
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol. , 08 August 2019
Sec. Clinical Diabetes
Volume 10 - 2019 | https://doi.org/10.3389/fendo.2019.00559
 Yan-yan Li1,2*
Yan-yan Li1,2* Xin-zheng Lu3
Xin-zheng Lu3 Xin-xing Yang2
Xin-xing Yang2 Hui Wang3
Hui Wang3 Hong-yu Geng4
Hong-yu Geng4 Ge Gong5
Ge Gong5 Yi-yang Zhan2
Yi-yang Zhan2 Hyun Jun Kim6
Hyun Jun Kim6 Zhi-jian Yang3
Zhi-jian Yang3Background: Although many studies indicate a positive correlation between GHRL gene Leu72Met polymorphism and an increased susceptibility to type 2 diabetes mellitus (T2DM), inconsistencies between independent studies still remain.
Objective: Considering the inconsistencies between them, we have performed the current meta-analysis study. The objective of this study is to better examine the correlation of the GHRL gene Leu72Met polymorphism and T2DM.
Methods: The current meta-analysis, involving 8,194 participants from 11 independent studies, was performed. A fixed effect model was used to evaluate the pooled odds ratios (ORs) and the corresponding 95% confidence intervals (95% CIs).
Results: A significant association was found between T2DM and GHRL gene Leu72Met polymorphism under recessive (OR: 1.33, 95% CI: 1.01–1.76, P = 0.04), and homozygous genetic models (OR: 1.34, 95% CI: 1.01–1.78, P = 0.04) in the whole population. The correlation was more distinct in our subgroup analysis of the Chinese population under recessive (OR: 1.52, 95% CI: 1.07–2.15, P = 0.02), dominant (OR: 1.70, 95% CI: 1.38–2.10, P < 0.00001), additive (OR: 1.16, 95% CI: 1.02–1.33, P = 0.02), and homozygous genetic models (OR: 1.54, 95% CI: 1.07–2.20, P = 0.02).
Conclusions: In short, GHRL gene Leu72Met polymorphism was significantly correlated with increased T2DM risk, particularly in the Chinese population. Individuals carrying the Met72 allele of GHRL Leu72Met gene polymorphism, particularly those of Chinese ancestry, may be more susceptible to developing T2DM disease.
Type 2 diabetes mellitus (T2DM) is characterized by hyperglycemia in the context of insulin resistance and impaired insulin secretion. Its long-term complications include abnormal protein and lipid metabolism and multisystem damage (1). T2DM is a multi-genic disease caused by the synergistic interactions between hereditary and environmental factors. Recently multiple genes determining the presence of T2DM have been reported as ATP binding cassette transporter 1 (ABCA 1) (2), β-defensin 1 (3), 5- lipoxygenase (ALOX5) (4), solute carrier family 30 member 8 (SLC30A8) (5), ADIPOQ, KCNJ11, TCF7L2 (6), superoxide dismutase 2 (SOD2) (7), interleukin-10 (IL-10) (8), paraoxonase 2 (PON2) gene (9), and GHRL (Ghrelin) (10). While hereditary factors play an important role in the T2DM development, clarification of their exact mechanisms depend on the identification of T2DM susceptibility genes.
GHRL (Ghrelin) is a promising susceptibility gene for T2DM. First discovered by Kojima in 1999, the GHRL gene encodes for a 28 amino acid brain-gut peptide hormone, ghrelin (10). Although ghrelin is primarily synthesized and secreted by X/A-like oxyntic cells of the stomach, it is also produced within the hypothalamus and pancreas (11). Ghrelin is also synthesized by other tissues, such as small intestine, kidney, heart, adipose tissue, and so on (12). As the endogenous ligand of the growth-hormone secretagogue receptor (GHS-R), ghrelin promotes adenohypophysis to release growth hormone, but also has roles in appetite regulation, fat accumulation, gut motility, and energy balance (13). Additionally, ghrelin may play a role in the blood glucose and insulin regulation as decreased plasma ghrelin levels was associated with T2DM (14).
The GHRL gene, located in 3p25-26, contains 4 exons and 3 introns. The four exons encode preproghrelin with 117 amino acids, of which, the first and second encode mature ghrelin with 28 amino acids (15). Most of GHRL polymorphisms studied to date are located in the promoter and in the coding regions of the gene and some of them have implications for gene activity (16). The polymorphism most strongly associated with T2DM is the Leu72Met polymorphism (+408C>A) and by switching cytosine (C) in the second exon to adenine (A), the mutation replaces the leucine (Leu) at the 72nd amino acid with a methionine (Met).
Though many studies examining the association between the GHRL gene Leu72Met polymorphism and T2DM have been conducted, the conclusions of these studies have often been contradictory. In 2006, Mager et al. reported that Finnish subjects with the Leu72Leu genotype had a decreased risk for T2DM development and that the Met72 allele might be a contributory factor to T2DM (17). In 2012, Wang et al. reproduced the result in China (18). On the contrary, in 2008, Berthold et al. observed that German subjects with Met72+ genotypes had a significantly reduced risk of T2DM (OR 0.63, 95% CI 0.42–0.95, P = 0.026) (19). In 2006, Choi et al. reported that GHRL gene Leu72Met polymorphism was not found to be significantly associated with susceptibility to T2DM in the Korean population (20).
The current meta-analysis including 4,299 T2DM cases and 3,895 controls was carried out to evaluate the association of T2DM and GHRL Leu72Met gene polymorphism (Supplement S1).
We searched databases, such as the China National Knowledge Infrastructure, the China Biological Medicine Database, Pubmed, Embase, and the Web of Science, using the terms, “GHRL,” “Leu72Met,” “type 2 diabetes mellitus,” and “polymorphism.” The search deadline was on December 17, 2018 and the publication date of the resultant studies ranged from 2005 to 2012.
The selected studies had to conform to the following inclusion criteria: (a) assessment of the association between T2DM and GHRL Leu72Met gene polymorphism. (b) fasting plasma glucose (FPG) is used in the diagnosis of T2DM as proposed by the American Diabetes Association (2005). The FPG level ≥7.0 mmol/L or the 2 h plasma glucose of oral glucose tolerance test ≥11.1 mmol/L. No genetic relationships are present between the participants of the study. (c) Adopted data is derived from case-control or cohort studies published in the official journals or postgraduate dissertations. (d) In the individual studies, the control group genotype member should follow the Hardy-Weinberg equilibrium (HWE).
The data present in the studies was extracted according to classic protocol. Several investigators performed the meta-analysis. Two investigators retrieved the studies in duplicate and a third served as the arbitrator to settle differences between the two investigators and helped form a consensus. The excluded researches included those that failed to satisfy inclusion criteria, were repeated in other publications present in the analysis, or supplied inadequate information. Same data presented in different papers, but derived from the same group of authors was used only once. Items, such as the name of the first author, publication date, research region, genotypes numbers, and a whole number of subjects were included in the extracted data.
Four genetic models were used in the meta-analysis: recessive (MM vs. LL+LM), dominant (LM+MM vs. LL), homozygous (MM vs. LL), and additive (M vs. L) genetic models. The association of GHRL gene Leu72Met polymorphism and T2DM was analyzed using odds ratios (ORs) and their corresponding 95% confidence intervals (CIs). The heterogeneity among the studies was calculated by the chi-square-based Q-test (P < 0.05) (22). If heterogeneity were present, the random-effect model using the DerSimonian and Laird method would be employed (23). But in the case that heterogeneity was not detected, the fixed-effect model using the Mantel-Haenszel method would be adopted (24). The pooled OR was estimated by Z-test (P < 0.05).
The Fisher's exact test was used to assess the HWE (P < 0.05) and the funnel plot, to estimate potential publication bias. Egger's linear regression test on the natural logarithm scale of the OR was used to evaluate the funnel plot symmetry (P < 0.05) (25). The Stata 11.0 software (StataCorp, College Station, TX, USA) was used to perform the statistical analyses.
Of the 19 manuscripts produced by the search 11 satisfied the inclusion criteria. Among the eight discarded studies, three papers were repeated publications, two were reviews, and the remaining three were not related to the topic in question. None of the studies deviated from the HWE. The data listed in Table 1 was drawn from 4,299 T2DM patients and 3,895 controls (1, 17, 19, 20, 26–32) (Supplement S2). The participants of the studies represent six countries: China, Denmark, Finland, France, Germany, and Korea. For our analysis, participants from Denmark, Finland, France, Germany, and Korea were categorized into the non-Chinese subgroup. Within the Chinese subgroup, participants hailed from five different provinces: Beijing, Shanghai, Guangdong, Heilongjiang, and Gansu.
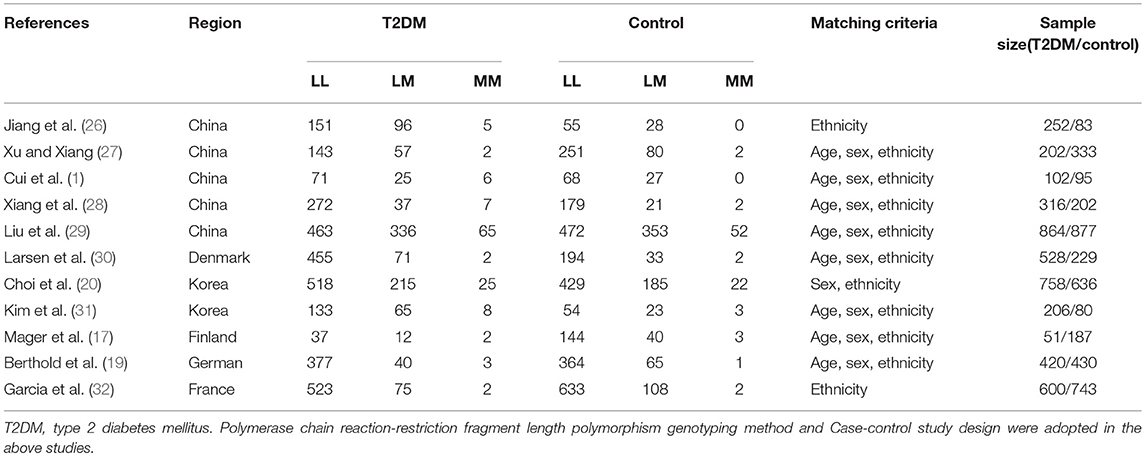
Table 1. Characteristics of the investigated studies of the association between type 2 diabetes mellitus (T2DM) and GHRL gene Leu72Met (L72M) polymorphism.
In our analysis of the whole population, we found a significant correlation between the GHRL Leu72Met gene polymorphism and T2DM under recessive (OR: 1.33, 95% CI: 1.01–1.76, P = 0.04) and homozygous genetic models (OR: 1.34, 95% CI: 1.01–1.78, P = 0.04). No significant correlation was found under dominant (OR: 1.16, 95% CI: 0.90–1.50, P = 0.26) or additive genetic models (OR: 1.03, 95% CI: 0.94–1.13, P = 0.14). In our subgroup analysis of the non-Chinese population, we found no significant correlation was detected under recessive (OR: 1.05, 95% CI: 0.66–1.68, P = 0.83), dominant (OR: 0.87, 95% CI: 0.71–1.07, P = 0.19), homozygous (OR: 1.05, 95% CI: 0.65–1.68, P = 0.85), and additive genetic models (OR: 0.91, 95% CI: 0.80–1.04, P = 0.18; Table 2, Figures 1–4). On the other hand, our subgroup analysis of the Chinese population found a more pronounced correlation between the GHRL Leu72Met gene polymorphism and T2DM under recessive (OR: 1.52, 95% CI: 1.07–2.15, P = 0.02), dominant (OR: 1.70, 95% CI: 1.38–2.10, P < 0.00001), additive (OR: 1.16, 95% CI: 1.02–1.33, P = 0.02), and homozygous (OR: 1.54, 95% CI: 1.07–2.20, P = 0.02).
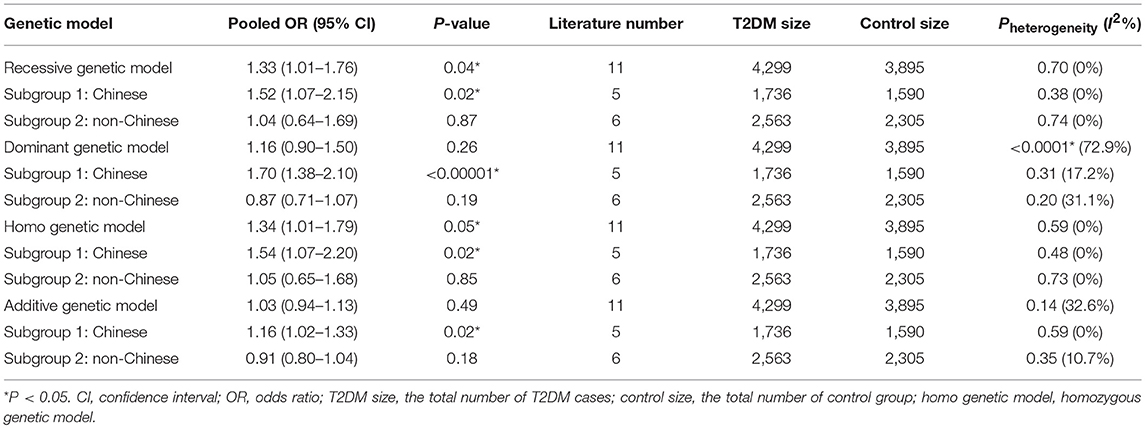
Table 2. Summary of meta-analysis of association of type 2 diabetes mellitus (T2DM) and GHRL gene Leu72Met (L72M) polymorphism.
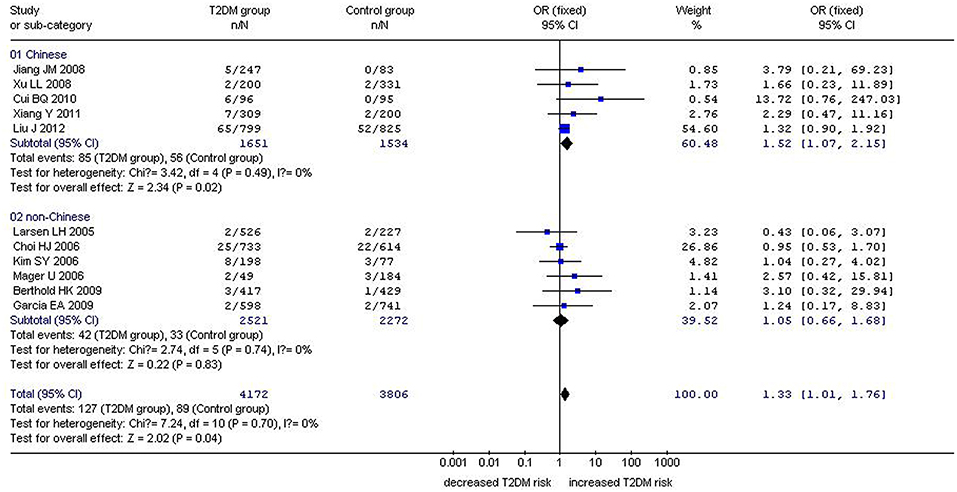
Figure 1. Forest plot of T2DM associated with GHRL gene Leu72Met (L72M) polymorphism under a recessive genetic model (MM vs. LM+LL). Under the recessive genetic model, a significant correlation between the GHRL Leu72Met gene polymorphism and T2DM was found (OR: 1.33, 95% CI: 1.01–1.76, P = 0.04). A more pronounced correlation between them was found (OR: 1.52, 95% CI: 1.07–2.15, P = 0.02) in the Chinese population. No significant correlation was detected in the non-Chinese population (OR: 1.05, 95% CI: 0.66–1.68, P = 0.83).
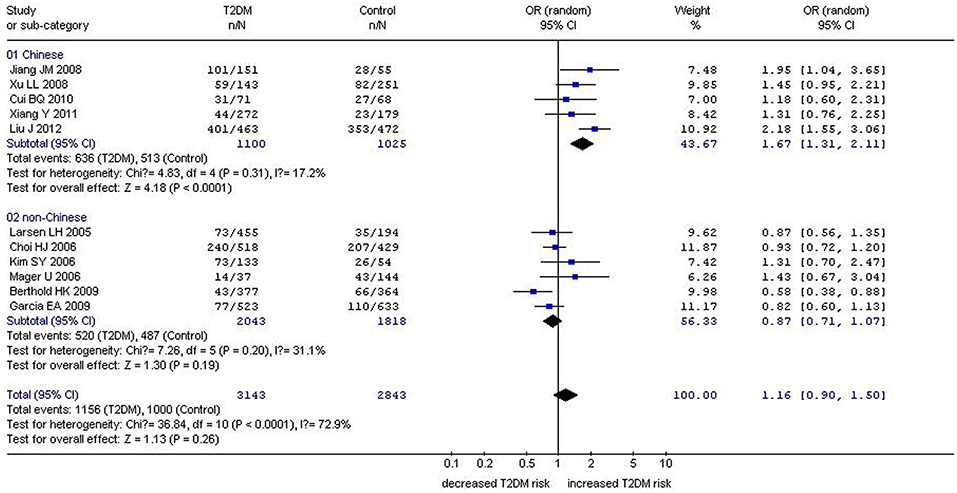
Figure 2. Forest plot of T2DM associated with GHRL gene Leu72Met (L72M) polymorphism under a dominant genetic model (LM+MM vs. LL). Under the dominant genetic model, no significant correlation was found (OR: 1.16, 95% CI: 0.90–1.50, P = 0.26). A significant correlation between them was found (OR: 1.70, 95% CI: 1.38–2.10, P < 0.00001) in the Chinese population. No significant correlation was detected in the non-Chinese population (OR: 0.87, 95% CI: 0.71–1.07, P = 0.19).
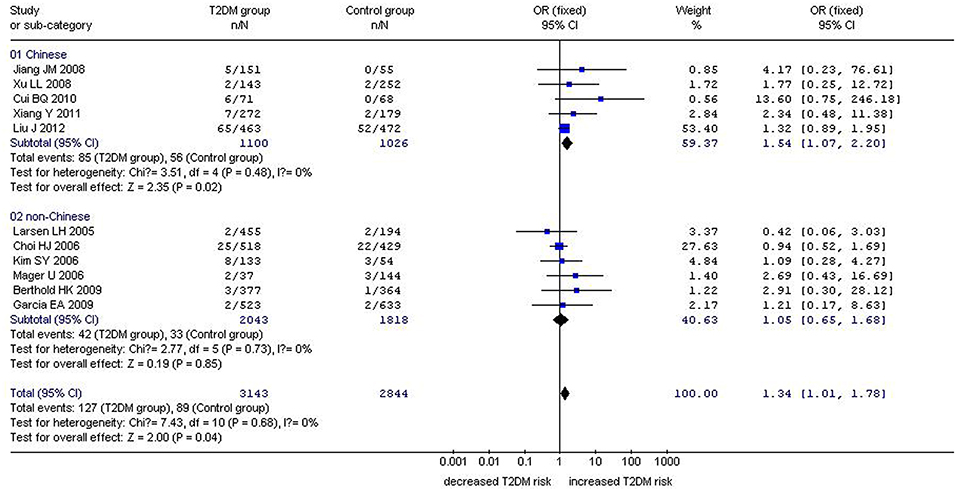
Figure 3. Forest plot of T2DM associated with GHRL gene Leu72Met (L72M) polymorphism under a homozygous genetic model (MM vs. LL). Under the homozygous genetic model, a significant correlation between the GHRL Leu72Met gene polymorphism and T2DM was found (OR: 1.34, 95% CI: 1.01–1.78, P = 0.04). A more pronounced correlation between them was found (OR: 1.54, 95% CI: 1.07–2.20, P = 0.02) in the Chinese population. No significant correlation was detected in the non-Chinese population (OR: 1.05, 95% CI: 0.65–1.68, P = 0.85).
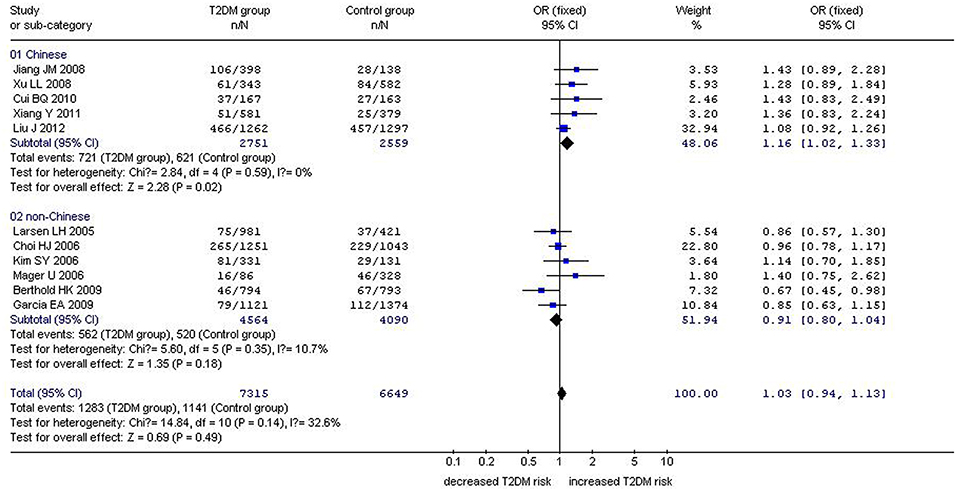
Figure 4. Forest plot of T2DM associated with GHRL gene Leu72Met (L72M) polymorphism under an additive genetic model (M vs. L). Under the additive genetic model, no significant correlation was found (OR: 1.03, 95% CI: 0.94–1.13, P = 0.14). A significant correlation between them was found (OR: 1.16, 95% CI: 1.02–1.33, P = 0.02) in the Chinese population. No significant correlation was detected in the non-Chinese population (OR: 0.91, 95% CI: 0.80–1.04, P = 0.18).
We used the funnel plot and Egger's test to assess for publication bias among the individual studies. The symmetry of the funnel plot (Figure 5) and the lack of significant difference detected by the Egger's test imply no publication bias is present in the current meta-analysis by using the recessive genetic model (T = −0.61, P = 0.563).
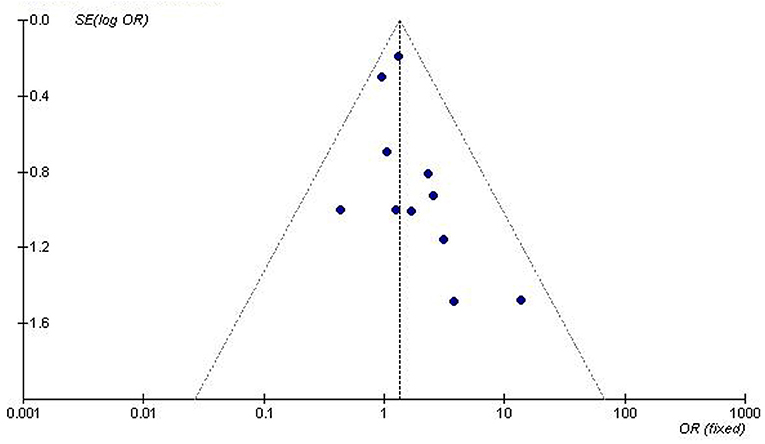
Figure 5. Funnel plot for studies of the association of T2DM associated with GHRL gene Leu72Met (L72M) polymorphism under a recessive genetic model (LM+MM vs. LL). The dot distribution in the funnel plot is symmetrical which imply no publication bias is present in the current meta-analysis by using the recessive genetic model. The horizontal and vertical axis correspond to the OR and confidence limits. OR, odds ratio; SE, standard error.
In the current meta-analysis, we observed a significant correlation in the whole population between the GHRL gene Leu72Met polymorphism and T2DM under recessive (OR: 1.33) and homozygous genetic models (OR: 1.34). While correlation in the non-Chinese population was not significant under any of the genetic models (P > 0.05), correlation in the Chinese population was significant under all four genetic models—recessive (OR: 1.52), dominant (OR: 1.70), homozygous (OR: 1.54), and additive genetic models (OR: 1.16). Though the Met72 allele of GHRL Leu72Met gene polymorphism has no significant effect on the T2DM susceptibility in the non-Chinese population, data suggested that the mutation might be strongly associated with the increased T2DM risk within the Chinese people. The significant difference between the two populations might be attributable to the difference in region and ethnicity.
Ghrelin has been shown to takes part in glucose metabolism which could promote insulin production in rats (13). Ghrelin secretion can also be inhibited by insulin. For example, obese individuals with elevated levels of plasma glucose had decreased ghrelin levels due to hyperinsulinemia. It has also been observed that the plasma ghrelin concentrations were negatively correlated with fasting insulin level, hypertension, insulin resistance and T2DM prevalence (33, 34). Levels of circulating ghrelin were even reduced in the healthy offspring of T2DM (35). Animal experiments and clinical researches have shown that the low ghrelin level could serve as the prediction indicator for obesity and T2DM (14). In 2002, Saad et al. reported that ghrelin administration reduced insulin secretion in human (36).
Although the GHRL gene Leu72Met polymorphism does not alter the sequence of mature ghrelin, changes to mRNA stability or protein synthesis may contribute to T2DM development by affecting ghrelin secretion and activity. In 2008, Zavarella et al. observed a significant decreasing trend from Leu/Leu to Leu/Met and to Met/Met homozygous subjects in fasting insulin levels, triglycerides, and HOMA-IR index and an increase trend in ghrelin levels. Their observations suggested that the Met72 variant might play a protective role in modulating insulin resistance. They also speculated that an unknown Met72 functional variant in linkage disequilibrium could increase the ghrelin levels and probably improve insulin sensitivity (37).
Ghrelin is present in the bloodstream in two major forms: desacyl and acylated ghrelin (38). Under physiological conditions, the ratio of acylated/des-acyl ghrelin ranges from 1:3 to 1:4, but this ratio varies under pathological conditions, including T2DM. In this regard, several reports have found that insulin resistance is associated with a reduction in circulating des-acyl ghrelin, while acylated ghrelin levels are increased (39, 40) or unchanged (41, 42). In addition, opposite effects of ghrelin isoforms on hepatic glucose metabolism have been found with des-acyl form suppressing hepatocyte glucose release as well as antagonizing the acylated ghrelin-induced increase in hepatic glucose output in vitro (43). In 2004, Vivenza et al. reported that GHRL gene polymorphisms (Arg51Gln, Leu72Met, and Gln90Leu), do not have a relevant impact in the secretion of total and acylated ghrelin (44).
Liao et al. in 2013 reported a protective effect from T2DM in the Caucasians population, but no correlation between the genetic mutation and T2DM in the overall population (45). As only three studies were included in the Caucasian subgroup and six of the whole population, this finding may be inaccurate. A 2012 meta-analysis by Liu et al. found no correlation from a relative large Chinese population, but also found that FPG levels of T2DM patients with Leu72Leu genotype were significantly higher than that of other variant genotypes (Leu72Met and Met72Met) (29). Though Liu's data alone did not find statistically significant correlation, once their work was incorporated into the current meta-analysis, the final results indicated a significant correlation between them. Because of the relatively limited sample size in Liu's individual study compared to that of the current meta-analysis, it is possible that the relationship between GHRL gene Leu72Met polymorphism with T2DM susceptibility was simply undetected by Liu's individual study. In 2012, Wang et al. also performed a similar meta-analysis of the Chinese population and came to a similar conclusion as the current meta-analysis (18). Because two of the five studies in Wang's paper were conducted by the same authors and had significant overlaps in data, their meta-analysis may not be as sound as the one presented here.
There are inevitably some limitations in the current meta-analysis. The current meta-analysis still lacks large-scale studies on the correlation between the GHRL gene Leu72Met polymorphism and T2DM. The ghrelin level was influenced not only by the GHRL gene Leu72Met polymorphism, but also by other gene polymorphism as Arg52Gln, and unhealthy dietary habits (46). As the genetic susceptibility model of T2DM was predominantly minor gene model, the genetic heterogeneity caused the T2DM susceptibility being influenced by races, regions, environment, and ancestry (47).
Finally, GHRL gene Leu72Met polymorphism was significantly associated with the increased T2DM risk, especially among the Chinese people. Individuals carrying the Met72 allele of GHRL Leu72Met gene polymorphism, particularly those of Chinese ancestry, may be more susceptible to developing T2DM disease. The above conclusion may open a novel window in guiding us to formulate a new T2DM therapy, especially in the Chinese T2DM population. Considering the limitations of this study, more large-scale researches are still necessary to clarify the conclusion in the long run.
The authors report no relationships that could be construed as a conflict of interest.
YL: conceived and designed the experiments, statistical analyses, and paper writing. YL, GG, HG, and HW: performed the experiments. YL, XY, and XL: analyzed the data. YL and HW: contributed reagents, material, and analysis tools. YL and HK: wrote the manuscript. YL and XL: reference collection and data management. YL, YZ, and ZY: study design.
This research was supported by the National Natural Science Foundation of China (NSFC 81100073 to YL), Jiangsu Overseas Research & Training Program for University Prominent Young & Middle-aged Teachers and Presidents (2014), Excellent Young and Middle-Aged Teachers Assistance Program of Nanjing Medical University for YL (2013–2015, JX2161015034), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). This work was also funded by the Natural Science Foundation of Jiangsu Province (BK 2012648 to HW), six talent peaks project in Jiangsu Province (2015-WSN-033).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Thank all our colleagues working in the Clinical Research Center and the Department of geriatrics, the First Affiliated Hospital of Nanjing Medical University.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2019.00559/full#supplementary-material
1. Cui BQ, He ZY, Lin YZ. Relationship between Leu72Met polymorphism of preproghrelin gene and type 2 diabetes mellitus in Han ethnics of Guangzhou. J Chongqing Med Univ. (2010) 35:1067–70.
2. Hasan MM, Hosen MB, Rahman MM, Howlader MZH, Kabir Y. Association of ATP binding cassette transporter 1 (ABCA 1) gene polymorphism with type 2 diabetes mellitus (T2DM) in Bangladeshi population. Gene. (2018) 688:151–4. doi: 10.1016/j.gene.2018.12.003
3. Martinez-Rios MA, Vargas-Alarcon G, Peña-Duque MA, Perez-Mendez O, Rodriguez-Perez JM, Perez-Hernandez N, et al. The−44 C/G (rs1800972) polymorphism of the β-defensin 1 is associated with increased risk of developing type 2 diabetes mellitus. Mol Genet Genomic Med. (2019) 7:e00509. doi: 10.1002/mgg3.509
4. Nejatian N, Häfner AK, Shoghi F, Badenhoop K, Penna-Martinez M. 5- lipoxygenase (ALOX5): genetic susceptibility to type 2 diabetes and vitamin D effects on monocytes. J Steroid Biochem Mol Biol. (2018) 187:52–7. doi: 10.1016/j.jsbmb.2018.10.022
5. Wang Y, Duan L, Yu S, Liu X, Han H, Wang J, et al. Association between “solute carrier family 30 member 8” (SLC30A8) gene polymorphism and susceptibility to type 2 diabetes mellitus in Chinese Han and minority populations: an updated meta-analysis. Asia Pac J Clin Nutr. (2018) 27:1374–90. doi: 10.6133/apjcn.201811_27(6).0025
6. Isakova J, Talaibekova E, Vinnikov D, Saadanov I, Aldasheva N. ADIPOQ, KCNJ11 and TCF7L2 polymorphisms in type 2 diabetes in Kyrgyz population: a case-control study. J Cell Mol Med. (2018) 23:1628–31. doi: 10.1111/jcmm.14061
7. Işikli A, Kubat-Üzüm A, Satman I, Matur Z, Öge AE, Küçükali CI, et al. A SOD2 polymorphism is associated with abnormal quantitative sensory testing in type 2 diabetic patients. Noro Psikiyatr Ars. (2018) 55:276–9. doi: 10.29399/npa.23027
8. Shu Y, Chen Y, Luo H, Li H, Tang J, Liang Y, et al. The roles of IL-10 gene polymorphisms in diabetes mellitus and their associated complications: a meta-analysis. Horm Metab Res. (2018) 50:811–5. doi: 10.1055/a-0651-5051
9. Ren H, Tan SL, Liu MZ, Banh HL, Luo JQ. Association of PON2 gene polymorphisms (Ser311Cys and Ala148Gly) with the risk of developing type 2 diabetes mellitus in the Chinese Population. Front Endocrinol. (2018) 9:495. doi: 10.3389/fendo.2018.00495
10. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. (1999) 402:656–60. doi: 10.1038/45230
11. Lu S, Guan JL, Wang QP, Uehara K, Yamada S, Goto N, et al. Immunocytochemical observation of ghrelin-containing neurons in the rat arcuate nucleus. Neurosci Lett. (2002) 321:157–60. doi: 10.1016/S0304-3940(01)02544-7
12. Chen CY, Asakawa A, Fujimiya M, Lee SD, Inui A. Ghrelin gene products and the regulation of food intake and gut motility. Pharmacol Rev. (2009) 61:430–81. doi: 10.1124/pr.109.001958
13. Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. (2005) 85:495–522. doi: 10.1152/physrev.00012.2004
14. Pöykkö SM, Kellokoski E, Hörkkö S, Kauma H, Kesäniemi YA, Ukkola O. Low plasma ghrelin is associated with insulin resistance, hypertension, and the prevalence of type 2 diabetes. Diabetes. (2003) 52:2546–53. doi: 10.2337/diabetes.52.10.2546
15. Kanamoto N, Akamizu T, Tagami T, Hataya Y, Moriyama K, Takaya K, et al. Genomic structure and characterization of the 5'-flanking region of the human ghrelin gene. Endocrinology. (2004) 145:4144–53. doi: 10.1210/en.2003-1718
16. Mora M, Adam V, Palomera E, Blesa S, Díaz G, Buquet X, et al. Ghrelin gene variants influence on metabolic syndrome components in aged Spanish population. PLoS ONE. (2015) 10:e0136931. doi: 10.1371/journal.pone.0136931
17. Mager U, Lindi V, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, et al. Association of the Leu72Met polymorphism of the ghrelin gene with the risk of Type 2 diabetes in subjects with impaired glucose tolerance in the Finnish Diabetes Prevention Study. Diabet Med. (2006) 23:685–9. doi: 10.1111/j.1464-5491.2006.01870.x
18. Wang YF, Liu J, Liu J, Zhang SL, Guo Q, Tian LM, et al. Meta-analysis of the association between ghrelin gene Leu72met polymorphism and type 2 diabetes mellitus in Chinese people. J Lanzhou Univ. (2012) 38:39–42. doi: 10.3969/j.issn.1000-2812.2012.04.010
19. Berthold HK, Giannakidou E, Krone W, Mantzoros CS, Gouni-Berthold I. The Leu72Met polymorphism of the ghrelin gene is associated with a decreased risk for type 2 diabetes. Clin Chim Acta. (2009) 399:112–16. doi: 10.1016/j.cca.2008.09.022
20. Choi HJ, Cho YM, Moon MK, Choi HH, Shin HD, Jang HC, et al. Polymorphisms in the ghrelin gene are associated with serum high-density lipoprotein cholesterol level and not with type 2 diabetes mellitus in Koreans. J Clin Endocrinol Metab. (2006) 91:4657–63. doi: 10.1210/jc.2005-2549
21. Li YY, Wang LS, Lu XZ, Yang ZJ, Wang XM, Zhou CW, et al. CDKAL1 gene rs7756992 A/G polymorphism and type 2 diabetes mellitus: a meta-analysis of 62,567 subjects. Sci Rep. (2013) 3:3131. doi: 10.1038/srep03131
22. Cochran WG. The effectiveness of adjustment by subclassification in removing bias in observational studies. Biometrics. (1968) 24:295–313. doi: 10.2307/2528036
23. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
24. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. (1959) 22:719–48.
25. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
26. Jiang JM, Sun YN, Liu LM, Zheng TS, Wang NS, Wang F. Relationship between Leu72Met polymorphism of preproghrelin gene and type 2 diabetes mellitus and diabetic nephropathy. J Shanghai Jiaotong Univ. (2008) 28:863–6. doi: 10.3969/j.issn.1674-8115.2008.07.030
27. Xu LL, Xiang HD. Association of human ghrelin gene polymorphism with type 2 diabetes mellitus. Basic Clin Med. (2008) 28:44–47. doi: 10.3969/j.issn.1001-6325.2008.01.010
28. Xiang Y, Tan N, Zhang Z, Li Q, Xu J, Gu M. Relationship between ghrelin gene Leu72Met polymorphism and type 2 diabetes mellitus. Chin J Diabetes. (2011) 19:729–31. doi: 10.3969/j.issn.1006-6187.2011.10.003
29. Liu J, Liu J, Tian LM, Liu JX, Bing YJ, Zhang JP, et al. Association of ghrelin Leu72Met polymorphism with type 2 diabetes mellitus in Chinese population. Gene. (2012) 504:309–12. doi: 10.1016/j.gene.2012.03.025
30. Larsen LH, Gjesing AP, Sørensen TI, Hamid YH, Echwald SM, Toubro S, et al. Mutation analysis of the preproghrelin gene: no association with obesity and type 2 diabetes. Clin Biochem. (2005) 38:420–4. doi: 10.1016/j.clinbiochem.2005.01.008
31. Kim SY, Jo DS, Hwang PH, Park JH, Park SK, Yi HK, et al. Preproghrelin Leu72Met polymorphism is not associated with type 2 diabetes mellitus. Metabolism. (2006) 55:366–70. doi: 10.1016/j.metabol.2005.09.011
32. Garcia EA, King P, Sidhu K, Ohgusu H, Walley A, Lecoeur C, et al. The role of ghrelin and ghrelin-receptor gene variants and promoter activity in type 2 diabetes. Eur J Endocrinol. (2009)161:307–15. doi: 10.1530/EJE-09-0122
33. Erdmann J, Lippl F, Wagenpfeil S, Schusdziarra V. Differential association of basal and postprandial plasma ghrelin with leptin, insulin, and type 2 diabetes. Diabetes. (2005) 54:1371–8. doi: 10.2337/diabetes.54.5.1371
34. McLaughlin T, Abbasi F, Lamendola C, Frayo RS, Cummings DE. Plasma ghrelin concentrations are decreased ininsulin-resistant obese adults relative to equally obese insulin-sensitive controls. J Clin Endocrinol Metab. (2004) 89:1630–5. doi: 10.1210/jc.2003-031572
35. Østergård T, Hansen TK, Nyholm B, Gravholt CH, Djurhuus CB, Hosoda H, et al. Circulating ghrelin concentrations are reduced in healthy offspring of Type 2 diabetic subjects, and are increased in women independent of a family history of Type 2 diabetes. Diabetologia. (2003) 46:134–6. doi: 10.1007/s00125-002-0985-4
36. Saad MF, Bernaba B, Hwu CM, Jinagouda S, Fahmi S, Kogosov E, et al. Insulin regulates plasma ghrelin concentration. J Clin Endocrinol Metab. (2002) 87:3997–4000. doi: 10.1210/jcem.87.8.8879
37. Zavarella S, Petrone A, Zampetti S, Gueorguiev M, Spoletini M, Mein CA, et al. A new variation in the promoter region, the−604 C>T, and the Leu72Met polymorphism of the ghrelin gene are associated with protection to insulin resistance. Int J Obes. (2008) 32:663–8. doi: 10.1038/sj.ijo.0803766
38. Hosoda H, Kojima M, Matsuo H, Kangawa K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun. (2000) 279:909–13. doi: 10.1006/bbrc.2000.4039
39. Rodríguez A, Gómez-Ambrosi J, Catalán V, Becerril S, Sáinz N, Gil MJ, et al. Association of plasma acylated ghrelin with blood pressure and left ventricular mass in patients with metabolic syndrome. J Hypertens. (2010) 28:560–7. doi: 10.1097/HJH.0b013e328334327c
40. Ezquerro S, Mocha F, Frühbeck G, Guzmán-Ruiz R, Valentí V, Mugueta C, et al. Ghrelin reduces TNF-α-induced human hepatocyte apoptosis, autophagy, and pyroptosis: role in obesity-associated NAFLD. J Clin Endocrinol Metab. (2019) 104:21–37. doi: 10.1210/jc.2018-01171
41. St-Pierre DH, Karelis AD, Coderre L, Malita F, Fontaine J, Mignault D, et al. Association of acylated and nonacylated ghrelin with insulin sensitivity in overweight and obese postmenopausal women. J Clin Endocrinol Metab. (2007) 92:264–9. doi: 10.1210/jc.2006-1603
42. Barazzoni R, Zanetti M, Ferreira C, Vinci P, Pirulli A, Mucci M, et al. Relationships between desacylated and acylated ghrelin and insulin sensitivity in the metabolic syndrome. J Clin Endocrinol Metab. (2007) 92:3935–40. doi: 10.1210/jc.2006-2527
43. Gauna C, Delhanty PJ, Hofland LJ, Janssen JA, Broglio F, Ross RJ, et al. Ghrelin stimulates, whereas des-octanoyl ghrelin inhibits, glucose output by primary hepatocytes. J Clin Endocrinol Metab. (2005) 90:1055–60. doi: 10.1210/jc.2004-1069
44. Vivenza D, Rapa A, Castellino N, Bellone S, Petri A, Vacca G, et al. Ghrelin gene polymorphisms and ghrelin, insulin, IGF-I, leptin and anthropometric data in children and adolescents. Eur J Endocrinol. (2004) 151:127–33. doi: 10.1530/eje.0.1510127
45. Liao N, Xie ZK, Huang J, Xie ZF. Association between the ghrelin Leu72Met polymorphism and type 2 diabetes risk: a meta-analysis. Gene. (2013) 517:179–83. doi: 10.1016/j.gene.2012.12.094
46. Zhang SL, Liu J, Guo Q, Liu JX, Jin LY. Association of Arg51Gln and Leu72Met polymorphisms of ghrelin gene polymorphism with type 2 diabetes mellitus and lipid in Muslins in Gansu province. J Lanzhou Univ. (2011) 37:21–4. doi: 10.3969/j.issn.1000-2812.2011.01.005
Keywords: GHRL, Leu72Met, polymorphism, type 2 diabetes mellitus, T2DM
Citation: Li Y, Lu X, Yang X, Wang H, Geng H, Gong G, Zhan Y, Kim HJ and Yang Z (2019) GHRL Gene Leu72Met Polymorphism and Type 2 Diabetes Mellitus: A Meta-Analysis Involving 8,194 Participants. Front. Endocrinol. 10:559. doi: 10.3389/fendo.2019.00559
Received: 10 May 2018; Accepted: 29 July 2019;
Published: 08 August 2019.
Edited by:
Ondřej Šeda, Charles University, CzechiaReviewed by:
Dubravka Jurišić ErŽen, University of Rijeka, CroatiaCopyright © 2019 Li, Lu, Yang, Wang, Geng, Gong, Zhan, Kim and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan-yan Li, bHl5bmptdTEyM0AxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.