- 1Amsterdam University College, University of Amsterdam and Vrije Universiteit, Amsterdam, Netherlands
- 2Department of Periodontology, Academic Centre for Dentistry Amsterdam, Amsterdam University College, University of Amsterdam and Vrije Universiteit, Amsterdam, Netherlands
Introduction: It is beneficial for all biomedical track courses to train students early in their educational career in reading biomedical literature. A shortcoming of many biomed track courses during undergraduate education is that laboratory techniques necessary for fully understanding further reading of biomedical articles are not part of courses early in the curriculum. To bridge this gap, an educational investment is needed that will create confident and competent readers of scientific biomedical literature. All consecutive courses in the biomedical track may benefit from such an investment. Probably, the nescience of techniques needed for protein detection, which are part of virtually all composite figures in cell biological articles, forms the basis of such a gap. Activating forms of education were shown to be effective and are increasingly implemented in higher education.
Methods: Here, the implementation of a flipped classroom approach for explaining ELISA, Immunohistochemistry, Western Blotting and flow cytometry as four common basic protein detection methods is described. The successfulness of the educational approach was assessed in the exam, where a comparison was made between the experts’ and receivers’ grades. Students gave feedback on whether this method made them more confident and competent readers of biomedical literature.
Results: Experts on the four techniques were successful in conveying their field of expertise since exam performances on the specific techniques were equally good between experts and receivers. The flipped classroom activity made students more confident (65% agreed vs. 18% disagreed) and more competent (79% agreed vs. 12% disagreed).
Conclusion: A simple and time-efficient intervention early in their educational career, using a flipped classroom approach has resulted in self-reported confident and competent readers of scientific cell biological literature.
1. Introduction: background and rationale for the educational activity innovation
Teaching Molecular Cell Biology is the teaching of life at its fundamental aspects. How do cells integrate signals from the outside world, how do cells respond, and what are the internal cellular specifics of response? One could teach such a course by only using educationally well-designed and thorough textbooks such as Essential Cell Biology (Alberts et al., 2019). However, one can only understand cell biology when focusing on the ‘How do we know’ question, bringing along the history of science, the experiments that were crucial in the elucidating of mechanisms and cell biological insights. As a bridge to the novel world of discoveries made at the cell biological level, students should be encouraged to take the next step: to read for themselves and explore current articles from higher journals, such as Cell, Nature and Science. In my decade-long experience of teaching Molecular Cell Biology, I have noticed that there is a threshold to be taken by undergraduate students for actively reading biomedical literature. Since cell biology is a very visual science with its micrographs, blots and bar diagrams, students should become more confident and competent in analyzing figures and concepts from these journals. This can only be achieved when knowledge is deepened and interpretative skills are developed on the figures that are presented. As a consequence, students should be familiarized with the techniques that detect nucleic acid sequences and proteins. For the latter, one could focus on the most common ones: ELISA, Western blotting, immunohistochemistry and flow cytometry.
2. Background of the course
Molecular Cell Biology is taught in the first semester of the second year at Amsterdam University College (AUC). AUC is an international, English-taught honor’s bachelor college. Molecular Cell Biology is a 200 level course and is a prerequisite for the biomedical track. Entry requirement was successful completion of the first-year course “Introduction to Biology” or “Human body I.” Most of the enrolled students pursue a career in medicine or biomedical sciences. It is a full semester (September–December), 6 European Credits (EC) course, where 1 EC = a study load of 28 h, with two times 2 hours of class per week. Each class contains a maximum of 25 students, the course is given to two groups of students. Chapters from Essential Cell Biology (Alberts et al., 2019) are leading and account for approximately 75% of the classes.Classes also focus on what is not explicitly mentioned in the book, like cell biological models and methods to detect nucleic acids and proteins. Students commit to five assignments: three exams (each 22% of the final grade), a presentation (14% of the final grade) and an essay (20% of the final grade) on a cell biological topic (De Vries, 2021). The latter two assignments are performed by duos. The course is taught within the biomedical track, where courses like Immunology, Infectious Diseases, courses on Anatomy, Epigenetics, Bioinformatics, Cancer Biology, various Neurology courses and lab courses like Molecular techniques and Cell Physiology Lab (De Vries et al., 2019) prepare students for admittance to follow-up Master’s degrees in biomedical sciences and for a career in medicine. One the three learning outcomes is linked to competence and confidence in reading scientific literature: “The student is able to apply the fundamental knowledge of cell biology and molecular techniques to critically evaluate scientific literature.” The positioning of Molecular Cell Biology in the first semester of the second year makes it a strategic place for making competent and confident readers of biomedical literature. Students will then benefit from this for all subsequent courses in the biomedical track.
3. Pedagogical framework(s), pedagogical principles, competencies/standards underlying the educational activity
3.1. The flipped classroom
Flipped classroom is an educational method of activated learning. Instead of frontal, teacher-guided lessons, students are responsible for preparing (learning) and teaching the other students about what they have learned. The role of the teacher is rather that of a coach; meaning that students are much more engaged in the course (Farmer, 2018). A full cycle of the flipped classroom method contains pre-class preparation, in-class activities, after-class activities and student assessment of learning (Persky and McLauglin, 2017). According to a recent meta-analysis, student perceptions about this method are generally positive (Chen et al., 2017), albeit that the effectiveness is inconclusive in the same meta-analysis (Chen et al., 2017). Another meta-analysis reported that flipped classrooms significantly improved students’ learning compared to traditional teaching methods in health profession education (Hew and Lo, 2018).
4. Learning environment; learning objectives; pedagogical format
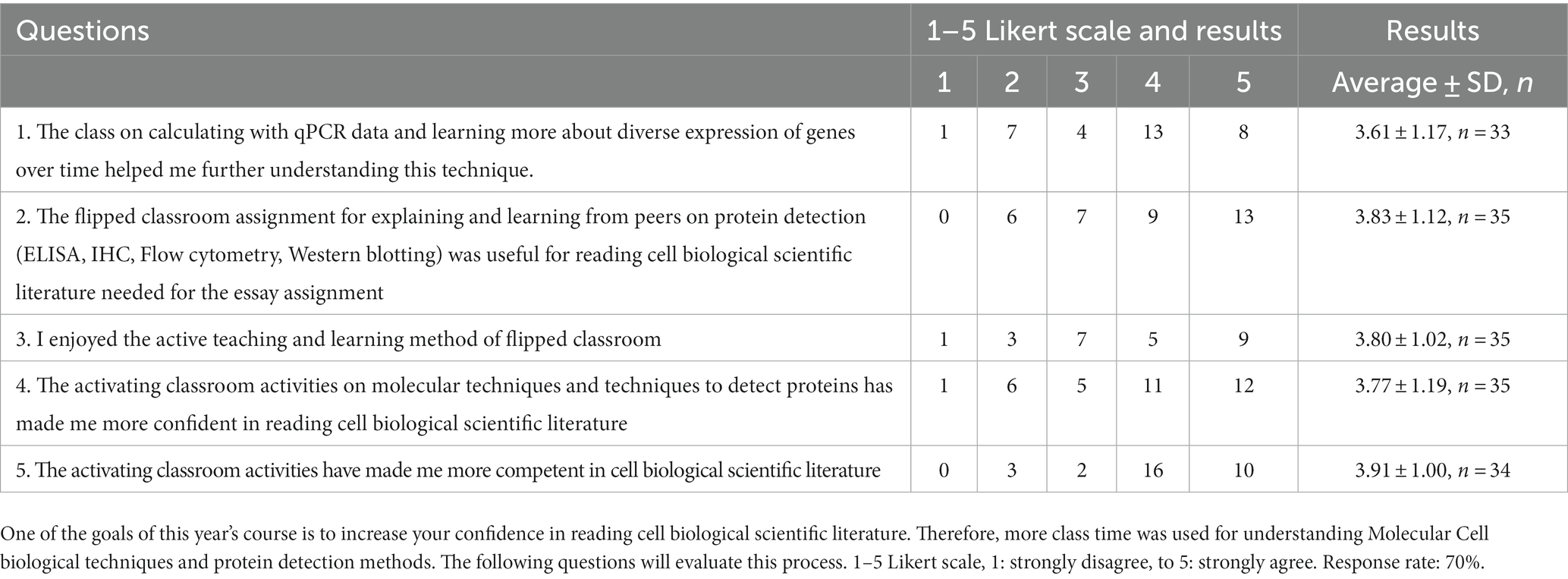
Since one of the learning objectives was to make more competent and confident readers of cell biological literature at the level of reading, Cell, Nature and Science articles, I decided to spread and spend class-time for achieving this learning objective during several occasions spread over the semester, since repetition of a concept is important for internalization (Trninic, 2018; Wiggins et al., 2021). In fact, one could distinguish three steps of making students confident and competent readers of cell biological literature They are listed below (4.1–4.3) and as a flow-diagram (Figure 1).
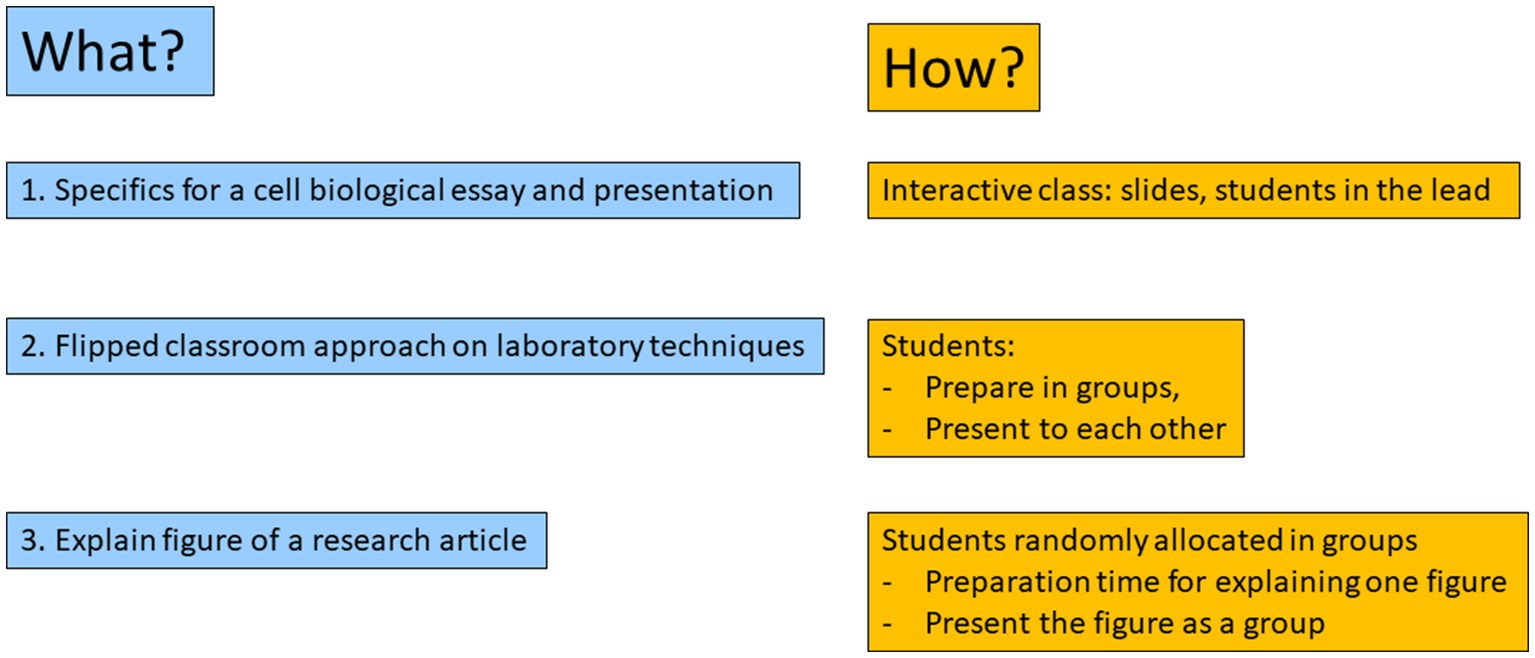
Figure 1. Three steps of making students competent and confident readers of cell-biological literature. Diagram show the three steps (paragraphs 4.1–4.3) that were undertaken over time to make confident and competent readers.
4.1. What are the requirements for a cell biological presentation and essay?
Confidence and competence in reading cell biological literature is immediately applied in my course, since students have an essay assignment within the course, with a deadline at the end. AUC students are taught to present during Academic Writing Skills, a first year course. Within my course, I prepared students for one hour to further develop their specific academic writing, tailored to molecular cell biology. In an interactive session, I persuaded them to think out loud about what is specifically required for a Molecular Cell Biology presentation of 12 min and a Molecular Cell Biology essay of 1,500 words. Together, the group came up with aspects like: show integration of research data, try to keep it simple, reduce and present only essentials of the very composite figures of Cell, Nature and Science papers. Furthermore, cell biological literature is full of abbreviations that have to be addressed and explained to a layman audience with some experience in cell biology. Another pitfall is the use of schemes with too dense information that is probably not all relevant for a presentation or essay. I advised my students to keep it simple, otherwise the main message would not come across (Gillen, 2006). I also explained that the 1,500 word essay should NOT be written in a review-article-style, and instructed the students to describe the specifics (Round and Cambell, 2013) of up to three research articles and link these. A special assignment on the research question was posted at 1/3 of the semester with a go/no-go and comments by me were posted on Canvas. Each duo had to phrase a research question that applied to both the presentation and the essay. Within this assignment, at least three research papers had to be shown. Students were given time to read the rubrics, followed by ranking three anonymized essays from previous years and a short debate on why they were ranked as such.
4.2. Flipped classroom design for learning about protein detection techniques
Students were instructed in a 17 min short clip with PowerPoint slides on the purpose of the flipped classroom: to learn about the various protein detection techniques. For all techniques, a short video clip from the internet and a mind map with terms that are relevant to the technique was provided. Groups of 6 students were randomly formed per technique by the instructor and students had to finish an unfinished PowerPoint presentation on ELISA, Western blotting, Immunohistochemistry or Flowcytometry. A presentation of 20 min by the experts to the receivers had to be prepared for the next class. Each presentation had to contain the following:
• Explain the principle. Feel free to use videos from the internet.
• When would you use your technique?
• Find an example in the scientific literature and present.
• Write a little narrative on one PowerPoint slide that includes all terms provided in the mind map.
Students presented in groups of 6 as experts on the technique (Figure 2) and the non-experts were encouraged to ask questions after the presentation.
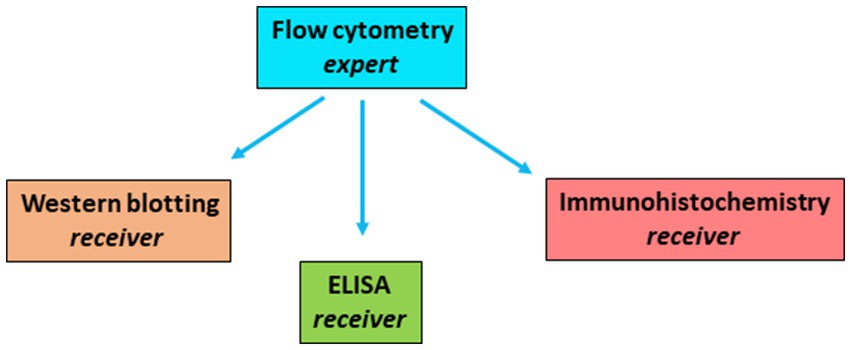
Figure 2. Expert group conveying there expertise. Expert groups of ELISA, Western blotting, immunohistochemistry and flowcytometry were formed. Expertise was conveyed to the non-experts in a 20 min presentation. An example is shown of the Flowcytometry group presenting to the others, but vice versa, the ELISA, Immunohistochemistry and Western blotting teams took turns to present as expert group.
The exam had one larger question on the protein detection techniques, results per technique were analyzed per expert and per receiver/non-experts (t-test).
4.3. Practice presentation of cell biological figures
It is important to practice in showing scientific data. After a presentation on the strengths and limitations of cell biological methods, students were divided into six groups to prepare a short, one-or-two minute presentation on the six figures from an article on bone-marrow-on-a chip (Sieber et al., 2018). The figures were added in a PowerPoint presentation where students indicated the essence of the images. Figures were on a graphical abstract of the study design, on qPCR results (what is the logic of choosing these genes, what is a housekeeping gene), results on immunohistochemistry (why was it important for the authors to show these proteins), colony forming units of hematopoietic cells (what is the principle here?) and flow cytometry data (without any advanced knowledge about this technique: what is shown here and why?).
5. Results to date/assessment (processes and tools)
5.1. Construction and outcomes of the exam
To test the knowledge of all techniques in the exam, an equal point distribution of 4 points on each of the techniques was assigned. The questions were on (a) recognizing and being able to describe the application of the various specific terms. Studying the mind map per technique would help here (6 out of 16 points, 1.5 point per technique). Examples were: HCl for stopping ELISA, forward scatter, being cell size aspect in flowcytometry, tissue section for staining tissue in immunohistochemistry and SDS for equal negative loading of proteins in western blot. In a “Keeping control” question (b) the various positive and negative controls per technique were asked (2 out of 16 points, 0.5 point per technique). Finally, (c) for applying, various insight questions were asked per technique (8 out of 16 points, 2 points per technique).
I hypothesized that if the flipped classroom was successful in getting all aligned for the protein detection techniques, the experts would score as well in the exam as the receivers. Results are shown in Figure 3. No significant differences were observed between the experts and receivers for any of the techniques, demonstrating that the learning of the experts was as good as the learning of the receivers.
Figure 3. Exam results by expert groups and by receivers. Exam results per technique were calculated for the experts and for the receivers. No differences were observed for experts (n = 12) and receivers (n = 36) for any of the techniques. In this exam, questions on ELISA and Western blotting scored better in this exam. Results are shown as mean +/− S.D.
5.2. Flipped classroom-learned protein detection techniques makes more confident and competent readers of molecular cell biological scientific literature
Finally, students were asked questions about the various aspects of activating class work in a questionnaire with 1–5 Likert scale questions (Table 1 Figure 4) during a mid-term evaluation. Students voluntarily filled-out the questionnaire, identity of students was not tracible. Furthermore, filling out or not had no repercussions. It also included feedback on a qPCR calculus session (question 1) with real research results. In general, most students scored the higher ticks of the 5-point Likert scale with 4 (agree) and 5 (strongly agree) for all questions (Table 1 Figure 4). The final goal, that knowledge on the 4 protein detection techniques and an interactive workshop on qPCR has made students more confident (question 4) and more competent (question 5) in reading cell biological scientific literature was achieved, since these questions got a positive reply of 23/35 or 65% for confidence and 26/34 or 79% for competence. In the open question “What have you found to be most useful in class?,” 6 students replied: focus on techniques, 7 replied: the flipped classroom approach. Two specific comments on this were: “I dug deeper in understanding my designated topic which made studying other related topics in the book easier,” and “The flipped classroom modules which allow me to take control of my own learning” Two students thought that the flipped classroom was the least useful in class.
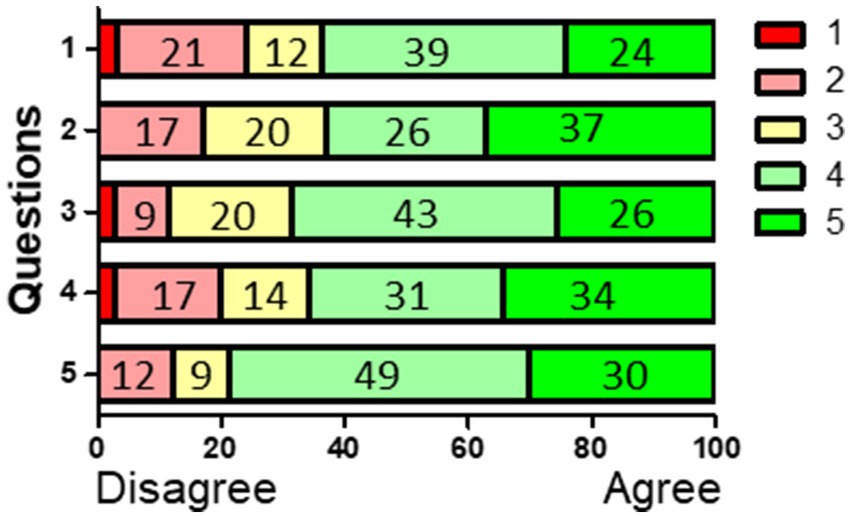
Figure 4. Answers to questions 1–5 in percentages (see also Table 1). The 1–5 Likert score of Table 1 were recalculated as percentages. N = 33–35, depending on the question.
6. Discussion on the practical implications, objectives and lessons learned
Learning Molecular Cell Biology from the textbook has probably become rather abstract and not as complete as when finding the connection with everyday research. Indeed, such courses invite opportunities to connect with everyday scientific discoveries (Round and Cambell, 2013). Scientifically, Molecular Cell Biology is a quickly expanding field and it can take advantage of the knowledge of single mutations that are known to cause diseases to explain for instance signaling pathways (Schoenmaker et al., 2020) and of the landscape of all genes that are regulated by certain proteins and are now know thanks to the Human Genome Project (Gonzaga-Jauregui et al., 2012). The specific gap between textbook knowledge and reading scientific literature along the course, has been previously acknowledged (Wiegant et al., 2011). In this rather humble and short research article, I show the steps I undertook to teach students how to read and how to report on cell biological scientific literature.
Although it is always a challenge for scientists who enjoy teaching to determine how to split their time, I believe that university college and university students should be ignited also by the typical scientist-teachers. As already proposed by DebBurman (2002), one should bridge the gap between theoretical courses and the laboratory (De Vries et al., 2019), where discoveries are being made.
The approach that was undertaken here, has definitely some limitations. First of all, concerning the results on competence and confidence in Table 1 and Figure 4, did not take a baseline measurement, which is important for such type of research (Ferron and Sentovich, 2002; Glymour et al., 2005). Second, the results are from a relatively small group and only collected once. Third, there is a relatively large standard deviation in performance of some of the exam questions (Figure 3).
Interpretation of research data is only possible when knowing the ins-and-outs of the techniques used to achieve those. Knowledge of protein detection techniques and subsequent confidence and competence in reading scientific biomedical literature gained early in the curriculum is crucial for later courses in the biomedical track.
7. Acknowledgment of any conceptual, methodological, environmental, or material constraints
One could point out as a shortcoming that the present study is relatively limited in size and that only one, relatively intuitive approach was described. A longer follow-up could be interesting, since I foresee that also other subsequent courses in the biomedical track could benefit from a deeper knowledge of cell biological techniques at are essential for reading figures from scientific journals. Also, the questionnaire did not study before and after. Despite these shortcomings, 79% of the students stated that they were more competent readers of cell biological scientific literature. Whether the competence is a lasting achievement, could be tested during the last semester of the bachelor’s.
My approach focused on investing time during the course to the goal of making more confident and competent readers of cell biological journals. Spaced repetition is a proven method for retention of knowledge (Gonzaga-Jauregui et al., 2012). For educational programmes that have frequent assessments, it is always a challenge to retain knowledge after examination (Renoult et al., 2019). I therefore hope that the step-by-step approach, with emphasis on learning the protein detection methods in a flipped classroom manner, will lead to lasting knowledge. In the case of reading biomedical papers, I hope that a foundation is laid for four essential protein detection techniques ELISA, Western blotting, immunohistochemistry and flowcytometry. Not only to the benefit of the undergraduate degree, but also for life as a biomedical researcher or as a medical doctor.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for this study in accordance with the local legislation and institutional requirements. Written informed consent from the program evaluation focus group participants was not required to participate in this study in accordance with the national legislation and the institutional requirements. Participation in the questionaire was voluntary, and choice to participate or not had no consequence for the students. Identity was not tracible.
Author contributions
TV designed and executed the study and wrote the article.
Acknowledgments
The help of Keith Groot, educationalist at ACTA is acknowledged for his expert input in the mid-term evaluation questionnaire. The author would like to thank my students of the 2022 class of Molecular Cell Biology for their participation and their willingness to fill out the questionnaire. In particular, the author would like to thank Aidan Lowrie, Ayden Teye, Lydia Woodfork and Lotte van der Zanden, four native speakers of English, for their proofreading and suggestions on the pre-final version of the manuscript.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alberts, B. D., Hopkin, K., Johnson, A., Lewis, J., Raff, M., Roberts, K., et al. (2019). Essential Cell Biology. Milton Park: Taylor and Francis. 734p
Chen, F., Liu, A. M., and Martinelli, S. M. (2017). A systematic review of the effectiveness of flipped classrooms in medical education. Med. Educ. 51, 585–597. doi: 10.1111/medu.13272
De Vries, T. J. (2021). The pandemic that has forced teachers to go online. Zooming in on tips for online teaching. Front. Educ. 6:647445. doi: 10.3389/feduc.2021.647445
De Vries, T. J., Schoenmaker, T., van Veen, H. A., Hogervorst, J., Krawczyk, P. M., Moonen, C. G. J., et al. (2019). The challenge of teaching essential immunology laboratory skills to undergraduates in one month—experience of an Osteoimmunology course on TLR activation. Front. Immunol. 10:1822. doi: 10.3389/fimmu.2019.01822
DebBurman, S. K. (2002). Learning how scientists work: experiential research projects to promote cell biology learning and scientific process skills. Cell Biol. Educ. 1, 154–172. doi: 10.1187/cbe.02-07-0024
Farmer, R. (2018). The what, the how and the why of the flipped classroom. Innov. Pract. High. Educ. 3, 14–31.
Ferron, J., and Sentovich, C. (2002). Statistical power of randomization tests used with multiple-baseline designs. J. Exp. Educ. 70, 165–178. doi: 10.1080/00220970209599504
Gillen, G. M. (2006). Criticism and interpretation: teaching the persuasive aspects of research articles. CBE Life Sci. Educ. 5, 34–38. doi: 10.1187/cbe.05-08-0101
Glymour, M. M., Weuve, J., Berkman, L. F., Kawachi, I., and Robins, J. M. (2005). When is baseline adjustment useful in analyses of change? An example with education and cognitive change. Am. J. Epidemiol. 162, 267–278. doi: 10.1093/aje/kwi187
Gonzaga-Jauregui, C., Lupski, J. R., and Gibbs, R. A. (2012). Human genome sequencing in health and disease. Annu. Rev. Med. 63, 35–61. doi: 10.1146/annurev-med-051010-162644
Hew, K. F., and Lo, C. K. (2018). Flipped classroom improves student learning in health professions education: a meta-analysis. BMC Med. Educ. 18:38. doi: 10.1186/s12909-018-1144-z
Persky, A. M., and McLauglin, J. E. (2017). The flipped classroom – from theory to practice in health professional education. Am. J. Pharm. Educ. 81:118. doi: 10.5688/ajpe816118
Renoult, L., Irish, M., Moscovitch, M., and Rugg, M. D. (2019). From knowing to remembering: the semantic-episodic distinction. Trends Cogn. Sci. 23, 1041–1057. doi: 10.1016/j.tics.2019.09.008
Round, J. E., and Cambell, M. (2013). Figure facts: encouraging undergraduates to take a data-centered approach to Reading primary literature. CBE Life Sci. Educ. 12, 39–46. doi: 10.1187/cbe.11-07-0057
Schoenmaker, T., Deng, D., and de Vries, T. J. (2020). Tailored teaching for specialized (Para-)medical students - experience from incorporating a relevant genetic disease throughout a course of molecular cell biology. Front. Public Health 8:224. doi: 10.3389/fpubh.2020.00224
Sieber, S., Wirth, L., Cavak, N., Koenigsmark, M., Marx, U., Lauster, R., et al. (2018). Bone marrow-on-a-chip: long-term culture of human haematopoietic stem cells in a three-dimensional microfluidic environment. J. Tissue Eng. Regen. Med. 12, 479–489. doi: 10.1002/term.2507
Trninic, D. (2018). Instruction, repetition, discovery: restoring the historical educational role of practice. Instr. Sci. 46, 133–153. doi: 10.1007/s11251-017-9443-z
Walsh, M. M., Krusmark, M. A., Jastrembski, T., Hansen, D. A., Honn, K. A., and Gunzelmann, G. (2022). Enhancing learning and retention through the distribution of practice repetitions across multiple sessions. Mem. Cogn. 51, 455–472. doi: 10.3758/s13421-022-01361-8. Online ahead of print,
Wiegant, F., Scager, K., and Boonstra, J. (2011). An undergraduate course to bridge the gap between textbooks and scientific research. CBE Life Sci. Educ. 10, 83–94. doi: 10.1187/cbe.10-08-0100
Keywords: flipped classroom, molecular cell biology, protein detection methods, undergraduate, ELISA, flow cytometry, Western blotting (WB), immunohistochemistry
Citation: de Vries TJ (2023) Making confident and competent readers of Cell, Nature and Science papers using a flipped classroom approach to introduce protein detection techniques. Front. Educ. 8:1144010. doi: 10.3389/feduc.2023.1144010
Edited by:
Jouhanna Menegaz, Santa Catarina State University, BrazilReviewed by:
Andressa Parente, Federal University of Pará, BrazilLenilton Silva Da Silveira Júnior, Liga Contra o Câncer, Brazil
Copyright © 2023 de Vries. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Teun J. de Vries, dGV1bi5kZXZyaWVzQGFjdGEubmw=
 Teun J. de Vries
Teun J. de Vries