- 1Department of Cardiovascular Medicine, The Second Affiliated Hospital of Nanchang University, Nanchang, China
- 2Department of Cardiovascular Medicine, The First Affiliated Hospital of Nanchang University, Nanchang, China
- 3Department of Endocrinology and Metabolism, The Second Affiliated Hospital of Nanchang University, Nanchang, China
- 4Department of Cardiovascular Medicine, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
Background: A network meta-analysis of randomized controlled trials (RCTs) was conducted to explore the cardiovascular outcomes of all the kind and dosages of sodium-glucose cotransport-2 (SGLT2) inhibitors in type 2 diabetes mellitus (T2DM) patients.
Method and Result: The Cochrane Library, PubMed, and Embase databases were systematically searched for studies to compare the therapeutic effects of different SGLT2 inhibitors in T2DM patients. The effect measurements estimate chosen were odds ratios (ORs) and their corresponding 95% confidence interval (CI). Forty-seven RCTs involving a total of 70574 participants were eligible for direct and indirect comparisons. In direct comparison, treatment with dapagliflozin 5mg showed significantly lower risk of all-cause mortality compared with treatment with dapagliflozin 2.5mg (OR 0.09, 95% CI 0.01-0.70). According to NMA, interestingly, empagliflozin 10mg/25mg, and canagliflozin 100mg was associated with significantly lower risks of all-cause mortality compared with placebo (OR of 0.70, 95% CI 0.58-0.85; 0.69, 95% CI 0.57-0.84; and 0.83, 95% CI 0.73-0.95, respectively). Compared with placebo, dapagliflozin 10mg, empagliflozin 10mg and 25mg displayed the lower risks for cardiovascular events (OR 0.78, 95% CI 0.44-1.00; OR 0.47, 95% CI 0.22-0.93; and 0.43, 95% CI 0.24-0.74, respectively) by direct comparison. Moreover, canagliflozin 100/300mg showed significantly higher risks of cardiovascular events compared with empagliflozin 10mg (OR of 4.83, 95% CI 1.14-20.46 and 5.31, 95% CI 1.26-22.34, respectively) and empagliflozin 25mg (4.23, 95% CI 1.13-15.83 and 4.65, 95% CI 1.25-17.27, respectively) according to NMA. There were non-significant differences among all interventions in volume depletion in traditional pairwise meta-analysis. While in NMA, canagliflozin 100/300mg were associated with significantly increased risks of volume depletion compared with placebo (OR of 1.47, 95% CI 1.08-1.99 and 2.19, 95% CI 1.66-2.90, respectively).
Conclusion: In the limitations of the NMA, this study showed that empagliflozin might be better than other SGLT2 inhibitors with low risks of all-cause mortality and cardiovascular events in patients with T2DM suggesting the need for ad hoc RCTs.
1 Introduction
Type 2 diabetes mellitus (T2DM) affects roughly 451 million adults in 2017 worldwide, these figures were expected to increase to almost 700 million by 2045 (1). T2DM is one of the most important risk factors of cardiovascular disease (CVD) (2, 3), the present of both T2DM and CVD is correlated with higher mortality rate despite advances in treatment (4). Enormous studies have shown that glucose lowing therapy failed to reduce the rates of death, although metabolism benefits were shown in these studies (5). In addition, some antihypergycemic agents increase the risk of all-cause mortality and major adverse cardiovascular events (MACEs) in T2DM patients with established CVD or CVD risk factors (6–8). Thus, novel strategies to improve prognosis and reduce mortality in T2DM patients are needed.
Sodium-glucose cotransport-2 (SGLT2) inhibitors, which reduce blood glucose levels in an insulin-independent manner in T2DM patients (9), are correlated with improvement of many metabolic and hemodynamic abnormalities (10). Moreover, it is important to note that SGLT2 inhibition is associated with reduced aortic stiffness (11) and cardiac structure and function improvement (12). Because of the beneficial cardiometabolic/hemodynamic profile induced by SGLT2 inhibitors treatment, clinical studies investigated the efficacy and safety of this class of drugs in T2DM patients (13–15). However, debate continues as to whether all SGLT2 inhibitors that exert similar cardioprotective effects.
Network meta-analysis (NMA) offers the potential to assess multiple therapeutic strategies simultaneously within a single framework and to rank treatments based on efficacy and safety (16). In the current paper, we conducted an NMA of randomized controlled trials (RCTs) for the first time to explore cardiovascular outcomes of different kind and dosages of SGLT2 inhibitors in T2DM patients.
2 Methods
2.1 Data Sources and Search Strategy
We conducted a systematic search up to October 1, 2020, without any language restriction, using PubMed, Embase, the Cochrane Library, and Clinical trials. We searched studies with key words and Medical Subject Headings that covered “diabetic” or “diabetes” or “Type 2 Diabetes Mellitus” or “T2MD” and “sodium-glucose co-transporter 2 inhibitors”, or “SGLT 2 inhibitors” or “SGLT2 inhibitors” or “sodium-glucose transporter inhibitors” or “canagliflozin” or “dapagliflozin” or “empagliflozin” or “ipragliflozin” or “remogliflozin” or “tofogliflozin” or “sergliflozin”. We also reviewed the corresponding reference list of each retrieved article to identify any relevant studies that may be neglected. The meta-analysis was conducted and reported according to the Preferred Reporting Items of Systematic Reviews and Meta-Analyses (PRISMA) guidelines (17). The search strategies are provided in Supplementary Table S1.
2.2 Selection Criteria
We collected all RCTs to compare the therapeutic effects of different SGLT2 inhibitors in T2MD patients in this network meta-analysis. Inclusion criteria of the studies were as follows: (a) T2MD patients treatment with SGLT2 inhibitors, (b) study design was an RCT of the treatment group (SGLT2 inhibitors) and control group, (c) studies with outcomes of “all-cause death”, “all-cause mortality”, “myocardial infarction”, “nonfatal myocardial infarction”, “nonfatal stroke”, “cardiovascular death”, “hypertension”, “hypotension”, “volume depletion”, “dehydration”, or “hypovolemia”. The detail was shown in the Table 1.
The criteria for exclusion were as follows: (a) studies such as systemic reviews, comments, case reports, conference abstracts, and editorials, (b) subjects with an eGFR level lower than 30 mL/min per 1.73m (2), and (c) articles that had no data on T2DM patients.
Included trials reported comparisons of 10 interventions (placebo, dapagliflozin 2.5mg, 5mg, 10mg; empagliflozin 10mg, 25 mg; and canagliflozin 100mg, 300 mg). NMA integrates data from direct comparisons of treatments within trials and from indirect comparisons of interventions assessed against a common comparator in separate trials to compare all investigated treatments.
2.3 Data Extraction and Quality Assessment
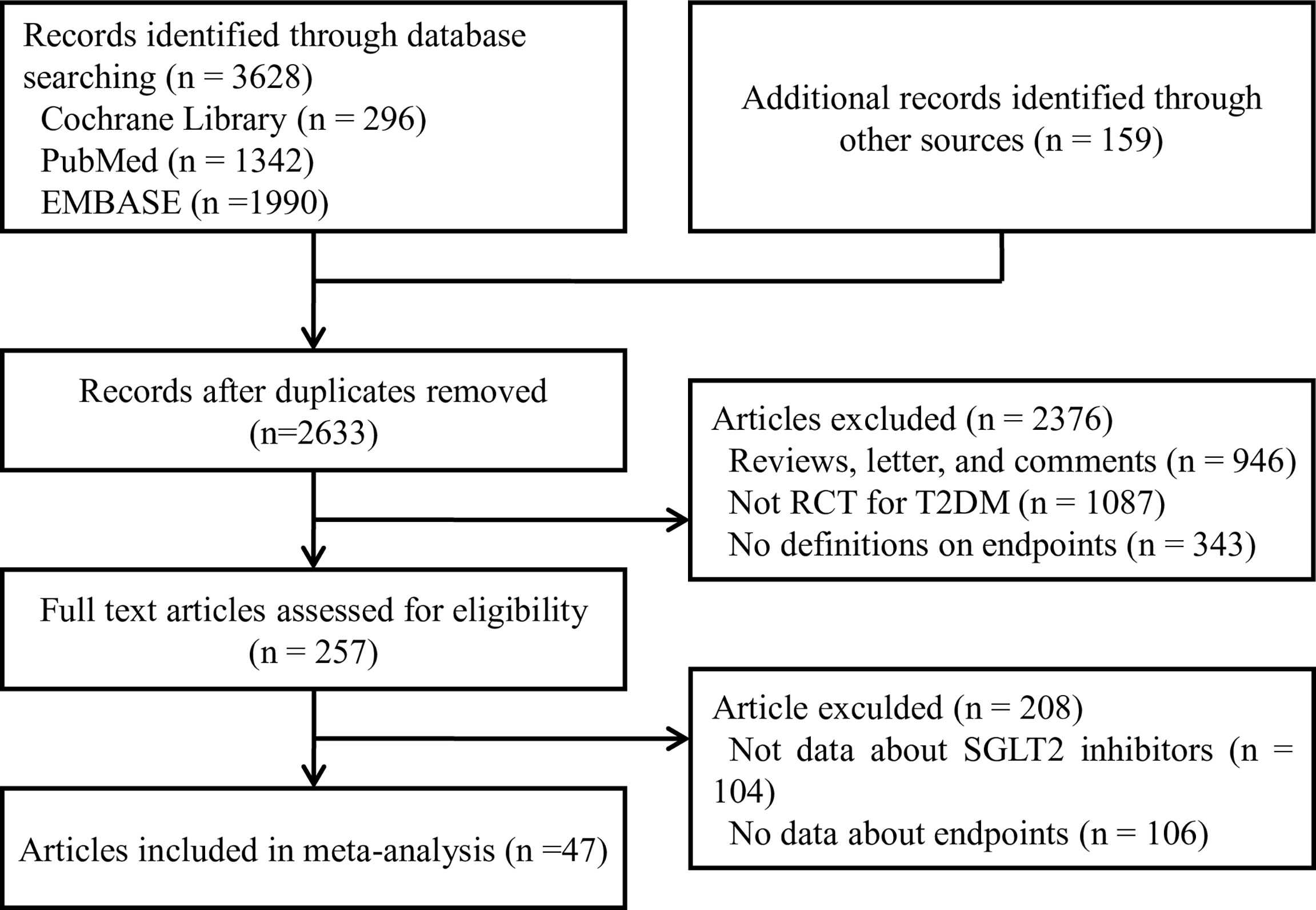
Two authors (J-Y and Y-PP) extracted data and accessed quality independently in an electronic database. The investigators cross-checked the data and reached a consensus on any discrepancies through discussion. Disagreements were resolved through discussions or referral to other authors (S-W and W-QH). Reference lists of identified trials and review articles were manually scanned to identify related research references at the same time as indicated in Figure 1.
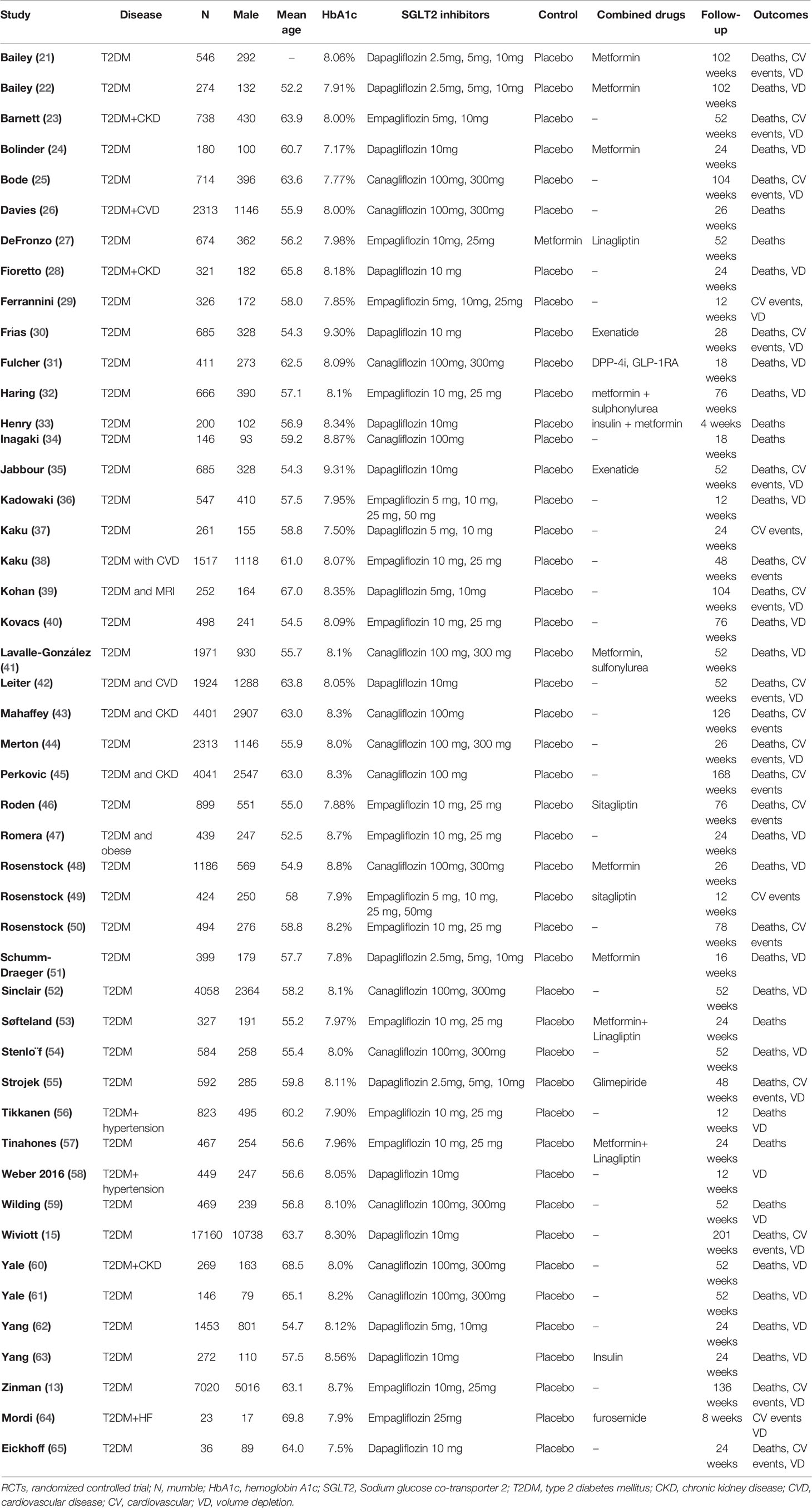
The extracted data included the first author’s name, year of publication, clinical characteristics, HbA1C% level, sample size, the number of males, doses of treatment, control, combined drugs, follow-up duration, the outcomes of all-cause mortality, cardiovascular events, volume depletion. Cardiovascular events included “myocardial infarction”, “nonfatal myocardial infarction”, “nonfatal stroke”, “cardiovascular death”, and “hypertension”.
2.4 Risk of Bias Assessment
Two independent reviewers (J-Y and Y-PP) assessed the methodological quality of included trials using a slightly adapted version of the risk of bias approach by using Review Manager 5.3 (Cochrane Collaboration, Oxford, UK) risk of bias tool including four sections: selection, performance, detection, attrition, reporting, and other bias. The publication bias assessment was performed via Deek’s funnel plot asymmetry.
2.5 Statistical Analysis
The data were abstracted and analyzed by STATA (version 14.0, Stata MP) and Review Manager (version 5.3, Cochrane Collaboration, Copenhagen, Denmark). The odds ratios (ORs) and their corresponding 95% confidence interval (CI) were used to compare different medications with respect to various clinical outcomes. For each analysis, we generated 50000 simulations for each of the 2 sets of different initial values and discarded the first 20000 simulations as the burn-in period. The stability of the results was obtained by sensitivity analyses by discarding each study sequentially. Convergence was checked using trace plots and the Brooks-Gelman-Rubin (18). To rank the treatments for an outcome, we used the surface under the cumulative ranking area (SUCRA) probabilities (19). Thus, a larger SUCRA score might indicate a higher probability of the end point event. We also used Loop-specific inconsistency (used in Stata and R software) to assess the inconsistency that is the actual difference between direct and indirect comparisons (20).
3 Results
3.1 Description of Included Studies
We identified 3787 unique records from our searches. Forty-seven RCTs involving a total of 70574 participants were eligible for this NMA. The selection process details are shown in Figure 1. The trials included were issued up to September 2020. Table 2 summarizes the essential baseline characteristics of these included studies (detail in Supplementary Table S2). Of 43 studies, all studies reported the end point event of all-cause death, 25 studies submitted data on cardiovascular events, and 30 studies provided data on volume depletion. The number of patients included in every study ranged from 35 to 17160, and the follow-up for patients ranged from 4 to 201 weeks. The risk of bias in studies contributing to the primary outcomes was generally low (Supplementary Table S3).
A network plot of treatment comparisons for NMA is shown in Figure 2. There are 8 interventions for all-cause death, cardiovascular events and volume depletion. The size of the nodes (blue circles) corresponds to the sample size of the interventions. The comparisons are linked by a straight line, of which the thickness corresponds to the number of trials that assessed the comparison. As shown in the network plot, the number of interventions varied in different subjects.
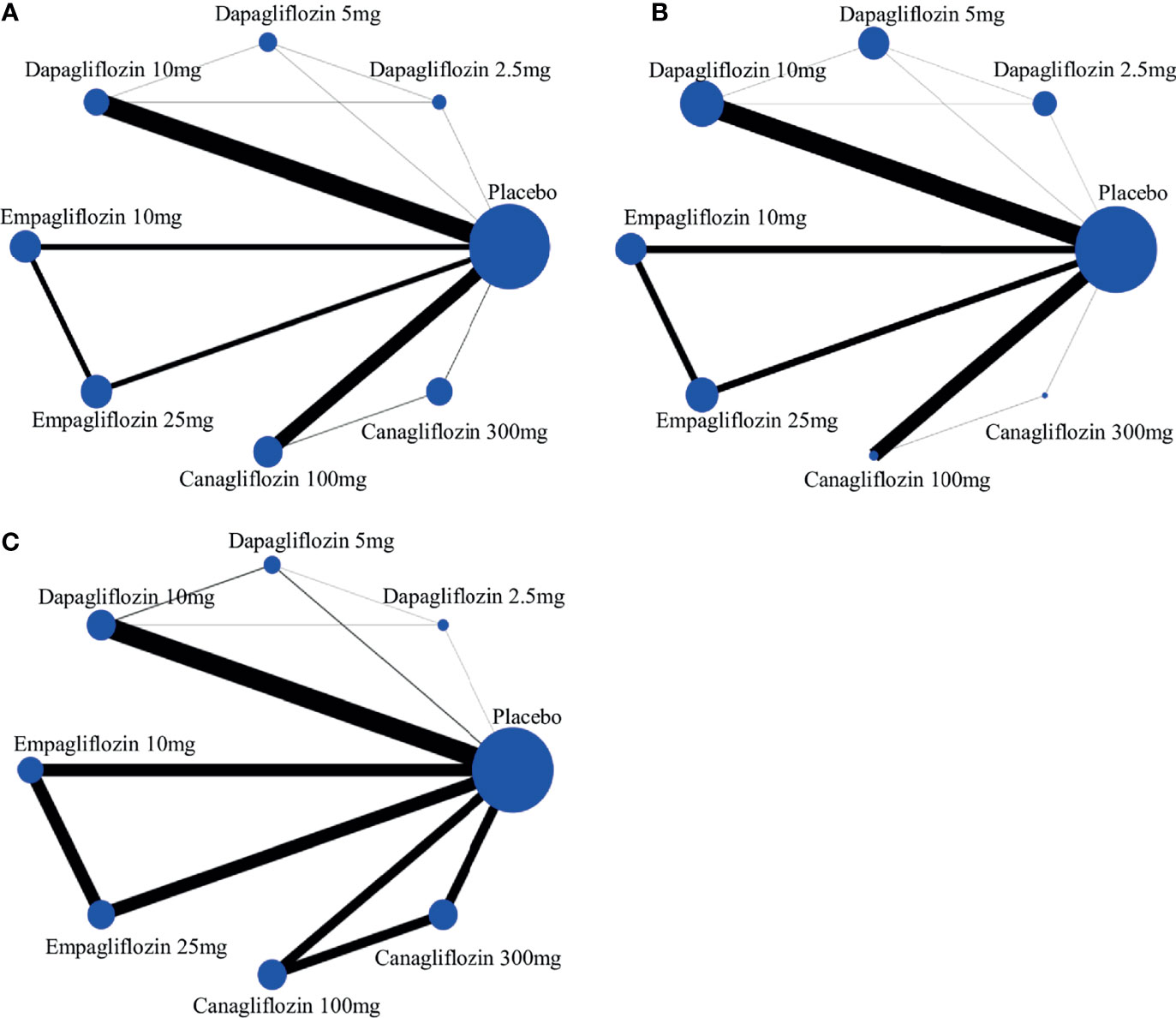
Figure 2 The evidence network of studies reporting (A) all-cause death, (B) cardiovascular event and (C) volume depletion. The size of the nodes (blue circles) corresponds to the overall sample size of the corresponding intervention. Each line represents the direct comparison between the two interventions, and its thickness corresponds to the number of trials that assessed the comparison.
3.2 The Outcome
3.2.1 All-Cause Mortality
We performed a series of traditional pairwise meta-analysis and NMA to summarize the results of trials directly and indirectly comparing the same classes of SGLT2 inhibitors. In direct comparison, there were non-significant differences among all interventions in all-cause mortality, expected Dapagliflozin 5mg vs 2.5mg (OR 0.09, 95% CI 0.01-0.70). According to NMA, interestingly, empagliflozin 10mg/25mg, and canagliflozin 100mg was associated with significantly lower risks of all-cause mortality compared with placebo (OR of 0.70, 95% CI 0.58-0.85; 0.69, 95% CI 0.57-0.84; and 0.83, 95% CI 0.73-0.95, respectively). Moreover, empagliflozin 10mg/25mg was leaded to significantly lower risks of all-cause mortality compared with dapagliflozin 10mg (OR of 0.75, 95% CI 0.60-0.95; 0.74, 95% CI 0.59-0.93, respectively), as depicted in Table 3A.
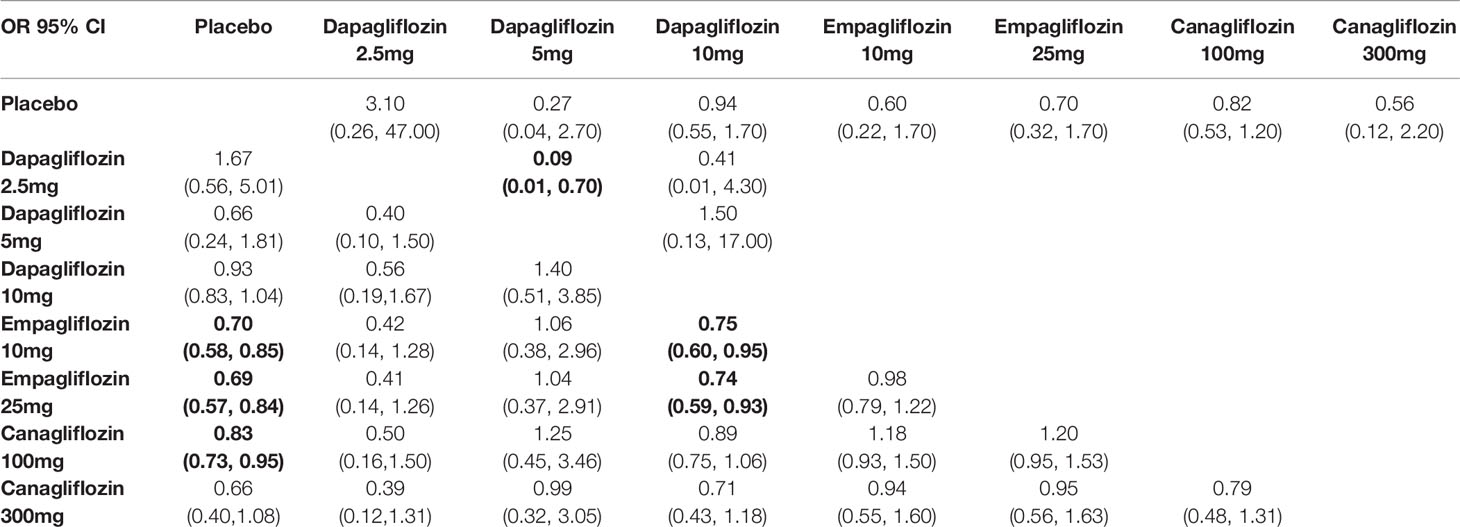
Table 3A Summary of results from network meta-analysis and traditional pairwise meta-analysis on all-cause death.
The comparative effects of different class and doses of SGLT2 inhibitors in reducing mortality by SUCRA probabilities and incidence rate of each intervention was shown in Table 4. The NMA suggested that higher dosage of empagliflozin (25 mg once daily) was associated with the lowest probability of achieving at all-cause mortality (SUCRA, 23.9%), followed by canagliflozin 300 mg (SUCRA, 24.4%), and empagliflozin 10 mg (SUCRA, 26.3%). However, dapagliflozin 2.5mg was associated with the highest probability of all-cause death (SUCRA, 89.7%).
3.2.2 Cardiovascular Events
In traditional pairwise meta-analysis, compared with placebo, dapagliflozin 10mg, empagliflozin 10mg and 25mg displayed the lowest risks for cardiovascular events (OR 0.78, 95% CI 0.44-1.00; OR 0.47, 95% CI 0.22-0.93; and 0.43, 95% CI 0.24-0.74, respectively). According to NMA, canagliflozin 100/300mg was associated with significantly higher risks of cardiovascular events compared with empagliflozin 10mg (OR of 4.83, 95% CI 1.14-20.46 and 5.31, 95% CI 1.26-22.34, respectively) and empagliflozin 25mg (4.23, 95% CI 1.13-15.83 and 4.65, 95% CI 1.25-17.27, respectively) (Table 3B). Empagliflozin 25mg and dapagliflozin 5mg ranked the best and second (SUCRA of 21.8% and 23.9%, respectively), followed by empagliflozin 10mg (SUCRA of 30.9%). In addition, canagliflozin 300 mg was ranked the least effective treatment in reducing cardiovascular events (Table 4).
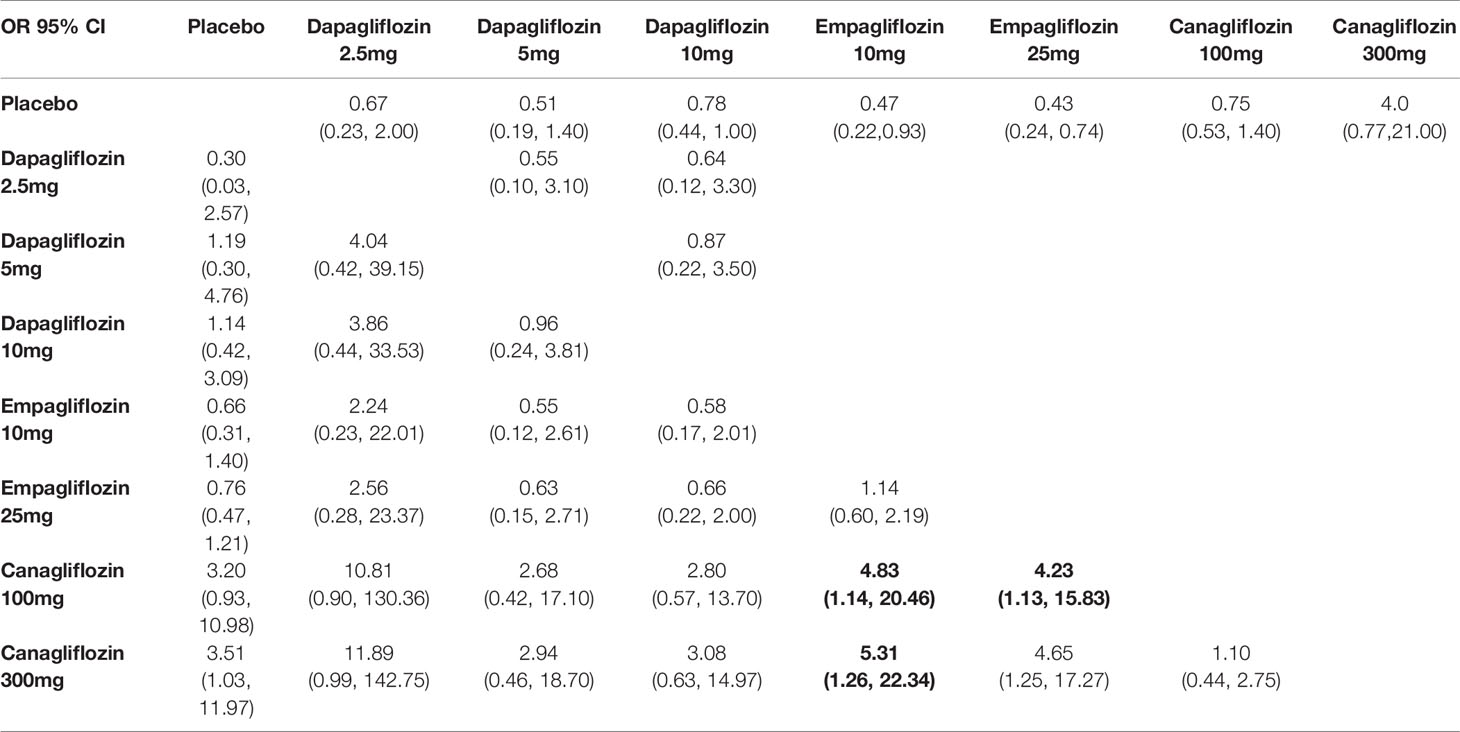
Table 3B Summary of results from network meta-analysis and traditional pairwise meta-analysis on cardiovascular events.
3.2.3 Volume Depletion
There were non-significant differences among all interventions in volume depletion in traditional pairwise meta-analysis. While in NMA, canagliflozin 100/300mg were associated with significantly increased risks of volume depletion compared with placebo (OR of 1.47, 95% CI 1.08-1.99 and 2.19, 95% CI 1.66-2.90, respectively). The incidence of volume depletion induced by canagliflozin 300mg was significantly higher than that induced by dapagliflozin 5/10mg, empagliflozin 10/25mg and canagliflozin 100mg. These NMA results are illustrated in Table 3C.
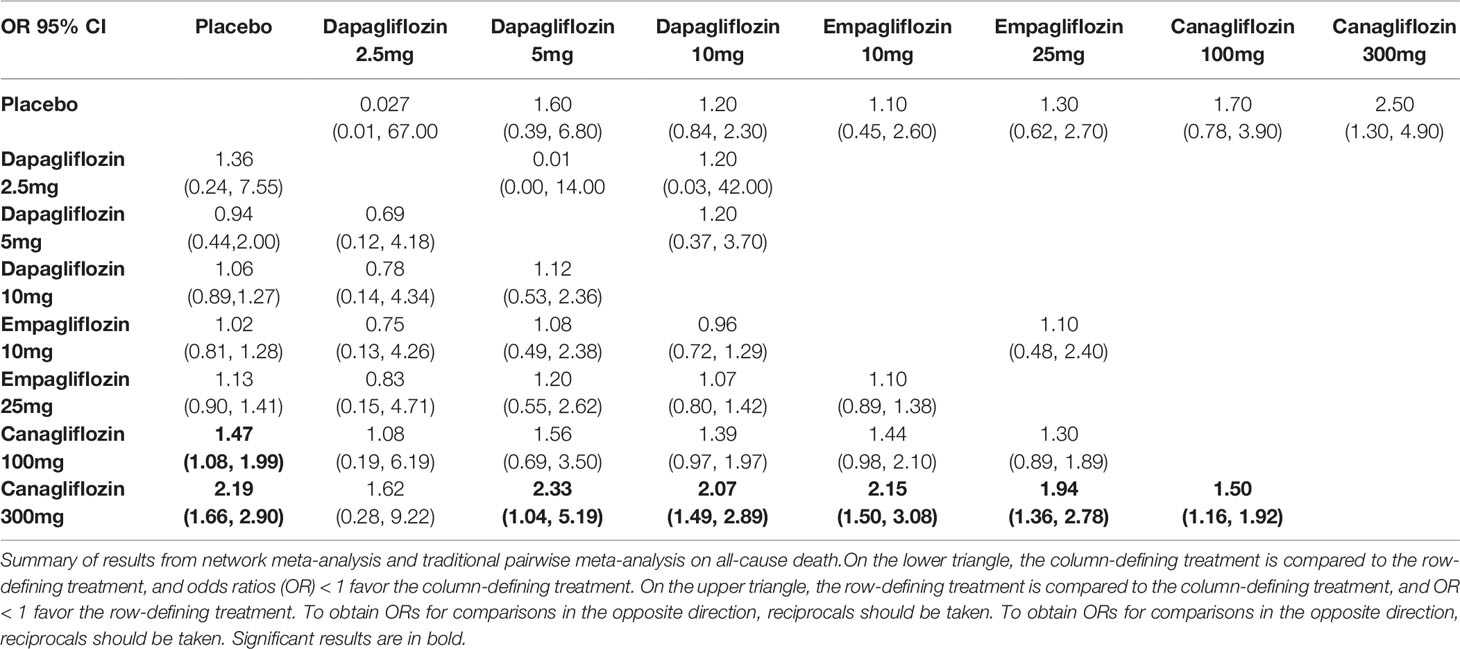
Table 3C Summary of results from network meta-analysis and traditional pairwise meta-analysis on volume depletion.
Canagliflozin 300mg had the highest probabilities of being ranked first with respect to volume depletion (SUCRA 95.5%), whereas canagliflozin 100mg had the second highest probability (SUCRA 75.1%). Both dapagliflozin 5mg and empagliflozin 10mg shown smallest cumulative probabilities for volume depletion, with all values lower 30% (SUCRA 29.9% and 30.5%, respectively), as depicted in Table 4.
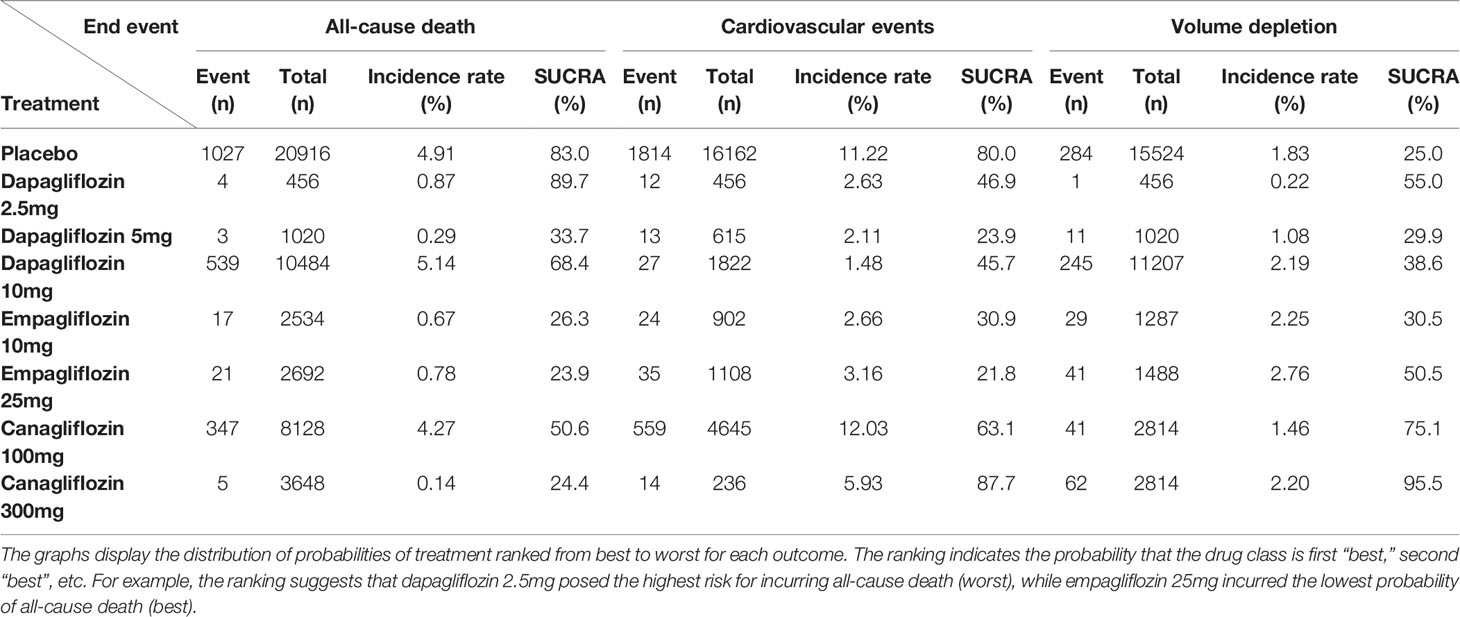
Table 4 Incidence rate and SUCRA for the efficacy of treatments to induce end points in the T2DM patients.
3.3 Exploration of Inconsistency, Sensitivity Analysis, and Publication Bias
Treatment from network meta-analysis evidence in general did not demonstrate evidence of statistical inconsistency (Supplementary Figure S1). As shown in Supplementary Figure S1, the inconsistency plot consists of two triangular loops and four quadrangular loops. The IF values of all loops were truncated at zero, and P value > 0.05 verified their consistency statistically.
A sensitivity analysis was conducted to examine the impact of studies according to the treatment effects on the outcomes of all-cause death, cardiovascular events, and volume depletion. We performed an analysis in T2DM used Bayesian, and there was no significant difference in the different methods (Supplementary Table S3). No significant publication bias was detected in the funnel plot (Supplementary Figure S2).
4 Discussion
In recent years, clinical trials revealed the cardioprotective effects of SGLT2 inhibitors in T2DM patients (13–15). Moreover, SGLT-2 inhibitors were most likely to rank best for all-cause/cardiac mortality and the outcomes of heart failure and myocardial infarction compared with dipeptidyl peptidase 4 inhibitors and glucagon-like peptide 1 agonists (66). However, no studies have directly or simultaneously compared the efficacy and safety of all the kind and dosages of SGLT2 inhibitors. In the present meta-analysis, we grouped all available SGLT2 inhibitors together, including dapagliflozin 2.5mg/5mg/10mg, empagliflozin 10mg/25mg, and canagliflozin 100mg/300mg. Since only few studies allowed direct comparisons, indirect comparisons were further conducted to identified the effects of different SGLT2 inhibitors on all-cause mortality, cardiovascular events and volume depletion, respectively. We demonstrated that dose of dapagliflozin 5mg was associated with all-cause mortality reduction, rather than other SGLT2 inhibitors by direct comparison. According to NMA, empagliflozin 10mg/25mg, and canagliflozin 100mg was associated with significantly lower risks of all-cause mortality compared with placebo. Moreover, empagliflozin 10mg/25mg was leaded to significantly lower risks of all-cause mortality compared with dapagliflozin 10mg. Dapagliflozin 10mg, empagliflozin 10mg and 25mg displayed the lower risks for cardiovascular events compared with placebo. In addition, it seems likely that canagliflozin 100/300mg showed significantly higher risks of cardiovascular events compared with empagliflozin 10mg/25mg according to NMA. Finally, we suggested that treatment with canagliflozin 100/300mg were associated with significantly increased risks of volume depletion compared with placebo by NMA.
In recent years, numerous studies investigated the role of SGLT2 inhibitor on mortality reduction. For instance, In EMPA-REG OUTCOME trial, Zinman and coworkers revealed that the rate of all-cause mortality was significantly lower in patients who received empagliflozin 10mg/25mg than controlled group (13). More recently, however, CANVAS (Canagliflozin Cardiovascular Assessment Study) Program which is an integrated analysis of CANVAS and CANVAS-R (Canagliflozin Cardiovascular Assessment Study-Renal) (14), and DECLARE–TIMI 58 trial (15) failed to show the positive effect of canagliflozin 100mg/300 mg and dapagliflozin 10mg on reducing the rate of all-cause death, respectively. The major limitations of CANVAS Program are lack of events number and discontinuation of randomized therapy, which may result in underestimation of benefits effect of canagliflozin on all-cause mortality reduction. The present meta-analysis grouped all available SGLT2 inhibitors for direct and indirect comparison. Since only few studies allowed direct comparisons between SGLT2 inhibitors, NMA were conducted and found that dapagliflozin 5mg was associated with lower risk of all-cause mortality. Moreover, the positive role of empagliflozin 10mg/25mg and canagliflozin 100mg on reducing all-cause mortality was demonstrated by NMA (as shown in Table 3). Although the potential factors that responsible for mortality reduction of canagliflozin are need to be further investigated, it may be relevant to identify that canagliflozin has less SGLT2 selectively and may induce more glucosuria (67), suggesting great volume depletion by canagliflozin and rise in hematocrit and hemoconcentration could increase blood viscosity. On the other hand, it has been reported that canagliflozin impacts activation of AMP-kinase in mitochondrial function, which is helpful to keep the balance of cellular energy metabolism (68). As an alternative explanation, we selected 43 studies investigating the efficacy of SGLT2 inhibitors on mortality rate in 66819 T2DM patients and included a total of 2470 deaths, data reporting may have differed between these studies. These finding highlighted that more clinical trials are urgently needed to explore the specific cardioprotective role of SGLT2 inhibitors in T2DM patients, even though they have similar effects.
For patients with T2DM, an important goal of currently treatment is reducing the rate of cardiovascular events. In recent years, clinical trials investigated the role of SGLT2 inhibitors on MACE and suggested that the particular clinical benefits of SGLT2 inhibition may rely on the baseline characteristics of patient population. For instance, the positive effect of empagliflozin on cardiovascular mortality and MACEs rate was revealed in EMPA-REG OUTCOME trial which included T2DM patients with established CVD (13). However, dapagliflozin treatment resulted in lower rate of cardiovascular death rather than total MACEs in DECLARE-TIMI 58 trial which included 41.64% T2DM with CVD patients (15). Moreover, canagliflozin significantly reduce the rate of MACEs in CANVAS Program which included 66% T2DM with CVD patients (14). Recently, a meta-analysis included these three clinical trials and their secondary analyses revealed that SGLT2 inhibitors reduce MACEs by 11% in patients with established CVD. Moreover, they found that the effect of empagliflozin on cardiovascular death was more pronounced than that of canagliflozin or dapagliflozin (69). In currently paper, we suggested that dapagliflozin 10mg, empagliflozin 10mg/25mg and canagliflozin 100mg could exert similar role in reducing the risk of cardiovascular events in T2DM patients, highlighting that SGLT2 inhibitors may be considered to manage T2DM in patients not only with established CVD but also in patients without CVD but at elevated risk. Additionally, we found that empagliflozin 10mg/25mg rank the best and second choice in regarding to cardiovascular events risk reduction by SUCRA probabilities.
It has been reported that SGLT2 inhibition decrease sodium reabsorption and increases urinary sodium excretion (70). In addition to exert multiple metabolic effects including reduce HbA1c, change caloric balance and weight loss, the glucosuria/natriuretic effects of SGLT2 inhibitors are also account for, at least in part, the positive role of it on reducing the rate of hospitalization for heart failure. However, it is important to recognize that these effects may have protective and injurious potential. For instance, natriuresis may also result in increasing risk of volume depletion, such as hypotension and syncope, and promoting neurohormonal activation and tissue ischemia in the periphery (71). According to our analysis, we found that higher dosage of canagliflozin (300 mg once daily) was associated with increased the rate of volume depletion among all SGLT2 inhibitors included in this study, suggesting that the potential of higher dosage of canagliflozin induced volume depletion may account for the neutralize results in all-cause mortality.
We acknowledge several limitations of our study. First, we used aggregated study-level data rather than individual participant data, and the different studies have been conducted in different population. Second, we could not include data from several trials such as DECLARE-TIMI 58, CANVAS Program, EMPA-REG OUTCOME due to data was limited in these studies. Third, we did not perform a subgroup analysis of cardiovascular mortality caused by SGLT2 inhibitors in T2DM patients. Then, although the heterogeneity in the network analysis was low, it is likely that the low power to detect heterogeneity is due to limited data for some dosage SGLT2 inhibitors. Finally, the NMA results do not meet the assumptions of homogeneity of direct evidence and/or transitivity, therefore, several larger multi-center RCTs which investigated effects between SGLT2 inhibitors including patients with similar clinical characteristics need to be implemented to achieve more robust results.
5 Conclusion
In the limitations of the NMA, this study showed that empagliflozin 10mg/25mg once daily might be better than other SGLT2 inhibitors with low risks of all-cause mortality and cardiovascular events in patients with T2DM suggesting the need for ad hoc RCTs.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author Contributions
YJ, PY, and LF performed the meta-analysis. LS was responsible for the statistical analysis. WS provided editing assistance. QW prepared the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82160093), the incubation project of the National Natural Science Foundation of the Second Affiliated Hospital of Nanchang University (No. 2021YNFY2021), the Jiangxi Graduate Innovation Special Fund (No. YC2019-B039), the Jiangxi Provincial Health Commission Project (No. 202210663), and the State Scholarship Fund of the China Scholarship Council (grant number 201906820003).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.802992/full#supplementary-material
Abbreviations
RCTs, Randomized controlled trials; SGLT2, Sodium-glucose cotransport-2; T2DM, Type 2 diabetes mellitus; ORs, Odds ratios; CVD, Cardiovascular disease; MACEs, Major adverse cardiovascular events; NMA, Network meta-analysis; SUCRA, The surface under the cumulative ranking area; CI, Confidence interval; CANVAS, Canagliflozin cardiovascular assessment study.
References
1. Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: Global Estimates of Diabetes Prevalence for 2017 and Projections for 2045. Diabetes Res Clin Pract (2018) 138:271–81. doi: 10.1016/j.diabres.2018.02.023
2. Emerging Risk Factors C, Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, et al. Diabetes Mellitus, Fasting Blood Glucose Concentration, and Risk of Vascular Disease: A Collaborative Meta-Analysis of 102 Prospective Studies. Lancet (2010) 375(9733):2215–22. doi: 10.1016/S0140-6736(10)60484-9
3. Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of Cardiovascular Disease in Type 2 Diabetes: A Systematic Literature Review of Scientific Evidence From Across the World in 2007-2017. Cardiovasc Diabetol (2018) 17(1):83. doi: 10.1186/s12933-018-0728-6
4. Emerging Risk Factors C, Di Angelantonio E, Kaptoge S, Wormser D, Willeit P, Butterworth AS, et al. Association of Cardiometabolic Multimorbidity With Mortality. Jama (2015) 314(1):52–60. doi: 10.1001/jama.2015.7008
5. Lexis CP, van der Horst IC, Lipsic E, Wieringa WG, de Boer RA, van den Heuvel AF, et al. Effect of Metformin on Left Ventricular Function After Acute Myocardial Infarction in Patients Without Diabetes: The GIPS-III Randomized Clinical Trial. Jama (2014) 311(15):1526–35. doi: 10.1001/jama.2014.3315
6. Fitchett DH, Udell JA, Inzucchi SE. Heart Failure Outcomes in Clinical Trials of Glucose-Lowering Agents in Patients With Diabetes. Eur J Heart Fail (2017) 19(1):43–53. doi: 10.1002/ejhf.633
7. Son JW, Kim S. Dipeptidyl Peptidase 4 Inhibitors and the Risk of Cardiovascular Disease in Patients With Type 2 Diabetes: A Tale of Three Studies. Diabetes Metab J (2015) 39(5):373–83. doi: 10.4093/dmj.2015.39.5.373
8. Groop PH, Cooper ME, Perkovic V, Hocher B, Kanasaki K, Haneda M, et al. Linagliptin and its Effects on Hyperglycaemia and Albuminuria in Patients With Type 2 Diabetes and Renal Dysfunction: The Randomized MARLINA-T2D Trial. Diabetes Obes Metab (2017) 19(11):1610–9. doi: 10.1111/dom.13041
9. Gallo LA, Wright EM, Vallon V. Probing SGLT2 as a Therapeutic Target for Diabetes: Basic Physiology and Consequences. Diabetes Vasc Dis Res (2015) 12(2):78–89. doi: 10.1177/1479164114561992
10. Abdul-Ghani MA, Norton L, Defronzo RA. Role of Sodium-Glucose Cotransporter 2 (SGLT 2) Inhibitors in the Treatment of Type 2 Diabetes. Endocr Rev (2011) 32(4):515–31. doi: 10.1210/er.2010-0029
11. Cherney DZ, Perkins BA, Soleymanlou N, Har R, Fagan N, Johansen OE, et al. The Effect of Empagliflozin on Arterial Stiffness and Heart Rate Variability in Subjects With Uncomplicated Type 1 Diabetes Mellitus. Cardiovasc Diabetol (2014) 13:28. doi: 10.1186/1475-2840-13-28
12. Verma S, Garg A, Yan AT, Gupta AK, Al-Omran M, Sabongui A, et al. Effect of Empagliflozin on Left Ventricular Mass and Diastolic Function in Individuals With Diabetes: An Important Clue to the EMPA-REG OUTCOME Trial? Diabetes Care Dec (2016) 39(12):e212–3. doi: 10.2337/dc16-1312
13. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med (2015) 373(22):2117–28. doi: 10.1056/NEJMoa1504720
14. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med (2017) 377(7):644–57. doi: 10.1056/NEJMoa1611925
15. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med (2019) 380(4):347–57. doi: 10.1056/NEJMoa1812389
16. Lu G, Ades AE. Combination of Direct and Indirect Evidence in Mixed Treatment Comparisons. Stat Med (2004) 23(20):3105–24. doi: 10.1002/sim.1875
17. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. Ann Internal Med (2009) 151(4):W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136
18. Brooks SP, Gelman A. General Methods for Monitoring Convergence of Iterative Simulations. J Comput Graph Stat (1998) 7(4):434–55. doi: 10.1080/10618600.1998.10474787
19. Salanti G, Ades AE, Ioannidis JP. Graphical Methods and Numerical Summaries for Presenting Results From Multiple-Treatment Meta-Analysis: An Overview and Tutorial. J Clin Epidemiol (2011) 64(2):163–71. doi: 10.1016/j.jclinepi.2010.03.016
20. Spiegelhalter DJ, Best NG, Carlin BP, van der Linde A. Bayesian Measures of Model Complexity and Fit. J R Stat Soc: Ser b (Stat Methodol) (2002) 64(4):583–639. doi: 10.1111/1467-9868.00353
21. Bailey CJ, Gross JL, Hennicken D, Iqbal N, Mansfield TA, List JF. Dapagliflozin Add-on to Metformin in Type 2 Diabetes Inadequately Controlled With Metformin: A Randomized, Double-Blind, Placebo-Controlled 102-Week Trial. BMC Med (2013) 11:43. doi: 10.1186/1741-7015-11-43
22. Bailey CJ, Morales Villegas EC, Woo V, Tang W, Ptaszynska A, List JF. Efficacy and Safety of Dapagliflozin Monotherapy in People With Type 2 Diabetes: A Randomized Double-Blind Placebo-Controlled 102-Week Trial. Diabetic Med J Br Diabetic Assoc (2015) 32:531–41. doi: 10.1111/dme.12624
23. Barnett AH, Mithal A, Manassie J, Jones R, Rattunde H, Woerle HJ, et al. Efficacy and Safety of Empagliflozin Added to Existing Antidiabetes Treatment in Patients With Type 2 Diabetes and Chronic Kidney Disease: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Diabetes Endocrinol (2014) 2:369–84. doi: 10.1016/S2213-8587(13)70208-0
24. Bolinder J, Ljunggren O, Kullberg J, Johansson L, Wilding J, Langkilde AM, et al. Effects of Dapagliflozin on Body Weight, Total Fat Mass, and Regional Adipose Tissue Distribution in Patients With Type 2 Diabetes Mellitus With Inadequate Glycemic Control on Metformin. J Clin Endocrinol Metab (2012) 97:1020–31. doi: 10.1210/jc.2011-2260
25. Bode B, Stenlof K, Harris S, Sullivan D, Fung A, Usiskin K, et al. Long-Term Efficacy and Safety of Canagliflozin Over 104 Weeks in Patients Aged 55-80 Years With Type 2 Diabetes. Diabetes Obes Metab (2015) 17:294–303. doi: 10.1111/dom.12428
26. Davies MJ, Merton K, Vijapurkar U, Yee J, Qiu R. Efficacy and Safety of Canagliflozin in Patients With Type 2 Diabetes Based on History of Cardiovascular Disease or Cardiovascular Risk Factors: A Post Hoc Analysis of Pooled Data. Cardiovasc Diabetol (2017) 16:40. doi: 10.1186/s12933-017-0517-7
27. DeFronzo RA, Lewin A, Patel S, Liu D, Kaste R, Woerle HJ, et al. Combination of Empagliflozin and Linagliptin as Second-Line Therapy in Subjects With Type 2 Diabetes Inadequately Controlled on Metformin. Diabetes Care (2015) 38:384–93. doi: 10.2337/dc14-236
28. Fioretto P, Del Prato S, Buse JB, Goldenberg R, Giorgino F, Reyner D, et al. Efficacy and Safety of Dapagliflozin in Patients With Type 2 Diabetes and Moderate Renal Impairment (Chronic Kidney Disease Stage 3A): The DERIVE Study. Diabetes Obes Metab (2018) 20:2532–40. doi: 10.1111/dom.13413
29. Ferrannini E, Seman L, Seewaldt-Becker E, Hantel S, Pinnetti S, Woerle HJ. A Phase IIb, Randomized, Placebo-Controlled Study of the SGLT2 Inhibitor Empagliflozin in Patients With Type 2 Diabetes. Diabetes Obes Metab (2013) 15:721–8. doi: 10.1111/dom.12081
30. Frias JP, Guja C, Hardy E, Ahmed A, Dong F, Ohman P, et al. Exenatide Once Weekly Plus Dapagliflozin Once Daily Versus Exenatide or Dapagliflozin Alone in Patients With Type 2 Diabetes Inadequately Controlled With Metformin Monotherapy (DURATION-8): A 28 Week, Multicentre, Double-Blind, Phase 3, Randomised Controlled Trial. Lancet Diabetes Endocrinol (2016) 4:1004–16. doi: 10.1016/S2213-8587(16)30267-4
31. Fulcher G, Matthews DR, Perkovic V, de Zeeuw D, Mahaffey KW, Mathieu C, et al. Efficacy and Safety of Canagliflozin When Used in Conjunction With Incretin-Mimetic Therapy in Patients With Type 2 Diabetes. Diabetes Obes Metab (2016) 18:82–91. doi: 10.1111/dom.12589
32. Haering HU, Merker L, Christiansen AV, Roux F, Salsali A, Kim G, et al. Empagliflozin as Add-on to Metformin Plus Sulphonylurea in Patients With Type 2 Diabetes. Diabetes Res Clin Practice (2015) 110:82–90. doi: 10.1111/dom.12589
33. Henry RR, Strange P, Zhou R, Pettus J, Shi L, Zhuplatov SB, et al. Effects of Dapagliflozin on 24-Hour Glycemic Control in Patients With Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Technol Ther (2018) 20(11):715–24. doi: 10.1089/dia.2018.0052
34. Inagaki N, Harashima S, Maruyama N, Kawaguchi Y, Goda M, Iijima H. Efficacy and Safety of Canagliflozin in Combination With Insulin: A Double-Blind, Randomized, Placebo-Controlled Study in Japanese Patients With Type 2 Diabetes Mellitus. Cardiovasc Diabetol (2016) 15:89. doi: 10.1186/s12933-016-0407-4
35. Jabbour SA, Frias JP, Hardy E, Ahmed A, Wang H, Ohman P, et al. Safety and Efficacy of Exenatide Once Weekly Plus Dapagliflozin Once Daily Versus Exenatide or Dapagliflozin Alone in Patients With Type 2 Diabetes Inadequately Controlled With Metformin Monotherapy: 52-Week Results of the DURATION-8 Randomized Controlled Trial. Diabetes Care (2018) 41:2136–46. doi: 10.2337/dc18-0680
36. Kadowaki T, Haneda M, Inagaki N, Terauchi Y, Taniguchi A, Koiwai K, et al. Empagliflozin Monotherapy in Japanese Patients With Type 2 Diabetes Mellitus: A Randomized, 12-Week, Double-Blind, Placebo-Controlled, Phase II Trial. Adv Ther (2014) 31:621–38. doi: 10.1007/s12325-014-0126-8
37. Kaku K, Kiyosue A, Inoue S, Ueda N, Tokudome T, Yang J, et al. Efficacy and Safety of Dapagliflozin Monotherapy in Japanese Patients With Type 2 Diabetes Inadequately Controlled by Diet and Exercise. Diabetes Obes Metab (2014) 16:1102–10. doi: 10.1111/dom.12325
38. Kaku K, Lee J, Mattheus M, Kaspers S, George J, Woerle HJ, et al. Empagliflozin and Cardiovascular Outcomes in Asian Patients With Type 2 Diabetes and Established Cardiovascular Disease- Results From EMPA-REG OUTCOME((R)). Circ J Off J Jpn Circ Soc (2017) 81:227–34. doi: 10.1111/dom.12325
39. Kohan DE, Fioretto P, Tang W, List JF. Long-Term Study of Patients With Type 2 Diabetes and Moderate Renal Impairment Shows That Dapagliflozin Reduces Weight and Blood Pressure But Does Not Improve Glycemic Control. Kidney Int (2014) 85:962–71. doi: 10.1038/ki.2013.356
40. Kovacs CS, Seshiah V, Merker L, Christiansen AV, Roux F, Salsali A, et al. Empagliflozin as Add-On Therapy to Pioglitazone With or Without Metformin in Patients With Type 2 Diabetes Mellitus. Clin Ther (2015) 37:1773–88.e1. doi: 10.1016/j.clinthera.2015.05.511
41. Lavalle-Gonzalez FJ, Eliaschewitz FG, Cerdas S, Chacon Mdel P, Tong C, Alba M. Efficacy and Safety of Canagliflozin in Patients With Type 2 Diabetes Mellitus From Latin America. Curr Med Res Opin (2016) 32:427–39. doi: 10.1185/03007995.2015.1121865
42. Leiter LA, Cefalu WT, de Bruin TW, Gause-Nilsson I, Sugg J, Parikh SJ. Dapagliflozin Added to Usual Care in Individuals With Type 2 Diabetes Mellitus With Preexisting Cardiovascular Disease: A 24-Week, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study With a 28-Week Extension. J Am Geriatr Soc (2014) 62:1252–62. doi: 10.1185/03007995.2015.1121865
43. Mahaffey KW, Jardine MJ, Bompoint S, Cannon CP, Neal B, Heerspink HJL, et al. Canagliflozin and Cardiovascular and Renal Outcomes in Type 2 Diabetes Mellitus and Chronic Kidney Disease in Primary and Secondary Cardiovascular Prevention Groups. Circulation (2019) 140:739–50. doi: 10.1161/CIRCULATIONAHA.119.042007
44. Merton K, Davies MJ, Vijapurkar U, Inman D, Meininger G. Achieving the Composite Endpoint of HbA1c, Body Weight, and Systolic Blood Pressure Reduction With Canagliflozin in Patients With Type 2 Diabetes. Curr Med Res Opin (2018) 34:313–18. doi: 10.1080/03007995.2017.1391759
45. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med (2019) 380:2295–306. doi: 10.1056/NEJMoa1811744
46. Roden M, Merker L, Christiansen AV, Roux F, Salsali A, Kim G, et al. Safety, Tolerability and Effects on Cardiometabolic Risk Factors of Empagliflozin Monotherapy in Drug-Naive Patients With Type 2 Diabetes: A Double-Blind Extension of a Phase III Randomized Controlled Trial. Cardiovasc Diabetol (2015) 14:154. doi: 10.1186/s12933-015-0314-0
47. Romera I, Gomis R, Crowe S, de Pablos-Velasco P, Aranda U, Garcia A, et al. Empagliflozin in Combination With Oral Agents in Young and Overweight/Obese Type 2 Diabetes Mellitus Patients: A Pooled Analysis of Three Randomized Trials. J Diabetes Complications (2016) 30:1571–76. doi: 10.1016/j.jdiacomp.2016.07.016
48. Rosenstock J, Chuck L, Gonzalez-Ortiz M, Merton K, Craig J, Capuano G, et al. Initial Combination Therapy With Canagliflozin Plus Metformin Versus Each Component as Monotherapy for Drug-Naive Type 2 Diabetes. Diabetes Care (2016) 39:353–62. doi: 10.2337/dc15-1736
49. Rosenstock J, Seman LJ, Jelaska A, Hantel S, Pinnetti S, Hach T, et al. Efficacy and Safety of Empagliflozin, a Sodium Glucose Cotransporter 2 (SGLT2) Inhibitor, as Add-on to Metformin in Type 2 Diabetes With Mild Hyperglycaemia. Diabetes Obes Metab (2013) 15:1154–60. doi: 10.1111/dom.12185
50. Rosenstock J, Jelaska A, Zeller C, Kim G, Broedl UC, Woerle HJ, et al. Impact of Empagliflozin Added on to Basal Insulin in Type 2 Diabetes Inadequately Controlled on Basal Insulin: A 78-Week Randomized, Double-Blind, Placebo-Controlled Trial. Diabetes Obes Metab (2015) 17:936–48. doi: 10.1111/dom.12503
51. Schumm-Draeger PM, Burgess L, Koranyi L, Hruba V, Hamer-Maansson JE, de Bruin TW. Twice-Daily Dapagliflozin Co-Administered With Metformin in Type 2 Diabetes: A 16-Week Randomized, Placebo-Controlled Clinical Trial. Diabetes Obes Metab (2015) 17:42–51.
52. Sinclair AJ, Bode B, Harris S, Vijapurkar U, Shaw W, Desai M, et al. Efficacy and Safety of Canagliflozin in Individuals Aged 75 and Older With Type 2 Diabetes Mellitus: A Pooled Analysis. J Am Geriatr Soc (2016) 64:543–52. doi: 10.1111/jgs.14028
53. Softeland E, Meier JJ, Vangen B, Toorawa R, Maldonado-Lutomirsky M, Broedl UC. Empagliflozin as Add-On Therapy in Patients With Type 2 Diabetes Inadequately Controlled With Linagliptin and Metformin: A 24-Week Randomized, Double-Blind, Parallel-Group Trial. Diabetes Care (2017) 40:201–9. doi: 10.2337/dc16-1347
54. Stenlof K, Cefalu WT, Kim KA, Jodar E, Alba M, Edwards R, et al. Long-Term Efficacy and Safety of Canagliflozin Monotherapy in Patients With Type 2 Diabetes Inadequately Controlled With Diet and Exercise: Findings From the 52-Week CANTATA-M Study. Curr Med Res Opinion (2014) 30:163–75. doi: 10.1185/03007995.2013.850066
55. Strojek K, Yoon KH, Hruba V, Sugg J, Langkilde AM, Parikh S. Dapagliflozin Added to Glimepiride in Patients With Type 2 Diabetes Mellitus Sustains Glycemic Control and Weight Loss Over 48 Weeks: A Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Trial. Diabetes Ther Res Treat Educ Diabetes Rel Disord (2014) 5:267–83. doi: 10.1007/s13300-014-0072-0
56. Tikkanen I, Narko K, Zeller C, Green A, Salsali A, Broedl UC, et al. Empagliflozin Reduces Blood Pressure in Patients With Type 2 Diabetes and Hypertension. Diabetes Care (2015) 38:420–8. doi: 10.2337/dc14-1096
57. Tinahones FJ, Gallwitz B, Nordaby M, Gotz S, Maldonado-Lutomirsky M, Woerle HJ, et al. Linagliptin as Add-on to Empagliflozin and Metformin in Patients With Type 2 Diabetes: Two 24-Week Randomized, Double-Blind, Double-Dummy, Parallel-Group Trials. Diabetes Obes Metab (2017) 19:266–74. doi: 10.2337/dc14-1096
58. Weber MA, Mansfield TA, Cain VA, Iqbal N, Parikh S, Ptaszynska A. Blood Pressure and Glycaemic Effects of Dapagliflozin Versus Placebo in Patients With Type 2 Diabetes on Combination Antihypertensive Therapy: A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Study. Lancet Diabetes Endocrinol (2016) 4:211–20. doi: 10.1016/S2213-8587(15)00417-9
59. Wilding JP, Charpentier G, Hollander P, Gonzalez-Galvez G, Mathieu C, Vercruysse F, et al. Efficacy and Safety of Canagliflozin in Patients With Type 2 Diabetes Mellitus Inadequately Controlled With Metformin and Sulphonylurea: A Randomised Trial. Int J Clin Pract (2013) 67:1267–82. doi: 10.1111/ijcp.12322
60. Yale JF, Bakris G, Cariou B, Nieto J, David-Neto E, Yue D, et al. Efficacy and Safety of Canagliflozin Over 52 Weeks in Patients With Type 2 Diabetes Mellitus and Chronic Kidney Disease. Diabetes Obes Metab (2014) 16:1016–27. doi: 10.1111/dom.12348
61. Yale JF, Xie J, Sherman SE, Garceau C. Canagliflozin in Conjunction With Sulfonylurea Maintains Glycemic Control and Weight Loss Over 52 Weeks: A Randomized, Controlled Trial in Patients With Type 2 Diabetes Mellitus. Clin Ther (2017) 39:2230–42.e2. doi: 10.1016/j.clinthera.2017.10.003
62. Zhao Q, Kim T, Pang J, Sun W, Yang X, Wang J, et al. A Novel Function of CXCL10 in Mediating Monocyte Production of Proinflammatory Cytokines. J Leukocyte Biol (2017) 102:1271–80. doi: 10.1189/jlb.5A0717-302
63. Yang W, Ma J, Li Y, Li Y, Zhou Z, Kim JH, et al. Dapagliflozin as Add-on Therapy in Asian Patients With Type 2 Diabetes Inadequately Controlled on Insulin With or Without Oral Antihyperglycemic Drugs: A Randomized Controlled Trial. J Diabetes (2018) 10:589–99. doi: 10.1111/1753-0407.12634
64. Mordi NA, Mordi IR, Singh JS, McCrimmon RJ, Struthers AD, Lang CC. Renal and Cardiovascular Effects of SGLT2 Inhibition in Combination With Loop Diuretics in Patients With Type 2 Diabetes and Chronic Heart Failure: The RECEDE-CHF Trial. Circulation (2020) 142(18):1713–24. doi: 10.1161/CIRCULATIONAHA.120.048739
65. Eickhoff MK, Olsen FJ, Frimodt-Moller M, Diaz LJ, Faber J, Jensen MT, et al. Effect of Dapagliflozin on Cardiac Function in People With Type 2 Diabetes and Albuminuria - A Double Blind Randomized Placebo-Controlled Crossover Trial. J Diabetes Complications (2020) 34:107590. doi: 10.1161/CIRCULATIONAHA.120.048739
66. Zheng SL, Roddick AJ, Aghar-Jaffar R, Shun-Shin MJ, Francis D, Oliver N, et al. Association Between Use of Sodium-Glucose Cotransporter 2 Inhibitors, Glucagon-Like Peptide 1 Agonists, and Dipeptidyl Peptidase 4 Inhibitors With All-Cause Mortality in Patients With Type 2 Diabetes: A Systematic Review and Meta-Analysis. Jama (2018) 319(15):1580–91. doi: 10.1001/jama.2018.3024
67. Sha S, Polidori D, Farrell K, Ghosh A, Natarajan J, Vaccaro N, et al. Pharmacodynamic Differences Between Canagliflozin and Dapagliflozin: Results of a Randomized, Double-Blind, Crossover Study. Diabetes Obes Metab (2015) 17(2):188–97. doi: 10.1111/dom.12418
68. Hawley SA, Ford RJ, Smith BK, Gowans GJ, Mancini SJ, Pitt RD, et al. The Na+/Glucose Cotransporter Inhibitor Canagliflozin Activates AMPK by Inhibiting Mitochondrial Function and Increasing Cellular AMP Levels. Diabetes (2016) 65(9):2784–94. doi: 10.2337/db16-0058
69. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 Inhibitors for Primary and Secondary Prevention of Cardiovascular and Renal Outcomes in Type 2 Diabetes: A Systematic Review and Meta-Analysis of Cardiovascular Outcome Trials. Lancet (2019) 393(10166):31–9. doi: 10.1016/S0140-6736(18)32590-X
70. Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a Glucose-Regulating Drug With Diuretic Properties in Subjects With Type 2 Diabetes. Diabetes Obes Metab (2013) 15(9):853–62. doi: 10.1111/dom.12127
Keywords: type 2 diabetes mellitus, SGLT2 inhibitors, cardiovascular events, meta-analysis, empagliflozin
Citation: Jiang Y, Yang P, Fu L, Sun L, Shen W and Wu Q (2022) Comparative Cardiovascular Outcomes of SGLT2 Inhibitors in Type 2 Diabetes Mellitus: A Network Meta-Analysis of Randomized Controlled Trials. Front. Endocrinol. 13:802992. doi: 10.3389/fendo.2022.802992
Received: 27 October 2021; Accepted: 13 January 2022;
Published: 16 March 2022.
Edited by:
David H. Wagner, University of Colorado Denver, United StatesReviewed by:
Martin Yussman, University of Colorado, United StatesMario Luca Morieri, University of Padua, Italy
Copyright © 2022 Jiang, Yang, Fu, Sun, Shen and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen Shen, MTgxNDY2MjIxOTdAMTYzLmNvbQ==; Qinghua Wu, bmN3cWhAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Yu Jiang1,2†
Yu Jiang1,2† Pingping Yang
Pingping Yang Linghua Fu
Linghua Fu Wen Shen
Wen Shen