- 1Center for Affective Neuroscience, Development, Learning and Education, and Brain and Creativity Institute, and Rossier School of Education, University of Southern California, Los Angeles, CA, United States
- 2Neuroscience Graduate Program and Department of Psychology, University of Southern California, Los Angeles, CA, United States
Anticipating what adolescents will remember is a common goal in education research, but what tools allow us to predict adolescents' memory without interrupting the learning process as it naturally occurs? To attempt to identify neurophysiological markers of deep processing that may predict long-term retention, here we conducted an exploratory study by adding a cued recall probe to the last wave of data collection in a longitudinal psychosocial and neuroimaging study of 65 urban adolescents. Five years prior, and again 3 years prior, participants had reacted to the same emotionally evocative true stories during a videotaped interview that allowed us to measure eye-blink rate (EBR), and again during fMRI scanning. We analyzed EBR and neural data from the initial story exposure. We found that memory for a story was predicted by both EBR (a proxy for striatal dopamine) and default mode network neural activity to that story (involved in integrative memory and processing of emotional feelings). EBR and default mode network activity were uncorrelated and explained additive variance. Though more work is needed, our study contributes preliminary supportive evidence linking EBR and neural activity trial-by-trial to long-term memory in a naturalistic task. The analyses suggest that including EBR, a non-invasive, portable, and inexpensive measure that can be coded from high-quality video recording, could be useful in future studies of adolescents' learning.
Introduction
The utility for education research of measuring learners' physiology in natural settings and the aspects of neurophysiology that may be most relevant to studies of learning and memory remain open questions (Immordino-Yang and Christodoulou, 2014; Dahlstrom-Hakki et al., 2019). One measure that has not received much attention among education researchers, but that may be useful for predicting learning, is eye blink rate (EBR), which can be measured inexpensively, non-invasively, and in natural settings. Controlled laboratory studies of blinks and eye behavior have been shown to index a range of physiological processes with psychological implications relevant to education and learning, such as arousal, executive functioning, memory, and depth of cognitive processing (Eckstein et al., 2017). In fieldwork with students it would be difficult to distinguish, for example, between spontaneous and volitional contributions to EBR. However, this may not be necessary for EBR to contribute useful information—a possibility we explore here.
Spontaneous EBR (as opposed to volitional blinking) is an effective proxy for the presence of the neurotransmitter dopamine in the striatum (Jongkees and Colzato, 2016; Eckstein et al., 2017). Though a direct link between EBR and long-term memory is not yet well-established, striatal dopamine is associated with arousal, attention, reward-driven learning, sequence learning, motivational signaling, and, most relevant to the present study, long-term memory formation (Wise, 2004; Badgaiyan et al., 2007; Shohamy and Adcock, 2010; Nyberg et al., 2016; Rieckmann et al., 2018). Dopamine may influence episodic memory formation and retrieval through interactions with the hippocampus before, during, and after events, and it may expand the scope of what an individual attends to and encodes (Shohamy and Adcock, 2010; Clewett and Murty, 2019; Thorp et al., 2020). Higher spontaneous EBR has been related to greater learning in a variety of simple learning paradigms (e.g., visuomotor sequence learning, infant reward learning, and learned patterns of attention shifts; Tharp and Pickering, 2011; Slagter et al., 2015; San Anton et al., 2018; Tummeltshammer et al., 2019). Here we explored whether adolescents' EBR as they described their feelings to a series of stories in an interview predicts their long-term memory for the stories, and investigated the predictive power of EBR relative to a neural measure known to be associated with memory. To do this, we added a memory probe to the last wave of an ongoing longitudinal study, and calculated EBR from the videos when adolescents had first reacted to the stories (5 years prior). We were interested to know whether EBR might be a measure of physiology that could be assessed naturalistically to predict long-term memory, and therefore may be a potentially useful tool for educational researchers.
In addition to indexing striatal dopamine, spontaneous EBR, and other ocular behaviors may be related to memory formation because they may index deep processing associated with cognitive elaboration—when people are thinking deeply, they may spontaneously or intentionally blink more frequently or even close or avert their eyes (Glenberg et al., 1998; Doherty-Sneddon et al., 2002; Doherty-Sneddon and Phelps, 2005; Chermahini and Hommel, 2010; Yang et al., 2018; Ranti et al., 2020). Extensive evidence has accrued over nearly two centuries that engaging deeply with content (e.g., Craik and Tulving, 1975; Pressley, 1982; Schacter and Graf, 1986; Symons and Johnson, 1997) and experiencing curiosity and other intense emotions can promote memory (Levine and Pizarro, 2004; Kang et al., 2009; Pekrun, 2011; Gruber et al., 2014; Fandakova and Gruber, 2020).
EBR may also be related to memory indirectly because it may promote the gating of visual information so that a neural network associated with both external disengagement and memory can activate (Yang et al., 2018). Specifically, spontaneous EBR, and visual gating more generally, have been associated with activity in the brain's default mode network (DMN) (Nakano et al., 2013; Yang et al., 2018). The DMN is a connected network of neural regions that activates in the absence of motoric or perceptual demands from the environment and is involved in various kinds of complex and integrative thinking, including processing narratives with social-psychological and moral implications (Raichle et al., 2001; Immordino-Yang et al., 2009, 2012; Raichle, 2015; Kaplan et al., 2017). Notably, the posterior-medial DMN is important for episodic and narrative memory (Nielsen et al., 2005; Sestieri et al., 2011; Immordino-Yang et al., 2012; Amlien et al., 2018). DMN connectivity has been linked to more frequent episodic memory recall during social processing of stories (Yang et al., 2013). Patterns of activation and deactivation in DMN regions during memory encoding and retrieval predict memory performance (Daselaar et al., 2004, 2009; Kim, 2010; Huijbers et al., 2011; White et al., 2013). We sought to test whether naturalistic measures of EBR would be related to DMN activity in our dataset and if and how EBR and DMN activity would independently and together predict long-term memory. Such connections between naturalistic measures that are inexpensive and minimally invasive, and measures like neural functioning requiring highly specialized equipment like fMRI, could lead to important advances in the toolkit of educational researchers aiming to study neurophysiological functioning in the field.
Despite the fact that EBR might provide an easily measurable real-time index of learning and insights into the neurochemical basis of memory, especially for socially-relevant processing, to our knowledge spontaneous EBR has only been utilized once in a non-clinical adolescent population (Barkley-Levenson and Galvan, 2017), and this was to study reward decisions. We know of no studies using EBR during an active learning task with a non-clinical adolescent sample.
Multi-year studies of adolescents, which are useful for measuring long-term memory, are difficult to conduct and infrequently undertaken, especially with diverse community adolescents from low-SES settings (Rad et al., 2018). In this exploratory study we examine the predictive power of EBR on long-term memory for social stories by leveraging a unique, 5-year longitudinal data set initially designed to assess a community sample of adolescents' psychosocial and neural development (see https://candle.usc.edu/). Learning from social stories is a useful context in which to study long-term memory because sharing stories about ourselves and others is a ubiquitous and untutored activity in which people revel, and it is an effective means to learn about others and the world (Dunbar et al., 1997; Ochs and Capps, 2009). We have found previously that adolescents' deep, abstract talk as they react to stories is related to their feeling of remembering stories (Gotlieb et al., in Preparation), and that their abstract talk is associated with activation patterns in the DMN (Gotlieb et al., in Preparation).
Given previous research suggesting a link between EBR and DMN functioning (Nakano et al., 2013), we first tested whether when participants' EBR was higher while responding to a story, they were more likely to show higher DMN activity when reacting to that story in the fMRI scanner (Hypothesis 1). Controlling for general effects of age and intelligence on memory (Ofen et al., 2007; Healey et al., 2014), we then tested whether EBR to a story predicted memory for that story ~5 years after initial exposure to it (H2a). We also tested whether DMN activity predicted memory for the stories (H2b). Finally, we tested whether EBR would predict memory above and beyond DMN activity (H3).
Methods
This study was part of a larger mixed-methods, multicultural longitudinal investigation. As such recruitment procedures were designed to enroll a representative sample of our target population, and other tasks and analyses unrelated to the present study were conducted with these participants (e.g., studies of participants' anatomical brain development, functional brain development, and social-cognitive processing; including Butler et al., 2018; Gotlieb et al., in Preparation).
Participants
Recruitment procedures: Los Angeles public high schools serving low-socioeconomic status (SES) communities with high proportions of immigrant families were identified as recruitment sites. In schools that agreed to participate, teachers distributed IRB-approved recruitment fliers to their classes. Participants were selected in the order that they contacted researchers, until the recruitment goals were met. Inclusion criteria included: being in good academic standing and not under any disciplinary action; not using any illegal drugs or alcohol, nicotine, or marijuana; not having neurological or psychiatric disorders or history of physical or emotional abuse or neglect; being right handed with normal hearing and normal or corrected-to-normal vision; meeting health criteria for MRI research; having at least one parent who immigrated to the U.S. as an adult; identifying as a person of color. The sample was selected to be as balanced for gender as possible.
Sample: 65 adolescents, aged 14–18 years old (M = 15.8, SD = 1.06), agreed to participate and received parental consent. Of these, 51 participants reported receiving free or reduced-price school lunches, indicating that their households earn at or below 185% of the federal poverty line (U. S. Department of Agriculture, 2019). Thirty six participants identified as female and 29 identified as male. Thirty four participants identified as Latinx, 29 as East Asian American, and 2 as African American.
Memory data were collected slightly <5 years after initial data collection (M = 4.73 years after initial data collection, SD = 0.61 years) and slightly <3 years after participants were re-exposed to the stories as part of the larger research project activities, unrelated to the present study (M = 2.70 years after wave 2 data collection, SD = 0.64 years). Fifty five participants completed the memory probe at a mean age of 20.6 years old (SD = 0.96). Of the returning participants, roughly half (29) identified as female. Twenty five participants identified as Latinx, 28 as East Asian American, and 2 as African American. Forty one of the returning participants had previously reported receiving free or reduced-price school lunches. Attrition of 10 participants was generally due to having moved outside of the region or country, or to loss of communication with the researchers.
On the day of data collection, all participants, and parents gave written informed consent or assent, consistent with the University of Southern California's Institutional Review Board requirements. All participants were compensated at each round of data collection.
Procedure
Initial Data Collection: Task-Based Eye Blink Rate and Functional Magnetic Resonance Imaging
We adapted the social-emotional video interviewing protocol used by Immordino-Yang et al. (2009). As described in Gotlieb et al, (in Preparation), during a 2-h, private video-taped interview, an experimenter shared 40 true narrative stories about non-famous adolescents from around the world. Narratives began with the interviewer saying, “This is a story about a boy/girl who…,” followed by the interviewer telling a scripted story, and showing an ~1-min documentary-style video featuring the protagonist. The interviewer then asked each participant, “How does this story make you feel?” Participants were encouraged to be as candid as possible. Unbeknownst to the participants, the narratives were designed to fall into four categories with ten stories each, similar to Immordino-Yang et al. (2009). The stories were designed to be: (1) emotionally evocative stories about non-famous adolescents' admirable triumphs over adversity; (2) ordinary descriptions of everyday life events; (3) about physically painful injuries; and (4) about skillful accomplishments. Notably, participants were never told that they would be asked to recall the narratives beyond the experiment day.
The interviewer comfortably avoided eye contact while participants were talking during the interview. To accomplish this, the interviewer gazed down to write notes as participants responded to the story narratives (see also methods in Yang et al., 2018). Interviews were videotaped from about 2.5 feet away with the camera facing the participant directly. To standardize light levels and time of day, which can affect blink rate, the interviews always commenced between 9 and 11 a.m. in a softly and naturally lit room. Participants wore any vision-correction devices that they would typically wear, including contact lenses, or glasses. Participants were instructed to get a good night's sleep the night before the experiment and to avoid consuming caffeine on the morning of the experiment, as lack of sleep and caffeine consumption can affect EBR. Participants were not told that their blinks would be counted.
Following the interview, participants completed a Blood Oxygen Level Dependent (BOLD) functional Magnetic Resonance Imagining (fMRI) scan. During the scan, participants saw a 5-s reminder clip of each of the narrative stories from the interview with a summary sentence in both auditory and written form. Then participants saw 13 s of gray screen and indicated with four handheld buttons how emotional the stories made them feel, which, for the purpose of this analysis, serves as a compliance check. Fixation periods ranging from 2 to 6 s separated trials. Narrative stories were presented in one of two counterbalanced fixed pseudo-random orders. Each narrative story was presented twice over the course of the experiment, but never twice in the same run. This resulted in 80 trials divided into four runs, each of which was slightly <7 min. After all stories were presented, participants completed a 7-min resting-state scan.
MRI Data Acquisition
Scanning was conducted using a 3T Siemens Trio scanner with a 12-channel matrix head coil at the University of Southern California Dana and David Dornsife Neuroimaging Center. Functional scans were acquired using a T2*-weighted echo-planar imaging (EPI) sequence (TR = 2 s, TE = 25 ms, flip angle = 90°, acquisition matrix: 64 × 64, FOV = 192 mm) with a voxel resolution of 3 × 3 × 3 mm. Forty one continuous transverse slices were acquired in interleaved order to cover the whole brain and brain stem. Anatomical images were acquired using a magnetization-prepared rapid acquisition gradient echo sequence (TI = 800 ms, TR = 2,530 ms, TE = 3.09 ms, flip angle = 10°, isotropic voxel resolution of 1 mm; acquisition dimensions: 256 × 256 × 176).
Memory Data Collection
At the final wave of data collection, slightly <5 years after initial data collection, participants completed an online survey of their memory for the stories from the interviews. They received the online survey via electronic communication (i.e., email, text message, and/or social media direct message).
Other Data Collection: IQ and Standardized Eye Blink Rate
As part of the larger research project, 2 years after initial data collection the interview, with the same narrative stories, was repeated, as was the fMRI scan with those stories. During that same round of data collection participants completed the vocabulary and matrix reasoning subtests of the Wechsler Abbreviated Scale of Intelligence.
Midway through this wave of data collection we sought to validate the wave 1 EBR measure. The 37 participants who had not yet participated in this wave of data collection read aloud from a computer screen an engaging 1,600-word excerpt of a New York Times article (Suskind, 2014) while they were videotaped from about 2.5 feet away. Participants were alone while doing so. They completed the task in the late afternoon in a naturally lit room.
Measures and Scoring
Eye Blink Rate While Responding to Stories
Trained coders counted, from video recordings of the interviews, the number of blinks a participant made while responding to each narrative story, beginning when the participant began explaining how the story made him or her feel and ending when they finished talking. EBR was calculated as blinks per minute (BPM). Responses that lasted 3 s or less [e.g., “(it makes me feel) good.”] were not coded.
A second set of coders, blind to the first coders' EBR scores, determined EBR for all narrative stories from a randomly selected group of 20 participants. We calculated the interclass correlation coefficient (ICC) for EBR based on the two sets of scores for this sample of participants. The single measure ICC (from a two-way random, absolute agreement model) was 0.82, p < 0.001. Given this strong agreement, analyses were performed using the original EBR scores for all participants.
EBR was calculated for each story. Then an average EBR across all stories was calculated for each participant and used to validate the interview-based EBR against EBR during the standardized reading task.
Eye Blink Rate During a Standardized Reading Task
Consistent with standard practice for measuring EBR (Bentivoglio et al., 1997; Doughty, 2001), we also calculated participants' blink rate while they read aloud the aforementioned New York Times passage. Standard readability tests suggested that the passage was an appropriate reading-level for participants (Clay, 1991; Jahner, 2017).
Two independent coders counted the total number of blinks captured in the first 7 min of reading. The ICC between the two coders was 0.98, p < 0.001 and scores from the two coders were averaged.
Default Mode Network Activity
fMRI Data Pre-Processing
SPM12 (SCR_007037) and MATLAB 2015b were used to perform standard preprocessing of fMRI data (Wellcome Department of Cognitive Neurology, London, UK; MathWorks, Inc., Natick, MA, USA). Functional images were slice timing and motion corrected, and were co-registered to the anatomical image. Co-registered images were examined for each participant in the native space, and anatomical images were normalized to the Montreal Neurological Institute space using the segmentation procedure. The resulting normalization transformation was applied to the functional images. The functional images were resampled into an isotropic voxel resolution of 2x2x2 mm and smoothed using an 8 mm full width at half maximum Gaussian kernel. The Artifact Detection Tool (ART; SCR_005994; https://www.nitrc.org/projects/artifact_detect/) was used to identify outlier scans. Trials with excessive motion (i.e., scans with scan-to-scan composite movement exceeding one mm of movement or normalized scan-to-scan global signal change exceeding a z-score of nine) were excluded.
DMN Identification
We utilized DMN ROIs as defined in Gotlieb et al, (in Preparation). These were derived from a whole-brain voxel-wise regression analysis of functional activation during the task. To locate the DMN, we included as a covariate a verbal measure of cognitive processing taken from the interview, that we had previously shown is related to DMN activity in our task (Yang et al., 2018). We imposed a statistical threshold of p < 0.005 and a cluster extent threshold of 65 voxels. We selected DMN ROIs based on visual inspection of positive significant results that corresponded to key DMN hubs based on previous literature (e.g., Andrews-Hanna et al., 2010). We created 6-mm spheres centered at the peak effects in the clusters in the dorsomedial prefrontal cortex, ventromedial prefrontal cortex, and posteroinferior posteromedial cortices. See Figure 1.
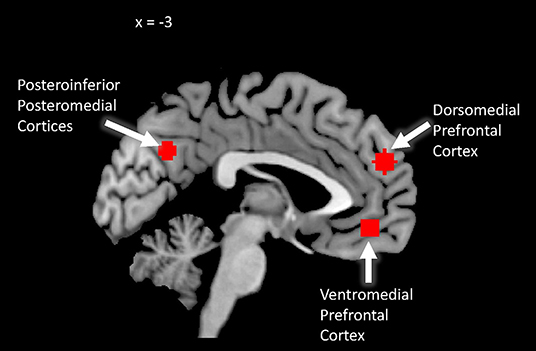
Figure 1. Default mode network region of interest map. Sagittal view of regions of interest in the default mode network derived from whole-brain neural correlates of a verbal measure of cognitive processing previously shown to relate to DMN activity (Yang et al., 2018; see also Gotlieb et al, in Preparation). Each circle represents a 6 mm spherical ROI.
Modeling Story-by-Story DMN Activity
Participants' responses to each narrative story was modeled by averaging TRs 4-8, which corresponded with the period 6–16 s post video onset, from both presentations of the stimulus. We previously identified that time window as the appropriate period for capturing emotional responses in this task (Immordino-Yang et al., 2009). This resulted in one value per narrative story per participant, per ROI. However, due to motion, some narratives were excluded. This approach replicated previously used methods (Immordino-Yang et al., 2014; Yang et al., 2018).
Memory
Cued recall was measured using a computer-based survey with still images of each of the 40 story protagonists presented individually. Participants selected a one-sentence description, among five possibilities, that best summarized the story represented by the image of the protagonists. The five options included one correct response, and four other responses similar to the types of stories told. Each option was substantially different from all other options such that if the participant remembered the gist of the story he or she should be able to identify the correct answer. All answers were designed and piloted to seem plausible given the image displayed. For each story, participants received a zero score if the multiple-choice question was answered incorrectly and a score of one if it was answered correctly. Across all participants' response to all stories, 73% were recalled correctly.
IQ- Wechsler Abbreviated Scale of Intelligence
Participants completed orally the vocabulary and matrix reasoning subtests of the Wechsler Abbreviated Scale of Intelligence—second edition (Wechsler, 2013). The test was administered by a trained researcher to each participant individually and privately in a quiet room. Due to time constraints one participant only completed the vocabular subtest. That participant's overall IQ was imputed based on how far that subtest score was from the mean for that subtest. Age-normed scores were within the normal range (M = 104.10, SD = 11.88).
Missing Data
Beyond missing data due to attrition, we also have missing fMRI data from one participant due to excessive sleepiness during scanning. Due to motion, for 12 participants fMRI data was excluded for 1 or 2 of the narratives. For 4 additional participants fMRI data was excluded from 3 to 7 of the narratives.
For 49 participants EBR data is available for 35 or more of narratives. The remaining 16 participants had usable EBR data for more than half of the narratives. Missing EBR data was due to excessively short responses that were excluded from analyses and some missing video recordings because of a malfunctioning video recorder.
One participant answered only half of the questions on the memory survey. We have missing IQ data from one participant who did not return when IQ was tested.
Analysis Plan
We examined all hypotheses using a series of generalized estimating equation models (IBM SPSS 25, SPSS Inc., Chicago, IL, USA; SCR_002865) as these allow us to nest within a participant his or her EBR and DMN activity in response to a story. These models provide unbiased parameter estimates that account for within-participant correlations of repeated measures—e.g., base rates of how memorable a given story was, and base rates of how likely a participant was to report remembering a story they had seen (Ballinger, 2004). We use binary logistic response functions for all memory analyses as the outcome variable involved binary (i.e., correct or incorrect) responses to memory prompts. In testing the relative predictive power of EBR, we used standardized z-scores of EBR and DMN activity. In all tests of effects on memory we control for age and IQ. Age and IQ were not correlated with average EBR or average DMN activity (all r < 0.14, all p > 0.29). We control also for the order in which the 40 stories were presented to participants (in one of two pseudo-random orders; to account for primacy or recency effects), and to which of four types of narrative stories the participant was reacting (since more emotionally-evocative categories of stories might be more memorable).
Results
Establishing Eye Blink Rate Measure
EBR while reading (M = 15.06 blinks per minute (BPM), SD = 12.16) and during the interview (M = 28.92 BPM, SD = 13.13 BPM) are on the high-end of a normal blink rate based on research with adult populations (Doughty, 2001). Typical EBR for adolescents is not well-established. Average EBR while participants were reacting to the social stories was correlated with EBR from the standardized reading task, r (35) = 0.51, p = 0.001. This suggests that EBR during the interview is a fair measure of inter-individual variability in EBR.
Among our participants, as is typical in previous EBR research, there was a substantial amount of inter-individual variability in EBR (McMonnies, 2010). Although average EBR while responding to all the stories was approximately normally distributed, there was one participant whose EBR was more than 3 standard deviations above the mean. This higher-than-average EBR is consistent with suggestions that there may be a group of people with frequent eye blink activity (Doughty and Naase, 2006). Given that previous literature has not documented a non-clinical adolescent population's task-based EBR and that this participant was neurologically normal, we believe that the participant's EBR may still represent typical variability in EBR, and we do not exclude the participant from analyses reported here. Other research on EBR (e.g., Nakano et al., 2013) has excluded participants with high EBRs similar to this participant's, which may make it more challenging to understand how the full range of variability in EBR contributes to psychological and neurological traits. All EBR results were tested without that participant and significant results remain significant and trends remain in the same direction.
H1: EBR Was Unrelated to DMN Activity
Hypothesis 1 was not supported. Controlling for narrative story category, narrative story presentation order, and within subject effects, EBR and DMN activity were unrelated on a story-by-story basis, p = 0.30.
H2: EBR and DMN Activity Each Independently Predicted Memory
Hypothesis 2a and b about main effects of EBR and DMN activity were supported. EBR predicted memory, Wald χ2(1, N = 1903 stories, nested in 54 participants, with data from responses to 20–40 stories each) = 7.96, b = 0.02, SE = 0.006, 95% CI [0.01, 0.03], p = 0.01, exp(B) = 1.02.
DMN activity also predicted memory, Wald χ2(1, N = 2,068 stories, nested in 53 participants, with data from responses to 19–40 stories each) = 6.72, b = 0.26, SE = 0.16, 95% CI [0.10, 0.74], p = 0.01, exp(B) = 1.53.
H3: EBR and DMN Activity Predicted Memory Controlling for Effects of One Another
Since we found main effects of EBR and DMN activity on memory, we tested for additive effects of standardized scores of EBR and DMN activity on memory. Both EBR controlling for DMN activity and DMN activity controlling for EBR predicted memory, Wald χ2(1, N = 1,837 stories, nested in 53 participants, with data from responses to 19–40 stories each) = 7.18, b = 0.24, SE = 0.09, 95% CI [0.07, 0.42], p = 0.01, exp(B) = 1.27 (EBR); Wald χ2(1, N = 1,837) = 9.06, b = 0.17, SE = 0.06, 95% CI [0.06, 0.29], p = 0.003, exp(B) = 1.19 (DMN activity).
Discussion
Much can be learned about learning itself from physiological measures that tap into mechanisms and processes that underlie thinking and learning. However, for educational researchers to utilize neuropsychological research in classroom contexts, tools, and measures are needed that do not require the kind of controlled context that is possible in laboratories but rarely in schools (Immordino-Yang and Christodoulou, 2014; Immordino-Yang and Gotlieb, 2017). Working with a community sample of urban adolescents of color, a large and growing demographic group that is underrepresented in research (Rad et al., 2018), here we attempted to bridge the gap between a relatively naturalistic measure, eye-blink rate collected using a simple video camera, and a measure only possible to collect in a laboratory with highly specialized equipment, namely functional neuroimaging. Using a post-hoc design, we sought to test the utility of the naturalistic measure and the neuroimaging measure in a longitudinal study of memory for story stimuli. We found that, even controlling for adolescents' full-scale IQ, both measures predicted trial-by-trial memory for individual stories after the 5-year delay, and that each contributed to explaining different variance. Though EBR measurement is time consuming and does require some methodological considerations in a school context and so is not a silver-bullet, this study demonstrates the utility of EBR methods in studying processing that leads to long-term memory. In so doing, the study offers a methodological proof-of-concept that EBR holds promise as a tool for educational researchers.
Our study also adds to the body of literature connecting the DMN's activity to long-term memory formation, and extends this literature to an adolescent population engaging in a naturalistic social learning task. The fact that trial-by-trial DMN activity as a participant reacted to a story predicted memory for that story above and beyond EBR reaffirms the value of cross-modal studies of adolescents' learning in the field and laboratory (Immordino-Yang and Christodoulou, 2014; Immordino-Yang and Gotlieb, 2017).
Arguably among the most important topics in adolescent education today is the need to promote and measure deep processing of complex information, which is associated with deeper understanding and long-term retention (Perkins, 2014; Immordino-Yang and Knecht, 2020). Deep processing is notoriously difficult to measure and study objectively, especially without disrupting the learning process. Recent laboratory and neuroimaging research is revealing the underlying neurological mechanisms that support this kind of thinking and the necessary conditions (Immordino-Yang et al., 2012; Immordino-Yang, 2016; Yang et al., 2018). But to connect this laboratory research to studies of deep learning in real-world school environments, new tools are needed. The present study provides compelling evidence for the value of continued exploration of EBR as a non-invasive tool for researchers to prospectively gauge youths' learning and memory from their physiology. Developing new, objective, physiological indicators of learning may facilitate understanding the neurobiological mechanisms by which learning occurs under various conditions. Connecting EBR measures to gaze aversion, another spontaneous measure of ocular behavior that has been associated with DMN activation and deep cognitive processing (Yang et al., 2018), could also be useful with adolescents. With careful consideration of ethical and user-experience considerations, it could possibly also be incorporated into artificial intelligence technologies to help teachers and learners predict what students will remember or refine digital learning technologies to promote deep thinking.
For memory researchers working in educational contexts, EBR may complement or confer advantages over typical psychological and neural strategies for identifying deep processing and predicting memory. Psychological predictors of memory during learning, such as assessing the depth with which someone is processing information with surveys or interviews, can disrupt the flow of the learning experience and can be biased by participants' ability or inclination to report about the depth of their processing (Duckworth and Yeager, 2015). EBR, however, can be measured unobtrusively and without self-reporting biases. Despite its potential usefulness and common use in research on clinical populations and in research with adults, infants, and other species, EBR has been underutilized in educational research and in research with adolescents more generally (Doughty, 2001; Eckstein et al., 2017).
Especially as interest in mixed-methods research among education and psychology researchers continues to increase (Molina-Azorin and Fetters, 2017), and as more research into adolescent learning explores the physiological instantiation of educationally-relevant processes (e.g., Chang and Beilock, 2016), strategically designed research studies that move between school and laboratory contexts could make unique contributions to understanding the neurobiology of learning. For example, because dopamine is involved in the experience of curiosity (DeYoung, 2013) and curiosity is a powerful driver of learning (Engel, 2015), it would be interesting to examine whether EBR measured during a classroom-based learning task is related to reports of curiosity about the topic and predicts comprehension and retention. Alternatively, because dopamine may be associated with stress under certain conditions, EBR may be an effective way of indexing who might perform poorly in high-pressure situations (e.g., testing), with EBR potentially predicting more “choking” under pressure (van de Groep et al., 2017). At a psychological and behavioral level, EBR is an involuntary, and veridical facial measure that can be used to study emotion, attention, cognitive control, and interest (Dreisbach et al., 2005; Siegle et al., 2008; Colzato et al., 2009; Oh et al., 2012; Kret, 2015; Kruis et al., 2016; Nakano and Miyazaki, 2019).
Because we added measures to an ongoing project that was not designed specifically to address long-term memory, there are some limitations in the present study. Participants had been exposed to the stories twice when we measured memory, so it is not possible to know whether the second exposure interacted with the first to impact long-term retention. In addition, because we aimed to study a community sample of youth in an ecologically valid way, we did not control for factors thought to impact tonic EBR, such as contact lens use, phase of the menstrual cycle and seasonal allergies (That said, though these factors could influence tonic EBR, our analyses control for EBR base-rate, and focus instead on how intrapersonal trial-by-trial fluctuations in EBR predict memory for particular stimuli). Future research should replicate the findings with a range of populations to ensure the representativeness of our sample. Finally, as is to be expected with 5-year longitudinal research engaging low-SES youths, 10 of 65 participants lost touch or moved away before the study was complete. As for all studies, attrition poses a potential threat to validity and suggests the need for replication.
It is worth noting again that it is not certain what the EBR measure in our study is indexing neurobiologically. Though a large literature exists, recently there has been growing debate about the exact nature of the link between spontaneous EBR and striatal dopamine (Dang et al., 2017; Sescousse et al., 2018; Unsworth et al., 2019). Even if evidence continues to grow for the lack of a relationship between spontaneous EBR and dopamine, it is possible that EBR might support memory processes by facilitating the gating of perceptual information and creating micro-moments for deeper processing and encoding. Also, we did not find the hypothesized relationship between EBR and DMN activity in the brain in our data. This is possibly because, as Nakano et al. (2013) suggest, the relationship between EBR and DMN activity (and between EBR and dopamine) is likely driven by unconscious spontaneous blinks, which we did not have the resolution in our data to distinguish. Additional research into the neural processes underlying EBR could be useful, as well as research about the mechanism connecting EBR and memory when EBR is measured in a more precise way in a laboratory setting.
In conclusion, though our study has notable limitations and should be replicated, it offers an initial examination of the possible utility of EBR in studying deep processing that leads to memory. Measurements of the activity of the eyes have been used for centuries to investigate the bodily and psychological processes that constitute the mind (Wade, 2010). Perhaps it is time to re-view these ocular measures and utilize them in educational contexts.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, upon request, without undue reservation, while protecting the identities of research participants.
Ethics Statement
The studies involving human participants were reviewed and approved by University of Southern California Institutional Review Board. Written informed consent to participate in this study was provided by participants and their parents/legal guardian.
Author Contributions
RG, X-FY, and MHI-Y contributed to the design and interpretation of the work. RG, X-FY, and MHI-Y, with help from research assistants, acquired the data for this study. RG and X-FY processed data and conducted analyses. RG drafted the manuscript and X-FY and MHI-Y contributed to its critical revision. MHI-Y conceptualized the overarching study of adolescent social-emotional development, acquired the main funding that made this work possible, and supervised the project.
Funding
We are grateful for the generous funding for this work provided by the National Science Foundation Career grant 11519520 to MHI-Y, the Raikes Foundation grant #61405837-118286 to MHI-Y & Camille Farrington, NSF GRFP award to RG, USC Provost's Research and Teaching Fellowships to RG, and the USC Dean's Research Assistantship Awards in Urban Education to RG.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful for Erik Jahner's collection of reading data, Rodrigo Rivero's help collecting IQ data, and Christa Simone's help conducting video interviews about the emotionally-evocative stories at initial data collection. We are grateful to several research assistants who helped with various tasks involved in the production of this manuscript, most notably recording eye blinks. The authors acknowledge that a version of this manuscript first appeared in RG's doctoral dissertation.
References
Amlien, I. K., Sneve, M. H., Vidal-Piñeiro, D., Walhovd, K. B., and Fjell, A. M. (2018). The lifespan trajectory of the encoding-retrieval flip: a multimodal examination of medial parietal cortex contributions to episodic memory. J. Neurosci. 38, 8666–8679. doi: 10.1523/JNEUROSCI.1702-17.2018
Andrews-Hanna, J. R., Reidler, J. S., Sepulcre, J., Poulin, R., and Buckner, R. L. (2010). Functional-anatomic fractionation of the brain's default network. Neuron 65, 550–562. doi: 10.1016/j.neuron.2010.02.005
Badgaiyan, R. D., Fischman, A. J., and Alpert, N. M. (2007). Striatal dopamine release in sequential learning. Neuroimage 38, 549–556. doi: 10.1016/j.neuroimage.2007.07.052
Ballinger, G. A. (2004). Using generalized estimating equations for longitudinal data analysis. Organizat. Res. Methods 7, 127–150. doi: 10.1177/1094428104263672
Barkley-Levenson, E., and Galvan, A. (2017). Eye blink rate predicts reward decisions in adolescents. Dev. Sci. 20:e12412. doi: 10.1111/desc.12412
Bentivoglio, R., Bressman, S. B., Cassetta, E., Carretta, D., Tonali, P., and Albanese, A. (1997). Analysis of blink rate patterns in normal subjects. Movement Disord. 12, 1028–1034. doi: 10.1002/mds.870120629
Butler, O., Yang, X. F., Laube, C., Kühn, S., and Immordino-Yang, M. H. (2018). Community violence exposure correlates with smaller gray matter volume and lower IQ in urban adolescents. Human Brain Mapp. 39, 2088–2097. doi: 10.1002/hbm.23988
Chang, H., and Beilock, S. L. (2016). The math anxiety-math performance link and its relation to individual and environmental factors: a review of current behavioral and psychophysiological research. Curr. Opin. Behav. Sci. 10, 33–38. doi: 10.1016/j.cobeha.2016.04.011
Chermahini, S. A., and Hommel, B. (2010). The (b) link between creativity and dopamine: spontaneous eye blink rates predict and dissociate divergent and convergent thinking. Cognition 115, 458–465. doi: 10.1016/j.cognition.2010.03.007
Clay, M. M. (1991). Becoming Literate: The Construction of Inner Control. Heinemann Educational Books.
Clewett, D., and Murty, V. P. (2019). Echoes of emotions past: How neuromodulators determine what we recollect. Eneuro 6:ENEURO.0108-18.2019. doi: 10.1523/ENEURO.0108-18.2019
Colzato, L. S., van den Wildenberg, W. P., van Wouwe, N. C., Pannebakker, M. M., and Hommel, B. (2009). Dopamine and inhibitory action control: evidence from spontaneous eye blink rates. Exp. Brain Res. 196, 467–474. doi: 10.1007/s00221-009-1862-x
Craik, F. I., and Tulving, E. (1975). Depth of processing and the retention of words in episodic memory. J. Exp. Psychol. 104:268. doi: 10.1037/0096-3445.104.3.268
Dahlstrom-Hakki, I., Asbell-Clarke, J., and Rowe, E. (2019). Showing is knowing: The potential and challenges of using neurocognitive measures of implicit learning in the classroom. Mind Brain Educat. 13, 30–40. doi: 10.1111/mbe.12177
Dang, L. C., Samanez-Larkin, G. R., Castrellon, J. J., Perkins, S. F., Cowan, R. L., Newhouse, P. A., et al. (2017). Spontaneous eye blink rate (EBR) is uncorrelated with dopamine D2 receptor availability and unmodulated by dopamine agonism in healthy adults. eNeuro 4:ENEURO.0211-17.2017. doi: 10.1523/ENEURO.0211-17.2017
Daselaar, S. M., Prince, S. E., and Cabeza, R. (2004). When less means more: deactivations during encoding that predict subsequent memory. Neuroimage 23, 921–927. doi: 10.1016/j.neuroimage.2004.07.031
Daselaar, S. M., Prince, S. E., Dennis, N. A., Hayes, S. M., Kim, H., et al. (2009). Posterior midline and ventral parietal activity is associated with retrieval success and encoding failure. Front. Human Neurosci. 3:13. doi: 10.3389/neuro.09.013.2009
DeYoung, C. G. (2013). The neuromodulator of exploration: a unifying theory of the role of dopamine in personality. Front. Human Neurosci. 7:762. doi: 10.3389/fnhum.2013.00762
Doherty-Sneddon, G., Bruce, V., Bonner, L., Longbotham, S., and Doyle, C. (2002). Development of gaze aversion as disengagement from visual information. Dev. Psychol. 38:438. doi: 10.1037/0012-1649.38.3.438
Doherty-Sneddon, G., and Phelps, F. G. (2005). Gaze aversion: a response to cognitive or social difficulty?. Memory Cognit. 33, 727–733. doi: 10.3758/BF03195338
Doughty, M. J. (2001). Consideration of three types of spontaneous eyeblink activity in normal humans: during reading and video display terminal use, in primary gaze, and while in conversation. Optometry Vision Sci. 78, 712–725. doi: 10.1097/00006324-200110000-00011
Doughty, M. J., and Naase, T. (2006). Further analysis of the human spontaneous eye blink rate by a cluster analysis-based approach to categorize individuals with ‘normal' vs. ‘frequent' eye blink activity. Eye Contact Lens 32, 294–299. doi: 10.1097/01.icl.0000224359.32709.4d
Dreisbach, G., Müller, J., Goschke, T., Strobel, A., Schulze, K., Lesch, K. P., et al. (2005). Dopamine and cognitive control: the influence of spontaneous eyeblink rate and dopamine gene polymorphisms on perseveration and distractibility. Behav. Neurosci. 119:483. doi: 10.1037/0735-7044.119.2.483
Duckworth, A. L., and Yeager, D. S. (2015). Measurement matters: assessing personal qualities other than cognitive ability for educational purposes. Educat. Res. 44, 237–251. doi: 10.3102/0013189X15584327
Dunbar, R. I., Marriott, A., and Duncan, N. D. (1997). Human conversational behavior. Human Nature 8, 231–246. doi: 10.1007/BF02912493
Eckstein, M. K., Guerra-Carrillo, B., Singley, A. T. M., and Bunge, S. A. (2017). Beyond eye gaze: what else can eyetracking reveal about cognition and cognitive development? Dev. Cognit. Neurosci. 25, 69–91. doi: 10.1016/j.dcn.2016.11.001
Engel, S. (2015). The Hungry Mind: The Origins of Curiosity in Childhood. Cambridge, MA: Harvard University Press.
Fandakova, Y., and Gruber, M. J. (2020). States of curiosity and interest enhance memory differently in adolescents and in children. Dev. Sci. e13005. doi: 10.1111/desc.13005
Glenberg, A. M., Schroeder, J. L., and Robertson, D. A. (1998). Averting the gaze disengages the environment and facilitates remembering. Memory Cogn. 26, 651–658. doi: 10.3758/BF03211385
Gruber, M. J., Gelman, B. D., and Ranganath, C. (2014). States of curiosity modulate hippocampus-dependent learning via the dopaminergic circuit. Neuron 84, 486–496. doi: 10.1016/j.neuron.2014.08.060
Healey, M. K., Crutchley, P., and Kahana, M. J. (2014). Individual differences in memory search and their relation to intelligence. J. Exp. Psychol. 143, 1553–1559. doi: 10.1037/a0036306
Huijbers, W., Pennartz, C. M., Cabeza, R., and Daselaar, S. M. (2011). The hippocampus is coupled with the default network during memory retrieval but not during memory encoding. PLoS ONE 6:e17463. doi: 10.1371/journal.pone.0017463
Immordino-Yang, M. H. (2016). Emotion, sociality, and the brain's default mode network: Insights for educational practice and policy. Policy Insights Behav. Brain Sci. 3, 211–219. doi: 10.1177/2372732216656869
Immordino-Yang, M. H., and Christodoulou, J. A. (2014). “Neuroscientific contributions to understanding and measuring emotions in educational contexts,” in International Handbook of Emotions in Education, eds R. Pekrun and L. Linnenbrink-Garcia (New York, NY: Taylor and Francis/Routledge), 607–624.
Immordino-Yang, M. H., Christodoulou, J. A., and Singh, V. (2012). Rest is not idleness: Implications of the brain's default mode for human development and education. Perspect. Psychol. Sci. 7, 352–364. doi: 10.1177/1745691612447308
Immordino-Yang, M. H., and Gotlieb, R. (2017). Embodied brains, social minds, cultural meaning: integrating neuroscientific and educational research on social-affective development. Am. Educat. Res. J. 54:344S−67S. doi: 10.3102/0002831216669780
Immordino-Yang, M. H., and Knecht, D. (2020) Building Meaning Builds Teens' Brains: Connecting Adolescents' Concrete Work to Big Ideas May Help Shape Their Neural Networks Over Time. Educational Leadership. Available online at: http://www.ascd.org/publications/educational-leadership/may20/vol77/num08/Building-Meaning-Builds-Teens'-Brains.aspx (accessed June 15, 2020).
Immordino-Yang, M. H., McColl, A., Damasio, H., and Damasio, A. (2009). Neural correlates of admiration and compassion. Proc. Natl. Acad. Sci. U.S.A. 106, 8021–8026. doi: 10.1073/pnas.0810363106
Immordino-Yang, M. H., Yang, X. F., and Damasio, H. (2014). Correlations between social-emotional feelings and anterior insula activity are independent from visceral states but influenced by culture. Front. Human Neurosci. 8:728. doi: 10.3389/fnhum.2014.00728
Jahner, E. E. (2017). Resting as Knowing: A Lagged Structure Analysis of Resting State fMRI with Application to Mind Wandering during Oral Reading. Ph.D. Dissertation: University of California, Riverside. Available online at: https://search.proquest.com/docview/2024187295?pq-origsite=gscholar (accessed March 9, 2020).
Jongkees, B. J., and Colzato, L. S. (2016). Spontaneous eye blink rate as predictor of dopamine-related cognitive function—A review. Neurosci. Biobehav. Rev. 71, 58–82. doi: 10.1016/j.neubiorev.2016.08.020
Kang, M. J., Hsu, M., Krajbich, I. M., Loewenstein, G., McClure, S. M., Wang, J. T. Y., et al. (2009). The wick in the candle of learning: epistemic curiosity activates reward circuitry and enhances memory. Psychol. Sci. 20, 963–973. doi: 10.1111/j.1467-9280.2009.02402.x
Kaplan, J. T., Gimbel, S. I., Dehghani, M., Immordino-Yang, M. H., Sagae, K., Wong, J. D., et al. (2017). Processing narratives concerning protected values: a cross-cultural investigation of neural correlates. Cereb. Cortex 27, 1428–1438. doi: 10.1093/cercor/bhv325
Kim, H. (2010). Dissociating the roles of the default-mode, dorsal, and ventral networks in episodic memory retrieval. Neuroimage 50, 1648–1657. doi: 10.1016/j.neuroimage.2010.01.051
Kret, M. E. (2015). Emotional expressions beyond facial muscle actions. A call for studying autonomic signals and their impact on social perception. Front. Psychol. 6:711. doi: 10.3389/fpsyg.2015.00711
Kruis, A., Slagter, H. A., Bachhuber, D. R., Davidson, R. J., and Lutz, A. (2016). Effects of meditation practice on spontaneous eyeblink rate. Psychophysiology 53, 749–758. doi: 10.1111/psyp.12619
Levine, L. J., and Pizarro, D. A. (2004). Emotion and memory research: a grumpy overview. Soc. Cogn. 22, 530–554. doi: 10.1521/soco.22.5.530.50767
McMonnies, C. W. (2010). “Blinking mechanisms,” in Encyclopedia of the Eye, eds D. A. Dartt, J. C. Besharse, and R. Dana (San Diego, CA: Elsevier Ltd.), 202–208. doi: 10.1016/B978-0-12-374203-2.00079-8
Molina-Azorin, J. F., and Fetters, M. D. (2017). The journal of mixed methods research starts a new decade: the first 10 years in review. J. Mixed Methods Res. 11, 143–155. doi: 10.1177/1558689817696365
Nakano, T., Kato, M., Morito, Y., Itoi, S., and Kitazawa, S. (2013). Blink-related momentary activation of the default mode network while viewing videos. Proc. Natl. Acad. Sci. U. S. A. 110, 702–706. doi: 10.1073/pnas.1214804110
Nakano, T., and Miyazaki, Y. (2019). Blink synchronization is an indicator of interest while viewing videos. Int. J. Psychophysiol. 135, 1–11. doi: 10.1016/j.ijpsycho.2018.10.012
Nielsen, F. Å., Balslev, D., and Hansen, L. K. (2005). Mining the posterior cingulate: segregation between memory and pain components. Neuroimage 27, 520–532. doi: 10.1016/j.neuroimage.2005.04.034
Nyberg, L., Karalija, N., Salami, A., Andersson, M., Wåhlin, A., Kaboovand, N., et al. (2016). Dopamine D2 receptor availability is linked to hippocampal–caudate functional connectivity and episodic memory. Proc. Natl. Acad. Sci. U.S.A. 113, 7918–7923. doi: 10.1073/pnas.1606309113
Ochs, E., and Capps, L. (2009). Living Narrative: Creating Lives in Everyday Storytelling. Harvard University Press.
Ofen, N., Kao, Y. C., Sokol-Hessner, P., Kim, H., Whitfield-Gabrieli, S., and Gabrieli, J. D. (2007). Development of the declarative memory system in the human brain. Nat. Neurosci. 10:1198. doi: 10.1038/nn1950
Oh, J., Jeong, S. Y., and Jeong, J. (2012). The timing and temporal patterns of eye blinking are dynamically modulated by attention. Human Movement Sci. 31, 1353–1365. doi: 10.1016/j.humov.2012.06.003
Pekrun, R. (2011). “Emotions as drivers of learning and cognitive development,” in New Perspectives on Affect and Learning Technologies, eds R. A. Calvo, and S. K. D'Mello (New York, NY: Springer), 23–39. doi: 10.1007/978-1-4419-9625-1_3
Perkins, D. (2014). Future Wise: Educating Our Children for a Changing World. San Francisco, CA: John Wiley and Sons.
Rad, M. S., Martingano, A. J., and Ginges, J. (2018). Toward a psychology of Homo sapiens: Making psychological science more representative of the human population. Proc. Natl. Acad. Sci. U.S.A. 115, 11401–11405. doi: 10.31234/osf.io/hfs3e
Raichle, M. E. (2015). The brain's default mode network. Annual Rev. Neurosci. 38, 433–447. doi: 10.1146/annurev-neuro-071013-014030
Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A., and Shulman, G. L. (2001). A default mode of brain function. Proc. Natl. Acad. Sci. U.S.A. 98, 676–682. doi: 10.1073/pnas.98.2.676
Ranti, C., Jones, W., Klin, A., and Shultz, S. (2020). Blink Rate patterns provide a reliable measure of individual engagement with scene content. Sci. Rep. 10, 1–10. doi: 10.1038/s41598-020-64999-x
Rieckmann, A., Johnson, K. A., Sperling, R. A., Buckner, R. L., and Hedden, T. (2018). Dedifferentiation of caudate functional connectivity and striatal dopamine transporter density predict memory change in normal aging. Proc. Natl. Acad. Sci. U.S.A. 115, 10160–10165. doi: 10.1073/pnas.1804641115
San Anton, E., Cleeremans, A., Destrebecqz, A., Peigneux, P., and Schmitz, R. (2018). Spontaneous eyeblinks are sensitive to sequential learning. Neuropsychologia 119, 489–500. doi: 10.1016/j.neuropsychologia.2018.09.007
Schacter, D. L., and Graf, P. (1986). Effects of elaborative processing on implicit and explicit memory for new associations. J. Exp. Psychol. 12:432. doi: 10.1037/0278-7393.12.3.432
Sescousse, G., Ligneul, R., van Holst, R. J., Janssen, L. K., de Boer, F., Janssen, M., et al. (2018). Spontaneous eye blink rate and dopamine synthesis capacity: preliminary evidence for an absence of positive correlation. Eur. J. Neurosci. 47, 1081–1086. doi: 10.1111/ejn.13895
Sestieri, C., Corbetta, M., Romani, G. L., and Shulman, G. L. (2011). Episodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analyses. J. Neurosci. 31, 4407–4420. doi: 10.1523/JNEUROSCI.3335-10.2011
Shohamy, D., and Adcock, R. A. (2010). Dopamine and adaptive memory. Trends Cogn. Sci. 14, 464–472. doi: 10.1016/j.tics.2010.08.002
Siegle, G. J., Ichikawa, N., and Steinhauer, S. (2008). Blink before and after you think: blinks occur prior to and following cognitive load indexed by pupillary responses. Psychophysiology 45, 679–687. doi: 10.1111/j.1469-8986.2008.00681.x
Slagter, H. A., Georgopoulou, K., and Frank, M. J. (2015). Spontaneous eye blink rate predicts learning from negative, but not positive, outcomes. Neuropsychologia 71, 126–132. doi: 10.1016/j.neuropsychologia.2015.03.028
Symons, C. S., and Johnson, B. T. (1997). The self-reference effect in memory: a meta-analysis. Psychol. Bull. 121:371. doi: 10.1037/0033-2909.121.3.371
Tharp, I. J., and Pickering, A. D. (2011). Individual differences in cognitive-flexibility: the influence of spontaneous eyeblink rate, trait psychoticism and working memory on attentional set-shifting. Brain Cogn. 75, 119–125. doi: 10.1016/j.bandc.2010.10.010
Thorp, J., Clewett, D., and Riegel, M. (2020). Two routes to incidental memory under arousal: dopamine and norepinephrine. J. Neurosci. 40, 1790–1792. doi: 10.1523/JNEUROSCI.2698-19.2020
Tummeltshammer, K., Feldman, E. C., and Amso, D. (2019). Using pupil dilation, eye-blink rate, and the value of mother to investigate reward learning mechanisms in infancy. Dev. Cogn. Neurosci. 36:100608. doi: 10.1016/j.dcn.2018.12.006
Unsworth, N., Robison, M. K., and Miller, A. L. (2019). Individual differences in baseline oculometrics: examining variation in baseline pupil diameter, spontaneous eye blink rate, and fixation stability. Cognit. Affect. Behav. Neurosci. 19, 1074–1093. doi: 10.3758/s13415-019-00709-z
U. S. Department of Agriculture Food Nutrition Service. (2019). Child Nutrition Programs: Income Eligibility Guidelines. Available online at: https://www.fns.usda.gov/cnp/fr-032019 (accessed at: July 1, 2019–June 30, 2020)
van de Groep, I. H., de Haas, L. M., Schutte, I., and Bijleveld, E. (2017). Spontaneous eye blink rate (EBR) predicts poor performance in high-stakes situations. Int. J. Psychophysiol. 119, 50–57. doi: 10.1016/j.ijpsycho.2017.01.009
Wechsler, D. (2013). WASI -II: Wechsler abbreviated scale of intelligence - second edition. J. Psychoeducat. Assessment 31, 337–341. doi: 10.1177/0734282912467756
White, T. P., Jansen, M., Doege, K., Mullinger, K. J., Park, S. B., Liddle, E. B., et al. (2013). Theta power during encoding predicts subsequent memory performance and default mode network deactivation. Human Brain Mapp. 34, 2929–2943. doi: 10.1002/hbm.22114
Wise, R. A. (2004). Dopamine, learning and motivation. Nat. Rev. Neurosci. 5, 483–494. doi: 10.1038/nrn1406
Yang, X., Bossman, J., Schiffhauer, B., Jordan, M., and Immordino-Yang, M. H. (2013). Intrinsic default mode network connectivity predicts spontaneous verbal descriptions of autobiographical memories during social processing. Front. Cognit. Sci. 3:592. doi: 10.3389/fpsyg.2012.00592
Keywords: default mode network (DMN), psychophysiology, memory, eye blink rate (EBR), deep learning
Citation: Gotlieb RJM, Yang X-F and Immordino-Yang MH (2021) Measuring Learning in the Blink of an Eye: Adolescents' Neurophysiological Reactions Predict Long-Term Memory for Stories. Front. Educ. 5:594668. doi: 10.3389/feduc.2020.594668
Received: 14 August 2020; Accepted: 09 December 2020;
Published: 07 January 2021.
Edited by:
Zachary Hawes, University of Toronto, CanadaReviewed by:
Alejandro Lorenzo-Lledó, University of Alicante, SpainEmily Barkley-Levenson, Hofstra University, United States
Copyright © 2021 Gotlieb, Yang and Immordino-Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mary Helen Immordino-Yang, SW1tb3JkaW5AdXNjLmVkdQ==
 Rebecca J. M. Gotlieb
Rebecca J. M. Gotlieb Xiao-Fei Yang
Xiao-Fei Yang Mary Helen Immordino-Yang
Mary Helen Immordino-Yang