- 1Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
- 2Department of Medicine, National University Hospital, Singapore, Singapore
- 3National University Centre for Organ Transplantation, National University Hospital, Singapore, Singapore
Objective: Non-alcoholic fatty liver disease (NAFLD) is a very common disorder among patients with type 2 diabetes and may share causal relationship. Type 2 diabetes is a risk factor for progression and potential poor outcomes in NAFLD patients. This meta-analysis aimed to analyze the current evidence of sodium-glucose co-transporter-2 inhibitors (SGLT2i), a glucose-lowering drug to improve NAFLD in patients with Type 2 Diabetes.
Methods: Medline, Embase and Cochrane Central Register of Controlled Trials were searched for articles examining efficacy of SGLT2i on treatments of NAFLD in type 2 diabetes in July 2020, and articles were sieved. Continuous data were extracted in the form of mean and standard deviation and were pooled with standardized mean difference (SMD).
Results: 10 articles involving 555 patients from seven randomized controlled trials (RCTs) and three cohort studies, were included in this meta-analysis. Our analysis revealed significant improvements in hepatic fat content (after treatment: -0.789 (-1.404 to -0.175), p = 0.012; compared with control: -0.923 (-1.562 to -0.285), p = 0.005), AST (After Treatment: -0.539 (-0.720 to -0.357), p < 0.001; compared with control: -0.421 (-0.680 to -0.161), p = 0.001), ALT (after treatment: -0.633 (-0.892 to -0.373), p < 0.001; compared with Control: -0.468 (-0.685 to -0.251), p < 0.001), body composition (BMI: after treatment: -0.225 (-0.456 to 0.005), p = 0.055; compared with Control: -1.092 (-2.032 to -0.153), p = 0.023), glycemic control (HbA1c: After Treatment: -0.701 (-1.098 to -0.303), p = 0.001; compared with control: -0.210 (-0.603 to 0.183), p = 0.295), lipid parameters (Triglycerides: after treatment: -0.230 (-0.409 to -0.052), p = 0.011; compared with control: -0.336 (-0.597 to -0.076), p = 0.011), inflammatory markers (serum ferritin: after treatment: -0.409 (-0.694 to -0.124), p = 0.005; compared with control: -0.814 (-1.688 to 0.059), p = 0.068) after SGLT2i treatment, and when compared against controls. There was a trend in the improvement in fibrosis markers after SGLT2i treatment.
Conclusions: SGLT2i is an effective treatment to improve NAFLD among patients with type 2 diabetes. Further studies are needed to understand the direct and indirect effects of SGLT2i on NAFLD and if SGLT2i could prevent the progression of NAFLD or NASH. SGLT2i could potentially be considered for patients with type 2 diabetes and NAFLD, if there are no contraindications.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common, but unappreciated liver disease in developed countries (1). It occurs when there is excessive fat accumulation in the liver without a history of significant alcohol consumption or other underlying causes which result in fat accumulation. NAFLD has two principle phenotypes, non-alcoholic fatty liver (NAFL), and non-alcoholic steatohepatitis (NASH), which are differentiated by the presence of inflammation and hepatocyte injury (2). NAFLD can subsequently progress to fibrosis and liver cirrhosis, and might lead to the development of liver cancer (3).
The rising prevalence of NAFLD mirrors the rising rates of obesity, and is closely associated with its complications of metabolic syndrome and type 2 diabetes (4–7). The current global prevalence of NAFLD is estimated about 25.24% (8). Among patients with type 2 diabetes, the prevalence of NAFL and NASH is as high as 55.5 and 37.3%, respectively (9). It has been shown that type 2 diabetes is an independent predictor for the progression of NAFL to NASH and liver fibrosis (10, 11). On the other hand, NAFLD is also associated with a higher risk of incident type 2 diabetes and diabetes-related complications, such as chronic kidney disease and retinopathy (12–14). With the global diabesity epidemic, it is projected that the prevalence of NAFLD and the cost of treating NAFLD will rise exponentially, with a study projecting the healthcare costs of NAFLD in United States to be $103 billion (15).
Sodium-glucose co-transporter 2 inhibitors (SGLT2i) are a relatively new class of glucose-lowering drugs, and has been shown to reduce mortality from cardiovascular disease, prevent hospitalization from heart failure and reduce progression of diabetic kidney disease (16). Several studies and meta-analyses have also shown that SGLT2i could improve liver steatosis (17, 18), but the focus is largely on canagliflozin. As such, the effects of the other SGLT2i treatments, such as dapagliflozin, empagliflozin, ipragliflozin and luseogliflozin remain largely unknown. While we believe that the effect of SGLT2i on NAFLD is of class effect, this remains speculative as there is no head-to-head comparison. This meta-analysis aimed to consolidate the current evidence of the effects of SGLT2i drugs on NAFLD among patients with type 2 diabetes.
Material and Methods
Search Strategy
This meta-analysis was conducted in accordance to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (19). Searches on electronic databases Medline, Embase and Cochrane Central Register of Controlled Trials were conducted on 21st June 2020. Keywords and thesaurus terms pertaining to “NAFLD” and “SGLT2 inhibitors” were used in the search. References of relevant articles were also searched manually for additional studies. The search strategy is attached in the Supplementary Material 1.
Selection Criteria and Eligibility Criteria
Articles identified from the search underwent a title and abstract sieve. A full-text review was then conducted independently by two authors and any discrepancies were discussed and addressed, until a full consensus was reached. The inclusion criteria for this meta-analysis is as follows: 1) a definite diagnosis of both NAFLD and Type 2 Diabetes in patients and 2) comparative studies comparing SGLT2i against anti-diabetic treatments. In addition, only original articles were included, excluding commentaries, conference abstracts, editorials and non-English studies.
Data Extraction and Outcomes
Data extraction was conducted independently by two authors. The data extracted from the studies include the author, year of publication, country of study, study design, dosage of SGLT2i and control treatments, sample size, demographics (gender composition, age, duration of diabetes, concomitant medication usage, comorbidities) and relevant primary data. For continuous variables, the mean and standard deviation (SD) were extracted. In the case where mean and SD were not available, the data was transformed according to existing formulae; using calculations from Hozo et al. for conversion from median and range (20), and calculations from Wan et al. for conversion from median and interquartile range (21). The outcomes of this meta-analysis include changes in body composition, metabolic parameters, adipokines and inflammatory markers, steatosis markers, fibrosis markers, as well as liver and renal biomarkers.
Statistical Analysis and Quality Assessment
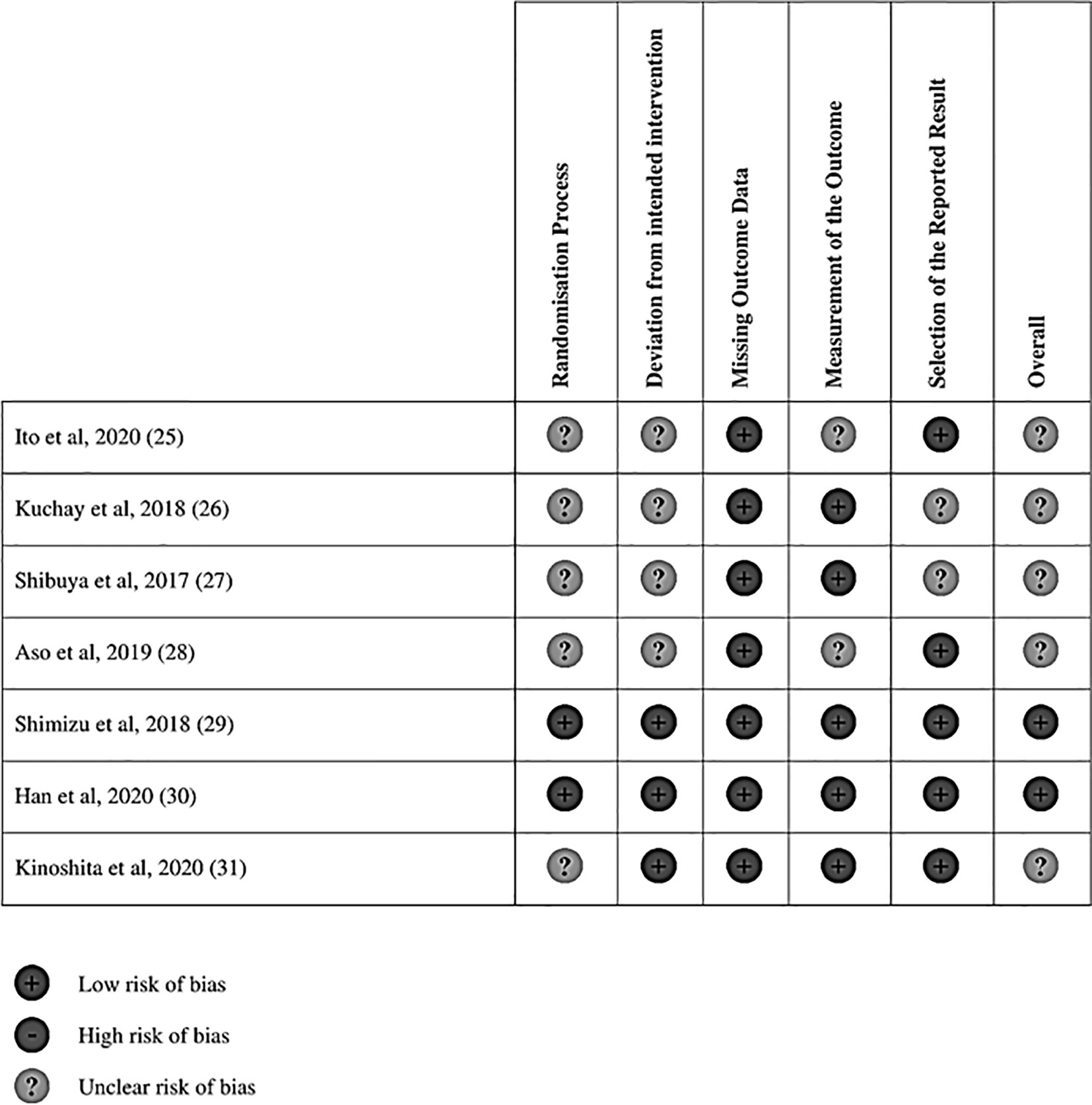
To account for the different units of analysis, the standard mean differences was preferred in the analysis (22). Continuous data was pooled with standardized mean difference (SMD). All analysis was conducted in STATA and p < 0.05 was considered statistically significant. Quality assessment of cohort studies was conducted via the Newcastle Ottawa Scale (NOS) while risk of bias assessment of randomized controlled trials (RCT) was carried out using Cochrane’s Risk of Bias 2 (RoB2) tool. The RoB2 tool assesses quality on 5 domains primarily the randomization process, deviations from intended interventions, missing outcome data, outcome measurements and reporting (23). The NOS assesses quality based on three main domains including selection, comparability and outcome (24).
Results
Baseline Characteristics
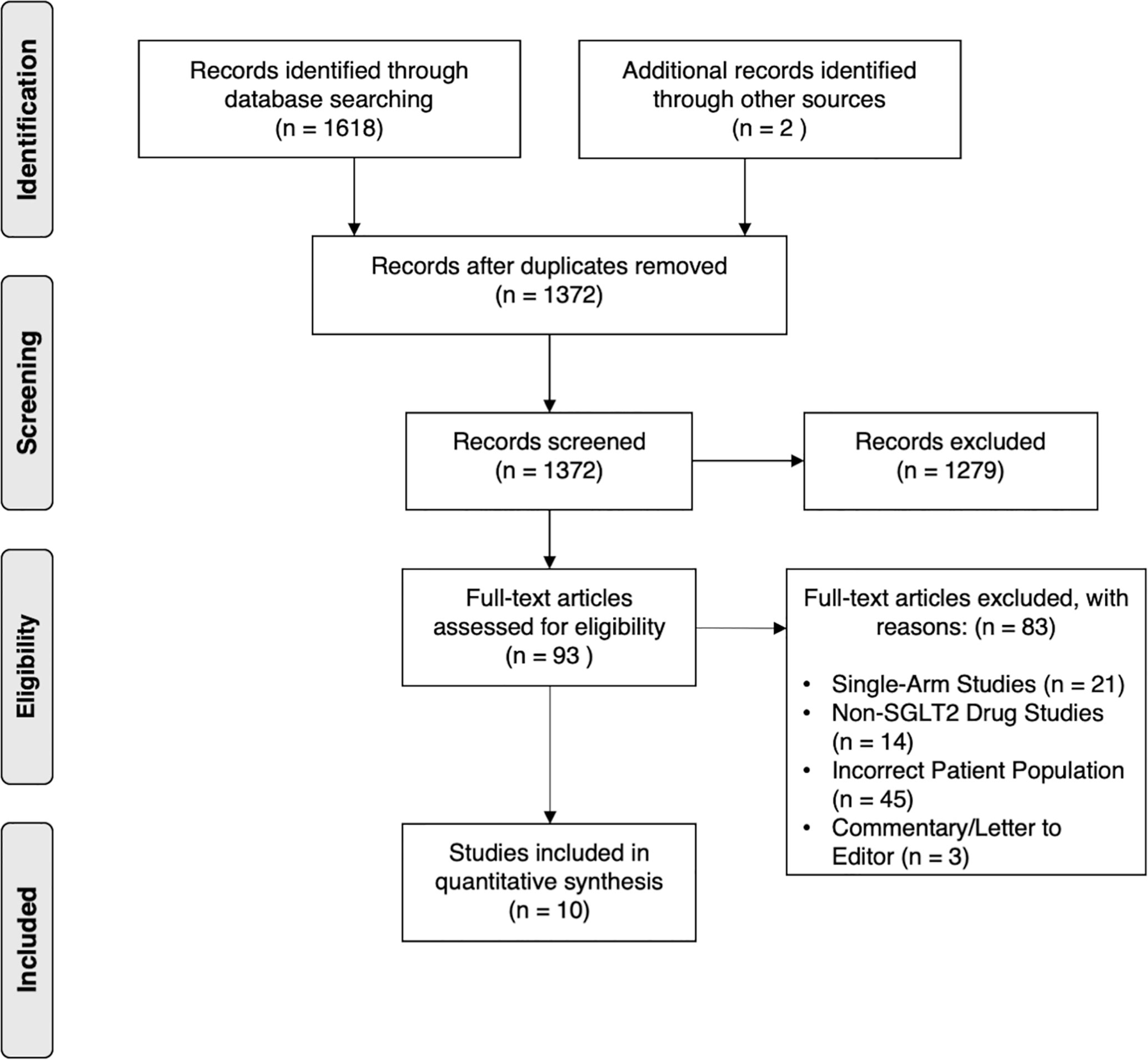
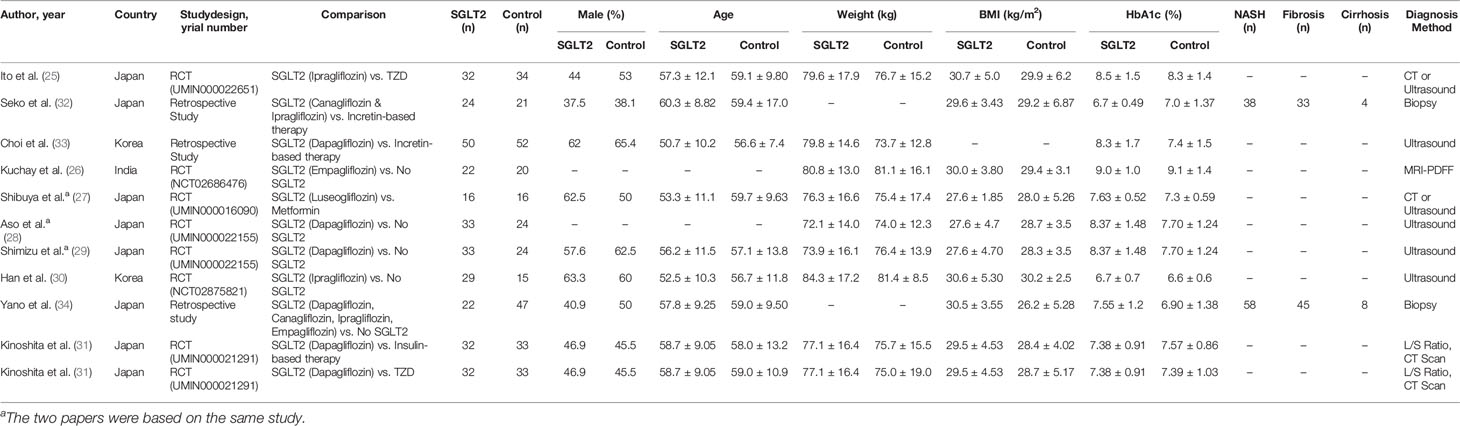
Electronic database searches identified 1,372 articles after duplicate removal. 93 articles were selected for full text review, of which 10 met the inclusion criteria, inclusive of 7 randomized controlled trials (RCT) (25–31). Figure 1 shows the flowchart of the review. Of the 10 articles, one compared SGLT2i to thiazolidinediones (TZD) (25), two compared SGLT2i to incretin-based therapies (32, 33), two compared SGLT2i to metformin (27), five compared SGLT2i to non-SGLT2i therapies (26, 28–30, 34), and one was a three-arm study which compared SGLT2i, TZD and insulin-based therapies (31). In total, there were 555 patients, with 260 on SGLT2i, 67 on TZD, 73 on incretin-based therapies, 33 on insulin-based therapies, 16 on metformin and the remaining 106 on non-SGLT2i treatments. The SGLT2i treatments consist of Canagliflozin, Dapagliflozin, Empagliflozin, Ipragliflozin and Luseogliflozin. All patients were clinically diagnosed with type 2 diabetes and NAFLD, with two studies reporting patients with NASH (n = 96), fibrosis (n = 78) and cirrhosis (n = 12) based on liver biopsy. The duration of treatment for patients in included studies ranged from twenty-four weeks to more than three years. The glycosylated hemoglobin (HbA1c) ranged from 6.0% to 12.0%. The characteristics of patients in included papers are presented in Table 1. Quality assessment of included articles are found in Supplementary Material 8 and Figure 2.
Changes in Body Composition
As expected, SGLT2i treatment reduced body weight, waist circumference, subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT). The reduction in body weight was significantly greater compared to controls (SMD: -2.317, CI: -3.576 to -1.057, p < 0.001), TZD (SMD: -4.817, CI: -9.201 to -0.433, p = 0.031), incretin-based therapies (SMD: -0.589, CI: -0.986 to -0.192, p = 0.004) and insulin-based therapies (SMD: -2.074, CI: -2.681 to -1.468, p < 0.001) (see Supplementary Material 2). The reduction in BMI was significantly greater when compared to controls (SMD: -1.092, CI: -2.032 to -0.153, p = 0.023) and metformin (SMD: -1.120, CI: -1.869 to -0.371, p = 0.003). The reduction of VAT was statistically significant after SGLT2i treatment (SMD: -0.277, CI: -0.511 to -0.043, p = 0.02) and was greater when compared to controls (SMD: -2.247, CI: -3.586 to -0.907, p = 0.001), insulin-based therapies (SMD: -1.179, CI: -1.707 to -0.651, p < 0.001) and metformin (SMD: -1.145, CI: -1.896 to -0.394, p = 0.003). The reduction in SAT reached statistical significance when compared against TZD (SMD: -6.347, CI: -7.547 to -5.146, p < 0.001).
Changes in Metabolic Parameters
SGLT2i treatment significantly decreased fasting glucose (SMD: -0.326, CI: -0.634 to -0.017, p = 0.039), HbA1c (SMD: -0.701, CI: -1.098 to -0.303, p = 0.001), triglyceride levels (SMD: -0.230, CI: -0.409 to -0.052, p = 0.011) (see Supplementary Material 3).
Compared to controls, SGLT2i treatment resulted in a significantly lower triglyceride levels (SMD: -0.336, CI: -0.597 to -0.076, p = 0.011, Figure 3). Compared to TZD, SGLT2i treatment had a greater reduction in total cholesterol levels (SMD: -1.545, CI: -2.096 to -0.993, p < 0.001). Compared to incretin-based therapy, SGLT2i treatment showed a greater reduction in fasting glucose (SMD: -0.841, CI: -1.321 to -0.360, p = 0.001). Compared to insulin-based therapies, SGLT2i treatment resulted in a significant higher HDL levels (SMD: 0.861, CI: 0.352 to 1.370, p = 0.001). Compared to metformin, SGLT2i treatment led to a greater reduction in HbA1c levels (SMD: -0.825, CI: -1.548 to -0.101, p = 0.026). The comparisons between SGLT2i treatment with other glucose-lowering agents on the indices of insulin resistance (fasting insulin, HOMA-IR, CPR, adipo-IR), beta-cell function (CPR index, HOMA-B), systolic and diastolic blood pressure (SBP), diastolic blood pressure (DBP), non-esterified fatty acids (NEFA) and low-density lipoprotein (LDL) levels were non-significant.
Changes in Adipokines and Inflammatory Markers
SGLT2i treatment resulted in an increase in adiponectin levels (SMD: 0.301, CI: 0.005 to 0.596, p = 0.046) (see Supplementary Material 4). There were no differences in the change in adiponectin levels for the comparison between SGLT2i treatment and TZD or insulin-based therapies. SGLT2i treatment significantly reduced soluble dipeptidyl peptidase 4 (sDPP-4) levels (SMD: -0.764, CI: -1.264 to -0.264, p = 0.003) and the reduction was greater when compared to controls (SMD: -0.638, CI: -1.177 to -0.099, p = 0.02).
Changes in Steatosis Markers
SGLT2i treatment significantly reduced hepatic fat content, as measured by the magnetic resonance imaging proton density fat fraction (MRI-PDFF) (SMD: -0.789, CI: -1.404 to -0.175, p = 0.012) and when compared to control (SMD: -0.923, CI:-1.562 to -0.285, p = 0.005). SGLT2i treatment significantly improved the liver-to-spleen (L/S) attenuation ratios as measured by computed tomography (CT) scans (SMD: 0.456, CI: 0.142 to 0.771, p = 0.004) (see Supplementary Material 5), and the improvement was greater compared to insulin-based therapies (SMD: 0.614, CI: 0.116 to 1.112, p = 0.016) or metformin (SMD: 1.957, CI: 1.105 to 2.809, p < 0.001). SGLT2i treatment had a greater reduction in CAP scores (SMD: -1.376, CI: -2.540 to -0.213, p = 0.02) when compared to controls. The hepatic steatosis index (HSI) was lower after SGLT2i treatment but did not reach statistical significance.
Changes in Fibrosis Markers
SGLT2i treatment also reduced the numerical measured fibrosis markers such as FIB-4 index, liver stiffness measurement (by transient elastography), Mac-2 Binding Protein but did not reach statistical significance (see Supplementary Material 6). There was no significant change in the NAFLD fibrosis score but significantly reduced NAFIC score (predicting NASH) (SMD: -0.569, CI: -1.062 to -0.077, p = 0.023) and serum ferritin levels (SMD: -0.409, CI: -0.694 to -0.124, p = 0.005) was found. Similarly, when compared to controls, SGLT2i treatment resulted in significant reductions in all the fibrosis markers but only reached statistical significance for NAFIC score (SMD: -0.692, CI: -1.233 to -0.15), p = 0.012). Compared to TZD, SGLT2i treatment resulted in a significantly lower FIB-4 index values (SMD: -0.780, CI: -1.281 to -0.279, p = 0.002).
Changes in Liver and Renal Biomarkers
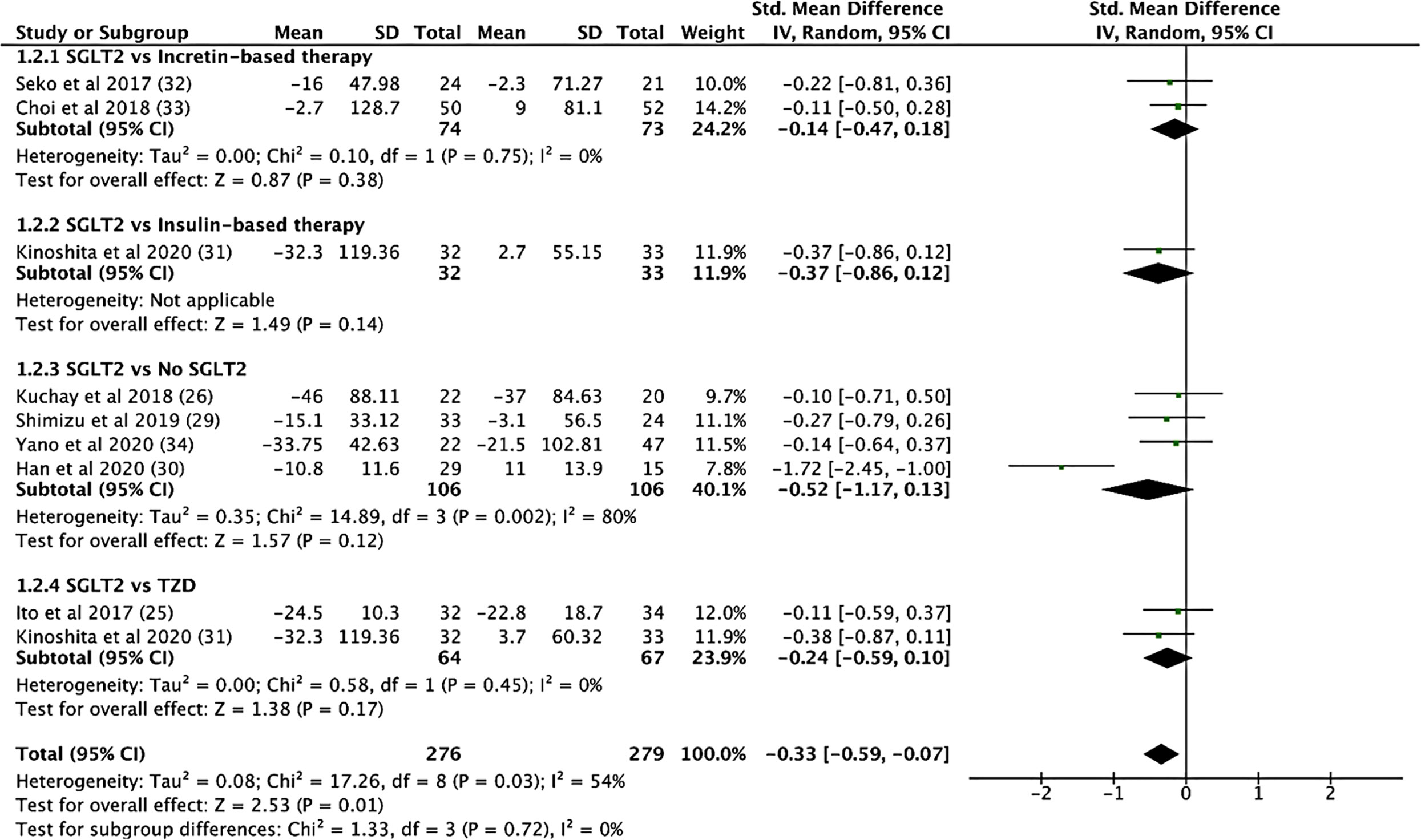
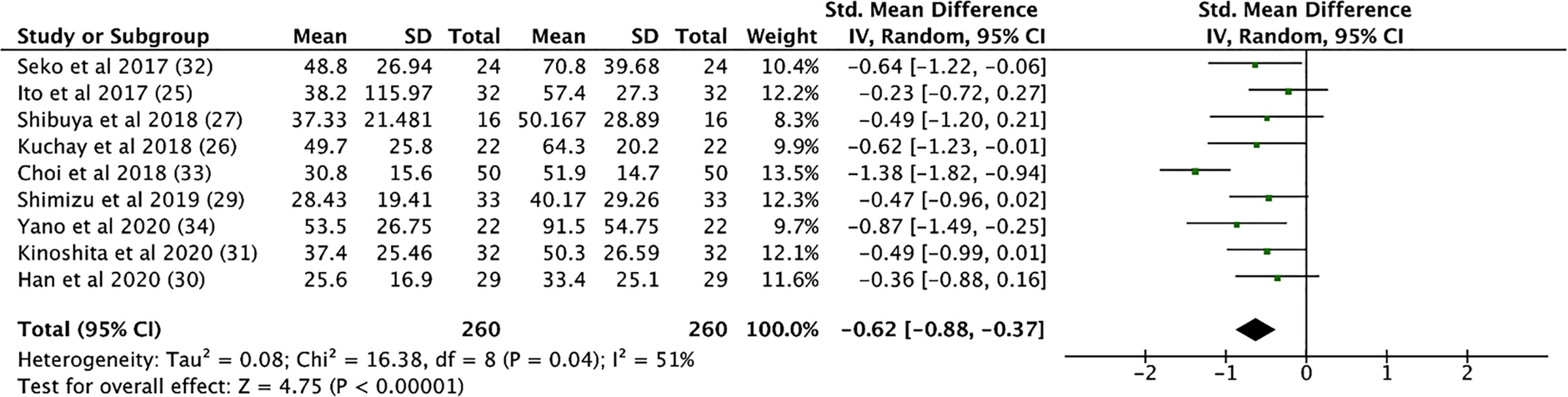
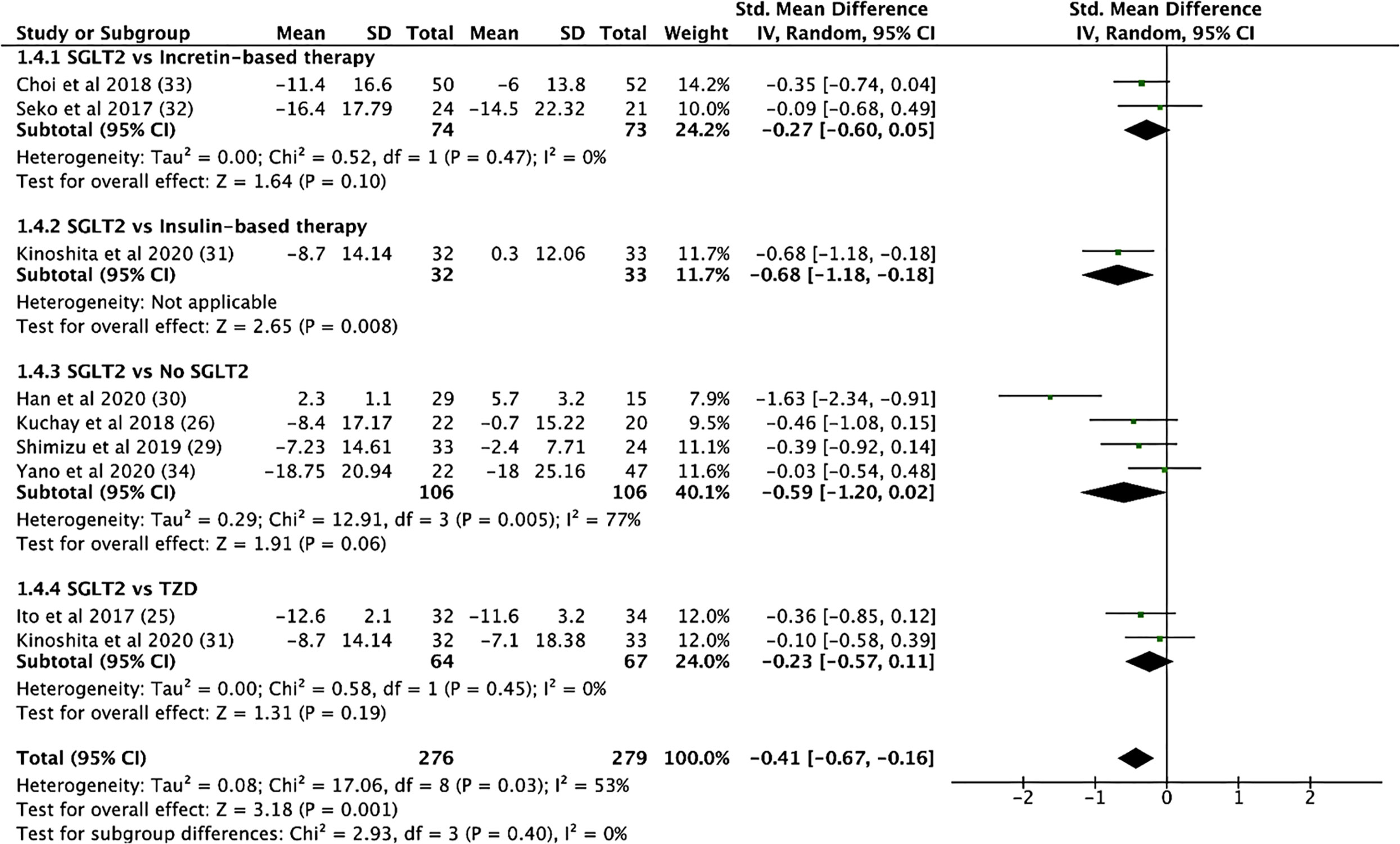
SGLT2i treatment significantly reduced aspartate aminotransferase (AST) levels (SMD: -0.539, CI: -0.720 to -0.357, p < 0.001), alanine transaminase (ALT) levels (SMD: -0.633, CI: -0.892 to -0.373, p < 0.001, Figure 4), and gamma-glutamyl transferase (GGT) levels (SMD: -0.330, CI: -0.530 to -0.129, p = 0.001) (see Supplementary Material 7). In addition, SGLT2i treatment also resulted in significant increases in albumin levels (SMD: 0.353, CI: 0.034 to 0.671, p = 0.03). Compared to controls, SGLT2i resulted in a significant decrease in ALT levels (SMD: -0.468, CI: -0.685 to -0.251, p < 0.001) and AST levels (SMD: -0.421, CI: -0.680 to -0.161, p = 0.001, Figure 5) and increase in albumin levels (SMD: 0.363, CI: 0.047 to 0.678, p = 0.024). Compared to insulin-based therapies, SGLT2i treatment had a significantly lower AST levels (SMD: -0.686, CI: -1.186 to -0.185, p = 0.007), ALT levels (SMD: -0.551, CI: -1.046 to -0.055, p = 0.029) and GGT levels (SMD: -0.639, CI: -1.138 to -0.140, p = 0.012). Total bilirubin, platelet count, uric acid levels and eGFR were largely unchanged.
Discussion
To the best of our knowledge, this is a comprehensive meta-analysis that analyses the effectiveness of SGLT2i on the improvement of NAFLD and hepatic fibrosis specific to type 2 diabetes population. While there are currently no established guidelines on the pharmacological treatment of NAFLD in type 2 diabetic patients (2), pioglitazone has been shown to improve the liver histology among pre-diabetics, diabetics and non-diabetics with NAFLD and NASH (35, 36). However, pioglitazone has some serious side effects including weight gain, fluid retention, hospitalization of heart failure, and bone loss (2). More recently, there have been reports on another oral glucose-lowering agent, SGLT2i, in improving NAFLD (37–39). In our analysis, SGLT2i was shown to result in significant improvement in hepatic steatosis, body composition, metabolic parameters and liver biomarkers, which indicates promise of SGLT2i as a treatment modality in patients with concurrent NAFLD and type 2 diabetes mellitus.
Unlike chronic kidney disease, where serum creatinine, urinary albumin-to-creatinine ratio and estimated glomerular filtration rate could be used to identify and as biomarkers for therapeutic efficacy, such biomarkers for NAFLD are less known. Liver biopsies are the gold standard to diagnose and prognosticate NAFLD and NASH (40), but are associated with significant risks to the patients (41). Thus, surrogate indicators such as MRI-PDFF, L/S ratios, CAP scores and HSI values have been used to quantify the degree of hepatic steatosis (42). Non-invasive markers of liver fibrosis such as FIB-4 index, NFS and TEM (42), and serum biomarkers such as ALT, AST and GGT have been associated with NAFLD and NASH (43, 44).
In this analysis, SGLT2i treatment was associated with improvement in hepatic steatosis as measured by MRI-PDFF, L/S attenuation ratios, CAP score and HSI values. In the animal studies, SGLT2i has been shown to decrease de novo lipogenesis and increase lipolysis, and this has been postulated as the mechanism on how SGLT2i improves hepatic steatosis (45). Studies have shown that SGLT2i results in elevation of glucagon and a change in the insulin-to-glucagon ratio, which favours lipolysis and ketogenesis in the liver (46). Metformin is a very potent oral glucose-lowering agent and can reduce hepatic glucose production and improve insulin sensitivity (47). In a meta-analysis, metformin improves liver function, HOMA-IR and BMI to some extent, but not histological response in NAFLD patients (48). Our results showed that SGLT2i had greater effectiveness in reducing hepatic steatosis compared to metformin. Insulin is an anabolic hormone that results in weight gain and increased lipogenesis (49). Juurinen L et al. showed that chronic insulin therapy for type 2 diabetic patients resulted in weight gain but a slight significant reduction in liver fat content with improved hepatic liver sensitivity (50). Compared with insulin-based therapies, SGLT2i had a greater effect on reducing hepatic fat content, in addition to that of significant weight loss and reduced visceral adipose tissue (51).
NAFIC score is a non-invasive scoring system for predicting NASH in Japanese NAFLD and is a derivative of serum ferritin, fasting insulin and plasma type IV collagen 7S concentrations (52). This study showed that SGLT2i significantly improved the NAFIC score and serum ferritin. It might indicate that serum ferritin could be a biomarker of choice to inform physicians on the therapeutic efficacy in improving NASH and liver fibrosis. The effect of SGLT2i on other known indicators of liver fibrosis such as in FIB-4 index and NFS, and both liver fibrosis and cirrhosis for liver stiffness measurements were largely non-significant. The stage of NAFLD (i.e. fatty infiltration, inflammation or fibrosis) or the duration of treatment might have influenced the therapeutic efficacy of SGLT2i on the biomarkers and other non-invasive measurements in this meta-analysis.
Pioglitazone has shown benefits in liver function, liver fat, and NASH resolution (35). In this meta-analysis, SGLT2i treatment had a larger decrease in FIB-4 index values compared to TZD-based therapy. Similar to the comparison with insulin-based therapy, the SGLT2i treatment showed a reduction in body weight and SAT compared to TZD-based therapy. Whether weight loss attributed to SGLT2i added advantage over TZD-based therapy is currently not known. The American Association for the Study of Liver Diseases (AASLD) recommended pharmacologic treatment such as pioglitazone in patients with biopsy-proven NASH and fibrosis, since patients without fibrosis generally have a favorable prognosis (2). Whether this recommendation will extend to SGLT2i awaits the outcomes from ongoing RCT on effectiveness of SGLT2i treatment on biopsy-proven NASH (53).
Consistent with the improvement in the NAFIC score, serum ferritin and steatosis biomarkers, there were significant improvements in the ALT and AST levels after SGLT2i treatments and when compared to controls or insulin-based therapy. While the accuracy of raised ALT and AST as biomarkers of NASH is low (54), they are commonly utilized as clinical indicators of hepatocellular injury and improvement in these levels indicates improvement in fatty liver or NASH (55).
SGLT2i treatment significantly reduced body weight and BMI, and improved body fat composition (SAT and VAT), which are consistent with current diabetic studies (56). Weight loss and reduction in VAT is strongly correlated with the decrease in hepatic fat (57), and is a key factor in the improvement in liver histology in NASH patients (58). Improvement in the body fat composition is also associated with an increase in adiponectin levels, an adipokine that is associated with improved insulin sensitivity (59). In our analysis, SGLT2i also resulted in improved insulin sensitivity, as measured by HOMA-IR, but did not reach statistical significance. While both Shimuzu et al. and Han et al. showed a significant decrease in the HOMA-IR after SGLT2i use (28, 30), the baseline values of the patients included in the two studies were lower compared to studies which had non-significant changes in HOMA-IR values (25, 31, 32). This could potentially indicate that the effects of SGLT2i is stronger in patients with higher baseline insulin sensitivity. However, further studies are required to determine the efficacy of SGLT2i on patients with differing insulin sensitivity levels. It is widely recognized that SGLT2i confer cardiorenal benefits among people with and without type 2 diabetes (60), but it is currently not clear whether the benefits of SGLT2i on cardiorenal protection will extend to NAFLD with and without type 2 diabetes.
Limitations
There are several limitations in this meta-analysis. The studies included in this meta-analysis were mainly from Asian countries, which resulted in less representation from the West. However, NAFLD is a common disease with general underlying pathophysiology of insulin resistance and metabolic syndrome and thus, we believe that the results of this study could be generalized to other regions in the world. In addition, we were only able to include 7 RCTs in this paper due to the limited number of clinical trials available, with 5 out of 7 RCTs deemed to have an unclear risk of bias. This indicates strong possibility of bias in the results of this analysis, and thus prompts the need for more clinical trials to better examine the efficacy of SGLT2i, especially vis-à-vis other anti-diabetic medication, on the improvement of NAFLD in patients with concomitant type 2 diabetes. The focus of this meta-analysis was on patients with concomitant NAFLD and type 2 diabetes, but we believe the effect of SGLT2i could be extended to patients with NAFLD without type 2 diabetes as the effect of SGLT2i on improving NAFLD is independent of glycaemia, similar to the cardiorenal protection. Due to the invasive nature of liver biopsy, many of the included studies did not report liver biopsy as a diagnostic tool but relied on ultrasonography, CT scans and MRI to diagnose NAFLD, which are less accurate in demonstrating improvements in NASH (61, 62). SGLT2i treatments have been known to result in a mean weight loss of 3% (63). In the LookAhead study (NCT00017953), a weight loss between 1 and 5% was associated with a mean 33.3% improvement in hepatic steatosis (64). Thus, we are not able to differentiate the direct effect to SGLT2i and the indirect effect of weight loss from SGLT2i on the improvement in NAFLD.
Conclusion
In conclusion, SGLT2i treatment improves hepatic steatosis, body composition and adiponectin levels and to some extent liver fibrosis. SGLT2i could be considered as a potential treatment strategy among type 2 diabetic patients with NAFLD after further discussions with physicians, if there are no contraindications. More studies are needed to understand the direct and indirect effects of SGLT2i on the prognosis and mortality from NAFLD.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
CW: Literature Search, data extraction, data analysis and interpretation, drafting and critical revisions of the manuscript. CY: Literature search, data extraction, and data analysis and interpretation. CN: Study conception, literature search, methodology, data analysis and interpretation, critical revision of article and final approval. YC: Data analysis and interpretation, critical revision of article and final approval. YL: Drafting of article and critical revision of article. AL: Critical revision of the article and final approval. MM: Data interpretation, critical revision of the article and final approval. CK: Study conception, methodology, data interpretation, critical revision of article and final approval. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer KV declared a shared affiliation with the authors to the handling editor at time of review.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2020.609135/full#supplementary-material
References
1. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol (2018) 15(1):11–20. doi: 10.1038/nrgastro.2017.109
2. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology (2018) 67(1):328–57. doi: 10.1002/hep.29367
3. Marengo A, Jouness RI, Bugianesi E. Progression and Natural History of Nonalcoholic Fatty Liver Disease in Adults. Clin Liver Dis (2016) 20(2):313–24. doi: 10.1016/j.cld.2015.10.010
4. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology (2018) 67(1):123–33. doi: 10.1002/hep.29466
5. Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med (2005) 143(10):722–8. doi: 10.7326/0003-4819-143-10-200511150-00009
6. Williamson RM, Price JF, Glancy S, Perry E, Nee LD, Hayes PC, et al. Prevalence of and risk factors for hepatic steatosis and nonalcoholic Fatty liver disease in people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes Care (2011) 34(5):1139–44. doi: 10.2337/dc10-2229
7. Muthiah MD, Sanyal AJ. Current management of non-alcoholic steatohepatitis. Liver Int (2020) 40(S1):89–95. doi: 10.1111/liv.14355
8. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology (2016) 64(1):73–84. doi: 10.1002/hep.28431
9. Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol (2019) 71(4):793–801. doi: 10.1016/j.jhep.2019.06.021
10. McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol (2015) 62(5):1148–55. doi: 10.1016/j.jhep.2014.11.034
11. Arrese M. Nonalcoholic fatty liver disease: liver disease: an overlooked complication of diabetes mellitus. Nat Rev Endocrinol (2010) 6(12):660–1. doi: 10.1038/nrendo.2010.173
12. Chang Y, Jung HS, Yun KE, Cho J, Cho YK, Ryu S. Cohort study of non-alcoholic fatty liver disease, NAFLD fibrosis score, and the risk of incident diabetes in a Korean population. Am J Gastroenterol (2013) 108(12):1861–8. doi: 10.1038/ajg.2013.349
13. Chen SC, Tsai SP, Jhao JY, Jiang WK, Tsao CK, Chang LY. Liver Fat, Hepatic Enzymes, Alkaline Phosphatase and the Risk of Incident Type 2 Diabetes: A Prospective Study of 132,377 Adults. Sci Rep (2017) 7(1):4649. doi: 10.1038/s41598-017-04631-7
14. Hazlehurst JM, Woods C, Marjot T, Cobbold JF, Tomlinson JW. Non-alcoholic fatty liver disease and diabetes. Metabolism (2016) 65(8):1096–108. doi: 10.1016/j.metabol.2016.01.001
15. Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology (2016) 64(5):1577–86. doi: 10.1002/hep.28785
16. Noureddin M, Muthiah MD, Sanyal AJ. Drug discovery and treatment paradigms in nonalcoholic steatohepatitis. Endocrinol Diabetes Metab (2020) 3(4):e00105. doi: 10.1002/edm2.105
17. Takase T, Nakamura A, Miyoshi H, Yamamoto C, Atsumi T. Amelioration of fatty liver index in patients with type 2 diabetes on ipragliflozin: an association with glucose-lowering effects. Endocr J (2017) 64(3):363–7. doi: 10.1507/endocrj.EJ16-0295
18. Li B, Wang Y, Ye Z, Yang H, Cui X, Wang Z, et al. Effects of Canagliflozin on Fatty Liver Indexes in Patients with Type 2 Diabetes: A Meta-analysis of Randomized Controlled Trials. J Pharm Pharm Sci (2018) 21(1):222–35. doi: 10.18433/jpps29831
19. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (2009) 339:b2535. doi: 10.1136/bmj.b2535
20. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol (2005) 5(1):13. doi: 10.1186/1471-2288-5-13
21. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol (2014) 14(1):135. doi: 10.1186/1471-2288-14-135
22. Takeshima N, Sozu T, Tajika A, Ogawa Y, Hayasaka Y, Furukawa TA. Which is more generalizable, powerful and interpretable in meta-analyses, mean difference or standardized mean difference? BMC Med Res Methodol (2014) 14(1):30. doi: 10.1186/1471-2288-14-30
23. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (2011) 343:d5928. doi: 10.1136/bmj.d5928
24. Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. (2000). Availabe at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Accessed July 31, 2020].
25. Ito D, Shimizu S, Inoue K, Saito D, Yanagisawa M, Inukai K, et al. Comparison of Ipragliflozin and Pioglitazone Effects on Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes: A Randomized, 24-Week, Open-Label, Active-Controlled Trial. Diabetes Care (2017) 40(10):1364–72. doi: 10.2337/dc17-0518
26. Kuchay MS, Krishan S, Mishra SK, Farooqui KJ, Singh MK, Wasir JS, et al. Effect of Empagliflozin on Liver Fat in Patients With Type 2 Diabetes and Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial (E-LIFT Trial). Diabetes Care (2018) 41(8):1801–8. doi: 10.2337/dc18-0165
27. Shibuya T, Fushimi N, Kawai M, Yoshida Y, Hachiya H, Ito S, et al. Luseogliflozin improves liver fat deposition compared to metformin in type 2 diabetes patients with non-alcoholic fatty liver disease: A prospective randomized controlled pilot study. Diabetes Obes Metab (2018) 20(2):438–42. doi: 10.1111/dom.13061
28. Aso Y, Kato K, Sakurai S, Kishi H, Shimizu M, Jojima T, et al. Impact of dapagliflozin, an SGLT2 inhibitor, on serum levels of soluble dipeptidyl peptidase-4 in patients with type 2 diabetes and non-alcoholic fatty liver disease. Int J Clin Pract (2019) 73(5):e13335. doi: 10.1111/ijcp.13335
29. Shimizu M, Suzuki K, Kato K, Jojima T, Iijima T, Murohisa T, et al. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes Metab (2019) 21(2):285–92. doi: 10.1111/dom.13520
30. Han E, Lee Y-H, Lee B-W, Kang ES, Cha B-S. Ipragliflozin Additively Ameliorates Non-Alcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Controlled with Metformin and Pioglitazone: A 24-Week Randomized Controlled Trial. J Clin Med (2020) 9(1):259 p. doi: 10.3390/jcm9010259
31. Kinoshita T, Shimoda M, Nakashima K, Fushimi Y, Hirata Y, Tanabe A, et al. Comparison of the effects of three kinds of glucose-lowering drugs on non-alcoholic fatty liver disease in patients with type 2 diabetes: A randomized, open-label, three-arm, active control study. J Diabetes Invest (2020) 11(6):1612–22. doi: 10.1111/jdi.13279
32. Seko Y, Sumida Y, Tanaka S, Mori K, Taketani H, Ishiba H, et al. Effect of sodium glucose cotransporter 2 inhibitor on liver function tests in Japanese patients with non-alcoholic fatty liver disease and type 2 diabetes mellitus. Hepatol Res (2017) 47(10):1072–8. doi: 10.1111/hepr.12834
33. Choi DH, Jung CH, Mok JO, Kim CH, Kang SK, Kim BY. Effect of Dapagliflozin on Alanine Aminotransferase Improvement in Type 2 Diabetes Mellitus with Non-alcoholic Fatty Liver Disease. Endocrinol Metab (Seoul) (2018) 33(3):387–94. doi: 10.3803/enm.2018.33.3.387
34. Yano K, Seko Y, Takahashi A, Okishio S, Kataoka S, Takemura M, et al. Effect of Sodium Glucose Cotransporter 2 Inhibitors on Renal Function in Patients with Nonalcoholic Fatty Liver Disease and Type 2 Diabetes in Japan. Diagn (Basel) (2020) 10(2):86. doi: 10.3390/diagnostics10020086
35. Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, et al. Long-Term Pioglitazone Treatment for Patients With Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus: A Randomized Trial. Ann Intern Med (2016) 165(5):305–15. doi: 10.7326/m15-1774
36. Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. or Placebo for Nonalcoholic Steatohepatitis. N Engl J Med (2010) 362(18):1675–85. doi: 10.1056/NEJMoa0907929
37. Inoue M, Hayashi A, Taguchi T, Arai R, Sasaki S, Takano K, et al. Effects of canagliflozin on body composition and hepatic fat content in type 2 diabetes patients with non-alcoholic fatty liver disease. J Diabetes Invest (2019) 10(4):1004–11. doi: 10.1111/jdi.12980
38. Itani T, Ishihara T. Efficacy of canagliflozin against nonalcoholic fatty liver disease: a prospective cohort study. Obes Sci Pract (2018) 4(5):477–82. doi: 10.1002/osp4.294
39. Chrysavgis L, Papatheodoridi A-M, Chatzigeorgiou A, Cholongitas E. The impact of Sodium Glucose co-transporter 2 inhibitors on non-alcoholic fatty liver disease. J Gastroenterol Hepatol (2020) In Press. doi: 10.1111/jgh.15202
40. Puri P, Sanyal AJ. Nonalcoholic fatty liver disease: Definitions, risk factors, and workup. Clin Liver Dis (Hoboken) (2012) 1(4):99–103. doi: 10.1002/cld.81
41. Patel K, Sebastiani G. Limitations of non-invasive tests for assessment of liver fibrosis. JHEP Rep (2020) 2(2):100067. doi: 10.1016/j.jhepr.2020.100067
42. Karanjia RN, Crossey MME, Cox IJ, Fye HKS, Njie R, Goldin RD, et al. Hepatic steatosis and fibrosis: Non-invasive assessment. World J Gastroenterol (2016) 22(45):9880–97. doi: 10.3748/wjg.v22.i45.9880
43. Obika M, Noguchi H. Diagnosis and Evaluation of Nonalcoholic Fatty Liver Disease. Exp Diabetes Res (2012) 2012:145754. doi: 10.1155/2012/145754
44. Mak KM, Mei R. Basement Membrane Type IV Collagen and Laminin: An Overview of Their Biology and Value as Fibrosis Biomarkers of Liver Disease. Anat Rec (Hoboken) (2017) 300(8):1371–90. doi: 10.1002/ar.23567
45. Basu D, Huggins L-A, Scerbo D, Obunike J, Mullick AE, Rothenberg PL, et al. Mechanism of Increased LDL (Low-Density Lipoprotein) and Decreased Triglycerides With SGLT2 (Sodium-Glucose Cotransporter 2) Inhibition. Arterioscler Thromb Vasc Biol (2018) 38(9):2207–16. doi: 10.1161/ATVBAHA.118.311339
46. Thomas MC, Cherney DZI. The actions of SGLT2 inhibitors on metabolism, renal function and blood pressure. Diabetologia (2018) 61(10):2098–107. doi: 10.1007/s00125-018-4669-0
47. Giannarelli R, Aragona M, Coppelli A, Del Prato S. Reducing insulin resistance with metformin: the evidence today. Diabetes Metab (2003) 29(4, Part 2):6S28–35. doi: 10.1016/S1262-3636(03)72785-2
48. Li Y, Liu L, Wang B, Wang J, Chen D. Metformin in non-alcoholic fatty liver disease: A systematic review and meta−analysis. BioMed Rep (2013) 1(1):57–64. doi: 10.3892/br.2012.18
49. Qaid MM, Abdelrahman MM. Role of insulin and other related hormones in energy metabolism—A review. Cogent Food Agric (2016) 2(1):1267691. doi: 10.1080/23311932.2016.1267691
50. Juurinen L, Tiikkainen M, Häkkinen A-M, Hakkarainen A, Yki-Järvinen H. Effects of insulin therapy on liver fat content and hepatic insulin sensitivity in patients with type 2 diabetes. Am J Physiol Endocrinol Metab (2007) 292(3):E829–E35. doi: 10.1152/ajpendo.00133.2006
51. Matsuzaka T, Shimano H. Molecular mechanisms involved in hepatic steatosis and insulin resistance. J Diabetes Invest (2011) 2(3):170–5. doi: 10.1111/j.2040-1124.2011.00111.x
52. Sumida Y, Yoneda M, Hyogo H, Yamaguchi K, Ono M, Fujii H, et al. A simple clinical scoring system using ferritin, fasting insulin, and type IV collagen 7S for predicting steatohepatitis in nonalcoholic fatty liver disease. J Gastroenterol (2011) 46(2):257–68. doi: 10.1007/s00535-010-0305-6
53. Takeshita Y, Kanamori T, Tanaka T, Kaikoi Y, Kita Y, Takata N, et al. Study Protocol for Pleiotropic Effects and Safety of Sodium–Glucose Cotransporter 2 Inhibitor Versus Sulfonylurea in Patients with Type 2 Diabetes and Nonalcoholic Fatty Liver Disease. Diabetes Ther (2020) 11(2):549–60. doi: 10.1007/s13300-020-00762-9
54. Muthiah MD, Sanyal AJ. Burden of Disease due to Nonalcoholic Fatty Liver Disease. Gastroenterol Clin North Am (2020) 49(1):1–23. doi: 10.1016/j.gtc.2019.09.007
55. McGill MR. The past and present of serum aminotransferases and the future of liver injury biomarkers. EXCLI J (2016) 15:817–28. doi: 10.17179/excli2016-800
56. Pereira MJ, Eriksson JW. Emerging Role of SGLT-2 Inhibitors for the Treatment of Obesity. Drugs (2019) 79(3):219–30. doi: 10.1007/s40265-019-1057-0
57. Koutoukidis DA, Astbury NM, Tudor KE, Morris E, Henry JA, Noreik M, et al. Association of Weight Loss Interventions With Changes in Biomarkers of Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. JAMA Intern Med (2019) 179(9):1262–71. doi: 10.1001/jamainternmed.2019.2248
58. Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, et al. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology (2015) 149(2):367–78.e5; quiz e14-5. doi: 10.1053/j.gastro.2015.04.005
59. Kelly KR, Navaneethan SD, Solomon TPJ, Haus JM, Cook M, Barkoukis H, et al. Lifestyle-induced decrease in fat mass improves adiponectin secretion in obese adults. Med Sci Sports Exerc (2014) 46(5):920–6. doi: 10.1249/MSS.0000000000000200
60. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet (2019) 393(10166):31–9. doi: 10.1016/s0140-6736(18)32590-x
61. Siddiqui MS, Harrison SA, Abdelmalek MF, Anstee QM, Bedossa P, Castera L, et al. Case definitions for inclusion and analysis of endpoints in clinical trials for nonalcoholic steatohepatitis through the lens of regulatory science. Hepatology (2018) 67(5):2001–12. doi: 10.1002/hep.29607
62. Food and Drug Administration. Noncirrhotic Nonalcoholic Steatohepatitis With Liver Fibrosis: Developing Drugs for Treatment. (2018). Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/noncirrhotic-nonalcoholic-steatohepatitis-liver-fibrosis-developing-drugs-treatment [Accessed August 20, 2020].
63. Ribola FA, Cançado FB, Schoueri JH, De Toni VF, Medeiros VH, Feder D. Effects of SGLT2 inhibitors on weight loss in patients with type 2 diabetes mellitus. Eur Rev Med Pharmacol Sci (2017) 21(1):199–211. PMID: 28121337
Keywords: hepatic fat, non-alcoholic fatty liver disease, sodium-glucose co-transporter-2 inhibitors, type 2 diabetes, meta-analysis
Citation: Wong C, Yaow CYL, Ng CH, Chin YH, Low YF, Lim AYL, Muthiah MD and Khoo CM (2021) Sodium-Glucose Co-Transporter 2 Inhibitors for Non-Alcoholic Fatty Liver Disease in Asian Patients With Type 2 Diabetes: A Meta-Analysis. Front. Endocrinol. 11:609135. doi: 10.3389/fendo.2020.609135
Received: 22 September 2020; Accepted: 14 December 2020;
Published: 11 February 2021.
Edited by:
Charumathi Sabanayagam, Singapore Eye Research Institute (SERI), SingaporeReviewed by:
Hidetaka Hamasaki, Hamasaki Clinic, JapanKavita Venkataraman, National University of Singapore, Singapore
Copyright © 2021 Wong, Yaow, Ng, Chin, Low, Lim, Muthiah and Khoo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chin Meng Khoo, bWRja2NtQG51cy5lZHUuc2c=
†These authors have contributed equally to this work
 Chloe Wong1†
Chloe Wong1† Clyve Yu Leon Yaow
Clyve Yu Leon Yaow Cheng Han Ng
Cheng Han Ng Yip Han Chin
Yip Han Chin Amanda Yuan Ling Lim
Amanda Yuan Ling Lim Chin Meng Khoo
Chin Meng Khoo