- 1Department of Laboratory Medicine, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Geriatrics and National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University, Chengdu, China
Background: Sarcopenia is an age-related and skeletal muscle disorder involving the loss of muscle mass or strength, and physiological function. Although the diagnostic indicators used in the different guidelines are for muscle mass, strength and physical performance, there are currently no uniform diagnostic criteria. Therefore, we aimed to explore the relationship between a series of biomarkers with sarcopenia in southwest China.
Methods: We included 4302 patients from West China Health and Aging Trend (WCHAT) study. Sarcopenia was defined according to the Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. Thyroxine、albumin、total protein、prealbumin、albumin to globulin ratio (A/G)、25(OH)VD、fasting insulin、adrenal cortisol、triglyceride、high-density lipoprotein、hemoglobin and aspartate aminotransferase to alanine aminotransferase ratio (AST/ALT) were measured. The receiver operating characteristic curves (ROC) were established to describe the predictive value for sarcopenia and we also used multivariate logistic regression analysis to identify risk factors of the disease.
Results: In terms of protein state, patients with sarcopenia had lower value in total protein, albumin, prealbumin, A/G than the control (P<0.001). Patients had lower value in triglyceride but higher value in high-density lipoprotein compared with the healthy in the indicators of lipid metabolism (P<0.001). In the aspect of hormone state, patients had lower free triiodothyronine, fasting insulin but higher free tetraiodothyronine and adrenal cortisol than the healthy (P<0.001). The fasting insulin level (AUC=0.686) and the AST/ALT ratio (AUC=0.682) were the best predictors of sarcopenia among biomarkers. The diagnostic performance of fasting insulin combined with the AST/ALT ratio (AUC=0.720) is equal to multiple indicators (AUC=0.742).
Conclusion: The fasting insulin combined with the AST/ALT ratio exhibits good diagnostic performance for sarcopenia.
Introduction
Sarcopenia is an age-related and skeletal muscle disorder involving the loss of muscle mass or strength and physiological function (1). It`s reported that the prevalence of sarcopenia according to the Asian Working Group for Sarcopenia (AWGS) 2014 criteria ranged from 5.5% to 25.7% (2, 3). With the aggravation of the aging population in our country and the increased adverse health outcomes associated with sarcopenia including frailty, falls, disability and mortality, it`s necessary for the clinic to make an early diagnosis and intervention. In addition to clarifying the relationship between aging and sarcopenia, nutrition, endocrine dysfunction, glucose, and lipid metabolism as well as metabolic syndrome (Mets) have received more and more attention. The pathophysiology of sarcopenia may be that 1) a decline in muscle fiber numbers with aging and an increase in fibrosis leads to muscle dysfunction; 2) the imbalance between muscle protein synthesis and regeneration leads to loss of skeletal muscle; 3) on the one hand, insulin resistance leads to the dysfunction of the use of glucose, on the other hand, it leads to muscle atrophy (4–6). Now, the diagnosis of sarcopenia requires objective measurements of muscle mass, muscle strength, and physical performance. Currently, dual-energy X-ray absorptiometry (DXA), and bioelectrical impedance analysis (BIA) were the most commonly used in Asia for appendicular skeletal muscle mass measurement. Handgrip strength was widely recommended to indicate skeletal muscle strength (1, 7, 8). Although the diagnostic indicators used in the different guidelines are for muscle mass, strength, and physical performance, there are currently no uniform diagnostic criteria and the results measured by different devices cannot be directly compared. Despite promising advances in evaluating muscle mass and strength, it`s hard to implement in some primary hospitals in China due to the lack of equipment. The physical performance including Short Physical Performance Battery (SPPB), 6-min walk test, and the stair climb power test (SCPT) were not available for some elderly patients either. Meanwhile, the mechanism of sarcopenia has not been fully characterized. Therefore, we used the data from the West China Health and Aging Trend (WCHAT) study to explore the relationship between clinical laboratory biomarkers with sarcopenia.
Methods
The current research is a cross-sectional analysis including baseline data of WCHAT study which was approved by the Ethical Review Committee (reference: 2017-445). The method of sampling is multi-stage cluster sampling and the response rate was 50.2% in the baseline data collection. And this research is supported by Grant No.2020YFC2005600/03 from the National Key R&D Program of China.
Study Participants
Date selected from baseline of the WCHAT study, which was initiated in 2018 and included 7536 people aged 50 or older in the west China, were used in this analysis (9, 10). The exclusion criteria for them were as follows: 1) cognitive impairment; 2) a recent history of malignancy; 3) missing data on the laboratory measurements; 4) Diabetes Mellitus. Finally, a total of 4120 elderly patients from various communities in Sichuan Province, southwest of China, were admitted into this study, 786 of whom were diagnosed with sarcopenia. All participants were willing to take part in this study and informed consent was signed. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Sichuan University.
Sarcopenia Assessment
We used the AWGS 2019 as the diagnostic criteria, which was widely exerted in the diagnosis of sarcopenia in Asia, considering both the loss in muscle mass, muscle strength as well as physical performance. According to the AWGS, appendicular muscle mass (male: <7.0kg/m2, female: <5.7 kg/m2) for BIA, is considered as a loss of muscle mass. The AWGS also suggests that handgrip strength of <28kg and <18kg for men and women is defined as low muscle strength. The 6 m walking test <1.0 m/s and SPPB ≤9 are recommended for the evaluation of physical ability. Patients are definitely diagnosed with sarcopenia when low muscle strength and poor muscle function are confirmed. Meanwhile, if low physical performance is also accompanied sarcopenia is considered to sever.
Specimen Collection
We collected specimen from the antecubital vein after an overnight fast. Biomarkers including fasting insulin, 25(OH)VD, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total protein (TP), prealbumin (PA), albumin (ALB), free triiodothyronine (FT3), free tetraiodothyronine (FT4), triglycerides (TG), high-density lipoprotein (HDL), very low-density lipoprotein (VLDL), plasm total cortisol (PTC) and hemoglobin (Hb) were measured. Except Hb was used with whole blood, the remaining blood was centrifuged at 3000rpm for 10 minutes to obtain serum. TP, ALB, FT3, FT4, TG, VLDL, HDL, PTC were measured by electrochemiluminescence (cobas e801, Roche). ALT and AST were measured by enzyme method (cobas e801, Roche). PA was measured by Turbidimetric inhibition immuno assay (IMMAGE 800, Beckman). Some clinic signs such as weight, height, calf circumference (CC), waistline, mid-arm circumference (MAC), thickness of triceps skinfold (TST), hip circumference (HIPL) were also measured. Trained interviewers collected questionnaire data through face-to-face, one-on-one personal interviews. Trained technicians performed the anthropometric and bioimpedance measurements.
Statistical Analysis
Kolmogorov-Smirnov test was used to confirm the normal distribution. Measurement data on normal distribution were expressed as mean ± standard deviation (M ± SD), and tested by an independent t-test. Abnormal distribution data were expressed as the median (interquartile range, IQR) and tested by the Mann-Whitney U test. Multivariate logistic regression was performed, including all univariately associated variables (P < 0.05). The analyses above were completed via SPSS software 25.0 (Chicago, IL, USA). All P-values were two-tailed and statistical significance was set at P < 0.05.
Results
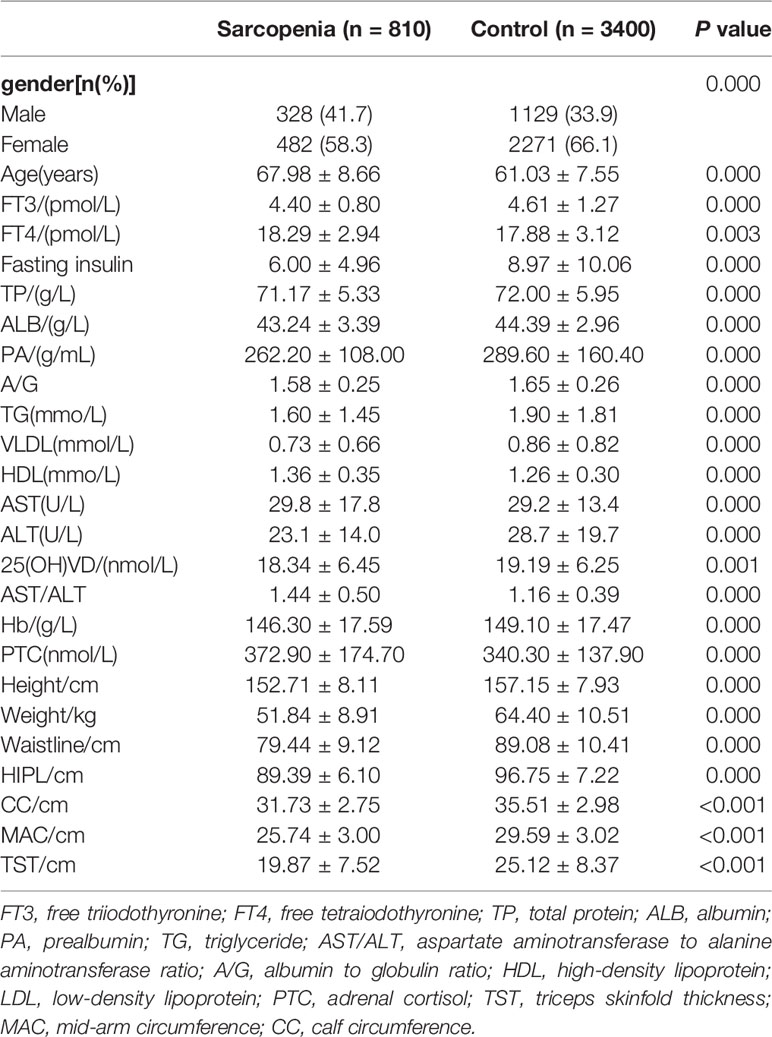
Characteristics of the Study Cohorts
Clinical characteristics of the cohorts are described in (Table 1) and (Supplementary Table 1). Patients with sarcopenia had lower value in height, weight, waistline, HIPL, CC, MAC and TST than the healthy (P<0.001). In terms of nutrition state, patients with sarcopenia had lower value in TP, ALB, and PA than the control (P<0.001). Meanwhile, the level of A/G ratio in the sarcopenia group was lower than in the control (P<0.001). Patients had lower value in TG and VLDL but higher value in HDL compared with the healthy in the indicators of lipid metabolism (P<0.001). In the aspect of hormone state, patients had lower FT3, fasting insulin but higher FT4 and PTC than the healthy (P<0.001).
Risk Factors for Sarcopenia
Based on the findings of the univariate analysis, the following parameters were entered in the multivariate logistic regression model: fasting insulin, AST/ALT ratio, TP, PA, ALB, A/G, FT3, FT4, TG, HDL, HGB PTC,25(OH)VD. After the adjustment, the odds ratios (ORs) of sarcopenia showed that fasting insulin (OR=0.904, [95%CI: 0.882-0.927] P=0.000), AST/ALT ratio (OR=1.920, [95%CI: 1.564-2.358] P=0.000), HDL (OR=1.917, [95%CI: 1.413-2.600] P=0.000), TG (OR=1.109, [95%CI: 1.007-1.220] P=0.035), PA (OR=0.998, [95%CI: 0.997-1.000] P=0.022), FT4 (OR=1.084, [95%CI: 1.047-1.122] P=0.000), PTC (OR=1.001, [95%CI: 1.000-1.002] P=0.005) and 25(OH)VD (OR=0.980, [95%CI: 0.966-0.994] P=0.006) were identified as risk factors for the sarcopenia (Table 2).
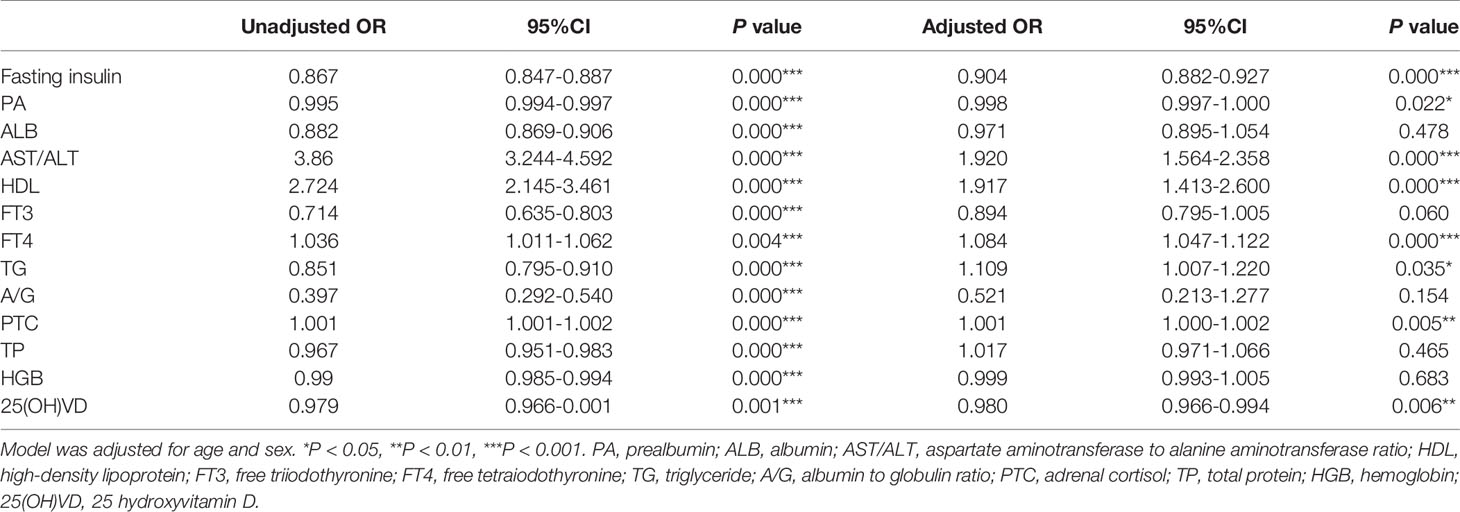
Table 2 Association between biochemical indicators and sarcopenia according to unadjusted and adjusted logistic regression models.
The Predictive Value of Indicators for Sarcopenia
We analyzed the diagnostic performance of indicators above through the receiver-operating characteristic curves (ROC). We found that fasting insulin AUC = 0.686 [95%CI: 0.670-0.707] and AST/ALT ratio AUC = 0.682 [95%CI: 0.662-0.703] had better predictive value than other parameters (Figure 1). The diagnostic efficacy of FT3 AUC = 0.574 [95%CI: 0.552-0.597] was significantly higher than that of FT4 AUC = 0.548 [95%CI: 0.525-0.570], and the difference was statistically significant P < 0.001. The index reflecting the protein levels, the diagnostic efficiency of sarcopenia from large to small was PA > ALB > A/G> TP, and the corresponding AUC were 0.608 [95%CI: 0.586-0.630], 0.604 [95%CI: 0.581-0.626)], 0.564 [95%CI: 0.542-0.586], 0.546 [95%CI: 0.523-0.568] respectively. In terms of lipid metabolism, AUC HDL = 0.581 [95%CI: 0.559-0.604] and TG = 0.564 [95%CI: 0.542-0.585]. Besides, 25 (OH) VD AUC = 0.542 [95%CI:0.519-0.564] has the lowest diagnostic efficacy for sarcopenia in our study (Supplementary Figure 1). The AUC of multiple factors including fasting insulin, AST/ALT ratio, PA, HDL, FT4, PTC and 25(OH)VD was 0.742 [95%CI: 0.722-0.761], and the combination of fasting insulin with AST/ALT ratio was 0.720 [95%CI: 0.701-0.740] (Figure 2).
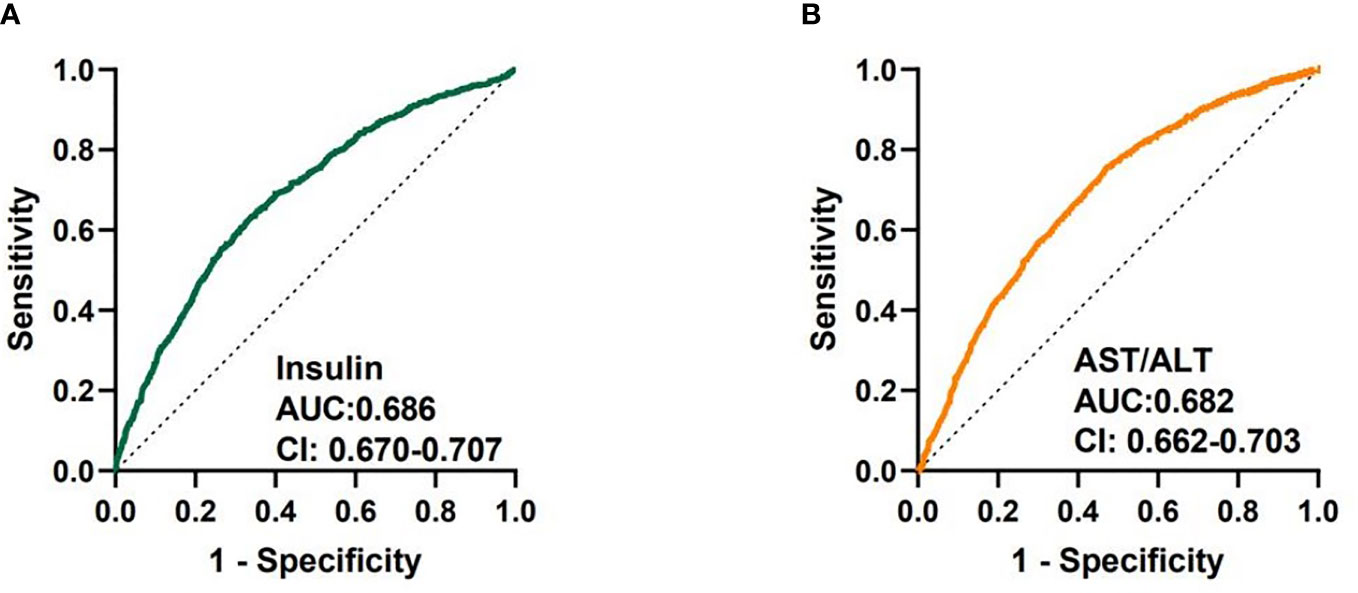
Figure 1 Receiver-operating characteristic curves (ROC) used for sarcopenia of fasting insulin and AST/ALT ratio. (A) ROC curve analysis of fasting insulin together with AUC and 95% confidence interval values. (B) ROC curve analysis of AST/ALT ratio together with AUC and 95% confidence interval values.
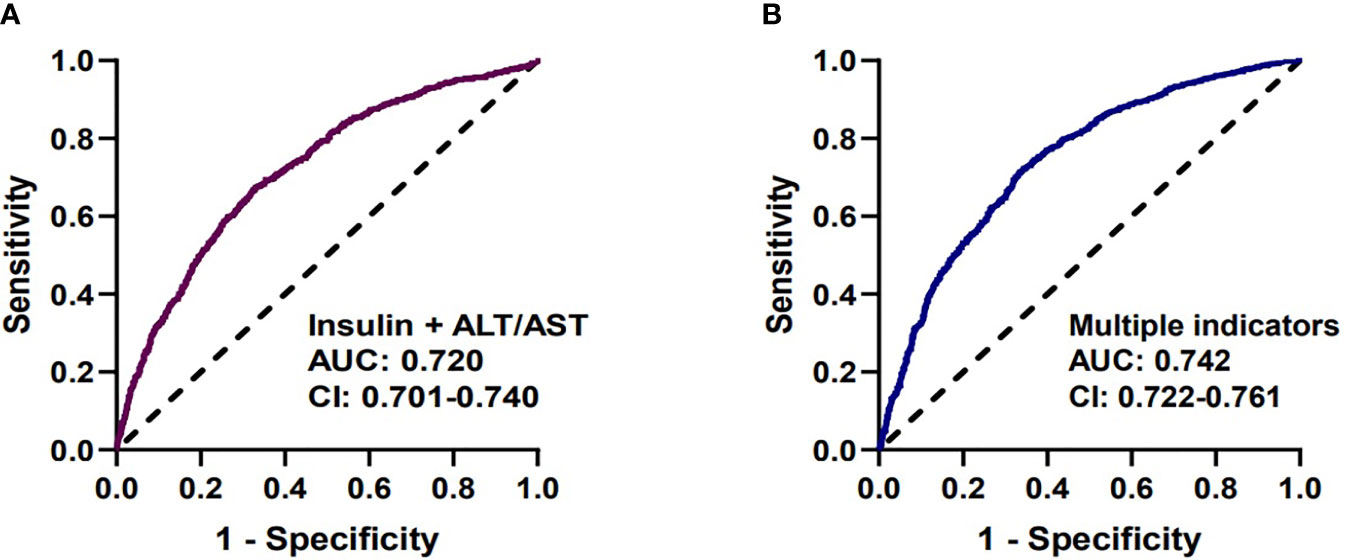
Figure 2 Receiver-operating characteristic curves (ROC) used for sarcopenia. (A) The predictive performance of insulin and AST/ALT (AUC=0.720 [95%CI: 0.701-0.740]). (B) The predictive performance of multiple factors, including fasting insulin, AST/ALT ratio, PA, HDL, FT4, PTC, 25(OH)VD (AUC=0.742 [95%CI: 0.722-0.761]).
Discussion
Sarcopenia is an age-dependent disorder. Skeletal muscle quality and function gradually decline with age, which not only reduces the quality of life of the elderly but also increases the social burden. Due to the different diagnostic criteria and study population, the prevalence of sarcopenia also varies greatly (11). It was reported that, according to the diagnostic criteria developed by the AWGS in 2014, the prevalence of sarcopenia is about 5.5% to 25.7%. The mechanism of sarcopenia not only includes the age, but also nutrition, chronic inflammation, and metabolic derangements. Therefore, we aimed to identify some biomarkers related to nutrition and hormone for the diagnosis of sarcopenia in this study. Patients with sarcopenia had lower value of A/G compared to the control. In our study, we found that patients with sarcopenia had lower values in TP, ALB, A/G, and PA than the healthy (P<0.001). Low albumin has been proved to lead to more antioxidant capacities, causing more oxidative damage and therefore muscle breakdown (12, 13). Many elderly people had an inadequate intake of protein due to the decline of digestive and masticatory function, resulting in the imbalance of protein metabolism (14, 15). Malnutrition is frequent in the older and there is an evidence that the supplementation of essential amino acid can prevent the older from sarcopenia (16, 17).
Meanwhile, low 25(OH)VD levels have been confirmed to lead to loss of muscle mass and grip strength through the reduction in II type muscle fiber synthesis identified as fast twitch, which was also consistent with our findings (18, 19). Several studies have already showed that 25(OH)VD could induce muscle cells proliferation, differentiation, and regulate muscle regeneration initiation (20, 21). Besides, Vitamin D could bind to the VDR receptor on muscle fibers and increases their size, improving muscle strength and physical performance. However, the diagnostic performance of 25(OH)VD was the lowest among the biomarkers. The reason may be that the baseline of 25(OH)VD was generally low and 25(OH)VD deficiency is frequent among the older in southwest China. Additionally, we also found that patients had higher levels of PTC. It was found that high levels of PTC could prompt protein degradation through inhibiting mTOR pathway, thus leading to muscle atrophy and affecting the normal physiological function of skeletal muscle (22–25). Patients with sarcopenia had lower levels of fasting insulin than the healthy. The research found that a lack of insulin or insulin resistance leads to accelerated development of sarcopenia. Insulin could mediate accretion of muscle mass through stimulating the mammalian target of rapamycin (mTOR) signaling pathway and promoting the phosphorylation of Akt, thus activating protein synthesis (26). They also showed that physiological hyperinsulinemia increases skeletal muscle protein synthesis (27). Surprisingly, there was a separation between the FT3 and FT4. We found that patients with sarcopenia had lower levels of FT3 but higher levels of FT4 than the healthy. As is known to all, the thyroid hormone plays an important role in regulating the growth and development of our brain and bones. It is also essential for skeletal muscle contractile function and muscle regeneration. Except for regulating the growth, FT3 also regulates skeletal muscle cells by regulating myosin expression (28, 29). Studies have reported that with the increase of age, FT3 will decrease while FT4 will remain stable or increase (30–33). Szlejf et al. revealed that subtle thyroid dysfunction was related to sarcopenia and FT3 had a negative association with muscle mass (29). Although the exact pathogenesis of sarcopenia is still unclear, it is certain that several factors related to age contribute to sarcopenia. Therefore, we inferred that thyroid function may have a certain correlation with the occurrence of sarcopenia, and the specific mechanism needed to be further studied. In the sarcopenia group we found that patients had lower levels of TG and VLDL but higher levels of HDL. Therefore, we inferred that lower TG levels may be a consequence of poor health status associated with sarcopenia (34). A randomized controlled trial revealed that treatment with medium-chain triglycerides could increase the muscle strength and function in elderly with sarcopenia (35). We made multivariate logistic regression analysis and evaluated the predictive performance through the AUC. We found the diagnostic performance of fasting insulin combined with the AST/ALT ratio 0.720 [95%CI: 0.701-0.740] was equal to multiple indicators 0.742 [95%CI 0.722-0.761]. From an economic point of view, we strongly recommend fasting insulin and AST/ALT ratio for screening for sarcopenia. Study showed that although non-alcoholic fatty liver disease (NAFLD) was the first cause of liver enzyme abnormalities, reduced ALT has been strongly associated with sarcopenia (36). Besides high serum AST with normal ALT could reflect widespread skeletal muscle pathology (37). Thus, AST to ALT ratio may be a good indicator for frailty and sarcopenia in older patients (38) and multivariate logistic regression model also showed that fasting insulin (OR=0.919, [95%CI: 0.878-0.922] P=0.000) and AST to ALT ratio (OR=2.771, [95%CI: 1.544-2.342] P=0.000) were risk factors for sarcopenia. Although TP, ALB and PA can all reflect the nutritional status of the body, ALB and PA are more recommended in the evaluation of the nutritional status of patients with sarcopenia, and their corresponding AUC is 0.604 and 0.609, respectively.
Strengths and Limitations
The current research is a cross-sectional study using baseline data of the WCHAT study and data were collected from four provinces in West China, including Yunnan, Guizhou, Sichuan, and Xinjiang. Although we demonstrated that a significant decrease in triglycerides, albumin, insulin, ALT, vitamin D, and an increase in HDL and AST in sarcopenic patients in the WCHAT study population as prior researches did, in our research we compared the indicators through the ROC curves from a medical laboratory perspective and identified the indictors that were most effective in diagnosing sarcopenia. However, there are also some limitations. Firstly, more data are needed to validate the accuracy of the diagnostic performance. Additionally, only biochemical indicators were included in this research, but other indicators represented immune functions were not available. We will collect new data to validate the accuracy and establish the diagnostic performance of immunological indicators for sarcopenia in future study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
MY: Conceptualization, Data curation, Formal analysis, Writing - original draft, Writing - review and editing. HZ: Data curation, Writing - review and editing. LH: Data curation, Writing - review and editing. YD: Data curation, Writing - review and editing. HW: Data curation, Writing - review and editing. QL and FD: Funding acquisition, Data curation, Writing - review and editing. JY and YH: Conceptualization, Writing - review and editing. All authors contributed to the article and approved the submitted version.
Funding
This research is supported by Grant National Key R&D Program of China (2020YFC2005600 and 2020YFC2005603), Sichuan Science and Technology Agency of Sichuan Province (2020YFS0185) and Sichuan Science and Technology Program (2019YFS0277).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.785045/full#supplementary-material
References
1. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing (2019) 48:16–31. doi: 10.1093/ageing/afy169
2. Yu R, Leung J, Woo J. Incremental Predictive Value of Sarcopenia for Incident Fracture in an Elderly Chinese Cohort: Results From the Osteoporotic Fractures in Men (MrOs) Study. J Am Med Directors Assoc (2014) 15:551–8. doi: 10.1016/j.jamda.2014.02.005
3. Yoshimura N, Muraki S, Oka H, Iidaka T, Kodama R, Kawaguchi H, et al. Is Osteoporosis a Predictor for Future Sarcopenia or Vice Versa? Four-Year Observations Between the Second and Third ROAD Study Surveys. Osteoporos Int (2017) 28:189–99. doi: 10.1007/s00198-016-3823-0
4. Rubio-Ruiz ME, Guarner-Lans V, Perez-Torres I, Soto ME. Mechanisms Underlying Metabolic Syndrome-Related Sarcopenia and Possible Therapeutic Measures. Int J Mol Sci (2019) 20:647–69. doi: 10.3390/ijms20030647
5. Zhang HQ, Lin S, Gao TL, Zhong F, Cai J, Sun YY, et al. Association Between Sarcopenia and Metabolic Syndrome in Middle-Aged and Older Non-Obese Adults: A Systematic Review and Meta-Analysis. Nutrients (2018) 10:364–76. doi: 10.3390/nu10030364
6. Morley JE, Malmstrom TK, Rodriguez-Mans L, Sinclair AJ. Frailty, Sarcopenia and Diabetes. J Am Med Directors Assoc (2014) 15:853–9. doi: 10.1016/j.jamda.2014.10.001
7. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Directors Assoc (2020) 21:300. doi: 10.1016/j.jamda.2019.12.012
8. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: Consensus Report of the Asian Working Group for Sarcopenia. J Am Med Directors Assoc (2014) 15:95–101. doi: 10.1016/j.jamda.2013.11.025
9. Zhao WY, Zhang Y, Hou LS, Xia X, Ge ML, Liu XL, et al. The Association Between Systemic Inflammatory Markers and Sarcopenia: Results From the West China Health and Aging Trend Study (WCHAT). Arch Gerontol Geriatr (2021) 92:104262. doi: 10.1016/j.archger.2020.104262
10. Zhang Y, Ge M, Zhao W, Hou L, Xia X, Liu X, et al. Association Between Number of Teeth, Denture Use and Frailty: Findings From the West China Health and Aging Trend Study. J Nutr Health Aging (2020) 24:423–8. doi: 10.1007/s12603-020-1346-z
11. Liu X, Hao Q, Hou L, Xia X, Zhao W, Zhang Y, et al. Ethnic Groups Differences in the Prevalence of Sarcopenia Using the AWGS Criteria. J Nutr Health Aging (2020) 24:665–71. doi: 10.1007/s12603-020-1381-9
12. Oettl K, Stauber RE. Physiological and Pathological Changes in the Redox State of Human Serum Albumin Critically Influence its Binding Properties. Br J Pharmacol (2007) 151:580–90. doi: 10.1038/sj.bjp.0707251
13. Derbre F, Gratas-Delamarche A, Gomez-Cabrera MC, Vina J. Inactivity-Induced Oxidative Stress: A Central Role in Age-Related Sarcopenia? Eur J Sport Sci (2014) 14(Suppl 1):S98–108. doi: 10.1080/17461391.2011.654268
14. Hai S, Cao L, Wang H, Zhou JH, Liu P, Yang Y, et al. Association Between Sarcopenia and Nutritional Status and Physical Activity Among Community-Dwelling Chinese Adults Aged 60 Years and Older. Geriatrics Gerontology Int (2017) 17:1959–66. doi: 10.1111/ggi.13001
15. Kim HK, Suzuki T, Saito K, Yoshida H, Kobayashi H, Kato H, et al. Effects of Exercise and Amino Acid Supplementation on Body Composition and Physical Function in Community-Dwelling Elderly Japanese Sarcopenic Women: A Randomized Controlled Trial. J Am Geriatrics Soc (2012) 60:16–23. doi: 10.1111/j.1532-5415.2011.03776.x
16. Naseeb MA, Volpe SL. Protein and Exercise in the Prevention of Sarcopenia and Aging. Nutr Res (2017) 40:1–20. doi: 10.1016/j.nutres.2017.01.001
17. Sieber CC. Malnutrition and Sarcopenia. Aging Clin Exp Res (2019) 31:793–8. doi: 10.1007/s40520-019-01170-1
18. Girgis CM, Clifton-Bligh RJ, Mokbel N, Cheng K, Gunton JE. Vitamin D Signaling Regulates Proliferation, Differentiation, and Myotube Size in C2C12 Skeletal Muscle Cells. Endocrinology (2014) 155:347–57. doi: 10.1210/en.2013-1205
19. Nagai T, Okano I, Ishikawa K, Kuroda T, Oshita Y, Tsuchiya K, et al. The Serum 25(OH)D Level and Hand Grip Strength for Fall Risk Assessment Among Osteoporotic Elderly Japanese Women. Arch Osteoporosis (2021) 16:42–9. doi: 10.1007/s11657-021-00901-0
20. Agergaard J, Trøstrup J, Uth J, Iversen JV, Boesen A, Andersen JL, et al. Does Vitamin-D Intake During Resistance Training Improve the Skeletal Muscle Hypertrophic and Strength Response in Young and Elderly Men? - A Randomized Controlled Trial. Nutr Metab (2015) 12:32. doi: 10.1186/s12986-015-0029-y
21. Remelli F, Vitali A, Zurlo A, Volpato S. Vitamin D Deficiency and Sarcopenia in Older Persons. Nutrients (2019) 11:2861–74. doi: 10.3390/nu11122861
22. Peeters GMEE, van Schoor NM, Visser M, Knol DL, Eekhoff EMW, de Ronde W, et al. Relationship Between Cortisol and Physical Performance in Older Persons. Clin Endocrinol (2007) 67:398–406. doi: 10.1111/j.1365-2265.2007.02900.x
23. Jellyman JK, Martin-Gronert MS, Cripps RL, Giussani DA, Ozanne SE, Shen QWW, et al. Effects of Cortisol and Dexamethasone on Insulin Signalling Pathways in Skeletal Muscle of the Ovine Fetus During Late Gestation. PloS One (2012) 7:e52363. doi: 10.1371/journal.pone.0052363
24. Yanagita I, Fujihara Y, Kitajima Y, Tajima M, Honda M, Kawajiri T, et al. A High Serum Cortisol/DHEA-S Ratio Is a Risk Factor for Sarcopenia in Elderly Diabetic Patients. J Endocrine Soc (2019) 3:801–13. doi: 10.1210/js.2018-00271
25. Shimizu N, Yoshikawa N, Ito N, Maruyama T, Suzuki Y, Takeda S, et al. Crosstalk Between Glucocorticoid Receptor and Nutritional Sensor mTOR in Skeletal Muscle. Cell Metab (2011) 13:170–82. doi: 10.1016/j.cmet.2011.01.001
26. Fujita S, Rasmussen BB, Cadenas JG, Drummond MJ, Glynn EL, Sattler FR, et al. Aerobic Exercise Overcomes the Age-Related Insulin Resistance of Muscle Protein Metabolism by Improving Endothelial Function and Akt/mammalian Target of Rapamycin Signaling. Diabetes (2007) 56:1615–22. doi: 10.2337/db06-1566
27. Fujita S, Glynn EL, Timmerman KL, Rasmussen BB, Volpi E. Supraphysiological Hyperinsulinaemia Is Necessary to Stimulate Skeletal Muscle Protein Anabolism in Older Adults: Evidence of a True Age-Related Insulin Resistance of Muscle Protein Metabolism. Diabetologia (2009) 52:1889–98. doi: 10.1007/s00125-009-1430-8
28. Salvatore D, Simonides WS, Dentice M, Zavacki AM, Larsen PR. Thyroid Hormones and Skeletal Muscle–New Insights and Potential Implications. Nat Rev Endocrinol (2014) 10:206–14. doi: 10.1038/nrendo.2013.238
29. Szlejf C, Suemoto CK, Janovsky C, Barreto SM, Diniz M, Lotufo PA, et al. Thyroid Function and Sarcopenia: Results From the ELSA-Brasil Study. J Am Geriatr Soc (2020) 68:1545–53. doi: 10.1111/jgs.16416
30. Clegg A, Hassan-Smith Z. Frailty and the Endocrine System. Lancet Diabetes Endo (2018) 6:743–52. doi: 10.1016/S2213-8587(18)30110-4
31. Lago-Sampedro AM, Gutierrez-Repiso C, Valdes S, Maldonado C, Colomo N, Almaraz MC, et al. Changes in Thyroid Function With Age: Results From the Pizarra Population-Based Longitudinal Study. Int J Clin Pract (2015) 69:577–87. doi: 10.1111/ijcp.12545
32. Yeap BB, Alfonso H, Chubb SAP, Walsh JP, Hankey GJ, Almeida OP, et al. Higher Free Thyroxine Levels are Associated With Frailty in Older Men: The Health In Men Study. Clin Endocrinol (2012) 76:741–8. doi: 10.1111/j.1365-2265.2011.04290.x
33. Yeap BB, Alfonso H, Hankey GJ, Flicker L, Golledge J, Norman PE, et al. Higher Free Thyroxine Levels are Associated With All-Cause Mortality in Euthyroid Older Men: The Health In Men Study. Eur J Endocrinol (2013) 169:401–8. doi: 10.1530/EJE-13-0306
34. Can B, Kara O, Kizilarslanoglu MC, Arik G, Aycicek GS, Sumer F, et al. Serum Markers of Inflammation and Oxidative Stress in Sarcopenia. Aging Clin Exp Res (2017) 29:745–52. doi: 10.1007/s40520-016-0626-2
35. Abe S, Ezaki O, Suzuki M. Medium-Chain Triglycerides (8:0 and 10:0) are Promising Nutrients for Sarcopenia: A Randomized Controlled Trial. Am J Clin Nutr (2019) 110:652–65. doi: 10.1093/ajcn/nqz138
36. Vespasiani-Gentilucci U, De Vincentis A, Ferrucci L, Bandinelli S, Antonelli Incalzi R, Picardi A. Low Alanine Aminotransferase Levels in the Elderly Population: Frailty, Disability, Sarcopenia, and Reduced Survival. J Gerontol A Biol Sci Med Sci (2018) 73:925–30. doi: 10.1093/gerona/glx126
37. Shibata M, Nakajima K, Higuchi R, Iwane T, Sugiyama M, Nakamura T. High Concentration of Serum Aspartate Aminotransferase in Older Underweight People: Results of the Kanagawa Investigation of the Total Check-Up Data From the National Database-2 (KITCHEN-2). J Clin Med (2019) 8:1282–91. doi: 10.3390/jcm8091282
Keywords: sarcopenia, AST/ALT ratio, fasting insulin, diagnostic performance, metabolism
Citation: Yin M, Zhang H, Liu Q, Ding F, Deng Y, Hou L, Wang H, Yue J and He Y (2021) Diagnostic Performance of Clinical Laboratory Indicators With Sarcopenia: Results From the West China Health and Aging Trend Study. Front. Endocrinol. 12:785045. doi: 10.3389/fendo.2021.785045
Received: 28 September 2021; Accepted: 12 November 2021;
Published: 10 December 2021.
Edited by:
Stefano Masiero, University of Padua, ItalyReviewed by:
Olga Savinova, New York Institute of Technology, United StatesJannis Papathanasiou, Medical University - Sofia, Bulgaria
Copyright © 2021 Yin, Zhang, Liu, Ding, Deng, Hou, Wang, Yue and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jirong Yue, yuejirong11@hotmail.com; Yong He, heyong1011@163.com
†These authors have contributed equally to this work
 Mengting Yin1†
Mengting Yin1†