
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Endocrinol., 23 April 2021
Sec. Cancer Endocrinology
Volume 12 - 2021 | https://doi.org/10.3389/fendo.2021.680305
This article is part of the Research TopicNIKE: Neuroendocrine Tumors, Innovation in Knowledge and EducationView all 11 articles
 Manuela Albertelli1,2†
Manuela Albertelli1,2† Federica Grillo2,3*†
Federica Grillo2,3*† Fabio Lo Calzo4,5
Fabio Lo Calzo4,5 Giulia Puliani6,7
Giulia Puliani6,7 Carmen Rainone4
Carmen Rainone4 Annamaria Anita Livia Colao4,8
Annamaria Anita Livia Colao4,8 Antongiulio Faggiano9 and NIKE group
Antongiulio Faggiano9 and NIKE groupDuring the 5th NIKE (Neuroendocrine tumors Innovation in Knowledge and Education) meeting, held in Naples, Italy, in May 2019, discussions centered on the understanding of pathology reports of gastroenetropancreactic neuroendocrine neoplasms. In particular, the main problem concerned the difficulty that clinicians experience in extrapolating relevant information from neuroendocrine tumor pathology reports. During the meeting, participants were asked to identify and rate issues which they have encountered, for which the input of an expert pathologist would have been appreciated. This article is a collection of the most rated questions and relative answers, focusing on three main topics: 1) morphology and classification; 2) Ki67 and grading; 3) immunohistochemistry. Patient management should be based on multidisciplinary decisions, taking into account clinical and pathology-related features with clear comprehension between all health care professionals. Indeed, pathologists require clinical details and laboratory findings when relevant, while clinicians require concise and standardized reports. In keeping with this last statement, the minimum requirements in pathology datasets are provided in this paper and should be a baseline for all neuroendocrine tumor professionals.
During the 5th NIKE (Neuroendocrine tumors Innovation in Knowledge and Education) meeting, held in Naples, Italy, in May 2019, discussions centered on the understanding of pathology reports in gastroenetropancreactic (GEP) neuroendocrine neoplasms (NENs). In particular, the main problem concerned the difficulty clinicians (be they experts or not) have, in extrapolating relevant information from neuroendocrine tumor pathology reports. As the famous publication entitled “Clinicians are from Mars and pathologists are from Venus” (1), perfectly summed up, this is not a new issue. During the meeting, participants were asked to identify issues which they have encountered, for which the input of an expert pathologist would have been appreciated. This article is a collection of the most rated questions, focusing on three main topics: 1) morphology and classification; 2) Ki67 and grading; 3) immunohistochemistry.
A series of questions on various aspects of pathology were proposed to a panel of 36 experts in the field of GEP-NENs (including endocrinologists, pathologists, oncologists, gastroenterologists, surgeons, radiologists and laboratory clinicians; see acknowledgment section). All questions are summarized in Table 1S, along with the rate of votes obtained during the poll (participants could select a total of 8 questions), while the top scored questions are answered below.
Classification systems for NENs have varied over time, each one emphasizing different aspects including function, morphology, site, size and extension of primary tumor, presence of metastases.
With the 2010 GEP NEN WHO classification (2), a morphology and proliferation-based classification system was introduced. It focused on the morphologic distinction between well differentiated (WD neuroendocrine tumors - NET) and poorly differentiated (PD neuroendocrine carcinomas - NEC) neoplasms, as already suggested in the WHO 2000 classification (3). WD-NETs are composed of uniform neoplastic cells, with organoid, trabecular or ribbon-like architecture, round/oval nuclei with “salt and pepper” chromatin and with low nuclear-cytoplasmic ratio. They present secretory granules responsible for intense and diffuse staining for general neuroendocrine markers (synaptophysin and chromogranin) (Figures 1A–D). Nucleoli are inconspicuous and little or no atypia is seen. Mitoses are rare/uncommon and necrosis is also generally absent. PD-NECs, are either of large cell or small cell type (or mixed), with pleomorphic and atypical nuclei, solid growth pattern and abundant non-ischemic necrosis, arranged to form either ‘‘map-like” or ‘‘spotty” necrosis. Mitoses are plentiful and often atypical (4) and proliferation index is extremely high (Figures 1E–H).
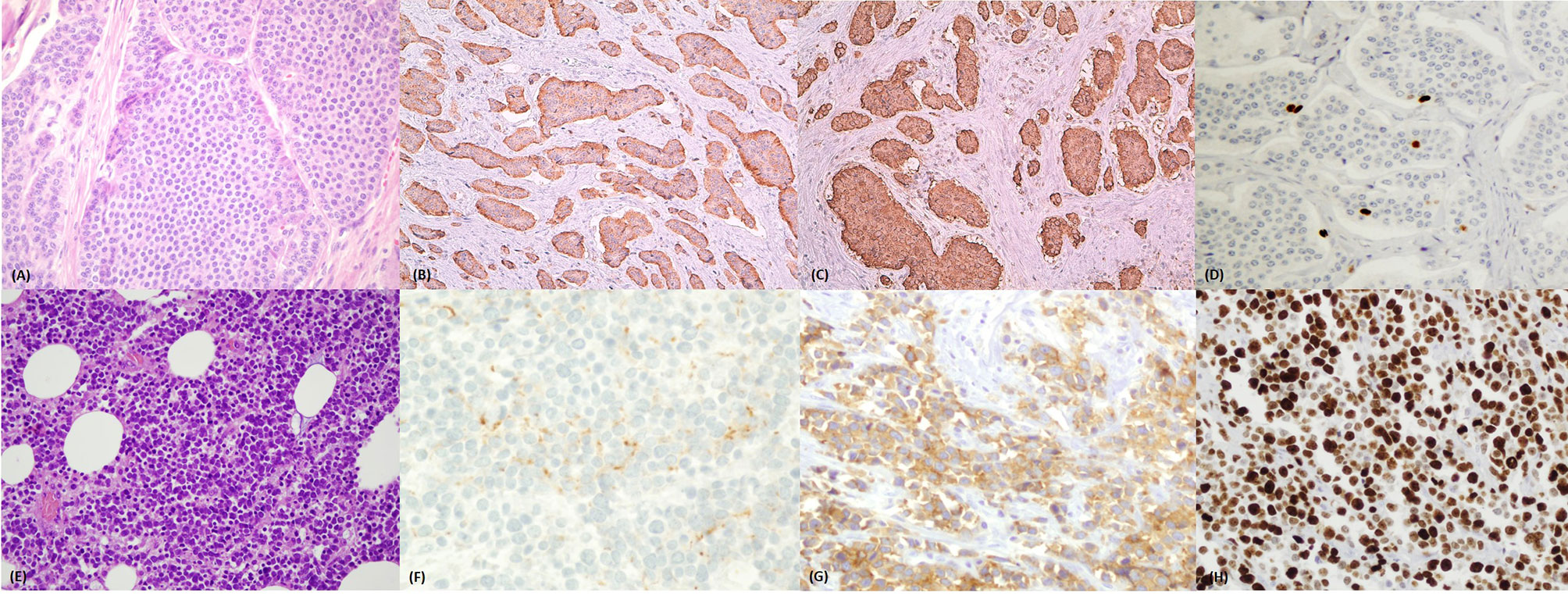
Figure 1 (A–D) Well differentiated neuroendocrine tumor of the ileum. (A) Haematoxylin and eosin stained section (magnification 40x) of a well differentiated ileal neuroendocrine tumor with organoid insular architecture and monomorphic cells with ample eosinophilic cytoplasm and uniform nuclei. (B) Chromogranin A positivity and (C) synaptophysin positivity by immunohistochemistry. (D) Ki67 immunostaining showing rare positive nuclei (stained brown) with <3% proliferation ratio – grade 1. (E–H) Poorly differentiated neuroendocrine carcinoma of the colon. (E) Haematoxylin and eosin stained section (magnification 40x) of a poorly differentiated neuroendocrine carcinoma showing solid structure and small/moderate atypical cells with scanty cytoplasm and hyperchromatic nuclei. (F) Focal dot like positivity for Chromogranin A but diffuse, cytoplasmic expression of synaptophysin (G). (H) Ki67 immunostaining showing diffusely positive nuclei (stained brown) with 90% proliferation ratio – grade 3.
The second aspect of the 2010 WHO classification, which has now become paramount for patient management, is grade, based on mitotic index and/or Ki67 index (see question 3). Initially, categories comprised G1 and G2 WD-NETs and G3 PD-NECs. Some G3 neoplasms were, however, found to be morphologically well differentiated (perhaps with focal areas of greater atypia) but with proliferation indexes greater than 20% [around 45% (5), usually no higher than 50-60%] (6, 7). Subsequent studies have shown that WD G3 neoplasms are a separate category showing better survival compared to PD G3 carcinomas (but worse compared to G2 NET) (8), somatostatin receptor positivity (9), Gallium-PET positivity (with associated possible FGD-PET positivity) and, at the molecular level, mutation profiles similar to WD G1/G2 tumors (10). The landmark study by Sorbye et al (11) reported differences in response to chemotherapy between G3 NENs with Ki67 < 55% compared to > 55%; this study unfortunately failed to review the morphology of the accrued cases.
A revised common classification system of GEP-NENs was therefore proposed for pancreatic NENs in 2017 (12) and extended to all digestive NENs in 2019 (13).
With regards to stage, the 2017 edition of the UICC/AJCC staging manual has specified site specific TNM systems for well differentiated GEP NETs including gastric, duodenal/ampullary, jejunal/ileal, appendiceal, colonic/rectal and pancreatic NETs. The use of this updated system should be standard in all pathology reports.
Mixed neuroendocrine/non-neuroendocrine neoplasms have been described in all organs of the digestive system, with highest frequency in the colon and a diagnostic requirement is that both components be at least 30% of the lesion (though this cut off is arbitrary and not evidence-based). The WHO 2010 classification recommended the term mixed adeno-neuroendocrine carcinoma (MANEC) (2) for such tumors however, this term, does not adequately cover the heterogeneity of possible combinations of neuroendocrine (WD or PD) and non-neuroendocrine (adenocarcinoma, squamous cell carcinoma or adenoma for example) phenotypes.
For this reason, the 2017-2019 WHO classifications changed the term to mixed neuroendocrine and non-neuroendocrine neoplasms (MiNEN). These neoplasms can be stratified into different prognostic categories according to the grade of malignancy of each component: low-grade MiNENs (adenoma and a WD-NET, called MANETs (14); high-grade MiNENs, (PD-NEC with adenocarcinoma, called MANEC or squamous carcinoma in the esophagus or anal canal); intermediate grade neoplasms, composed of adenocarcinoma and NET (15, 16).
In general, the most aggressive cell population drives clinical behavior and this should be considered for therapeutic strategy (17). Recent studies on digestive system MiNENs have shown that prognosis is driven mostly by the NEC component when present, and often, it is this component which metastasizes (18). The Ki67 proliferative index of the neuroendocrine component appears to be the key prognostic factor with differences in survival if Ki67 is above 55% (17). Similarly, Ki67 of 55% seems to be important in composite lung large cell neuroendocrine carcinomas also (19).
With regards to origin, a few studies have demonstrated that both the high-grade NEC component and the non-neuroendocrine component probably derive from the same precursors as they show similar mutation profiles (20).
Grade represents a major prognostic factor (21, 22) and is evaluated on the basis of mitotic index and proliferative index (Ki67 immunostaining) evaluated on sections of tumor. Ki67 is a nuclear protein expressed in the active phases of the cell cycle (G1/S1/G2/M phases) and its function is as a biological surfactant to disperse mitotic chromosomes (23). Discordance between grade assessed by mitotic counting or by Ki67 index is often seen (about 30% of cases), and grade is usually higher when assessed by Ki67 (24, 25). In WHO 2017-2019 (12, 13), grade cut offs have been slightly modified between G1 and G2 so that no grey zone (between 2 and 3%) exists; the distinction between G1 and G2 tumors is now <3% Ki67 index and <2 mitosis/10 high power fields (HPF).
The suggested number of cells in hot spots of expression which should be counted has changed over the years, from 2000 cells in the WHO 2010 to 400-500 cells in the WHO 2017-2019. Furthermore, methods of evaluation of Ki67 have come under scrutiny in recent years as not all methods are equally reliable (26). ‘Eye-ball’ estimation has proved to be unreliable while optimal methods include automated counting by image analyser, manual eye-counting and manual count of camera-captured image. The accuracy and reproducibility of these methods vary in different studies (27, 28).
Besides technical aspects, other possible limitations of Ki67 index assessment derive from the small quantity of tissue available, such as small biopsies (29, 30) and, even more so, in case of cytologic samples. Several studies have focused on the comparison of grading evaluation using endoscopic ultrasound-guided fine needle aspiration and surgical pathology in pancreatic NEN, identifying the correct identification of grade G2 NET as the principal limitation of cytology with both over and undegrading of lesions (31–34). Overall, agreement between cytology and definitive histologic examination was extremely variable in all studies ranging from as low as 34% (31) to close to 100% (35). While it is true that cytology may not be able to accurately predict Ki67 proliferation index in the intermediate range (distinction between G1 from G2 WD-NETs), it is reliable in identifying very proliferative tumors (36) and clinicians should be aware of this.
With regards to intratumoral heterogeneity of grade in NENs, this can be seen (up to 77% of patients in a study in small bowel NENs (37)) and may be related to multifocality and size, when primary tumor > 1 cm, making the staining of Ki67 sufficient only in the largest lesion (38).
When considering change in grade, this has been shown to occur between the primary and metastatic sites and between synchronous/metachronous metastases (39). The first published study on this topic identified 49 patients with metastatic GEP-NEN, showing a discrepancy in grade between sites in 39% of cases, especially in distant compared to locoregional metastases (39). Further studies have demonstrated an overall discordance rate between primary and metastatic tumour of between 1/3-1/2, both with regards to increase (including from G1 to G3) and decrease in grade from primary to metastatic sites (40). Importantly, increased grade in metastatic sites is associated with lower progression free survival and overall survival (41–43).
In conclusion, it is very important for the clinician to be aware of the possibility of change of grade between sites and over time and it may become useful to re-evaluate grade on a new biopsy.
Five somatostatin receptor (SSTR) subtypes have been identified; moreover, two forms of the SSTR2, A and B, are transcripted by alternative splicing, with SSTR2A being the most highly expressed (44). SSTR2 and SSTR5 are the most expressed subtypes and their expression on the membrane of neoplastic cells is the rationale for the use of somatostatin analogues (SSA) and peptide receptor radionuclide therapy in WD-NENs (45, 46). In most cases, functional imaging with 68-Ga-DOTATOC/DOTANOC/DOTATATE PET CT permits the in vivo evaluation of receptor expression (47); as an alternative, the presence of SSTR can be demonstrated by immunohistochemistry. SSTR2A monoclonal antibody has shown high sensitivity/specificity and can be used in formalin-fixed and paraffin-embedded tissues. To standardize the interpretation of immunostaining, Volante et al. proposed a score considering the subcellular pattern and the extension of positive neoplastic cell population (48) with demonstrated high interlaboratory and interobserver SSTR2A immunostaining agreement (49).
Clinicians should be aware of the availability of SSTR2 receptor evaluation in those patients who have not undergone pre-operatory nuclear imaging when, for example, the diagnosis of NEN is made after surgery as recommended by ENETS (36).
SSTR2A expression has been shown to be higher in low-grade NENs and decreased in high grade lesions, both in digestive (50) and in lung (51) neoplasms. Studies have proposed a correlation between the downregulation of SSTR2 expression and NEN growth and progression (52) as well as differences in expression in metastatic sites compared to primary (53). SSTR2A expression may also be correlated with prognosis [WD-NETs with high expression of SSTR2 are associated with longer overall survival (54–56)].
While several studies have evaluated the expression of all SSTR subtypes in NEN (57, 58), this profiling is not part of the routine immunohistochemical evaluation. Notwithstanding this, two aspects seem very promising for future applications: the expression of SSTR5, for predicting the additional value of new SSA pasireotide (59) and the identification of the truncated variant of SSTR5 which seems associated with worse prognosis and low response to SSA (60).
A frequent clinical setting (between 9-19% of NENs) is a patient with multiple liver metastases which show WD-NET and for which the clinician requires, not only a diagnosis of histotype and grade, but an indication of origin as well (61).
Determining the origin of the tumor by histologic features alone is often impossible. The typical neuroendocrine markers used in clinical practice, chromogranin and synaptophysin, do not indicate a specific primary, therefore, further immunohistochemical testing may be required to help pathologists identify primary site. Only in WD-NETs are transcription factors useful and these may be differentially expressed in the bowel (CDX2), lung (TTF1) or pancreas (PAX8, ISL1, PDX1). PD-NECs do not express transcription factors with reliability and these should not be used to identify origin (e.g. TTF1 is often expressed in PD-NECs of any site, including the digestive system).
CDX2 is a nuclear homeobox transcription factor responsible for development of all (neuroendocrine and non-neuroendocrine) intestinal epithelial cells. High prevalence of CDX2 expression was found in ileal (86%) and colonic (75%) NETs while no expression was found in NETs of gastric origin, lung, skin, ovary and thymus (62). CDX2 expression has however been reported in a low percentage of pancreatic NETs (pNETs) (15-26%) (62, 63). Worthy of note is that CDX2 has been shown to be expressed in up to 98% of appendiceal and rectal NETs which originate from enterochromaffin cells (serotonin producing) but not from L-cell NETs (which may be found at both sites) (64).
TTF1 is a transcriptional factor expressed in tissues from the thyroid and lung. Immunohistochemical TTF1 staining is commonly used to identify NET of pulmonary origin as it is highly specific (100%) for pulmonary NET with a lower sensibility, ranging from 35% to 53% (62, 63). OPT – orthopedia homeobox (65) is an extremely useful lung NET marker which is positive in 80% of bronchopulmonary carcinoids and shows much higher sensitivity (80.2% sensitivity and 99.4% specificity) compared to TTF1.
Paired-box genes (PAX) encode a family of nine transcription factors (PAX1-9) important for embryogenesis and organogenesis. PAX8 was found to be expressed in 56-74% of pNET (66, 67), However, specificity is hindered by PAX expression in NETs from the duodenum (75%), stomach (10%) (67, 68).
ISL1 is a transcription factor expressed in pancreatic islet cells and has been shown to be expressed in primary GEP-NETs and, less so, in pulmonary NENs: 59-90% pancreatic, 89% duodenum, 0-16% lung, 0-16% ileum, 0% gastric (69–71). Overall, ISL1 should not be considered entirely specific for pNETs, (overall sensitivity - 69-90% and specificity - 78-88%) considering that sensitivity ranges fall to 67-76% in metastatic pNETs (while specificity increases to 89-98%).
Finally, the sensibility and sensitivity of Pancreatic and Duodenal Homeobox 1 (PDX1) and its role in characterization of NETs is discordant. While some studies found a relatively high specificity and sensibility of PDX1 for pNET (72% expression in primary pNET and 100% in metastatic pNET, with a specificity of 92% and 75% respectively) (72), others demonstrated staining of PDX1 in the rectum, stomach, duodenum, appendix (and rarely in the lung and small bowel) and low percentages of expression in pNET (30%) (62, 73).
An important issue with pancreatic markers is that appendiceal/rectal L-cell tumors often express pancreatic markers such as ISL1, PDX1 and PAX8, as shown above. To overcome this potential pitfall, recent studies have shown that special AT-rich sequence binding protein-2 (SATB2), a transcription factor binding protein, may be used as a specific marker for appendiceal/rectal NETs (it is not expressed in pancreatic/duodenal NETs) (74). Lastly, to distinguish rectal and appendiceal L-cell NETs, positivity for prostatic acid phosphatase confirms rectal origin.
Various immunopanels have been proposed in the literature to identify site of origin, based on differential use of transcription factors and hormone/amine products (61, 69, 73, 75, 76). An immunohistochemical panel demonstrating TTF1 positivity, negativity for CDX2, ISL1 and PDX1 supports a diagnosis of pulmonary NEN. In this setting calcitonin and CEA expression study can help pathologist to distinguish medullary thyroid carcinoma and pulmonary NEN (61, 69). Conversely, an immunohistochemical panel showing strong and diffuse positive staining for CDX2 and negativity for TTF1, ISL1 and PDX1 favors a midgut origin (usually ileal or appendiceal) (61, 69, 73). An immunohistochemical panel demonstrating TTF1 negativity, negative or weak staining for CDX2, ISL1 and PDX1 positivity suggests a NEN originating from the pancreas or duodenum (61, 72) (the distinction between a pancreatic versus a duodenal NEN is challenging). An immunohistochemical panel demonstrating TTF1 and PDX1 negativity, negative or weak staining for CDX2 and ISL1 positivity suggests a L-cell NEN (61, 70, 72). Despite the use of multiple markers primary tumor detection often remains challenging and requires clinical and radiologic information to reach the final diagnosis.
New prognostic immunomarkers, have been recently proposed in NEN. Most of these markers have been principally investigated in pNET and their role in NENs of different sites still remains to be established.
Cytokeratin-19 (CK19) has been shown to be a prognostic factor for NEN even though its prognostic role seems to vary depending on the subtype of pNET. Indeed, CK19 has been identified as a prognostic factor in pNET, excluding insulinomas, with evidence of correlation between CK19 expression and a more aggressive phenotype (77). CK19 has been shown to be an independent prognostic factor (78) with a 5-year survival of all CK19 negative cases of 100%, with a drop to 47% in CK19 positive neoplasms as confirmed by a recent meta-analysis (79).
Insulinoma associated protein 1 (INSM1), a nuclear transcription factor, is a sensitive and well-validated marker for neuroendocrine differentiation (80). Preliminary studies suggest the potential utility of INSM1 as a prognostic factor, as INSM1 expression seems to correlate with more malignant behavior and with greater propensity of metastasis in gastrointestinal NENs (81).
c-KIT, a tyrosine kinase receptor of the platelet derived growth factor subfamily, was found to be a negative independent prognostic marker in pNET with adverse prognosis in c-KIT positive NENs (82).
The prognostic role of DAXX/ATRX expression is more controversial. Some studies have shown loss of expression of DAXX/ATRX to be associated with more aggressive behavior and shorter disease-free survival (83, 84). In contrast, other observations appear to show an improved overall survival in tumors showing loss of DAXX/ATRX (85, 86).
In conclusion, patient management should be based on multidisciplinary decisions based on precise and specific comprehension of information and communication. Clinicians require an understanding of classification systems (which change over time) and the importance of novel markers which may aid in diagnosis and prognosis as well as concise and standardized pathology reports. In keeping with this last statement, an example of the minimum requirements in pathology datasets is shown in Table 1 and should be a baseline for all neuroendocrine tumor professionals (87).

Table 1 Minimum and optional requirements for a pathology report of gastroenteropancreatic neuroendocrine neoplasm [adapted from Volante et al. (87)].
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
MA and FG conceived the study, wrote and finalized the manuscript. GP, FL, and CR contributed to the collection of information and references, writing of the manuscript and approval of the final manuscript. AF and AC supervised and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the Italian Ministry of Education, University and Research (MIUR): PRIN 2017Z3N3YC.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This review is part of the ‘Neuroendocrine Tumors Innovation Knowledge and Education’ project led by Prof. Annamaria Colao and Prof. Antongiulio Faggiano, which aims at increasing the knowledge on NET. We would like to acknowledge all the Collaborators of the “NIKE” project:
Barbara Altieri – Wurzburg; Luigi Barrea – Napoli; Filomena Bottiglieri - Napoli; Severo Campione – Napoli; Federica De Cicco – Napoli; Sergio Di Molfetta - Bari; Alessandra Dicitore – Milano; Carlotta Dolci - Milano, Tiziana Feola - Roma; Giuseppe Fanciulli – Sassari; Diego Ferone - Genova; Francesco Ferraù - Messina; Marco Gallo - Torino; Elisa Giannetta - Roma; Erika Grossrubatscher – Milano; Elia Guadagno - Napoli; Valentina Guarnotta - Palermo; Andrea M. Isidori - Roma; Andrea Lania - Milano; Andrea Lenzi - Roma; Pasquale Malandrino - Catania; Erika Messina - Messina; Roberta Modica - Napoli; Giovanna Muscogiuri - Napoli; Luca Pes - Sassari, Genoveffa Pizza - Avellino; Riccardo Pofi - Roma; Paola Razzore – Torino; Laura Rizza - Roma; Manila Rubino - Milano; Rosa Maria Ruggieri - Messina; Emilia Sbardella – Roma; Franz Sesti - Roma, Mary Anna Venneri - Roma; Giovanni Vitale – Milano; Maria Chiara Zatelli - Ferrara.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.680305/full#supplementary-material
1. Powsner SM, Costa J, Homer RJ. Clinicians are From Mars and Pathologists are From Venus. Arch Pathol Lab Med (2000) 124(7):1040–6. doi: 10.5858/2000-124-1040-CAFMAP
2. Rindi G, Arnold R, Bosman F, Capella C, Klimstra D, Klöppel G, et al. Nomenclature and Classification of Neuroendocrine Neoplasms of the Digestive System. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System, 4th. Lyon: IARC Press (2010). p. 13–14.
3. Capella C, Solcia E, Sobin L. Pathology and Genetics of Tumours of the Digestive System. Hamilton SR, Aaltonen LA, editors. Lyon: IARC Press. (2010). p. 77–8220.
4. Basturk O, Tang L, Hruban RH, Adsay V, Yang Z, Krasinskas AM, et al. Poorly Differentiated Neuroendocrine Carcinomas of the Pancreas: A Clinicopathologic Analysis of 44 Cases. Am J Surg Pathol (2014) 38(4):437–47. doi: 10.1097/PAS.0000000000000169
5. Han X, Xu X, Ma H, Ji Y, Wang D, Kuang T, et al. Clinical Relevance of Different WHO Grade 3 Pancreatic Neuroendocrine Neoplasms Based on Morphology. Endocr Connect (2018) 7(2):355–63. doi: 10.1530/EC-17-0388
6. Coriat R, Walter T, Terris B, Couvelard A, Ruszniewski P. Gastroenteropancreatic Well-Differentiated Grade 3 Neuroendocrine Tumors: Review and Position Statement. Oncologist (2016) 21(10):1191–9. doi: 10.1634/theoncologist.2015-0476
7. Fazio N, Milione M. Heterogeneity of Grade 3 Gastroenteropancreatic Neuroendocrine Carcinomas: New Insights and Treatment Implications. Cancer Treat Rev (2016) 50:61–7. doi: 10.1016/j.ctrv.2016.08.006
8. Tang LH, Basturk O, Sue JJ, Klimstra DS. A Practical Approach to the Classification of WHO Grade 3 (G3) Well-differentiated Neuroendocrine Tumor (Wd-NET) and Poorly Differentiated Neuroendocrine Carcinoma (Pd-NEC) of the Pancreas. Am J Surg Pathol (2016) 40(9):1192–202. doi: 10.1097/PAS.0000000000000662
9. Konukiewitz B, Schlitter AM, Jesinghaus M, Pfister D, Steiger K, Segler A, et al. Somatostatin Receptor Expression Related to TP53 and RB1 Alterations in Pancreatic and Extrapancreatic Neuroendocrine Neoplasms With a Ki67-index Above 20. Mod Pathol (2017) 30(4):587–98. doi: 10.1038/modpathol.2016.217
10. Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, et al. The 2019 WHO Classification of Tumours of the Digestive System. Histopathology (2020) 76(2):182–8. doi: 10.1111/his.13975
11. Sorbye H, Welin S, Langer SW, Vestermark LW, Holt N, Osterlund P, et al. Predictive and Prognostic Factors for Treatment and Survival in 305 Patients With Advanced Gastrointestinal Neuroendocrine Carcinoma (WHO G3): The NORDIC NEC Study. Ann Oncol (2013) 24(1):152–60. doi: 10.1093/annonc/mds276
12. Klöppel G, Couvelard A, Hruban R, Klimstra D, Komminoth P, Osamura R, et al. WHO Classification of Neoplasms of the Neuroendocrine Pancreas. In: Lloyd RV, Osamura RY, Klöppel G, Rosai JL, editors. WHO Classification of Tumors of Endocrine Organs. Lyon, France: IARC Press (2017).
13. Klimstra D, Kloppel G, LA Rosa S, Rindi G, WHO Classification of Tumours Editorial Board. Classification of Neuroendocrine Neoplasms of the Digestive System. In: . Who Classification of Tumours of the Digestive System, 5. Lyon: IARC Press (2019). p. 16–22.
14. La Rosa S, Uccella S, Molinari F, Savio A, Mete O, Vanoli A, et al. Mixed Adenoma Well-Differentiated Neuroendocrine Tumor (MANET) of the Digestive System: An Indolent Subtype of Mixed Neuroendocrine-Nonneuroendocrine Neoplasm (Minen). Am J Surg Pathol (2018) 42(11):1503–12. doi: 10.1097/PAS.0000000000001123
15. La Rosa S, Marando A, Sessa F, Capella C. Mixed Adenoneuroendocrine Carcinomas (Manecs) of the Gastrointestinal Tract: An Update. Cancers (Basel) (2012) 4(1):11–30. doi: 10.3390/cancers4010011
16. La Rosa S, Sessa F, Uccella S. Mixed Neuroendocrine-Nonneuroendocrine Neoplasms (Minens): Unifying the Concept of a Heterogeneous Group of Neoplasms. Endocr Pathol (2016) 27(4):284–311. doi: 10.1007/s12022-016-9432-9
17. Milione M, Maisonneuve P, Pellegrinelli A, Grillo F, Albarello L, Spaggiari P, et al. Ki67 Proliferative Index of the Neuroendocrine Component Drives MANEC Prognosis. Endocr Relat Cancer (2018) 25(5):583–93. doi: 10.1530/ERC-17-0557
18. Tanaka T, Kaneko M, Nozawa H, Emoto S, Murono K, Otani K, et al. Diagnosis, Assessment, and Therapeutic Strategy for Colorectal Mixed Adenoneuroendocrine Carcinoma. Neuroendocrinology (2017) 105(4):426–34. doi: 10.1159/000478743
19. Milione M, Maisonneuve P, Grillo F, Mangogna A, Centonze G, Prinzi N, et al. Ki-67 Index of 55% Distinguishes Two Groups of Bronchopulmonary Pure and Composite Large Cell Neuroendocrine Carcinomas With Distinct Prognosis. Neuroendocrinology (2020) 211–4. doi: 10.1159/000508376
20. Jesinghaus M, Konukiewitz B, Keller G, Kloor M, Steiger K, Reiche M, et al. Colorectal Mixed Adenoneuroendocrine Carcinomas and Neuroendocrine Carcinomas are Genetically Closely Related to Colorectal Adenocarcinomas. Mod Pathol (2017) 30(4):610–9. doi: 10.1038/modpathol.2016.220
21. Philips P, Kooby DA, Maithel S, Merchant NB, Weber SM, Winslow ER, et al. Grading Using Ki-67 Index and Mitotic Rate Increases the Prognostic Accuracy of Pancreatic Neuroendocrine Tumors. Pancreas (2018) 47(3):326–31. doi: 10.1097/MPA.0000000000000990
22. Grillo F, Albertelli M, Annunziata F, Boschetti M, Caff A, Pigozzi S, et al. Twenty Years of Gastroenteropancreatic Neuroendocrine Tumors: Is Reclassification Worthwhile and Feasible? Endocrine (2016) 53(1):58–62. doi: 10.1007/s12020-015-0734-3
23. Cuylen S, Blaukopf C, Politi AZ, Muller-Reichert T, Neumann B, Poser I, et al. Ki-67 Acts as a Biological Surfactant to Disperse Mitotic Chromosomes. Nature (2016) 535(7611):308–12. doi: 10.1038/nature18610
24. McCall CM, Shi C, Cornish TC, Klimstra DS, Tang LH, Basturk O, et al. Grading of Well-Differentiated Pancreatic Neuroendocrine Tumors is Improved by the Inclusion of Both Ki67 Proliferative Index and Mitotic Rate. Am J Surg Pathol (2013) 37(11):1671–7. doi: 10.1097/PAS.0000000000000089
25. van Velthuysen ML, Groen EJ, van der Noort V, van de Pol A, Tesselaar ME, Korse CM. Grading of Neuroendocrine Neoplasms: Mitoses and Ki-67 are Both Essential. Neuroendocrinology (2014) 100(2-3):221–7. doi: 10.1159/000369275
26. van Velthuysen ML, Groen EJ, Sanders J, Prins FA, van der Noort V, Korse CM. Reliability of Proliferation Assessment by Ki-67 Expression in Neuroendocrine Neoplasms: Eyeballing or Image Analysis? Neuroendocrinology (2014) 100(4):288–92. doi: 10.1159/000367713
27. Reid MD, Bagci P, Ohike N, Saka B, Erbarut Seven I, Dursun N, et al. Calculation of the Ki67 Index in Pancreatic Neuroendocrine Tumors: A Comparative Analysis of Four Counting Methodologies. Mod Pathol (2015) 28(5):686–94. doi: 10.1038/modpathol.2014.156
28. Tang LH, Gonen M, Hedvat C, Modlin IM, Klimstra DS. Objective Quantification of the Ki67 Proliferative Index in Neuroendocrine Tumors of the Gastroenteropancreatic System: A Comparison of Digital Image Analysis With Manual Methods. Am J Surg Pathol (2012) 36(12):1761–70. doi: 10.1097/PAS.0b013e318263207c
29. Grillo F, Valle L, Ferone D, Albertelli M, Brisigotti MP, Cittadini G, et al. Ki-67 Heterogeneity in Well Differentiated Gastro-Entero-Pancreatic Neuroendocrine Tumors: When is Biopsy Reliable for Grade Assessment? Endocrine (2017) 57(3):494–502. doi: 10.1007/s12020-017-1364-8
30. Sugimoto S, Hotta K, Shimoda T, Imai K, Ito S, Yamaguchi Y, et al. Can the Ki-67 Labeling Index in Biopsy Specimens Predict the World Health Organization Grade of Rectal Neuroendocrine Tumors? Dig Dis (2018) 36(2):118–22. doi: 10.1159/000484083
31. Abi-Raad R, Lavik JP, Barbieri AL, Zhang X, Adeniran AJ, Cai G. Grading Pancreatic Neuroendocrine Tumors by Ki-67 Index Evaluated on Fine-Needle Aspiration Cell Block Material. Am J Clin Pathol (2020) 153(1):74–81. doi: 10.1093/ajcp/aqz110
32. Boutsen L, Jouret-Mourin A, Borbath I, van Maanen A, Weynand B. Accuracy of Pancreatic Neuroendocrine Tumour Grading by Endoscopic Ultrasound-Guided Fine Needle Aspiration: Analysis of a Large Cohort and Perspectives for Improvement. Neuroendocrinology (2018) 106(2):158–66. doi: 10.1159/000477213
33. Hwang HS, Kim Y, An S, Kim SJ, Kim JY, Kim SY, et al. Grading by the Ki-67 Labeling Index of Endoscopic Ultrasound-Guided Fine Needle Aspiration Biopsy Specimens of Pancreatic Neuroendocrine Tumors can Be Underestimated. Pancreas (2018) 47(10):1296–303. doi: 10.1097/MPA.0000000000001157
34. Weynand B, Borbath I, Bernard V, Sempoux C, Gigot JF, Hubert C, et al. Pancreatic Neuroendocrine Tumour Grading on Endoscopic Ultrasound-Guided Fine Needle Aspiration: High Reproducibility and Inter-Observer Agreement of the Ki-67 Labelling Index. Cytopathology (2014) 25(6):389–95. doi: 10.1111/cyt.12111
35. Grosse C, Noack P, Silye R. Accuracy of Grading Pancreatic Neuroendocrine Neoplasms With Ki-67 Index in Fine-Needle Aspiration Cellblock Material. Cytopathology (2019) 30(2):187–93. doi: 10.1111/cyt.12643
36. Perren A, Couvelard A, Scoazec JY, Costa F, Borbath I, Delle Fave G, et al. Enets Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Pathology: Diagnosis and Prognostic Stratification. Neuroendocrinology (2017) 105(3):196–200. doi: 10.1159/000457956
37. Shi C, Gonzalez RS, Zhao Z, Koyama T, Cornish TC, Hande KR, et al. Liver Metastases of Small Intestine Neuroendocrine Tumors: Ki-67 Heterogeneity and World Health Organization Grade Discordance With Primary Tumors. Am J Clin Pathol (2015) 143(3):398–404. doi: 10.1309/AJCPQ55SKOCYFZHN
38. Numbere N, Huber AR, Shi C, Cates JMM, Gonzalez RS. Should Ki67 Immunohistochemistry be Performed on All Lesions in Multifocal Small Intestinal Neuroendocrine Tumours? Histopathology (2019) 74(3):424–9. doi: 10.1111/his.13771
39. Grillo F, Albertelli M, Brisigotti MP, Borra T, Boschetti M, Fiocca R, et al. Grade Increases in Gastroenteropancreatic Neuroendocrine Tumor Metastases Compared to the Primary Tumor. Neuroendocrinology (2016) 103(5):452–9. doi: 10.1159/000439434
40. Adesoye T, Daleo MA, Loeffler AG, Winslow ER, Weber SM, Cho CS. Discordance of Histologic Grade Between Primary and Metastatic Neuroendocrine Carcinomas. Ann Surg Oncol (2015) 22 Suppl 3:S817–21. doi: 10.1245/s10434-015-4733-7
41. Keck KJ, Choi A, Maxwell JE, Li G, O’Dorisio TM, Breheny P, et al. Increased Grade in Neuroendocrine Tumor Metastases Negatively Impacts Survival. Ann Surg Oncol (2017) 24(8):2206–12. doi: 10.1245/s10434-017-5899-y
42. Richards-Taylor S, Tilley C, Jaynes E, Hu H, Armstrong T, Pearce NW, et al. Clinically Significant Differences in Ki-67 Proliferation Index Between Primary and Metastases in Resected Pancreatic Neuroendocrine Tumors. Pancreas (2017) 46(10):1354–8. doi: 10.1097/MPA.0000000000000933
43. Dhall D, Mertens R, Bresee C, Parakh R, Wang HL, Li M, et al. Ki-67 Proliferative Index Predicts Progression-Free Survival of Patients With Well-Differentiated Ileal Neuroendocrine Tumors. Hum Pathol (2012) 43(4):489–95. doi: 10.1016/j.humpath.2011.06.011
44. Reubi JC, Waser B, Schaer JC, Laissue JA. Somatostatin Receptor sst1-sst5 Expression in Normal and Neoplastic Human Tissues Using Receptor Autoradiography With Subtype-Selective Ligands. Eur J Nucl Med (2001) 28(7):836–46. doi: 10.1007/s002590100541
45. Caplin ME, Pavel M, Cwikla JB, Phan AT, Raderer M, Sedlackova E, et al. Anti-Tumour Effects of Lanreotide for Pancreatic and Intestinal Neuroendocrine Tumours: The CLARINET Open-Label Extension Study. Endocr Relat Cancer (2016) 23(3):191–9. doi: 10.1530/ERC-15-0490
46. Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med (2017) 376(2):125–35. doi: 10.1056/NEJMoa1607427
47. Sundin A, Arnold R, Baudin E, Cwikla JB, Eriksson B, Fanti S, et al. Enets Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Radiological, Nuclear Medicine & Hybrid Imaging. Neuroendocrinology (2017) 105(3):212–44. doi: 10.1159/000471879
48. Volante M, Brizzi MP, Faggiano A, La Rosa S, Rapa I, Ferrero A, et al. Somatostatin Receptor Type 2A Immunohistochemistry in Neuroendocrine Tumors: A Proposal of Scoring System Correlated With Somatostatin Receptor Scintigraphy. Mod Pathol (2007) 20(11):1172–82. doi: 10.1038/modpathol.3800954
49. Kasajima A, Papotti M, Ito W, Brizzi MP, La Salvia A, Rapa I, et al. High Interlaboratory and Interobserver Agreement of Somatostatin Receptor Immunohistochemical Determination and Correlation With Response to Somatostatin Analogs. Hum Pathol (2018) 72:144–52. doi: 10.1016/j.humpath.2017.11.008
50. Wada H, Matsuda K, Akazawa Y, Yamaguchi Y, Miura S, Ueki N, et al. Expression of Somatostatin Receptor Type 2A and PTEN in Neuroendocrine Neoplasms Is Associated With Tumor Grade But Not With Site of Origin. Endocr Pathol (2016) 27(3):179–87. doi: 10.1007/s12022-016-9436-5
51. Righi L, Volante M, Tavaglione V, Bille A, Daniele L, Angusti T, et al. Somatostatin Receptor Tissue Distribution in Lung Neuroendocrine Tumours: A Clinicopathologic and Immunohistochemical Study of 218 ‘Clinically Aggressive’ Cases. Ann Oncol (2010) 21(3):548–55. doi: 10.1093/annonc/mdp334
52. Fotouhi O, Zedenius J, Hoog A, Juhlin CC. Regional Differences in Somatostatin Receptor 2 (SSTR2) Immunoreactivity is Coupled to Level of Bowel Invasion in Small Intestinal Neuroendocrine Tumors. Neuro Endocrinol Lett (2018) 39(4):305–9.
53. Mai R, Kaemmerer D, Trager T, Neubauer E, Sanger J, Baum RP, et al. Different Somatostatin and CXCR4 Chemokine Receptor Expression in Gastroenteropancreatic Neuroendocrine Neoplasms Depending on Their Origin. Sci Rep (2019) 9(1):4339. doi: 10.1038/s41598-019-39607-2
54. Nielsen K, Binderup T, Langer SW, Kjaer A, Knigge P, Grondahl V, et al. P53, Somatostatin Receptor 2a and Chromogranin A Immunostaining as Prognostic Markers in High Grade Gastroenteropancreatic Neuroendocrine Neoplasms. BMC Cancer (2020) 20(1):27. doi: 10.1186/s12885-019-6498-z
55. Qian ZR, Li T, Ter-Minassian M, Yang J, Chan JA, Brais LK, et al. Association Between Somatostatin Receptor Expression and Clinical Outcomes in Neuroendocrine Tumors. Pancreas (2016) 45(10):1386–93. doi: 10.1097/MPA.0000000000000700
56. Wang Y, Wang W, Jin K, Fang C, Lin Y, Xue L, et al. Somatostatin Receptor Expression Indicates Improved Prognosis in Gastroenteropancreatic Neuroendocrine Neoplasm, and Octreotide Long-Acting Release is Effective and Safe in Chinese Patients With Advanced Gastroenteropancreatic Neuroendocrine Tumors. Oncol Lett (2017) 13(3):1165–74. doi: 10.3892/ol.2017.5591
57. Herrera-Martinez AD, Gahete MD, Pedraza-Arevalo S, Sanchez-Sanchez R, Ortega-Salas R, Serrano-Blanch R, et al. Clinical and Functional Implication of the Components of Somatostatin System in Gastroenteropancreatic Neuroendocrine Tumors. Endocrine (2018) 59(2):426–37. doi: 10.1007/s12020-017-1482-3
58. de Herder WW, Hofland LJ, van der Lely AJ, Lamberts SW. Somatostatin Receptors in Gastroentero-Pancreatic Neuroendocrine Tumours. Endocr Relat Cancer (2003) 10(4):451–8. doi: 10.1677/erc.0.0100451
59. Sawicka-Gutaj N, Owecki M, Ruchala M. Pasireotide - Mechanism of Action and Clinical Applications. Curr Drug Metab (2018) 19(10):876–82. doi: 10.2174/1389200219666180328113801
60. Sampedro-Nunez M, Luque RM, Ramos-Levi AM, Gahete MD, Serrano-Somavilla A, Villa-Osaba A, et al. Presence of sst5TMD4, a Truncated Splice Variant of the Somatostatin Receptor Subtype 5, is Associated to Features of Increased Aggressiveness in Pancreatic Neuroendocrine Tumors. Oncotarget (2016) 7(6):6593–608. doi: 10.18632/oncotarget.6565
61. Bellizzi AM. Assigning Site of Origin in Metastatic Neuroendocrine Neoplasms: A Clinically Significant Application of Diagnostic Immunohistochemistry. Adv Anat Pathol (2013) 20(5):285–314. doi: 10.1097/PAP.0b013e3182a2dc67
62. Srivastava A, Hornick JL. Immunohistochemical Staining for CDX-2, Pdx-1, NESP-55, and TTF-1 can Help Distinguish Gastrointestinal Carcinoid Tumors From Pancreatic Endocrine and Pulmonary Carcinoid Tumors. Am J Surg Pathol (2009) 33(4):626–32. doi: 10.1097/PAS.0b013e31818d7d8b
63. Saqi A, Alexis D, Remotti F, Bhagat G. Usefulness of CDX2 and TTF-1 in Differentiating Gastrointestinal From Pulmonary Carcinoids. Am J Clin Pathol (2005) 123(3):394–404. doi: 10.1309/UKN6PVRKXHG422DA
64. Koo J, Zhou X, Moschiano E, De Peralta-Venturina M, Mertens RB, Dhall D. The Immunohistochemical Expression of Islet 1 and PAX8 by Rectal Neuroendocrine Tumors Should be Taken Into Account in the Differential Diagnosis of Metastatic Neuroendocrine Tumors of Unknown Primary Origin. Endocr Pathol (2013) 24(4):184–90. doi: 10.1007/s12022-013-9264-9
65. Nonaka D, Papaxoinis G, Mansoor W. Diagnostic Utility of Orthopedia Homeobox (OTP) in Pulmonary Carcinoid Tumors. Am J Surg Pathol (2016) 40(6):738–44. doi: 10.1097/PAS.0000000000000621
66. Haynes CM, Sangoi AR, Pai RK. PAX8 is Expressed in Pancreatic Well-Differentiated Neuroendocrine Tumors and in Extrapancreatic Poorly Differentiated Neuroendocrine Carcinomas in Fine-Needle Aspiration Biopsy Specimens. Cancer Cytopathol (2011) 119(3):193–201. doi: 10.1002/cncy.20136
67. Sangoi AR, Ohgami RS, Pai RK, Beck AH, McKenney JK, Pai RK. PAX8 Expression Reliably Distinguishes Pancreatic Well-Differentiated Neuroendocrine Tumors From Ileal and Pulmonary Well-Differentiated Neuroendocrine Tumors and Pancreatic Acinar Cell Carcinoma. Mod Pathol (2011) 24(3):412–24. doi: 10.1038/modpathol.2010.176
68. Long KB, Srivastava A, Hirsch MS, Hornick JL. Pax8 Expression in Well-Differentiated Pancreatic Endocrine Tumors: Correlation With Clinicopathologic Features and Comparison With Gastrointestinal and Pulmonary Carcinoid Tumors. Am J Surg Pathol (2010) 34(5):723–9. doi: 10.1097/PAS.0b013e3181da0a20
69. Schmitt AM, Riniker F, Anlauf M, Schmid S, Soltermann A, Moch H, et al. Islet 1 (Isl1) Expression is a Reliable Marker for Pancreatic Endocrine Tumors and Their Metastases. Am J Surg Pathol (2008) 32(3):420–5. doi: 10.1097/PAS.0b013e318158a397
70. Koo J, Mertens RB, Mirocha JM, Wang HL, Dhall D. Value of Islet 1 and PAX8 in Identifying Metastatic Neuroendocrine Tumors of Pancreatic Origin. Mod Pathol (2012) 25(6):893–901. doi: 10.1038/modpathol.2012.34
71. Graham RP, Shrestha B, Caron BL, Smyrk TC, Grogg KL, Lloyd RV, et al. Islet-1 is a Sensitive But Not Entirely Specific Marker for Pancreatic Neuroendocrine Neoplasms and Their Metastases. Am J Surg Pathol (2013) 37(3):399–405. doi: 10.1097/PAS.0b013e31826f042c
72. Chan ES, Alexander J, Swanson PE, Jain D, Yeh MM. Pdx-1, CDX-2, Ttf-1, and CK7: A Reliable Immunohistochemical Panel for Pancreatic Neuroendocrine Neoplasms. Am J Surg Pathol (2012) 36(5):737–43. doi: 10.1097/PAS.0b013e31824aba59
73. Yang Z, Klimstra DS, Hruban RH, Tang LH. Immunohistochemical Characterization of the Origins of Metastatic Well-Differentiated Neuroendocrine Tumors to the Liver. Am J Surg Pathol (2017) 41(7):915–22. doi: 10.1097/PAS.0000000000000876
74. Zhao LH, Chen C, Mao CY, Xiao H, Fu P, Xiao HL, et al. Value of SATB2, ISL1, and TTF1 to Differentiate Rectal From Other Gastrointestinal and Lung Well-Differentiated Neuroendocrine Tumors. Pathol Res Pract (2019) 215(7):152448. doi: 10.1016/j.prp.2019.152448
75. Bellizzi AM. Immunohistochemistry in the Diagnosis and Classification of Neuroendocrine Neoplasms: What can Brown do for You? Hum Pathol (2020) 96:8–33. doi: 10.1016/j.humpath.2019.12.002
76. Maxwell JE, Sherman SK, Stashek KM, O’Dorisio TM, Bellizzi AM, Howe JR. A Practical Method to Determine the Site of Unknown Primary in Metastatic Neuroendocrine Tumors. Surgery (2014) 156(6):1359–65; discussion 65-6. doi: 10.1016/j.surg.2014.08.008
77. La Rosa S, Rigoli E, Uccella S, Novario R, Capella C. Prognostic and Biological Significance of Cytokeratin 19 in Pancreatic Endocrine Tumours. Histopathology (2007) 50(5):597–606. doi: 10.1111/j.1365-2559.2007.02662.x
78. Deshpande V, Fernandez-del Castillo C, Muzikansky A, Deshpande A, Zukerberg L, Warshaw AL, et al. Cytokeratin 19 is a Powerful Predictor of Survival in Pancreatic Endocrine Tumors. Am J Surg Pathol (2004) 28(9):1145–53. doi: 10.1097/01.pas.0000135525.11566.b4
79. Cen D, Chen J, Li Z, Zhao J, Cai X. Prognostic Significance of Cytokeratin 19 Expression in Pancreatic Neuroendocrine Tumor: A Meta-Analysis. PloS One (2017) 12(11):e0187588. doi: 10.1371/journal.pone.0187588
80. Rooper LM, Sharma R, Li QK, Illei PB, Westra WH. Insm1 Demonstrates Superior Performance to the Individual and Combined Use of Synaptophysin, Chromogranin and CD56 for Diagnosing Neuroendocrine Tumors of the Thoracic Cavity. Am J Surg Pathol (2017) 41(11):1561–9. doi: 10.1097/PAS.0000000000000916
81. Rosenbaum JN, Guo Z, Baus RM, Werner H, Rehrauer WM, Lloyd RV. Insm1: A Novel Immunohistochemical and Molecular Marker for Neuroendocrine and Neuroepithelial Neoplasms. Am J Clin Pathol (2015) 144(4):579–91. doi: 10.1309/AJCPGZWXXBSNL4VD
82. Zhang L, Smyrk TC, Oliveira AM, Lohse CM, Zhang S, Johnson MR, et al. KIT is an Independent Prognostic Marker for Pancreatic Endocrine Tumors: A Finding Derived From Analysis of Islet Cell Differentiation Markers. Am J Surg Pathol (2009) 33(10):1562–9. doi: 10.1097/PAS.0b013e3181ac675b
83. Marinoni I, Kurrer AS, Vassella E, Dettmer M, Rudolph T, Banz V, et al. Loss of DAXX and ATRX are Associated With Chromosome Instability and Reduced Survival of Patients With Pancreatic Neuroendocrine Tumors. Gastroenterology (2014) 146(2):453–60. doi: 10.1053/j.gastro.2013.10.020
84. Singhi AD, Liu TC, Roncaioli JL, Cao D, Zeh HJ, Zureikat AH, et al. Alternative Lengthening of Telomeres and Loss of DAXX/ATRX Expression Predicts Metastatic Disease and Poor Survival in Patients With Pancreatic Neuroendocrine Tumors. Clin Cancer Res (2017) 23(2):600–9. doi: 10.1158/1078-0432.CCR-16-1113
85. Park JK, Paik WH, Lee K, Ryu JK, Lee SH, Kim YT. DAXX/ATRX and MEN1 Genes are Strong Prognostic Markers in Pancreatic Neuroendocrine Tumors. Oncotarget (2017) 8(30):49796–806. doi: 10.18632/oncotarget.17964
86. Jiao Y, Shi C, Edil BH, de Wilde RF, Klimstra DS, Maitra A, et al. Daxx/Atrx, MEN1, and mTOR Pathway Genes are Frequently Altered in Pancreatic Neuroendocrine Tumors. Science (2011) 331(6021):1199–203. doi: 10.1126/science.1200609
Keywords: neuroendocrine neoplasms (NENs), neuroendocrine classification, immunohistochemistry, pathology, morphology, grade, Ki67
Citation: Albertelli M, Grillo F, Lo Calzo F, Puliani G, Rainone C, Colao AAL, Faggiano A and NIKE group (2021) Pathology Reporting in Neuroendocrine Neoplasms of the Digestive System: Everything You Always Wanted to Know but Were Too Afraid to Ask. Front. Endocrinol. 12:680305. doi: 10.3389/fendo.2021.680305
Received: 14 March 2021; Accepted: 07 April 2021;
Published: 23 April 2021.
Edited by:
Michele Caraglia, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Boccellino Mariarosaria, University of Campania Luigi Vanvitelli, ItalyCopyright © 2021 Albertelli, Grillo, Lo Calzo, Puliani, Rainone, Colao, Faggiano and NIKE group. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Federica Grillo, ZmVkZXJpY2EuZ3JpbGxvQHVuaWdlLml0
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.