- 1Department of Medical Research, Kuang Tien General Hospital, Taichung, Taiwan
- 2Department of Endocrinology and Metabolism, Kuang Tien General Hospital, Taichung, Taiwan
- 3Department of Nutrition and Institute of Biomedical Nutrition, Hung Kuang University, Taichung, Taiwan
- 4Department of Pathology, School of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
- 5Department of Pathology, Kaohsiung Municipal Ta-Tung Hospital, Kaohsiung, Taiwan
- 6Department of Obstetrics and Gynecology, Ditmanson Medical Foundation Chia-Yi Christian Hospital, Chiayi, Taiwan
- 7Department of Pathology and Laboratory Medicine, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan
- 8Division of Endocrinology and Metabolism, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung, Taiwan
- 9Department of Medicine, School of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan
- 10National Chung Hsing University, Taichung, Taiwan
- 11Department of Post-Baccalaureate Medicine, College of Medicine, National Chung Hsing University, Taichung, Taiwan
Introduction: We investigated the associations of exposure to particulate matter with an aerodynamic diameter less than 2.5 μm (PM2.5) and several gaseous pollutants with risk of gestational diabetes mellitus (GDM) in Taiwan.
Methods: We retrospectively identified pregnant women who underwent a two-step approach to screen for GDM between 2006 and 2014. Information on concentrations of air pollutants (including PM2.5, sulfur dioxide [SO2], nitrogen oxides [NOx], and ozone [O3]) were collected from a single fixed-site monitoring station. We conducted logistic regression analyses to determine the associations between exposure to air pollutants and risk of GDM.
Results: A total of 11210 women were analyzed, and 705 were diagnosed with GDM. Exposure to PM2.5 during the second trimester was associated with a nearly 50% higher risk of GDM (odds ratio [OR] 1.47, 95% CI 0.96 to 2.24, p=0.077). The associations were consistent in the two-pollutant model (PM2.5 + SO2 [OR 1.73, p=0.038], PM2.5 + NOx [OR 1.52, p=0.064], PM2.5 + O3 [OR 1.96, p=0.015]), and were more prominent in women with age <30 years and body mass index <25 kg/m2 (interaction p values <0.01).
Discussion: Exposure to PM2.5 was associated with risk of GDM, especially in women who were younger or had a normal body mass index.
Introduction
Air pollution is a serious global problem that increases disease burden and shortens life expectancy, especially in low- and middle-income countries (LMIC) (1–3). Global mortality attributable to exposure to particulate matter with an aerodynamic diameter less than 2.5 μm (PM2.5) increased by 20% from 1990 to 2015, accounting for 7.6% of total deaths in 2015 (2). Moreover, exposure to air pollutants has been associated with subclinical inflammation and insulin resistance (4). This may explain the association between exposure to PM2.5 and risk of incident diabetes (5).
As air pollution is a critical environmental issue in east and south Asia (2, 6), the number of people with incident diabetes and hyperglycemia in pregnancy is continuously increasing in these regions (7–10). The prevalence of diabetes in people aged 20-79 years significantly increased from 7.15% in 2005 to 10.10% in 2014 (p<0.001) in Taiwan (10). The numbers for incidence of diabetes were 11.9 per 10000 persons and 14.5 per 10000 persons (p<0.001), respectively (10). Similarly, the prevalence of GDM in Taiwan significantly increased from 7.6% in 2004 to 13.4% in 2015 (9). Physiologic insulin resistance could be noted in normal pregnancy, and gestational diabetes mellitus (GDM) may develop due to inadequate adaptation of insulin secretion (11, 12). In this context, GDM is often diagnosed in women with risk factors of abnormal glucose regulation.
The Diabetes Association of the Republic of China (DAROC) (http://www.endo-dm.org.tw/dia/) recommends universal screening for GDM for pregnant women with no history of diabetes at gestational week 24-28 using either one-step or two-step approach (13–15). Since chronic exposure to air pollutants may induce insulin resistance (4), exposure to PM2.5 has been associated with risk of GDM in a number of studies (16–18). However, there are inconsistent findings (19, 20) and data from east and south Asian countries are limited (13, 21). The Chiayi City is a mid-sized city located on the plains of southwestern Taiwan at a height of 69 m above sea level. Concentrations of air pollutants collected from a fixed-site monitoring station in this city could be representative of the residents’ ambient exposure to air pollutants. In this study, we aimed to investigate the associations of exposures to PM2.5 and several gaseous pollutants with risk of GDM in a group of pregnant women who underwent standard screening procedures in a hospital in the Chiayi City in Taiwan.
Materials and methods
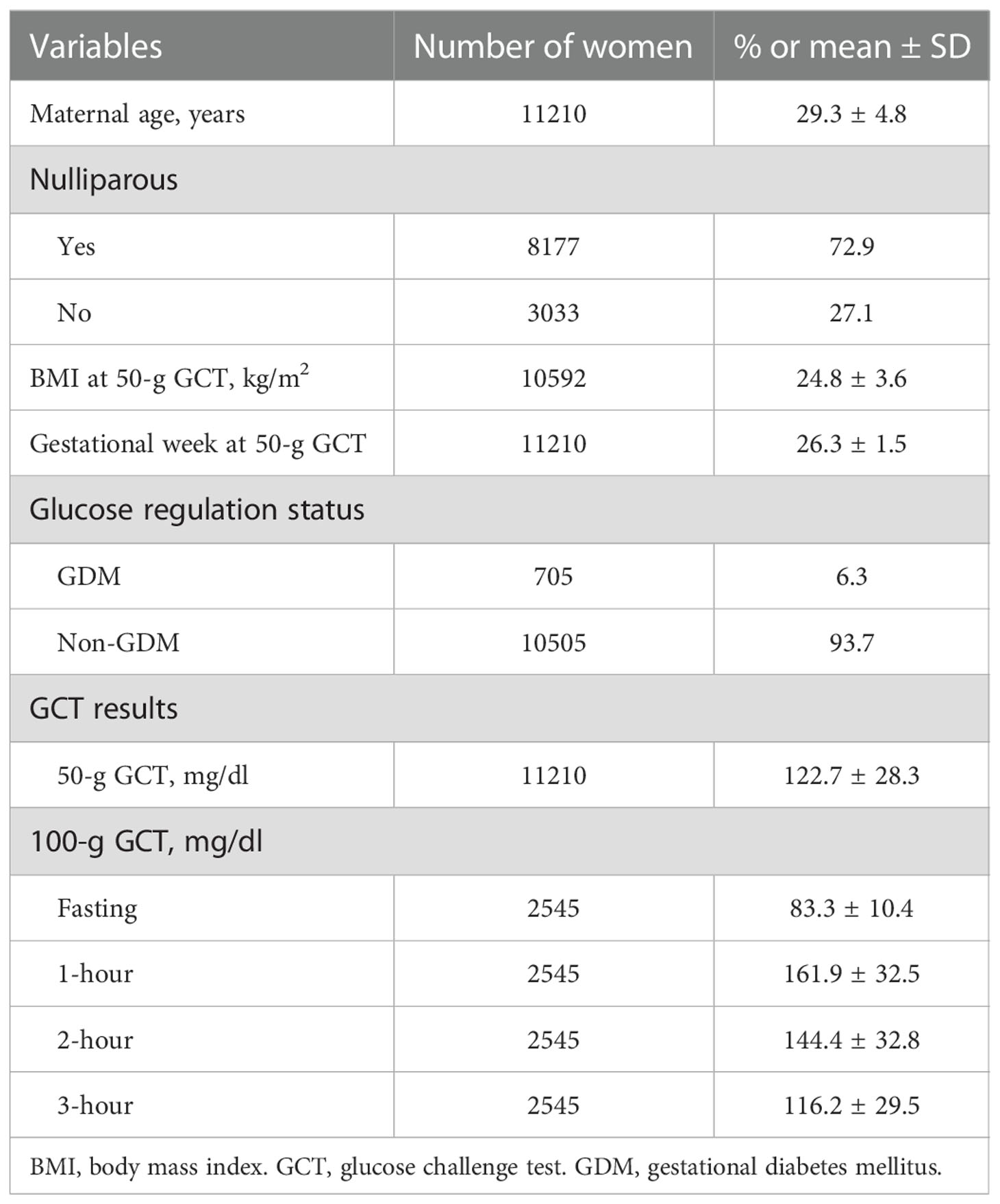
This study was conducted in accordance with the Declaration of Helsinki, and was approved (approval number: CYCH IRB No. 100006) by the Institutional Review Board of Ditmanson Medical Foundation Chia-Yi Christian Hospital. Informed consent was waived due to the retrospective study design and de-identification of data used in the analyses. We retrospectively identified pregnant women with no history of diabetes who underwent a two-step approach to screen for GDM (14, 15) at the Department of Obstetrics and Gynecology in the Ditmanson Medical Foundation Chia-Yi Christian Hospital between 2006 and 2014. Information on nulliparous/parous and body mass index (BMI) was collected from the electronic medical records.
All women underwent a 50-g glucose challenge test (GCT) at 24-28 weeks of gestation (Figure 1) (14, 15, 22). If the 1-hour plasma glucose after the 50-g GCT was ≥ 140 mg/dl, the women undertook a 100-g oral glucose tolerance test (OGTT) (23) before 30 weeks of gestation. The 100-g OGTT was conducted in the morning after an overnight fast at an outpatient clinic. Plasma glucose levels were examined at 4 time points (fasting, and 1 hour, 2 hours, and 3 hours after the 100-g OGTT). GDM was diagnosed if there were two or more of the plasma glucose levels higher than the cutoff values (≥ 95, 180, 155, and 140 mg/dl, respectively) based on international criteria (14, 15, 22, 23). All venous plasma glucose levels were determined using the hexokinase-G6PDH method with a Hitachi 7170 automatic analyzer (Hitachi Co., Tokyo, Japan).
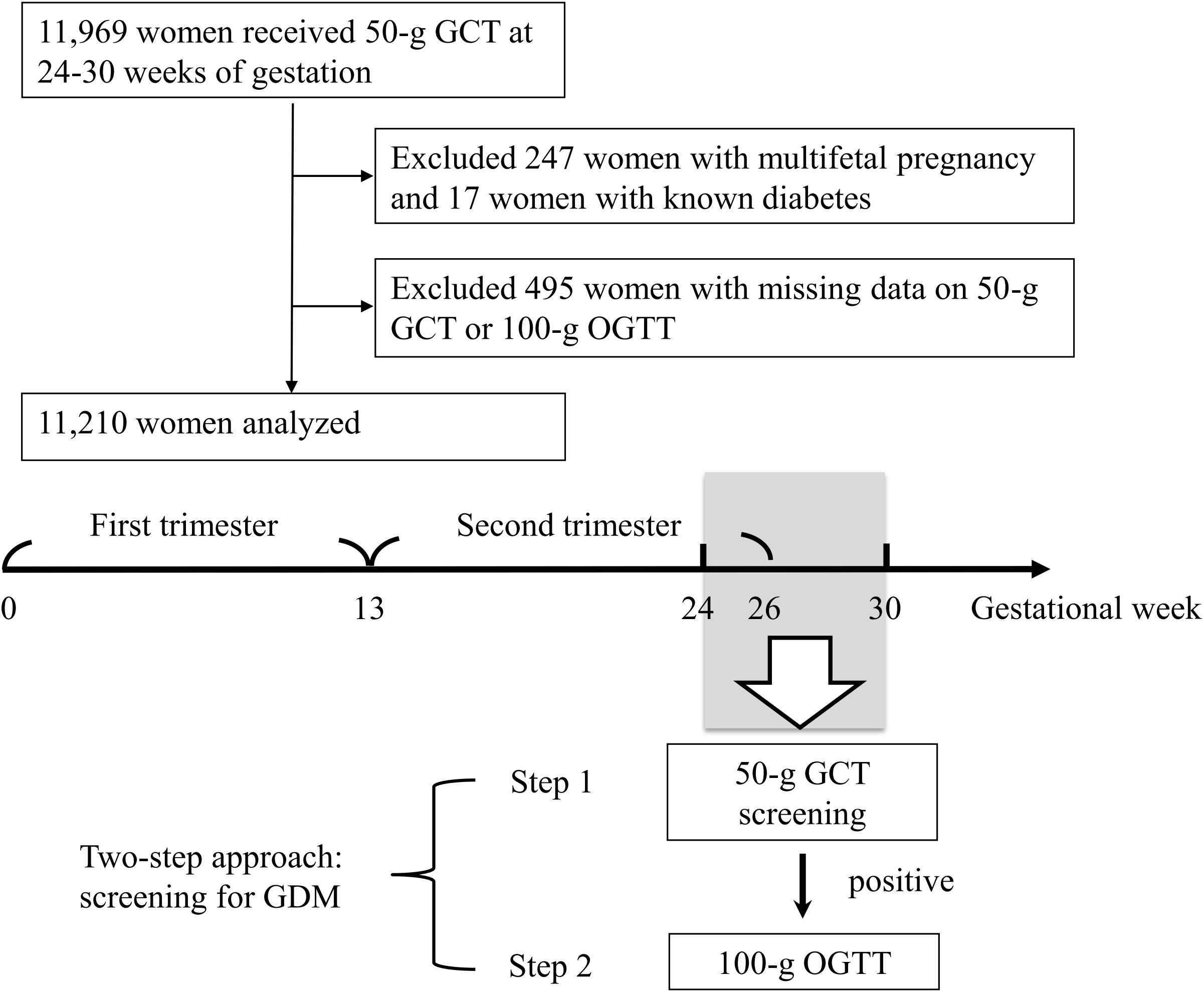
Figure 1 Enrollment of the study population. GCT, glucose challenge test. GDM, gestational diabetes mellitus. OGTT, oral glucose tolerance test.
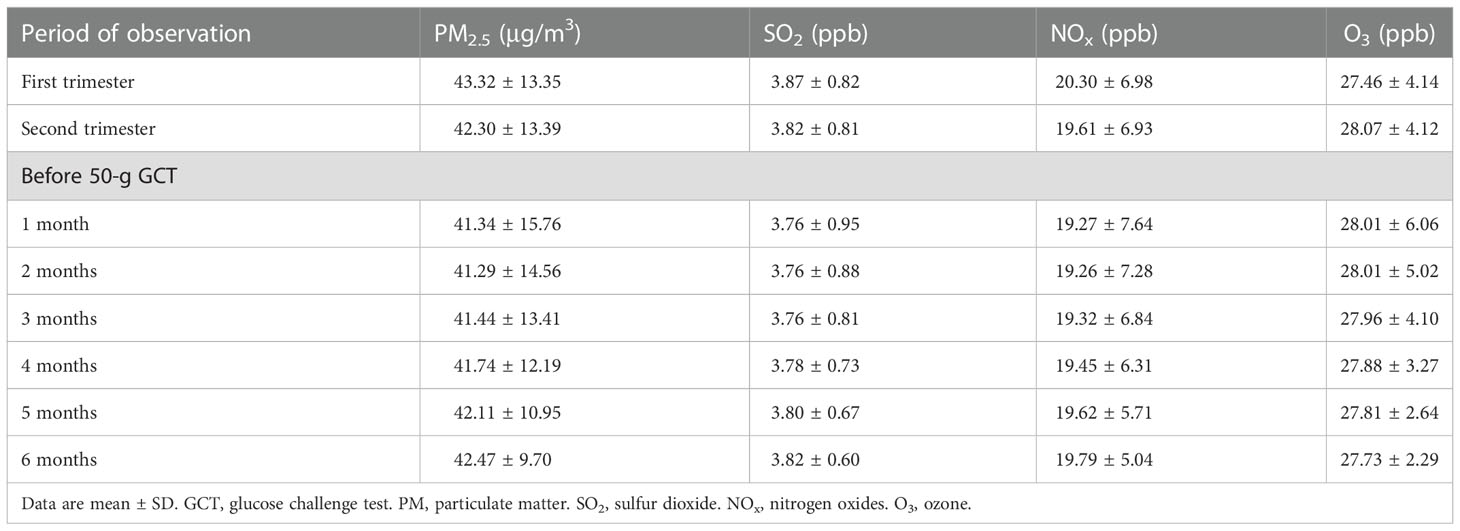
Information on concentrations of air pollutants was collected from a single fixed-site monitoring station (Chiayi City station), and the temporal distribution of exposure was used for analyses. All study participants were residents of Chiayi City. The city is located on the plains of southwestern Taiwan at a height of 69 m above sea level (https://twinfo.ncl.edu.tw/tiqry/hypage.cgi?HYPAGE=search/search_res.hpg&dtd_id=4&g=0&sysid=00000014). The east-west and north-south diameters of the city measure 15.8 and 10.5 km, respectively. Hence, the data from the Chiayi City station were considered representative of the residents’ ambient exposure to air pollutants. Concentrations of air pollutants (including PM2.5, sulfur dioxide [SO2], nitrogen oxides [NOx], and ozone [O3]) and meteorological measurements (temperature and humidity) were collected (https://airtw.epa.gov.tw/ENG/default.aspx). We calculated the daily average concentrations of air pollutants for analyses (24, 25). To examine the associations between exposure to air pollutants at various periods and risk of GDM, the mean concentrations of air pollutants during the first (1-13 gestational weeks) and the second (14-26 gestational weeks) trimester were determined. We also calculated different time windows of exposure to air pollutants (1-6 months) before the 50-g GCT.
All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). A two-sided p value of <0.05 was considered statistically significant. We conducted logistic regression analyses to determine the associations between exposure to air pollutants (PM2.5, SO2, NOx, and O3) and risk of GDM. The models were adjusted for age, nulliparous/parous, BMI, season/year, and the moving averages of humidity and temperature. We also investigated the associations between exposure to two pollutants (PM2.5 + SO2, PM2.5 + NOx, and PM2.5 + O3) and risk of GDM. Last, the aforementioned associations were examined in various subgroups (age <30 vs. ≥ 30 years, nulliparous yes vs. no, BMI <25 vs. ≥ 25 kg/m2).
Results
A total of 11210 women were analyzed (mean age 29.3 ± 4.8 years, mean BMI 24.8 ± 3.6 kg/m2, 72.9% nulliparous, Table 1). All women underwent a 50-g GCT at 24-28 weeks of gestation, and 2545 of them had a 1-hour plasma glucose ≥ 140 mg/dl. A 100-g OGTT was conducted for these women before 30 weeks of gestation, and 705 were diagnosed with GDM. The mean concentrations of air pollutants during the first trimester, the second trimester, and 1-6 months before the 50-g GCT are shown in Table 2.
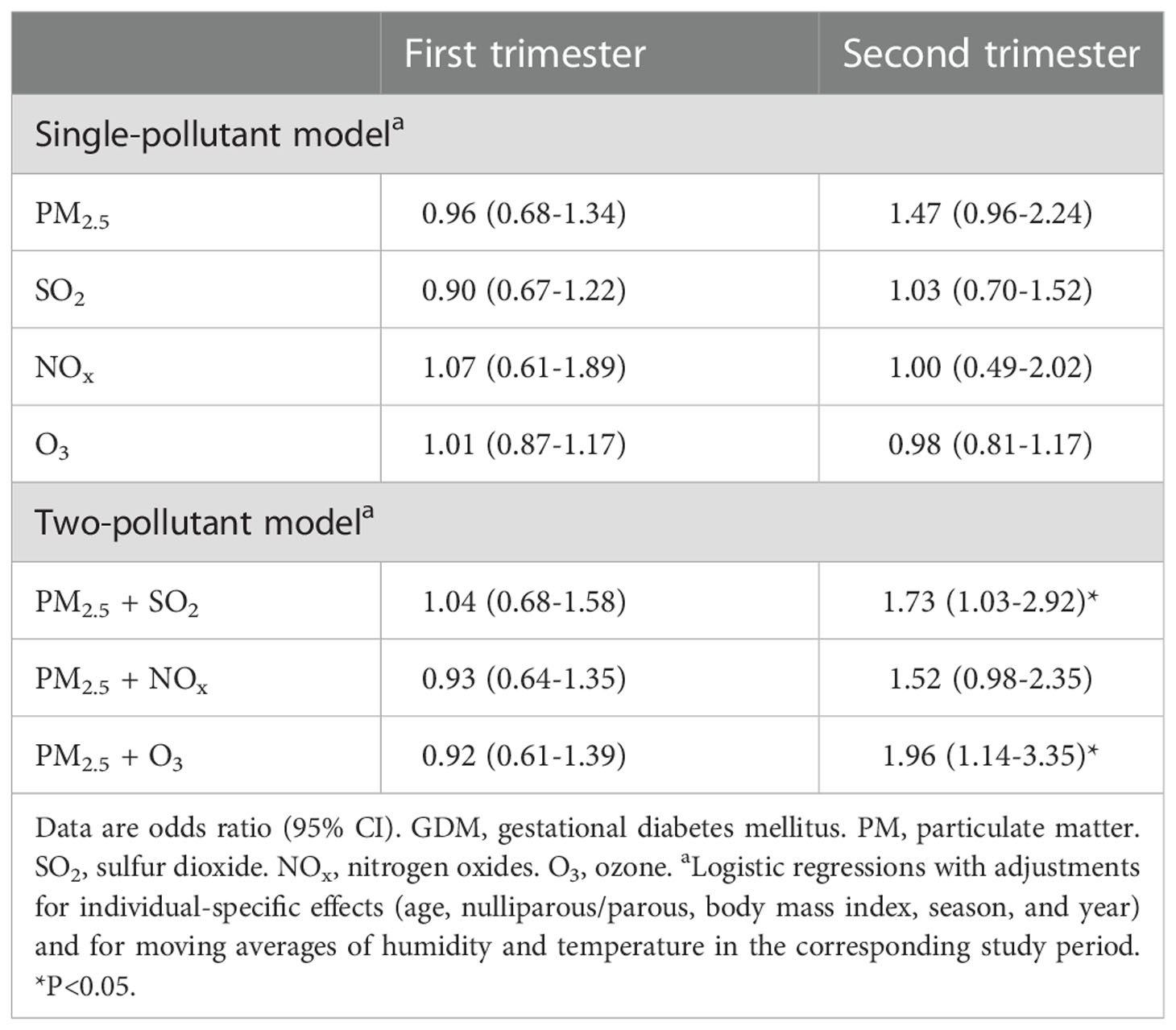
Table 3 shows the associations between mean concentrations of air pollutants during the first and second trimester and diagnosis of GDM. Exposure to air pollutants during the first trimester was not associated with risk of GDM. In contrast, exposure to PM2.5 during the second trimester was associated with a nearly 50% higher risk of GDM (odds ratio 1.47, 95% CI 0.96 to 2.24, p=0.077). The associations were consistent in the two-pollutant model (PM2.5 + SO2 [odds ratio 1.73, 95% CI 1.03 to 2.92, p=0.038], PM2.5 + NOx [odds ratio 1.52, 95% CI 0.98 to 2.35, p=0.064], PM2.5 + O3 [odds ratio 1.96, 95% CI 1.14 to 3.35, p=0.015]).
Figure 2 shows the associations between exposure to air pollutants during the second trimester and GDM among various subgroups (age <30 vs. ≥ 30 years, nulliparous yes vs. no, BMI <25 vs. ≥ 25 kg/m2). The associations were more prominent in women with age <30 years, nulliparous, and BMI <25 kg/m2. Interaction p values were <0.01 for the age and BMI subgroups.
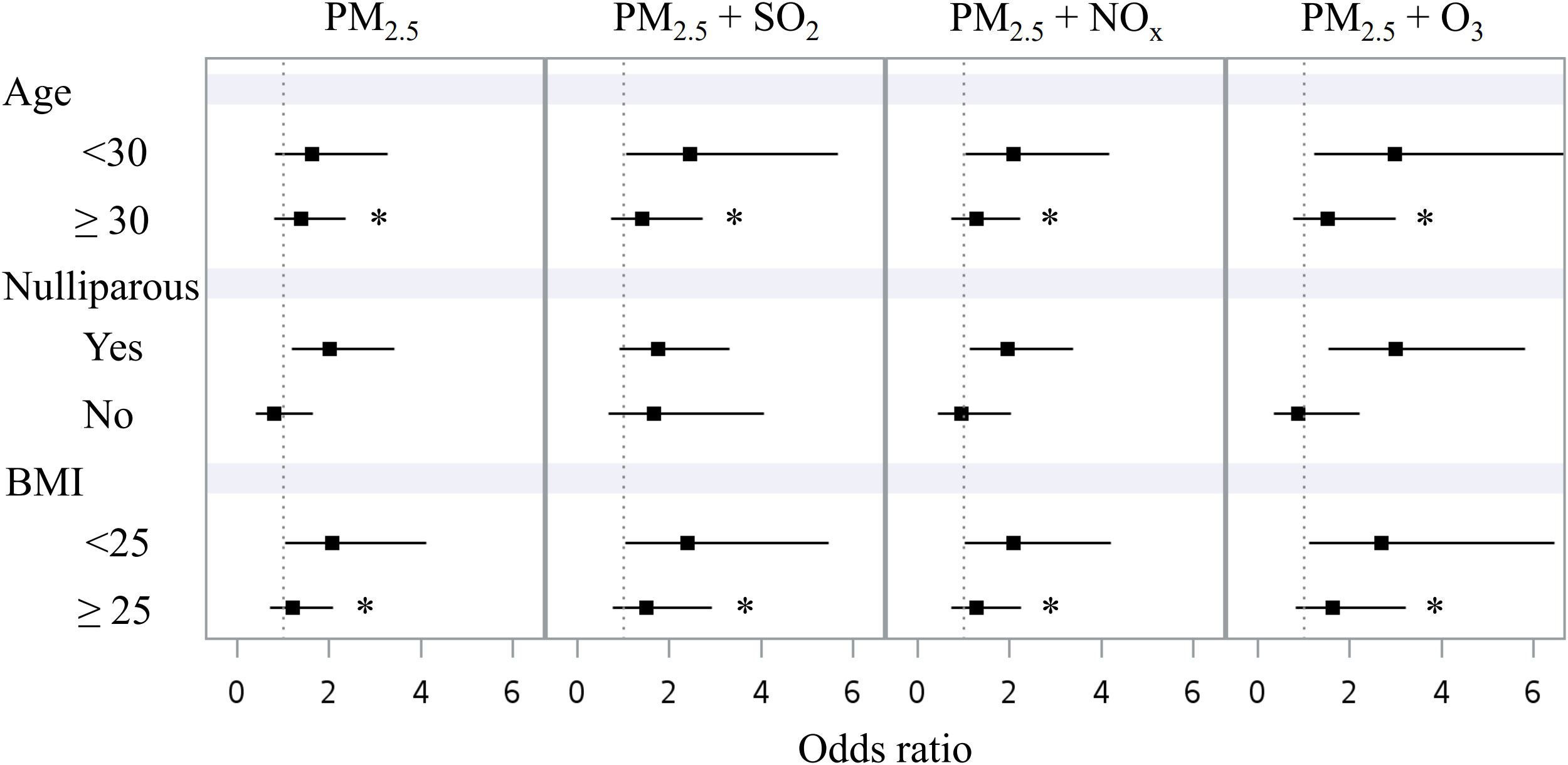
Figure 2 Forest plot displaying the associations between mean concentrations of air pollutants in the second trimester and GDM in different subgroups: age (<30 vs. ≥ 30 years), nulliparous (yes vs. no), and BMI (<25 vs. ≥ 25 kg/m2). BMI, body mass index. GDM, gestational diabetes mellitus. PM2.5, particulate matter with an aerodynamic diameter less than 2.5 μm. SO2, sulfur dioxide. NOx, nitrogen oxides. O3, ozone. *p interaction <0.01.
Table 4 shows the associations between exposure to air pollutants 1-6 months before the 50-g GCT and GDM. There were no significant associations when exposure to PM2.5 for 1-3 months prior to the 50-g GCT was examined. However, we observed significant associations between exposure to PM2.5 for 4-6 months prior to the 50-g GCT and GDM. There were no significant associations between exposure to SO2, NOx, O3, and GDM in this model.
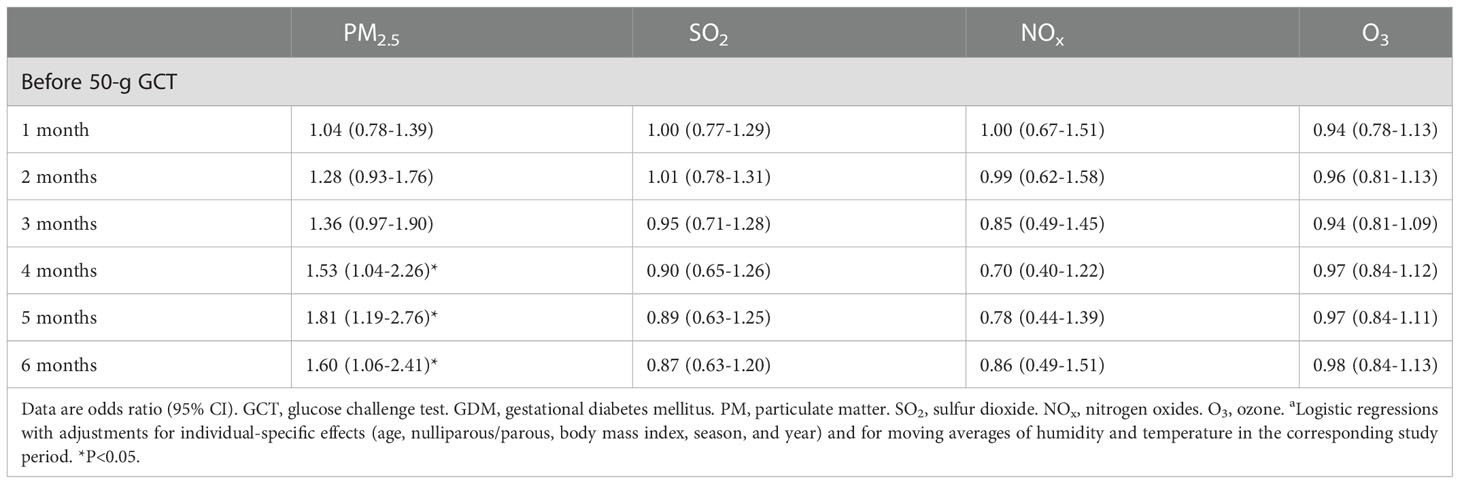
Table 4 Associations between mean concentrations of air pollutants during a defined period and GDMa.
Discussion
In this study, we demonstrated that exposure to PM2.5 was associated with risk of GDM in women with no history of diabetes. The association was significant when considering the exposure in the second trimester (especially in the two-pollutant model, Table 3) or for 4-6 months before the 50-g GCT (Table 4). In the subgroup analyses, an association was observed in women who had an age <30 years or a BMI <25 kg/m2 (Figure 2). Our findings suggest that exposure to PM2.5 was associated with risk of GDM, especially in women who were younger or had a normal body mass index. These results may have implications for the healthcare of patients with GDM in regions with serious air pollution (2, 6) and a high prevalence of hyperglycemia in pregnancy (7–9).
Exposure to air pollutants has been associated with risk of GDM, although there are inconsistent findings. In a recent study (26), PM2.5 exposure during pregnancy was associated with a higher risk of GDM in a large US cohort. In contrast, no association was noted between PM2.5 exposure in the first or second trimester and risk of GDM in another US cohort (20). Differences in the methods used to diagnose GDM, investigations of various gaseous pollutants, and different covariates adjusted in the analytic models may partly explain the inconsistent results in previous studies (16–20, 26–29). All study participants were screened for GDM using the standard “two-step approach” (14, 15) in this study, and our findings are consistent with a recent meta-analysis (18) in which an association between the second trimester exposure to PM2.5 and risk of GDM was observed.
Air pollution is a serious issue in east and south Asia (2, 6) where the prevalence of GDM is high (7–9). Investigations on the associations between exposure to air pollutants and GDM in these regions are important, but relevant data are limited (13, 21). The level of PM2.5 in a study conducted in the US (20) was around 10-11 μg/m3. In a previous study in Taiwan (21) based on data from all 76 fixed-site air quality monitoring station, the level was around 30 μg/m3. The authors reported a significant association between PM2.5 exposure and risk of GDM. In this study, the mean level of PM2.5 was around 41-43 μg/m3 (Table 2). Using the same screening approach, the rate of GDM in the study conducted in the US (20) was 3.4% (vs. 6.3% in our cohort). We suggest that exposure to air pollutants may contribute to the high prevalence of abnormal glucose regulation during pregnancy in east and south Asia (7–9, 13, 21). Our findings were consistent with recent studies in China (30–32). In these studies, the median level of PM2.5 was around 70-80 μg/m3 (30, 31).
The significant associations between the two-pollutant models and GDM (Table 3) are not surprising, since the gaseous pollutants (SO2, NOx, and O3) have been associated with abnormal glucose regulation in pregnancy (33–39). It is interesting to note that the association was mainly observed in women with an age <30 years or a BMI <25 kg/m2 (Figure 2). Insulin resistance associated with weight gain during pregnancy may contribute to the development of abnormal glucose regulation (11, 12). Furthermore, exposure to PM2.5 has been shown to exaggerate insulin resistance in animal models (40, 41). This effect might be more prominent in people at a relatively low insulin resistance state (younger or leaner), which merits further investigations.
This study has several limitations. First, the causality of exposure to air pollutants and GDM cannot be confirmed with a retrospective study design. Second, there were considerable variations in the interactions between the environment and human subjects (42). The bias in the quantification of human exposure to air pollutants might have confounded our results. Nevertheless, monitoring individual exposure to air pollution in a large cohort is difficult in a real-world setting. Our cohort was selected from a single medical center and all of the study participants resided in Chiayi City (a mid-sized city located on the plains of southwestern Taiwan). In this context, using data from the Chiayi City station to estimate exposure to ambient air pollutants may have minimized the aforementioned bias. Final, we acknowledge that some factors may influence the effects of exposure to air pollutants on glucose regulation, such as dietary, genetic, and occupational factors (43–45). These factors were not examined in this study, and should be considered when interpreting our results.
Conclusion
In summary, we demonstrated that exposure to PM2.5 was associated with risk of GDM, especially in women who were younger or had a normal body mass index. Our results are consistent with previous studies conducted in regions with high levels of PM2.5 (30–32). The differences in associations among subgroups require further investigations.
The aforementioned findings may have implications for the healthcare of patients with GDM in east and south Asia where the air pollution is serious. Universal screening for GDM in pregnant women with no history of diabetes may be required. National policy and efforts to improve air quality might help reduce the prevalence of abnormal glucose regulation in these regions.
Data availability statement
The datasets presented in this article are not readily available because of privacy/ethical restrictions. Requests to access the datasets should be directed to Jun-SW, anN3YW5nQHZnaHRjLmdvdi50dw==.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of Ditmanson Medical Foundation Chia-Yi Christian Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Y-HY, C-CC, PW, and M-CL contributed to conception and design of the study. Y-HY, Y-CW, Jyh-SW, and Jun-SW organized the database. Y-HY and M-CL performed the statistical analysis. Y-HY, C-CC, and Jun-SW wrote the first draft of the manuscript. PW, M-CL, Y-CW, and Jyh-SW reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Kuang Tien General Hospital (KTGH) Research Funding [grant number KTGH106-1, 2017; grant number KTGH107-2, 2018]. The sponsors had no role in the design, execution, interpretation, or writing of the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: A systematic analysis for the global burden of disease study 2015. Lancet (2016) 388:1659–724. doi: 10.1016/S0140-6736(16)31679-8
2. Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the global burden of diseases study 2015. Lancet (2017) 389:1907–18. doi: 10.1016/S0140-6736(17)30505-6
3. Liu C, Chen R, Sera F, Vicedo-Cabrera AM, Guo Y, Tong S, et al. Ambient particulate air pollution and daily mortality in 652 cities. N Engl J Med (2019) 381:705–15. doi: 10.1056/NEJMoa1817364
4. Wolf K, Popp A, Schneider A, Breitner S, Hampel R, Rathmann W, et al. KORA-study group. association between long-term exposure to air pollution and biomarkers related to insulin resistance, subclinical inflammation, and adipokines. Diabetes (2016) 65:3314–26. doi: 10.2337/db15-1567
5. Bowe B, Xie Y, Li T, Yan Y, Xian H, Al-Aly Z. The 2016 global and national burden of diabetes mellitus attributable to PM2·5 air pollution. Lancet Planet Health (2018) 2:e301–12. doi: 10.1016/S2542-5196(18)30140-2
6. World Health Organization: World Health Assembly . Available at: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (Accessed May 23, 2022).
7. International Diabetes Federation. IDF diabetes atlas (2021). Available at: https://diabetesatlas.org/atlas/tenth-edition/ (Accessed May 23, 2022).
8. Guariguata L, Linnenkamp U, Beagley J, Whiting DR, Cho NH. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res Clin Pract (2014) 103:176–85. doi: 10.1016/j.diabres.2013.11.003
9. Su FL, Lu MC, Yu SC, Yang CP, Yang CC, Tseng ST, et al. Increasing trend in the prevalence of gestational diabetes mellitus in Taiwan. J Diabetes Investig (2021) 12:2080–8. doi: 10.1111/jdi.13595
10. Sheen YJ, Hsu CC, Jiang YD, Huang CN, Liu JS, Sheu WH. Trends in prevalence and incidence of diabetes mellitus from 2005 to 2014 in Taiwan. J Formos Med Assoc (2019) 118 Suppl 2:S66–73. doi: 10.1016/j.jfma.2019.06.016
11. Kühl C. Insulin secretion and insulin resistance in pregnancy and GDM. implications for diagnosis and management. Diabetes (1991) 40 Suppl 2:18–24. doi: 10.2337/diab.40.2.s18
12. Barbour LA, McCurdy CE, Hernandez TL, Kirwan JP, Catalano PM, Friedman JE. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care (2007) 30 Suppl 2:S112–9. doi: 10.2337/dc07-s202
13. Lu MC, Wang P, Cheng TJ, Yang CP, Yan YH. Association of temporal distribution of fine particulate matter with glucose homeostasis during pregnancy in women of chiayi city, Taiwan. Environ Res (2017) 152:81–7. doi: 10.1016/j.envres.2016.09.023
14. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol (1982) 144:768–73. doi: 10.1016/0002-9378(82)90349-0
15. Hillier TA, Ogasawara KK, Pedula KL, Vesco KK. Markedly different rates of incident insulin treatment based on universal gestational diabetes mellitus screening in a diverse HMO population. Am J Obstet Gynecol (2013) 209:e1–9:440. doi: 10.1016/j.ajog.2013.06.044
16. Hu H, Ha S, Henderson BH, Warner TD, Roth J, Kan H, et al. Association of atmospheric particulate matter and ozone with gestational diabetes mellitus. Environ Health Perspect (2015) 123:853–9. doi: 10.1289/ehp.1408456
17. Jo H, Eckel SP, Chen JC, Cockburn M, Martinez MP, Chow T, et al. Associations of gestational diabetes mellitus with residential air pollution exposure in a large southern California pregnancy cohort. Environ Int (2019) 130:104933. doi: 10.1016/j.envint.2019.104933
18. Tang X, Zhou JB, Luo F, Han Y, Heianza Y, Cardoso MA, et al. Air pollution and gestational diabetes mellitus: Evidence from cohort studies. BMJ Open Diabetes Res Care (2020) 8:e000937. doi: 10.1136/bmjdrc-2019-000937
19. Fleisch AF, Gold DR, Rifas-Shiman SL, Koutrakis P, Schwartz JD, Kloog I, et al. Air pollution exposure and abnormal glucose tolerance during pregnancy: the project viva cohort. Environ Health Perspect (2014) 122:378–83. doi: 10.1289/ehp.1307065
20. Fleisch AF, Kloog I, Luttmann-Gibson H, Gold DR, Oken E, Schwartz JD. Air pollution exposure and gestational diabetes mellitus among pregnant women in Massachusetts: A cohort study. Environ Health (2016) 15:40. doi: 10.1186/s12940-016-0121-4
21. Shen HN, Hua SY, Chiu CT, Li CY. Maternal exposure to air pollutants and risk of gestational diabetes mellitus in Taiwan. Int J Environ Res Public Health (2017) 14:1604. doi: 10.3390/ijerph14121604
22. Wang P, Lu MC, Yu CW, Wang LC, Yan YH. Influence of food intake on the predictive value of the gestational diabetes mellitus screening test. Obstet Gynecol (2013) 121:750–8. doi: 10.1097/AOG.0b013e31828784d3
23. American Diabetes Association 2. classification and diagnosis of diabetes: Standards of medical care in diabetes-2022. Diabetes Care (2022) 45(Suppl 1):S17–38. doi: 10.2337/dc22-S002
24. Chuang KJ, Yan YH, Cheng TJ. Effect of air pollution on blood pressure, blood lipids, and blood sugar: A population-based approach. J Occup Environ Med (2010) 52:258–62. doi: 10.1097/JOM.0b013e3181ceff7a
25. Chuang KJ, Yan YH, Chiu SY, Cheng TJ. Long-term air pollution exposure and risk factors for cardiovascular diseases among the elderly in Taiwan. Occup Environ Med (2011) 68:64–8. doi: 10.1136/oem.2009.052704
26. Sun Y, Li X, Benmarhnia T, Chen JC, Avila C, Sacks DA, et al. Exposure to air pollutant mixture and gestational diabetes mellitus in southern California: Results from electronic health record data of a large pregnancy cohort. Environ Int (2022) 158:106888. doi: 10.1016/j.envint.2021.106888
27. Cheng X, Ji X, Yang D, Zhang C, Chen L, Liu C, et al. Associations of PM2.5 exposure with blood glucose impairment in early pregnancy and gestational diabetes mellitus. Ecotoxicol Environ Saf (2022) 232:113278. doi: 10.1016/j.ecoenv.2022.113278
28. Pedersen M, Olsen SF, Halldorsson TI, Zhang C, Hjortebjerg D, Ketzel M, et al. Gestational diabetes mellitus and exposure to ambient air pollution and road traffic noise: A cohort study. Environ Int (2017) 108:253–60. doi: 10.1016/j.envint.2017.09.003
29. He D, Wu S, Zhao H, Qiu H, Fu Y, Li X, et al. Association between particulate matter 2.5 and diabetes mellitus: A meta-analysis of cohort studies. J Diabetes Investig (2017) 8:687–96. doi: 10.1111/jdi.12631
30. Ye B, Zhong C, Li Q, Xu S, Zhang Y, Zhang X, et al. The associations of ambient fine particulate matter exposure during pregnancy with blood glucose levels and gestational diabetes mellitus risk: A prospective cohort study in wuhan, China. Am J Epidemiol (2020) 189:1306–15. doi: 10.1093/aje/kwaa056
31. Liu R, Zhang J, Chu L, Zhang J, Guo Y, Qiao L, et al. Association of ambient fine particulate matter exposure with gestational diabetes mellitus and blood glucose levels during pregnancy. Environ Res (2022) 214(Pt 3):114008. doi: 10.1016/j.envres.2022.114008
32. Yu G, Ao J, Cai J, Luo Z, Martin R, Donkelaar AV, et al. Fine particular matter and its constituents in air pollution and gestational diabetes mellitus. Environ Int (2020) 142:105880. doi: 10.1016/j.envint.2020.105880
33. Lin Q, Zhang S, Liang Y, Wang C, Wang C, Wu X, et al. Ambient air pollution exposure associated with glucose homeostasis during pregnancy and gestational diabetes mellitus. Environ Res (2020) 190:109990. doi: 10.1016/j.envres.2020.109990
34. Zhang H, Zhao Y. Ambient air pollution exposure during pregnancy and gestational diabetes mellitus in shenyang, China: a prospective cohort study. Environ Sci pollut Res Int (2021) 28:7806–14. doi: 10.1007/s11356-020-11143-x
35. Choe SA, Eliot MN, Savitz DA, Wellenius GA. Ambient air pollution during pregnancy and risk of gestational diabetes in new York city. Environ Res (2019) 175:414–20. doi: 10.1016/j.envres.2019.04.030
36. Liu WY, Lu JH, He JR, Zhang LF, Wei DM, Wang CR, et al. Combined effects of air pollutants on gestational diabetes mellitus: A prospective cohort study. Environ Res (2022) 204(Pt D):112393. doi: 10.1016/j.envres.2021.112393
37. Rammah A, Whitworth KW, Symanski E. Particle air pollution and gestational diabetes mellitus in Houston, Texas. Environ Res (2020) 190:109988. doi: 10.1016/j.envres.2020.109988
38. Yao M, Liu Y, Jin D, Yin W, Ma S, Tao R, et al. Relationship betweentemporal distribution of air pollution exposure and glucose homeostasis during pregnancy. Environ Res (2020) 185:109456. doi: 10.1016/j.envres.2020.109456
39. Robledo CA, Mendola P, Yeung E, Männistö T, Sundaram R, Liu D, et al. Preconception and early pregnancy air pollution exposures and risk of gestational diabetes mellitus. Environ Res (2015) 137:316–22. doi: 10.1016/j.envres.2014.12.020
40. Sun Q, Yue P, Deiuliis JA, Lumeng CN, Kampfrath T, Mikolaj MB, et al. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation (2009) 119:538–46. doi: 10.1161/CIRCULATIONAHA.108.799015
41. Yan YH, Chou CC, Lee CT, Liu JY, Cheng TJ. Enhanced insulin resistance in diet-induced obese rats exposed to fine particles by instillation. Inhal Toxicol (2011) 23:507–19. doi: 10.3109/08958378.2011.587472
42. Steinle S, Reis S, Sabel CE. Quantifying human exposure to air pollution–moving from static monitoring to spatio-temporally resolved personal exposure assessment. Sci Total Environ (2013) 443:184–93. doi: 10.1016/j.scitotenv.2012.10.098
43. Hehua Z, Yang X, Qing C, Shanyan G, Yuhong Z. Dietary patterns and associations between air pollution and gestational diabetes mellitus. Environ Int (2021) 147:106347. doi: 10.1016/j.envint.2020.106347
44. Rajagopalan S, Park B, Palanivel R, Vinayachandran V, Deiuliis JA, Gangwar RS, et al. Metabolic effects of air pollution exposure and reversibility. J Clin Invest (2020) 130:6034–40. doi: 10.1172/JCI137315
Keywords: air pollution, gestational diabetes mellitus, hyperglycemia, particulate matter, pregnancy
Citation: Yan Y-H, Chien C-C, Wang P, Lu M-C, Wei Y-C, Wang J-S and Wang J-S (2023) Association of exposure to air pollutants with gestational diabetes mellitus in Chiayi City, Taiwan. Front. Endocrinol. 13:1097270. doi: 10.3389/fendo.2022.1097270
Received: 13 November 2022; Accepted: 30 December 2022;
Published: 16 January 2023.
Edited by:
Mohammed Hussen Bule, Ambo University, EthiopiaReviewed by:
Farhad Saravani, Tehran University of Medical Sciences, IranZahra Bayrami, Tehran University of Medical Sciences, Iran
Ahmed Hassan Albelbeisi, Israa University, Palestine
Copyright © 2023 Yan, Chien, Wang, Lu, Wei, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun-Sing Wang, anN3YW5nQHZnaHRjLmdvdi50dw==
†These authors have contributed equally to this work and share first authorship
 Yuan-Horng Yan1,2,3†
Yuan-Horng Yan1,2,3† Yu-Ching Wei
Yu-Ching Wei Jun-Sing Wang
Jun-Sing Wang