- 1Rochester Skin Lymphoma Medical Group, Fairport, NY, United States
- 2Department of Dermatology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, United States
- 3Department of Dermatology, Mayo Clinic Arizona, Phoenix, AZ, United States
- 4Inova Schar Cancer Institute, VA, United States
- 5Department of Dermatology, University of Arkansas for Medical Sciences, Little Rock, AK, United States
- 6Soligenix, Inc., Princeton, NJ, United States
Cutaneous T-cell lymphoma (CTCL) is a rare type of non-Hodgkin lymphoma of the skin, where at later stages skin-homing malignant T-cells affect lymph nodes, blood, and visceral organs. Even though early CTCL does not affect survival, it can progress to more advanced stages of disease and have a significant effect on the quality of life of patients. Although expectant management is a treatment consideration in early disease stages, most patients cycle through different skin-directed therapies throughout their lifetime. It can become a challenge to manage the serious and accumulating risk of side effects of these therapies, including various skin cancers and skin damage. Adverse effects from topical therapies limit their long-term utility. Thus, there is an unmet need for well-characterized therapies that have a rapid onset of action and minimal long-term/cumulative side effect profile. Most recently, the results of a Phase 3 study of topical HyBryte™ as a potential treatment for CTCL demonstrated its efficacy and safety profile. This article summarizes what is known about HyBryte™, focuses on its mechanism of action, and highlights its effectiveness, safety, and tolerability in the context of other current FDA-approved topical therapies for CTCL.
1 Introduction
Cutaneous T-cell lymphoma (CTCL) is listed as a rare cancer in the National Organization for Rare Disorders (NORD): Rare Diseases Database (Munoz, 2013) and has been recognized as a serious and orphan indication by the Federal Food and Drug Administration (FDA), as shown by the awarding of Fast Track and Orphan designations granted to several products for the treatment of CTCL, including HyBryte™. CTCL is an incurable cancer that is slowly progressive and requires chronic maintenance therapies (Berg et al., 2017). The skin develops red scaly patches and plaques that are created by infiltrating skin-homing lymphocytes. Progression involves expansion of skin patches/plaques/erythema, ulcerative nodules, and tumors, spread to the lymph nodes, the blood, and rarely into other organs (Hristov et al., 2021).
Early-stage treatment mainly consists of skin-directed therapies to manage symptoms and improve quality of life while limiting toxicity, as the disease itself does not limit survival in early stages (Kim et al., 1996). Treatment options in early-stage disease most commonly include expectant management, topical/intralesional corticosteroids, phototherapy (psoralen plus ultraviolet-A (PUVA), narrow band ultraviolet-B (nbUVB), topical nitrogen mustard, imiquimod, topical bexarotene, localized radiotherapy and/or total skin electron beam therapy (TSEB) (Trautinger et al., 2006; Prince et al., 2009). Despite the effectiveness of these treatments, there is an unmet medical need as patients continually transition between various treatment modalities to manage the serious and accumulating risks of side effects, including various skin cancers and skin damage (Stern et al., 1979; Licata et al., 1995; Man et al., 2005; Black and Gavin, 2006; Lindahl et al., 2013).
It is the side effect profiles of these agents that limit their long-term utility (Vonderheid et al., 1989; Nijsten and Stern, 2003). All therapies for CTCL are either approved as second-line therapies due to their potential toxicities or are used off-label. None of them have been characterized in randomized, placebo-controlled clinical trials. Therefore, well-characterized therapies that have a rapid onset of action and minimal long-term/cumulative side effect profile are crucial to the treatment of CTCL. Most recently, the results of a randomized, placebo-controlled clinical trial on the safety and effectiveness of HyBryte™ (research name: SGX301) were reported (Kim et al., 2022). HyBryte™ is a potential treatment for CTCL with a mild to moderate side effect profile, with most common side effects being transient and resolved with time, such as skin erythema, pain, and pruritus. These results are exciting, as if approved, it may provide patients with CTCL an additional skin directed treatment that is safe to use over time. Thus, in this manuscript, we aim to summarize the mechanism of action, effectiveness, safety and tolerability of HyBryte™ in early-stage CTCL and discuss the results of the Phase three FLASH (Fluorescent Light and Hypericin Study) study in the context of other treatments for early CTCL.
2 Use of HyBryte™
HyBryte™ is an ointment-based agent that is applied selectively to patches and plaques of patients with CTCL and covered for 18–24 h, as it is activated by visible light. After this timeframe, the patient receives phototherapy via a light device capable of producing visible light with consistent wavelengths. Treatment is patient-response directed, and dosing gradually increased over time as tolerated by the patient. The Phase three FLASH study included up to three 6-week treatment cycles for up to a total of 18 weeks of treatment for 166 patients.
3 Mechanisms of action
The active ingredient in HyBryte™ is hypericin, a known photosensitizer, which is synthetically produced for use as a 0.25% ointment and stimulated with safe, visible light in the 500–650 nm wavelength range. Several proteins and genes involved in cell growth, apoptosis, necrosis, autophagy, angiogenesis, cell cycle arrest, and the formation of cellular colonies contribute to its several antitumor effects in many different cancers (Dong et al., 2021). Hypericin has a well-described affinity for tumor cells and in the context of CTCL, it is absorbed by malignant T-cells, inducing apoptosis of these cells after activation with white light and UVA (Chung et al., 1994; Fox et al., 1998; Noell et al., 2011; Xu et al., 2019; Damke et al., 2020). Hypericin is taken up by cells and appears to be transported to cytoplasmic organelles including the endoplasmic reticulum, lysosomes, and mitochondria (Ali and Olivo, 2002). Hypericin has been shown to exert anti-tumor effects in the dark and after photoactivation (Blank et al., 2004). When activated by visible light, hypericin releases its induced energy partly by exciting cytoplasmic oxygen to its singlet state (Thomas et al., 1992) and by producing superoxide radicals (Verebová et al., 2020). This triggers apoptosis through the intrinsic mitochondrial pathway (Garg et al., 2013; Garg and Agostinis, 2014), and not through a direct nuclear interaction, and bypasses the extrinsic pathway that has been shown to be inactivated in several cancers including CTCL (Durán and Song, 1986; Johnson and Pardini, 1998; Blank et al., 2003; Chan et al., 2009; Garg and Agostinis, 2014). Hypericin 0.25% ointment was not detected in the blood after topical application, thus interactions with other drugs, such as those known to be present for St. John’s Wort, should not be a concern (Jendželovská et al., 2016; Kim et al., 2022).
There are several benefits to the use of visible light in HyBryte™ photodynamic therapy. The use of red-yellow visible wavelengths enables deeper penetration of the activating light than UV light commonly used in phototherapy, potentially allowing treatment of thicker and/or deeper lesions in CTCL (Figure 1). Moreover, the use of visible light also reduces the carcinogenic risk associated with UV light. Similar to what has been reported for other types of cancers, even when not activated by light, hypericin can also have antiproliferative effects (Fox et al., 1998; Jendželovská et al., 2016). However, these effects are significantly stronger when activated by light (Fox et al., 1998).
4 Effectiveness of HyBryte™ in CTCL
In the large, randomized, placebo-controlled study of HyBryte™ in CTCL (Figure 2), patients were randomized 2:1 to receive 0.025% HyBryte™ ointment or a placebo-matched ointment (Kim et al., 2022). All patients received visible light treatments, starting at 5 J/cm2 and gradually increased by 1 J/cm2 at each biweekly visit until light erythema was observed (maximum light dose was 12 J/cm2). The ointment was applied 18–24 h prior to light therapy and ointment treated lesions were kept covered (clothing or bandages) until the light therapy was completed at the physician’s office. Twice a week treatment in each cycle (up to 3 cycles) was undertaken for 6 weeks, and then the lesion scores were again assessed after a 2-week rest period to allow the transient drug staining and transient erythema to subside and light-induced erythema to fade. After the initial 6 weeks, all placebo-randomized subjects were allowed to cross-over to the HyBryte™ treatment arm and treatment for both study arms was re-started at 5 J/cm2. Cycle 3 was optional and for compassionate use. In Cycle 3, subjects were allowed to treat all of their CTCL lesions, starting at the maximal light dose tolerated in Cycle 2 with the ability to increase the dose by 1 J/cm2 until light erythema was observed or maximal light dose was achieved. The primary objective was to evaluate response to treatment after Cycle 1 (6 weeks of treatment). Treatment response was defined as a 50% or more improvement in the cumulative modified Composite Assessment of Index Lesion Severity (mCAILS) score (Olsen et al., 2011), from baseline to week 8. mCAILS is a composite scoring system that assesses erythema, scaling, plaque elevation and surface area for individual lesions (Kim et al., 2022). The second objective was to evaluate the effect of a second cycle of treatment of lesions previously treated with HyBryte™ in Cycle 1 and to further expand on the data obtained in Cycle 1 for those who were randomized to the placebo control arm of the study and crossed over. Subjects randomized to both treatment arms were comparable in terms of sex, age, race, tumor stage, disease duration, and baseline mCAILS scores. While there was no statistical difference among treatment groups based on prior therapies received, patients that were naïve to treatment as well as those who had received multiple therapies were enrolled. Most patients were Caucasian, had a mean age of 58–59 years old, and were either stage IA or IB (5 patients were stage IIA in the HyBryte™ arm). A total of 166 evaluable subjects were recruited across 39 treatment sites in the United States.
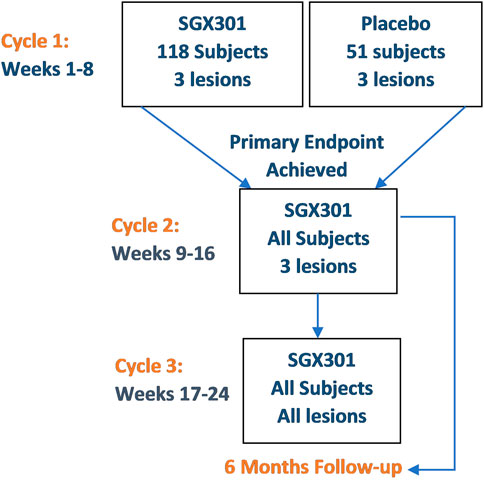
FIGURE 2. HPN-CTCL-01 study design (Kim et al., 2022).
There was a statistically significant difference in treatment response rate between the HyBryte™ and placebo treated arms over the first 6-week Cycle. This is notably different from other topical treatments, that can take months to yield an effect (e.g., mechlorethamine) (Lessin et al., 2013). Moreover, as expected with photodynamic therapy, the treatment response rate continued to increase over each of the succeeding treatment cycles, with 49% of patients who elected to receive all 3 Cycles (18 weeks) of therapy achieving a >50% reduction in the mCAILS score of their index lesions (Table 1).
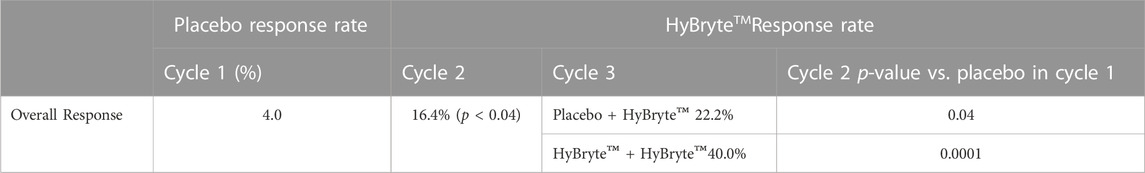
TABLE 1. HyBryte™(topical synthetic hypericin activated with visible light) Response Rate by Subject (Kim et al., 2022).
More generally, the average change in Cycle 1 in cumulative mCAILS score was 24% across all subjects, meaning that subjects had at least a 24% decrease in index lesion scores from baseline. This further improved to a 37% decrease after Cycle 2, indicating again continued patient response to treatment and correspondingly, a lack of disease progression.
This meaningful rapid response profile was demonstrated in a rigorously defined placebo-controlled setting, which is very rare in the context of CTCL clinical trials. Results documented in this strict study design allow patients and physicians to confidently recognize treatment utility. Treating physicians may be able to more quickly assess response to therapy and thereby fast-track treatment planning and avoid long unsuccessful trials of various therapeutic modalities. Shorter clinical follow up directly benefits patients, by alleviating some of the anxiety from waiting for months or more to see a response and may also improve their quality of life with a faster response time. This can be supported by the higher rate of patients who discontinued treatment in the placebo group in the Phase three study of HyBryte™ in CTCL (Kim et al., 2022). Unfortunately, due to the lack of rigor in assessing other treatment modalities, comparative statements regarding the efficacy of other treatment modalities is very difficult. Nevertheless, there is some clinical trial data available for mechlorethamine and bexarotene gel.
No prior therapeutics have demonstrated efficacy with a similar or better response rate (i.e., 16%) over such a short interval of time (i.e., 6 weeks of treatment). Mechlorethamine gel (non-inferiority trial between pharmacy prepared (i.e., compounded) and centrally prepared formulations of mechlorethamine with a non-inferiority margin of 25%) did not show responses until at least 8 weeks of continuous treatment and took almost 13 weeks of continuous treatment to reach a 16% response rate (Lessin et al., 2013). Bexarotene gel also did not achieve 16% until at least day 60 (9 weeks of continuous treatment) (Heald et al., 2003). Even when treating for months, physicians do not expect to see maximal responses. Indeed, it has become standard practice to treat patients with FDA-approved mechlorethamine, bexarotene gel, phototherapy, or other therapies until disease progression or side effects limit, rather than in a fixed schedule of time.
Another benefit of this study was the response rate evaluation in Cycle 1, which represents a rigorously defined parameter in the context of the clinical study design. Unlike other studies, the timeline for response was measured from the first application of study drug and light (5 J/cm2). The light dose was then gradually increased (to a maximum of 12 J/cm2), as dictated by the individual skin responses of the patient. Many patients did not achieve their maximal response until midway through Cycle 1. Alternatively measuring response rate from the first optimal dose of drug + light, equivalent to the first application of mechlorethamine or bexarotene gel, would consequently yield an even faster assessment of onset of action.
5 Efficacy in thick/deep cutaneous tissues
5.1 Folliculotropic mycosis fungoides
Folliculotropic MF (FMF) is a more aggressive form of MF with malignant cells present deeper in the skin, surrounding hair follicles (Mehta-Shah et al., 2020). The limited reach of skin-directed therapies into deeper tissue frequently results in reduced effectiveness. Topical HyBryte™’s maximal absorption between 500 and 650 nm in wavelength is known to penetrate significantly deeper than ultraviolet light (as depicted in Figure 1). (Alexander-S et al., 2020) Current recommendations from the National Comprehensive Cancer Network Guidelines for the treatment of FMF are to move to systemic agents due to current lack of efficacy of available skin-directed therapies (Mehta-Shah et al., 2020). Although response in this subject population may need further study, the absorption spectrum of HyBryte™ may be beneficial to patients with deeper lesions such as in folliculotropic MF, giving them a topical alternative to systemic therapy which can be more costly and dangerous.
5.2 Efficacy in patch and plaque disease
Plaques are thicker lesions, and much like folliculotropic MF, have been associated with a worse prognosis (Agar et al., 2010; Talpur et al., 2012). Furthermore, the thickness of lesions is associated with a worsened response to skin directed therapy, including with UV light therapy (Gökdemir et al., 2006). In the Phase three study of HyBryte™, it was found that therapy was as effective in thicker plaques as in patches. Response rates for patch lesions were 18% and 25% for plaques after Cycle 1. Likewise, these rates went up to 37% and 42% for patch and plaque disease, respectively (Kim et al., 2022).
5.3 Efficacy across race/other demographics
Another striking finding that may warrant further study is that there were 4/27 (15%) Black patients and 3/84 (4%) Caucasian patients that achieved a complete clinical response. This is an advantage over some therapies that are less effective in those with darker skin tones, such as UVB light phototherapy (Nikolaou et al., 2018).
6 HyBryte™ is safe and well tolerated
The combination of targeted topical drug therapy with targeted light therapy using visible red-yellow spectrum light yields a benign safety profile. HyBryte™ is non-mutagenic and even when administered intravenously) at much higher doses, does not cause significant adverse events other than those related to photoactivity (Gulick et al., 1999; Jacobson et al., 2001). Thus, the compound itself is benign. This is in contrast to the application of the standard therapies, e.g., topical nitrogen mustard or oral psoralen, which are associated with mutagenesis leading to melanoma and non-melanoma skin cancers (Vonderheid et al., 1989; Nijsten and Stern, 2003). The use of visible (vs UV) light significantly reduces the risk of skin damage and skin cancers. Furthermore, lack of dependence on blue light spectrum minimizes the potential for ocular damage caused by blue light (Tosini et al., 2016). This is an advantage over other types of phototherapy that induce photodamage and incidence of skin cancers in at least 27% of patients with early-stage disease (Querfeld et al., 2005).
Another advantage is that synthetic hypericin is preferentially absorbed by T-cells and even more specifically, malignant T-cells (Penjweini et al., 2014). Most importantly, even after up to 18 weeks (36 applications) of treatment, over multiple body regions, no systemic absorption of hypericin was observed in the blood (detection limit 5 ng/mL) after 18 weeks of treatment. Hypericin also has less cutaneous adverse events compared to other treatments. During the Phase three clinical trial investigating the effectiveness and safety of HyBryte™, three significant adverse events related to treatment were observed. One patient opted to discontinue treatment in Cycle 1 due to intense pain at the application site, another had to temporarily halt treatment because of severe erythema, and the third experiences application-site pain that eventually resolved without any intervention. Overall, all patients who received hypericin treatment throughout the study had a discontinuation rate due to AEs of 1.2% (Kim et al., 2022).
In contrast, the clinical study with mechlorethamine gel recorded a 22% discontinuation rate due to moderate or severe adverse events (AEs) with about two-thirds of dropouts occurring within the first 90 days and temporary suspension of treatment occurred in 34% of patients. Overall, 70% of patients ≥65 years of age in the mechlorethamine trial experienced cutaneous AEs and 38% discontinued treatment. Additionally, mechlorethamine treatment also created lifestyle issues for patients since treated areas of skin cannot be in contact with other people (Pharmaceuticals Helsinn, 2013). Similarly, topical bexarotene also has increased risks. Severe treatment related adverse events happened in 21% of patients on topical bexarotene. Treatment limiting toxic effects for the 1% formulation was found in 19% of patients. All treatment related toxicities (with the exception of trigeminal neuralgia) were at application sites associated with dermal irritation. Four patients (6%) withdrew from the study due to a possible treatment related adverse event (Breneman et al., 2002).
HyBryte™ has potentially fewer long term side effects than other topical treatment modalities, such as topical corticosteroids, which are commonly used in CTCL, but can cause significant damage to the skin. Known side effects include skin atrophy, striae, telangiectasias, and acne. Some patients may also experience hypertrichosis, delayed wound healing, and pigmentation changes. Moreover, some can also be systemically absorbed when applied in large amounts (Hengge et al., 2006). Systemic absorption has also been seen with topical Imiquimod, causing increased risk of upper respiratory infections, back pain, fatigue, fevers, headaches, and flu-like symptoms in >1% of patients (Pharmaceuticals Taro, 2004). In contrast, no HyBryte™ levels have been detected in the blood of CTCL patients (Kim et al., 2022). In comparison to these side effect profiles, HyBryte™ is safe and well tolerated.
Treatment decisions are driven not only by efficacy but also by safety and tolerability. HyBryte™ has a distinct response profile from other treatments used for CTCL, with an active ingredient that is not mutagenic and a light source that is not carcinogenic allowing for extensive treatment periods. This unique combination of efficacy and safety also offers an alternative treatment for patients who may be unable to access other treatments. For example, the use of mechlorethamine can be limited for patients who have children in their household and the use of UV light may be limited for patients who have a history of other skin damage or skin cancer. Ultimately, the low rate of AEs will further enhance treatment compliance and hopefully provide a long-term treatment option for patients with early stage CTCL.
7 Conclusion
In the designated patient population (patients with Stage IA, IB or IIA CTCL), the results of the HyBryte™ Phase three FLASH study, including both the rapid onset efficacy (16%, p = 0.04) following 6 weeks of therapy, sustained efficacy (40% response rate after 12 weeks of treatment, p < 0.0001), ability to address patient populations which otherwise have been resistant to treatment (plaque lesions, folliculotropic MF, African-American patients) and overall safety, make this a promising therapy for the treatment of early stage CTCL.
Author contributions
BP: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. CA-S: Data curation, Formal Analysis, Project administration, Validation, Writing–original draft, Writing–review and editing. EK: Writing–review and editing. AM: Writing–review and editing. JD: Writing–review and editing. HW: Writing–review and editing. ATR: Writing–review and editing. OD: Conceptualization, Formal Analysis, Writing–review and editing. AH: Writing–review and editing. CS: Writing–review and editing. RS: Writing–review and editing. CP: Conceptualization, Data curation, Formal Analysis, Writing–review and editing. AHR: Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Soligenix funded the publisher’s fee.
Conflict of interest
Authors ATR, OD, AH, CS, RS, and CP were employed by Soligenix, Inc. and Author BP was employed by Rochester Skin Lymphoma Medical Group.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fddsv.2023.1298453/full#supplementary-material
References
Agar, N. S., Wedgeworth, E., Crichton, S., Mitchell, T. J., Cox, M., Ferreira, S., et al. (2010). Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J. Clin. Oncol. 28 (31), 4730–4739. doi:10.1200/JCO.2009.27.7665
Alexander-Savino, C., Gilmore, E., Alhafnawi, M., and Poligone, B. (2020). Response in a patient with refractory folliculotropic mycosis fungoides to a topical hypericin cream activated with fluorescent light, Proceedings of the 4th World Congress of Cutaneous Lymphomas, February 2020, Barcelona, Spain.
Ali, S. M., and Olivo, M. (2002). Bio-distribution and subcellular localization of Hypericin and its role in PDT induced apoptosis in cancer cells. Int. J. Oncol. 21 (3), 531–540. doi:10.3892/ijo.21.3.531
Berg, S., Villasenor-Park, J., Haun, P., and Kim, E. J. (2017). Multidisciplinary management of mycosis fungoides/sézary syndrome. Curr. Hematol. Malig. Rep. 12 (3), 234–243. doi:10.1007/s11899-017-0387-9
Black, R. J., and Gavin, A. T. (2006). Photocarcinogenic risk of narrowband ultraviolet B (TL-01) phototherapy: early follow-up data. Br. J. Dermatol 154 (3), 566–567. doi:10.1111/j.1365-2133.2005.07085.x
Blank, M., Lavie, G., Mandel, M., Hazan, S., Orenstein, A., Meruelo, D., et al. (2004). Antimetastatic activity of the photodynamic agent hypericin in the dark. Int. J. cancer 111 (4), 596–603. doi:10.1002/ijc.20285
Blank, M., Mandel, M., Keisari, Y., Meruelo, D., and Lavie, G. (2003). Enhanced ubiquitinylation of heat shock protein 90 as a potential mechanism for mitotic cell death in cancer cells induced with hypericin. Cancer Res. 63 (23), 8241–8247.
Breneman, D., Duvic, M., Kuzel, T., Yocum, R., Truglia, J., and Stevens, V. J. (2002). Phase 1 and 2 trial of bexarotene gel for skin-directed treatment of patients with cutaneous T-cell lymphoma. Archives Dermatology 138 (3), 325–332. doi:10.1001/archderm.138.3.325
Chan, P. S., Koon, H. K., Wu, Z. G., Wong, R. N., Lung, M. L., Chang, C. K., et al. (2009). Role of p38 MAPKs in hypericin photodynamic therapy-induced apoptosis of nasopharyngeal carcinoma cells. Photochem Photobiol. 85 (5), 1207–1217. doi:10.1111/j.1751-1097.2009.00572.x
Chung, P. S., Saxton, R. E., Paiva, M. B., Rhee, C. K., Soudant, J., Mathey, A., et al. (1994). Hypericin uptake in rabbits and nude mice transplanted with human squamous cell carcinomas: study of a new sensitizer for laser phototherapy. Laryngoscope 104 (12), 1471–1476. doi:10.1288/00005537-199412000-00008
Damke, G., Damke, E., de Souza Bonfim-Mendonça, P., Ratti, B. A., de Freitas Meirelles, L. E., da Silva, V. R. S., et al. (2020). Selective photodynamic effects on cervical cancer cells provided by P123 Pluronic®-based nanoparticles modulating hypericin delivery. Life Sci. 255, 117858. doi:10.1016/j.lfs.2020.117858
Dong, X., Zeng, Y., Zhang, Z., Fu, J., You, L., He, Y., et al. (2021). Hypericin-mediated photodynamic therapy for the treatment of cancer: a review. J. Pharm. Pharmacol. 73 (4), 425–436. doi:10.1093/jpp/rgaa018
Durán, N., and Song, P. S. (1986). Hypericin and its photodynamic action. Photochem Photobiol. 43 (6), 677–680. doi:10.1111/j.1751-1097.1986.tb05646.x
Fox, F. E., Niu, Z., Tobia, A., and Rook, A. H. (1998). Photoactivated hypericin is an anti-proliferative agent that induces a high rate of apoptotic death of normal, transformed, and malignant T lymphocytes: implications for the treatment of cutaneous lymphoproliferative and inflammatory disorders. J. Invest. Dermatol 111 (2), 327–332. doi:10.1046/j.1523-1747.1998.00278.x
Garg, A. D., and Agostinis, P. (2014). ER stress, autophagy and immunogenic cell death in photodynamic therapy-induced anti-cancer immune responses. Photochem Photobiol. Sci. 13 (3), 474–487. doi:10.1039/c3pp50333j
Garg, A. D., Dudek, A. M., Ferreira, G. B., Verfaillie, T., Vandenabeele, P., Krysko, D. V., et al. (2013). ROS-induced autophagy in cancer cells assists in evasion from determinants of immunogenic cell death. Autophagy 9 (9), 1292–1307. doi:10.4161/auto.25399
Gökdemir, G., Barutcuoglu, B., Sakiz, D., and Köşlü, A. (2006). Narrowband UVB phototherapy for early-stage mycosis fungoides: evaluation of clinical and histopathological changes. J. Eur. Acad. Dermatol Venereol. 20 (7), 804–809. doi:10.1111/j.1468-3083.2006.01635.x
Gulick, R. M., McAuliffe, V., Holden-Wiltse, J., Crumpacker, C., Liebes, L., Stein, D. S., et al. (1999). Phase I studies of hypericin, the active compound in St. John's Wort, as an antiretroviral agent in HIV-infected adults. AIDS Clinical Trials Group Protocols 150 and 258. Ann. Intern Med. 130 (6), 510–514. doi:10.7326/0003-4819-130-6-199903160-00015
Heald, P., Mehlmauer, M., Martin, A. G., Crowley, C. A., Yocum, R. C., Reich, S. D., et al. (2003). Topical bexarotene therapy for patients with refractory or persistent early-stage cutaneous T-cell lymphoma: results of the phase III clinical trial. J. Am. Acad. Dermatol 49 (5), 801–815. doi:10.1016/s0190-9622(03)01475-0
Hengge, U. R., Ruzicka, T., Schwartz, R. A., and Cork, M. J. (2006). Adverse effects of topical glucocorticosteroids. J. Am. Acad. Dermatol 54 (1), 1–15. doi:10.1016/j.jaad.2005.01.010
Hristov, A. C., Tejasvi, T., and Ryan, A. W. (2021). Cutaneous T-cell lymphomas: 2021 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 96 (10), 1313–1328. doi:10.1002/ajh.26299
Jacobson, J. M., Feinman, L., Liebes, L., Ostrow, N., Koslowski, V., Tobia, A., et al. (2001). Pharmacokinetics, safety, and antiviral effects of hypericin, a derivative of St. John's wort plant, in patients with chronic hepatitis C virus infection. Antimicrob. Agents Chemother. 45 (2), 517–524. doi:10.1128/AAC.45.2.517-524.2001
Jendželovská, Z., Jendželovský, R., Kuchárová, B., and Fedoročko, P. (2016). Hypericin in the light and in the dark: two sides of the same coin. Front. Plant Sci. 7, 560. doi:10.3389/fpls.2016.00560
Johnson, S. A., and Pardini, R. S. (1998). Antioxidant enzyme response to hypericin in EMT6 mouse mammary carcinoma cells. Free Radic. Biol. Med. 24 (5), 817–826. doi:10.1016/s0891-5849(97)00364-x
Kim, E. J., Mangold, A. R., DeSimone, J. A., Wong, H. K., Seminario-Vidal, L., Guitart, J., et al. (2022). Efficacy and safety of topical hypericin photodynamic therapy for early-stage cutaneous T-cell lymphoma (mycosis fungoides): the FLASH phase 3 randomized clinical trial. JAMA Dermatol 158 (9), 1031–1039. doi:10.1001/jamadermatol.2022.2749
Kim, Y. H., Jensen, R. A., Watanabe, G. L., Varghese, A., and Hoppe, R. T. (1996). Clinical stage IA (limited patch and plaque) mycosis fungoides. A long-term outcome analysis. Arch. Dermatol 132 (11), 1309–1313. doi:10.1001/archderm.132.11.1309
Lessin, S. R., Duvic, M., Guitart, J., Pandya, A. G., Strober, B. E., Olsen, E. A., et al. (2013). Topical chemotherapy in cutaneous T-cell lymphoma: positive results of a randomized, controlled, multicenter trial testing the efficacy and safety of a novel mechlorethamine, 0.02%, gel in mycosis fungoides. JAMA Dermatol 149 (1), 25–32. doi:10.1001/2013.jamadermatol.541
Licata, A. G., Wilson, L. D., Braverman, I. M., Feldman, A. M., and Kacinski, B. M. (1995). Malignant melanoma and other second cutaneous malignancies in cutaneous T-cell lymphoma. The influence of additional therapy after total skin electron beam radiation. Arch. Dermatol 131 (4), 432–435. doi:10.1001/archderm.131.4.432
Lindahl, L. M., Fenger-Gron, M., and Iversen, L. (2013). Topical nitrogen mustard therapy in patients with mycosis fungoides or parapsoriasis. J. Eur. Acad. Dermatol Venereol. 27 (2), 163–168. doi:10.1111/j.1468-3083.2011.04433.x
Man, I., Crombie, I. K., Dawe, R. S., Ibbotson, S. H., and Ferguson, J. (2005). The photocarcinogenic risk of narrowband UVB (TL-01) phototherapy: early follow-up data. Br. J. Dermatol 152 (4), 755–757. doi:10.1111/j.1365-2133.2005.06537.x
Mehta-Shah, N., Horwitz, S. M., Ansell, S., Ai, W. Z., Barnes, J., Barta, S. K., et al. (2020). NCCN Guidelines insights: primary cutaneous lymphomas, version 2.2020. J. Natl. Compr. Canc Netw. 18 (5), 522–536. doi:10.6004/jnccn.2020.0022
Munoz, J. (2013). Mycosis fungoides: national organization for rare diseases: rare disease Database. Available from: https://rarediseases.org/rare-diseases/mycosis-fungoides/#complete-report.
Nijsten, T. E., and Stern, R. S. (2003). The increased risk of skin cancer is persistent after discontinuation of psoralen+ultraviolet A: a cohort study. J. Invest. Dermatol 121 (2), 252–258. doi:10.1046/j.1523-1747.2003.12350.x
Nikolaou, V., Sachlas, A., Papadavid, E., Economidi, A., Karambidou, K., Marinos, L., et al. (2018). Phototherapy as a first-line treatment for early-stage mycosis fungoides: the results of a large retrospective analysis. Photodermatol. Photoimmunol. Photomed. 34 (5), 307–313. doi:10.1111/phpp.12383
Noell, S., Mayer, D., Strauss, W. S., Tatagiba, M. S., and Ritz, R. (2011). Selective enrichment of hypericin in malignant glioma: pioneering in vivo results. Int. J. Oncol. 38 (5), 1343–1348. doi:10.3892/ijo.2011.968
Olsen, E. A., Whittaker, S., Kim, Y. H., Duvic, M., Prince, H. M., Lessin, S. R., et al. (2011). Clinical end points and response criteria in mycosis fungoides and sézary syndrome: a consensus statement of the international society for cutaneous lymphomas, the United States cutaneous lymphoma consortium, and the cutaneous lymphoma task force of the European organisation for research and treatment of cancer. J. Clin. Oncol. 29 (18), 2598–2607. doi:10.1200/JCO.2010.32.0630
Penjweini, R., Smisdom, N., Deville, S., and Ameloot, M. (2014). Transport and accumulation of PVP-Hypericin in cancer and normal cells characterized by image correlation spectroscopy techniques. Biochim. Biophys. Acta 1843 (5), 855–865. doi:10.1016/j.bbamcr.2014.01.016
Pharmaceuticals Helsinn (2013). Package insert online: food and drug administration. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/202317lbl.pdf.
Pharmaceuticals Taro (2004). Imiquimod cream DailyMed. Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=59af8153-4cf8-4f22-9000-f239798d52d1.
Prince, H. M., Whittaker, S., and Hoppe, R. T. (2009). How I treat mycosis fungoides and Sézary syndrome. Blood 114 (20), 4337–4353. doi:10.1182/blood-2009-07-202895
Querfeld, C., Rosen, S. T., Kuzel, T. M., Kirby, K. A., Roenigk, H. H., Prinz, B. M., et al. (2005). Long-term follow-up of patients with early-stage cutaneous T-cell lymphoma who achieved complete remission with psoralen plus UV-A monotherapy. Archives Dermatology 141 (3), 305–311. doi:10.1001/archderm.141.3.305
Stern, R. S., Thibodeau, L. A., Kleinerman, R. A., Parrish, J. A., and Fitzpatrick, T. B. (1979). Risk of cutaneous carcinoma in patients treated with oral methoxsalen photochemotherapy for psoriasis. N. Engl. J. Med. 300 (15), 809–813. doi:10.1056/NEJM197904123001501
Talpur, R., Singh, L., Daulat, S., Liu, P., Seyfer, S., Trynosky, T., et al. (2012). Long-term outcomes of 1,263 patients with mycosis fungoides and Sézary syndrome from 1982 to 2009. Clin. Cancer Res. 18 (18), 5051–5060. doi:10.1158/1078-0432.CCR-12-0604
Thomas, C., MacGill, R. S., Miller, G. C., and Pardini, R. S. (1992). Photoactivation of hypericin generates singlet oxygen in mitochondria and inhibits succinoxidase. Photochem Photobiol. 55 (1), 47–53. doi:10.1111/j.1751-1097.1992.tb04208.x
Tosini, G., Ferguson, I., and Tsubota, K. (2016). Effects of blue light on the circadian system and eye physiology. Mol. Vis. 22, 61–72.
Trautinger, F., Knobler, R., Willemze, R., Peris, K., Stadler, R., Laroche, L., et al. (2006). EORTC consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome. Eur. J. Cancer 42 (8), 1014–1030. doi:10.1016/j.ejca.2006.01.025
Verebová, V., Beneš, J., and Staničová, J. (2020). Biophysical characterization and anticancer activities of photosensitive phytoanthraquinones represented by hypericin and its model compounds. Molecules 25 (23), 5666. doi:10.3390/molecules25235666
Vonderheid, E. C., Tan, E. T., Kantor, A. F., Shrager, L., Micaily, B., and Van Scott, E. J. (1989). Long-term efficacy, curative potential, and carcinogenicity of topical mechlorethamine chemotherapy in cutaneous T cell lymphoma. J. Am. Acad. Dermatol 20 (3), 416–428. doi:10.1016/s0190-9622(89)70051-7
Keywords: CTCL, photodynamic therapy, mycosis fungoides, cancer, drug discovery, immunology, rare disease (RD)
Citation: Poligone B, Alexander-Savino CV, Kim EJ, Mangold AR, Desimone J, Wong HK, Rumage AT, Donini O, Haulenbeek A, Schaber CJ, Straube R, Pullion C and Rook AH (2023) HyBryte™ use in early-stage cutaneous T-cell lymphoma. Front. Drug Discov. 3:1298453. doi: 10.3389/fddsv.2023.1298453
Received: 21 September 2023; Accepted: 23 October 2023;
Published: 27 November 2023.
Edited by:
Deepak Balak, Leiden University Medical Center (LUMC), NetherlandsReviewed by:
Jean Leandro Dos Santos, São Paulo State University, BrazilCopyright © 2023 Poligone, Alexander-Savino, Kim, Mangold, Desimone, Wong, Rumage, Donini, Haulenbeek, Schaber, Straube, Pullion and Rook. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brian Poligone, YnBvbGlnb25lQHJvY2x5bXBob21hLmNvbQ==
†Present address: Carolina V. Alexander-Savino, Department of Dermatology, University of North Carolina, Chapel Hill, NC, United States
 Brian Poligone
Brian Poligone Carolina V. Alexander-Savino
Carolina V. Alexander-Savino Ellen J. Kim2
Ellen J. Kim2 Henry K. Wong
Henry K. Wong Christopher Pullion
Christopher Pullion