- 1San Raffaele IRCSS, Rome, Italy
- 2Department of Pharmaceutical Sciences, University of Perugia, Perugia, Italy
- 3Department of Medicine and Surgery, University of Perugia, Perugia, Italy
- 4San Raffaele Research Center, Sulmona, L’Aquila, Italy
Despite significant advances in diagnosis and treatment over recent decades, cardiovascular disease (CVD) remains one of the leading causes of morbidity and mortality in Western countries. This persistent burden is partly due to the incomplete understanding of fundamental pathogenic mechanisms, which limits the effectiveness of current therapeutic interventions. In this context, recent evidence highlights the pivotal role of immuno-inflammatory activation by the gut microbiome in influencing cardiovascular disorders, potentially opening new therapeutic avenues. Indeed, while atherosclerosis has been established as a chronic inflammatory disease of the arterial wall, accumulating data suggest that immune system regulation and anti-inflammatory pathways mediated by gut microbiota metabolites play a crucial role in a range of CVDs, including heart failure, pericardial disease, arrhythmias, and cardiomyopathies. Of particular interest is the emerging understanding of how tryptophan metabolism—by both host and microbiota—converges on the Aryl hydrocarbon Receptor (AhR), a key regulator of immune homeostasis. This review seeks to enhance our understanding of the role of the immune system and inflammation in CVD, with a focus on how gut microbiome-derived tryptophan metabolites, such as indoles and their derivatives, contribute to cardioimmunopathology. By exploring these mechanisms, we aim to facilitate the development of novel, microbiome-centered strategies for combating CVD.
1 Introduction
Cardioimmunology is a field of study that focuses on the interactions between the cardiovascular system and the immune system (1–5). It explores how the immune system can impact various cardiovascular diseases, such as atherosclerosis, myocardial infarction, cardiomyopathies, arrhythmias and heart failure. Research in cardioimmunology aims to understand the complex mechanisms by which immune cells and molecules contribute to both the pathogenesis and resolution of cardiovascular diseases (CVD) (6). By uncovering these interactions, scientists hope to develop new strategies for preventing and treating cardiovascular conditions through targeted immunomodulatory therapies (7).
Cardioimmunology, indeed, is a relatively new and rapidly growing field that focuses on the intersection of CVD and the immune system (8). It explores the complex interactions between the immune system and the cardiovascular system, as well as the role of inflammation in the development and progression of various cardiovascular conditions (9, 10). In the last decade, the systemic dimension has emerged as a predictor of CVD, alongside cardiomyopathies and arrhythmias. Immunomodulation in the local microenvironment, metabolism and mitochondria of the cardiac tissue are highly responsive to the environment (10–15). Experiments involving factors such as gut microbial composition (e.g., considering the host-microbiome dyad as a “superorganism”), the circadian clock (16) or hypoxia (17) have been shown to impact tissue function, leading to conditions such as cardiometabolic disorders, myocarditis, arrhythmias along with tissue remodeling notably with occurrence of fibrosis. Similarly, systemic inflammation, immune cells and oxidative stress also contribute to the pathogenesis of hypertension, which is a significant risk factor for CVD, through vascular inflammation and microvascular remodeling (18).
Mapping cell destinies, depleting and regenerating immune cells in experimental models of heart disease, along with analyzing the human heart at a cellular level, significantly enhance our comprehension of the intricate communication between immune and non-immune cells within the heart (19, 20). Although the immediate immune reaction is crucial for triggering inflammation and repairing tissue after damage, prolonged activation leads to harmful effects, influencing negative changes in the heart’s structure and function. Identifying the precise roles of immune cells within the cardiac setting opens up novel avenues for adjusting immune responses to manage inflammation effectively in cases of heart diseases.
2 Key aspects of cardioimmunology
The heart incorporates immune cells as crucial cellular elements that engage in communication with resident cardiac cells in situations of homeostasis, cardiac injury, and remodeling (21, 22). These discoveries play a pivotal role in shaping and broadening the emerging domain of cardioimmunology. In this analysis, we examine the latest literature related to this subject and deliberate on the ongoing and prospective initiatives aimed at propelling this field ahead. Some of the crucial aspects of cardioimmunology are depicted in Figure 1 and listed below.
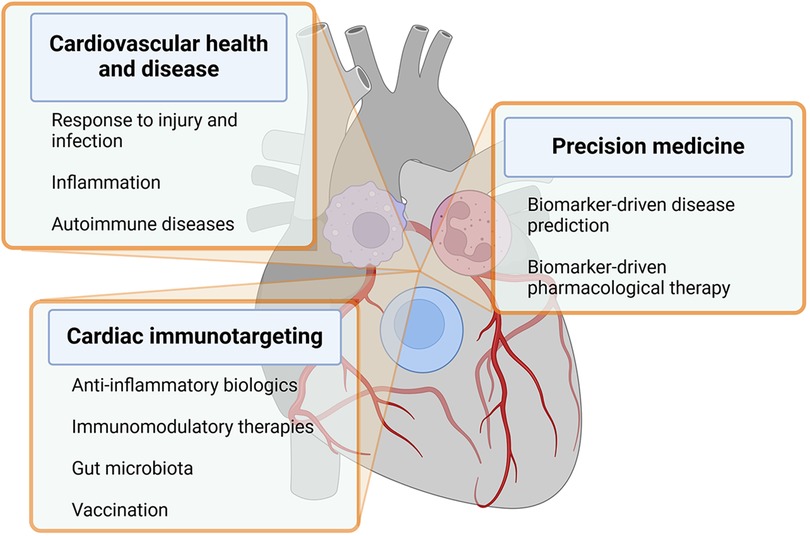
Figure 1. The figure recapitulates key aspects of cardioimmunology within text boxes departing from immune cells overlying a human heart. Details are described in the text. Created with BioRender.com.
(a) Inflammation and cardiovascular disease: Chronic inflammation is now recognized as a key driver of CVD, including atherosclerosis, cardiomyopathies, arrhythmias, heart failure, and myocarditis. Immune cells and inflammatory mediators play a crucial role in the development of these conditions (12, 23).
(b) Immune cells in cardiovascular health and disease: Immune cells such as macrophages, T cells, and neutrophils play important roles in maintaining cardiovascular health and responding to injury or infection in the heart and blood vessels (22).
(c) Immunomodulatory therapies for CVD: Researchers are exploring the potential of targeting the immune system to develop novel therapies for CVD (24). This includes investigating the use of anti-inflammatory agents, immune-modulating drugs, and biologics to reduce inflammation and improve outcomes in patients with heart disease (25–27).
(d) Biomarkers of inflammation in CVD: Biomarkers of inflammation, such as C-reactive protein and IL-6, are used to assess the level of inflammation in patients with CVD. Monitoring these biomarkers can help guide treatment decisions and predict outcomes (28).
(e) Cardiovascular complications of autoimmune diseases: Some autoimmune diseases, such as rheumatoid arthritis and systemic lupus erythematosus, are associated with an increased risk of cardiovascular complications due to chronic inflammation and immune system dysregulation (29).
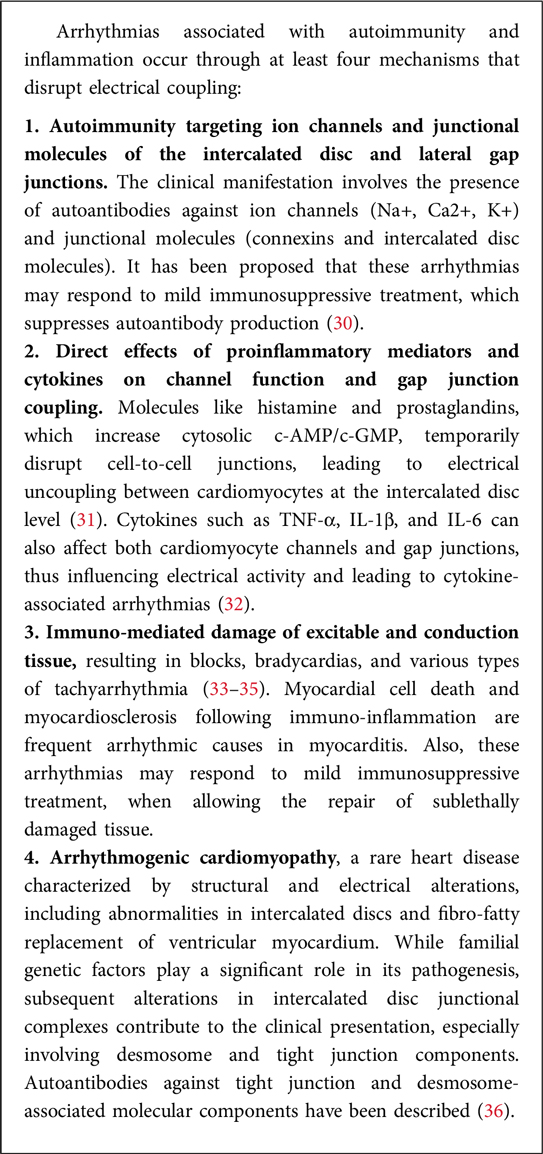
(f) Ongoing directions: Current research in cardioimmunology aims to further elucidate the mechanisms underlying the crosstalk between the immune system and the cardiovascular system, identify new therapeutic targets, and develop personalized approaches to prevent and treat CVD based on immunological profiles (10). Paradigmatic in this context are the mechanistic studies linking autoimmunity and inflammation to the development of arrhythmia (Box 1).
Overall, cardioimmunology represents an exciting and promising area of research that has the potential to improve our understanding of CVD and lead to the development of innovative therapies to combat these conditions. In particular, some emerging areas of particular interest are represented by:
(i) The role of the gut microbiota and products thereof. The gut microbiota plays a pivotal role in educating and modulating the immune system, influencing the balance between pro-inflammatory and anti-inflammatory responses. On the one hand, dysbiosis of the gut microbiota, characterized by alterations in microbial composition and function, has been linked to immune dysregulation and chronic low-grade inflammation, which are key drivers of atherosclerosis, hypertension, and other cardiovascular conditions. Understanding the interconnections between the cardiovascular and the gut microbiota opens avenues for developing novel therapeutic strategies (37, 38). On the other hand, the intricate crosstalk between the gut microbiota and the cardiovascular system underscores the importance of considering the gut as a key player in cardioimmunology (39). By elucidating the mechanisms by which the gut microbiota influences immune responses, inflammation, and metabolism in the context of cardiovascular health, researchers may uncover novel therapeutic targets and strategies for the prevention and management of several pathologic conditions, including CVD (39, 40).
(ii) The potential role of anti-inflammatory biologics in CVD. The gut microbiota produces a myriad of bioactive metabolites and signaling molecules that can impact immune function and cardiovascular health. Short-chain fatty acids (SCFAs), produced through the fermentation of dietary fibers by gut bacteria, have been shown to exert immunomodulatory effects by regulating the differentiation and activity of immune cells, thereby influencing inflammation in the cardiovascular system (41). Beyond SCFAs, the gut microbiota generates a diverse array of metabolites, including trimethylamine N-oxide (TMAO), lipopolysaccharides (LPS), and bile acids (BAs), which have been implicated in the pathogenesis of CVD (42). On the one hand, the exploration of anti-inflammatory biologics in cardiovascular diseases, particularly anti-inflammatory and immunomodulatory indole derivatives, represents an exciting avenue for research and therapeutic development (43). While there is evidence suggesting their potential benefits, ongoing studies and further clinical trials are necessary to establish their role in specific patient populations and to refine treatment strategies. On the other hand, chronic inflammation is a hallmark of many cardiovascular diseases, and the gut microbiota has been implicated in the regulation of inflammatory pathways that contribute to vascular dysfunction and atherogenesis. Through the activation of toll-like receptors and nucleotide-binding oligomerization domain-like receptors, gut-derived microbial products can trigger inflammatory cascades that promote the development of cardiovascular pathology. Some biologics that target inflammatory pathways, such as TNF-α inhibitors or IL-1 inhibitors (26, 27, 44), may have indirect heart-protective effects by reducing systemic inflammation, which is a risk factor for cardiovascular disease (45). Overall, targeting the gut microbiota represents a promising avenue for the development of novel therapeutic strategies in cardioimmunology. Approaches such as probiotics, prebiotics, postbiotics, and fecal microbiota transplantation offer the potential to modulate the gut microbiota composition and activity, thereby influencing immune responses and mitigating cardiovascular risk factors (46–48).
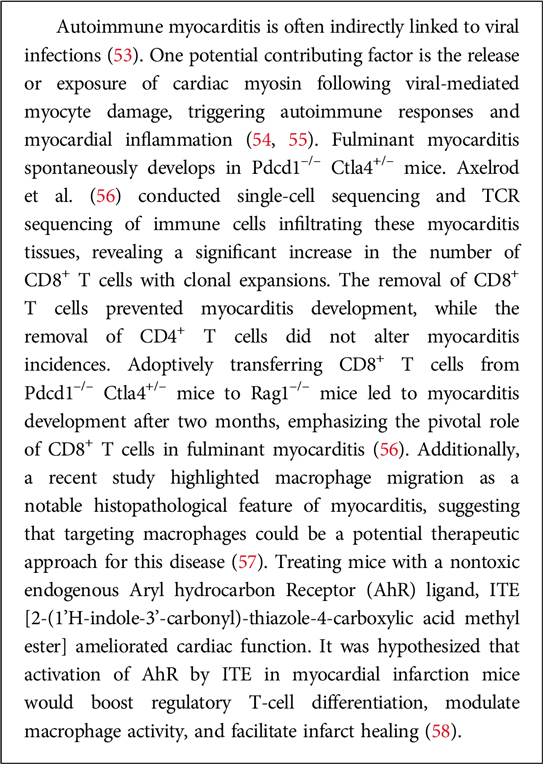
(iii) Vaccination and cardiovascular health. Some research in cardioimmunology explores the impact of vaccinations on cardiovascular health. For instance, vaccines targeting infectious agents may not only prevent infections but also impact the risk of cardiovascular events associated with inflammation. In particular, vaccination plays a crucial role in maintaining overall health, including cardiovascular health. While vaccines primarily target specific infections, their impact can extend beyond preventing the targeted diseases. Here are some aspects of vaccination and cardiovascular health (49). A prototypic example is represented by influenza vaccine: Influenza infections can lead to respiratory complications, and severe cases may have cardiovascular implications. By preventing the flu, the vaccine helps reduce the risk of influenza-related cardiovascular events. Not secondarily, COVID-19, caused by the SARS-CoV-2 virus, has been associated with various cardiovascular complications. These include myocarditis, pericarditis, and an increased risk of blood clot formation. COVID-19 vaccination has been shown to be effective in preventing severe illness and complications, including those related to the cardiovascular system (50). Nevertheless, the very vaccine, owing to its formulation (i.e., mRNA in lipid nanoparticles) can exert direct effects on the cardiovascular system (51, 52). Relevant in this regard are experimental studies on autoimmune myocarditis. Box 2 provides some information in this regard.
3 Human and gut microbiota synergy on tryptophan utilization
Both humans and gut microbiota feed on dietary Trp for proteogenesis and other functions that rely of the degradation of this essential amino acid. The phylogenesis of Trp catabolism across different organisms provides insights into the evolutionary advantages and diverse functions of this pathway (59). Trp catabolism is a highly conserved process that has evolved over time, and its existence across various organisms suggests its importance in adaptation and survival (60–62). Here are some key points regarding the phylogenesis of Trp catabolism: (a) Universal presence: Trp catabolism is found in a wide range of organisms, including bacteria, fungi, plants, and animals. This universality indicates that the pathway has been evolutionarily conserved and is fundamental to the biology of diverse life forms (60); (b) Metabolic regulation: The regulation of Trp catabolism has likely evolved as a mechanism for organisms to adapt to changing environmental conditions, nutrient availability, and immune responses (63). In many cases, the regulation of Trp catabolism is responsive to external stimuli, such as stress, infection, or inflammation, highlighting its role in the adaptive response to various challenges; (c) Host-pathogen dynamics: The evolutionary arms race between hosts and pathogens has likely shaped the development and diversification of Trp catabolism. Hosts may have evolved this pathway as a defense mechanism to limit nutrient availability for pathogens, while some pathogens have developed strategies to exploit or manipulate Trp metabolism for their benefit (64); (d) Immunomodulation and tolerance: The immunomodulatory effects of Trp catabolism, including the generation of metabolites with immunosuppressive properties, suggest that this pathway has evolved to play a role in regulating the immune response (65). Tolerance induction, mediated by Trp catabolism, could have provided an evolutionary advantage by preventing excessive inflammation and immunopathology, contributing to the host’s ability to coexist with commensal microorganisms; (e) Nutrient sensing and energy metabolism: Trp catabolism is not only involved in immune responses but is also linked to broader metabolic processes. The breakdown of Trp can provide precursors for the synthesis of other molecules, and its regulation may be tied to overall energy metabolism.
Therefore, as to the question of why an organism would want to destroy an essential amino acid like Trp, it is important to note that the goal is not necessarily the destruction of Trp but rather the modulation of its levels (63, 66–69). The evolutionary advantage lies in the ability to dynamically regulate Trp availability in response to specific environmental and physiological cues. By controlling Trp levels, organisms can influence their own metabolism, respond to stressors, and shape the host-microbe interaction in ways that promote survival and adaptation. It is interesting to note that bacteria that eat worms use NAD as a ‘food signal’ to open their mouths but, if NAD is unavailable, they stop reproducing and enter a developmental and reproductive arrest phase, mediated by serotonin, to survive (60, 70). In the context of host defense, limiting the availability of essential nutrients like Trp can be a strategic means of thwarting the growth and proliferation of pathogens (Figure 2).
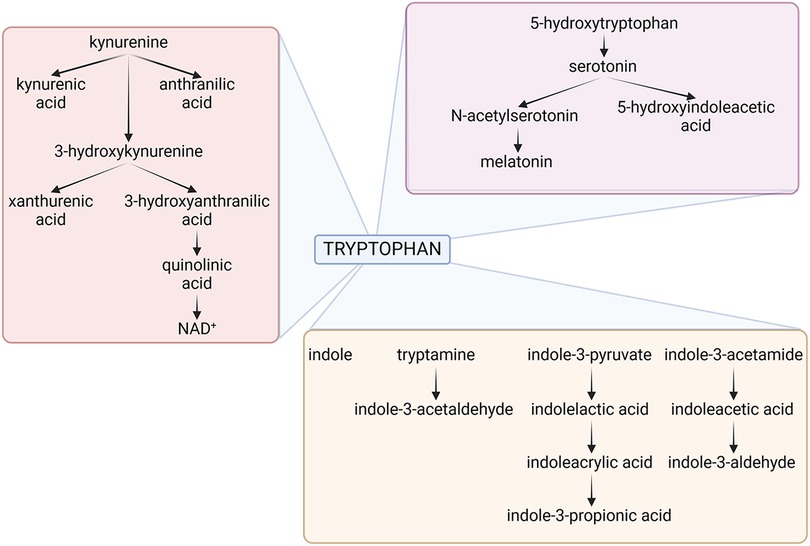
Figure 2. The figure depicts the three main tryptophan metabolic pathways of host and microbial origin, namely the kynurenine (pink box), serotonin (violet box) and indole (brown box) pathways. Details are described in the text. NAD, Nicotinamide Adenine Dinucleotide. Created with BioRender.com.
Much like their hosts, bacteria can metabolize Trp along all three pathways, influencing the so-called “brain-gut-microbiota axis”. Bacteroides spp., Clostridium spp., Escherichia coli, Lactobacillus spp., Bifidobacterium spp., Akkermansia muciniphila, Faecalibacterium prausnitzii, Prevotella spp., Ruminococcus spp., all degrade Trp in the gut via the kynurenine pathway (71). There is some evidence that several of the same species may likewise induce serotonin in the gut. Escherichia coli, Clostridium Spp., Bacteroides spp., Akkermansia muciniphila, Clostridioides difficile, Enterococcus spp., Proteus spp., Citrobacter spp., and Klebsiella spp. will instead produce a preponderance of indole or derivatives thereof (72). It follows that—if host and gut bacteria—both metabolize Trp there must be competition for the substrate, yet flexible balance for local and systemic homeostasis (69).
4 Tryptophan and the heart
Gut microbiota and microbiota-derived metabolites have been increasingly recognized for their potential impact on CVD, including hypertension, heart failure, myocardial infarction, arrhythmia, atherosclerosis, and myocarditis. Evidence from recent studies has shown that gut microbiota contributes to the development of myocarditis, an inflammatory disease that can result in myocardial damage and the emerging field of cardioimmunology (37, 73, 74). The metabolites produced by gut microbiota can affect the immune system and have an impact on cardiovascular health (10, 40). This is in line with the long-established notion that gut microbiota-dependent metabolites serve as a link connecting the dynamic balance between the host and the gut microbiota (75). In addition to microbial-derived SCFAs, BAs and TMAO, a number of host and microbial metabolites derived from the essential amino acid tryptophan (Trp) degradation has been implicated in cardiovascular diseases (10, 76).
The kynurenine pathway is a metabolic pathway that plays a crucial role in Trp degradation (77–82). This pathway is primarily associated with immune regulation and has been implicated in various physiological and pathological processes, including immune homeostasis and cardiovascular integrity (83). The pathway’s contributions to immune homeostasis include (a) Regulation of T-cell responses: The kynurenine pathway is a key player in the modulation of immune responses. One of its main functions is the regulation of T cell activity. Specifically, the production of kynurenine metabolites can influence T cell differentiation and function (84); (b) Immunosuppressive effects: Certain metabolites of the kynurenine pathway, such as kynurenine itself, have immunosuppressive properties. They can inhibit the proliferation of T cells and promote the generation of regulatory T cells, which are crucial for maintaining immune tolerance and preventing autoimmune reactions (82). As regards the cardiovascular system, additional functions are: (c) Regulation of vascular function: Components of the kynurenine pathway, including kynurenine and its derivatives, have been implicated in vascular function by affecting, for instance, endothelial function and blood vessel integrity (85); (d) Control of inflammation and atherosclerosis. The immunomodulatory effects of the kynurenine pathway may influence the inflammatory processes associated with cardiovascular conditions (86–88). As a matter of fact, dysregulation of the kynurenine pathway has been implicated in cardiovascular diseases in which the kynurenine and the [Kyn]/[Trp]-ratio were associated with an increased risk of developing cardiovascular disease (10, 76) while higher Trp levels were associated with a tendency toward lower incident risk of mortality (76).
The serotonin pathway also plays an important role in the degradation of Trp with effects that go beyond its role as neurotransmitter in the central nervous system to include, among others, the regulation the immune and cardiovascular functions. In the periphery, serotonin is a major regulator of vasoreactivity, by directly inducing vasoconstriction in large arteries and veins and exerting a vasodilatory effect in arterioles via nitric oxide release and vascular smooth muscle relaxation (89). Aggregating platelets release serotonin that may contribute to the etiology of spasm in cerebral, digital and coronary vessels, and in the maintenance of the elevated peripheral resistance in arterial hypertension (90). Increased concentrations of serotonin were indeed associated with an increased risk of cardiovascular damage and disease (76, 91).
Finally, the impact of indoles, produced by bacterial degradation of Trp, has gained attention for their dual influence at the pathogen-host interface (84, 92–94). In cardiology, metabolites in the indole pathway did not show consistent associations with cardiovascular outcomes, although higher concentrations of some end products of the indole pathway have been reported to be associated with a lower risk of atherosclerosis (76, 95). For instance, the indole-3-aldehyde (3-IAld) metabolite was found to be lower, different from others, in patients with myocardial infarction (96). The section below delves into the manifold influence of indole metabolites on the host metabolism and immunity, specifically examining their systemic effects from the standpoint of cardioimmunology.
4.1 Indoles and systemic immunity
Indole is a heterocyclic organic compound with a bicyclic structure consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. It is found in various natural products such as the amino acid tryptophan and the hormone serotonin. Indole derivatives have been studied in relation to their effects on the cardiovascular system (Box 3).
4.2 Indole and the aryl hydrocarbon receptor: the case of indole-3-aldehyde, 3-IAld
The Aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor that plays a crucial role in regulating various physiological processes, including immune responses, xenobiotic metabolism, and maintenance of cellular homeostasis (106–108). AhR is a member of the basic helix-loop-helix/Per-ARNT-Sim (bHLH-PAS) protein family and is primarily localized in the cytoplasm in its inactive state. AhR activation occurs when it binds to specific ligands, leading to its translocation into the nucleus, where it forms a complex with its partner, the AhR nuclear translocator. This complex then binds to specific DNA sequences known as xenobiotic response elements in the promoter regions of target genes, thereby regulating their expression.
The gut microbiota has been implicated in the production of AhR ligands (106–110). These ligands are often derived from dietary components and microbial metabolism. Indole derivatives are prominent examples of AhR ligands produced by gut microbiota through the breakdown of dietary tryptophan. Additionally, certain metabolites of tryptophan, such as kynurenine and kynurenic acid, can also activate AhR. The activation of AhR by these microbial-derived ligands has been associated with various immunomodulatory effects. AhR activation in immune cells can regulate the balance between pro-inflammatory and anti-inflammatory responses, impacting the development and function of immune cells. Furthermore, AhR activation in the gut has been linked to the maintenance of intestinal barrier integrity and the regulation of mucosal immune responses (111–113). The interaction between the gut microbiota and AhR highlights the intricate relationship between the microbiome, dietary factors, and host physiology, emphasizing the importance of these interactions in shaping immune and metabolic functions (114). As mentioned above, degradation of Trp by bacteria or enterocytes generates several AhR-binding molecules, mainly serotonin, tryptamine and indoles, including: indole, indole-3-propionic acid (IPA), indole-3-acetic acid (IAA), indole-3-aldehyde (3-IAld), indole-3-acetaldehyde (IAAld), indole-3-lactic acid (ILA), indole acrylic acid and others, including tryptamine and skatole (115). Thus, the co-evolution of microbial communities with mammalian hosts has led to interconnected metabolic pathways that intricately influence both physiological and pathological processes. Trp derivatives, arising from both host and microbial sources, exemplify this metabolic complexity. Among these derivatives, 3-IAld stands out as a metabolite produced by the gut microbiota (92, 116–119).
Initially recognized for its role as an agonist of the AhR, particularly in promoting epithelial barrier functions, this compound has now been implicated in a myriad of activities across various pathological conditions. Along this direction, our group has been involved for some time in turning microbial AhR agonists into therapeutic agents via drug delivery systems (116, 118). By deciphering how signaling molecules, such as 3-IAld, interact with AhR may pave the way for novel therapeutics in inflammatory human diseases, for the realization of which drug delivery platforms are instrumental. So far, three synthetic AhR agonists—laquinimod, tranilast and benvitimod—have been investigated in phase I–III clinical trials. The trials involved patients with autoimmune conditions, such as Crohn’s disease, rheumatoid arthritis, asthma, atopic dermatitis or multiple sclerosis (120). However, to our knowledge, none of them was tested in patients with cardiovascular diseases. A 3-IAld dry powder for inhalation was formulated and assessed for its effectiveness in comparison to oral and intranasal delivery methods in experimental lung inflammation (44). The resulting inhalable dry powder demonstrated: (i) suitability for pulmonary administration, (ii) favorable toxicological safety, and (iii) superior efficacy over alternative administration routes (oral and intranasal) in reducing inflammatory and disease scores. This research advocates for the utilization of 3-IAld inhalable dry powders as a promising and innovative therapeutic approach for targeting inflammation in pulmonary diseases and, likely, cardiopulmonary conditions (121, 122).
In conclusion, AhR plays a crucial role not only in detoxification but also in various physiological processes, particularly in maintaining vascular homeostasis. Despite its high expression in the endothelium, there is a lack of comprehensive understanding of AhR’s function in this context. There is a definite need for consolidating existing knowledge regarding AhR’s involvement in the endothelium and its implications for cardiovascular health (122). Whatever, modulating AhR signaling emerges as a potential therapeutic target for addressing vascular disorders whereby systemic low-grade inflammation reduces circulating endothelial progenitor cells (123). In this regard, it is of interest that while indoxyl sulfate promoted vascular inflammation, IPA and 3-IAld had protective effects (117).
5 Future perspectives and conclusions
Accumulated evidence substantiates the notion that a triad comprising microbiota, Trp, and AhR is a targeted axis in several cardiovascular conditions (Figure 3).
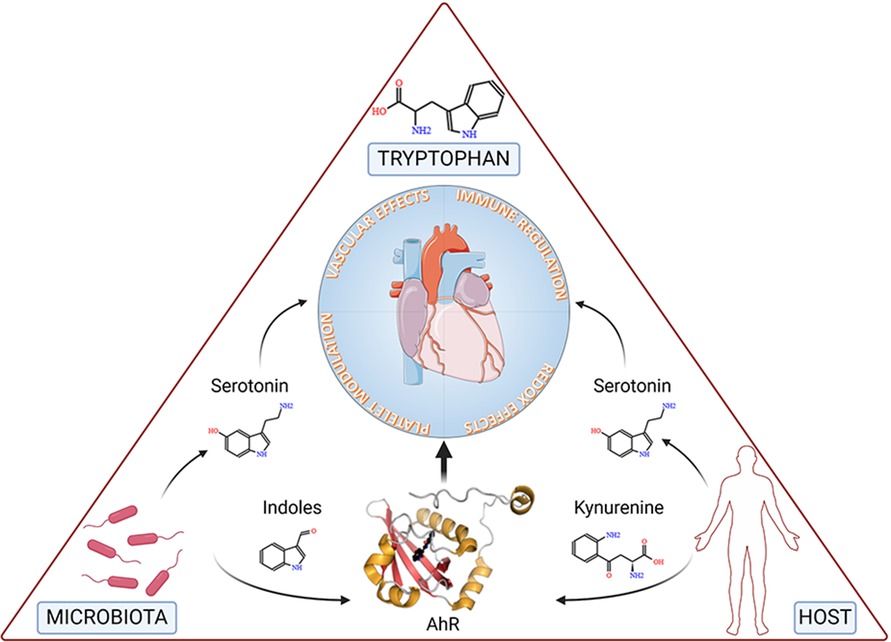
Figure 3. The figure depicts tryptophan, the microbiota and the host at the vertices of a triangle representing the triad involved in cardiovascular health and disease. Specifically, microbial- and host-dependent degradation of tryptophan results in the production of metabolites, including serotonin, indoles and kynurenine, the latter two working as ligands of the Aryl Hydrocarbon Receptor (AhR), ultimately affecting key functions of the cardiovascular system. Details are described in the text. Created with BioRender.com.
Presently, most treatments primarily address symptoms, focusing on enhancing compromised functionality. However, therapeutic options capable of influencing cellular degeneration or pathology remain elusive. Should a significant role of abnormal intestinal flora composition in specific phenotypes be confirmed, a promising therapeutic avenue could emerge by optimizing pharmacological therapy of CVD with Trp supplementation and/or medications altering the Trp metabolic pathways. Nevertheless, variations among individuals, coupled with the impact of comorbidities, dietary patterns, medications, infections, and lifestyle, can alter gut microbiota composition. Hence, future research demands meticulous investigation using a rigorous experimental framework, taking these factors into account (124). Indeed, despite the potential promise of precisely defined and tailored diets in influencing the microbiome and inflammation, their practical applicability in the general population remains questionable as it is the potential long-lasting benefits of fecal microbiota transplantation in CVD (125).
Additionally, the potential value of the measurement of Trp metabolites as screening and diagnostic tools for a broader population, indicate that mechanistic studies are required to understand the role of Trp metabolism in CVD with the goal to identify new diagnostic and therapeutic options.
Lastly, delving into research on microbiota, Trp catabolic pathways and AhR signaling has uncovered insights into the mechanisms of intestinal distress (126, 127). Despite being underappreciated, a role for the intestinal barrier dysfunction in CVD has become evident in light of its occurrence in hypertension, coronary artery disease, atherosclerosis, heart failure, and myocardial infarction (128). Thus, testing indole derivatives as intestinal barrier-targeted compounds may lead to their potential new class of CVD therapeutics.
Author contributions
MR: Conceptualization, Writing – review & editing. MP: Writing – review & editing. CC: Writing – review & editing. SG: Writing – review & editing. MR: Writing – review & editing. EG: Conceptualization, Writing – review & editing. LR: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by MicroTher (ERC-2018-PoC-813099 to LR).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gergely TG, Drobni ZD, Kallikourdis M, Zhu H, Meijers WC, Neilan TG, et al. Immune checkpoints in cardiac physiology and pathology: therapeutic targets for heart failure. Nat Rev Cardiol. (2024) 21(7):443–62. doi: 10.1038/s41569-023-00986-9
2. Thackeray JT, Lavine KJ, Liu Y. Imaging inflammation past, present, and future: focus on cardioimmunology. J Nucl Med. (2023) 64:39s–48. doi: 10.2967/jnumed.122.264865
3. Thorp EB. Cardiac macrophages and emerging roles for their metabolism after myocardial infarction. J Clin Invest. (2023) 133:e171953. doi: 10.1172/JCI171953
4. Saleh D, Jones RTL, Schroth SL, Thorp EB, Feinstein MJ. Emerging roles for dendritic cells in heart failure. Biomolecules. (2023) 13:1535. doi: 10.3390/biom13101535
5. Zambrano MA, Alcaide P. Immune cells in cardiac injury repair and remodeling. Curr Cardiol Rep. (2023) 25:315–23. doi: 10.1007/s11886-023-01854-1
6. Wong A, Hamidzada H, Epelman S. A cardioimmunologist’s toolkit: genetic tools to dissect immune cells in cardiac disease. Nat Rev Cardiol. (2022) 19:395–413. doi: 10.1038/s41569-022-00701-0
7. Galajda N, Meznerics FA, Mátrai P, Fehérvári P, Lengyel AS, Kolonics MV, et al. Reducing cardiovascular risk in immune-mediated inflammatory diseases: tumour necrosis factor inhibitors compared to conventional therapies-A systematic review and meta-analysis. J Eur Acad Dermatol Venereol. (2024) 38(6):1070–88. doi: 10.1111/jdv.19900
8. Swirski FK, Nahrendorf M. Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat Rev Immunol. (2018) 18:733–44. doi: 10.1038/s41577-018-0065-8
9. Lazzerini PE, Hamilton RM, Boutjdir M. Editorial: cardioimmunology: inflammation and immunity in cardiovascular disease. Front Cardiovasc Med. (2019) 6:181. doi: 10.3389/fcvm.2019.00181
10. Russo MA, Garaci E, Frustaci A, Fini M, Costantini C, Oikonomou V, et al. Host-microbe tryptophan partitioning in cardiovascular diseases. Pharmacol Res. (2023) 198:106994. doi: 10.1016/j.phrs.2023.106994
11. Suffee N, Le Goff W, Chen J. Editorial: cardiometabolic diseases and inflammatory responses. Front Immunol. (2024) 15:1384022. doi: 10.3389/fimmu.2024.1384022
12. Henein MY, Vancheri S, Longo G, Vancheri F. The role of inflammation in cardiovascular disease. Int J Mol Sci. (2022) 23:12906. doi: 10.3390/ijms232112906
13. Fredman G, Serhan CN. Specialized pro-resolving mediators in vascular inflammation and atherosclerotic cardiovascular disease. Nat Rev Cardiol. (2024) doi: 10.1038/s41569-023-00984-x
14. Toldo S, Abbate A. The role of the NLRP3 inflammasome and pyroptosis in cardiovascular diseases. Nat Rev Cardiol. (2024) 21:219–37. doi: 10.1038/s41569-023-00946-3
15. Riksen NP, Bekkering S, Mulder WJM, Netea MG. Trained immunity in atherosclerotic cardiovascular disease. Nat Rev Cardiol. (2023) 20:799–811. doi: 10.1038/s41569-023-00894-y
16. Crnko S, Du Pre BC, Sluijter JPG, Van Laake LW. Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat Rev Cardiol. (2019) 16:437–47. doi: 10.1038/s41569-019-0167-4
17. Bilo G, Gatterer H, Torlasco C, Villafuerte FC, Parati G. Editorial: hypoxia in cardiovascular disease. Front Cardiovasc Med. (2022) 9:990013. doi: 10.3389/fcvm.2022.990013
18. Guzik TJ, Nosalski R, Maffia P, Drummond GR. Immune and inflammatory mechanisms in hypertension. Nat Rev Cardiol. (2024) 21(6):396–416. doi: 10.1038/s41569-023-00964-1
19. Simões FC, Riley PR. Immune cells in cardiac repair and regeneration. Development. (2022) 149:dev199906. doi: 10.1242/dev.199906
20. Su X, Wang L, Ma N, Yang X, Liu C, Yang F, et al. Immune heterogeneity in cardiovascular diseases from a single-cell perspective. Front Cardiovasc Med. (2023) 10:1057870. doi: 10.3389/fcvm.2023.1057870
21. Kologrivova I, Shtatolkina M, Suslova T, Ryabov V. Cells of the immune system in cardiac remodeling: main players in resolution of inflammation and repair after myocardial infarction. Front Immunol. (2021) 12:664457. doi: 10.3389/fimmu.2021.664457
22. Steffens S, Nahrendorf M, Madonna R. Immune cells in cardiac homeostasis and disease: emerging insights from novel technologies. Eur Heart J. (2022) 43:1533–41. doi: 10.1093/eurheartj/ehab842
23. Attiq A, Afzal S, Ahmad W, Kandeel M. Hegemony of inflammation in atherosclerosis and coronary artery disease. Eur J Pharmacol. (2024) 966:176338. doi: 10.1016/j.ejphar.2024.176338
24. Jyotsna F, Ikram J, Nageeta F, Komal F, Anjlee F, Patel H, et al. Unlocking the potential of immunotherapy in cardiovascular disease: a comprehensive review of applications and future directions. Cureus. (2023) 15:e42790. doi: 10.7759/cureus.42790
25. Booz GW, Altara R, Zouein FA. Editorial: immunomodulatory approaches in cardiovascular diseases. Front Cardiovasc Med. (2022) 9:873452. doi: 10.3389/fcvm.2022.873452
26. Ridker PM, Everett BM, Thuren T, Macfadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. (2017) 377:1119–31. doi: 10.1056/NEJMoa1707914
27. Thompson PL, Nidorf SM. Anti-inflammatory therapy with canakinumab for atherosclerotic disease: lessons from the cantos trial. J Thorac Dis. (2018) 10:695–8. doi: 10.21037/jtd.2018.01.119
28. Liu Y, Guan S, Xu H, Zhang N, Huang M, Liu Z. Inflammation biomarkers are associated with the incidence of cardiovascular disease: a meta-analysis. Front Cardiovasc Med. (2023) 10:1175174. doi: 10.3389/fcvm.2023.1175174
29. Mesa A, Giménez M, Perea V, Serés-Noriega T, Boswell L, Blanco J, et al. Severe hypoglycemia and hypoglycemia awareness are associated with preclinical atherosclerosis in patients with type 1 diabetes without an estimated high cardiovascular risk. Diabetes Metab Res Rev. (2024) 40:e3785. doi: 10.1002/dmrr.3785
30. Frustaci A, Chimenti C, Pieroni M, Salvatori L, Morgante E, Sale P, et al. Cell death, proliferation and repair in human myocarditis responding to immunosuppressive therapy. Mod Pathol. (2006) 19:755–65. doi: 10.1038/modpathol.3800594
31. Frustaci A, Caldarulo M, Di Rienzo V, Russo MA, Gentiloni N. Antiarrhythmic effect of H-2 antihistamines. Chest. (1991) 99:262–3. doi: 10.1378/chest.99.1.262
32. Lazzerini PE, Capecchi PL, Laghi-Pasini F, Boutjdir M. Autoimmune channelopathies as a novel mechanism in cardiac arrhythmias. Nat Rev Cardiol. (2017) 14:521–35. doi: 10.1038/nrcardio.2017.61
33. Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. (1997) 96:1180–4. doi: 10.1161/01.CIR.96.4.1180
34. Frustaci A, Priori SG, Pieroni M, Chimenti C, Napolitano C, Rivolta I, et al. Cardiac histological substrate in patients with clinical phenotype of brugada syndrome. Circulation. (2005) 112:3680–7. doi: 10.1161/CIRCULATIONAHA.105.520999
35. Chimenti C, Russo MA, Carpi A, Frustaci A. Histological substrate of human atrial fibrillation. Biomed Pharmacother. (2010) 64:177–83. doi: 10.1016/j.biopha.2009.09.017
36. Austin KM, Trembley MA, Chandler SF, Sanders SP, Saffitz JE, Abrams DJ, et al. Molecular mechanisms of arrhythmogenic cardiomyopathy. Nat Rev Cardiol. (2019) 16:519–37. doi: 10.1038/s41569-019-0200-7
37. Rahman MM, Islam F, Or-Rashid MH, Mamun AA, Rahaman MS, Islam MM, et al. The gut microbiota (microbiome) in cardiovascular disease and its therapeutic regulation. Front Cell Infect Microbiol. (2022) 12:903570. doi: 10.3389/fcimb.2022.903570
38. Rashid S, Sado AI, Afzal MS, Ahmed A, Almaalouli B, Waheed T, et al. Role of gut microbiota in cardiovascular diseases—a comprehensive review. Ann Med Surg (Lond). (2024) 86:1483–9. doi: 10.1097/MS9.0000000000001419
39. Nesci A, Carnuccio C, Ruggieri V, D'alessandro A, Di Giorgio A, Santoro L, et al. Gut Microbiota and cardiovascular disease: evidence on the metabolic and inflammatory background of a Complex relationship. Int J Mol Sci. (2023) 24:9087. doi: 10.3390/ijms24109087
40. Shi B, Li H, He X. Advancing lifelong precision medicine for cardiovascular diseases through gut microbiota modulation. Gut Microbes. (2024) 16:2323237. doi: 10.1080/19490976.2024.2323237
41. Lu Y, Zhang Y, Zhao X, Shang C, Xiang M, Li L, et al. Microbiota-derived short-chain fatty acids: implications for cardiovascular and metabolic disease. Front Cardiovasc Med. (2022) 9:900381. doi: 10.3389/fcvm.2022.900381
42. Kirk D, Costeira R, Visconti A, Khan Mirzaei M, Deng L, Valdes AM, et al. Bacteriophages, gut bacteria, and microbial pathways interplay in cardiometabolic health. Cell Rep. (2024) 43:113728. doi: 10.1016/j.celrep.2024.113728
43. Fragoulis GE, Soulaidopoulos S, Sfikakis PP, Dimitroulas T, D Kitas G. Effect of biologics on cardiovascular inflammation: mechanistic insights and risk reduction. J Inflamm Res. (2021) 14:1915–31. doi: 10.2147/JIR.S282691
44. Puccetti M, Pariano M, Stincardini C, Wojtylo P, Schoubben A, Nunzi E, et al. Pulmonary drug delivery technology enables anakinra repurposing in cystic fibrosis. J Control Release. (2023) 353:1023–36. doi: 10.1016/j.jconrel.2022.11.043
45. Abbate A, Toldo S, Marchetti C, Kron J, Van Tassell BW, Dinarello CA. Interleukin-1 and the inflammasome as therapeutic targets in cardiovascular disease. Circ Res. (2020) 126:1260–80. doi: 10.1161/CIRCRESAHA.120.315937
46. Ji J, Jin W, Liu SJ, Jiao Z, Li X. Probiotics, prebiotics, and postbiotics in health and disease. MedComm. (2023) 4:e420. doi: 10.1002/mco2.420
47. Ciernikova S, Sevcikova A, Drgona L, Mego M. Modulating the gut microbiota by probiotics, prebiotics, postbiotics, and fecal microbiota transplantation: an emerging trend in cancer patient care. Biochim Biophys Acta Rev Cancer. (2023) 1878:188990. doi: 10.1016/j.bbcan.2023.188990
48. Wang W, Fan J, Zhang C, Huang Y, Chen Y, Fu S, et al. Targeted modulation of gut and intra-tumor microbiota to improve the quality of immune checkpoint inhibitor responses. Microbiol Res. (2024) 282:127668. doi: 10.1016/j.micres.2024.127668
49. Yedlapati SH, Mendu A, Tummala VR, Maganti SS, Nasir K, Khan SU. Vaccines and cardiovascular outcomes: lessons learned from influenza epidemics. Eur Heart J Suppl. (2023) 25:A17–24. doi: 10.1093/eurheartjsupp/suac110
50. Pepera G, Tribali MS, Batalik L, Petrov I, Papathanasiou J. Epidemiology, risk factors and prognosis of cardiovascular disease in the coronavirus disease 2019 (COVID-19) pandemic era: a systematic review. Rev Cardiovasc Med. (2022) 23:28. doi: 10.31083/j.rcm2301028
51. Centers for Disease, C. & Prevention. Myocarditis and Pericarditis After mRNA Covid-19 Vaccination. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/myocarditis.html (accessed October 10, 2023).
52. Yonker LM, Swank Z, Bartsch YC, Burns MD, Kane A, Boribong BP, et al. Circulating spike protein detected in post-COVID-19 mRNA vaccine myocarditis. Circulation. (2023) 147:867–76. doi: 10.1161/CIRCULATIONAHA.122.061025
53. Stephenson E, Savvatis K, Mohiddin SA, Marelli-Berg FM. T-cell immunity in myocardial inflammation: pathogenic role and therapeutic manipulation. Br J Pharmacol. (2017) 174:3914–25. doi: 10.1111/bph.13613
54. Neu N, Rose NR, Beisel KW, Herskowitz A, Gurri-Glass G, Craig SW. Cardiac myosin induces myocarditis in genetically predisposed mice. J Immunol. (1987) 139:3630–6. doi: 10.4049/jimmunol.139.11.3630
55. Lazzerini PE, Capecchi PL, El-Sherif N, Laghi-Pasini F, Boutjdir M. Emerging arrhythmic risk of autoimmune and inflammatory cardiac channelopathies. J Am Heart Assoc. (2018) 7:e010595. doi: 10.1161/JAHA.118.010595
56. Axelrod ML, Meijers WC, Screever EM, Qin J, Carroll MG, Sun X, et al. T cells specific for α-myosin drive immunotherapy-related myocarditis. Nature. (2022) 611:818–26. doi: 10.1038/s41586-022-05432-3
57. Toita R, Kawano T, Murata M, Kang J-H. Bioinspired macrophage-targeted anti-inflammatory nanomedicine: a therapeutic option for the treatment of myocarditis. Mater Sci Eng C. (2021) 131:112492. doi: 10.1016/j.msec.2021.112492
58. Seong E, Lee JH, Lim S, Park EH, Kim E, Kim CW, et al. Activation of aryl hydrocarbon receptor by ite improves cardiac function in mice after myocardial infarction. J Am Heart Assoc. (2021) 10:e020502. doi: 10.1161/JAHA.120.020502
59. Grohmann U, Puccetti P. The coevolution of IDO1 and AhR in the emergence of regulatory T-cells in mammals. Front Immunol. (2015) 6:58. doi: 10.3389/fimmu.2015.00058
60. Grohmann U, Mondanelli G, Belladonna ML, Orabona C, Pallotta MT, Iacono A, et al. Amino-acid sensing and degrading pathways in immune regulation. Cytokine Growth Factor Rev. (2017) 35:37–45. doi: 10.1016/j.cytogfr.2017.05.004
61. Gargaro M, Scalisi G, Manni G, Briseño CG, Bagadia P, Durai V, et al. Indoleamine 2,3-dioxygenase 1 activation in mature cDC1 promotes tolerogenic education of inflammatory cDC2 via metabolic communication. Immunity. (2022) 55:1032–1050.e14. doi: 10.1016/j.immuni.2022.05.013
62. Iacono A, Pompa A, De Marchis F, Panfili E, Greco FA, Coletti A, et al. Class IA PI3Ks regulate subcellular and functional dynamics of IDO1. Embo Rep. (2020) 21:e49756. doi: 10.15252/embr.201949756
63. Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. (2013) 34:137–43. doi: 10.1016/j.it.2012.10.001
64. Castro-Portuguez R, Sutphin GL. Kynurenine pathway, NAD(+) synthesis, and mitochondrial function: targeting tryptophan metabolism to promote longevity and healthspan. Exp Gerontol. (2020) 132:110841. doi: 10.1016/j.exger.2020.110841
65. Mellor AL, Munn DH. Tryptophan catabolism prevents maternal T cells from activating lethal anti-fetal immune responses. J Reprod Immunol. (2001) 52:5–13. doi: 10.1016/S0165-0378(01)00118-8
66. Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, Bianchi R, et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol. (2011) 12:870–8. doi: 10.1038/ni.2077
68. Bello C, Heinisch PP, Mihalj M, Carrel T, Luedi MM. Indoleamine-2,3-dioxygenase as a perioperative marker of the immune system. Front Physiol. (2021) 12:766511. doi: 10.3389/fphys.2021.766511
69. Klaessens S, Stroobant V, De Plaen E, Van Den Eynde BJ. Systemic tryptophan homeostasis. Front Mol Biosci. (2022) 9:897929. doi: 10.3389/fmolb.2022.897929
70. Mylenko M, Boland S, Penkov S, Sampaio JL, Lombardot B, Vorkel D, et al. NAD+ is a food component that promotes exit from dauer diapause in caenorhabditis elegans. PLoS One. (2016) 11:e0167208. doi: 10.1371/journal.pone.0167208
71. Iwaniak P, Owe-Larsson M, Urbańska EM. Microbiota, tryptophan and aryl hydrocarbon receptors as the target triad in Parkinson’s disease—a narrative review. Int J Mol Sci. (2024) 25:2915. doi: 10.3390/ijms25052915
72. Ye X, Li H, Anjum K, Zhong X, Miao S, Zheng G, et al. Dual role of indoles derived from intestinal microbiota on human health. Front Immunol. (2022) 13:903526. doi: 10.3389/fimmu.2022.903526
73. Sanchez-Gimenez R, Ahmed-Khodja W, Molina Y, Peiró OM, Bonet G, Carrasquer A, et al. Gut microbiota-derived metabolites and cardiovascular disease risk: a systematic review of prospective cohort studies. Nutrients. (2022) 14:2654. doi: 10.3390/nu14132654
74. Chen X, Zhang H, Ren S, Ding Y, Remex NS, Bhuiyan MS, et al. Gut microbiota and microbiota-derived metabolites in cardiovascular diseases. Chin Med J (Engl). (2023) 136:2269–84. doi: 10.1097/CM9.0000000000002206
75. Levy M, Blacher E, Elinav E. Microbiome, metabolites and host immunity. Curr Opin Microbiol. (2017) 35:8–15. doi: 10.1016/j.mib.2016.10.003
76. Teunis CJ, Stroes ESG, Boekholdt SM, Wareham NJ, Murphy AJ, Nieuwdorp M, et al. Tryptophan metabolites and incident cardiovascular disease: the Epic-Norfolk prospective population study. Atherosclerosis. (2023) 387:117344. doi: 10.1016/j.atherosclerosis.2023.117344
77. Grohmann U, Fallarino F, Puccetti P. Tolerance, DCs and tryptophan: much ado about IDO. Trends Immunol. (2003) 24:242–8. doi: 10.1016/S1471-4906(03)00072-3
78. Fallarino F, Grohmann U, You S, Mcgrath BC, Cavener DR, Vacca C, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor ζ-chain and induce a regulatory phenotype in naive T Cells. J Immunol. (2006) 176:6752–61. doi: 10.4049/jimmunol.176.11.6752
79. Zelante T, Fallarino F, Bistoni F, Puccetti P, Romani L. Indoleamine 2,3-dioxygenase in infection: the paradox of an evasive strategy that benefits the host. Microbes Infect. (2009) 11:133–41. doi: 10.1016/j.micinf.2008.10.007
80. Matteoli G, Mazzini E, Liev L, Mileti E, Fallarino F, Puccetti P, et al. Gut CD103+dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut. (2010) 59:595. doi: 10.1136/gut.2009.185108
81. Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, et al. Ctla-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. (2002) 3:1097–101. doi: 10.1038/ni846
82. Puccetti P, Grohmann U. IDO and regulatory T cells: a role for reverse signalling and non-canonical NF-κB activation. Nat Rev Immunol. (2007) 7:817–23. doi: 10.1038/nri2163
83. Gaspar R, Halmi D, Demjan V, Berkecz R, Pipicz M, Csont T. Kynurenine pathway metabolites as potential clinical biomarkers in coronary artery disease. Front Immunol. (2021) 12:768560. doi: 10.3389/fimmu.2021.768560
84. Romani L, Fallarino F, De Luca A, Montagnoli C, D’angelo C, Zelante T, et al. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. (2008) 451:211–5. doi: 10.1038/nature06471
85. Ala M, Eftekhar SP. The footprint of kynurenine pathway in cardiovascular diseases. Int J Tryptophan Res. (2022) 15:11786469221096643. doi: 10.1177/11786469221096643
86. Sudar-Milovanovic E, Gluvic Z, Obradovic M, Zaric B, Isenovic ER. Tryptophan metabolism in atherosclerosis and diabetes. Curr Med Chem. (2022) 29:99–113. doi: 10.2174/0929867328666210714153649
87. Ramprasath T, Han YM, Zhang D, Yu CJ, Zou MH. Tryptophan catabolism and inflammation: a novel therapeutic target for aortic diseases. Front Immunol. (2021) 12:731701. doi: 10.3389/fimmu.2021.731701
88. Song P, Ramprasath T, Wang H, Zou MH. Abnormal kynurenine pathway of tryptophan catabolism in cardiovascular diseases. Cell Mol Life Sci. (2017) 74:2899–916. doi: 10.1007/s00018-017-2504-2
89. Van Nueten JM, Janssens WJ, Vanhoutte PM. Serotonin and vascular reactivity. Pharmacol Res Commun. (1985) 17:585–608. doi: 10.1016/0031-6989(85)90067-0
90. Cerrito F, Lazzaro MP, Gaudio E, Arminio P, Aloisi G. 5HT2-receptors And serotonin release: their role in human platelet aggregation. Life Sci. (1993) 53:209–15. doi: 10.1016/0024-3205(93)90671-O
91. Ma Y, Liang X, Li C, Li R, Tong X, Zhang R, et al. 5-HT(2A) Receptor and 5-HT degradation play a crucial role in atherosclerosis by modulating macrophage foam cell formation, vascular endothelial cell inflammation, and hepatic steatosis. J Atheroscler Thromb. (2022) 29:322–36. doi: 10.5551/jat.58305
92. Zelante T, Puccetti M, Giovagnoli S, Romani L. Regulation of host physiology and immunity by microbial indole-3-aldehyde. Curr Opin Immunol. (2021) 70:27–32. doi: 10.1016/j.coi.2020.12.004
93. Puccetti M, Xiroudaki S, Ricci M, Giovagnoli S. Postbiotic-enabled targeting of the host-microbiota-pathogen interface: hints of antibiotic decline? Pharmaceutics. (2020) 12:624. doi: 10.3390/pharmaceutics12070624
94. Kumar A, Sperandio V. Indole signaling at the host-microbiota-pathogen interface. mBio. (2019) 10:e01031-19. doi: 10.1128/mBio.01031-19
95. Cason CA, Dolan KT, Sharma G, Tao M, Kulkarni R, Helenowski IB, et al. Plasma microbiome-modulated indole- and phenyl-derived metabolites associate with advanced atherosclerosis and postoperative outcomes. J Vasc Surg. (2018) 68:1552–62.e7. doi: 10.1016/j.jvs.2017.09.029
96. Lu Y, Chong J, Shen S, Chammas JB, Chalifour L, Xia J. Trpnet: understanding tryptophan metabolism across gut microbiome. Metabolites. (2021) 12:10. doi: 10.3390/metabo12010010
97. Pulakazhi Venu VK, Saifeddine M, Mihara K, Tsai YC, Nieves K, Alston L, et al. The pregnane X receptor and its microbiota-derived ligand indole 3-propionic acid regulate endothelium-dependent vasodilation. Am J Physiol Endocrinol Metab. (2019) 317:E350–61. doi: 10.1152/ajpendo.00572.2018
98. Siddique S, Ahmad KR, Nawaz SK, Ahmad SN, Ali R, Inayat I, et al. Evaluation of the anti-inflammatory, analgesic, anti-pyretic and anti-ulcerogenic potentials of synthetic indole derivatives. Sci Rep. (2023) 13:8639. doi: 10.1038/s41598-023-35640-4
99. Zhang SS, tan QW, Guan LP. Antioxidant, anti-inflammatory, antibacterial, and analgesic activities and mechanisms of quinolines, indoles and related derivatives. Mini Rev Med Chem. (2021) 21:2261–75. doi: 10.2174/1389557521666210111145011
100. Lee J, Lee W, Kim MA, Hwang JS, Na M, Bae JS. Inhibition of platelet aggregation and thrombosis by indole alkaloids isolated from the edible insect protaetia brevitarsis seulensis (kolbe). J Cell Mol Med. (2017) 21:1217–27. doi: 10.1111/jcmm.13055
101. Hajra S, Patra AR, Basu A, Bhattacharya S. Prevention of doxorubicin (DOX)-induced genotoxicity and cardiotoxicity: effect of plant derived small molecule indole-3-carbinol (I3C) on oxidative stress and inflammation. Biomed Pharmacother. (2018) 101:228–43. doi: 10.1016/j.biopha.2018.02.088
102. Li Q, Xia B, Wu J, Yuan X, Lu X, Huang C, et al. Indole-3-carbinol (I3C) protects the heart from ischemia/reperfusion injury by inhibiting oxidative stress, inflammation, and cellular apoptosis in mice. Front Pharmacol. (2022) 13:924174. doi: 10.3389/fphar.2022.924174
103. Singh N, Aggarwal RC, Singh CP. Cardiovascular activity of indole derivatives by incorporating oxadiazole, azetidinone and thiazolidinone moieties: a review. Int J Drug Dev Res. (2014) 6:30–9.
104. Ramakrishna K, Krishnamurthy S. Indole-3-carbinol ameliorated the isoproterenol-induced myocardial infarction via multimodal mechanisms in wistar rats. Nat Prod Res. (2022) 36:6044–9. doi: 10.1080/14786419.2022.2041632
105. Gu P, Wu Y, Lu W. New perspectives on the role and therapeutic potential of melatonin in cardiovascular diseases. Am J Cardiovasc Drugs. (2024) 24(2):171–95. doi: 10.1007/s40256-024-00631-x
106. Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, et al. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature. (2008) 453:65–71. doi: 10.1038/nature06880
107. Stockinger B, Di Meglio P, Gialitakis M, Duarte JH. The aryl hydrocarbon receptor: multitasking in the immune system. Annu Rev Immunol. (2014) 32:403–32. doi: 10.1146/annurev-immunol-032713-120245
108. Gutiérrez-Vázquez C, Quintana FJ. Regulation of the immune response by the aryl hydrocarbon receptor. Immunity. (2018) 48:19–33. doi: 10.1016/j.immuni.2017.12.012
109. Bessede A, Gargaro M, Pallotta MT, Matino D, Servillo G, Brunacci C, et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature. (2014) 511:184–90. doi: 10.1038/nature13323
110. Moura-Alves P, Faé K, Houthuys E, Dorhoi A, Kreuchwig A, Furkert J, et al. AhR sensing of bacterial pigments regulates antibacterial defence. Nature. (2014) 512:387–92. doi: 10.1038/nature13684
111. Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. (2013) 39:372–85. doi: 10.1016/j.immuni.2013.08.003
112. Pernomian L, Duarte-Silva M, De Barros Cardoso CR. The aryl hydrocarbon receptor (AhR) as a potential target for the control of intestinal inflammation: insights from an immune and bacteria sensor receptor. Clin Rev Allergy Immunol. (2020) 59:382–90. doi: 10.1007/s12016-020-08789-3
113. Dong F, Perdew GH. The aryl hydrocarbon receptor as a mediator of host-microbiota interplay. Gut Microbes. (2020) 12:1859812. doi: 10.1080/19490976.2020.1859812
114. Han H, Safe S, Jayaraman A, Chapkin RS. Diet-host-microbiota interactions shape aryl hydrocarbon receptor ligand production to modulate intestinal homeostasis. Annu Rev Nutr. (2021) 41:455–78. doi: 10.1146/annurev-nutr-043020-090050
115. Hou Y, Li J, Ying S. Tryptophan metabolism and gut microbiota: a novel regulatory axis integrating the microbiome, immunity, and cancer. Metabolites. (2023) 13:1166. doi: 10.3390/metabo13111166
116. Puccetti M, Pariano M, Wojtylo P, Schoubben A, Giovagnoli S, Ricci M. Turning microbial AhR agonists into therapeutic agents via drug delivery systems. Pharmaceutics. (2023) 15:506. doi: 10.3390/pharmaceutics15020506
117. Paeslack N, Mimmler M, Becker S, Gao Z, Khuu MP, Mann A, et al. Microbiota-derived tryptophan metabolites in vascular inflammation and cardiovascular disease. Amino Acids. (2022) 54:1339–56. doi: 10.1007/s00726-022-03161-5
118. Puccetti M, Pariano M, Costantini C, Giovagnoli S, Ricci M. Pharmaceutically active microbial AhR agonists as innovative biodrugs in inflammation. Pharmaceuticals (Basel). (2022) 15:336. doi: 10.3390/ph15030336
119. Puccetti M, Giovagnoli S, Zelante T, Romani L, Ricci M. Development of novel indole-3-aldehyde-loaded gastro-resistant spray-dried microparticles for postbiotic small intestine local delivery. J Pharm Sci. (2018) 107:2341–53. doi: 10.1016/j.xphs.2018.04.023
120. Modoux M, Rolhion N, Mani S, Sokol H. Tryptophan metabolism as a pharmacological target. Trends Pharmacol Sci. (2021) 42:60–73. doi: 10.1016/j.tips.2020.11.006
121. Puccetti M, Gomes Dos Reis L, Pariano M, Costantini C, Renga G, Ricci M, et al. Development and in vitro-in vivo performances of an inhalable indole-3-carboxaldehyde dry powder to target pulmonary inflammation and infection. Int J Pharm. (2021) 607:121004. doi: 10.1016/j.ijpharm.2021.121004
122. Guerra-Ojeda S, Suarez A, Valls A, Verdú D, Pereda J, Ortiz-Zapater E, et al. The role of aryl hydrocarbon receptor in the endothelium: a systematic review. Int J Mol Sci. (2023) 24:13537. doi: 10.3390/ijms241713537
123. Mannarino MR, Bianconi V, Scalisi G, Franceschini L, Manni G, Cucci A, et al. A tryptophan metabolite prevents depletion of circulating endothelial progenitor cells in systemic low-grade inflammation. Front Immunol. (2023) 14:964660. doi: 10.3389/fimmu.2023.964660
124. Fernstrom JD. A perspective on the safety of supplemental tryptophan based on its metabolic Fates123. J Nutr. (2016) 146:2601S–8. doi: 10.3945/jn.115.228643
125. Pakmehr A, Mousavi SM, Ejtahed HS, Hoseini-Tavassol Z, Siadat SD, Hasani-Ranjbar S, et al. The effect of fecal microbiota transplantation on cardiometabolic risk factors: a systematic review and meta-analysis. Clin Ther. (2024) 46:e87–e100. doi: 10.1016/j.clinthera.2023.11.015
126. Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology. (2017) 112:399–412. doi: 10.1016/j.neuropharm.2016.07.002
127. Parolisi S, Montanari C, Borghi E, Cazzorla C, Zuvadelli J, Tosi M, et al. Possible role of tryptophan metabolism along the microbiota-gut-brain axis on cognitive & behavioral aspects in phenylketonuria. Pharmacol Res. (2023) 197:106952. doi: 10.1016/j.phrs.2023.106952
Keywords: cardioimmunology, cardiovascular diseases, tryptophan, indole-3-aldehyde, Aryl hydrocarbon receptor
Citation: Russo MA, Puccetti M, Costantini C, Giovagnoli S, Ricci M, Garaci E and Romani L (2024) Human and gut microbiota synergy in a metabolically active superorganism: a cardiovascular perspective. Front. Cardiovasc. Med. 11:1411306. doi: 10.3389/fcvm.2024.1411306
Received: 2 April 2024; Accepted: 26 September 2024;
Published: 11 October 2024.
Edited by:
Tharmarajan Ramprasath, Georgia State University, United StatesReviewed by:
Senthamizharasi Manivasagam, The University of Iowa, United StatesVasudevan Varadaraj, Hadassah Medical Center, Israel
Ashraf Yusuf Rangrez, University of Kiel, Germany
Copyright: © 2024 Russo, Puccetti, Costantini, Giovagnoli, Ricci, Garaci and Romani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luigina Romani, bHVpZ2luYS5yb21hbmlAdW5pcGcuaXQ=; bHVpZ2luYS5yb21hbmlAb3V0bG9vay5pdA==
 Matteo Antonio Russo
Matteo Antonio Russo Matteo Puccetti
Matteo Puccetti Claudio Costantini
Claudio Costantini Stefano Giovagnoli
Stefano Giovagnoli Maurizio Ricci
Maurizio Ricci Enrico Garaci4
Enrico Garaci4 Luigina Romani
Luigina Romani