
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Educ. , 30 October 2020
Sec. Educational Psychology
Volume 5 - 2020 | https://doi.org/10.3389/feduc.2020.540387
This article is part of the Research Topic Development, Wellbeing, and Lifelong Learning in Individuals with a Dual Sensory Loss View all 21 articles
 Gitta de Vaan1
Gitta de Vaan1 Roseriet Beijers2
Roseriet Beijers2 Mathijs P. J. Vervloed2*
Mathijs P. J. Vervloed2* Harry Knoors2,3
Harry Knoors2,3 Kitty A. Bloeming-Wolbrink4
Kitty A. Bloeming-Wolbrink4 Carolina de Weerth5
Carolina de Weerth5 Ludo Verhoeven2,3
Ludo Verhoeven2,3Background and Aims: Persons with combined sensory and intellectual disabilities are more sensitive to stress than people without disabilities, especially when they have an Autism Spectrum Disorder (ASD). Reversely, stress can also trigger ASD symptoms. The current study investigated the relationship between stress and ASD symptoms in this population.
Methods and Procedures: Participants (n = 46) were persons with combined sensory and intellectual disabilities. The presence of ASD was assessed with Observation of Autism in people with Sensory and Intellectual Disabilities (OASID). This assessment also served as a stressor. Stress levels were measured with salivary cortisol during the OASID assessment and on a control day.
Results: There were no differences in cortisol levels between participants with and without ASD, or between the OASID test day and control day. Cortisol levels were positively related to the presence of stereotyped and repetitive behaviors.
Conclusions: No differences were found in stress levels after administration of OASID between people with or without ASD based on the classification of OASID. Administration of OASID was found not to produce increases in cortisol. Cortisol levels were correlated with stereotyped and repetitive behaviors, which makes it likely that these behaviors are stress reactions.
Persons with a combination of an intellectual disability and sensory impairments with or without an Autism Spectrum Disorder (ASD) can be more susceptible to stress than people without impairments. Missing visual and auditory information from the environment can make situations more unpredictable, more difficult to interpret and to control, hence making these situations more stressful (see Dickerson and Kemeny, 2004; Corbett et al., 2006; Bloeming-Wolbrink et al., 2012). The experience of stress can be defined as a reaction that occurs when a person perceives a threat to their well-being. This reaction can be based on an actual threat or something that is interpreted as a threat (Morilak et al., 2005). This reaction may include psychological reactions, such as feelings of helplessness, and physiological reactions, such as increased heartrate, muscle tension and transpiration (Lovallo, 1997; Schuengel and Janssen, 2006). Also, the stress hormone cortisol is released. This hormone is often measured in saliva, urine or blood (Hellhammer et al., 2009).
Cortisol is the end product of the hypothalmic-pitutitary-adrencocortical (HPA) axis. When confronted with unpredictable, uncertain, and stressful situations, the HPA axis is activated and an elevated cortisol response can be seen. In addition to this stress reactive response, cortisol production on the HPA axis follows a well-defined circadian rhythm (Sapolsky et al., 2000, Simons et al., 2015).
The current study will compare persons with sensory and intellectual disabilities with and without symptoms of ASD on salivary cortisol responses and will assess the relationship between salivary cortisol responses and ASD characteristics. The relationship between ASD characteristics and stress in this population is expected to be complex and has never been studied before.
Not only are people with sensory and intellectual disabilities more susceptible to stress than people without disabilities, but they additionally lack the ability to adequately cope with stressful situations (Schuengel and Janssen, 2006), which means it takes them longer to recover from stress. For example, a typical way for children or adults with developmental disabilities to cope with the feeling of stress is to seek comfort with an attachment figure, such as a parent, or a trusted significant other person, such as a caregiver. Comfort seeking may be difficult for people with intellectual or multiple disabilities, because they are often less securely attached or they lack the behaviors to seek comfort. In general, the experience of stress, coping with stress, attachment behaviors and disabilities seem to be closely related in this population (Janssen et al., 2002; Schuengel and Janssen, 2006; Schuengel et al., 2013; Giltaij et al., 2016).
The circadian rhythm of cortisol is often atypical in people with visual impairments, as this rhythm is influenced by light perception (Lockley et al., 2007). Sterkenburg (2008) showed that in people with intellectual disabilities and visual impairment the cortisol morning peak is lower and the evening cortisol values are higher than in people without disabilities. This may be related to the attachment problems that were described earlier, since Sterkenburg (2008) found that an attachment-based intervention, that is an intervention that first improves bonding between client and therapist before starting Applied Behavior Analysis, led to more typical cortisol patterns in people with intellectual disabilities and visual impairments. Contrarily, the cortisol curves of people with intellectual disabilities and congenital deafblindness were found to be quite normal in a small study by Bloeming-Wolbrink et al. (2012).
Not only people with intellectual and sensory disabilities, but also people with ASD may be more susceptible to stress. Though they show similar daily patterns of salivary cortisol levels as healthy controls, there is more variability in reactive stress levels as measured by cortisol levels among persons with ASD (Corbett et al., 2008). In people with ASD the magnitude of the initial stress reaction to novel stimuli is larger than in typically developing persons (Corbett et al., 2006) and they are also known to show a more prolonged cortisol response and slower recovery from (social) stressors than people without ASD (Corbett et al., 2012; Spratt et al., 2012).
ASD may not only make people more prone to stress, but stress may also elicit behaviors that are topographically similar to behaviors characteristic of ASD. ASD consists of two major components, social communication and interaction on the one hand, and stereotyped and repetitive behavior on the other. Both of these behavioral components may be affected by stress. For example, when feeling stressed or helpless a person might revert to stereotyped behaviors (Kraijer, 2004) or social withdrawal (Rubin et al., 2013), both of which are also symptoms of ASD (Frith, 2003; American Psychiatric Association, 2013). So, on the one hand, the presence of ASD may cause people to be more stressed in specific situations (Corbett et al., 2006). On the other hand, stress may lead to more ASD typical behaviors in people with sensory and intellectual disabilities, regardless of the actual presence of ASD. This complicated relationship between ASD symptoms and stress reactions is even more complex in persons with sensory and intellectual disabilities, as they are known to show ASD typical behaviors regardless of the presence of stress or ASD (Hoevenaars-van den Boom et al., 2009; De Vaan et al., 2013, 2016; Dammeyer, 2014; Jure et al., 2016; Probst and Borders, 2017).
The goal of the present study is to begin to clarify the complex relationship between stress and ASD in people with both sensory and intellectual disabilities. These persons are hypothesized to experience higher stress because they miss visual and auditory information, making situations more unpredictable, uncertain, and stressful (Dickerson and Kemeny, 2004; Corbett et al., 2006; Bloeming-Wolbrink et al., 2012). In these types of situations, the HPA-axis is activated and cortisol is released (Sapolsky et al., 2000, Simons et al., 2015). Despite the fact that cortisol can easily be sampled in saliva, there is still a knowledge gap on cortisol responses to stressful situations in our target group of people with sensory and intellectual disabilities. Additionally, although the target group has been studied with regard to salivary cortisol levels before, this was always done in single case studies or very small samples (e.g., Sterkenburg, 2008; Bloeming-Wolbrink et al., 2012; Nelson et al., 2013). The current study is the first to include a relatively large group of persons with sensory impairments and intellectual disability.
The sample size and the use of multilevel statistics makes the results also more robust to potential problems of missing data. Furthermore, our study is the first to relate the hormonal stress reactions to the behavioral characteristics of participants with sensory and intellectual disabilities with and without symptoms of ASD. The current study hopes to give more insight in the relationship between salivary cortisol levels and the expression of ASD characteristics in persons with multiple disabilities.
This study has two research questions. The first question focuses on whether the stress reaction differs in people with combined sensory and intellectual disabilities with and without symptoms of ASD. Based on the literature we expect that persons with combined sensory and intellectual disabilities and ASD will show a stronger stress reaction and a slower recovery from stress than persons with combined sensory and intellectual disabilities without symptoms of ASD. The second question focuses on whether the stress reaction is related to autism-typical behaviors in persons with combined sensory and intellectual disabilities, regardless of the presence of an ASD diagnosis.
We expect to find a positive relationship between stress reactivity and autistic behavior, as experiencing stress could lead to behaviors that are also typical for ASD. These behaviors will be assessed on both of the two major components of ASD typical behaviors as described in the DSM-5 (American Psychiatric Association, 2013), “stereotyped and repetitive behavior” and “social communication and interaction.”
Participants were recruited in four residential facilities and three schools for people with combined sensory and intellectual disabilities. Participants were recruited by a contact person from each facility. The inclusion criteria were a moderate to profound intellectual disability combined with a visual impairment and an age between 5 and 55 years. Given the small total sample size it was decided to include both children and adults in the study as well as people who are deafblind. Information about visual impairments, auditory impairments and intellectual disabilities were retrieved directly from the participants' records kept at the facilities.
Sixty participants with combined sensory and intellectual disabilities were recruited for this study. In five cases legal representatives gave no consent to collect saliva, in four cases participants did not accept saliva sampling and in five cases not enough saliva was collected to analyze cortisol levels. Overall, saliva samples of 46 participants were included (a response rate of 77%).
Information about levels of intellectual, visual and auditory impairment and the number of males vs. females is shown in Table 1. The mean age of the 31 male and 15 female participants was 33.8 years (SD = 14.74, range 6–55). One third of the sample consisted of deafblind participants. Based on the number of ASD symptoms in the OASID instrument two groups were formed (for OASID see materials section). The no ASD group consisted of 22 persons (15 males) with a mean age of 35.7 (SD = 13.1), the ASD group consisted of 24 persons (16 males) with a mean age of 31.81 (SD = 16.2). Note that no formal total assessment common for the diagnosis of ASD was performed to confirm the presence of ASD. OASID was only used to experimentally divide the group in two.
This study was approved by the local Committee on Research Involving Human Subjects and conformed to the Ethical Principles for Medical Research Involving Human Subjects of the World Medical Association declaration of Helsinki (World Medical Association, 2013). Because of ethical and legal requirements parents or legal representatives were asked for informed consent before the study started.
The presence of ASD symptoms was assessed using Observation of Autism in people with Sensory and Intellectual Disabilities (OASID) (De Vaan et al., 2016, 2018). OASID is an assessment tool consisting of a semi-structured play session. During the play session the experimenter played five tasks with the participants using toys and games. The session is adapted to each individual by taking into account the participant's intellectual disabilities, sensory impairments and communication skills. The assessment lasted between 30 and 60 min.
The assessment was recorded on video and observed and scored afterwards, using a 40 item questionnaire. Each item was scored on a Likert scale from 0 to 2, where a higher score corresponded with more autistic behaviors. Item scores were added to calculate two scale scores, based on the two main criteria for ASD as described in DSM-5 (American Psychiatric Association, 2013): “Social Interaction and Communication” and “Repetitive and Stereotyped behavior.” Scores on both scales were used to assess the presence of ASD according to the guidelines of OASID (De Vaan et al., 2018).
OASID was found to be both a valid and reliable measurement tool to assess ASD symptoms in persons with a combination of intellectual disabilities and sensory impairments (De Vaan et al., 2016, 2018). In this study OASID was used to assess the presence of ASD symptoms, not to diagnose ASD. Though OASID was designed as a non-stressful measurement, it might be stressful for participants nevertheless, especially because of the unfamiliar researcher who served as the administrator. Therefore, the OASID play session was used as a potential stressor for the cortisol measure.
Levels of physiological stress were determined using cortisol measurements in saliva, following the protocol of Bloeming-Wolbrink et al. (2012). Saliva was collected using Salivettes, cotton rolls that were used to swab the participant's mouth. Saliva sampling was done by a familiar caregiver of the participant who was instructed on the procedures by letter and video. Caregivers were instructed to wear medical gloves in order to keep the cotton rolls sterile. The salivettes were stored in a fridge immediately after sampling, and frozen within a few days after sampling at −20°C until further analysis. Salivary cortisol was measured by the University Medical Center in Utrecht, the Netherlands, and was measured without extraction using an in house competitive radioimmunoassay employing a polyclonal anti-cortisol antibody (K7348). [1,2-3H(N)]-Hydrocortisone (PerkinElmer NET396250UC) was used as a tracer. The lower limit of detection was 1.0 nmol/l and inter- and intra-assay variations were below 10%.
Saliva was collected six times, three times on the OASID test day, and three times on a control day. Caregivers were instructed that the participants could not eat, drink (except water if necessary) or brush their teeth an hour before the saliva samples were taken (see also Bloeming-Wolbrink et al., 2012). On the test day, saliva samples were taken before the beginning of the OASID assessment (prestressor, T1), 35 min after the beginning of OASID (stress reaction, T2) and 75 min after the beginning of OASID; which is 35 min after the end of OASID (the average duration of OASID is 40 min - recovery, T3). A cortisol reaction is visible in saliva around 25 min after the stressor, although inter- individual differences exist. To take this inter-individual variation into account, the samples were taken 35 min after the end of the stressor. On the control day, saliva samples were taken at the same times as on the test day to assess the cortisol pattern during a standard day. To control for the cortisol awakening response (see Lovallo, 1997), all the OASID assessments were done after 11.00 A.M. For 29 participants all six samples were analyzed, for 5 participants there was one missing sample, for 4 participants there were two, for 6 participants there were three, for one participant there were four and for one participant there were five missing samples. The missing samples were not clearly related to a single moment of sampling.
After written consent was given, the OASID assessment was planned and caregivers were informed about the procedure. They were asked to be present during the assessment and to perform the saliva sampling. For the comfort of the participant, we chose to let familiar caregivers perform the saliva sampling instead of doing this ourselves as unfamiliar researchers. Before the assessment, the caregivers received information about the protocol in text and video, with instructions for saliva sampling. They also received all of the necessary materials, including medical gloves and salivettes, with some additional salivettes for practice. They were given the opportunity to ask questions about this procedure to the first author. The OASID assessment was performed and the saliva samples were taken. Saliva samples on the control day were taken at the exact same times as on the test day. This control day was before or after the test day and the same procedure was followed for collecting and storing cortisol. Saliva samples of all participants were stored in a freezer until analysis.
In the current study, an assessment session with an unfamiliar researcher served as the stressor. This session is a novel situation performed by an unfamiliar psychologist and includes social evaluation, and thus is potentially stressful, especially for participants with ASD (Dickerson and Kemeny, 2004). Although there was some variation in the materials used during the assessment with OASID, the content and duration (average duration 40 min) of the assessment were overall the same for all participants. The stress reactions were assessed by studying cortisol levels during and after the assessment and were compared with similar measures taken on a typical day to correct for individual variation in salivary cortisol.
The results were analyzed with mixed-model (multilevel) designs. First, all variables were checked for outliers (>3 SD difference from the mean). One outlier was detected for the cortisol measurements on the OASID test day, and four outliers for the cortisol measurements on the control day. Since an advantage of multilevel analyses is its robustness for missing data, these outliers were removed before analysis (Tabachnik and Fidell, 2007). All residuals were normally distributed.
To test whether the cortisol response to OASID differed between people with few and many ASD symptoms, two longitudinal regression analyses were performed using mixed-model (multilevel) designs. One analysis aimed to test the difference between people with few and many ASD symptoms, and the other analysis aimed to test whether the continuous scales of “Social interaction and communication” and “Repetitive and stereotyped behavior” were able to predict the people's cortisol response to the administration of OASID.
In these analyses, the three repeated cortisol measures (T1-T3) were used at Level 1 and nested within the participants at Level 2. To examine whether the nested structure was required, the intraclass correlation (ICC) was calculated using a null model for the area under the curve (AUC). The ICC for children's cortisol AUC measure was 0.7772, indicating that 77.72% of the variability in cortisol responses to the OASID was associated with differences between participants, meaning that multilevel analyses were applicable.
Thereafter, a build-up strategy was followed in which variables were added one-by-one to the model with random intercept (allowing the intercept of the regression line to vary per participant). After adding each variable, the change in deviance on the −2 log likelihood ratio scale after generalized least square estimations was assessed. Variables that did not improve the model, by significantly reducing the deviance, were excluded. Time (considered as a random factor, allowing the slope of the regression line to vary per participant) and quadratic time (to indicate a cortisol response to OASID) were entered into the model first.
Secondly, the confounders were entered into the time models. The following confounders were taken into account separately: all three cortisol measurements on the control day, sex, age, level of visual and auditory impairments, level of intellectual disability and time of the day that the OASID took place. Lastly, the predictors and the interactions between the predictors and time were entered into the model. To test whether cortisol response to OASID differed between people with few and many symptoms of ASD, the first multilevel model contained the predictor few or many ASD symptoms. To test whether the continuous scales of OASID, regardless of the presence of few or many ASD symptoms, were able to predict the participant's cortisol response to OASID, the second multilevel model contained the predictors “Social interaction and communication” and “Repetitive and stereotyped behavior” instead of the number of ASD symptoms.
Two groups were created based on OASID scores, no or few ASD symptoms (n = 22) and many ASD symptoms (n = 24). The latter group consisted of participants with mild, severe, and profound numbers of symptoms according to the OASID Manual (De Vaan et al., 2019). The mean cortisol level in nmol/l for each group and the moment of measurement is provided in Table 2. Independent samples T-tests revealed no differences in cortisol levels between the groups with few and many ASD symptoms for any of the cortisol measures on both the test and the control day. Also within groups no differences were found on cortisol levels between the different measurement times.
Multilevel analyses were performed to assess the effect of ASD on the cortisol response to the OASID administration. Table 3 represents the best fitting multilevel model. The predictors time and time quadratic were not significant, indicating that the OASID did not provoke a significant cortisol response for the whole group. Furthermore, the dummy indicating whether people had few or many ASD symptoms, was not significant. This indicates that the cortisol response levels of people with few and many ASD symptoms were similar to the assessment, so with no significant cortisol responses to the stressor administration of OASID. The cortisol measurements on the control day significantly predicted the matched cortisol responses to the day OASID was administered. Higher cortisol concentrations on the control day predicted higher cortisol concentrations on the OASID test day.
Another multilevel analysis was performed to assess how cortisol responses related to autistic behavior. Instead of dichotomizing the ASD diagnosis, the total scores on the two scales of OASID, “Social interaction and communication” and “Repetitive and stereotyped behavior,” were used. Table 4 represents the best fitting multilevel model predicting the cortisol response after administering OASID with the scores on the two OASID scales. More repetitive and stereotyped behavior during OASID administration significantly predicted higher cortisol concentrations on the OASID test day, after controlling for cortisol concentrations on the control day. No such relationship was found for the other ASD behavioral aspect, “Social interaction and communication.” Lastly, higher cortisol concentrations on the control day significantly predicted higher cortisol concentrations on the OASID test day.
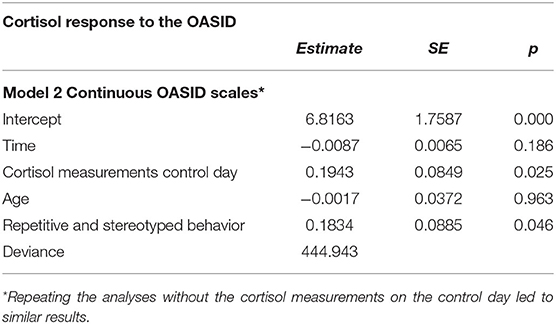
Table 4. Best fitting multilevel model studying the association between ASD symptoms measured with OASID scales and cortisol response.
The current study investigated whether cortisol responses to a stressor are related to ASD symptoms in people who have sensory impairments in combination with intellectual disabilities. Both people with ASD (Corbett et al., 2006; Bloeming-Wolbrink et al., 2012) and people with sensory and intellectual disabilities (Schuengel and Janssen, 2006) are more prone to experiencing stress in novel, unpredictable and uncontrollable situations. However, it is unknown what the additional effect of ASD symptoms is on stress levels when a person has these multiple disabilities. In addition, this study explored the relationship between salivary cortisol levels and the behavioral characteristics of ASD in this target population.
No differences in cortisol levels or cortisol responses were found between participants with few and many ASD symptoms, but a higher cortisol response was found to be related to more stereotyped and repetitive behaviors, one of the core characteristics of ASD.
Our multilevel analyses further confirmed the preliminary findings that cortisol levels did not differ between groups or moment of testing, as neither the assessment with OASID nor the presence of ASD predicted cortisol levels. The only significant predictor of cortisol levels on the OASID test day was the cortisol level on the control day. Baseline cortisol levels can vary between persons (Smyth et al., 1997; Bartels et al., 2003). This individual variation in salivary cortisol levels was the only significant predictor for salivary cortisol levels on an OASID test day. Neither the presence of many ASD symptoms, nor the assessment of OASID itself, was related to higher cortisol levels.
Possibly, OASID was too mild of a stressor for any of the participants to show stress reactions in the first place. We chose the administration of the OASID assessment as a stressor. However, OASID did not cause cortisol reactivity in either group, nor in the group as a whole. Neither of the groups showed a cortisol response to OASID, which could be the cause of not finding any differences between the groups on cortisol reactivity. Though the assessment was never designed to produce stress, in fact, precautions were taken to prevent stress (De Vaan et al., 2016), we still expected the session to be stressful to some extent. For example, because of the social evaluative aspects of the administration (Dickerson and Kemeny, 2004) and the fact that the session was with an unfamiliar researcher in an unfamiliar setting. The fact that OASID was not found to be stressful for participants could be seen as a limitation of this study because the intended stressor did not appear to be stressful. On a more positive note, this finding shows that OASID can measure symptoms of autism without producing physiological stress and that the precautions taken to prevent stress may have been successful.
In the second part of this study, we assessed if any of the behavioral aspects typical for ASD were related to stress. One behavioral domain of ASD, namely “stereotyped and repetitive behavior” was correlated with cortisol concentrations on the OASID test day, while controlling for cortisol concentrations of the control day. It is known that stress or anxiety can lead to stereotyped behavior (Leekam et al., 2011; Rodgers et al., 2012) but also that specific movements such as body rocking could be a possible way to cope with stress (Bloeming-Wolbrink et al., 2012). At the moment it is, given the design used in the current study, impossible to determine any causal relation between repetitive and stereotyped behavior and cortisol responses. We can only verify a correlation between both variables. The other aspect of ASD, “social interaction and communication,” was unrelated to cortisol levels. Although stress can lead to social withdrawal (Rubin et al., 2013) and negative feelings as a result of social evaluation may lead to stress (Dickerson and Kemeny, 2004), our data did not find an association between social behaviors and cortisol responses.
This study has some limitations. All six cortisol samples could only be analyzed for 29 participants. For the remaining 17 participants the number of missing measurements ranged from 1 to 5. For nine participants there were no usable cortisol samples, so their data were not included in the analyses. This shows that it was rather difficult to collect saliva in this target population. Missing values were caused by too little saliva and by the participants refusing to provide any saliva. Some participants did not accept the cotton swab in their mouths for more than a few seconds or did not accept it at all. Though multilevel analyses are robust against missing data, and we were still able study a relatively large group of 46 persons, the amount of missing cortisol samples represents a limitation.
Because our sample consisted of people with a moderate to profound intellectual disability (IQ < 50), combined with additional sensory impairments, there were only limited possibilities to communicate with them. For instance, to explain the intention of the saliva swab, and to persuade them to produce saliva. Though sampling of salivary cortisol is described as non-invasive and stress- free (Levine et al., 2007), this may not have been so for our study population. In fact, perhaps the persons that refused saliva sampling were stressed and only the non-stressed persons were therefore included in our study.
Another possible reason for the fact that we could not extract enough saliva might be due to staff not using the salivettes correctly. For reasons of comfort, the saliva sampling was done by familiar caregivers of the participant. However, these caregivers had no experience or expertise with saliva sampling. They received instructions on how to collect saliva by video and text, but they were not trained in person in sampling saliva. In order to increase the sampling success in future studies, it would be recommendable to give more training to the persons collecting saliva, or have the saliva collected by a more experienced researcher in the presence of a familiar caregiver. Despite these difficulties in collecting saliva in this multiple disabled population, we still believe it to be the best method to measure cortisol levels in this group. Cortisol can also be measured through blood sampling, but this is painful, expensive and requires medical staff (Levine et al., 2007). Other stress measures such as heart rate or skin conductance [see Meehan et al., 2002) require equipment to be placed on the body, which participants with multiple disabilities may not understand and reject. More recently a non-intrusive way of measuring skin conductance has, especially for persons with a visual and intellectual disability, been developed by Frederiks et al. (2015, 2019)]. This system was however not available at start of the current study. Finally, the communication difficulties of this population make it challenging if not impossible to validly use self-report scales to assess stress levels. Hence, salivary cortisol was the best way to measure cortisol responses in this target group at the time the study was done.
The current study revealed no differences in cortisol levels between people with few and many ASD symptoms and with a combination of sensory and intellectual disabilities. This implies that additional ASD symptoms does not lead to higher cortisol responses, and perhaps more stress, in these persons with multiply disabilities. We did however find stereotyped and repetitive behaviors were related to cortisol responses on OASID. Clinicians should take this into account when treating persons with multiple disabilities that show stereotyped behaviors, especially as this may be a self-regulating process of coping with experienced stress (Bloeming-Wolbrink et al., 2012). Stereotyped behavior in this sense is then a good warning signal for stress and treatment could then be focused on the reduction of stress instead of on reducing the stereotyped behaviors. This finding could also have strong implications for diagnosing ASD in this group. The observation of ASD typical behaviors such as stereotyped movements is not necessarily indicative of ASD but could be a symptom of stress. This is in line with earlier studies that have shown that in this target group the biggest differences between persons with and without ASD is on the social and communicative domain (Hoevenaars-van den Boom et al., 2009; De Vaan et al., 2016, 2018).
This study did not reveal differences in cortisol responses between participants with few and many ASD symptoms, but it has revealed a relationship between cortisol and a behavioral characteristic that is related to ASD, stereotyped and repetitive behavior. The OASID assessment did not provoke rises in cortisol concentrations in participants of this study. This could imply that OASID was not stressful and may be used without problems as an instrument in the assessment of ASD. At the same time, the results indicate that OASID cannot be used as an experimental stressor in future research. In order to compare cortisol responses in people with and without ASD, future studies should look at other ecologically relevant and ethically acceptable situations, occurring naturally, that are potentially more stressful events, such as medical examinations, visits to the dentist, or vaccinations. Finally, the collection of saliva in order to measure cortisol was challenging in this population. Nonetheless, we recommend this procedure over alternative stress measures, provided that the staff is well-trained in sampling saliva, to ensure comfortable sampling while preventing high numbers of missing values.
The datasets generated for this study are available on request to the corresponding author.
The studies involving human participants were reviewed and approved by Commissie Mensgebonden Onderzoek Arnhem-Nijmegen. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
GV conducted the study and wrote the first draft of the study together with RB. RB performed the statistical analysis and wrote the first draft on the result section. MV designed the study and edited the final draft of the manuscript. HK supervised the project and edited the whole manuscript. KB-W helped with designing the cortisol test protocol and helped with training and instructing care takers for the saliva sampling. CW helped with the cortisol study design and edited the whole manuscript. LV supervised the study and edited the whole manuscript. All authors contributed to the article and approved the submitted version.
This research was funded by ZonMw (grant number 60-00635-98-0081), Royal Dutch Kentalis and the Behavioral Science Institute, Radboud University Nijmegen. Funding sources were not involved in study design, data collection, writing of reports or decisions regarding publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders, DSM-5 5th edn. Washington, DC: American Psychiatric Publishing. doi: 10.1176/appi.books.9780890425596
Bartels, M., Van den Berg, M., Sluyter, F., Boomsma, D. I., and de Geus, E. J. C. (2003). Heritability of cortisol levels: review and simultaneous analysis of twin studies. Psychoneuroendocrinology 28, 121–137. doi: 10.1016/S0306-4530(02)00003-3
Bloeming-Wolbrink, K. A., Janssen, M. J., de Weerth, C., Ruijssenaars, W. A., Sweep, F. C., Eijsbouts, A., et al. (2012). Stress in adults with congenital deafblindness and an intellectual disability: information from their cortisol curves. Br. J. Vis. Impair. 30, 149–159. doi: 10.1177/0264619612456375
Corbett, B. A., Mendoza, S., Abdullah, M., Wegelin, J. A., and Levine, S. (2006). Cortisol circadian rhythms and response to stress in children with autism. Psychoneuroendocrinology 31, 59–68. doi: 10.1016/j.psyneuen.2005.05.011
Corbett, B. A., Mendoza, S., Wegelin, J. A., Carmean, V., and Levine, S. (2008). Variable cortisol circadian rhythms in children with autism and anticipatory stress. J. Psychiatr. Neurosci. 33, 227–234.
Corbett, B. A., Schupp, C. W., and Lanni, K. E. (2012). Comparing biobehavioral profiles across two social stress paradigms in children with and without autism spectrum disorders. Mol. Autism 3, 1–10. doi: 10.1186/2040-2392-3-13
Dammeyer, J. (2014). Symptoms of autism among children with congenital deafblindness. J. Autism Dev. Disord. 44, 1095–1102. doi: 10.1007/s10803-013-1967-8
De Vaan, G., Vervloed, M., Peters-Scheffer, N. C., van Gent, T., Knoors, H., and Verhoeven, L. (2016). Behavioral assessment of autism spectrum disorders in people with multiple disabilities. J. Intellect. Disabil. Res. 60, 101–112. doi: 10.1111/jir.12206
De Vaan, G., Vervloed, M., Peters-Scheffer, N. C., Van Gent, T., Knoors, H., and Verhoeven, L. (2018). Assessing autism spectrum disorder in people with sensory impairments combined with intellectual disabilities. J. Dev. Phys. Disabil. 30, 471–487. doi: 10.1007/s10882-018-9597-x
De Vaan, G., Vervloed, M. P. J., Knoors, H., and Verhoeven, L. (2013). “Autism spectrum disorders in people with sensory and intellectual disabilities symptom overlap and differentiating characteristics,” in Recent Advances in Autism Spectrum Disorders - Volume I: InTech, ed. M. Fitzgerald (London: IntechOpen Limited). doi: 10.5772/53714
De Vaan, G., Vervloed, M. P. J., Knoors, H., and Verhoeven, L. (2019). English Manual for OASID: Observation of Autism Spectrum Disorder in People with Sensory and Intellectual Disabilities. Unpublished manuscript. Nijmegen: Radboud University.
Dickerson, S. S., and Kemeny, M. E. (2004). Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol. Bull. 130, 355–391. doi: 10.1037/0033-2909.130.3.355
Frederiks, K., Croes, M., Chen, W., Oetomo, S. B., and Sterkenburg, P. (2015). Sense - a biofeedback system to support the interaction between parents and their child with the prader-willi syndrome: a pilot study. J. Ambient Intell. Smart Environ. 7, 449–459 doi: 10.3233/AIS-150327
Frederiks, K., Sterkenburg, P. S., Barakova, E., Peters, P. J. F., and Feijs, L. M. G. (2019). The effects of a bioresponse system on the joint attention behavior of adults with visual and severe or profound intellectual disabilities and their affective mutuality with their caregivers. J. Appl. Res. Intellect. Disabil. 32, 890–900. doi: 10.1111/jar.12581
Giltaij, H. P., Sterkenburg, P. S., and Schuengel, C. (2016). Adaptive behavior, comorbid psychiatric symptoms, and attachment disorders. Adv. Ment. Health Intellect. Disabil. 10, 82–91. doi: 10.1108/AMHID-07-2015-0035
Hellhammer, D. H., Wüst, S., and Kudielka, B. M. (2009). Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology 34, 163–171. doi: 10.1016/j.psyneuen.2008.10.026
Hoevenaars-van den Boom, M. A. A., Antonissen, A. C. F. M., Knoors, H., and Vervloed, M. P. J. (2009). Differentiating characteristics of deafblindness and autism in people with congenital deafblindness and profound intellectual disability. J. Intellect. Disabil. Res. 53, 548–558. doi: 10.1111/j.1365-2788.2009.01175.x
Janssen, C. G. C., Schuengel, C., and Stolk, J. (2002). Understanding challenging behavior in people with severe and profound intellectual disability: a stress-attachment model. J. Intellect. Disabil. Res. 46, 445–453. doi: 10.1046/j.1365-2788.2002.00430.x
Jure, R., Pogonza, R., and Rapin, I. (2016). Autism spectrum disorders (ASD) in blind children: very high prevalence, potentially better outlook. J. Autism Dev. Disord. 46, 749–759. doi: 10.1007/s10803-015-2612-5
Kraijer, D. (2004). Handboek Autismespectrumstoornissen en Verstandelijke Beperking [Handbook Autism spectrum Disorders and Intellectual Disability], Lisse: Harcourt.
Leekam, S. R., Prior, M. R., and Uljarevic, M. (2011). Restricted and repetitive behaviors in autism spectrum disorders: a review of research in the last decade. Psychol. Bull. 137, 562–593. doi: 10.1037/a0023341
Levine, A., Zagoory-Sharon, O., Feldman, R., Lewis, J. G., and Weller, A. (2007). Measuring cortisol in human psychobiological studies. Physiol. Behav. 90, 43–53. doi: 10.1016/j.physbeh.2006.08.025
Lockley, S. W., Arendt, J., and Skene, D. J. (2007). Visual impairment and circadiam rhythm disorders. Dialogues Clin. Neurosci. 9, 301–314.
Lovallo, W. R. (1997). Stress and Health: Biological and Psychological Interactions. London: SAGE Publications.
Meehan, M., Insko, B., Whitton, M., and Frederick, P., Brooks, J. (2002). Physiological measures of presence in stressful virtual environments. ACM Trans. Graph. 21, 645–652. doi: 10.1145/566654.566630
Morilak, D. A., Barrera, G., Echevarria, D. J., Garcia, A. S., Hernandez, A., Ma, S., et al. (2005). Role of brain norepinephrine in the behavioral response to stress. Progr. Neuro. Psychopharmacol. Biol. Psychiatr. 29, 1214–1224. doi: 10.1016/j.pnpbp.2005.08.007
Nelson, C., Greenfield, R. G., Hyte, H. A., and Schaffer, J. P. (2013). Stress, behavior, and children and youth who are deafblind. Res. Pract. Persons Severe Disabil. 38, 139–156. doi: 10.2511/027494813809330243
Probst, K. M., and Borders, C. M. (2017). Comorbid deafblindness and autism spectrum disorder—characteristics, differential diagnosis, and possible interventions. Rev. J. Autism Dev. Disord. 4, 95–117. doi: 10.1007/s40489-016-0100-2
Rodgers, J., Glod, M., Connolly, B., and McConachie, H. (2012). The relationship between anxiety and repetitive behaviors in autism spectrum disorder. J. Autism Dev. Disord. 42, 2404–2409. doi: 10.1007/s10803-012-1531-y
Rubin, K. H., Coplan, R. J., and Bowker, J. C. (2013). Social withdrawal in childhood. Annu. Rev. Psychol. 60, 141–171. doi: 10.1146/annurev.psych.60.110707.163642
Sapolsky, R. M., Romero, L. M., and Munck, A. U. (2000). How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrinol. Rev. 21, 55–89. doi: 10.1210/er.21.1.55
Schuengel, C., de Schipper, J. C., Sterkenburg, P. S., and Kef, S. (2013). Attachment, intellectual disabilities and mental health: research, assessment and intervention. J. Appl. Res. Intellect. Disabil. 26, 34–46. doi: 10.1111/jar.12010
Schuengel, C., and Janssen, C. G. C. (2006). People with mental retardation and psychopathology: stress, affect regulation and attachment: a review. Int. Rev. Res. Ment. Retard. 32, 229–260. doi: 10.1016/S0074-7750(06)32008-3
Simons, S. S. H., Beijers, R., Cillessen, A. H. N., and de Weerth, C. (2015). Development of the cortisol circadian rhythm in early childhood in the light of stress in early life. Psychoneuroendocrinology 62, 292–300. doi: 10.1016/j.psyneuen.2015.08.024
Smyth, J. M., Ockenfels, M. C., Gorin, A. A., Catley, D., Porter, L. S., Kirschbaum, C., et al. (1997). Individual differences in the diurnal cycle of cortisol. Psychoneuroendocrinology 22, 89–105. doi: 10.1016/S0306-4530(96)00039-X
Spratt, E. G., Nicholas, J. S., Brady, K. T., Carpenter, L. A., Hatcher, C. R., Meekins, K. A., et al. (2012). Enhanced cortisol response to stress in children in autism. J. Autism Dev. Disord. 42. 75–81. doi: 10.1007/s10803-011-1214-0
Sterkenburg, P. S. (2008). Intervening in Stress, Attachment and Challenging Behavior. Effects in Children with Multiple Disabilities. Unpublished doctoral dissertation. Amsterdam: Free University Amsterdam.
Tabachnik, B. G., and Fidell, L. S. (2007). Using Multivariate Statistics 5th edn. Ontaria: Pearson International.
World Medical Association (2013). “Declaration of helsinki - ethical principles for medical research involving human subjects,” in Adopted and amended by the World Medical Association's 64th General Assembly (Fortaleza). Available online at: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed October 13, 2020).
Keywords: sensory impairments, intellectual disabilities, multiple disabilities, stress, salivary cortisol, autism spectrum disorder
Citation: de Vaan G, Beijers R, Vervloed MPJ, Knoors H, Bloeming-Wolbrink KA, de Weerth C and Verhoeven L (2020) Associations Between Cortisol Stress Levels and Autism Symptoms in People With Sensory and Intellectual Disabilities. Front. Educ. 5:540387. doi: 10.3389/feduc.2020.540387
Received: 06 March 2020; Accepted: 30 September 2020;
Published: 30 October 2020.
Edited by:
Marleen J. Janssen, University of Groningen, NetherlandsReviewed by:
Catherine Anne Nelson, The University of Utah, United StatesCopyright © 2020 de Vaan, Beijers, Vervloed, Knoors, Bloeming-Wolbrink, de Weerth and Verhoeven. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mathijs P. J. Vervloed, bS52ZXJ2bG9lZEBwd28ucnUubmw=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.