- 1Department of Medicine, Division of Gastroenterology, Hepatology and Nutrition, University at Buffalo School of Medicine and Biomedical Sciences, Buffalo, NY, United States
- 2Department of Ophthalmology, University of Pittsburgh School of Medicine, Pittsburgh, PA, United States
- 3AiDEAS, Tallinn, Estonia
- 4Onassis Cardiac Surgery Center, Athens, Greece
- 5Department of Medicine, Aristotle University of Thessaloniki Medical School, Thessaloniki, Greece
Atrial fibrillation (AF) significantly increases the risk of stroke and heart failure, but is frequently asymptomatic and intermittent; therefore, its timely diagnosis poses challenges. Early detection in selected patients may aid in stroke prevention and mitigate structural heart complications through prompt intervention. Smartwatches, coupled with powerful artificial intelligence (AI)-enabled algorithms, offer a promising tool for early detection due to their widespread use, easiness of use, and potential cost-effectiveness. Commercially available smartwatches have gained clearance from the FDA to detect AF and are becoming increasingly popular. Despite their promise, the evolving landscape of AI-enabled smartwatch-based AF detection raises questions about the clinical value of this technology. Following the ongoing digital transformation of healthcare, clinicians should familiarize themselves with how AI-enabled smartwatches function in AF detection and navigate their role in clinical settings to deliver optimal patient care. In this review, we provide a concise overview of the characteristics of AI-enabled smartwatch algorithms, their diagnostic performance, clinical value, limitations, and discuss future perspectives in AF diagnosis.
Introduction
Atrial fibrillation (AF) constitutes the most common cardiac arrhythmia with a prevalence that is increasing. According to the Global Burden of Diseases study, the number of individuals affected by AF has doubled from 1990 to 2019, reaching approximately 60 million globally (1). This upward trajectory is projected to continue, with estimates suggesting that by 2030, the United States alone could see over 12.1 million people diagnosed with AF (2).
AF is associated with a fivefold increased risk of stroke, as well as an increased risk for heart failure (3, 4). However, its episodic manifestation and frequently asymptomatic nature, pose significant challenges for AF diagnosis (5). For instance, research data suggest that paroxysmal AF can be detected in 10%–20% of patients with cryptogenic strokes and extended monitoring with implantable devices is recommended in this population to increase sensitivity of AF detection (6–8). In selected asymptomatic patients, early AF detection could be helpful in preventing stroke using oral anticoagulation (OAC) or preventing heart failure using rhythm control strategies (4, 9).
Smartwatches empowered by artificial intelligence (AI) algorithms have emerged as a promising tool for early detection of AF (10, 11). Recent cost-effectiveness studies also underscore their potential to serve as a widespread tool for early arrhythmic detection (12). However, AF confirmation still requires electrocardiography (ECG) and widespread incorporation of smartwatches for AF detection has not been established (13–15).
In this review we first summarize the characteristics and performance of smartwatches that use AI-enabled algorithms for AF detection; secondly, we discuss their clinical applicability, the challenges around their use, and finally outline future directions in the field.
Smartwatch technology for AF detection
Photoplethysmography
A common technology for detecting AF with smartwatches is photoplethysmography (PPG). This technique involves illuminating the skin with a light-emitting diode (LED) and detecting the light reflected back with a photodetector (16). The intensity of the reflected light constitutes the PPG signal, which varies according to blood volume changes in the vessels throughout the cardiac cycle. Therefore, the PPG signal represents a pulse pressure waveform that enables passive, continuous, or semi-continuous heart rhythm monitoring measured at the wrist (17).
Since various neural, cardiac, and respiratory factors regulate blood flow, physiologic cardiovascular parameters such as heart rate, blood pressure, oxygen saturation, and respiratory rate could be derived from PPG signal analysis (18). Importantly, the peak of the PPG signal correlates closely with the R wave of the electrocardiogram (ECG) (19). As such, the PPG signal can provide R-R intervals, i.e., heart rate variability (20) and in AF, the PPG signal exhibits greater irregularity of pulse waveform (16). Assessment of PPG variability through a detection algorithm is used to detect AF.
Single-lead ECG
Another common method for detecting AF with smartwatches is based on the recording of a single-lead ECG. In this method, the watch's back acts as a positive electrode and the contralateral fingertip is placed on the crown, acting as a negative electrode (21). This creates a bipolar ECG-lead, simulating Einthoven's ECG lead I. The recorded ECG tracing is typically saved and can be analyzed either manually by physicians or automatically. In addition to single-lead ECG, other techniques further explore the use of a smartwatch-based wireless 6-lead limb-like ECG, which requires interpretation by cardiologists, combined with PPG to increase accuracy of AF detection (22).
Detection algorithms and machine learning
Detection algorithms may rely on traditional statistics or AI, particularly machine learning (ML) and deep learning (DL) algorithms (23). For commercially available smartwatches, the AI-empowered algorithms utilized for AF detection remain proprietary to each company (24, 25). Nonetheless, we will provide a brief overview of how similar algorithms described in the research setting function to aid physicians in understanding their mechanisms. PPG or ECG signals, once captured, undergo several preprocessing steps including noise reduction, normalization, and segmentation. Feature extraction focuses on both time-domain and frequency-domain attributes, which might include heart rate variability indices, the morphology of the PPG or ECG waveform, and temporal intervals between heartbeats. A tachogram, a visual representation of heart rate variability plotted against time, serves as a key component in the PPG-based AF detection process (26). Typically, a set number of irregular tachograms over a period of time is involved in the algorithm's decision to trigger an irregular pulse notification (IPN). ML models such as support vector machines and random forests analyze these engineered features to classify heart rhythms, benefitting from the clear delineation of feature-based input (27, 28). In contrast, DL models such as convolutional neural networks (CNNs) and recurrent neural networks (RNNs) can process raw or minimally preprocessed signal data (29, 30). These models are adept at automatically detecting complex patterns within large datasets, which is beneficial for identifying subtle and non-linear indicators of AF. CNNs, for instance, are useful for spatial pattern recognition within ECG signals, while RNNs excel at analyzing sequential data, capturing dynamic changes over time which are crucial for continuous ECG monitoring (31).
Challenges
PPG sensors can yield missing signals or inconclusive/unclassified rhythms. Missing signals are often due to poor sensor-skin contact. On the other hand, insufficient quality of the signal may be attributed to motion artifacts, such as from muscular motion. Overall, PPG sensors tend to underestimate AF detection at both higher and lower heart rates when compared to standard detection methods (32). Additionally, ectopic beats can further complicate AF detection by generating pulse irregularities (22). Although evidence remain inconclusive, skin pigmentation may also restrict PPG performance (33, 34). Moreover, the PPG signal may be weaker in elderly, due to pathologic or physiologic changes associated with aging including reduced peripheral blood flow, increased arterial wall stiffness, and skin changes (35, 36). On the other hand, there may be a low amplitude of P-waves with single-lead ECG, which challenges the classification of electrical activity (37). Lastly, limitations of using smartwatch to detect AF include the necessity for user cooperation and regular charging, depending on its battery life (10).
Similarly, AI models, despite their capabilities, require robust, diverse datasets for training to ensure effective performance across various demographic groups and conditions. Additionally, these models necessitate significant computational resources, particularly in DL frameworks, to process extensive data and learn through sophisticated layers of abstraction. The interpretability of these AI systems is also crucial, particularly in healthcare settings, where providers prefer algorithms that offer insights into their decision-making processes (38). This aligns with the increasing demand for explainable AI in clinical environments, highlighting another significant limitation.
Diagnostic performance of Ai-empowered smartwatches in AF detection
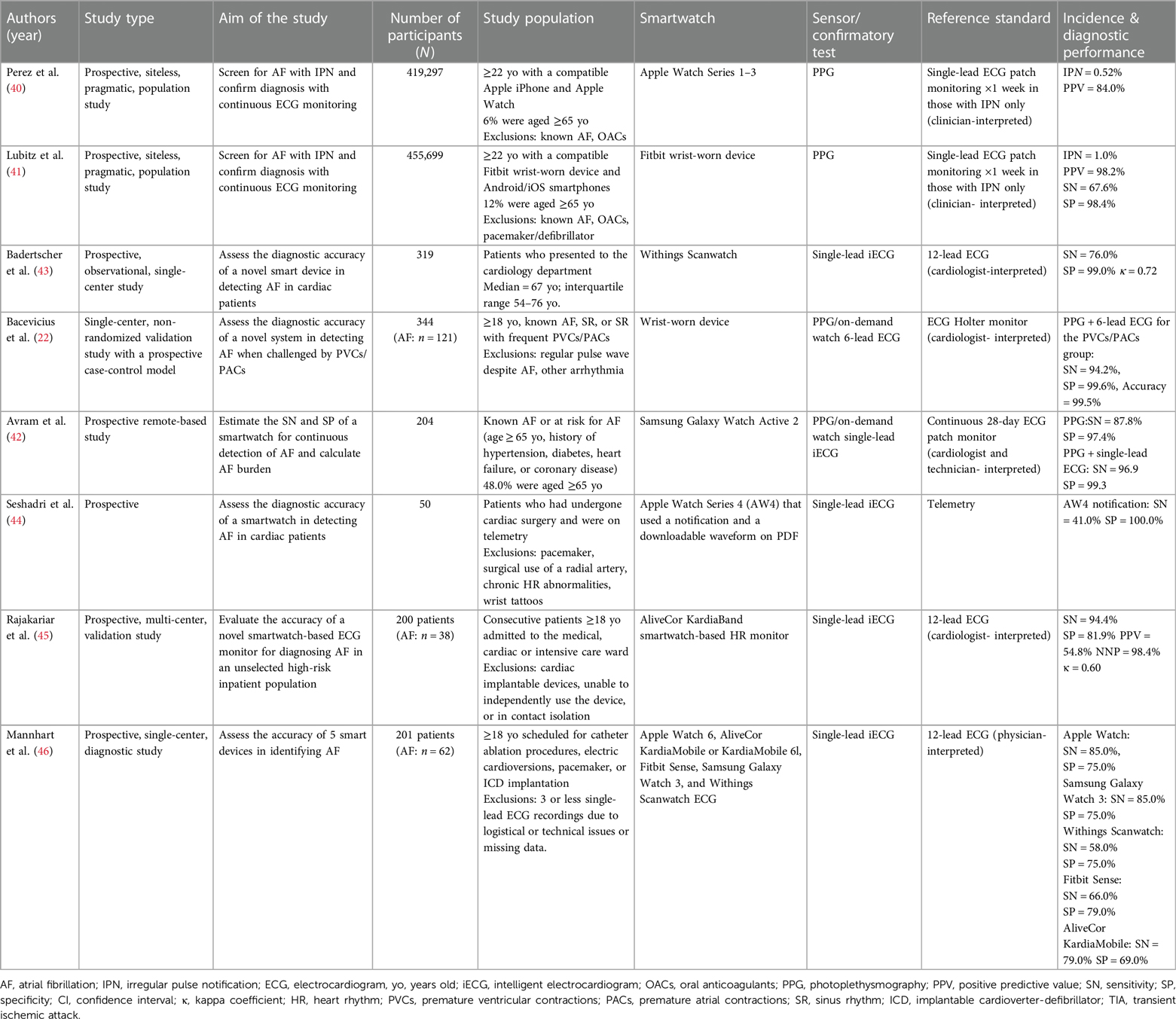
Currently, AF diagnosis requires that a physician reviews a standard 12-lead ECG or rhythm strips (9, 39). This also serves as the gold standard to determine the diagnostic accuracy of smartwatches. Table 1 summarizes key recent studies conducted from 2019 and onwards that evaluated the diagnostic performance of smartwatches for detecting AF.
Large-scale, pragmatic studies that were conducted for major smartwatch companies like Apple and Fitbit assessed the ability to screen for AF in the general population relying on PPG (40, 41). The Apple Heart Study evaluated over 400,000 individuals (mean age 41 ± 13 years, 42% women) for IPN with the Apple Watch indicating possible AF in that event (40). The duration of PGG monitoring and the criteria to triggering an IPN for AF varied significantly among studies assessing PPG sensors. PPG monitoring was more intermittent with the Apple algorithm, where the IPN required at least 5/6 consecutive irregular 1-minute tachograms within a 48-hour period (40). Conversely, the Fitbit algorithm allowed for more continuous monitoring and applied stricter notification criteria, requiring at least 30 min of irregular rhythm before notifying the user (41). Participants who received such an IPN notification received a single-lead ECG patch via mail to wear for 1 week, which was used to diagnose AF. Overall, only 0.52% of the study population received an IPN using the Apple algorithm, with the percentage correlating with increased age (≥65 years: IPN 3.2%). Similarly, the Fitbit Heart Study assessed the performance of the Fitbit Watch among over 400,000 participants (median age 47 years, interquartile range 35–58 years, 71% women), of whom 1% received an IPN (≥65 years: IPN 3.6%) (41). Of those who received an IPN in the Fitbit and Apple Heart studies, only up to 25% of all notified participants in these studies returned the ECG patch. 32.2% and 34.0%, respectively were confirmed to have AF lasting at least 30 s on the reference ECG patch. The positive predictive value (PPV) for AF, confirmed concurrently on the ECG patch, of the Apple algorithm was 84.0% (95% CI, 76.0%–92.0%). The PPV was lower among those over 65, i.e., 78% (95% CI, 64.0%–92.0%). The Fitbit algorithm yielded a sensitivity of 67.6%, specificity of 98.4%, and PPV of 98.2% (95% CI, 95.5%–99.5%), with a slight reduction among those aged ≥65 years at 97.0% (95% CI, 91.4%–99.4%).
Among studies conducted in research settings and among populations at high-risk for AF, the performance of PPG sensors varied with sensitivity ranging from 87.8% (42) to 94.2% (22) and specificity up to 99.1% (22). Other studies examined the performance of intelligent ECG (iECG), a smartwatch-based single-lead ECG with automatic AF detection function (43–46). AF was considered present if episodes lasted for at least 30 s. The sensitivity of automatic AF detection with iECG varied from as low as 41.0% among post-cardiac surgery patients (44) to 94.4% among high-risk inpatient populations (45), while the specificity ranged from 69.0% (46) to 100.0% (44). These performance indices should be interpreted with caution, as most of these studies employed intention-to-diagnose analyses, excluding a significant number of tracings that were deemed inconclusive. Badertscher et al. demonstrated a reduction in such inconclusive tracings from 14% to 4.1% after a cardiologist's review, and this was further supported by Mannhart et al. (43, 46). Avram et al. demonstrated that obtaining a confirmatory iECG one hour apart the first one improved the specificity (100.0%) without affecting sensitivity (96.0%). Moreover, novel AI algorithms have demonstrated excellent performance in reducing inconclusive classification rates (47). Lastly, in most studies, patients were instructed on how to use the technology for AF detection which may not reflect real-life circumstances or digital literacy, especially among the elderly.
Clinical impact of AF detection using Ai-empowered smartwatches
Potential benefits
The main potential advantage of detecting asymptomatic AF using smartwatches lies in the opportunity for early diagnosis and therapeutic intervention. Identifying AF among high risk, asymptomatic patients could lead to the initiation of OAC, potentially reducing the risk for stroke (4). The eBRAVE-AF trial showed that PPG-based screening more than doubled the detection rate of asymptomatic AF leading to subsequent OAC initiation (48). A recent meta-analysis demonstrated a significant reduction in stroke risk after OAC initiation in patients with asymptomatic AF detected by implantable cardiac devices (49). However, the results of studies investigating the specific clinical benefits of smartwatch-detected AF and the populations to which may apply, are pending (50). Additionally, increasing evidence suggests that early initiation of rhythm control not only lowers the risk of stroke, but also reduces the structural, long-term complications of AF, particularly HF (51, 52). Furthermore, identifying AF early could prompt the early identification and management of other concomitant cardiovascular conditions such as hypertension, underlying heart disease and diabetes mellitus (53). Lastly, population screening for AF using smartwatches is proposed as a more cost-effective approach compared to no screening or conventional screening (12). Nevertheless, no consensus has been reached yet regarding AF screening due to a lack of clinical data to support its potential benefits in asymptomatic patient groups at risk for AF and its complications (5, 9, 54).
Identifying populations for smartwatch-based AF detection
In the absence of relevant guidelines, it may be reasonable to introduce smartwatch AF screening in certain populations. For example, the 2020 ESC Guidelines for AF recommend AF screening for patients over 75 (5). Additionally, the same guidelines suggest screening for those at high stroke risk, though the optimal cut-offs on assessment tools, e.g., CHA2DS2-VASc score, for smartwatch-based AF detection require further clarification. Oppportunistic screening for AF is also suggested for patients aged 65 years and above, where smartwatches have already been incorporated as screening options (5). In contrast, the US Preventive Services Task Force implies that the current evidence is insufficient to assess the balance of benefits and harms for AF screening (54). It is noteworthy that AI algorithms utilizing Electronic Medical Records (PULsE-AI, FIND-AF) or ECGs (BEAGLE) may facilitate the identification of high-risk patients (55–57). However, it would be difficult to envision that smartwatches could be used for screening or substitute other established methods of AF detection in symptomatic patients at high risk for AF, such as those with cryptogenic stroke.
Clinical challenges and risks
Once the smartwatch indicates possible AF, the main question is how healthcare providers should proceed (10). In existing clinical studies, individuals were referred to contact a healthcare provider (40, 41, 58). Healthcare providers then confirmed the presence of AF through a standard ECG confirmatory test. This approach aligns with the 2020 ESC Guidelines for AF, which included smartwatches in the screening recommendations (5). However, the introduction of smartwatches for AF diagnosis poses risks for putting high stress on the healthcare system, particularly if extended population screening is implemented (59, 60). Therefore, it is crucial to optimize the parameters of smartwatch algorithms to minimize false positives and prevent physician overload. A study conducted among patients with known AF who utilized wearables, demonstrated increased engagement in follow-up healthcare, with no significant difference in pulse rates compared to those not using wearables (61). Further research into health outcomes will guide management for both patients and providers.
Data management
Smartwatch data management poses an ongoing challenge. The FDA does not classify smartwatches as medical devices; rather they are considered wellness tools, subject to expedited regulatory approval through the Digital Health Software Precertification (Pre-Cert) Program (62). This regulatory framework promotes the development of a large digital health market and the generation of a substantial volume of digital data (21). Despite this, smartwatch data have not yet been fully integrated into clinical workflows. While meaningful integration of smartwatch data into the existing Electronic Health Records (EHRs) will eventually become necessary, the specific type and volume of data to be shared with EHRs is yet to be determined. Consequently, there is an ongoing effort to endorse partnerships between startup organizations that specialize in integrating such data into EHRs with healthcare systems and insurance companies (63). It is also crucial to develop clear legislation regarding data ownership, ensure compliance with HIPAA regulations and address patient confidentiality, privacy, and security concerns (59, 64). Furthermore, attention is required concerning physician reimbursement, including the creation of standard documentation processes and billable codes related to the integration of smartwatch data into clinical workflows (65). With all these impending changes, it has been suggested that a “digital health counselor” could provide support and guide patients and providers throughout the initial transition phase of digital data integration into clinical workflows (59).
Conclusions and future directions
AI-empowered smartwatches have emerged as a potential screening tool for AF, the most common arrhythmia with an increasing prevalence. Potentially important advantages of this strategy include purely non-invasive nature, easiness of use, early detection of AF in high-risk asymptomatic individuals and reduction in AF burden. However, there is no consensus among pertinent societies regarding AF screening and the clinical impact of this strategy using smartwatches remains to be proven. Particular issues that have to be addressed are: identification of populations that could benefit from AF screening using smartwatches; optimization of AI algorithms to improve diagnostic accuracy and reduce confirmatory tests; data management of protected health care information including infrastructure for processing of large amounts of data, and clinical algorithms to assist with widespread application of AF screening via smartwatches. Large trials to address these questions are currently required and may expect the landscape of AF in the near future.
Author contributions
ZP: Writing – original draft, Writing – review & editing. DS: Writing – original draft, Writing – review & editing. SM: Writing – original draft, Writing – review & editing. DA: Writing – original draft, Writing – review & editing. ME: Writing – original draft, Writing – review & editing. PK: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer VV declared a shared affiliation with the author PK to the handling editor at the time of review.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396(10258):1204–22. doi: 10.1016/S0140-6736(20)30925-9
2. Prevention CfDCa. Atrial Fibrillation. (2022). Available online at: https://www.cdc.gov/heartdisease/atrial_fibrillation.htm (Accessed April 15, 2024).
3. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the framingham study. Stroke. (1991) 22(8):983–8. doi: 10.1161/01.STR.22.8.983
4. López-López JA, Sterne JAC, Thom HHZ, Higgins JPT, Hingorani AD, Okoli GN, et al. Oral anticoagulants for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis, and cost effectiveness analysis. Br Med J. (2017) 359:j5058. doi: 10.1136/bmj.j5058
5. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. (2021) 42(5):373–498. doi: 10.1093/eurheartj/ehaa612
6. Gladstone DJ, Spring M, Dorian P, Panzov V, Thorpe KE, Hall J, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. (2014) 370(26):2467–77. doi: 10.1056/NEJMoa1311376
7. Lakkireddy DR, Garg J, Ahmed A, Bawa D, Kabra R, Darden D, et al. LB-456091-2 dynamic data driven management of atrial fibrillation using implantable cardiac monitors—the monitor af study. Heart Rhythm. (2023) 20(7):1085–6. doi: 10.1016/j.hrthm.2023.04.041
8. Hart RG, Diener H-C, Coutts SB, Easton JD, Granger CB, O'Donnell MJ, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. (2014) 13(4):429–38. doi: 10.1016/S1474-4422(13)70310-7
9. Joglar JA, Chung MK, Armbruster AL, Benjamin EJ, Chyou JY, Cronin EM, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. (2024) 149(1):e1–e156. doi: 10.1161/CIR.0000000000001193
10. Bayoumy K, Gaber M, Elshafeey A, Mhaimeed O, Dineen EH, Marvel FA, et al. Smart wearable devices in cardiovascular care: where we are and how to move forward. Nat Rev Cardiol. (2021) 18(8):581–99. doi: 10.1038/s41569-021-00522-7
11. Dörr M, Nohturfft V, Brasier N, Bosshard E, Djurdjevic A, Gross S, et al. The WATCH AF trial: smartWATCHes for detection of atrial fibrillation. JACC Clin Electrophysiol. (2019) 5(2):199–208. doi: 10.1016/j.jacep.2018.10.006
12. Chen W, Khurshid S, Singer DE, Atlas SJ, Ashburner JM, Ellinor PT, et al. Cost-effectiveness of screening for atrial fibrillation using wearable devices. JAMA Health Forum. (2022) 3(8):e222419. doi: 10.1001/jamahealthforum.2022.2419
13. Wasserlauf J, You C, Patel R, Valys A, Albert D, Passman R. Smartwatch performance for the detection and quantification of atrial fibrillation. Circ Arrhythm Electrophysiol. (2019) 12(6):e006834. doi: 10.1161/CIRCEP.118.006834
14. Tran KV, Filippaios A, Noorishirazi K, Ding E, Han D, Mohagheghian F, et al. False atrial fibrillation alerts from smartwatches are associated with decreased perceived physical well-being and confidence in chronic symptoms management. Cardiol Cardiovasc Med. (2023) 7(2):97–107. doi: 10.26502/fccm.92920314
15. Sepehri Shamloo A, Bollmann A, Dagres N, Arya A, Hindricks G. Smart watch devices for atrial fibrillation screening. J Am Coll Cardiol. (2020) 75(11):1364–5. doi: 10.1016/j.jacc.2019.10.063
16. Charlton PH, Kyriaco PA, Mant J, Marozas V, Chowienczyk P, Alastruey J. Wearable photoplethysmography for cardiovascular monitoring. Proc IEEE Inst Electr Electron Eng. (2022) 110(3):355–81. doi: 10.1109/JPROC.2022.3149785
17. Pereira T, Tran N, Gadhoumi K, Pelter MM, Do DH, Lee RJ, et al. Photoplethysmography based atrial fibrillation detection: a review. NPJ Digit Med. (2020) 3:3. doi: 10.1038/s41746-019-0207-9
18. Nilsson LM. Respiration signals from photoplethysmography. Anesth Analg. (2013) 117(4):859–65. doi: 10.1213/ANE.0b013e31828098b2
19. Allen J. Photoplethysmography and its application in clinical physiological measurement. Physiol Meas. (2007) 28(3):R1. doi: 10.1088/0967-3334/28/3/R01
20. Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. (2017) 5:258. doi: 10.3389/fpubh.2017.00258
21. Foster KR, Torous J. The opportunity and obstacles for smartwatches and wearable sensors. IEEE Pulse. (2019) 10(1):22–5. doi: 10.1109/MPULS.2018.2885832
22. Bacevicius J, Abramikas Z, Dvinelis E, Audzijoniene D, Petrylaite M, Marinskiene J, et al. High specificity wearable device with photoplethysmography and six-lead electrocardiography for atrial fibrillation detection challenged by frequent premature contractions: doubleCheck-AF. Front Cardiovasc Med. (2022) 9:869730. doi: 10.3389/fcvm.2022.869730
23. Aschbacher K, Yilmaz D, Kerem Y, Crawford S, Benaron D, Liu J, et al. Atrial fibrillation detection from raw photoplethysmography waveforms: a deep learning application. Heart Rhythm O2. (2020) 1(1):3–9. doi: 10.1016/j.hroo.2020.02.002
24. Apple. Using Apple Watch for Arrhythmia Detection. (2020). Available online at: https://www.apple.com/healthcare/docs/site/Apple_Watch_Arrhythmia_Detection.pdf (Accessed April 15, 2024).
25. Lubitz SA, Faranesh AZ, Atlas SJ, McManus DD, Singer DE, Pagoto S, et al. Rationale and design of a large population study to validate software for the assessment of atrial fibrillation from data acquired by a consumer tracker or smartwatch: the fitbit heart study. Am Heart J. (2021) 238:16–26. doi: 10.1016/j.ahj.2021.04.003
26. University C. The Next Heartbeat. (2024). Available online at: https://www.thenextheartbeat.com/definitions-2/ (Accessed April 15, 2024).
27. Mei Z, Gu X, Chen H, Chen W. Automatic atrial fibrillation detection based on heart rate variability and spectral features. IEEE Access. (2018) 6:53566–75. doi: 10.1109/ACCESS.2018.2871220
28. Hirsch G, Jensen SH, Poulsen ES, Puthusserypady S. Atrial fibrillation detection using heart rate variability and atrial activity: a hybrid approach. Expert Syst Appl. (2021) 169:114452. doi: 10.1016/j.eswa.2020.114452
29. Chen X, Cheng Z, Wang S, Lu G, Xv G, Liu Q, et al. Atrial fibrillation detection based on multi-feature extraction and convolutional neural network for processing ECG signals. Comput Methods Programs Biomed. (2021) 202:106009. doi: 10.1016/j.cmpb.2021.106009
30. Mousavi S, Afghah F, Acharya UR. HAN-ECG: an interpretable atrial fibrillation detection model using hierarchical attention networks. Comput Biol Med. (2020) 127:104057. doi: 10.1016/j.compbiomed.2020.104057
31. Murat F, Sadak F, Yildirim O, Talo M, Murat E, Karabatak M, et al. Review of deep learning-based atrial fibrillation detection studies. Int J Environ Res Public Health. (2021) 18(21):11302. doi: 10.3390/ijerph182111302
32. Al-Kaisey AM, Koshy AN, Ha FJ, Spencer R, Toner L, Sajeev JK, et al. Accuracy of wrist-worn heart rate monitors for rate control assessment in atrial fibrillation. Int J Cardiol. (2020) 300:161–4. doi: 10.1016/j.ijcard.2019.11.120
33. Sañudo B, De Hoyo M, Muñoz-López A, Perry J, Abt G. Pilot study assessing the influence of skin type on the heart rate measurements obtained by photoplethysmography with the apple watch. J Med Syst. (2019) 43(7):195. doi: 10.1007/s10916-019-1325-2
34. Koerber D, Khan S, Shamsheri T, Kirubarajan A, Mehta S. Accuracy of heart rate measurement with wrist-worn wearable devices in Various skin tones: a systematic review. J Racial Ethn Health Disparities. (2023) 10(6):2676–84. doi: 10.1007/s40615-022-01446-9
35. Kelly RI, Pearse R, Bull RH, Leveque JL, de Rigal J, Mortimer PS. The effects of aging on the cutaneous microvasculature. J Am Acad Dermatol. (1995) 33(5 Pt 1):749–56. doi: 10.1016/0190-9622(95)91812-4
36. Meijer WT, Hoes AW, Rutgers D, Bots ML, Hofman A, Grobbee DE. Peripheral arterial disease in the elderly: the rotterdam study. Arterioscler Thromb Vasc Biol. (1998) 18(2):185–92. doi: 10.1161/01.ATV.18.2.185
37. Niu Y, Wang H, Wang H, Zhang H, Jin Z, Guo Y. Diagnostic validation of smart wearable device embedded with single-lead electrocardiogram for arrhythmia detection. Digit Health. (2023) 9:20552076231198682. doi: 10.1177/20552076231198682
38. Jo YY, Cho Y, Lee SY, Kwon JM, Kim KH, Jeon KH, et al. Explainable artificial intelligence to detect atrial fibrillation using electrocardiogram. Int J Cardiol. (2021) 328:104–10. doi: 10.1016/j.ijcard.2020.11.053
39. Steinberg JS, O'Connell H, Li S, Ziegler PD. Thirty-Second gold standard definition of atrial fibrillation and its relationship with subsequent arrhythmia patterns: analysis of a large prospective device database. Circ Arrhythm Electrophysiol. (2018) 11(7):e006274. doi: 10.1161/CIRCEP.118.006274
40. Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T, et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. (2019) 381(20):1909–17. doi: 10.1056/NEJMoa1901183
41. Lubitz SA, Faranesh AZ, Selvaggi C, Atlas SJ, McManus DD, Singer DE, et al. Detection of atrial fibrillation in a large population using wearable devices: the fitbit heart study. Circulation. (2022) 146(19):1415–24. doi: 10.1161/CIRCULATIONAHA.122.060291
42. Avram R, Ramsis M, Cristal AD, Nathan V, Zhu L, Kim J, et al. Validation of an algorithm for continuous monitoring of atrial fibrillation using a consumer smartwatch. Heart Rhythm. (2021) 18(9):1482–90. doi: 10.1016/j.hrthm.2021.03.044
43. Badertscher P, Lischer M, Mannhart D, Knecht S, Isenegger C, Du Fay de Lavallaz J, et al. Clinical validation of a novel smartwatch for automated detection of atrial fibrillation. Heart Rhythm O2. (2022) 3(2):208–10. doi: 10.1016/j.hroo.2022.02.004
44. Seshadri DR, Bittel B, Browsky D, Houghtaling P, Drummond CK, Desai MY, et al. Accuracy of apple watch for detection of atrial fibrillation. Circulation. (2020) 141(8):702–3. doi: 10.1161/CIRCULATIONAHA.119.044126
45. Rajakariar K, Koshy AN, Sajeev JK, Nair S, Roberts L, Teh AW. Accuracy of a smartwatch based single-lead electrocardiogram device in detection of atrial fibrillation. Heart. (2020) 106(9):665–70. doi: 10.1136/heartjnl-2019-316004
46. Mannhart D, Lischer M, Knecht S, du Fay de Lavallaz J, Strebel I, Serban T, et al. Clinical validation of 5 direct-to-consumer wearable smart devices to detect atrial fibrillation: bASEL wearable study. JACC Clin Electrophysiol. (2023) 9(2):232–42. doi: 10.1016/j.jacep.2022.09.011
47. Weidlich S, Mannhart D, Kennedy A, Doggart P, Serban T, Knecht S, et al. Reducing the burden of inconclusive smart device single-lead ECG tracings via a novel artificial intelligence algorithm. Cardiovasc Digit Health J. (2024) 5(1):29–35. doi: 10.1016/j.cvdhj.2023.12.003
48. Rizas KD, Freyer L, Sappler N, von Stülpnagel L, Spielbichler P, Krasniqi A, et al. Smartphone-based screening for atrial fibrillation: a pragmatic randomized clinical trial. Nat Med. (2022) 28(9):1823–30. doi: 10.1038/s41591-022-01979-w
49. McIntyre WF, Benz AP, Becher N, Healey JS, Granger CB, Rivard L, et al. Direct oral anticoagulants for stroke prevention in patients with device-detected atrial fibrillation: a study-level meta-analysis of the NOAH-AFNET 6 and ARTESiA trials. Circulation. (2024) 149(13):981–8. doi: 10.1161/CIRCULATIONAHA.123.067512
50. Kalla M, Fabritz L, Kirchhof P. SMART About watches: we need technical and biological validation of atrial fibrillation screening. JACC Clin Electrophysiol. (2019) 5(2):209–11. doi: 10.1016/j.jacep.2018.11.018
51. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. (2020) 383(14):1305–16. doi: 10.1056/NEJMoa2019422
52. Camm AJ, Naccarelli GV, Mittal S, Crijns HJGM, Hohnloser SH, Ma C-S, et al. The increasing role of rhythm control in patients with atrial fibrillation. J Am Coll Cardiol. (2022) 79(19):1932–48. doi: 10.1016/j.jacc.2022.03.337
53. Staerk L, Sherer JA, Ko D, Benjamin EJ, Helm RH. Atrial fibrillation: epidemiology, pathophysiology, and clinical outcomes. Circ Res. (2017) 120(9):1501–17. doi: 10.1161/CIRCRESAHA.117.309732
54. Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, et al. Screening for atrial fibrillation: uS preventive services task force recommendation statement. JAMA. (2022) 327(4):360–7. doi: 10.1001/jama.2021.23732
55. Hill NR, Groves L, Dickerson C, Ochs A, Pang D, Lawton S, et al. Identification of undiagnosed atrial fibrillation using a machine learning risk-prediction algorithm and diagnostic testing (PULsE-AI) in primary care: a multi-centre randomized controlled trial in England. Eur Heart J Digit Health. (2022) 3(2):195–204. doi: 10.1093/ehjdh/ztac009
56. Nadarajah R, Wu J, Hogg D, Raveendra K, Nakao YM, Nakao K, et al. Prediction of short-term atrial fibrillation risk using primary care electronic health records. Heart. (2023) 109(14):1072–9. doi: 10.1136/heartjnl-2022-322076
57. Noseworthy PA, Attia ZI, Behnken EM, Giblon RE, Bews KA, Liu S, et al. Artificial intelligence-guided screening for atrial fibrillation using electrocardiogram during sinus rhythm: a prospective non-randomised interventional trial. Lancet. (2022) 400(10359):1206–12. doi: 10.1016/S0140-6736(22)01637-3
58. Guo Y, Wang H, Zhang H, Liu T, Liang Z, Xia Y, et al. Mobile photoplethysmographic technology to detect atrial fibrillation. J Am Coll Cardiol. (2019) 74(19):2365–75. doi: 10.1016/j.jacc.2019.08.019
59. Ginsburg GS, Picard RW, Friend SH. Key issues as wearable digital health technologies enter clinical care. N Engl J Med. (2024) 390(12):1118–27. doi: 10.1056/NEJMra2307160
60. Wyatt KD, Poole LR, Mullan AF, Kopecky SL, Heaton HA. Clinical evaluation and diagnostic yield following evaluation of abnormal pulse detected using apple watch. J Am Med Inform Assoc. (2020) 27(9):1359–63. doi: 10.1093/jamia/ocaa137
61. Wang L, Nielsen K, Goldberg J, Brown JR, Rumsfeld JS, Steinberg BA, et al. Association of wearable device use with pulse rate and health care use in adults with atrial fibrillation. JAMA Netw Open. (2021) 4(5):e215821. doi: 10.1001/jamanetworkopen.2021.5821
62. Lee TT, Kesselheim ASUS. Food and drug administration precertification pilot program for digital health software: weighing the benefits and risks. Ann Intern Med. (2018) 168(10):730–2. doi: 10.7326/M17-2715
63. Dinh-Le C, Chuang R, Chokshi S, Mann D. Wearable health technology and electronic health record integration: scoping review and future directions. JMIR Mhealth Uhealth. (2019) 7(9):e12861. doi: 10.2196/12861
64. Services USDoHaH. The HIPAA Privacy Rule. (2022). Available online at: https://www.hhs.gov/hipaa/for-professionals/privacy/index.html (Accessed April 15, 2024).
Keywords: atrial fibrillation, smartwatches, photoplethysmography, single-lead electrocardiography, artificial intelligence, machine learning
Citation: Papalamprakopoulou Z, Stavropoulos D, Moustakidis S, Avgerinos D, Efremidis M and Kampaktsis PN (2024) Artificial intelligence-enabled atrial fibrillation detection using smartwatches: current status and future perspectives. Front. Cardiovasc. Med. 11:1432876. doi: 10.3389/fcvm.2024.1432876
Received: 14 May 2024; Accepted: 2 July 2024;
Published: 15 July 2024.
Edited by:
Patrick Badertscher, University Hospital of Basel, SwitzerlandReviewed by:
Vassilios Vassilikos, Aristotle University of Thessaloniki, Greece© 2024 Papalamprakopoulou, Stavropoulos, Moustakidis, Avgerinos, Efremidis and Kampaktsis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Polydoros N. Kampaktsis, pkampaktsis@yahoo.com
 Zoi Papalamprakopoulou
Zoi Papalamprakopoulou Dimitrios Stavropoulos2
Dimitrios Stavropoulos2 Polydoros N. Kampaktsis
Polydoros N. Kampaktsis