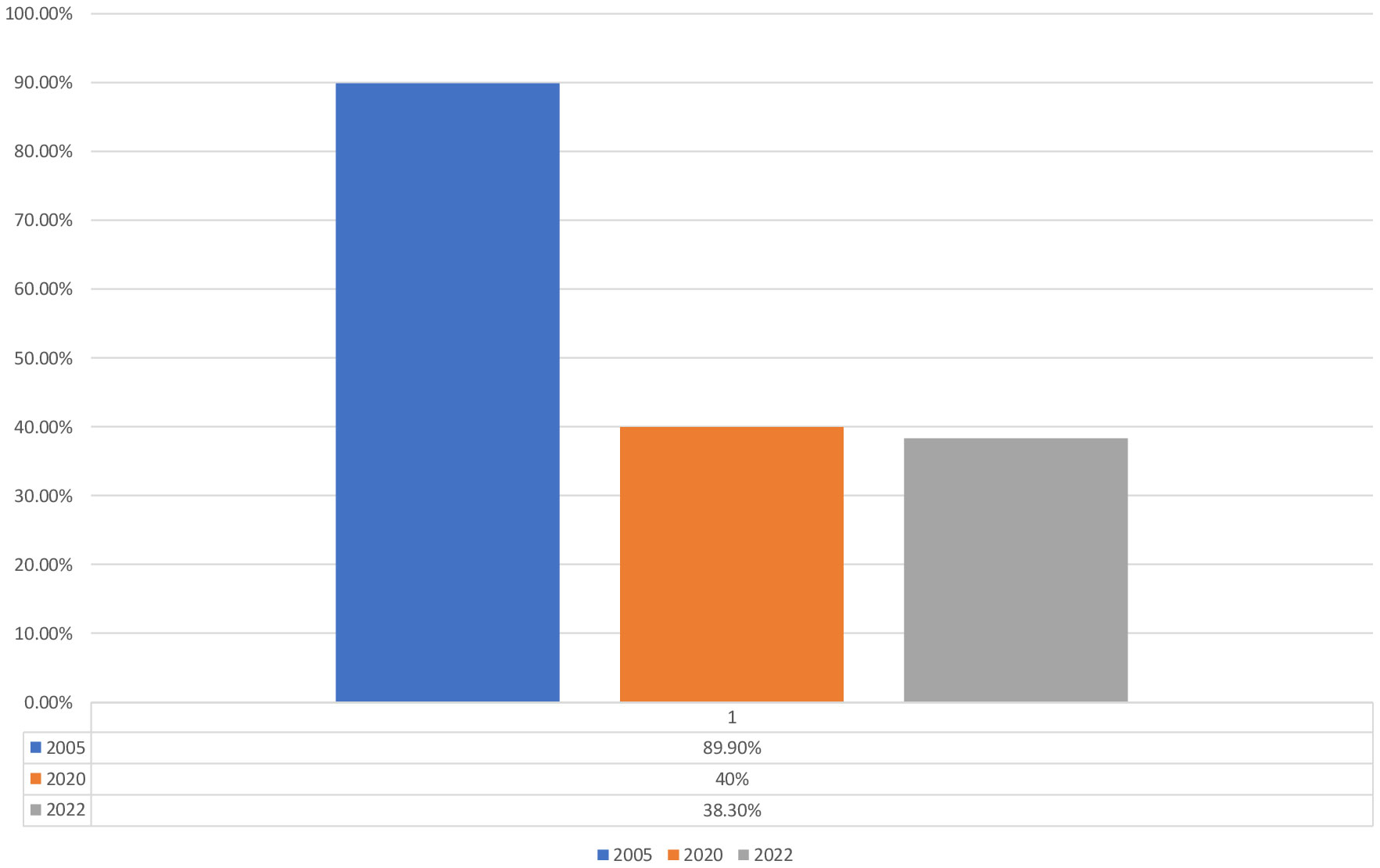
Figure 3 Trend of DKA in children and Youth with diabetes in Tanzania [Majaliwa, Edna., et al. “Diabetes care (2007);30.9: 2187-2192 (28); Kipasika, H., et al. “ Int J Diabetes Clin Res (2020); 7:126, Unpublished data].
Thus, several studies have shown the typical and quite pronounced problems with diabetes care in Tanzania (21, 23, 27–29, 36). In many other LMIC, efforts are made to improve the care and there are several reports showing that such work is possible (24, 33, 37–39). A rather modest improvement of care can have great importance (40). In Tanzania, the Tanzania Diabetes Association (TDA) has been collaborating with several partners to train paediatric endocrinologists and support specialized diabetes courses for medical officers. These trained professionals are working within the public and private sectors to enhance their capacity the and be master trainers for other healthcare workers at different levels of health system in the country to improve diabetes care for all people in Tanzania. So far eight paediatric endocrinologists have been trained in Tanzania.
To ensure long term sustainability of supply chain for the above-mentioned 38 designated type 1 diabetes clinics, TDA together with MoH and PORALG have engaged with Medical Store Department (MSD) to ensure strengthening logistic and supply chain for insulin and other commodities, so that they reach clinics timely and access to care and delivery of services for children and adolescents with type 1 diabetes is uninterrupted.
TDA through community awareness campaigns and health education, has emphasized lifestyle modification and importance of healthy diet, physical exercise, tobacco control and avoidance of excessive alcohol intake as a critical components toward prevention and control of diabetes and other NCDs in the general community. TDA works in collaboration with other organizations and Tanzania NCD Alliance (TANCDA).
There is an improved survival of children with T1D in Africa, including Tanzania. The blood glucose control is of particular importance since it leads to improved quality of life and if left untreated results in premature morbidity and mortality.
Much improvement has been seen in survival, case finding, improvement in mortality, and record keeping. However, many children are still being missed as well as the missed diagnois resulting in too early mortality. Achievement of glycemic control has been poor for a long time. There has been lack of basic need for glycemic control which has been improving progressively. Therefore, more work is needed on managing and taking care of children and youth to be able to manage the glycemia as efforts are being done to improve the supply of insulin and monitoring tools, adherence is part of something to be worked on so more than just these supplies (25, 41, 42). In most studies the psychological parts of children, youth and parents is an aspect which is missing in the African context. Stigma to diabetes is still a hindrance factor of medication adherence, hence increasing the rate of poor glycemic control. Most of the children and youth would not want to show that they have diabetes, neither in schools or in public places nor even in marriages. Another hindrance to get good metabolic control in the limited resource setting is the lack of care givers involvement in the management of diabetes of their children. Furthermore, most of the diet is starch, which may sometimes increase the need for very high dose of insulin, necessitating children to use high insulin doses even when they are out of the puberty phase.
Diabetes awareness, still a gap on limited resources setting, is one of the contributing factor for the early complications, hence more interventions are needed. There is also a-lack of devices for frequent blood glucose monitoring in African countries. To add to that salt is the poor record keeping such that, more often than not, diagnosis and initial symptoms might be missing even in a hospital. Despite the fact that the level of DKA at presentation, children are still presenting very late to the health facility with symptoms of diabetes.
Limited resource setting needs to bridge the gap on family involvement to improve the diabetes care beyond insulin supply and blood glucose monitoring. There is need to move toward patient-centered diabetes education. As most of the setting, Africa lacks psychologists or diabetes educators in the teams, so there is need to equip whoever is available, be it doctor, nurse, or pediatrician, to deal with psychological questions and provide education.
Metabolic control has been unachievable for decades. Now our focus should move from insulin for survival to insulin together with education and psychological support for good control and a good quality of life both for parents and their families. Overcoming the “last mile” is the next challenge to obtain optimum control.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Ethics committé. Linköping university and DaresSalaam. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
JL had the idea, designed the project and wrote the first draft, ME and KR both contributed with further data, and worked with the manuscript. All authors contributed to the article and approved the submitted version.
We are grateful to all those who do their best at the diabetes clinics in Tanzania despite meager resources, and to those organizations which support the diabetes care. There is no funding for this work, but JL is grateful to Barndiabetesfonden (The Swedish Child Diabetes Foundation) for making research on diabetes in children and adolescents possible in Sweden.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1043370/full#supplementary-material
1. Pociot F, Lernmark Å. Genetic risk factors for type 1 diabetes. Lancet (2016) 387(10035):2331–2339. doi: 10.1016/S0140-6736(16)30582-7
2. Rewers M, Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet (2016) 387(10035):2340–2348. doi: 10.1016/S0140-6736(16)30507-4
3. Pihoker C, Gilliam LK, Hampe CS, Lernmark A. Tuomilehto j. the emerging global epidemic of type 1 diabetes. Curr Diabetes Rep (2013) 13(6):795–804. doi: 10.1007/s11892-013-0433-5
4. Ludvigsson J, Carlsson A, Forsander G, Ivarsson S, Kockum I, Lernmark A, et al. C-peptide in the classification of diabetes in children and adolescents. Pediatr Diabetes (2012) 13(1):45–50. doi: 10.1111/j.1399-5448.2011.00807.x
5. Me C, Jefferies C, Dabelea D, Balde N, Seth A, Donaghue KC. Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr Diabetes (2014) 15:4–17. doi: 10.1111/pedi.12186
6. Lombard Z, Brune AE, Hoal EG, Babb C, Van Helden PD, Epplen JT, et al. HLA class II disease associations in southern Africa. Tissue Antigens (2006) 67(2):97–110. doi: 10.1111/j.1399-0039.2006.00530.x
7. Ibrahim TAM, Govender D, Abdullah MA, Noble JA, Hussien MO, Lane JA, et al. Clinical features, biochemistry, and HLA-DRB1 status in youth-onset type 1 diabetes in Sudan. Pediatr Diabetes (2021) 22(5):749–57. doi: 10.1111/pedi.13209
8. Piñero-Piloña A, Raskin P. Idiopathic type 1 diabetes. J Diabetes Complications (2001) 15:328–35. doi: 10.1016/S1056-8727(01)00172-6
9. Sobngwi E, Vexiau P, Levy V, Lepage V, Mauvais-Jarvis F, Leblanc H, et al. Metabolic and immunogenetic prediction of long-term insulin remission in African patients with atypical diabetes. Diabetes Med (2002) 19(10):832–5. doi: 10.1046/j.1464-5491.2002.00802.x
10. Balcha SA, Demisse AG, Mishra R, Vartak T, Cousminer DL, Hodge KM, et al. Type 1 diabetes in Africa: an immunogenetic study in the amhara of north-West Ethiopia. Diabetologia (2020) 63(10):2158–68. doi: 10.1007/s00125-020-05229-x
11. Balogun WO, Uloko AE, Owolabi MOl. Atypical diabetes presentations in Sub-Saharan Africa classification puzzle and possible role of precision medicine. West Afr J Med (2020) 37(5):574–82.
12. Tuomilehto J. The emerging global epidemic of type 1 diabetes. Curr Diabetes Rep (2013) 13(6):795–804. doi: 10.1007/s11892-013-0433-5
13. Ogle GD, James S, Dabelea D, Pihoker C, Svennson J, Maniam J, et al. Global estimates of incidence of type 1 diabetes in children and adolescents: Results from the international diabetes federation atlas, 10th edition. Diabetes Res Clin Pract (2022) 183:109083. doi: 10.1016/j.diabres.2021.109083
14. Harjutsalo V, Sund R, Knip M, Groop PH. Incidence of type 1 diabetes in Finland. JAMA (2013) 310(4):427–8. doi: 10.1001/jama.2013.8399
15. Ludvigsson J. Increasing incidence but decreasing awareness of type 1 diabetes in Sweden. Diabetes Care (2017) 40(10):e143–4. doi: 10.2337/dc17-1175
16. Hall V, Thomsen RW, Henriksen O, Lohse NN. Diabetes in Sub Saharan Africa 1999–2011: EPIDEMIOLOGY and public health implications. A systematic review. BMC (2011) 11:564. doi: 10.1186/1471-2458-11-564
17. Marshall SL, Edidin D, Arena VC, Becker DJ, Bunker CH, Gishoma C, et al. Epidemiology prevalence and incidence of clinically recognized cases of type 1 diabetes in children and adolescents in Rwanda, Africa. Diabetic Medicine (2015) 32:1186–92.
18. Sandy JL, Besançon S, Sidibé AT, Minkailou M, Togo A, Ogle GD, et al. Rapid increases in observed incidence and prevalence of type 1 diabetes in children and youth in Mali, 2007-2016. Pediatr Diabetes (2021) 22(4):545–51. doi: 10.1111/pedi.13191
19. Swai ABM, Lutale JL, McLarty DG. Prospective study of incidence of juvenile diabetes mellitus over 10 years in dar es salaam, Tanzania. BMJ (1993) 306:1570–2. doi: 10.1136/bmj.306.6892.1570
20. da Rocha Fernandes RJ, Ogurtsova K, Linnenkamp U, Guariguata L, Seuring T, Zhang P, et al. IDF diabetes atlas estimates of 2014 global health expenditures on diabetes. Diabetes Res Clin Pract (2016) 117:48–54. doi: 10.1016/j.diabres.2016.04.016
21. Jasem D, Majaliwa ES, Ramaiya K, Najem S, Swai ABM, Ludvigsson J. Incidence, prevalence and clinical manifestations at onset of juvenile diabetes in Tanzania. Diabetes Res Clin Pract (2019) 156. doi: 10.1016/j.diabres.2019.107817
22. Große J, Hornstein H, Manuwald U, Kugler J, Glauche I, Rothe U. Incidence of diabetic ketoacidosis of new-onset type 1 diabetes in children and adolescents in different countries correlates with human development index (HDI): An updated systematic review, meta-analysis, and meta-regression. Horm Metab Res (2018) 50(3):209–22.
23. Katte JC, Lemdjo G, Dehayem MY, Jones AG, McDonald TJ, Sobngwi E. Mortality amongst children and adolescents with type 1 diabetes in sub-Saharan Africa: The case study of the changing diabetes in children program in Cameroon. Pediatr Diabetes (2022) 23(1):33–7. doi: 10.1111/pedi.13294
24. McLarty RP, Alloyce JP, Chitema GG, Msuya LJ. Glycemic control, associated factors, acute complications of type 1 diabetes mellitus in children, adolescents and young adults in Tanzania. Endocrinol Diabetes Metab (2020) 4(2):e00200.
25. Fantahun B, Leulseged TW. Glycemic control among children with type 1 diabetes mellitus and its determinants in a resource-limited setting. J Pediatr Endocrinol Metab (2022) 35(6):813–7. doi: 10.1515/jpem-2022-0144
26. Beran D, Yudkin JS. Diabetes care in sub-Saharan Africa. Lancent (2006) 368:1689–95. doi: 10.1016/S0140-6736(06)69704-3
28. Majaliwa ES, Ramaiya K, Mpembeni R, Sanyiwa A, Mohn A, et al. Survey on acute and chronic complications in children and adolescents with type 1 diabetes at muhimbili national hospital in dar es salaam, Tanzania. Diabetes Care (2007) 30:2187–92. doi: 10.2337/dc07-0594
29. Najem S, Majaliwa ES, Ramaiya K, Swai ABM, Jasem D, Ludvigsson J. Glycemic control and complications of type 1 diabetes among children in Tanzania. J Clin Transl Endocrinol (2020) 23:100245.
30. Msanga D, Reis K, Kayange N, Bakalemwa R, Kidenya B, Hau D. Diabetic microvascular complications among children and adolescents in northwestern Tanzania: A cross-sectional study. Ann Glob Health (2020) 86(1):43. doi: 10.5334/aogh.2669
31. Shibeshi MS, Daba AK, Meiso KM, Tadesse BT. Glycemic control among children and adolescents with diabetes in southern Ethiopia: a cross-sectional study. BMC Endocr Disord (2022) 22(1):161. doi: 10.1186/s12902-022-01070-y
32. Essuman VA, Tagoe NN, Akpalu J, Essuman A, Sackey AH, Hayfron-Benjamin CF, et al. Morbidity and complications of diabetes mellitus in children and adolescents in Ghana: Protocol for a longitudinal study. JMIR Res Protoc (2021) 10(1):e21440. doi: 10.2196/21440
33. Adler AJ, Trujillo C, Schwartz L, Drown L, Pierre J, Noble C, et al. Experience of living with type 1 diabetes in a low-income country: A qualitative study from Liberia. BMJ Open (2021) 11(10):e049738. doi: 10.1136/bmjopen-2021-049738
34. Bille N, Byberg S, Gishoma C, Buch Kristensen K, Lund Christensen D. HbA1c variability and the development of nephropathy in individuals with type 1 diabetes mellitus from Rwanda. Diabetes Res Clin Pract (2021) 178:108929. doi: 10.1016/j.diabres.2021.108929
35. Patterson CC, Karuranga S, Salpea P, Saeedi P, Dahlquist G, Soltesz G, et al. Worldwide estimates of incidence, prevalence and mortality of type 1 diabetes in children and adolescents: Results from the international diabetes federation diabetes atlas, 9th edition. Diabetes Res Clin Pract [Internet] (2019) 157:107842. doi: 10.1016/j.diabres.2019.107842
36. Gill GV, Huddle KR, R M. Mortality and outcome of insulin-dependent diabetes in Soweto, south Africa. Diabetes Med (1995) 12:546–50. doi: 10.1111/j.1464-5491.1995.tb00539.x
37. Palmer T, Jennings HM, Shannon G, Salustri F, Grewal G, Chelagat W, et al. Improving access to diabetes care for children: An evaluation of the changing diabetes in children project in Kenya and Bangladesh. Pediatr Diabetes (2022) 23(1):19–32. doi: 10.1111/pedi.13277
38. Missambou Mandilou SV, Atipo-Ibara Ollandzobo LC, Kitemo Mpolo FLG, Ngoulou BPS. Psychosocial functioning and health related quality of life in children, adolescents and young adults with type 1 diabetes mellitus in Congo. Pediatr Diabetes (2021) 22(4):675–82. doi: 10.1111/pedi.13187
39. Gupta N, Coates MM, Shannon G, Salustri F, Grewal G, Chelagat W, et al. Availability of equipment and medications for non-communicable diseases and injuries at public first-referral level hospitals: A cross-sectional analysis of service provision assessments in eight low-income countries. BMJ Open (2020) 10(10):e038842. doi: 10.1136/bmjopen-2020-038842
40. Bahendeka S, Mutungi G, Tugumisirize F, Kamugisha A, Nyangabyaki C, Wesonga R, et al. Healthcare delivery for paediatric and adolescent diabetes in low resource settings: Type 1 diabetes clinics in Uganda. Glob Public Health (2019) 14(12):1869–83. doi: 10.1080/17441692.2019.1611897
41. Gregory GA, Guo J, Klatman EL, Ahmadov GA, Besançon S, Gomez ED. Costs and outcomes of "intermediate" vs "minimal" care for youth-onset type 1 diabetes in six countries. Pediatr Diabetes (2020) 21(4):628–36. doi: 10.1111/pedi.12988
Keywords: low income, resources, type 1 diabetes, children, ketoacidosis, complications, Africa
Citation: Ludvigsson J, Edna M and Ramaiya K (2023) Type 1 diabetes in low and middle-income countries - Tanzania a streak of hope. Front. Endocrinol. 14:1043370. doi: 10.3389/fendo.2023.1043370
Received: 13 September 2022; Accepted: 06 March 2023;
Published: 24 March 2023.
Edited by:
Mitchell Eugene Geffner, Children's Hospital of Los Angeles, United StatesReviewed by:
Tetyana Chaychenko, Kharkiv National Medical University, UkraineCopyright © 2023 Ludvigsson, Edna and Ramaiya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Johnny Ludvigsson, Sm9obm55Lkx1ZHZpZ3Nzb25AbGl1LnNl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.