- 1Department of Geriatric Psychiatry, Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Alzheimer's Disease and Related Disorders Center, Shanghai Jiao Tong University, Shanghai, China
- 3Department of Psychiatry, Hubei Provincial Hospital of TCM, Wuhan, China
Background: Previous studies have confirmed that APOE genotype is associated with lipid metabolism, but related studies are inconsistent. Therefore, we conducted this cross-sectional study to explore the associations between apolipoprotein E (APOE) genotypes and serum levels of fasting blood sugar, triglycerides, total cholesterol, high density lipoprotein, and low density lipoprotein in a cognitively normal aging Han Chinese population.
Methods: One hundred sixty-nine community elders with normal cognitive function were included in the study. Based on multiplex amplification refractory mutation system polymerase chain reaction (PCR), these subjects were divided into three groups: (1) E2/2 or E2/3 (APOE E2); (2) E3/3 (APOE E3); and (3) E2/4, E3/4, or E4/4 (APOE E4). Correlations of serum levels of fasting blood sugar, triglycerides, total cholesterol, high density lipoprotein, and low density lipoprotein with APOE genotypes were assessed.
Results: The results of Mann-Whitney analysis showed that the concentration of high density lipoprotein (HDL) in APOE E2 and E3 groups was higher than that in E4 groups (p < 0.05). Logistic regression analysis also suggested that a lower level of high density lipoprotein was associated with the E4 allele (adjusted odds ratio 0.164, 95% confidence interval 0.031~ 0.876, P = 0.034).
Conclusion: APOE E4 is associated with decreased serum high density lipoprotein concentration in healthy elderly. However, the above conclusions need to be further verified.
Introduction
Apolipoprotein E (APOE) is a polypeptide of 299 amino acids encoded by a gene on chromosome 19. It has three allele isoforms (APOE E2, APOE E3, APOE E4) differing in 112 and 158 amino acids positions (1). The APOE E4 allele, found in 13% of the general population, is considered as the predominant risk factor for late-onset Alzheimer's disease (AD); and APOE E3, present in most patients, provides an intermediate level of risk; whereas APOE E2, found in approximately 10% of the population, confers protection (2). The mechanistic link between APOE gene polymorphism and AD has been the focus of numerous studies (3–5), as APOE is one of the primary apolipoproteins in central nervous system (CNS) lipid metabolism (6), we speculate that APOE genotype may affect the pathogenesis of AD by altering lipid homeostasis (7). And increasing evidence also supports that the lipidation status of APOE plays an important role, more concretely, impacting Aβ aggregation, deposition, and clearance (8). In addition, APOE has been shown to directly bind tau, the other major proteinopathy of AD, in vitro (9), while neuronal expression of human APOE can result in tau hyperphosphorylation in vivo (10). So these data also suggest that APOE may directly influence tau pathology and tau-mediated neurodegeneration (10).
APOE exists in low-density lipoprotein (LDL) cholesterol as well as high-density lipoprotein (HDL) cholesterol, and HDL is thought to contribute to lipid transport from peripheral tissues to the liver (a process designated as reverse lipid transport) (11). A previous study (12) showed that APOE E4 was associated with elevated plasma HDL levels in normal elderly people, and this phenomenon was also demonstrated in patients with dementia (13). However, there were also studies showing that elevated serum HDL levels was associated with a significantly decreased risk of dementia (14, 15). What's more, other studies (16–18) supported that APOE E4 was only correlated with a higher serum of total cholesterol and low-density lipoprotein (LDL), but not HDL. Therefore, the conclusions of these studies were inconsistent. Since the relationship between APOE polymorphism with lipid-apolipoprotein blood profiles varies depending on the prevailing regional environmental parameters and ethnicity (19), and there are few similar studies in China, we recruited 169 elderly people from Chinese communities to explore the relationship between APOE genotypes and the levels of serum lipids (fasting blood sugar, triglycerides, total cholesterol, high density lipoprotein, and low density lipoprotein).
Materials and Methods
Participants
This cross-sectional study included 169 community elderly (age ranges from 60 to 90 years, with an average age of 69.74 ± 7.485; among them, 72 were males, accounting for 42.6%) with normal cognitive function and the method of sampling has been described in our previous studies (20). The inclusion criteria were as follows: (1) aged 60 or more; (2) normal cognitive function; (3) without major medical abnormalities, including central nervous system diseases and unstable, acute or life-threatening medical illness; (4) was able to cooperate and complete relevant inspections. Subjects with a history of major medical abnormalities (e.g., infection and cancer) and mental problems (e.g., schizophrenia, anxiety, depression, mild cognitive impairment (MCI) and dementia) or that might affect cognitive function as well as lipid metabolism were excluded. Through face-to-face interviews, we obtained general demographic data (for example, age and gender), daily living habits (smoking, drinking, drinking tea), and disease history (hypertension and diabetes) of the subjects.
Clinical Assessment and Cognitive Assessment
In order to exclude depression, mild cognitive impairment, dementia and other mental diseases, all the participants underwent a screening process that included physical and neurological examinations (by an experienced psychiatrist), a review of their medical history, laboratory tests and MRI scans. The Mini-Mental State Examination (MMSE) was used to assess the cognitive function of subjects. Due to the high educational level of most subjects, individuals with MMSE scores >25 were selected as subjects (21). At the same time, we also used the Global Deterioration Scale (GDS) (22) to eliminate depression.
Genotyping of APOE and Biochemical Detection of Blood Lipids
Genomic DNA was extracted from peripheral blood (Morning fasting whole blood) by using a Blood Genomic DNA Extraction Kit (Qiagen NV, Venlo, the Netherlands). And APOE genotype was determined by multiplex amplification refractory mutation system polymerase chain reaction (PCR). According to the methods previously described (23), these 169 subjects were divided into three groups, APOE E2 (ε2/ε2 and ε2/ε3, n = 25), APOE E3 (ε3/ε3, n = 111), and APOE E4 (ε2/ε4, ε3/ε4, and ε4/ε4, n = 33), and Supplementary Tables 1, 2 list the information about the gene distribution in detail. By using hexokinase method on an auto-analyzer (Dimension Xpand plus), we obtained the values of serum fasting blood glucose, triglyceride, cholesterol, high density lipoprotein, and low density lipoprotein.
The Research Ethical Committee of the affiliated mental health center of Shanghai jiaotong university school of medicine approved this study, and written informed consent was obtained from all participants before the study. All research processes was conducted in accordance with the principles of Declaration of Helsinki.
Statistical Analysis
Categorical variables were expressed as frequencies (%) and continuous variables were expressed as mean ± SD or Median. Single sample Kolmogorov-Smirnov test was used to test whether the data conforms to the normal distribution. One-way analysis of variance (ANOVA) Least—Significant Difference (LSD) was used to compare the data of normal distribution among the APOE E2 group, APOE E 3 group, and APOE E 4 group, while Kruskal-wallis H was used to compare the data of non-normal distribution among these three groups. Then Binary regression analysis was used to screen for possible related factors (At this stage, we divided the study population into APOE E4 group and non-APOE E4 group. And the related variables such as sex, age, and body mass index (BMI) were controlled). Two-tailed tests were used at a significance level of p < 0.05 for all analyses. The data were analyzed using SPSS 22.0 (IBM Corporation, Armonk, NY, USA).
Results
Characteristic of Subjects With Different APOE Genotypes
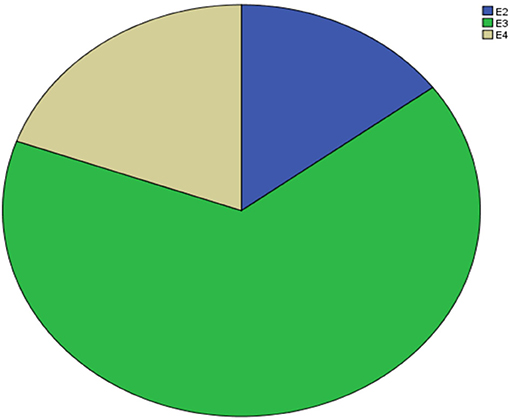
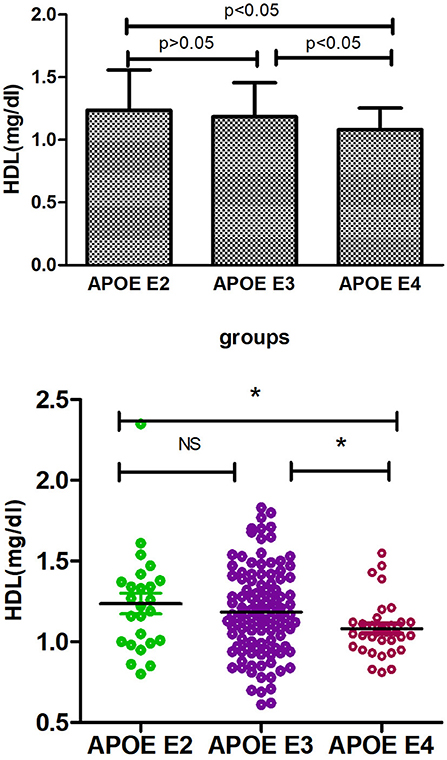
Supplementary Table 1 shows the results of allele and genotype frequencies. The frequencies of APOE E3 were greatest (65.7%), and that of APOE E2 and APOE E4 was 14.8 and 19.5%, respectively (Figure 1). Supplementary Table 2 shows the characteristic of subjects with different APOE genotypes. By using single sample Kolmogorov-Smirnov test, we found that BMI, cholesterol and low density lipoprotein (p > 0.05) were in normal distribution, while age, fasting blood sugar, triglyceride, and high density lipoprotein (p < 0.05) were in non-normal distribution. By using One-way analysis of variance (ANOVA) Least—Significant Difference (LSD) (Normal distribution) and Kruskal-wallis H (Non normal distribution), we found that there was statistical difference (p = 0.001) in high density lipoprotein among the three groups, while there was no significant difference (p > 0.05) in age, gender, BMI, hypertension, diabetes, smoker, drinkers, tea drinker, fasting blood sugar, triglyceride, cholesterol, and low density lipoprotein among the three groups. High density lipoprotein in APOE E4 group was significantly lower than that in APOE E2 group and APOE E3 group, while there was no statistical difference between APOE E2 group and APOE E3 group, Supplementary Table 3 and Figure 2 shows the results (by using Mann-Whitney U test). Then we divided 169 elderly people into APOE E4 group and non-APOE E4 group, by using binary regression analysis and controlling age, gender and BMI, we found that APOE E4 was significantly correlated with high density lipoprotein (OR = 0.164, p = 0.034. 95%CI 0.031~0.876) (Supplementary Table 4).
Discussion
APOE E4 is the major genetic risk factor for Alzheimer's disease (AD), increasing risk and decreasing age of disease onset (24), and a lot of research (25–27) has demonstrated the detrimental effects of APOE E4 in varying cellular contexts. APOE is closely related to circulating lipoproteins, specifically high-density lipoproteins, and very low-density lipoproteins (28), and plays an important role in catabolism and transport of lipoproteins (29). Cognitively normal individuals with APOE E4 have also been demonstrated alterations in cerebral fatty acid (FA) and carbohydrate metabolism congruent with AD patients (30, 31). Since both APOE E4 carriers and individuals with AD exhibit a state of cerebral lipid dyshomeostasis, we hypothesized that APOE may play a role in regulating lipid droplets (LD) metabolism (32).
In this cross-section study, we first investigated the distribution of APOE gene polymorphism in Chinese healthy aging adults, and found that the APOE E3 genotype and E3 allele were the most prevalent in the elderly people with normal cognition, which was consistent with other studies (18, 33). These differences of APOE allele frequency in the normal elderly population could indicate different disease risks; for example, the E4 allele that confers a higher risk of AD (34). Then we explored the effects of APOE polymorphisms on lipid metabolism (including fasting blood sugar, cholesterol, triglyceride, high density lipoprotein, and low density lipoprotein). By using Kruskal-wallis H test and Mann-Whitney U test, we found that the concentration of HDL in the serum of APOE E4 carriers was significantly lower than that of E2 and E3 carriers (p < 0.05), while there was no significant difference (p > 0.05) between E2 and E3 carriers. Similarly, there was also no significant difference (p > 0.05) in fasting blood sugar, triglyceride, cholesterol, and low density lipoprotein among the three groups. Finally, we further explored the correlation between APOE E4 genotype and HDL, and in this step, we combined E2 and E3 into non-E4 groups. By using binary logistic regression analysis, we found that APOE E4 was significantly related to HDL and independent of age, gender and BMI (adjusted odds ratio 0.164, 95% confidence interval 0.031~0.876, P = 0.034). So we concluded that APOE E4 was associated with the decrease of HDL in the serum of normal cognitive elderly.
A previous study has showed that AD patients at the late stage had significantly lower levels of high-density lipids than AD patients at the middle stage, which suggested that the lipid profile might be associated with the development of AD (35). Another research also suggested that low high density lipoprotein was considered as risk factors of dementia in elderly men (36). However, a similar study, also conducted in Chinese cognitively normal aging subjects, found that APOE E4 status was significantly correlated with a higher serum level of total cholesterol, but not with high density lipoprotein (18). Therefore, our results are not entirely consistent, and the discrepancy may be due to age differences (the age of our group was significantly lower than that of their group). In addition, genetic and environmental factors may also aggravate the differences (37, 38).
Based on these findings, we can explain why a lower serum level of high density lipoprotein will increase the risk of dementia. First, observational epidemiology studies have found that cardiovascular diseases are associated with AD risk (39), as high-density lipids is a protective factor against coronary atherosclerosis (40), so decreased high-density lipids concentration will increases the risk of coronary atherosclerosis. Second, lower levels of high density lipoprotein can increase the risk of stroke, atherosclerosis and an inflammatory state, which are all related to dementia (41). Third, macular degeneration in the elderly has been associated with impaired cognitive function, AD, and dementia (42). Zeaxanthin is absorbed together with intestinal fat and transported to the retina by high-density lipoproteins, which can as antioxidants, limiting oxidative damage to the retina cells, exerting a protective effect against Age-related macular degeneration (43). Therefore, APOE E4 might increase the risk of AD in the elderly by lowering serum levels of high density lipoprotein.
There are also containing some limitations: Our study is a cross-sectional study that fails to establish a causal link between APOE E4 and high density lipoprotein. Moreover, relatively small sample size reduces the reliability of research.
Conclusion
In conclusion, APOE E4 is associated with decreased serum high density lipoprotein concentration in Chinese healthy elderly. And further research is required to determine whether these links still exist in longitudinal studies.
Data Availability Statement
The datasets for this manuscript are not publicly available at the time of publication. Requests to access the datasets should be directed to SX, eGlhb3NoaWZ1QG1zbi5jb20=.
Ethics Statement
The studies involving human participants were reviewed and approved by The Research Ethical Committee of the affiliated mental health center of Shanghai jiaotong university school of medicine. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
WL, QQ, and LS contributed to the study concept and design. LY and YL acquired the data. XL and SX analyzed the data and drafted the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the National Key R&D Program of China (2017YFC1310501500), National Natural Science Foundation of China (number 81671402), and Clinical research center project of Shanghai Mental Health Center (CRC2017ZD02).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2019.00827/full#supplementary-material
References
1. Rall SC Jr, Mahley RW. role of apolipoprotein E genetic variants in lipoprotein disorders. J Intern Med. (1992) 231:653–9. doi: 10.1111/j.1365-2796.1992.tb01254.x
2. Ordovas JM, Litwack-Klein L, Wilson PW, Schaefer MM, Schaefer EJ. Apolipoprotein E isoform phenotyping methodology and population frequency with identification of apoE1 and apoE5 isoforms. J Lipid Res. (1987) 28:371–80.
3. Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. (1993) 261:921–3. doi: 10.1126/science.8346443
4. Shinohara M, Kanekiyo T, Yang L, Linthicum D, Shinohara M, Fu Y, et al. APOE2 eases cognitive decline during aging: clinical and preclinical evaluations. Ann Neurol. (2016) 79:758–74. doi: 10.1002/ana.24628
5. Arold S, Sullivan P, Bilousova T, Teng E, Miller CA, Poon WW, et al. Apolipoprotein E level and cholesterol are associated with reduced synaptic amyloid beta in Alzheimer's disease and apoE TR mouse cortex. Acta Neuropathol. (2012) 123:39–52. doi: 10.1007/s00401-011-0892-1
6. Pitas RE, Boyles JK, Lee SH, Hui D, Weisgraber KH. Lipoproteins and their receptors in the central nervous system. Characterization of the lipoproteins in cerebrospinal fluid and identification of apolipoprotein B,E(LDL) receptors in the brain. J Biol Chem. (1987) 262:14352–60.
7. Rebeck GW. Role of APOE on lipid homeostasis and inflammation in normal brains. J Lipid Res. (2017) 58:1493–9. doi: 10.1194/jlr.R075408
8. Hubin E, Verghese PB, van Nuland N, Broersen K. Apolipoprotein E associated with reconstituted high-density lipoprotein-like particles is protected from aggregation. FEBS Lett. (2019) 593:1144–53. doi: 10.1002/1873-3468.13428
9. Strittmatter WJ, Saunders AM, Goedert M, Weisgraber KH, Dong LM, Jakes R, et al. Isoform-specific interactions of apolipoprotein E with microtubule-associated protein tau: implications for Alzheimer disease. Proc Natl Acad Sci USA. (1994) 91:11183–6. doi: 10.1073/pnas.91.23.11183
10. Brecht WJ, Harris FM, Chang S, Tesseur I, Yu GQ, Xu Q, et al. Neuron-specific apolipoprotein e4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice. J Neurosci. (2004) 24:2527–34. doi: 10.1523/JNEUROSCI.4315-03.2004
11. Hoshino T, Kamino K, Matsumoto M. Gene dose effect of the APOE-epsilon4 allele on plasma HDL cholesterol level in patients with Alzheimer's disease. Neurobiol Aging. (2002);23:41–5. doi: 10.1016/S0197-4580(01)00252-4
12. Sing CF, Davignon J. Role of the apolipoprotein E polymorphism in determining normal plasma lipid and lipoprotein variation. Am J Hum Genet. (1985) 37:268–85.
13. Wehr H, Parnowski T, Puzynski S, Bednarska-Makaruk M, Bisko M, Kotapka-Minc S, et al. Apolipoprotein E genotype and lipid and lipoprotein levels in dementia. Dement Geriat Cogn Disord. (2000) 11:70–3. doi: 10.1159/000017217
14. Bonarek M, Barberger-Gateau P, Letenneur L, Deschamps V, Iron A, Dubroca B, et al. Relationships between cholesterol, apolipoprotein E polymorphism and dementia: a cross-sectional analysis from the PAQUID study. Neuroepidemiology. (2000) 19:141–8. doi: 10.1159/000026249
15. Merched A, Xia Y, Visvikis S, Serot JM, Siest G. Decreased high-density lipoprotein cholesterol and serum apolipoprotein AI concentrations are highly correlated with the severity of Alzheimer's disease. Neurobiol Aging. (2000) 21:27–30. doi: 10.1016/S0197-4580(99)00103-7
16. Liu HC, Hong CJ, Wang SJ, Fuh JL, Wang PN, Shyu HY, et al. ApoE genotype in relation to AD and cholesterol: a study of 2,326 Chinese adults. Neurology. (1999) 53:962–6. doi: 10.1212/WNL.53.5.962
17. Raygani AV, Rahimi Z, Kharazi H, Tavilani H, Pourmotabbed T. Association between apolipoprotein E polymorphism and serum lipid and apolipoprotein levels with Alzheimer's disease. Neuroscience Lett. (2006) 408:68–72. doi: 10.1016/j.neulet.2006.08.048
18. Tao QQ, Chen Y, Liu ZJ, Sun YM, Yang P, Lu SJ, et al. Associations between apolipoprotein E genotypes and serum levels of glucose, cholesterol, and triglycerides in a cognitively normal aging Han Chinese population. Clin Intervent Aging. (2014) 9:1063–7. doi: 10.2147/CIA.S62554
19. Boer JM, Feskens EJ, Schouten EG, Havekes LM, Seidell JC, Kromhout D. Lipid profiles reflecting high and low risk for coronary heart disease: contribution of apolipoprotein E polymorphism and lifestyle. Atherosclerosis. (1998) 136:395–402. doi: 10.1016/S0021-9150(97)00231-1
20. Xiao S, Li J, Tang M, Chen W, Bao F, Wang H, et al. Methodology of China's national study on the evaluation, early recognition, and treatment of psychological problems in the elderly: the China Longitudinal Aging Study (CLAS). Shanghai Arch Psychiatr. (2013) 25:91–8. doi: 10.3969/j.issn.1002-0829.2013.02.005
21. Katzman R, Zhang MY, Ouang-Ya-Qu, Wang ZY, Liu WT, Yu E, et al. A Chinese version of the Mini-Mental State Examination; impact of illiteracy in a Shanghai dementia survey. J Clin Epidemiol. (1988) 41:971–8. doi: 10.1016/0895-4356(88)90034-0
22. Reisberg B, Ferris SH, de Leon MJ, Crook T. Global deterioration scale for assessment of primary degenerative dementia. Am J Psychiatr. (1982) 139:1136–9. doi: 10.1176/ajp.139.9.1136
23. Donohoe GG, Salomäki A, Lehtimäki T, Pulkki K, Kairisto V. Rapid identification of apolipoprotein E genotypes by multiplex amplification refractory mutation system PCR and capillary gel electrophoresis. Clin Chem. (1999) 45:143–6.
24. Najm R, Jones EA, Huang Y. Apolipoprotein E4, inhibitory network dysfunction, and Alzheimer's disease. Mol Neurodegen. (2019) 14:24. doi: 10.1186/s13024-019-0324-6
25. Tong LM, Yoon SY, Andrews-Zwilling Y, Yang A, Lin V, Lei H, et al. Enhancing GABA signaling during middle adulthood prevents age-dependent GABAergic interneuron decline and learning and memory deficits in ApoE4 Mice. J Neurosci. (2016) 36:2316–22. doi: 10.1523/JNEUROSCI.3815-15.2016
26. Wang C, Najm R, Xu Q, Jeong DE, Walker D, Balestra ME, et al. Gain of toxic apolipoprotein E4 effects in human iPSC-derived neurons is ameliorated by a small-molecule structure corrector. Nat Med. (2018) 24:647–57. doi: 10.1038/s41591-018-0004-z
27. Chang S, ran Ma T, Miranda RD, Balestra ME, Mahley RW, Huang Y. Lipid- and receptor-binding regions of apolipoprotein E4 fragments act in concert to cause mitochondrial dysfunction and neurotoxicity. Proc Natl Acad Sci USA. (2005) 102:18694–9. doi: 10.1073/pnas.0508254102
28. Gregg RE, Zech LA, Schaefer EJ, Stark D, Wilson D, Brewer HB. Abnormal in vivo metabolism of apolipoprotein E4 in humans. J Clin Invest. (1986) 78:815–21. doi: 10.1172/JCI112645
29. Gupta MD, Girish MP, Sarkar PG, Gupta A, Kategari A, Bansal A, et al. Role of ApoE gene polymorphism and non-conventional biochemical risk factors among very young individuals (aged <35 years) presenting with acute myocardial infarction. Indian Heart J. (2018) 70 (Suppl. 3):S146–56. doi: 10.1016/j.ihj.2018.08.013
30. Brandon JA, Farmer BC, Williams HC, Johnson LA. APOE and Alzheimer's disease: neuroimaging of metabolic and cerebrovascular dysfunction. Front Aging Neurosci. (2018) 10:180. doi: 10.3389/fnagi.2018.00180
31. Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. Proc Natl Acad Sci USA. (2004) 101:284–9. doi: 10.1073/pnas.2635903100
32. Farmer BC, Kluemper J, Johnson LA. Apolipoprotein E4 alters astrocyte fatty acid metabolism and lipid droplet formation. Cells. (2019) 8:E182. doi: 10.3390/cells8020182
33. Donix M, Ercoli LM, Siddarth P, Brown JA, Martin-Harris L, Burggren AC, et al. Influence of Alzheimer disease family history and genetic risk on cognitive performance in healthy middle-aged and older people. Am J Geriat Psychiatry. (2012) 20:565–73. doi: 10.1097/JGP.0b013e3182107e6a
34. Yu JT, Ma XY, Wang YL, Sun L, Tan L, Hu N, et al. Genetic variation in clusterin gene and Alzheimer's disease risk in Han Chinese. Neurobiol Aging. (2013) 34:1921.e1917–23. doi: 10.1016/j.neurobiolaging.2013.01.010
35. Presećki P, Mück-Seler D, Mimica N, Pivac N, Mustapić M, Stipcević T, et al. Serum lipid levels in patients with Alzheimer's disease. Coll Antropol. (2011) 35(Suppl. 1):115–20.
36. Ancelin ML, Ripoche E, Dupuy AM, Barberger-Gateau P, Auriacombe S, Rouaud O, et al. differences in the associations between lipid levels and incident dementia. J Alzheimer's Dis. (2013) 34:519–28. doi: 10.3233/JAD-121228
37. Bernstein MS, Costanza MC, James RW, Morris MA, Cambien F, Raoux S, et al. Physical activity may modulate effects of ApoE genotype on lipid profile. Arterioscl Thromb Vasc Biol. (2002) 22:133–40. doi: 10.1161/hq0102.101819
38. Nicklas BJ, Ferrell RE, Bunyard LB, Berman DM, Dennis KE, Goldberg AP. Effects of apolipoprotein E genotype on dietary-induced changes in high-density lipoprotein cholesterol in obese postmenopausal women. Metabolism. (2002) 51:853–8. doi: 10.1053/meta.2002.33337
39. Attems J, Jellinger KA. overlap between vascular disease and Alzheimer's disease–lessons from pathology. Medicine. (2014) 12:206. doi: 10.1186/s12916-014-0206-2
40. Fonseca VA, Handelsman Y, Staels B. Colesevelam lowers glucose and lipid levels in type 2 diabetes: the clinical evidence. Diab Obesity Metabol. (2010) 12:384–92. doi: 10.1111/j.1463-1326.2009.01181.x
41. Zuliani G, Cavalieri M, Galvani M, Volpato S, Cherubini A, Bandinelli S, et al. Relationship between low levels of high-density lipoprotein cholesterol and dementia in the elderly. InChianti study. J Gerontol. (2010) 65:559–64. doi: 10.1093/gerona/glq026
42. Rong SS, Lee BY, Kuk AK, Yu XT, Li SS, Li J, et al. Comorbidity of dementia and age-related macular degeneration calls for clinical awareness: a meta-analysis. Br J Ophthal. (2019) 103:1777–83. doi: 10.1136/bjophthalmol-2018-313277
Keywords: APOE E4, cognitively normal aging, high density lipoprotein, Chinese, cognition
Citation: Li W, Li Y, Qiu Q, Sun L, Yue L, Li X and Xiao S (2019) Associations Between the Apolipoprotein E ε4 Allele and Reduced Serum Levels of High Density Lipoprotein a Cognitively Normal Aging Han Chinese Population. Front. Endocrinol. 10:827. doi: 10.3389/fendo.2019.00827
Received: 06 September 2019; Accepted: 12 November 2019;
Published: 05 December 2019.
Edited by:
James Harper, Sam Houston State University, United StatesReviewed by:
Sarah King, University of Sussex, United KingdomJennifer Rusted, University of Sussex, United Kingdom
Copyright © 2019 Li, Li, Qiu, Sun, Yue, Li and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xia Li, amFfMTAyM0Bob3RtYWlsLmNvbQ==; Shifu Xiao, eGlhb3NoaWZ1QG1zbi5jb20=
†These authors have contributed equally to this work
 Wei Li
Wei Li Yong Li
Yong Li Qi Qiu
Qi Qiu Lin Sun
Lin Sun Ling Yue
Ling Yue Xia Li
Xia Li Shifu Xiao
Shifu Xiao