- 1Department of Psychiatry and Psychology, Mayo Clinic, Jacksonville, FL, United States
- 2Department of Behavioral Health, Gundersen Health System, La Crosse, WI, United States
- 3Department of Psychiatry and Psychology and Division of Endocrinology, Diabetes, Metabolism, and Nutrition, Mayo Clinic, Rochester, MN, United States
Weight regain after bariatric surgery is associated with problematic eating behaviors that have either recurred after a period of improvement or are new-onset behaviors. Problematic eating behaviors after bariatric surgery have been conceptualized in different ways in the literature, such as having a food addiction and experiencing a loss of control of eating. The intersection of these constructs appears to be driven overeating defined as patients’ experiences of reduced control of their eating which results in overeating behavior. The purpose of this review is to define patient experiences of driven overeating through the behavioral expression of emotion-based eating, reward-based eating, and executive functioning deficits—namely impulsivity—which is associated with weight regain after having bariatric surgery. Delineating concepts in this way and determining treatment strategies accordingly may reduce distress related to the inevitable return of increased hunger, cravings, portion sizes, and tolerance for highly palatable foods after surgery. Along with standard behavioral weight maintenance strategies, topics including acceptance, motivation, emotion-based eating, reward-based/impulsive eating, physical activity, and self-compassion are discussed. These concepts have been adapted for patients experiencing weight regain after having bariatric surgery and may be particularly helpful in attenuating driven overeating and weight regain.
Introduction
For people who are living with obesity and have a body mass index (BMI) ≥35 kg/m2, bariatric surgery is often the most efficacious treatment for achieving and maintaining desired weight loss that is associated with improvements in health and quality of life (1). Unfortunately, providers and patients alike are often uneducated about the efficacy versus the risk of having bariatric surgery, and it remains an underutilized treatment for obesity (2). In examining long-term weight loss, the outcome will vary widely by type of procedure and by patient characteristics (3). A recent systematic review of the literature suggests that patients tend to regain up to 19% of maximum weight loss between 3 and 6 years after Roux-en-Y gastric bypass and potentially more after sleeve gastrectomy (4). Problematic eating behaviors before surgery—although common—are not reliable predictors of suboptimal weight loss and are rarely a contraindication for undergoing bariatric surgery (5, 6). However, weight regain in the long-term is associated with problematic eating behaviors that have either recurred after a period of improvement or are new-onset problems (7–10).
Bariatric surgery is now known to affect regions in the brain associated with motivation, reward, attention, and inhibition in response to food exposure (11). In the first year after surgery, patients may experience reduced cravings, changes in preference for highly palatable foods, and altered responses to environmental food cues (12). Beyond the first year, however, some patients report experiencing increased hunger, food cravings, portion sizes, and tolerance for highly palatable foods (13). Problematic eating behaviors are described in the literature in different, ways including hedonic eating, loss of control eating, food addiction, compulsive eating, binge eating episodes, reward-based eating, emotion-based eating, grazing, and other terms (14). Specifically, food addiction, which is reduced control over food intake with repeated unsuccessful attempts to control intake, and loss of control of eating, defined as the inability to stop or control what or how much food is consumed, have received substantial attention in the bariatric surgery literature (7, 8, 15–17). Still, a lack of consensus remains regarding the uniqueness of these constructs or their clinical utility in terms of diagnosis and treatment before and after having bariatric surgery (18, 19). The intersection of these constructs appears to be driven overeating or patients’ experiences of reduced control over their eating, which results in overeating behaviors (20–22). The purpose of this review is to define patient experiences of driven overeating through the behavioral expression of emotion-based eating, reward-based eating, and executive functioning deficits that may be associated with weight regain after having bariatric surgery. Delineating concepts in this way may help guide decision making regarding treatment strategies to attenuate weight regain.
Emotion-Based Eating
Modern societies have facilitated easy and unending access to highly caloric and palatable foods that can be consumed rapidly. With such easy access, emotion-based eating (i.e., comfort-eating) is prevalent in the general population and likely even more prevalent in those who have obesity (23). Indeed, a recent population-based study demonstrated that emotion-based eating appears to be a plausible pathway between depression and the development of obesity (24). In addition to depression, emotion-based eating is associated with many other factors, including emotion dysregulation, poor emotion regulation skills, low distress tolerance, dietary restraint, loss of control of eating, poor awareness of hunger/fullness signals, body image dissatisfaction, internalized shame, and low self-compassion (24–29). Specifically, a recent study found that patients seeking bariatric surgery who have high levels of internalized shame (e.g., feelings of inferiority, inadequacy, insignificance) and low levels of self-compassion (e.g., disapproving of perceived personal inadequacies, feeling alone in perceived failures) also report high levels of emotion-based eating (25). Thus, increasing self-compassion is likely an important intervention strategy for reducing emotion-based eating. Collectively, research has consistently associated emotion-based eating with longitudinal weight gain, less success with attempts at weight loss, and poorer weight loss maintenance (30–32).
For bariatric surgery patients specifically, emotion-based eating appears to reliably decrease in the first 18 months postoperatively (33, 34). Results beyond the 18-month timeframe are mixed, with some studies suggesting attenuation of emotion-based eating through 36 months after having bariatric surgery and other studies finding reoccurrence between 12 and 36 months after having bariatric surgery (33–39). Emotion-based eating may return postbariatric surgery due to inconsistency of support. While many programs require patients to go through a preoperative nutritional and psychological assessment that includes a focus on problematic eating behaviors, this professional support is often absent postbariatric surgery. Surveys have shown that long-term engagement with bariatric team support diminishes over time, leading to less opportunity for intervention when needed (40, 41). Another reason for a return to emotional eating after having bariatric surgery relates to patients’ dietary patterns and preferences in the first year after having bariatric surgery. While emotion-based eating generally leads people to consume highly palatable foods, patients typically avoid these foods due to care team recommendations and/or due to negative physiological responses to ingestion of high-sugar, high-fat foods (e.g., dumping syndrome) in the months following bariatric surgery. Over time, these physiological responses may diminish, leading to increased poor-quality food consumption (13, 42). A final mechanism in a patient’s return to emotion-based eating after having bariatric surgery may be related to improved emotional states over the short term, leading to less desire or need to use food to cope with negative emotions. Over time, as negative emotional states may return and the “honeymoon period” after having bariatric surgery ends, patients may find themselves craving food to manage negative emotions (43, 44). Taken together, the reoccurrence or new onset of emotion-based eating behavior appears to be an important risk factor for weight regain after having bariatric surgery. At postbariatric surgery follow-up visits, inquiring about emotion-based eating and screening for symptoms of depression and anxiety, as well as other concerning emotions like shame, dissatisfaction with body shape and low self-compassion may help identify patients at high risk for weight regain.
Reward-Based Eating
Despite societal messages which imply that behaviors necessary to induce weight loss are completely volitional (i.e., eat less and move more), food intake is predominantly determined by one’s neurobiological reward system (i.e., hypothalamus, limbic system, prefrontal cortex) (42). Moreover, gut-brain mechanisms (e.g., leptin and ghrelin regulation) are also strongly related to food intake (45). Thus, many in the obesity scientific community believe that biology, rather than will-power or personal responsibility, is the primary driver of daily decisions about food intake. Two primary components can impact a food’s rewarding influence: (1) “Liking” or experiencing sensory pleasure or enjoyment from food, and (2) “Wanting” or craving foods and being implicitly driven to eat them (46). Also called hedonic eating, or eating beyond what is needed for homeostasis, eating for taste and/or pleasure is associated with weight gain and obesity (47). Neuroimaging studies have found that individuals with higher body weight have enhanced activation of food reward regions in the brain, likely impacting food preference and food choice (45). Some research has also suggested that, compared to normal-weight control groups, those who have obesity have higher levels of reward-based eating (45, 48–51).
After having bariatric surgery, research has shown reduced activation of the brain’s reward systems, indicating that food is inherently less rewarding, and studies have found that reward-based eating is significantly reduced in the first 2 years after having bariatric surgery (52, 53). Postbariatric surgery patients tend to experience less liking of many foods (i.e., tastes and odor preferences changing) and less wanting (i.e., reduce cravings). Moreover, dopamine signaling changes in those who have undergone bariatric surgery, thereby reducing the inherent rewarding properties of many foods (54, 55). After having bariatric surgery, foods that are highly palatable and energy dense are most impacted by these neurobiological changes, which, in turn, decrease food intake (56–59). A recent study demonstrated that for both Roux-en-Y gastric bypass and sleeve gastrectomy, there is a decrease in preference for high fat and calorie-dense foods and an increase in healthy food intake following bariatric surgery (46).
In a qualitative study of weight loss and dietary changes postbariatric surgery, patients described the early months after having bariatric surgery as the “honeymoon period” where “weight loss is easy and surgery does the work” as appetite, hunger, and interest in food are diminished (43). A recent review of the literature on the relationship between bariatric surgery and food preferences highlighted changes in food preferences at varying time points postoperatively (42). In the first year, individuals who had undergone bariatric surgery experienced an increase in their energy intake from proteins, with a decrease in fat consumption through 2 years postoperatively. They also tended to choose more healthy foods and fewer foods containing high-sugar, high-fat, and high-salt content, as well as less calorically dense foods. Despite pleasure ratings for most foods decreasing immediately after bariatric surgery with continued avoidance of high-fat and high-sugar foods beyond the first year postbariatric surgery, food choices seem to eventually (~5 years postop) return to patients’ preferred foods preoperatively (42). More research is needed to confirm the mechanisms by which neurobiological food reward systems regress toward preoperative eating preferences and behavior and the time frame of these potential neurobiological changes. At follow-up visits postbariatric surgery, from a clinical care viewpoint, inquiring about reward-based eating and the return of preference for high-fat and high-sugar foods that can be consumed rapidly may help to identify patients who are at high risk of weight regain.
Executive Functioning
Impairment in brain systems that activate reward, emotion, impulsivity, motivation for food, decreased inhibitory control and memory functioning have all been implicated as possible pathways in the development of obesity (11). The frontal lobes of the brain are particularly complex as they have extensive and reciprocal connections that integrate information from all other brain regions and are involved in appetite regulation (60). Specifically, the prefrontal cortex is associated with executive functioning, higher-order cognitive processes required to plan and accomplish goals. Deficits in executive functioning have been consistently identified in people living with obesity (61, 62). Broadly defined, executive functioning comprises cognitive flexibility (adapting thinking in response to the environment), working memory (holding small amounts of information in the mind to accomplish complex tasks), verbal fluency (retrieving information from memory), decision making (choosing from multiple possibilities), planning (sequencing thoughts and actions for goal attainment), and inhibition of impulses (delaying gratification).
Impulsivity is a multifactorial personality trait influenced by both genetics and the environment, which is commonly considered to fall under the domain of executive functioning. Two dimensions of impulsivity that are potentially important for understanding eating behavior and obesity are reward-based motivation and behavioral disinhibition (63, 64). For individuals who have obesity and impulsivity, motivation may be connected to eating-related reward pathways in the brain; while behavioral disinhibition is characterized by the occurrence of spontaneous behavior with a little forethought, low effort toward self-regulation, and occurs without regard for consequences. Stated differently, some individuals who have obesity and impulsivity may have high sensitivity to food-related rewards. They may persistently seek out certain foods intended to intensify sensory impact—ultra-processed foods with high-fat, high-sugar, and high-salt content—in anticipation of experiencing reward (63, 65). Moreover, with repeated exposure to highly rewarding foods that are abundant in the environment, the inability to self-regulate consumption results in weight gain.
In addition to food-related reward sensitivity and behavioral inhibition dimensions of impulsivity, negative affect is likely to be another important factor. Specifically, bariatric surgery patients who have impulsive personality traits and symptoms of depression also tend to have high levels of reward-based eating after having bariatric surgery, which is associated with weight regain (66). Schag etal. (66) propose that the pathway between impulsivity and eating behavior is likely mediated by a negative mood. That is, food-related reward sensitivity and behavioral disinhibition of food intake are exacerbated by negative mood states, a construct known as “negative urgency.” Negative urgency, the tendency to act without thought or effort at self-regulation when in distress, is proposed as a third dimension of impulsivity that potentially contributes to weight regain after having bariatric surgery (64, 66). Symptoms of depression typically improve in the first year after having bariatric surgery, but these improvements may erode over time (44), and there is some evidence that many patients increase their antidepressant medications after having bariatric surgery (67). Therefore, patients who have trait impulsivity and experience reoccurrence or new-onset depression symptoms after having bariatric surgery may be particularly vulnerable to reward-based eating when exposed to high-fat and high-sugar content foods.
Bariatric surgery has been proposed as a treatment to improve executive functioning for patients living with obesity (11) (68). Significant improvements in executive functioning occur in the first year and may endure for up to 36 months after bariatric surgery (61, 69). Potential mechanisms for improvement are increased blood flow in the brain, reduced inflammation, alterations in gut hormones, and remission of sleep apnea, hypertension, and diabetes (68). Moreover, there is evidence to suggest that after bariatric surgery, patients who demonstrate the greatest reductions in impulsivity as measured by neuropsychologic testing before and after bariatric surgery also had the greatest reduction in BMI (70). However, with weight regain, increased adiposity, and return of medical comorbidities, there is a risk of reversal of improvements and cognitive decline (69). Research investigating weight regain and executive functioning beyond 4 years after surgery is limited, and clearly more needs to be learned about the long-term benefits of bariatric surgery on executive functioning (71).
Presently, there are no standardized neuropsychological screening tools for measuring executive functioning, including impulsivity postbariatric surgery (61). Screening may be recommended if reasons for nonadherence or poor self-care are unclear or related to deficits in basic comprehension of behaviors required for success postoperatively (72). Other areas of concern may be if patients describe deficits in response inhibition (i.e., impulsive choices regarding highly palatable foods) and deficits in reward processing, such as time discounting (70). That is, the rewards of engagement in weight reduction behaviors (e.g., choosing low-calorie foods) are perceived to be so distant in time that they cease to have any value, thereby exerting little influence on motivation in the present moment. Neuropsychological testing may also be indicated if a diagnosis of Attention Deficit Hyperactivity Disorder (ADHD) is suspected, as there is an association between ADHD and obesity (73).
Enhanced Behavioral Strategies for Driven Overeating
Maintaining weight loss requires substantial effort as patients experience biological and environmental pressures to regain weight (74). That is, after having achieved nadir weight loss, patients may be unprepared to experience increased hunger, desire for highly palatable foods, increased portion sizes, and driven overeating (13, 75, 76). Standard behavior modification strategies for weight loss have consistently included goal setting, self-monitoring, stimulus control, contingency management, problem solving, thought restructuring, social support, and stress management (77). Self-monitoring remains a core component in obesity treatment and the necessary strategies for weight maintenance are measuring body weight consistently, tracking food intake, and tracking activity level (78, 79).
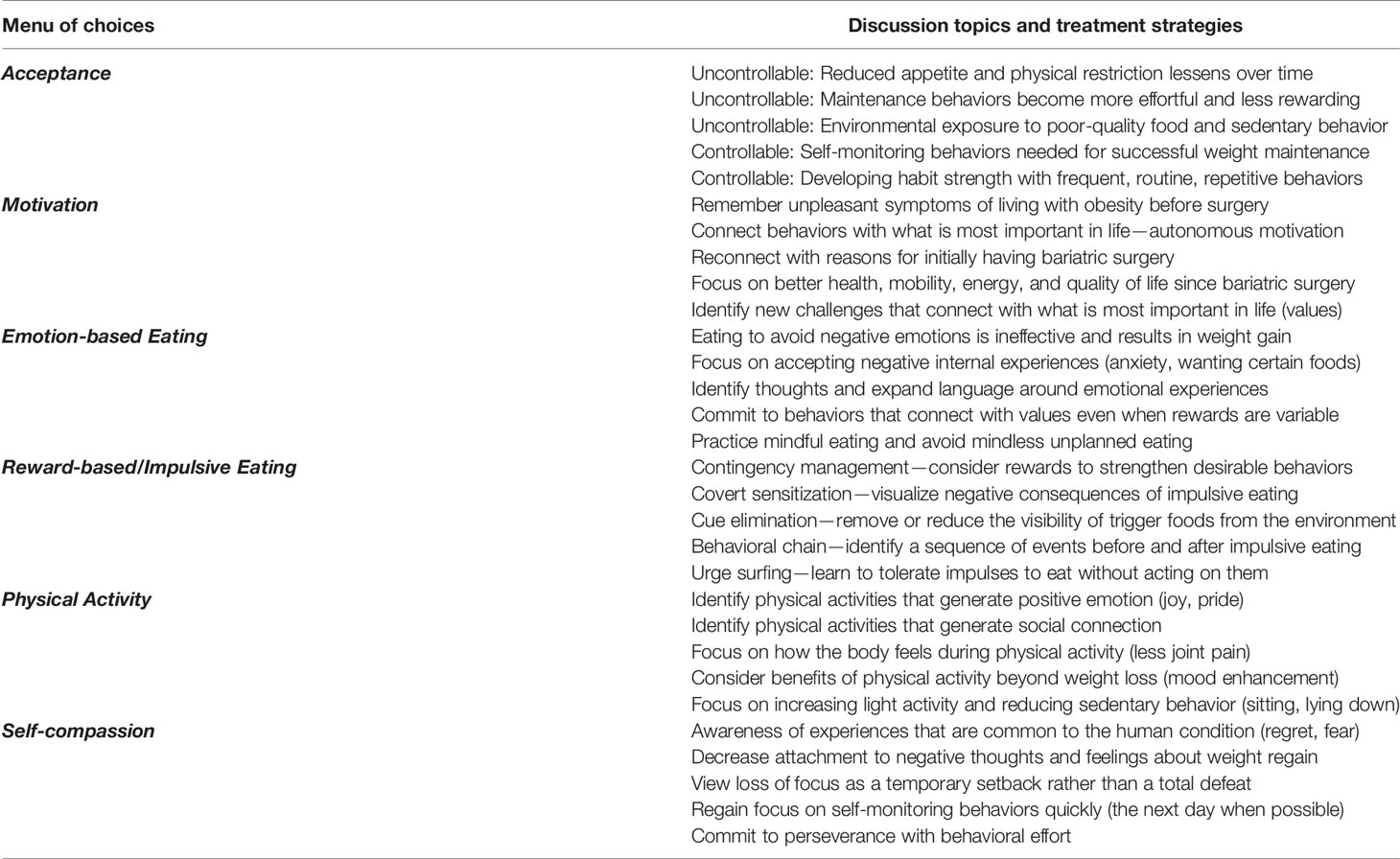
If patients experience the return of driven overeating after having bariatric surgery, strategies grounded in Acceptance and Commitment Therapy (ACT) may be useful for attenuating weight regain (80). Along with standard behavioral weight-loss strategies, topics discussed below regarding acceptance, motivation, emotional-based eating, reward-based/impulsive eating, physical activity, and self-compassion may also be useful. These concepts have been adapted for patients experiencing weight regain (>10% of nadir weight loss) after having bariatric surgery and may be particularly helpful in reducing driven overeating (81, 82). Effective Weight Loss Strategies: An Acceptance-Based Behavioral Approach is an excellent resource for multidisciplinary bariatric surgery care team providers, offering numerous experiential exercises and worksheets for patients (80). Providing a menu of treatment strategies may be most beneficial for supporting patients’ autonomy because not all strategies will be effective for every patient (83). Table 1 offers a menu of choices for discussion topics and treatment strategies for patients. Table 2 offers a patient vignette for a 47-year-old woman 18 months post-Roux-en-Y gastric bypass (BMI 27) maintaining a 34% reduction in initial body weight.

Table 2 Patient vignette for 47-year-old female 18 months post-Roux-en-Y gastric bypass (BMI 27) maintaining 34% reduction in initial body weight.
Promoting Acceptance
Because of the metabolic changes induced by bariatric surgery combined with behavioral effort, patients are typically rewarded with large weight loss and substantial improvements in health within the first year. However, when weight loss diminishes and stops, the powerful effects of reduced appetite and physical restriction induced by bariatric surgery are attenuated (13, 75, 76). Some postbariatric surgery patients may perceive these experiences as unexpected, distressing, and harbingers that their surgical weight loss tool is not working anymore, leaving them vulnerable to weight regain. Furthermore, behaviors that consistently resulted in large weight loss in the first year following bariatric surgery may appear to be more difficult and less rewarding once weight loss diminishes and stops (84, 85). For example, patients may grapple with daily decision fatigue about making healthy food choices or have difficulty with meal planning so that healthy food choices are easily accessible. They may also find it difficult to reduce sedentary behavior. Ultimately the rewards associated with weight loss, such as improved health, mobility, quality of life, and positive attention from others, have lessened and the need for the exertion of long-term focused behavioral effort becomes evident. Therefore, promoting acceptance that engaging in weight maintenance behaviors may become more effortful and less rewarding over time may be necessary (80). Similarly, promoting willingness means knowing that engaging in healthy eating and physical activity behaviors may be less rewarding than they once were, yet also acknowledging that they are necessary for weight maintenance and continued realization of health and quality-of-life improvements.
Promoting acceptance that there are some aspects of weight maintenance that are very challenging or even impossible to control may also be beneficial for some patients (74, 80). For many individuals, fully controlling their environmental exposure to poor-quality foods is unattainable, and the current physical activity environment is such that being sedentary has become the default behavior (86). In the same way, the occurrence of unforeseen life events that may reduce focus on the frequency or repetitiveness of healthy eating and activity behaviors is inevitable (87). For these reasons, one helpful strategy to regain focus may be reconnecting with behaviors that are under personal control, such as self-monitoring. A recent study of nonsurgery patients investigating weight maintenance self-monitoring behaviors compared weight loss maintainers (who lost greater than 24.5 kg and maintained a minimum of 9 kg reduction in weight for more than 3 years) to weight-stable individuals who had obesity (78). Weight loss maintainers commonly endorsed specific behaviors that included keeping a weight graph, setting daily intake goals, having lower-calorie foods easily accessible, choosing lower-calorie food options, measuring portions, and recording food intake. They also appeared to develop habit strength around healthy eating and physical activity. Healthy eating behaviors were done frequently, done as part of a daily routine, or repetitively and done automatically without having to think about them. Inquiring about what postbariatric surgery patients’ current habit strengths are, such as taking vitamins, staying hydrated, focusing on protein intake, or using a sit-to-stand desk at work may help regain focus and build confidence for adding additional weight maintenance behaviors. Regarding physical activity level, weight loss maintainers (BMI 27.6) reduced their sedentary behavior (i.e., sitting or lying down) and spent three more hours per day engaged in light- or moderate-intensity activities (i.e., walking, taking stairs) compared to weight-stable individuals who had obesity (BMI 38.9; total energy expenditure of 1,835 kcal/week vs. 785 kcals per week, respectively) (79). Therefore, reducing sedentary behavior and increasing light activity during leisure time may be a helpful strategy for maintaining weight loss, particularly for postbariatric patients who have low levels of activity and/or dislike engaging in higher intensity physical activities.
Strengthening Motivational Level for Healthy Living
Discovering autonomous motivation—attributing personal values to certain behaviors—may be especially helpful for sustaining behavioral effort to maintain weight loss even when it becomes more difficult and less rewarding (88). One way to do this is to help postbariatric patients reconnect with personal values or their ideas about what is important in life and what experiences have meaning for them. Helping them verbalize the reasons why they decided to have bariatric surgery may initially enhance their current motivational level for healthy living. Weight loss maintainers describe having unpleasant memories about how it was to live with obesity, and these memories can be targeted for high-quality motivation for sustaining behavior change (89). These negative preweight loss memories were primarily concerned with declining physical health as well as shame and embarrassment about body size and shape. Furthermore, weight loss maintainers found achieving improvements in their physical health like the remission of diabetes, lower blood pressure, taking fewer medications, and reduced pain with increased physical activity level as deeply rewarding. They also indicated achieving weight loss inspired increased self-confidence for pursuing personal goals and taking on new challenges (e.g., seeking a promotion at work) that were meaningful and consistent with their personal values. Best practices for conversations about health behavior changes may include a motivational interviewing communication style that includes asking open-ended questions, inviting a wide variety of responses, and offering reflective listening statements that evoke and strengthen the patients’ personal reasons for desiring change (90).
Reducing Emotional-Based Eating
One aspect of ACT that may be particularly helpful with emotion-based eating is accepting that experiencing thoughts (e.g., dissatisfaction with body shape), emotions (e.g., fear of weight regain, sadness, shame, regret), and physical sensations (e.g., food cravings, hunger) is inevitable (91). The conditioned behavior of eating in response to negative internal or private experiences may appear to be an effective strategy at the moment, but likely increases negative emotion and concern about weight gain in the long term. Eating for emotional comfort is not goal-directed or rational, exposes impulsivity, and ultimately results in undesirable consequences. Rather than trying to change negative thoughts to reduce the intensity of negative emotions (the core concept of cognitive behavioral therapy), patients instead focus on accepting and embracing negative internal experiences that were previously avoided as a normal part of the human experience. Moreover, instead of focusing on problem eating behaviors, patients commit to increasing behaviors that support personal values. In ACT, committed action is a concept that refers to maintaining behavior change knowing that the rewards experienced from these behaviors will be highly variable (80). Committed action may be strengthened by exploration of personal values associated with improved health, mobility, and quality of life, like being able to participate in physical activities with loved ones or improving work performance with increased mobility after weight loss.
Another component of ACT includes mindful eating (80). Mindfulness, operationally defined in the context of eating, is self-regulation of attention toward physical sensations like cravings, affective states such as anxious mood, and thoughts pertaining to liking and wanting certain foods (92). The second part of mindfulness is having a nonjudgmental attitude of acceptance and curiosity about food-related sensations, feelings, and thoughts. Mindfulness-based approaches have been used effectively for reducing problematic eating behaviors for individuals who have obesity and for bariatric surgery patients (93–95). Specific to weight regain after having bariatric surgery, one study conducted a randomized controlled trial of a 10-session mindfulness-based intervention compared to standard treatment in a sample of postbariatric surgery patients. The treatment was specifically designed to prevent weight gain after having bariatric surgery (95). The mindfulness-based intervention promoted acceptance and reduced reactivity to distress, including learning several different types of meditation (e.g., walking meditation, sitting meditation, or chair yoga) in session and during home practice, group sharing, and didactic sessions. After 6 months, the mindfulness intervention resulted in reduced emotion-based eating but did not result in weight loss. Mindfulness interventions designed to interrupt negative internal experiences and improve decision-making in the moment may help attenuate but not reverse weight gain. Nevertheless, mindfulness training is not yet a part of routine care for bariatric patients, and the long-term benefit and durability of such interventions are not known (96). Acquiring mindfulness skills requires experiential learning and consistent practice, which may not be appropriate or feasible for every patient.
Reducing Reward-Based/Impulsive Eating
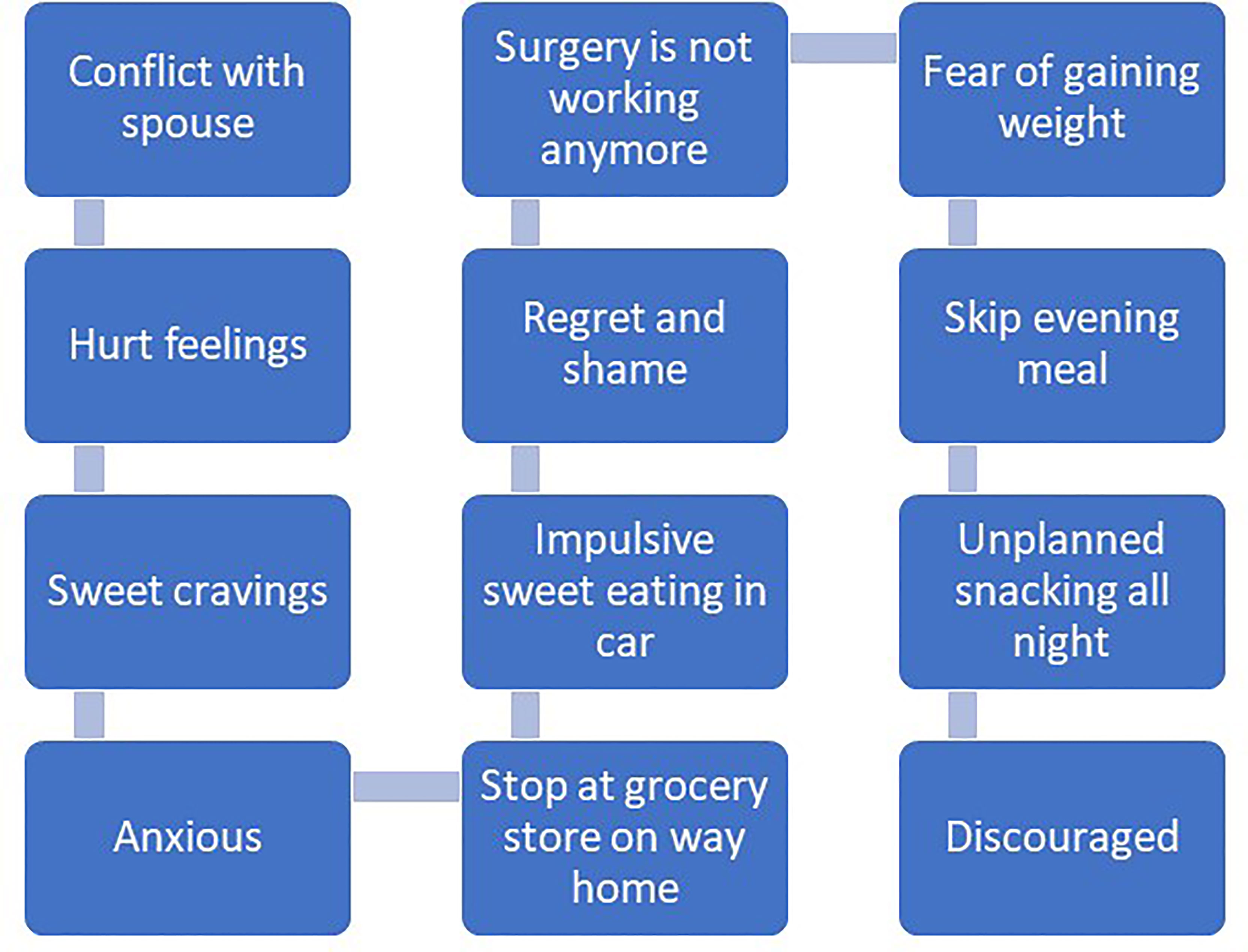
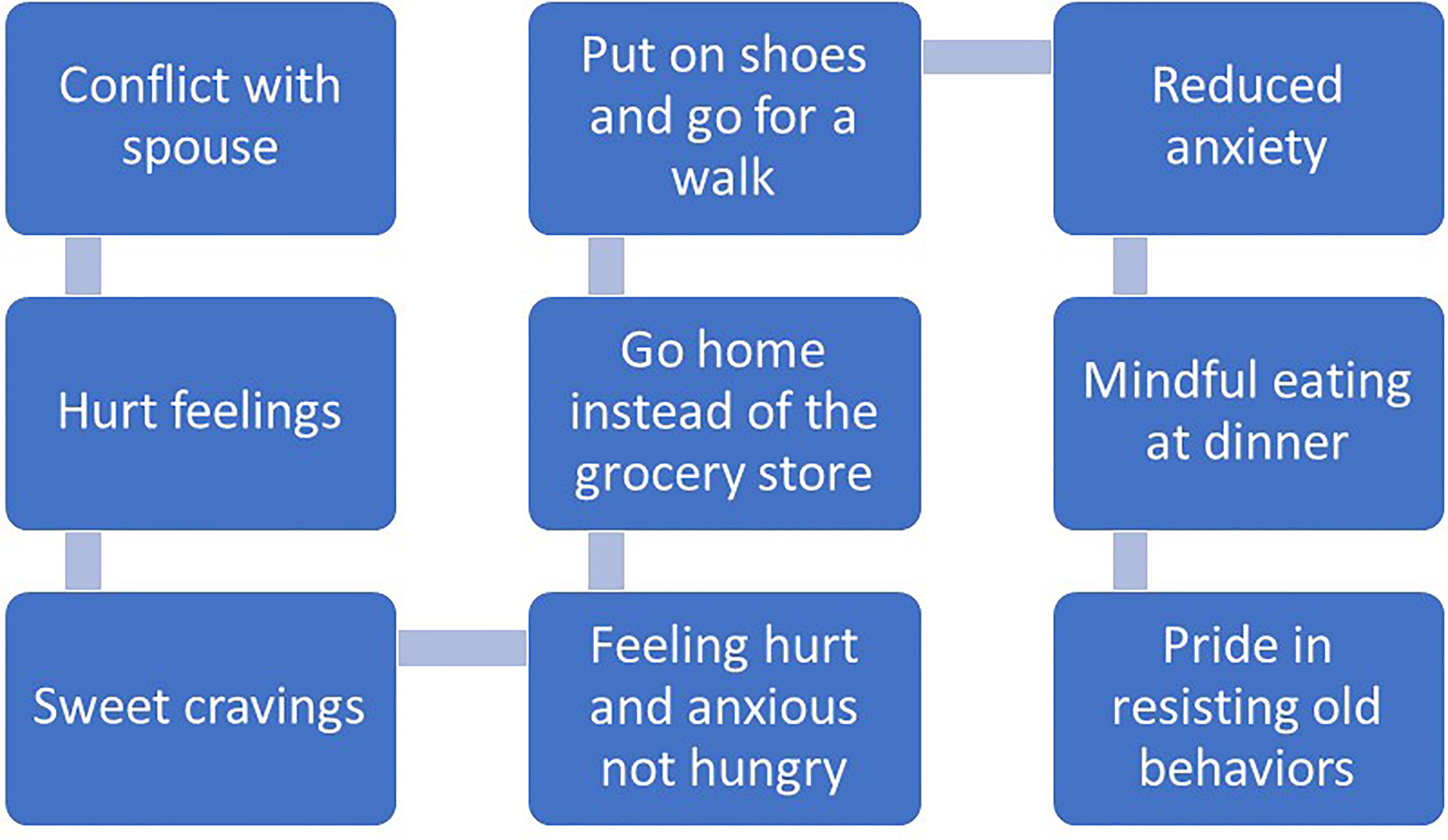
Recommendations for managing impulsivity with appetitive behaviors include several specific behavioral strategies (97). First, contingency management strategies involve implementing rewards or motivational incentives for desirable behaviors and removing rewards or incentives for undesirable behaviors. Presumably, the anticipated reward of consuming certain foods is to maintain problematic eating behaviors. Patients may consider other rewards that can be introduced or strengthened for performing desirable behaviors unrelated to eating. Second, covert sensitization is an imagery exercise designed to associate negative consequences with problem behaviors. Patients may imagine themselves in their usual environment where impulsive eating behavior may occur. Rather than focus on the positive rewards of impulsive eating at the moment (e.g., escape from negative emotion, distraction), patients are instructed to imagine the negative consequences of eating impulsively, like increased emotional distress that is associated with weight regain. If patients successfully reduce the frequency of impulsive eating, emotional distress and weight gain may be lessened over time. Third, cue elimination may be particularly effective for some patients as its purpose is to eliminate environmental cues that signal rewards for eating impulsively. Assisting patients with identifying which foods are highly rewarding, triggering impulsive eating behavior and developing management strategies for removal or reduced visibility of those foods may be beneficial. Fourth, assisting patients with behavioral chain analyses involves three critical steps for impulsivity management, including (1) identifying a sequence of events that occur immediately before and after impulsive eating, (2) identifying positive consequences that are maintaining impulsive eating as well as the associated negative consequences, and (3) identifying substitute behaviors that are less harmful but notably also perceived as rewarding. Figures 1, 2 offer examples of negative and positive behavioral chain analyses for emotion-based and impulsive eating, respectively.
Lastly, it is impossible to avoid foods associated with rewards completely, and experiencing urges, cravings, or impulses to eat is a normal part of the human experience. Therefore, developing management strategies for these inevitable situations is necessary. ACT incorporates concepts of defusion and urges surfing to manage impulsive eating behavior (80). Defusion is a concept that suggests viewing urges to eat as a normal part of an internal appetitive experience, and patients have a choice whether to act on the associated thoughts, feelings, and physical sensations. Stated differently, defusion encourages becoming aware of the actual thought process around impulsive eating and observing objectively rather than acting automatically on urges to eat. Similarly, urge surfing involves learning to tolerate impulses to eat without acting on them (80, 97). Patients are instructed to imagine urges to eat as ocean waves that build, reach peak intensity, and dissipate over time. Staying in contact with the present moment allows oneself to ride the ocean wave of thoughts, emotions, and physical sensations without judgment until the urge subsides. The goal eventually is that patients may develop what is known as psychological flexibility. That is, they are able to tolerate emotions, sensations, and thoughts, good or bad, and behave in accordance with their values of maintaining improved health and quality of life achieved after having bariatric surgery (98).
Promoting Physical Activity
Recent research suggests that there are themes associated with positive affect (i.e., excitement, determination, pride, sense of accomplishment, joy) while participating in physical activity after having bariatric surgery. These themes included enjoyment of the activity itself, social connection, mindfulness, and mastery of a skill (99). Patients may find it beneficial to reconnect with physical activities that they enjoy earlier in life or perhaps engage in novel activities they have not tried yet, such as yoga or rock climbing. Others may benefit from finding activities that involve connection with others such as a walking group, scheduling a fitness class, or participating in a fitness event like a charity 5-km walk. Mindfulness regarding how physical activity feels in the moment with a healthier body is also associated with positive affect, and many patients will resonate with the term, mindful movement. Patients may reflect on experiencing less joint pain, less shortness of breath, or ease of walking up stairs and focus instead on what their bodies can do after achieving weight loss.
Interestingly, a theme related to negative affect (i.e., frustration, fatigue, dislike) was all-or-nothing thinking about physical activity (99). For instance, focusing only on the negative aspects, having a general dislike of physical activity, or discounting efforts that fall short of personal goals were described as stressful and anxiety-provoking. Reminding patients that while goal setting around reaching a certain frequency, intensity, or time of physical activity is useful, there are other meaningful ways to incorporate activity, like focusing on reducing sedentary behavior during leisure time and increasing light activity throughout the day (79, 99). For patients who have sedentary work lives (e.g., telework), discussing strategies to limit or break up time spent sitting can be beneficial. Another theme that emerged as a barrier to physical activity was using weight loss as the primary motivator to be physically active (99). Considering the known benefits of physical activity beyond weight loss, such as improved metabolic health, fitness, strength, flexibility, balance, and mood enhancement, stress reduction and longevity may be helpful for some patients.
Promoting Self-Compassion
Destabilizing events that result in loss of control and focus on health behaviors are often unexpected and perhaps not immediately perceived as risk factors for weight gain. These events may include life challenges (e.g., prioritizing caregiving responsibilities over personal health, financial problems, housing insecurity, loss of employment and healthcare benefits), increased appetite, and changes in physical and mental health (e.g., prescribed weight-promoting medication) (13). The experience of weight regain after bariatric surgery is likely to induce feelings of disappointment, frustration, fear of regaining back to their heaviest weight, and shame (13, 75). Shame may be particularly detrimental. It is generated by fear of exposure to increasing adiposity, fear of judgment from others, silence, and secrecy, which may be associated with distancing from possible sources of support, including the bariatric surgery care team. Indeed, patients who have a high degree of internalized shame (e.g., feelings of inadequacy, inferiority) about having obesity may have the lowest levels of self-compassion (25).
Self-compassion is defined as increased self-kindness, awareness of experiences common to the human condition, and decreased attachment to negative thoughts and feelings in times of perceived failure, like weight regain (100). A recent investigation of bariatric surgery patients found that a high level of self-compassion presurgery was associated with less depression and greater body image satisfaction, quality of life, and confidence in one’s ability to control eating behaviors 12 months after having bariatric surgery (101). Findings from this study are supported by the larger body of research regarding self-compassion interventions and their impact on a wide variety of psychosocial outcomes. A recent meta-analysis found that there were large effect sizes for self-compassion interventions on improving maladaptive eating behaviors like binge eating and ruminative thinking patterns (102).
Practicing self-compassion in the context of weight maintenance may include acknowledging that loss of control and focusing on health behaviors is an inevitable part of long-term weight management (13, 89). Having days or weeks where there is less focus on the quality and quantity of food consumed or time spent engaging in physical activity should be expected as a normal part of the human experience. How one responds to the loss of focus on weight control behaviors appears to be critically important. Specifically, weight loss maintainers commonly suggested that having “perseverance” with behavioral effort and viewing loss of focus as a temporary setback rather than total defeat was beneficial (89). Moreover, rather than being self-critical, weight loss maintainers regained focus quickly—the next day when possible—by restarting self-monitoring behaviors. Unequivocally, weight loss maintainers accept that tracking food intake is a necessary part of lifestyle change and is critically important for long-term weight loss maintenance.
Finally, helping patients reduce self-judgment by putting into context the status of their current weight may increase self-compassion. Patients may become extremely self-critical about how much weight has been regained from their nadir weight loss. For example, with Roux-en-Y gastric bypass, maintaining a 25% reduction from presurgical weight is excellent, regardless of nadir percent total weight loss, and is often enough to sustain improvements in health status and quality of life (103). Striving to return to the lowest body weight achieved after bariatric surgery may unnecessarily induce emotional distress and self-criticism for some patients. Maintaining a so-called normal body weight or BMI is unnecessary after having bariatric surgery as cardiometabolic health, cancer risk, and life expectancy may improve dramatically even if BMI remains in the obesity range (104–106).
Conclusion
Identifying eating behaviors that may contribute to weight gain after having bariatric surgery remains challenging. The research literature is consistent that presurgical problematic eating behaviors typically improve in the first 12 months after having bariatric surgery, yet some patients will experience the return of these problematic eating behaviors or new-onset problematic eating behaviors in the long term. The construct of driven overeating or reduced control overeating appears to be the commonality among different types of problematic eating behaviors described in the literature. Specifically, patient experiences of driven overeating through a behavioral expression of emotion-based eating, reward-based eating, and executive functioning deficits—namely impulsivity—are associated with weight regain after bariatric surgery. Delineating concepts in this way and determining treatment strategies accordingly may reduce distress related to the inevitable return of increased hunger, cravings, portion sizes, and tolerance for highly palatable foods after surgery. Along with standard behavioral weight-loss strategies, topics such as acceptance, motivation, emotional-based eating, reward-based/impulsive eating, physical activity, and self-compassion may also be useful to the postbariatric surgery patient. These concepts have been adapted for patients experiencing weight regain after having bariatric surgery and may be particularly helpful in managing driven overeating. Providing a menu of treatment strategies may be most beneficial for supporting patients’ autonomy because not all strategies will be effective for every patient.
Author Contributions
GA and AK conceptualized and wrote the original draft of the manuscript. MC reviewed and edited the manuscript. All authors have read and approved the final draft of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Courcoulas AP, Johnson E, Arterburn DE, Haneuse S, Herrinton LJ, Fisher DP, et al. Reduction in Long-Term Mortality After Sleeve Gastrectomy and Gastric Bypass Compared to Non-Surgical Patients With Severe Obesity. Ann Surg (2021) 13:10.1097/SLA.0000000000005155. doi: 10.1097/SLA.0000000000005155
2. Ames GE, Maynard MR, Collazo-Clavell ML, Clark MM, Grothe KB, Elli EF. Rethinking Patient and Medical Professional Perspectives on Bariatric Surgery as a Medically Necessary Treatment. Mayo Clin Proc (2020) 95:527–40. doi: 10.1016/j.mayocp.2019.09.019
3. Courcoulas AP, Christian NJ, Belle SH, Berk PD, Flum DR, Garcia L, et al. Longitudinal Assessment of Bariatric Surgery, C.Weight Change and Health Outcomes at 3 Years After Bariatric Surgery Among Individuals With Severe Obesity. JAMA (2013) 310:2416–25. doi: 10.1001/jama.2013.280928
4. King WC, Hinerman AS, Courcoulas AP. Weight Regain After Bariatric Surgery: A Systematic Literature Review and Comparison Across Studies Using a Large Reference Sample. Surg Obes Relat Dis (2020) 16:1133–44. doi: 10.1016/j.soard.2020.03.034
5. Koball AM, Clark MM, Collazo-Clavell M, Kellogg T, Ames G, Ebbert J, et al. The Relationship Among Food Addiction, Negative Mood, and Eating-Disordered Behaviors in Patients Seeking to Have Bariatric Surgery. Surg Obes Relat Dis (2016) 12:165–70. doi: 10.1016/j.soard.2015.04.009
6. Brode CS, Mitchell JE. Problematic Eating Behaviors and Eating Disorders Associated With Bariatric Surgery. Psychiatr Clin North Am (2019) 42:287–97. doi: 10.1016/j.psc.2019.01.014
7. Ivezaj V, Wiedemann AA, Grilo CM. Food Addiction and Bariatric Surgery: A Systematic Review of the Literature. Obes Rev (2017) 18:1386–97. doi: 10.1111/obr.12600
8. Conceicao EM, de Lourdes M, Pinto-Bastos A, AR Vaz, Brandao I, Ramalho S. Problematic Eating Behaviors and Psychopathology in Patients Undergoing Bariatric Surgery: The Mediating Role of Loss of Control Eating. Int J Eat Disord (2018) 51:507–17. doi: 10.1002/eat.22862
9. King WC, Belle SH, Hinerman AS, Mitchell JE, Steffen KJ, Courcoulas AP. Patient Behaviors and Characteristics Related to Weight Regain After Roux-En-Y Gastric Bypass: A Multicenter Prospective Cohort Study. Ann Surg (2020) 272:1044–52. doi: 10.1097/SLA.0000000000003281
10. Mauro M, Papelbaum M, Brasil MAA, Carneiro JRI, Coutinho ESF, Coutinho W, et al. Is Weight Regain After Bariatric Surgery Associated With Psychiatric Comorbidity? A Systematic Review and Meta-Analysis. Obes Rev (2019) 20:1413–25. doi: 10.1111/obr.12907
11. Lin Z, Qu S. Legend of Weight Loss: A Crosstalk Between the Bariatric Surgery and the Brain. Obes Surg (2020) 30:1988–2002. doi: 10.1007/s11695-020-04474-8
12. Alamuddin N, Vetter ML, Ahima RS, Hesson L, Ritter S, Minnick A, et al. Changes in Fasting and Prandial Gut and Adiposity Hormones Following Vertical Sleeve Gastrectomy or Roux-En-Y-Gastric Bypass: An 18-Month Prospective Study. Obes Surg (2017) 27:1563–72. doi: 10.1007/s11695-016-2505-5
13. Tolvanen L, Christenson A, Surkan PJ, Lagerros YT. Patients' Experiences of Weight Regain After Bariatric Surgery. Obes Surg (2022) 32(5):1498–1507. doi: 10.1007/s11695-022-05908-1
14. Nightingale BA, Cassin SE. Disordered Eating Among Individuals With Excess Weight: A Review of Recent Research. Curr Obes Rep (2019) 8:112–27. doi: 10.1007/s13679-019-00333-5
15. Grilo CM, Ivezaj V, Duffy AJ, Gueorguieva R. Randomized Controlled Trial of Treatments for Loss-Of-Control Eating Following Bariatric Surgery. Obes (Silver Spring) (2021) 29:689–97. doi: 10.1002/oby.23124
16. Smith KE, Orcutt M, Steffen KJ, Crosby RD, Cao L, Garcia L, et al. Loss of Control Eating and Binge Eating in the 7 Years Following Bariatric Surgery. Obes Surg (2019) 29:1773–80. doi: 10.1007/s11695-019-03791-x
17. Holgerson AA, Clark MM, Ames GE, Collazo-Clavell ML, Kellogg TA, Graszer KM, et al. Association of Adverse Childhood Experiences and Food Addiction to Bariatric Surgery Completion and Weight Loss Outcome. Obes Surg (2018) 28:3386–92. doi: 10.1007/s11695-018-3370-1
18. Finlayson G. Food Addiction and Obesity: Unnecessary Medicalization of Hedonic Overeating. Nat Rev Endocrinol (2017) 13:493–8. doi: 10.1038/nrendo.2017.61
19. Lee PC, Dixon JB. Food for Thought: Reward Mechanisms and Hedonic Overeating in Obesity. Curr Obes Rep (2017) 6:353–61. doi: 10.1007/s13679-017-0280-9
20. Tanofsky-Kraff M. Is Driven Overeating Best Captured by Food Addiction, Loss of Control Eating, or Something Else? In: Obesity Week The Obesity Society (2021). p. 1–5.
21. Byrne ME, Shank LM, Altman DR, Swanson TN, Ramirez E, Moore NA, et al. Inhibitory Control and Negative Affect in Relation to Food Intake Among Youth. Appetite (2021) 156:104858. doi: 10.1016/j.appet.2020.104858
22. Byrne ME, Tanofsky-Kraff M, Lavender JM, Parker MN, Shank LM, Swanson TN, et al. Bridging Executive Function and Disinhibited Eating Among Youth: A Network Analysis. Int J Eat Disord (2021) 54:721–32. doi: 10.1002/eat.23476
23. Gibson EL. The Psychobiology of Comfort Eating: Implications for Neuropharmacological Interventions. Behav Pharmacol (2012) 23:442–60. doi: 10.1097/FBP.0b013e328357bd4e
24. Konttinen H, Van Strien T, Mannisto S, Jousilahti P, Haukkala A. Depression, Emotional Eating and Long-Term Weight Changes: A Population-Based Prospective Study. Int J Behav Nutr Phys Act (2019) 16:28. doi: 10.1186/s12966-019-0791-8
25. Braun TD, Gorin AA, Puhl RM, Stone A, Quinn DM, Ferrand J, et al. Shame and Self-Compassion as Risk and Protective Mechanisms of the Internalized Weight Bias and Emotional Eating Link in Individuals Seeking Bariatric Surgery. Obes Surg (2021) 31:3177–87. doi: 10.1007/s11695-021-05392-z
26. Geller S, Levy S, Goldzweig G, Hamdan S, Manor A, Dahan S, et al. Psychological Distress Among Bariatric Surgery Candidates: The Roles of Body Image and Emotional Eating. Clin Obes (2019) 9:e12298. doi: 10.1111/cob.12298
27. Van Strien T. Causes of Emotional Eating and Matched Treatment of Obesity. Curr Diabetes Rep (2018) 18:35. doi: 10.1007/s11892-018-1000-x
28. Devlin MJ, King WC, Kalarchian MA, White GE, Marcus MD, Garcia L, et al. Eating Pathology and Experience and Weight Loss in a Prospective Study of Bariatric Surgery Patients: 3-Year Follow-Up. Int J Eat Disord (2016) 49:1058–67. doi: 10.1002/eat.22578
29. Koball AM, Himes SM, Sim L, Clark MM, Collazo-Clavell ML, Mundi M, et al. Distress Tolerance and Psychological Comorbidity in Patients Seeking Bariatric Surgery. Obes Surg (2016) 26:1559–64. doi: 10.1007/s11695-015-1926-x
30. Koenders PG, Van Strien T. Emotional Eating, Rather Than Lifestyle Behavior, Drives Weight Gain in a Prospective Study in 1562 Employees. J Occup Environ Med (2011) 53:1287–93. doi: 10.1097/JOM.0b013e31823078a2
31. Ricca V, Castellini G, Mannucci E, lo Sauro C, Ravaldi C, Rotella CM, et al. Comparison of Individual and Group Cognitive Behavioral Therapy for Binge Eating Disorder. A Randomized, Three-Year Follow-Up Study. Appetite (2010) 55:656–65. doi: 10.1016/j.appet.2010.09.019
32. Wedin S, Madan A, Correll J, Crowley N, Malcolm R, Karl Byrne T, et al. Emotional Eating, Marital Status and History of Physical Abuse Predict 2-Year Weight Loss in Weight Loss Surgery Patients. Eat Behav (2014) 15:619–24. doi: 10.1016/j.eatbeh.2014.08.019
33. Dymek MP, le Grange D, Neven K, Alverdy J. Quality of Life and Psychosocial Adjustment in Patients After Roux-En-Y Gastric Bypass: A Brief Report. Obes Surg (2001) 11:32–9. doi: 10.1381/096089201321454088
34. Wong LY, Zafari N, Churilov L, Stammers L, Price S, Ekinci EI, et al. Change in Emotional Eating After Bariatric Surgery: Systematic Review and Meta-Analysis. BJS Open (2020) 4(6):995–1014. doi: 10.1002/bjs5.50318
35. Nasirzadeh Y, Kantarovich K, Wnuk S, Okrainec A, Cassin SE, Hawa R, et al. Binge Eating, Loss of Control Over Eating, Emotional Eating, and Night Eating After Bariatric Surgery: Results From the Toronto Bari-PSYCH Cohort Study. Obes Surg (2018) 28:2032–9. doi: 10.1007/s11695-018-3137-8
36. Laurenius A, Larsson I, Bueter M, Melanson KJ, Bosaeus I, Forslund HB, et al. Changes in Eating Behaviour and Meal Pattern Following Roux-En-Y Gastric Bypass. Int J Obes (Lond) (2012) 36:348–55. doi: 10.1038/ijo.2011.217
37. Papalazarou A, Y0annakoulia M, Kavouras SA, Komesidou V, Dimitriadis G, Papakonstantinou A, et al. Lifestyle Intervention Favorably Affects Weight Loss and Maintenance Following Obesity Surgery. Obes (Silver Spring) (2010) 18:1348–53. doi: 10.1038/oby.2009.346
38. Gill H, Kang S, Lee Y, Rosenblat JD, Brietzke E, Zuckerman H, et al. The Long-Term Effect of Bariatric Surgery on Depression and Anxiety. J Affect Disord (2019) 246:886–94. doi: 10.1016/j.jad.2018.12.113
39. Subramaniam K, Low WY, Lau PC, Chin KF, Chinna K, Kosai NR, et al. Eating Behaviour Predicts Weight Loss Six Months After Bariatric Surgery: A Longitudinal Study. Nutrients (2018) 10(11):1616. doi: 10.3390/nu10111616
40. Moroshko I, Brennan L, O'brien P. Predictors of Attrition in Bariatric Aftercare: A Systematic Review of the Literature. Obes Surg (2012) 22:1640–7. doi: 10.1007/s11695-012-0691-3
41. Jurgensen JA, Reidt W, Kellogg T, Mundi M, Shah M, Collazo Clavell ML. Impact of Patient Attrition From Bariatric Surgery Practice on Clinical Outcomes. Obes Surg (2019) 29:579–84. doi: 10.1007/s11695-018-3565-5
42. Guyot E, Dougkas A, Nazare JA, Bagot S, Disse E, Iceta S. A Systematic Review and Meta-Analyses of Food Preference Modifications After Bariatric Surgery. Obes Rev (2021) 22:e13315. doi: 10.1111/obr.13315
43. Lynch A. "When the Honeymoon is Over, the Real Work Begins:" Gastric Bypass Patients' Weight Loss Trajectories and Dietary Change Experiences. Soc Sci Med (2016) 151:241–9. doi: 10.1016/j.socscimed.2015.12.024
44. Smith KE, Mason TB, Cao L, Crosby RD, Steffen KJ, Garcia L, et al. Trajectories of Depressive Symptoms and Relationships With Weight Loss in the Seven Years After Bariatric Surgery. Obes Res Clin Pract (2020) 14:456–61. doi: 10.1016/j.orcp.2020.08.007
45. Guerrero-Hreins E, Foldi CJ, Oldfield BJ, Stefanidis A, Sumithran P, Brown RM. Gut-Brain Mechanisms Underlying Changes in Disordered Eating Behaviour After Bariatric Surgery: A Review. Rev Endocr Metab Disord (2021). doi: 10.1007/s11154-021-09696-4
46. Guyot E, Nazare JA, Oustric P, Robert M, Disse E, Dougkas A, et al. Food Reward After Bariatric Surgery and Weight Loss Outcomes: An Exploratory Study. Nutrients (2022) 14(3):449. doi: 10.3390/nu14030449
47. Kringelbach ML. Food for Thought: Hedonic Experience Beyond Homeostasis in the Human Brain. Neuroscience (2004) 126:807–19. doi: 10.1016/j.neuroscience.2004.04.035
48. Schultes B, Ernst B, Wilms B, Thurnheer M, Hallschmid M. Hedonic Hunger is Increased in Severely Obese Patients and is Reduced After Gastric Bypass Surgery. Am J Clin Nutr (2010) 92:277–83. doi: 10.3945/ajcn.2009.29007
49. Faulconbridge LF, Ruparel K, Loughead J, Allison KC, Hesson LA, Fabricatore AN, et al. Changes in Neural Responsivity to Highly Palatable Foods Following Roux-En-Y Gastric Bypass, Sleeve Gastrectomy, or Weight Stability: An fMRI Study. Obes (Silver Spring) (2016) 24:1054–60. doi: 10.1002/oby.21464
50. Frank S, Veit R, Sauer H, Enck P, Friederich HC, Unholzer T, et al. Dopamine Depletion Reduces Food-Related Reward Activity Independent of BMI. Neuropsychopharmacology (2016) 41:1551–9. doi: 10.1038/npp.2015.313
51. Goldstone AP, Miras AD, Scholtz S, Jackson S, Neff KJ, Penicaud L, et al. Link Between Increased Satiety Gut Hormones and Reduced Food Reward After Gastric Bypass Surgery for Obesity. J Clin Endocrinol Metab (2016) 101:599–609. doi: 10.1210/jc.2015-2665
52. Makaronidis JM, Neilson S, Cheung WH, Tymoszuk U, Pucci A, Finer N, et al. Reported Appetite, Taste and Smell Changes Following Roux-En-Y Gastric Bypass and Sleeve Gastrectomy: Effect of Gender, Type 2 Diabetes and Relationship to Post-Operative Weight Loss. Appetite (2016) 107:93–105. doi: 10.1016/j.appet.2016.07.029
53. Kittrell H, Graber W, Mariani E, Czaja K, Hajnal A, Di Lorenzo PM. Taste and Odor Preferences Following Roux-En-Y Surgery in Humans. PloS One (2018) 13:e0199508. doi: 10.1371/journal.pone.0199508
54. Dunn JP, Cowan RL, Volkow ND, Feurer ID, Li R, Williams DB, et al. Decreased Dopamine Type 2 Receptor Availability After Bariatric Surgery: Preliminary Findings. Brain Res (2010) 1350:123–30. doi: 10.1016/j.brainres.2010.03.064
55. Steele KE, Prokopowicz GP, Schweitzer MA, Magunsuon TH, Lidor AO, Kuwabawa H, et al. Alterations of Central Dopamine Receptors Before and After Gastric Bypass Surgery. Obes Surg (2010) 20:369–74. doi: 10.1007/s11695-009-0015-4
56. Holsen LM, Davidson P, Cerit H, Hye T, Moondra P, Haimovici F, et al. Neural Predictors of 12-Month Weight Loss Outcomes Following Bariatric Surgery. Int J Obes (Lond) (2018) 42:785–93. doi: 10.1038/ijo.2017.190
57. Ochner CN, Laferrere B, Afifi L, Atalayer D, Geliebter A, Teixeira J. Neural Responsivity to Food Cues in Fasted and Fed States Pre and Post Gastric Bypass Surgery. Neurosci Res (2012) 74:138–43. doi: 10.1016/j.neures.2012.08.002
58. Miras AD, Jackson RN, Jackson SN, Goldstone AP, Olbers T, Hackenberg T, et al. Gastric Bypass Surgery for Obesity Decreases the Reward Value of a Sweet-Fat Stimulus as Assessed in a Progressive Ratio Task. Am J Clin Nutr (2012) 96:467–73. doi: 10.3945/ajcn.112.036921
59. Olivo G, Zhou W, Sundbom M, Zhukovsky C, Hogenkamp P, Nikontovic L, et al. Resting-State Brain Connectivity Changes in Obese Women After Roux-En-Y Gastric Bypass Surgery: A Longitudinal Study. Sci Rep (2017) 7(1):6616. doi: 10.1038/s41598-017-06663-5
60. Gluck ME, Viswanath P, Stinson EJ. Obesity, Appetite, and the Prefrontal Cortex. Curr Obes Rep (2017) 6:380–8. doi: 10.1007/s13679-017-0289-0
61. Thiara G, Cigliobianco M, Muravsky A, Paoli RA, Mansur R, Hawa R, et al. Evidence for Neurocognitive Improvement After Bariatric Surgery: A Systematic Review. Psychosomatics (2017) 58:217–27. doi: 10.1016/j.psym.2017.02.004
62. Yang Y, Shields GS, Guo C, Liu Y. Executive Function Performance in Obesity and Overweight Individuals: A Meta-Analysis and Review. Neurosci Biobehav Rev (2018) 84:225–44. doi: 10.1016/j.neubiorev.2017.11.020
63. Giel KE, Teufel M, Junne F, Zipfel S, Schag K. Food-Related Impulsivity in Obesity and Binge Eating Disorder-A Systematic Update of the Evidence. Nutrients (2017) 9(11):1170. doi: 10.3390/nu9111170
64. Gullo MJ, Loxton NJ, Dawe S. Impulsivity: Four Ways Five Factors are Not Basic to Addiction. Addict Behav (2014) 39:1547–56. doi: 10.1016/j.addbeh.2014.01.002
65. Monteiro CA, Cannon G, Levy RB, Moubarac JC, Louzada ML, Rauber F, et al. Ultra-Processed Foods: What They are and How to Identify Them. Public Health Nutr (2019) 22:936–41. doi: 10.1017/S1368980018003762
66. Schag K, Mack I, Giel KE, Olschlager S, Skoda EM, von Feilitzsch M, et al. The Impact of Impulsivity on Weight Loss Four Years After Bariatric Surgery. Nutrients (2016) 8(11):721. doi: 10.3390/nu8110721
67. Cunningham JL, Merrell CC, Sarr M, Somers KJ, Mcalpine D, Reese M, et al. Investigation of Antidepressant Medication Usage After Bariatric Surgery. Obes Surg (2012) 22:530–5. doi: 10.1007/s11695-011-0517-8
68. Nota MHC, Vreeken D, Wiesmann M, Aarts EO, Hazebroek EJ, Kiliaan AJ. Obesity Affects Brain Structure and Function- Rescue by Bariatric Surgery? Neurosci Biobehav Rev (2020) 108:646–57. doi: 10.1016/j.neubiorev.2019.11.025
69. Alosco ML, Galioto R, Spitznagel MB, Strain G, Devlin M, Cohen R, et al. Cognitive Function After Bariatric Surgery: Evidence for Improvement 3 Years After Surgery. Am J Surg (2014) 207:870–6. doi: 10.1016/j.amjsurg.2013.05.018
70. Kulendran M, Borovoi L, Purkayastha S, Darzi A, Vlaev I. Impulsivity Predicts Weight Loss After Obesity Surgery. Surg Obes Relat Dis (2017) 13:1033–40. doi: 10.1016/j.soard.2016.12.031
71. Bianciardi E, Raimondi G, Samela T, Innamorati M, Contini LM, Procenesi L, et al. Neurocognitive and Psychopathological Predictors of Weight Loss After Bariatric Surgery: A 4-Year Follow-Up Study. Front Endocrinol (Lausanne) (2021) 12:662252. doi: 10.3389/fendo.2021.662252
72. Sogg S, Lauretti J, West-Smith L. Recommendations for the Presurgical Psychosocial Evaluation of Bariatric Surgery Patients. Surg Obes Relat Dis (2016) 12:731–49. doi: 10.1016/j.soard.2016.02.008
73. Cortese S, Moreira-Maia CR, St Fleur D, Morcillo-Penalver C, Rohde LA, Faraone SV. Association Between ADHD and Obesity: A Systematic Review and Meta-Analysis. Am J Psychiatry (2016) 173:34–43. doi: 10.1176/appi.ajp.2015.15020266
74. Maclean PS, Blundell JE, Mennella JA, Batterham RL. Biological Control of Appetite: A Daunting Complexity. Obes (Silver Spring) (2017) 25 Suppl 1:S8–S16. doi: 10.1002/oby.21771
75. Groven KS, Glenn NM. The Experience of Regaining Weight Following Weight Loss Surgery: A Narrative-Phenomenological Exploration. Health Care Women Int (2016) 37:1185–202. doi: 10.1080/07399332.2016.1195386
76. Engstrom M, Forsberg A. Wishing for Deburdening Through a Sustainable Control After Bariatric Surgery. Int J Qual Stud Health Well-being (2011) 6(1). doi: 10.3402/qhw.v6i1.5901
77. Wadden TA, Foster GD. Behavioral Treatment of Obesity. Med Clin North Am (2000) 84(441–61):vii. doi: 10.1016/s0025-7125(05)70230-3
78. Phelan S, Halfman T, Pinto AM, Foster GD. Behavioral and Psychological Strategies of Long-Term Weight Loss Maintainers in a Widely Available Weight Management Program. Obes (Silver Spring) (2020) 28:421–8. doi: 10.1002/oby.22685
79. Roake J, Phelan S, Alarcon N, Keadle SK, Rethorst CD, Foster GD. Sitting Time, Type, and Context Among Long-Term Weight-Loss Maintainers. Obes (Silver Spring) (2021) 29:1067–73. doi: 10.1002/oby.23148
80. Forman EM, Butryn ML. Effective Weight Loss: An Acceptance-Based Behavioral Approach. New York, NY: Oxford University Press (2016).
81. Bradley LE, Forman EM, Kerrigan SG, Butryn ML, Herbert JD, Sarwer DB. A Pilot Study of an Acceptance-Based Behavioral Intervention for Weight Regain After Bariatric Surgery. Obes Surg (2016) 26:2433–41. doi: 10.1007/s11695-016-2125-0
82. Bradley LE, Forman EM, Kerrigan SG, Goldstein SP, Butryn ML, Thomas JG, et al. Project HELP: A Remotely Delivered Behavioral Intervention for Weight Regain After Bariatric Surgery. Obes Surg (2017) 27:586–98. doi: 10.1007/s11695-016-2337-3
83. Newman AK, Herbozo S, Russell A, Eisele H, Zasadzinski L, Hassan C, et al. Psychosocial Interventions to Reduce Eating Pathology in Bariatric Surgery Patients: A Systematic Review. J Behav Med (2021) 44:421–36. doi: 10.1007/s10865-021-00201-5
84. Mckee H, Ntoumanis N, Smith B. Weight Maintenance: Self-Regulatory Factors Underpinning Success and Failure. Psychol Health (2013) 28:1207–23. doi: 10.1080/08870446.2013.799162
85. Kwasnicka D, Dombrowski SU, White M, Sniehotta F. Theoretical Explanations for Maintenance of Behaviour Change: A Systematic Review of Behaviour Theories. Health Psychol Rev (2016) 10:277–96. doi: 10.1080/17437199.2016.1151372
86. Cohen DA. Obesity and the Built Environment: Changes in Environmental Cues Cause Energy Imbalances. Int J Obes (Lond) (2008) 32 Suppl 7:S137–42. doi: 10.1038/ijo.2008.250
87. Torres SJ, Nowson CA. Relationship Between Stress, Eating Behavior, and Obesity. Nutrition (2007) 23:887–94. doi: 10.1016/j.nut.2007.08.008
88. Teixeira PJ, Carraca EV, Marques MM, Rutter H, Oppert JM, De Bourdeaudhuij I, et al. Successful Behavior Change in Obesity Interventions in Adults: A Systematic Review of Self-Regulation Mediators. BMC Med (2015) 13:84. doi: 10.1186/s12916-015-0323-6
89. Phelan S, Roake J, Alarcon N, Ng SM, Glanz H, Cardel MI, et al. In Their Own Words: Topic Analysis of the Motivations and Strategies of Over 6,000 Long-Term Weight-Loss Maintainers. Obes (Silver Spring) (2022) 30:751–61. doi: 10.1002/oby.23372
90. Ames GE, Clark MM, Grothe KB, Collazo Clavell ML. Talking to Patients About Bariatric Surgery: Guiding Meaningful Conversations and Evoking Commitment to Change. Bariatric Times (2015) 12:16–24.
91. Weineland S, Arvidsson D, Kakoulidis TP, Dahl J. Acceptance and Commitment Therapy for Bariatric Surgery Patients, a Pilot RCT. Obes Res Clin Pract (2012) 6:e1–e90. doi: 10.1016/j.orcp.2011.04.004
92. Bishop SR, Lau M, Shapiro S, Carlson L, Anderson ND, Carmody J, et al. Mindfulness: A Proposed Operational Definition. Clin Psychol: Sci Pract (2004) 11:230–41. doi: 10.1093/clipsy.bph077
93. Radin RM, Epel ES, Daubenmier J, Moran P, Schleicher S, Kristeller J, et al. Do Stress Eating or Compulsive Eating Influence Metabolic Health in a Mindfulness-Based Weight Loss Intervention? Health Psychol (2020) 39:147–58. doi: 10.1037/hea0000807
94. Felske AN, Williamson TM, Rash JA, Telfer JA, Toivonen KI, Campbell T. Proof of Concept for a Mindfulness-Informed Intervention for Eating Disorder Symptoms, Self-Efficacy, and Emotion Regulation Among Bariatric Surgery Candidates. Behav Med (2020) 14:1–14. doi: 10.1080/08964289.2020.1828255
95. Chacko SA, Yeh GY, Davis RB, Wee CC. A Mindfulness-Based Intervention to Control Weight After Bariatric Surgery: Preliminary Results From a Randomized Controlled Pilot Trial. Complement Ther Med (2016) 28:13–21. doi: 10.1016/j.ctim.2016.07.001
96. Pellegrini M, Carletto S, Scumaci E, Ponzo V, Ostacoli L, Bo S. The Use of Self-Help Strategies in Obesity Treatment. A Narrative Review Focused on Hypnosis and Mindfulness. Curr Obes Rep (2021) 10:351–64. doi: 10.1007/s13679-021-00443-z
97. Farmer FR, Golden JA. The Forms and Functions of Impulsive Actions: Implications for Behavioral Assessment and Therapy. Int J Behav Consultation Ther (2009) 5:12–30. doi: 10.1037/h0100870
98. Weineland S, Hayes SC, Dahl J. Psychological Flexibility and the Gains of Acceptance-Based Treatment for Post-Bariatric Surgery: Six-Month Follow-Up and a Test of the Underlying Model. Clin Obes (2012) 2:15–24. doi: 10.1111/j.1758-8111.2012.00041.x
99. Feig EH, Harnedy LE, Golden J, Thorndike AN, Huffman JC, Psaros C. A Qualitative Examination of Emotional Experiences During Physical Activity Post-Metabolic/Bariatric Surgery. Obes Surg (2022) 32:660–70. doi: 10.1007/s11695-021-05807-x
100. Neff KD. The Development and Validation of a Scale to Measure Self-Compassion. Self and Identity. Self Identity (2003) 2:223–50. doi: 10.1080/15298860309027
101. Pyykko JE, Aydin O, Gerdes VEA, Acherman YIZ, Groen AK, van De Laar AW, et al. Psychological Functioning and Well-Being Before and After Bariatric Surgery; What is the Benefit of Being Self-Compassionate? Br J Health Psychol (2022) 27:96–115. doi: 10.1111/bjhp.12532
102. Ferrari M, Hunt C, Harrysuncker A, Abbott MJ, Beath AP, Einstien DA. Self-Compassion Interventions and Psychosocial Outcomes: A Meta-Analysis of RCTs. Mindfulness (2019) 10:1455–73. doi: 10.1007/s12671-019-01134-6
103. Sjostrom L. Review of the Key Results From the Swedish Obese Subjects (SOS) Trial - a Prospective Controlled Intervention Study of Bariatric Surgery. J Intern Med (2013) 273:219–34. doi: 10.1111/joim.12012
104. Hong YR, Kelly AS, Johnson-Mann C, Lemas DJ, Cardel MI. Degree of Cardiometabolic Risk Factor Normalization in Individuals Receiving Bariatric Surgery: Evidence From NHANES 2015-2018. Diabetes Care (2021) 44:e57–8. doi: 10.2337/dc20-2748
105. Carlsson LMS, Sjoholm K, Jacobson P, Andersson-Assarsson JC, Svensson PA, Taube M, et al. Life Expectancy After Bariatric Surgery in the Swedish Obese Subjects Study. N Engl J Med (2020) 383:1535–43. doi: 10.1056/NEJMoa2002449
Keywords: obesity, behavioral treatment, bariatric surgery, reward-based eating, emotional-based eating, impulsivity, weight regain, driven overeating
Citation: Ames GE, Koball AM and Clark MM (2022) Behavioral Interventions to Attenuate Driven Overeating and Weight Regain After Bariatric Surgery. Front. Endocrinol. 13:934680. doi: 10.3389/fendo.2022.934680
Received: 02 May 2022; Accepted: 16 June 2022;
Published: 18 July 2022.
Edited by:
Alpana Shukla, Weill Cornell Medical College, United StatesReviewed by:
Ninoska Peterson, Cleveland Clinic, United StatesRachel Goldman, New York University, United States
Copyright © 2022 Ames, Koball and Clark. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gretchen E. Ames, YW1lcy5ncmV0Y2hlbkBtYXlvLmVkdQ==
 Gretchen E. Ames
Gretchen E. Ames Afton M. Koball2
Afton M. Koball2