- 1Graduate School, Jiangxi University of Chinese Medicine, Nanchang, Jiangxi, China
- 2Department of Gastroenterology, The Affiliated Hospital of Jiangxi University of Chinese Medicine, Nanchang, Jiangxi, China
Objective: The purpose of this study was to investigate the causal relationship between gastroesophageal reflux disease (GERD) and hypertension using a two-sample Mendelian randomization analysis.
Methods: The associated data of GERD with hypertension were derived from the genome-wide association study (GWAS) database, and two-sample Mendelian randomization (MR) analysis was performed using methods including inverse variance weighting (IVW), MR-Egger, and weighted median (WM) to investigate the causal association between GERD and hypertension.
Results: A total of 16 single nucleotide polymorphisms (SNPs) strongly associated with GERD were screened out, and the IVW suggested a causal relationship between GERD and hypertension (OR: 1.057; 95% CI: 1.044–1.071; P < 0.05). The weighted median also showed a similar relationship (OR: 1.051, 95% CI: 1.032–1.07; P < 0.05). In addition, no heterogeneity or horizontal pleiotropy was observed, suggesting a robustness of the outcome.
Conclusion: There is a positive causal relationship between GERD and hypertension.
1 Introduction
Hypertension is an important comorbidity and risk factor for cardiovascular disease (CVD) and is one of the leading causes of death due to CVD (1–3). It has been reported that about one-third of adults worldwide suffer from hypertension, and the prevalence is increasing year by year (4–6). The prevention and treatment of hypertension have become a major public health problem to be solved. Even though patients actively cope with it through improvements in lifestyle and medication, the percentage of hypertension treated and controlled satisfactorily remains low (7). This phenomenon arises from the intricate nature of the mechanisms governing blood pressure regulation, and a comprehensive comprehension of the etiology of hypertension remains elusive within the current body of knowledge.
Gastroesophageal reflux disease (GERD) is a common gastrointestinal disease that is caused by the reflux of gastric contents into the esophagus and mainly manifests as heartburn and reflux (8, 9). Besides, GERD is clinically correlated with many symptoms and diseases outside of the GI (gastrointestinal) tract in addition to the common GI symptoms (10, 11). A study has shown that GERD is closely related to the progression of hypertension and may be one of the important risk factors (12). Li et al. (13) found that the incidence of hypertension in GERD patients was significantly higher than that in non-GERD patients. After omeprazole antacid treatment, the incidence of hypertension in patients was significantly reduced, suggesting that PPI drugs could help hypertensive patients return to normal blood pressure. In addition, the study showed that antacids significantly reduced the blood pressure in patients with essential hypertension, suggesting that the treatment of GERD may help normalize the blood pressure in hypertensive patients. However, another study revealed no correlation between hypertension and GERD (14). Therefore, the relationship between GERD and hypertension remains controversial.
For the first time, the Mendelian randomization (MR) method was used to investigate whether there is a causal relationship between GERD and hypertension. MR is a statistical method based on whole-genome sequencing data, which can effectively reduce bias and is used to expose causal relationships (15). Compared with estimates from observational studies, MR estimates are less susceptible to potential reverse causality and confusion, as genetic variants are randomly distributed at conception and largely independent of environmental and life factors (16).
2 Research methods and materials
2.1 Study design
To investigate the causal relationship between GERD and hypertension, firstly, we derived the associated data of GERD with hypertension from the publicly available GWAS database, screened out qualified single nucleotide polymorphisms (SNPs), and determined the causal association between GERD and the risk of onset of hypertension by using various statistical methods.
2.2 Data sources
The GERD-related data were obtained from the IEU (Integrative Epidemiology Unit) open GWAS database at https://gwas.mrcieu.ac.uk/datasets/ebi-a-GCST90000514/. The sample size of this dataset was 602,604, which included 129,080 patients with GERD and 473,524 control cases, all of European origin, and the number of SNPs was 2,320,781. Data on hypertension were obtained from the IEU open GWAS database at https://gwas.mrcieu.ac.uk/datasets/ukb-b-12493/. The database has a sample size of 463,010, including 54,358 hypertensive patients and 408,652 control cases, all of European origin, and the number of SNPs was 9,851,867.
2.3 Selection of instrument variables
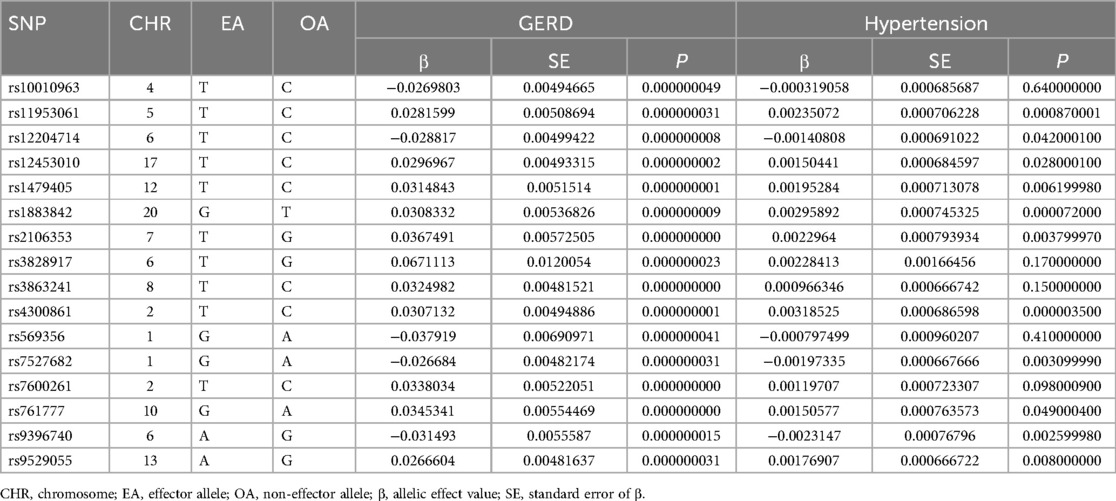
The following three assumptions for the two-sample Mendelian randomization (17) analysis were fulfilled: (1) The correlation assumption: the SNPs were strongly associated with exposure; (2) The independence assumption: the SNPs were not associated with confounders; and (3) The exclusivity assumption: the SNPs were not associated with the outcome see Figure 1. We screened out SNPs that were strongly associated with GERD (P < 5 × 10−8) from the database, ensuring that the correlation assumption was met. Additionally, we excluded SNPs with chain disequilibrium to ensure an r2 value less than 0.001 and a genetic distance greater than 10,000 kb, while maintaining the independence among SNPs (18). We used the PhenoScanner database (www.phenoscanner.medschl.cam.ac.uk) to identify and exclude SNPs associated with the confounders, ensuring that the independence assumption was met. We also calculated the F-statistics to assess the presence of a weak instrument bias. To further validate the association assumption, we used the computational formula F = [(N-K-1)/K] × [R2/(1-R2)], where N refers to the sample size of the exposure factors, K refers to the number of instrument variables, and R2 represents the variation percentage of the exposure factors explained by the instrument variables. We selected instrument variables with F greater than 10 (19), during the analysis, SNPS with palindromic structure were automatically excluded, and instrumental variables significantly correlated with GERD were finally obtained.
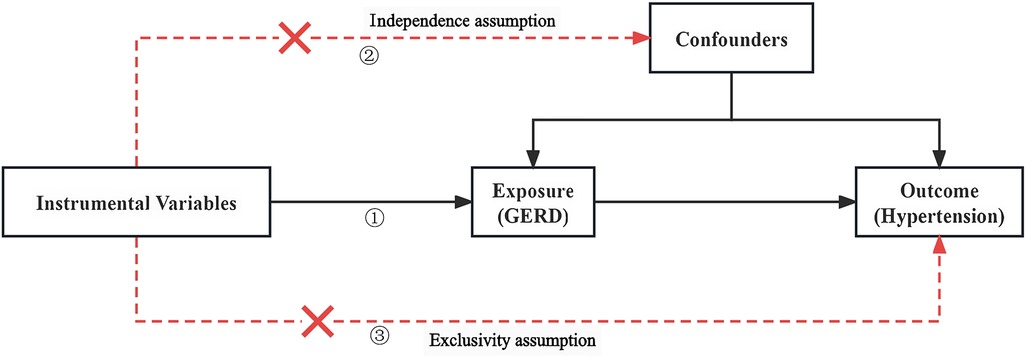
Figure 1. Two-sample Mendelian diagrammatic model. (1) Correlation hypothesis: SNPs are strongly associated with exposure; (2) Independence hypothesis: SNPs are not associated with confounders; (3) Exclusivity hypothesis: SNPs are not associated with outcomes.
2.4 Statistical processing
To estimate the causal effect between GERD and hypertension, we employed the methods of inverse variance weighted (IVW), MR-Egger, and weighted median (WM), with IVW being the predominant method (20). In addition, we assessed the heterogeneity of instrument variables using Cochran's Q-test, P > 0.05 indicates no heterogeneity. To determine the presence of outliers and horizontal pleiotropy, we conducted the MR-PRESSO (Mendelian randomization pleiotropy residual sum and outlier) test. Identify any factors that may lead to horizontal pleiotropy using the MR-PRESSO method, and if outliers are found, delete them and perform MR analysis again. The intercept term of MR-Egger regression was employed to test for potential horizontal pleiotropy of SNPs (21). P < 0.05 indicates the existence of horizontal pleiotropy, indicating that instrumental variables can affect outcomes through factors other than exposure, which violates the independence assumption of the three major hypotheses. Lastly, we used the leave-one-out method to evaluate the magnitude of the impact of individual SNPs on the results of causal association estimation, thereby further validating the robustness of the findings (22).
R 4.2.2 software, TwoSampleMR software package, and MRPRESSO software package were used for statistical processing. When P < 0.05, the value was considered statistically significant.
2.5 Ethics
For this study, we used data obtained from publicly available databases and there were no ethical issues.
3 Results
3.1 Final instrument variables included in MR analysis
We obtained 16 SNPs, and all the F-values included in the MR analysis were greater than 10, indicating that there was no weak instrument bias and that the outcomes were reliable, as shown in Table 1.
3.2 MR results
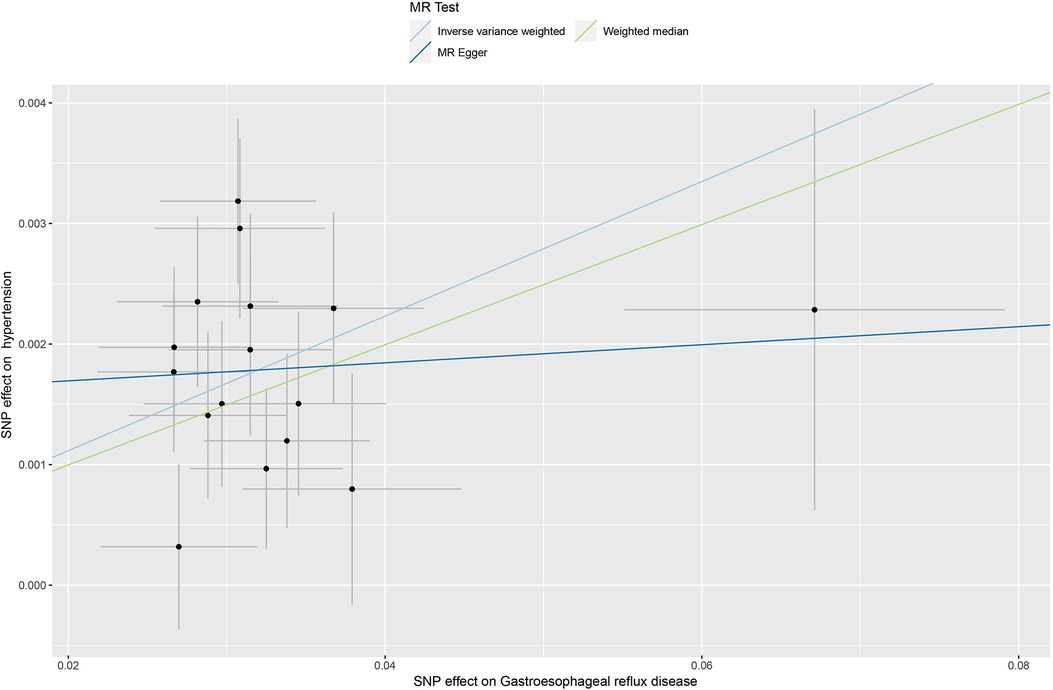
The inverse variance weighted (IVW) method estimated an odds ratio (OR) of 1.057 [95% confidence interval (95% CI): 1.044–1.071; P < 0.05], indicating a causal relationship between GERD and hypertension. Similarly, the weighted median method showed an OR of 1.051 [95% CI: 1.032–1.07; P < 0.05] supporting a comparable relationship. Furthermore, the MR Egger regression analysis indicated that the final results were not affected (OR: 1.007, 95% CI: 0.932–1.089; P > 0.05) see Figure 2 for details. The results demonstrated a strong relationship between GERD and the occurrence of hypertension.
3.3 Sensitivity analysis
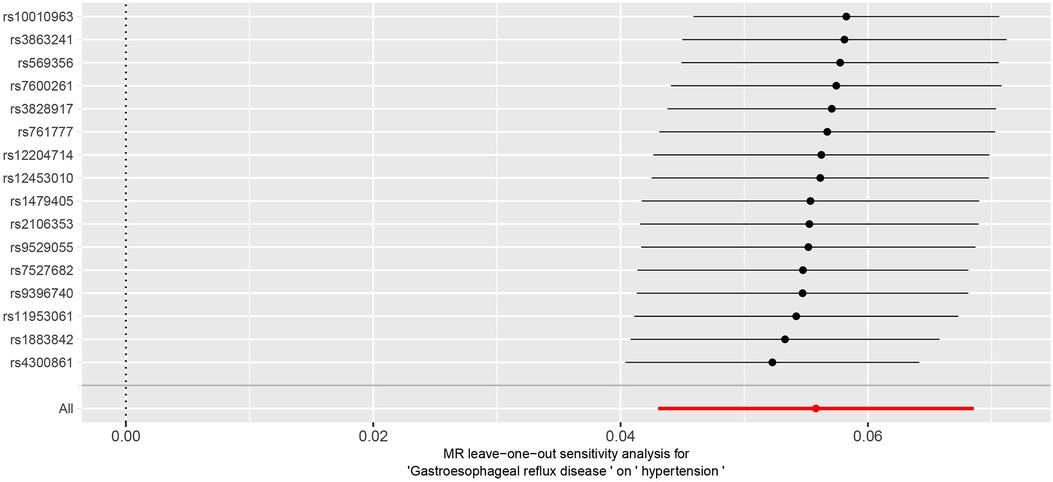
The Cochran Q-test results yielded a P-value of 0.2, indicating the absence of heterogeneity. The MR-Egger intercept term was close to 0 (P = 0.2), indicating that the results of this study were minimally affected by horizontal pleiotropy and therefore robust see Figure 3 for details. Additionally, MR PRESSO suggested the absence of outliers and horizontal pleiotropy (P = 0.4). Finally, the IVW method was tested using the leave-one-out method, and no single SNP significantly affected the robustness of the results, further confirming the reliability of the final results see Figure 4.
4 Discussion
GERD is a prevalent epidemic that has reached alarming levels of 10%–20% in Western countries (23). In the East Asian region, which historically had the lowest rate worldwide, the prevalence of GERD has also been increasing over time (24). Meanwhile, a study revealed that the number of adults with hypertension has increased from 648 million in 1990 to 1,278 million in 2019, indicating an upward trend (25). Consequently, the prevention and treatment of GERD and hypertension have been increasingly emphasized. A growing body of literature has also revealed a possible direct or indirect relationship between the two. By retrospectively analyzing a GERD database of 1,052 patients who had undergone laparoscopic fundoplication (LF), Hu et al. (26) found that a large proportion of patients with GERD combined with hypertension were stabilized in the normal range postoperatively after treatment with LF. The control of hypertension on average was significantly lower in all patients included in the study, which suggests that hypertension may be secondary to gastroesophageal reflux in some patients. Another study (27) found that rabeprazole can increase human plasma nitric oxide level, and nitric oxide has the function of vasodilating, anti-inflammatory, and inhibiting smooth muscle cell growth, and can effectively improve blood pressure level, especially for patients with gastroesophageal reflux disease accompanied by hypertension. GERD is also an important risk factor for cardiovascular diseases (CVD), and it has been reported in the literature that among patients with GERD, the incidence of cardiovascular diseases such as coronary artery disease (CAD), atrial fibrillation (AF), and acute myocardial infarction (AMI) is significantly higher than that in patients without GERD (28–30). In this study, we found a positive causal relationship between GERD and hypertension using the Mendelian randomization method. This finding suggests that GERD is an important risk factor for hypertension, which has significant implications for the prevention, diagnosis, and treatment of hypertension.
The specific mechanism by which GERD increases the risk of hypertension is currently unknown, and the available research suggests the following possible explanations. Since the activity of the digestive tract is closely related to the autonomic nerves, sympathetic excitation caused by esophageal stimulation during GERD episodes may be one of the explanations for GERD-induced hypertension (31–33). There is another theory that has been proposed to explain the relationship between hypertension and GERD. The theory suggests that the increased secretion of gastric acid during the onset of GERD in patients with GERD stimulates the activation of caudal solitary complex (cSC) neurons. The cSC neurons exhibit dual functionality, encompassing digestive reflexes and the modulation of cardiac and pulmonary functions. These functions manifest in gastrointestinal symptoms characterized by a reduction in the tone of the lower esophageal sphincter (LES), resulting in the reflux of gastric contents. Additionally, cSC neurons influence cardiac and pulmonary regulation by precipitating an elevation in the proportion of carbonic acid within the bloodstream, thereby inducing an increase in blood pressure via the mechanism of hypercapnia (34). Through studies on animal models and molecular data, it has been found that many inflammatory mediators such as IL-8, IL-6, and IL-1β, among others, exist in the pathogenesis of GERD. The main mechanism of esophageal injury caused by GERD may be the cytokine-mediated immune response, and these inflammatory mediators have been continuously discovered and studied in recent years (35). Meanwhile, as researchers have gained a deeper understanding of inflammatory mediators, numerous studies have demonstrated that inflammatory responses are also involved in the progression of hypertension (36). Thus, long-term chronic inflammation may contribute to the progression of hypertension by producing inflammatory mediators and participating in angiogenesis, while both innate and adaptive immunity can contribute to elevated blood pressure by triggering vascular inflammation and microvascular remodeling (37). Therefore, the involvement of GERD in the progression of hypertension through inflammatory cytokines is also one of the possible mechanisms by which GERD causes hypertension.
There may be mechanisms other than those mentioned above for GERD-induced hypertension. Only a limited number of studies have investigated the potential link between GERD and hypertension, and existing studies on the association between GERD and hypertension are mostly observational and lack in-depth analysis at the genetic level. Consequently, it is imperative to delve deeper into the underlying mechanisms responsible for GERD-induced hypertension, to elucidate the intricate signaling pathways associated with this phenomenon. Such an investigation holds the potential to offer valuable insights into the etiological framework of hypertension and may unveil prospective therapeutic targets for clinical intervention. This study provides theoretical support for this endeavor. The bidirectional feedback between GERD and hypertension can be investigated in subsequent studies to explore whether there is a causal relationship between hypertension and the development of GERD, thus further elucidating the pathogenic relation between GERD and hypertension.
This study has some limitations: First, the samples included in the study were of European origin, and it cannot be proved that the same results can be obtained in other regions and ethnic groups; second, since the data used are not original, we can only estimate the approximate causal relationship between the two, not determine their specific causal link; third, this study only produced statistical results and did not further investigate the mechanism by which GERD causes hypertension.
5 Conclusion
In summary, the two-sample MR method was used in this study for the first time to infer the causal relationship between GERD and the development of hypertension and produced a preliminary conclusion that a positive causal relationship exists between the two. Although the specific mechanism was not elucidated, this study provides another perspective on the risk factors for the development of hypertension. Timely diagnosis and treatment of GERD can significantly improve and reduce the development of associated hypertension and deserves further research and application.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
This study was conducted with approval from the Ethics Committee of The Affiliated Hospital of Jiangxi University of Chinese Medicine. This study was conducted in accordance with the declaration of Helsinki. Informed consent was obtained from all participants.
Author contributions
WeiL: Conceptualization, Formal Analysis, Validation, Writing – review & editing. QW: Conceptualization, Formal Analysis, Validation, Writing – review & editing. WenL: Formal Analysis, Validation, Writing – review & editing. XL: Data curation, Formal Analysis, Validation, Writing – original draft. ZL: Conceptualization, Formal Analysis, Validation, Writing – original draft. QD: Conceptualization, Funding acquisition, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Jiangxi Provincial Department of Education Technology Research Project (GJJ2200944). The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
GERD, gastroesophageal reflux; GWAS, genome-wide association study; IVW, inverse variance weighted; WM, weighted median; MR, Mendelian randomization; SNPs, single-nucleotide polymorphisms; LF, laparoscopic fundoplication; CAD, coronary artery disease; AF, atrial fibrillation; AMI, acute myocardial infarction; cSC, caudal solitary complex; LES, lower esophageal sphincter.
References
1. Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 international society of hypertension global hypertension practice guidelines. Hypertension. (2020) 75(6):1334–57. doi: 10.1161/HYPERTENSIONAHA.120.15026
2. Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. (2020) 75(2):285–92. doi: 10.1161/HYPERTENSIONAHA.119.14240
3. Di Palo KE, Barone NJ. Hypertension and heart failure: prevention, targets, and treatment. Heart Fail Clin. (2020) 16(1):99–106. doi: 10.1016/j.hfc.2019.09.001
4. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1,479 population-based measurement studies with 19·1 million participants [published correction appears in lancet. 2020 Sep 26;396(10255):886]. Lancet. (2017) 389(10064):37–55. doi: 10.1016/S0140-6736(16)31919-5
5. Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, et al. Heart disease and stroke statistics-2023 update: a report from the American Heart Association. Circulation. (2023) 147(8):e93–621. doi: 10.1161/CIR.0000000000001123
6. Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. (2020) 16(4):223–37. doi: 10.1038/s41581-019-0244-2
7. Kotseva K, De Backer G, De Bacquer D, Rydén L, Hoes A, Grobbee D, et al. Lifestyle and impact on cardiovascular risk factor control in coronary patients across 27 countries: results from the European Society of Cardiology ESC-EORP EUROASPIRE V registry. Eur J Prev Cardiol. (2019) 26(8):824–35. doi: 10.1177/2047487318825350
8. Fass R, Boeckxstaens GE, El-Serag H, Rosen R, Sifrim D, Vaezi MF. Gastro-oesophageal reflux disease. Nat Rev Dis Primers. (2021) 7(1):55. doi: 10.1038/s41572-021-00287-w
9. Maret-Ouda J, Markar SR, Lagergren J. Gastroesophageal reflux disease: a review. JAMA. (2020) 324(24):2536–47. doi: 10.1001/jama.2020.21360
10. Hunt R, Armstrong D, Katelaris P, Afihene M, Bane A, Bhatia S, et al. World gastroenterology organisation global guidelines: GERD global perspective on gastroesophageal reflux disease. J Clin Gastroenterol. (2017) 51(6):467–78. doi: 10.1097/MCG.0000000000000854
11. Li Y, Duan Z. Updates in interaction of gastroesophageal reflux disease and extragastroesophageal digestive diseases. Expert Rev Gastroenterol Hepatol. (2022) 16(11–12):1053–63. doi: 10.1080/17474124.2022.2056018
12. Gudlaugsdottir S, Verschuren W, Dees J, Stijnen T, Wilson J. Hypertension is frequently present in patients with reflux esophagitis or Barrett’s esophagus but not in those with non-ulcer dyspepsia. Eur J Intern Med. (2002) 13(6):369. doi: 10.1016/s0953-6205(02)00090-0
13. Li ZT, Ji F, Han XW, Wang L, Yue YQ, Wang ZG. The role of gastroesophageal reflux in provoking high blood pressure episodes in patients with hypertension. J Clin Gastroenterol. (2018) 52(8):685–90. doi: 10.1097/MCG.0000000000000933
14. Kubota S, Nakaji G, Shimazu H, Odashiro K, Maruyama T, Akashi K. Further assessment of atrial fibrillation as a risk factor for gastroesophageal reflux disease: a multicenter questionnaire survey. Intern Med. (2013) 52(21):2401–7. doi: 10.2169/internalmedicine.52.0923
15. Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. Br Med J. (2018) 362:k601. doi: 10.1136/bmj.k601
16. Palmer TM, Sterne JA, Harbord RM, Lawlor DA, Sheehan NA, Meng S, et al. Instrumental variable estimation of causal risk ratios and causal odds ratios in Mendelian randomization analyses. Am J Epidemiol. (2011) 173(12):1392–403. doi: 10.1093/aje/kwr026
17. Sang N, Gao RC, Zhang MY, Wu ZZ, Wu ZG, Wu GC. Causal relationship between sleep traits and risk of systemic lupus erythematosus: a two-sample Mendelian randomization study. Front Immunol. (2022) 13:918749. doi: 10.3389/fimmu.2022.918749
18. Chen X, Kong J, Diao X, Cai J, Zheng J, Xie W, et al. Depression and prostate cancer risk: a Mendelian randomization study. Cancer Med. (2020) 9(23):9160–7. doi: 10.1002/cam4.3493
19. Burgess S, Thompson SG. Bias in causal estimates from Mendelian randomization studies with weak instruments. Stat Med. (2011) 30(11):1312–23. doi: 10.1002/sim.4197
20. Chen X, Kong J, Pan J, Huang K, Zhou W, Diao X, et al. Kidney damage causally affects the brain cortical structure: a Mendelian randomization study. EBioMedicine. (2021) 72:103592. doi: 10.1016/j.ebiom.2021.103592
21. Ellingjord-Dale M, Papadimitriou N, Katsoulis M, Yee C, Dimou N, Gill D, et al. Coffee consumption and risk of breast cancer: a Mendelian randomization study. PLoS One. (2021) 16(1):e0236904. doi: 10.1371/journal.pone.0236904
22. Hartwig FP, Borges MC, Horta BL, Bowden J, Davey Smith G. Inflammatory biomarkers and risk of schizophrenia: a 2-sample Mendelian randomization study. JAMA Psychiatry. (2017) 74(12):1226–33. doi: 10.1001/jamapsychiatry.2017.3191
23. Tabrez ES, Hussain A, Rao S, Jagadeesh A, Peela JR, Tabrez SSM. Gastroesophageal reflux disease: a review of symptomatic management. Crit Rev Eukaryot Gene Expr. (2018) 28(1):87–92. doi: 10.1615/CritRevEukaryotGeneExpr.2018022767
24. Tan VP, Wong BC, Wong WM, Leung WK, Tong D, Yuen MF, et al. Gastroesophageal reflux disease: crosssectional study demonstrating rising prevalence in a Chinese population. J Clin Gastroenterol. (2016) 50(1):e1–7. doi: 10.1097/MCG.0000000000000304
25. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1,201 population-representative studies with 104 million participants [published correction appears in lancet. 2022 Feb 5;399(10324):520]. Lancet. (2021) 398(10304):957–80. doi: 10.1016/S0140-6736(21)01330-1
26. Hu Z, Chen M, Wu J, Song Q, Yan C, Du X, et al. Improved control of hypertension following laparoscopic fundoplication for gastroesophageal reflux disease. Front Med. (2017) 11(1):68–73. doi: 10.1007/s11684-016-0490-7
27. Swiatkowski M, Budzyński J, Kłopocka M, Grad K, Pulkowski G, Augustyńska B, et al. The effect of eight weeks of rabeprazole therapy on nitric oxide plasma level and esophageal pH and motility and motility nitric oxide plasma level in patients with erosive esophagitis. Med Sci Monit. (2004) 10(2):CR46–51.14737042
28. Song JH, Kim YS, Choi SY, Yang SY. Association between gastroesophageal reflux disease and coronary atherosclerosis. PLoS One. (2022) 17(5):e0267053. doi: 10.1371/journal.pone.0267053
29. Huang TC, Lo LW, Yamada S, Chou YH, Lin WL, Chang SL, et al. Gastroesophageal reflux disease and atrial fibrillation: insight from autonomic cardiogastric neural interaction. J Cardiovasc Electrophysiol. (2019) 30(11):2262–70. doi: 10.1111/jce.14181
30. Eisa M, Sandhu A, Prakash R, Ganocy SJ, Fass R. The risk of acute myocardial infarction in patients with gastroesophageal reflux disease. J Neurogastroenterol Motil. (2020) 26(4):471–6. doi: 10.5056/jnm19192
31. Linz D, Hohl M, Vollmar J, Ukena C, Mahfoud F, Böhm M. Atrial fibrillation and gastroesophageal reflux disease: the cardiogastric interaction. Europace. (2017) 19(1):16–20. doi: 10.1093/europace/euw092
32. Silvestro M, Dovetto FM, Corvino V, Apisa P, Malesci R, Tessitore A, et al. Enlarging the spectrum of cluster headache: extracranial autonomic involvement revealed by voice analysis. Headache. (2021) 61(9):1452–9. doi: 10.1111/head.14222
33. Drummond GR, Vinh A, Guzik TJ, Sobey CG. Immune mechanisms of hypertension. Nat Rev Immunol. (2019) 19(8):517–32. doi: 10.1038/s41577-019-0160-5
34. Dean JB. Theory of gastric CO2 ventilation and its control during respiratory acidosis: implications for central chemosensitivity, pH regulation, and diseases causing chronic CO2 retention. Respir Physiol Neurobiol. (2011) 175(2):189–209. doi: 10.1016/j.resp.2010.12.001
35. Pinto D, Plieschnegger W, Schneider NI, Geppert M, Bordel H, Höss GM, et al. Carditis: a relevant marker of gastroesophageal reflux disease. Data from a prospective central European multicenter study on histological and endoscopic diagnosis of esophagitis (histoGERD trial). Dis Esophagus. (2019) 32(1):10.1093/dote/doy073. doi: 10.1093/dote/doy073
36. Xiao L, Harrison DG. Inflammation in hypertension. Can J Cardiol. (2020) 36(5):635–47. doi: 10.1016/j.cjca.2020.01.013
Keywords: causal inference, gastroesophageal reflux disease, hypertension, Mendelian randomization, gastroenterology
Citation: Li W, Wang Q, Li W, Liu X, Li Z and Dai Q (2024) Study of the causal relationship between gastroesophageal reflux disease and hypertension through two-sample Mendelian randomization analysis. Front. Cardiovasc. Med. 11:1326348. doi: 10.3389/fcvm.2024.1326348
Received: 23 October 2023; Accepted: 5 September 2024;
Published: 24 September 2024.
Edited by:
Iain Brownlee, Northumbria University, United KingdomReviewed by:
Aneesh Dhore-Patil, Houston Methodist Hospital, United StatesMariana Floria, Grigore T. Popa University of Medicine and Pharmacy, Romania
Copyright: © 2024 Li, Wang, Li, Liu, Li and Dai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi Dai, ZGFpcWlkcUAxMjYuY29t
 Weige Li1
Weige Li1 Qi Dai
Qi Dai