
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 14 September 2022
Sec. Endocrinology of Aging
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.975647
This article is part of the Research Topic(Osteo)Sarcopenia & Sarcopenic ObesityView all 8 articles
Background: With the advancement of world population aging, age-related osteoporosis (OP) and sarcopenia (SP) impose enormous clinical and economic burden on society. Evidence from accumulating studies indicates that they mutually influence one another. However, an observational study may be affected by potential confounders. Meanwhile, a Mendelian randomization (MR) study can overcome these confounders to assess causality.
Objectives: The aim of this study was to evaluate the causality between OP and SP, informing new strategies for prevention, diagnosis, and treatment of osteosarcopenia.
Methods: Instrumental variables (IVs) at the genome‐wide significance level were obtained from published summary statistics, and the inverse variance weighted method and several other MR methods were conducted to evaluate the bi-directional causality between SP and OP. Myopia was analyzed as a negative control outcome to test the validity of IVs.
Results: Femoral neck bone mineral density (FN BMD), lumbar spine BMD (LS BMD), and forearm BMD (FA BMD) had a direct causal effect on appendicular lean mass (ALM) [FA BMD-related analysis: odds ratio (OR) = 1.028, 95% confidence interval (CI) = (1.008,1.049), p = 0.006; FN BMD-related analysis: OR (95% CI) = 1.131 (1.092,1.170), p = 3.18E-12; LS BMD-related analysis: OR (95% CI) = 1.080 (1.062,1.098), p = 2.86E-19]. ALM had a significant causal effect on LS BMD [OR (95% CI) = (1.033,1.147), p = 0.001]. There was no evidence for causal association between BMD and low grip strength.
Conclusions: OP and SP might mutually have a significant causal effect on each other. Our results supported the idea that the patient with severe OP was more susceptible to lose ALM and severe ALM loss might reduce LS BMD.
With the global aging of the population, the prevalence of osteoporosis (OP) and sarcopenia (SP) is increasing rapidly, which is positively associated with increased risk of fractures, reduced quality of life, and early death (1). They have caused a serious global public health problem, imposing enormous clinical and economic burden on society. OP and SP are both geriatric chronic disease with high incidence, and they are generally more prone to coexist in the same old person. Osteosarcopenia was proposed by Duque and colleagues to describe this overlap in 2017 (2). Among the community-dwelling older people, osteosarcopenia had widely ranging prevalence rates of approximately 5%–37% (≥65 years); meanwhile, osteosarcopenic individuals demonstrated poorer nutritional status than OP or SP alone (3). Considerable lines of evidence numerically indicated a close relationship between OP and SP on the basis of observational studies (4, 5). A recent meta-analysis study showed that OP was an associated factor of SP (6). However, two systematic reviews of RCTs reported that higher protein supplementation was only associated with lumbar spine bone mineral density (LS BMD) (7, 8). These conflicting results make it difficult to infer the causality between OP and SP, particularly when unmeasured potential confounders are involved, such as age, fat, and exercise, which can lead to OP and SP. Evaluating the causality between OP and SP can inform new strategies for prevention, diagnosis, and treatment of osteosarcopenia.
Mendelian randomization (MR) is a valid approach for causal inference using genetic variants as instrument variables (IVs), which can effectively overcome the confounding bias of traditional epidemiological studies (9). To the best of the authors’ knowledge, no MR has been investigated between OP and SP. Actually, nor did any randomized controlled trial (RCT) directly evaluate the bi-directional relation. Therefore, we performed a bi-directional two-sample MR analysis to address the associations between OP (measured as BMD) and SP (measured as body lean mass and low grip strength).
Valid MR analysis is based on three assumptions: (1) the used genetic IVs are robustly associated with exposure; (2) the selected IVs are not associated with potential confounders; and (3) the IVs can only affect the risk of outcome dependently through exposure (10). This bi-directional MR analysis was performed in two steps: OP was investigated as exposure while SP-related traits were investigated as outcome in the first step, whereas the second step was reversed. Figure 1 shows an overview of the three assumptions and study design.
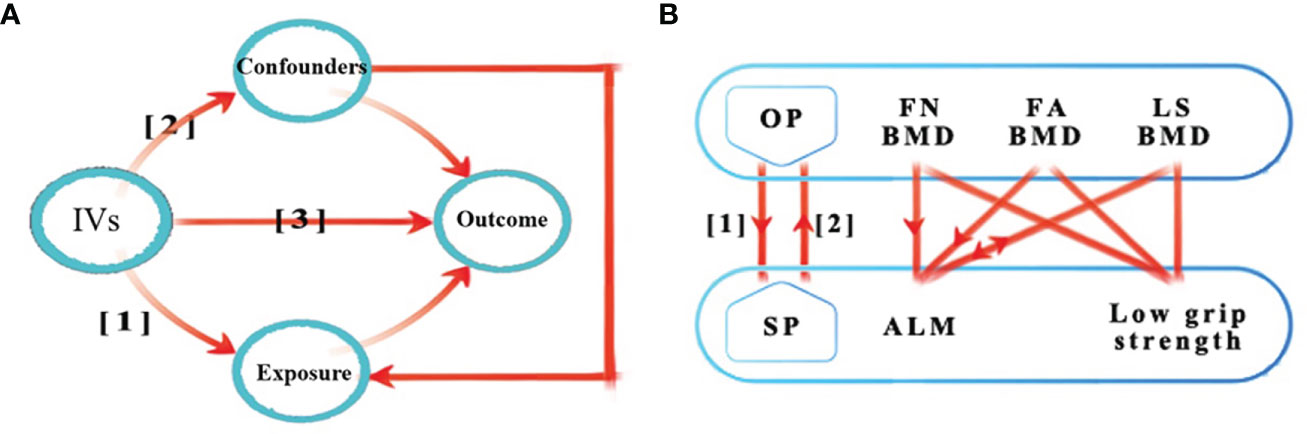
Figure 1 Schematic representation of the three assumptions and study design. (A) (1) The used genetic IVs are robustly associated with exposure; (2) The slected IVs are not associated with potential confounders; (3) The IVs can only affect the risk of outcome dependently through exposure. (B) This bidirectional MR analysis was performed in two steps: osteoporosis was studied as exposure while sarcopenia-related traits were studied as outcome in the first step, whereas the second step was reversed. The arrows indicate direction of causality in our results. IVs, instrumental variables; OP, osteoporosis; SP, sarcopenia; FA, forearm; FN, femoral neck; LS, lumbar spine; BMD, bone mineral density; ALM, appendicular lean mass; MR, mendelian randomization.
Clinically, femoral neck BMD (FN BMD), LS BMD, and forearm BMD (FA BMD) have been widely used as measurable and powerful predictors of OP. BMD is highly heritable and associated with common genetic variants (11). Related GWAS summary statistics were derived from the Genetic Factors for Osteoporosis (GEFOS) consortium (http://www.gefos.org/?q=content/data-release-2015) (12). The genetic values in 53,236 individuals of European ancestry were corrected for sex, age, age2, and weight, and standardized. Appendicular lean mass (ALM) is potentially important as a measure of muscle mass in older people (13). The ALM-related values were quantified by the sum of fat-free mass with 450,243 UK Biobank cohort participants (https://www.ebi.ac.uk/gwas/publications/33097823), adjusted for appendicular fat mass, age, age2, the top 10 principal components, and other covariates (14). The summary‐level statistics of low grip strength were obtained from a muscle weakness-related meta‐analysis study (https://www.ebi.ac.uk/gwas/publications/33510174) (15). The data included 256,523 individuals of European descent and were adjusted for sex, age, and population substructure. More details for phenotype and modeling, genotype quality control, and related association analysis can be found in the original publications (12, 14, 15).
In accordance with the three assumptions for MR analysis, independent single-nucleotide polymorphisms (SNPs) associated with the exposure at the genome‐wide significance level (p < 5 × 10-8) were selected as instrumental SNPs (clumping r2 = 0.001 and kb = 10,000) (16). The association values of the corresponding SNPs were also obtained from outcome GWAS summary statistics. However, only three SNPs were selected for FA BMD so that we relaxed the criteria to 1 × 10−6 for selecting FA-related IVs. After harmonizing, we calculated the F statistic to evaluate the strength of selected IVs (https://sb452.shinyapps.io/overlap) (17). Genetic IV with F statistics >10 indicated a good strength of instrument to alleviate potential bias in MR analysis.
The inverse variance weighted (IVW) method was performed to evaluate the bi-directional relation between SP and OP as the main statistical approach (https://mrcieu.github.io/TwoSampleMR/). The IVW method was considered as the most accurate method for estimating the causal relationship if no clear evidence for the presence of directional pleiotropy (p for MR-Egger intercept > 0.05) (18). When there was insufficient evidence of heterogeneity (p for MR-heterogeneity > 0.05) in these selected IVs, a random-effects model was conducted; otherwise, a fixed-effects model was assumed. A weighted median method was also conducted, which can generate effective causal estimates when at least 50% valid IVs were present in all the selected IVs (19). Robust Adjusted Profile Score (RAPS) could eliminate bias and assess causal relationship, even when there were hundreds of weak IVs (20). MR-PRESSO tested for pleiotropy and detected the outliers. After that, the IVW method was repeated (21). Considering multiple testing of OP and SP-related traits, we applied a conservative approach (Bonferroni) by adjusting the p-values (p = 0.05/(2×3) = 0.008).
To test the robustness of our results, several sensitivity analyses were performed. Heterogeneity across IVs was evaluated by Cochran’s Q statistic. MR pleiotropy test was employed to perform MR Egger and returns intercept values to assess horizontal pleiotropy. The MR Steiger test was conducted to examine whether the assumption that exposure causes outcome was valid. Considering that various confounders were closely associated with the pathogenesis of OP and SP, we conservatively culled SNPs, which were closely related to whole-body fat mass, physical activity, and vitamin D levels at the genome‐wide significance level. Data related to confounders were derived from the GWAS Catalog (https://www.ebi.ac.uk/gwas) and GWAS summary data (https://gwas.mrcieu.ac.uk/). We repeated the MR analysis after excluding confounding SNPs.
To the best of the authors’ knowledge, there was no evidence of a link between myopia and OP or SP. Myopia was analyzed as a negative control outcome. Summary‐level data for myopia were obtained from the FinnGen biobank (https://www.finngen.fi/en), including 252,923 individuals of European ancestry.
All the MR tests were performed with the R packages “TwosampleMR”, “MendelianRandomization”, and “MRPRESSO” in the R statistical software (Version 4.1.2).
In the first stage, the F statistics of FA BMD, FN BMD, and LS BMD were calculated and the results were 10.71, 33.38, and 26.41, respectively. Obviously, all the values were larger than 10, which indicated that the selected IVs were powerful enough to eliminate potential bias. The variance explained by the IVs we selected for FA BMD, FN BMD, and LS BMD was calculated through the MR Steiger test (Supplementary Table 1). The influence of OP on low grip strength was studied. A total of 15, 20, and 22 LD-independent and appropriate IVs were selected from GWASs for FA BMD, FN BMD, and LS BMD, respectively (Supplementary Table 2). As shown in Table 1, the IVW results suggested that BMD had no causal effect on low grip strength [FA BMD-related analysis: odds ratio (OR) = 1.006, 95% confidence interval (CI) = (0.945,1.071), p = 0.857; FN BMD-related analysis: OR (95% CI) = 0.971 (0.915,1.030), p = 0.322; LS BMD-related analysis: 0.988 (0.939,1.040), p = 0.655]. The MR pleiotropy test showed no horizontal pleiotropy and no outlier IV was identified in the MR‐PRESSO analysis. In total, all MR analyses supported the idea that OP had no significant causal effect on low grip strength.

Table 1 Mendelian randomization estimates for BMD on sarcopenia-related traits with all selected IVs.
The influence of OP on ALM was also studied. A total of 16, 20, and 22 IVs were obtained for FA BMD, FN BMD, and LS BMD, respectively (Supplementary Table 2). The MR pleiotropy test detected horizontal pleiotropy in FN BMD-related IVs (intercept = 0.028, p = 0.010) and MR-PRESSO detected several potential pleiotropic IVs for BMD (Supplementary Table 3). After the outliers were removed, the IVW results indicated that BMD had a significant causal effect on ALM [FA BMD-related analysis: OR (95% CI) = 1.028 (1.008,1.049), p = 0.006; FA BMD-related analysis: OR (95% CI) = 1.131 (1.092,1.170), p = 3.18E-12; LS BMD-related analysis: OR (95% CI) = 1.080 (1.062,1.098), p = 2.86E-19]. Other MR analysis results are shown in Table 1. In total, most MR analyses supported the notion that OP had a significant negative causal effect on ALM.
In stage 1, no IV was removed because they had no intersection with confounding SNPs (Supplementary Table 4).
In the second stage, the F statistics of low grip strength and ALM were computed and the results were 43.70 and 17.22, respectively. The variance explained by the IVs we selected for low grip strength and ALM were provided in Supplementary Table 1.
The influence of low grip strength on OP was studied. A total of 10, 10, and 10 LD-independent IVs at the genome‐wide significance level were selected from GWASs for low grip strength (Supplementary Table 5). As shown in Table 2, the IVW results suggested that low grip strength had no causal effect on OP [FA BMD-related analysis: OR (95% CI) = 1.191 (0.943,1.505), p = 0.142; FN BMD-related analysis: OR (95% CI) = 0.952 (0.811,1.116), p = 0.543; LS BMD-related analysis: 0.887 (0.776,1.013), p = 0.077]. The MR pleiotropy test showed no horizontal pleiotropy and no outlier IV was identified in the MR‐PRESSO analysis. In total, all MR analyses supported the idea that low grip strength had no significant causal effect on OP.
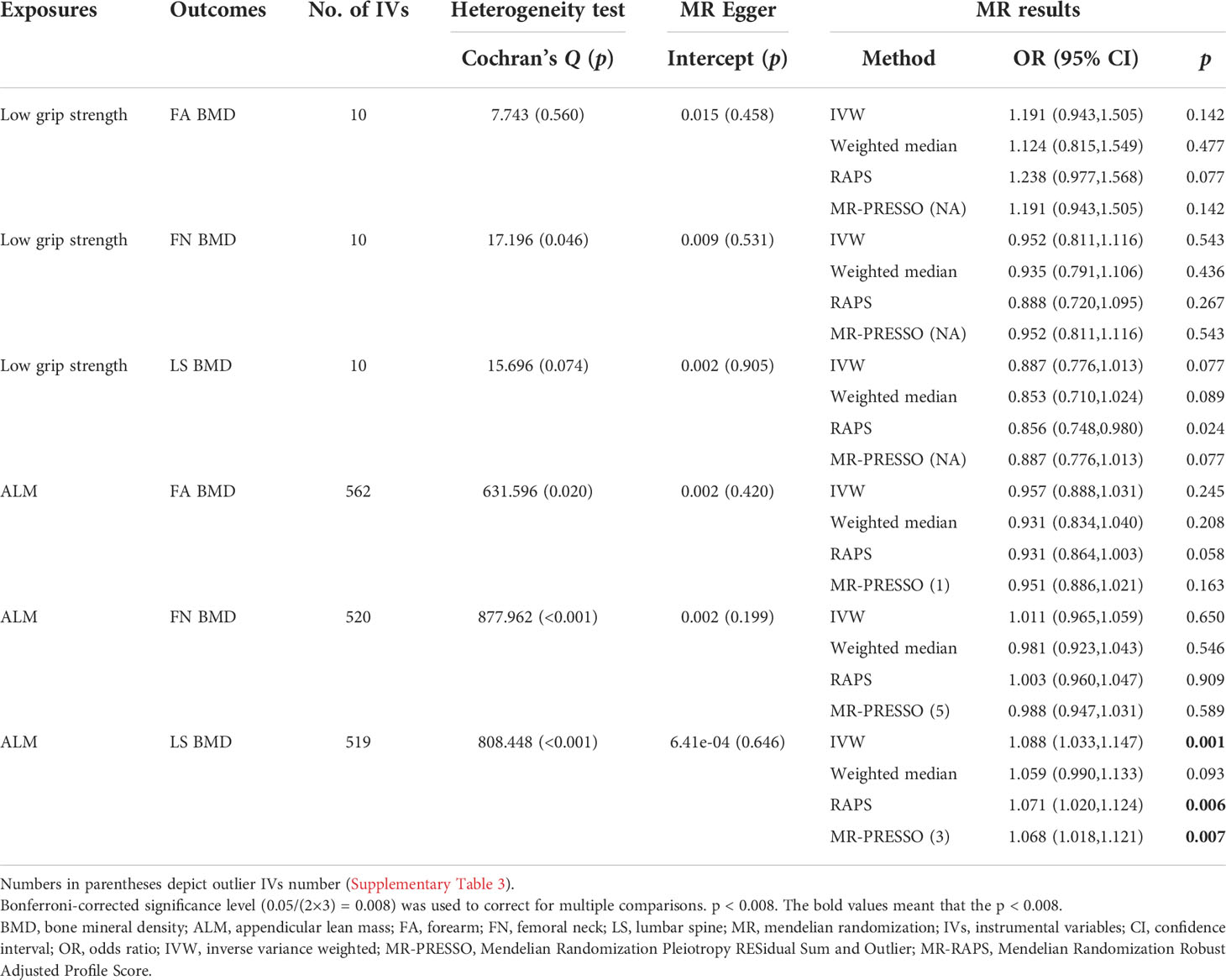
Table 2 Mendelian randomization estimates for sarcopenia-related traits on BMD with all selected IVs.
The influence of ALM on OP was also studied. A total of 560, 520, and 519 appropriate IVs were obtained for ALM, respectively (Supplementary Table 5). The MR pleiotropy test showed no horizontal pleiotropy, but MR-PRESSO detected several potential pleiotropic IVs for ALM (Supplementary Table 3). In total, combined with the MR results detailed above, the results of the MR analyses supported the notion that ALM had no significant causal effect on FA BMD or FN BMD while it identified a significant negative causal effect of ALM on LS BMD, consistent with the IVW results [FA BMD-related analysis: OR (95% CI) = 0.957 (0.888,1.031), p = 0.245; FN BMD-related analysis: OR (95% CI) = 1.011 (0.965,1.059), p = 0.650; LS BMD-related analysis: 1.088 (1.033,1.147), p = 0.001].
In stage 2, several IVs were removed because they had a significant intersection with confounding SNPs (Supplementary Table 4). However, the significance of MR analysis results and horizontal pleiotropy were exactly the same as before (Table 3). The negative control analysis results indicated that FA BMD, FN BMD, LS BMD, low grip strength, and ALM were not relevant to myopia such that the IVs we selected were appropriate (Supplementary Tables 1, 6, and 7).
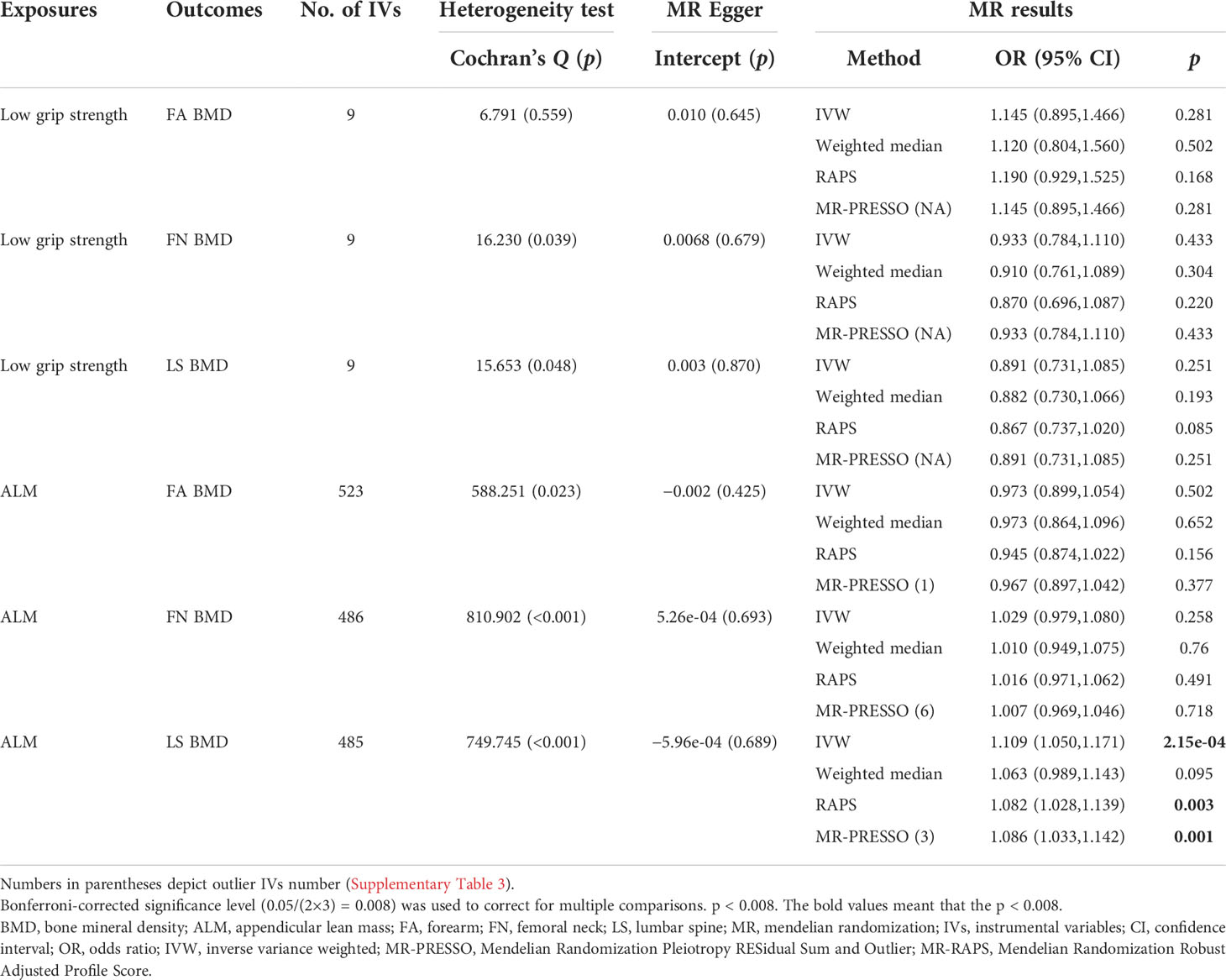
Table 3 Mendelian randomization estimates for sarcopenia-related traits on BMD after removing confounding IVs.
Based on our results, we successfully concluded that OP and SP might mutually had a significant causal effect on each other, identifying the significant positive causal effect of FA BMD, FN BMD, and LS BMD on ALM and the significant positive causal effect of ALM on LS BMD. However, there was no evidence for causal association between BMD and low grip strength. To our knowledge, this is the first bi-directional MR study to investigate causality between OP and SP, considering potential confounders.
Despite these differences, two studies with similar themes at the genetic level are present. It was observed that there was no significant genetic correlation between low grip strength and osteoporotic fracture risk after multiple-testing correction (15), whose result was not in conflict with our assessment of the causality between OP and low grip strength. It was worth noting that low grip strength was a major criterion in SP definition, which indicated that the causality between OP and SP was only partially proved in our results. Additional studies with better MR methods and data would be needed to verify the causality between OP and low grip strength in the future. Pei briefly conducted an MR analysis predicting a causal effect of ALM on fracture (14) while our result indicated that ALM had a significant causal effect on LS BMD, but not FA or FN BMD. Considering that protein supplementation can effectively increase lean mass, we broadened our search to explore more RCT evidence (22). Two meta-analyses involving RCTs and prospective cohort studies suggested that higher compared with lower protein intake had a protective effect on LS BMD, but no effect on total hip, femoral neck, or total body BMD (7). Our conclusion so far is consistent with these findings of relevant studies at the gene level or RCT.
Many previous observation studies have demonstrated the positive correlation between SP and OP (23–25). According to data from the OsteoSys study, 90% of the sarcopenic patients demonstrated low BMD while only few patients with low BMD demonstrated SP (24). A prospective study of 168,682 UK biobank participants demonstrated that pre-sarcopenic men and sarcopenic women had a higher risk of developing OP (25). Our bi-directional MR study further complements previous studies and provided evidence of causality between OP and SP. Bone and muscle are closely connected spatially, and mechanical signals are transmitted from muscle strength to coordinate BMD and muscle mass (26). A recent retrospective study also indicated that chair rising test maximum force and grip strength were positively correlated with cortical geometric and microarchitectural parameters at all measured sites (27). Moreover, accumulating evidence suggested that bone and muscle can secrete a variety of cytokines to modulate each other, including myostatin, irisin, interleukin 6, osteocalcin, RANKL, and osteoprotegerin (28). Notably, as muscle ages, pathophysiological processes in muscle function present as selective loss of fast motor neurons while progressive loss of skeletal muscle mass presents as atrophy of muscle fibers, loss of number of muscle fibers, and reduced number of satellite cells (29). However, a large study including 20,400 adults aged 60 years and over showed that telomere length was not associated with low ALM, low BMD, or low grip strength (30). Anyways, resistance and endurance exercises, creatine monohydrate supplementation, and intake of protein and vitamin D have protective effects on aging muscle and bone (31, 32). Our MR result indicated that OP had a significant causal effect on ALM instead of low grip strength, which provided more information on the mechanisms of muscle–bone crosstalk.
This study is the first bi-directional MR study to investigate causality between OP and SP. Several MR analysis methods were performed to ensure the accuracy and validity of our results; finally, largely consistent results were obtained, which made our results more reliable. The additional negative control, combined with the MR Steiger test, was incorporated to ensure the validity of IVs we selected. Furthermore, to satisfy the second assumptions of MR, we conservatively culled confounding SNPs and the conclusions remain the same and valid. Nevertheless, this study still has several potential limitations. Only the summary‐level statistics were extracted so that we did not evaluate the effect depending on different age and gender separately. Our results indicated that individuals with OP were prone to lose ALM and that severe ALM loss could reduce LS BMD. Meanwhile, it also showed that ALM had no significant causal effect on FA BMD or FN BMD, which warranted scrupulous consideration. Further MR studies with a larger sample size or RCTs are needed to obtain more accurate results. Although three main confounders were removed, other confounders may still work through the second assumptions of MR. Malnutrition should also be considered, which could help us understand the causality between OP and SP in our study. Regrettably, we did not find a qualified statistic of malnutrition. Considering that we should not infer causality from correlation, we only conservatively culled confounding SNPs at the genome‐wide significance level in the absence of relevant data support.
In conclusion, OP and SP might mutually have a significant causal effect on each other. We identified the significant positive causal effect of FA BMD, FN BMD, and LS BMD on ALM and the significant positive causal effect of ALM on LS BMD. There was no evidence for the causal association between BMD and low grip strength.
The datasets presented in this study can be found in online repositories. The websites for these datasets have been provided in the article.
CL had the idea and drafted the final manuscript. NYL performed data analysis. YX created the figure. YZZ gave constructive suggestions during the process. TX and HL drafted the final manuscript and finally approved the version to be published. All authors agreed to be accountable for all aspects of the work.
This work was supported by Research Project of Human Health Commission (grant number 202204073071).
All data used in this study were obtained from openly available databases and consortiums. We express our sincere appreciation to them.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.975647/full#supplementary-material
1. Laskou F, Fuggle NR, Patel HP, Jameson K, Cooper C, Dennison E. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire cohort study. J Cachexia Sarcopenia Muscle (2022) 13(1):220–9. doi: 10.1002/jcsm.12870
2. Hirschfeld HP, Kinsella R, Duque G. Osteosarcopenia: Where bone, muscle, and fat collide. Osteoporos Int (2017) 28(10):2781–90. doi: 10.1007/s00198-017-4151-8
3. Kirk B, Zanker J, Duque G. Osteosarcopenia: Epidemiology, diagnosis, and treatment-facts and numbers. J Cachexia Sarcopenia Muscle (2020) 11(3):609–18. doi: 10.1002/jcsm.12567
4. Lee DY, Shin S. Association of sarcopenia with osteopenia and osteoporosis in community-dwelling older Korean adults: A cross-sectional study. J Clin Med (2021) 11(1):129. doi: 10.3390/jcm11010129
5. Xu B, Guo Z, Jiang B, Zhang K, Zhu W, Lian X, et al. Factors affecting sarcopenia in older patients with chronic diseases. Ann Palliat Med (2022) 11(3):972–83. doi: 10.21037/apm-22-201
6. Gao Q, Hu K, Yan C, Zhao B, Mei F, Chen F, et al. Associated Factors Sarcopenia Community-Dwelling Older Adults: A Systematic Rev Meta-Analysis Nutrients (2021) 13(12):4291. doi: 10.3390/nu13124291
7. Shams-White MM, Chung M, Du M, Fu Z, Insogna KL, Karlsen MC, et al. Dietary protein and bone health: A systematic review and meta-analysis from the national osteoporosis foundation. Am J Clin Nutr (2017) 105(6):1528–43. doi: 10.3945/ajcn.116.145110
8. Tsagari A. Dietary protein intake and bone health. J Frailty Sarcopenia Falls (2020) 5(1):1–5. doi: 10.22540/JFSF-05-001
9. Davey Smith G, Hemani G. Mendelian randomization: Genetic anchors for causal inference in epidemiological studies. Hum Mol Genet (2014) 23(R1):R89–98. doi: 10.1093/hmg/ddu328
10. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through egger regression. Int J Epidemiol (2015) 44(2):512–25. doi: 10.1093/ije/dyv080
11. Styrkarsdottir U, Halldorsson BV, Gretarsdottir S, Gudbjartsson DF, Walters GB, Ingvarsson T, et al. New sequence variants associated with bone mineral density. Nat Genet (2009) 41(1):15–7. doi: 10.1038/ng.284
12. Zheng HF, Forgetta V, Hsu YH, Estrada K, Rosello-Diez A, Leo PJ, et al. Whole-genome sequencing identifies EN1 as a determinant of bone density and fracture. Nature (2015) 526(7571):112–7. doi: 10.1038/nature14878
13. Cawthon PM, Peters KW, Shardell MD, McLean RR, Dam TT, Kenny AM, et al. Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. J Gerontol A Biol Sci Med Sci (2014) 69(5):567–75. doi: 10.1093/gerona/glu023
14. Pei YF, Liu YZ, Yang XL, Zhang H, Feng GJ, Wei XT, et al. The genetic architecture of appendicular lean mass characterized by association analysis in the UK biobank study. Commun Biol (2020) 3(1):608. doi: 10.1038/s42003-020-01334-0
15. Jones G, Trajanoska K, Santanasto AJ, Stringa N, Kuo CL, Atkins JL, et al. Genome-wide meta-analysis of muscle weakness identifies 15 susceptibility loci in older men and women. Nat Commun (2021) 12(1):654. doi: 10.1038/s41467-021-20918-w
16. Clarke L, Zheng-Bradley X, Smith R, Kulesha E, Xiao C, Toneva I, et al. The 1000 genomes project: Data management and community access. Nat Methods (2012) 9(5):459–62. doi: 10.1038/nmeth.1974
17. Palmer TM, Lawlor DA, Harbord RM, Sheehan NA, Tobias JH, Timpson NJ, et al. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Methods Med Res (2012) 21(3):223–42. doi: 10.1177/0962280210394459
18. Holmes MV, Ala-Korpela M, Smith GD. Mendelian randomization in cardiometabolic disease: Challenges in evaluating causality. Nat Rev Cardiol (2017) 14(10):577–90. doi: 10.1038/nrcardio.2017.78
19. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol (2016) 40(4):304–14. doi: 10.1002/gepi.21965
20. Zhao Q, Wang J, Hemani G, Bowden J. Small DS. Stat inference two-sample summary-data Mendelian randomization using robust adjusted profile score. Ann Statist (2020) 48(3):1742–69. doi: 10.1214/19-AOS1866
21. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet (2018) 50(5):693–8. doi: 10.1038/s41588-018-0099-7
22. Gielen E, Beckwée D, Delaere A, De Breucker S, Vandewoude M, Bautmans I. Sarcopenia Guidelines Development Group of the Belgian Society of Gerontology and Geriatrics (BSGG). Nutritional interventions to improve muscle mass, muscle strength, and physical performance in older people: An umbrella review of systematic reviews and meta-analyses. Nutr Rev (2021) 79(2):121–47. doi: 10.1093/nutrit/nuaa011
23. Nielsen BR, Abdulla J, Andersen HE, Schwarz P, Suetta C. Sarcopenia and osteoporosis in older people: A systematic review and meta-analysis. Eur Geriatr Med (2018) 9(4):419–34. doi: 10.1007/s41999-018-0079-6
24. Pourhassan M, Buehring B, Stervbo U, Rahmann S, Molder F, Rutten S, et al. Osteosarcopenia, an asymmetrical overlap of two connected syndromes: Data from the OsteoSys study. Nutrients (2021) 13(11):3786. doi: 10.3390/nu13113786
25. Petermann-Rocha F, Ferguson LD, Gray SR, Rodriguez-Gomez I, Sattar N, Siebert S, et al. Association of sarcopenia with incident osteoporosis: A prospective study of 168,682 UK biobank participants. J Cachexia Sarcopenia Muscle (2021) 12(5):1179–88. doi: 10.1002/jcsm.12757
26. Avin KG, Bloomfield SA, Gross TS, Warden SJ. Biomechanical aspects of the muscle-bone interaction. Curr Osteoporos Rep (2015) 13(1):1–8. doi: 10.1007/s11914-014-0244-x
27. Simon A, Schafer HS, Schmidt FN, Sturznickel J, Amling M, Rolvien T. Compartment-specific effects of muscle strength on bone microarchitecture in women at high risk of osteoporosis. J Cachexia Sarcopenia Muscle (2022). doi: 10.1002/jcsm.13044
28. Zhang L, Sun Y. Muscle-bone crosstalk in chronic obstructive pulmonary disease. Front Endocrinol (Lausanne) (2021) 12:724911. doi: 10.3389/fendo.2021.724911
29. Mahindran E, Law JX, Ng MH, Nordin F. Mesenchymal stem cell transplantation for the treatment of age-related musculoskeletal frailty. Int J Mol Sci (2021) 22(19):10542. doi: 10.3390/ijms221910542
30. Kirk B, Kuo CL, Xiang M, Duque G. Associations between leukocyte telomere length and osteosarcopenia in 20,400 adults aged 60 years and over: Data from the UK biobank. Bone (2022) 161:116425. doi: 10.1016/j.bone.2022.116425
31. Candow DG, Chilibeck PD, Forbes SC, Fairman CM, Gualano B, Roschel H. Creatine supplementation for older adults: Focus on sarcopenia, osteoporosis, frailty and cachexia. Bone (2022) 162:116467. doi: 10.1016/j.bone.2022.116467
Keywords: osteoporosis, sarcopenia, mendelian randomization, osteosarcopenia, aging
Citation: Liu C, Liu N, Xia Y, Zhao Z, Xiao T and Li H (2022) Osteoporosis and sarcopenia-related traits: A bi-directional Mendelian randomization study. Front. Endocrinol. 13:975647. doi: 10.3389/fendo.2022.975647
Received: 22 June 2022; Accepted: 22 August 2022;
Published: 14 September 2022.
Edited by:
Yannis Dionyssiotis, General University Hospital of Patras, GreeceReviewed by:
Renqing Zhao, Yangzhou University, ChinaCopyright © 2022 Liu, Liu, Xia, Zhao, Xiao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Li, bGlodWl4QGNzdS5lZHUuY24=; Tao Xiao, eGlhb3Rhb3h5bEBjc3UuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.