- 1Cardiology Unit, San Giovanni di Dio Hospital, Azienda USL Toscana Centro, Florence, Italy
- 2Cardiology Unit, Santa Croce Hospital, Moncalieri, Italy
- 3Department of Clinical Trial, Le Scotte Hospital, University of Siena, Siena, Italy
- 4Department of Internal Medicine, Cardiology and Electrophysiology Unit, Azienda USL Toscana Centro, Florence, Italy
- 5Arrhythmia and Electrophysiology Unit, Careggi University Hospital, Florence, Italy
- 6Department of Clinical and Experimental Medicine, Careggi University Hospital, Florence, Italy
- 7Department of Cardiosciences, Azienda Ospedaliera San Camillo-Forlanini, Rome, Italy
- 8Department of Medical Biotechnologies, Division of Cardiology, University of Siena, Le Scotte Hospital, Siena, Italy
- 9Cardiovascular Diseases Unit, Cardio Thoracic and Vascular Department, Le Scotte Hospital, University of Siena, Siena, Italy
Background: Cardiac resynchronization therapy (CRT) is an established treatment in selected patients suffering from heart failure with reduced ejection fraction (HFrEF). It has been proposed that myocardial fibrosis and inflammation could influence CRT “response” and outcome. Our study investigated the long-term prognostic significance of cardiac biomarkers in HFrEF patients with an indication for CRT.
Methods: Consecutive patients referred for CRT implantation were retrospectively evaluated. The soluble suppression of tumorigenicity 2 (sST2), galectin-3 (Gal-3), N-terminal portion of the B-type natriuretic peptide (NT-proBNP), and estimated glomerular filtration rate (eGFR) were measured at baseline and after 1 year of follow-up. Multivariate analyses were performed to evaluate their correlation with the primary composite outcome of cardiovascular mortality and heart failure hospitalizations at a mean follow-up of 9 ± 2 years.
Results: Among the 86 patients enrolled, 44% experienced the primary outcome. In this group, the mean baseline values of NT-proBNP, Gal-3, and sST2 were significantly higher compared with the patients without cardiovascular events. At the multivariate analyses, baseline Gal-3 [cut-off: 16.6 ng/ml, AUC: 0.91, p < 0.001, HR 8.33 (1.88–33.33), p = 0.005] and sST2 [cut-off: 35.6 ng/ml AUC: 0.91, p < 0.001, HR 333 (250–1,000), p = 0.003] significantly correlated with the composite outcome in the prediction models with high likelihood. Among the parameters evaluated at 1-year follow-up, sST2, eGFR, and the variation from baseline to 1-year of Gal-3 levels showed a strong association with the primary outcome [HR 1.15 (1.08–1.22), p < 0.001; HR: 0.84 (0.74–0.91), p = 0.04; HR: 1.26 (1.10–1.43), p ≤ 0.001, respectively]. Conversely, the echocardiographic definition of CRT response did not correlate with any outcome.
Conclusion: In HFrEF patients with CRT, sST2, Gal-3, and renal function were associated with the combined endpoint of cardiovascular death and HF hospitalizations at long-term follow-up, while the echocardiographic CRT response did not seem to influence the outcome of the patients.
1. Introduction
Despite the significant advances in medical treatment, the prognosis in heart failure with reduced ejection fraction (HFrEF) remains poor, and the use of markers for outcome prediction remains scarce. Cardiac resynchronization therapy (CRT) proved to reduce mortality and heart failure (HF) hospitalizations in patients with left bundle branch block (LBBB) and left ventricular ejection fraction (LVEF) ≤35%, still symptomatic on top of optimal medial therapy (1). However, given that not all patients seem to equally benefit from CRT, the concept of “response” has been developed: various definitions, mainly based on clinical or echocardiographic modifications following CRT implantation, have tried to identify the subgroup of HF patients that gains the greatest advantage from resynchronization therapy. The aim is to optimize the candidates’ selection and the cost/benefit ratio of a relatively expensive tool (2). The degree of myocardial inflammation and fibrosis can impair the efficiency of resynchronization by affecting left ventricle (LV) adverse remodeling and outcome, becoming the main determinant of the so-called “CRT response” (3). Clinical and imaging assessments alone, performed before CRT implantation, are not able to fully evaluate the state of cardiomyocytes and myocardial extracellular matrix. Conversely, some biomarkers, such as the soluble suppression of tumorigenicity 2 (sST2), galectin-3 (Gal-3), and N-terminal portion of the B-type natriuretic peptide (NT-proBNP), have been related with myocardial fibrosis, inflammation, and congestion, which are affecting the prognosis in HF patients (4–6). Chronic kidney disease and HF may amplify pathophysiologic mechanisms that lead to a dangerous vicious cycle. It is still unclear whether the dynamic change of renal function after CRT implantation directly contributes to a poor outcome or whether eGFR only marks the advances of cardiac and renal dysfunction (7).
The associations between the variations of the mentioned biomarkers, renal function, CRT response, and cardiovascular (CV) outcome have not been systematically evaluated in contemporary cohorts. Thus, the aim of our study is to investigate the potential relationship of cardiac biomarkers, CRT response, and long-term outcome in a cohort of patients with HFrEF undergoing CRT implantation.
2. Materials and methods
2.1. Study design and participants
We retrospectively evaluated consecutive patients undergoing implantation of CRT pacing (CRT-P) or CRT and defibrillation (CRT-D) in our institution “Azienda Ospedaliera-Universitaria Careggi” from November 2010 to January 2012. According to current guidelines, the patients were addressed for implantation when affected by symptomatic HFrEF (New York Heart Association class II to ambulatory class IV) despite optimal medical therapy, LV systolic dysfunction with ejection fraction ≤35%, and QRS width ≥130 ms together with LBBB morphology (8). The presence of a LBBB was defined in case of QRS ≥ 130 ms; QS or rS complex in V1 to V2; monophasic and notched or slurred R waves in I, aVL, V5, or V6; and absent Q waves in leads V5 and V6 (9). A three pacing-lead device was implanted in each patient and was programmed to obtain the highest percentage of biventricular stimulation (≥90% of total beats). This study excluded patients with a QRS morphology different from LBBB or already carriers of a right-sided pacing system, either pacemaker or implantable defibrillator. The implantation of transvenous CRT systems was performed according to standard techniques, preferring the basal position of the lateral veins for LV lead placement avoiding the apical segment (10), and placing the right atrial and ventricular leads preferably at the atrial appendage and at the apex (11). No quadripolar LV leads were implanted since they were not available at that time in our institution. The CRTs were programmed by senior electrophysiology specialists according to current guidelines and manufacturer specifications (12). Our study is in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by our local institutional review board. Informed consents were obtained from all the patients.
2.2. Laboratory assessment
Gal-3, sST2, NT-proBNP, creatinine, and estimated glomerular filtration rate (eGFR calculated with the CKD-EPI formula) were measured at baseline and at 12 months after CRT implantation. The delta (Δ) was considered as the difference between the biomarkers at baseline and 1-year follow-up.
All blood samples obtained from the patients were collected with a sterile disposable syringe containing EDTA. They were analyzed using the Alere Triage BNP Test. This test is an immunoassay in a single-use plastic cartridge containing a monoclonal antibody for BNP, labeled with a fluorescent dye and BNP. Plasma BNP was measured with Triage BNP Test (Biosite Inc., San Diego, CA, United States). The human galectin-3 ELISA is an enzyme-linked immunosorbent assay for the quantitative detection of human galectin-3 (Platinum Elisa, eBioscience, San Diego, CA, United States). The assay was performed measuring the protein in EDTA plasma. Aliquots of serum samples were stored at temperature ranging from 2° to 8°, and the human galectin-3 level were determined after 24 h. Each sample was manually measured, and it has been assayed in duplicate; a calibration curve was built making serial dilution, starting from a value of 25,000 ng/ml to a value of 0.39 ng/ml. The final reading was realized using a specific scanner (DV 990 BV 4/6, N.T. laboratory Rome, Italy). The Presage sST2 assay is a quantitative sandwich monoclonal ELISA in a 96-well microtiter plate format for the measurement of sST2 in serum, EDTA plasma, or heparin plasma. The Presage sST2 assay utilizes two mAbs against ST2. A mouse monoclonal antihuman sST2 antibody is coated onto the surface of the microtiter plate wells and acts as the capture antibody to bind sST2 molecules in the solution. A second mouse monoclonal antihuman sST2 antibody is provided in the solution and functions as the tracer antibody for detecting ST2 molecules that bounded to the capture antibody (Critical Diagnostics, San Diego, CA, United States).
2.3. Echocardiography
All patients underwent a cardiologic evaluation and echocardiographic study at baseline, before CRT implantation, and at 1-year follow-up. The responders were defined by the reduction of LV end-systolic volume ≥15% at 1-year follow-up. The echocardiographic evaluation was interpreted and independently reviewed by three senior cardiologists according to the instructions provided by the American Society of Echocardiography (13). The LV volumes and LVEF were calculated using the apical two- and four-chamber views by the Simpson biplane formula. The pulsed-Doppler transmitral flow velocity was used to obtain the early diastolic velocity (E wave), late diastolic velocity (A wave), and their ratio (E/A), and the deceleration time of E wave. The tissue Doppler imaging (TDI) was used to collect the early diastolic myocardial velocity (e’) at the septal and lateral level and the average E/e’ ratio. The M-mode was then used to obtain the values of tricuspid annular plane systolic excursion (TAPSE). The delta (Δ) was defined as the difference between the echocardiographic data (LV volumes, LVEF) at baseline and 1-year follow-up.
2.4. Outcome definition
The primary clinical outcome was assessed using a composite clinical endpoint consisting of CV mortality and HF hospitalization. CV decease includes death that result from an acute myocardial infarction, sudden cardiac death, HF, stroke, CV procedures, CV hemorrhage, and other CV causes. The secondary outcomes were cardiovascular mortality, HF hospitalizations, and the first episode of sustained rapid ventricular tachyarrhythmias > 180 beats/min detected and terminated or recorded by the CRT device. All such events are routinely registered in our database at each outpatient visit and following consultations in the emergency room of hospital wards. The mean follow-up was 9 ± 2 years.
2.5. Statistical analysis
The continuous variables reported as mean ± standard deviation (SD) or as median were compared between patients with CV events and patients without CV events using the Student's t-test or non-parametric tests, as appropriate. The χ2 or Fisher exact test was used to compare non-continuous variables expressed as proportions. The categorical variables reported as percentages were compared between groups using the chi-squared test (or a Fisher exact test when any expected cell count was <5). The predictive parameters of the outcomes were determined by analyzing the receiver operating characteristic (ROC) curves to obtain the best cut-off values. The survival analyses and curves were performed using the Kaplan–Meier method. A Cox regression modeling was performed to assess the factors associated with the composite outcome, CV death or HF hospitalization: multivariate analyses included covariates in a rate of 1:10 with the events recorded. Given the relative low numbers of events at follow-up, we built different prognostic models including at least one clinical, one echocardiographic, and one laboratory parameter. The ones with the highest log-likelihood were then selected. P-values are two-sided and considered significant when <0.05. All analyses were performed using IBM SPSS Statistics for Macintosh, Version 26.0 (IBM Corp., Armonk, NY, United States).
3. Results
3.1. Baseline characteristics, biomarkers, and primary outcome
A total of 86 patients fulfilled the inclusion criteria and were enrolled in the current study. The mean age was 70 ± 9 years, mean QRS duration 165 ± 21 ms, and LVEF 26 ± 6%, and 43% of them had ischemic cardiomyopathy (Table 1). The biomarker levels according to HF etiology (non-ischemic vs. ischemic) are shown in Supplementary Table S1. The patients with non-ischemic etiology showed lower levels of sST2 and better renal function both at baseline and during follow-up compared with patients with ischemic etiology.
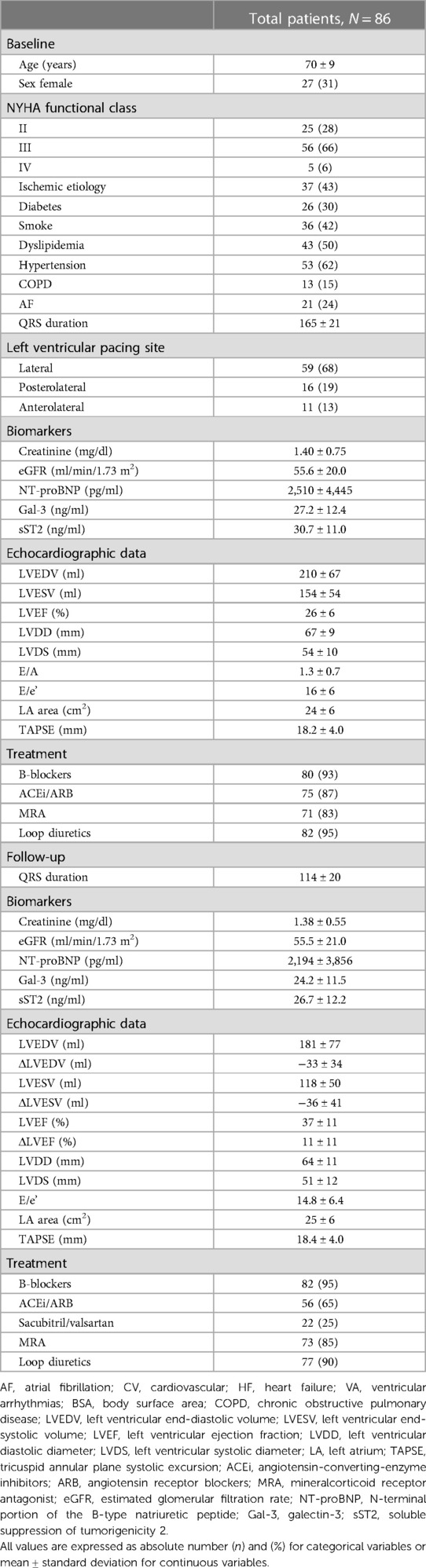
Table 1. Baseline and follow-up clinical, biomarkers, and echocardiographic characteristics of the population enrolled.
At a median follow-up of 9 ± 2 years, 38 patients (44%) experienced any component of the primary outcome: considering the single outcomes, 33 (38%) were hospitalized for HF and 20 (23%) died. Moreover, 15 (17%) experienced an episode of ventricular arrhythmia. Table 2 shows the differences of the baseline characteristics of the study groups in relation to the composite primary outcome.
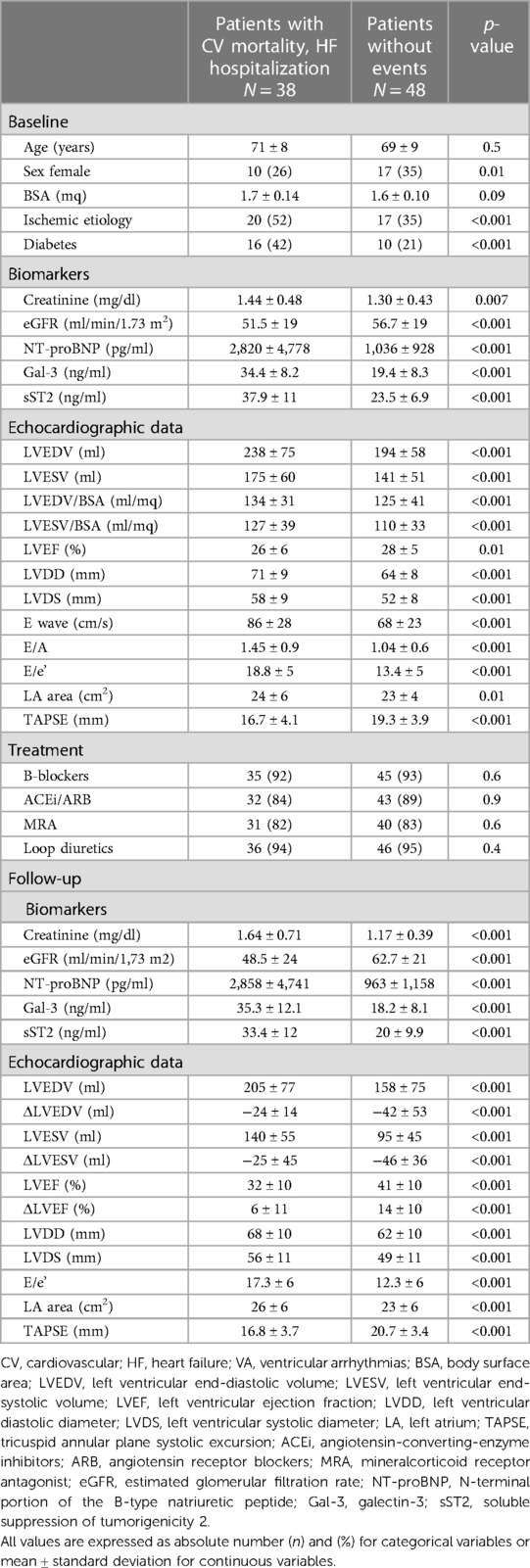
Table 2. Baseline and follow-up clinical, biomarkers, and echocardiographic characteristics of the patients with CV mortality and HF hospitalization vs. patients without CV events.
In the group with clinical events, the concentrations of all the biomarkers analyzed were significantly higher, as shown by the mean values of creatinine (1.44 ± 0.48 vs. 1.30 ± 0.43 mg/dl, p < 0.001), NT-proBNP (2,820 ± 4,778 vs. 1,036 ± 928 pg/ml, p < 0.001), Gal-3 (34.4 ± 8.2 vs. 19.4 ± 8.3 mg/ml p < 0.001), and sST2 (37.9 ± 11 vs. 23.5 ± 6.9 ng/ml, p < 0.001).
Considering the relative low numbers of events at follow-up, several multivariate analyses including at least one clinical, one echocardiographic, and one laboratory parameter were considered. Table 3 includes some of the multivariate analyses among those showing the highest log-likelihood. Baseline Gal-3 and sST2 cut-off values with the highest AUC at the ROC curve analysis were identified (Gal-3 cut-off: 16.6 ng/ml, AUC: 0.91, p < 0.001; sST2 cut-off: 35.6 ng/ml AUC: 0.91, p < 0.001) and maintained a strong correlation with the outcome at the multivariate analyses [HR 8.33 (1.88–33.33), p = 0.005 and HR 333 (250–1,000), p = 0.003, respectively]. In these “prediction models”, E/e’ was also statistically significant; conversely, no clinical variable maintained its correlation with the outcome, including ischemic etiology, as shown in Supplementary Table S2.
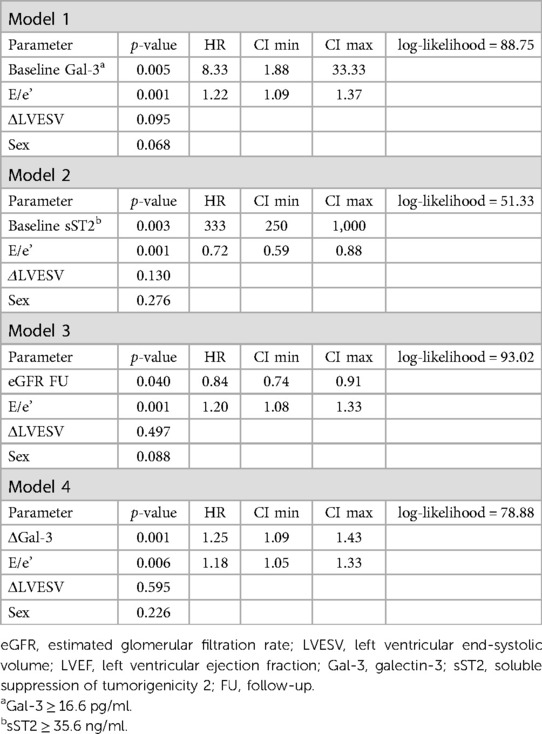
Table 3. Prediction models with multivariable risk analyses for CV death and HF hospitalization (primary outcome).
The Kaplan–Meier curve built with the same cut-off values of sST2 is shown in Figure 1. The survival curves were then created using the combination of the cut-off values of sST2 and Gal-3 found in our analysis. As displayed in Figure 2, the patients with both high baseline sST2 and Gal-3 had the lowest survival probability.
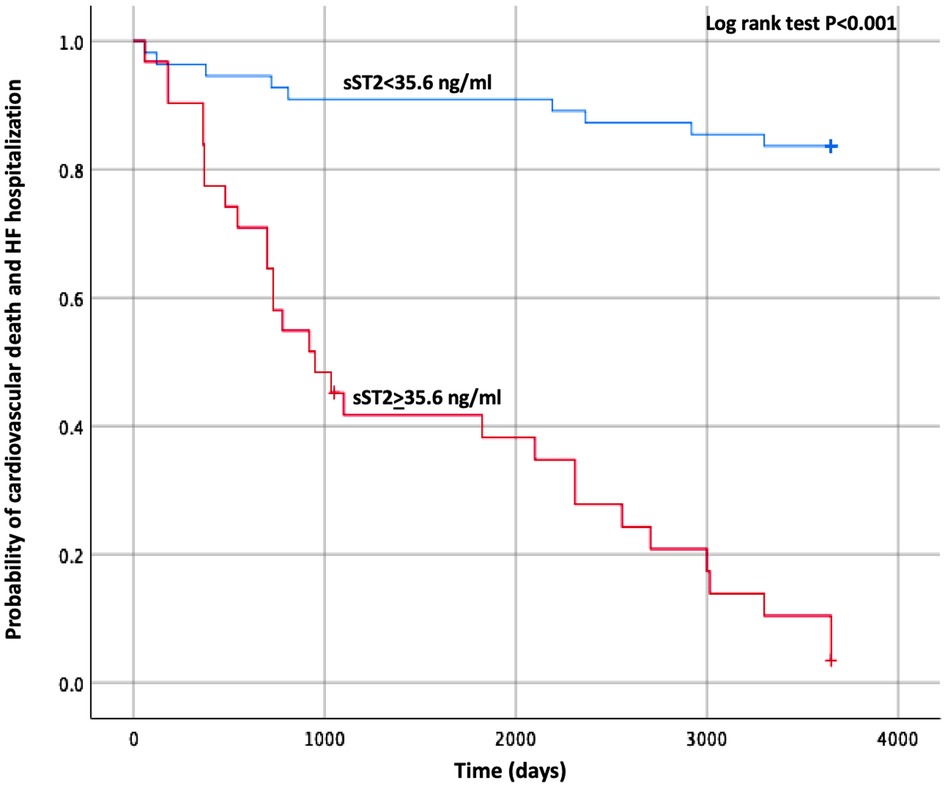
Figure 1. Kaplan–Meier estimates of the cumulative probability of CV death and HF hospitalization by ST2 cut-off. CV, cardiovascular; HF, heart failure; ST2, suppression of tumorigenicity 2.
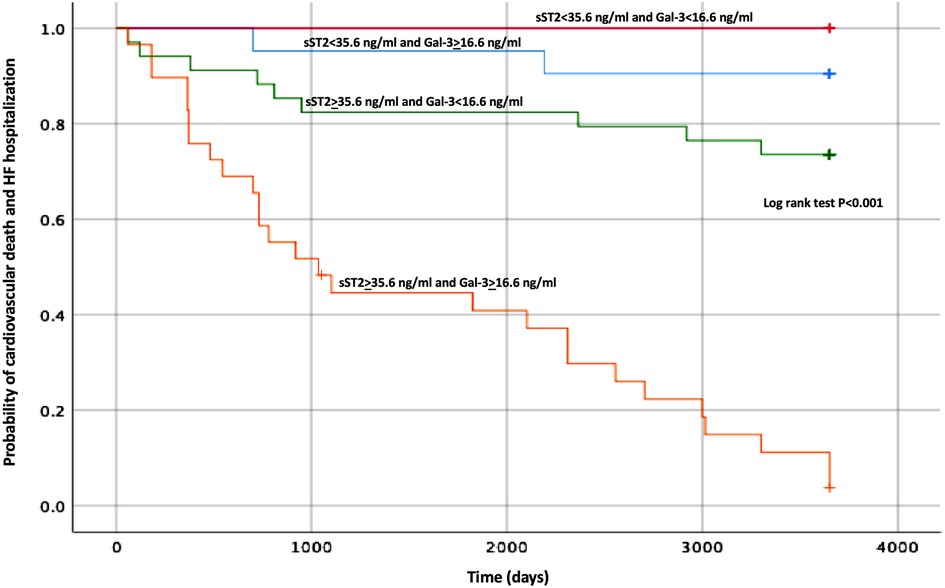
Figure 2. Kaplan–Meier estimates of the cumulative probability of CV death and HF hospitalization by Gal-3 and ST2 levels. CV, cardiovascular; HF, heart failure; ST2, suppression of tumorigenicity 2; Gal-3, galectin-3.
NT-proBNP was not significantly related with the composite outcome when considered singularly in various prediction models, but the parameter obtained by its combination with sST2 (both considered as categorical variables) proved to be significant [HR 7.69 (3.13–20), p < 0.001, log-likelihood = 56.16] and showed a high prediction performance (AUC 0.85).
The laboratory values obtained at 12 months confirmed the same trend presented at baseline, with higher levels of all cardiac biomarkers in the patients with CV events. At the multivariate analyses, sST2 [HR 1.15 (1.08–1.22), p < 0.001] and the ΔGal-3 [HR 1.26 (1.10–1.43), p ≤ 0.001] maintained their prognostic value at follow-up (Table 3). Interestingly, the baseline eGFR values did not significantly correlate with the composite outcome, as opposed to the values obtained at 1-year follow-up [HR: 0.84 (0.74–0.91), p = 0.04].
3.2. Predictive role of biomarkers and secondary outcomes
Concerning the secondary endpoints, baseline Gal-3 and sST2 maintained their significant association with both HF hospitalizations and CV mortality alone at the multivariate analyses, as shown in Supplementary Table S3, S4. The best predictor of CV mortality was Gal-3 with a cut-off value of 33.6 pg/ml (AUC 0.91 p < 0.001), and the relative Kaplan–Meier curve is shown in Figure 3.
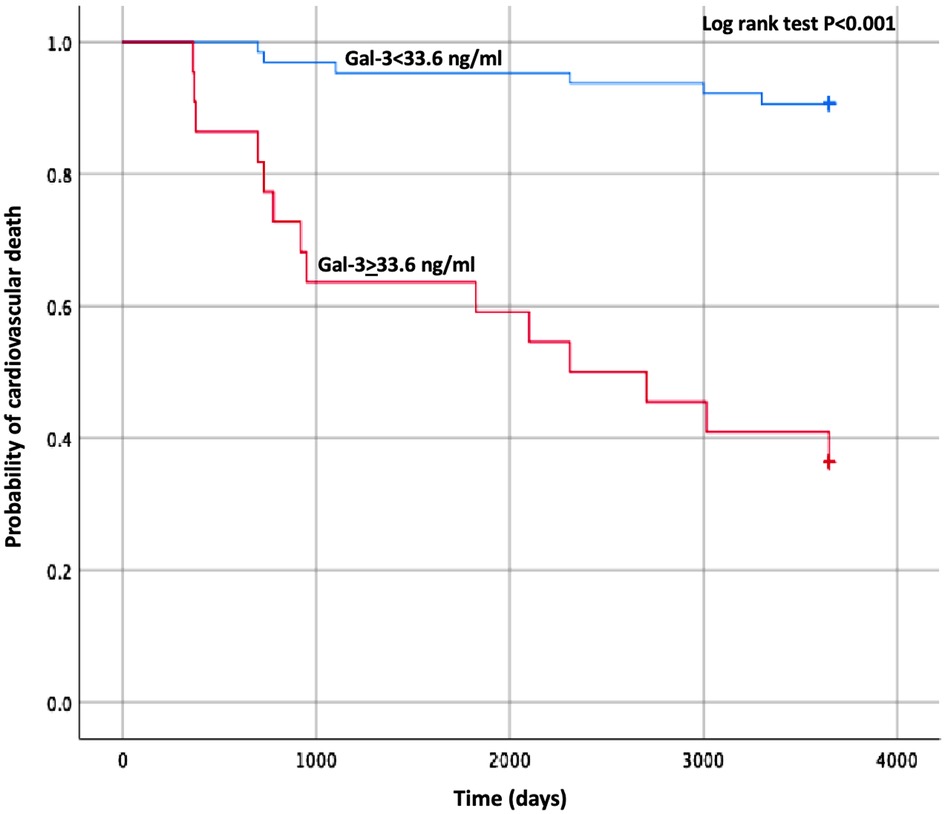
Figure 3. Kaplan–Meier estimates of the cumulative probability of cardiovascular death by Gal-3 cut-off. Gal-3, galectin-3.
As for the composite outcome, even if NT-proBNP alone was not significant, the parameter obtained by its combination with baseline sST2 (AUC 0.88, p < 0.001) showed good outcome prediction at the multivariate analysis [HR for HF hospitalizations 3.23 (1.89–4.35), p < 0.001, log-likelihood = 58.26; HR for CV mortality 3.33 (1.39–5.88), p = 0.010, log-likelihood = 59.37].
When biomarkers were evaluated at 1 year, the prediction model built with ΔGal-3 was predictive for the single components of secondary outcomes [HR for HF hospitalizations 1.19 (1.07–1.33), p = 0.001; HR for CV mortality 1.14 (1.04–1.26), p = 0.005]. The eGFR at follow-up maintained its prognostic role for cardiovascular mortality [HR 0.84 (0.80–0–90), p = 0.002].
Finally, no single predictor for the outcome of ventricular arrhythmias was significant at the multivariate analysis.
3.3. LV dimensions, CRT response, and outcomes
The difference between the left ventricular end-systolic volume (LVESV) at baseline and follow-up (ΔLVESV) did not correlate with primary and secondary outcome in all prognostic models when including biomarkers at the multivariate analyses, as shown in Table 3 and Supplementary Tables S2–S4. Accordingly, the echocardiographic definition of CRT response did not relate with the single components of the composite outcome in various prediction models and, among the total of 52 (62%) patients who showed LV reverse remodeling and were considered responders to CRT, no difference in terms of events rate was recorded.
4. Discussion
4.1. Biomarkers and outcome
In this paper concerning HFrEF patients undergoing CRT implantation, a significant correlation between biomarkers of myocardial inflammation, congestion, and fibrosis, together with renal function and the composite long-term outcome of HF hospitalization and CV mortality was found. Gal-3 and sST2 showed the highest power in the prediction models for CV death and HF hospitalization, also when analyzed as single endpoints. This finding supports the theory that inflammation and fibrosis can contribute to the course of the disease even when HF reaches advanced stages, indicating an ongoing myocardial damage and portending poor prognosis (14).
Although NT-proBNP, sST2, Gal-3, and eGFR, when considered individually, have already been demonstrated to have a prognostic role in HF (15–17), to the best of our knowledge, a combination of these parameters and its variations during the course of the disease were not tested in the candidates to CRT implantation in the long-term follow-up.
The sST2 was included in the Biomarker CRT score, developed in a sub-analysis of the SMART-AV trial, due to its additive predictive value of CRT response when considered against a composite of clinical variables (18). Its concentrations have been shown to predict sudden cardiac death in patients with HFrEF and provided complementary information to NT-proBNP (19). Moreover, serial measurements of sST2 provided incremental information to baseline levels, reflecting changes in myocardial remodeling over time and an increased risk of CV death (20).
Similarly, Gal-3 is a soluble beta-galactoside-binding lectin that has been related to inflammation and fibroblast activation; its effect on myocardial fibrosis, CV stiffness, and immune response modulation seems to determine pathological myocardial remodeling (21). High Gal-3 values have been related with CRT response at 6 months and with CV outcome at 48 months. Serial measurements have shown a prognostic role in acute HF, independently from BNP values (22). In a sub-analysis of CARE-HF, Gal-3 was an independent predictor of death from any cause or an unplanned hospitalization for a major CV event, even if it did not predict the response to CRT if considered as a separated outcome (23). Interestingly, in our population, the patients who met the primary outcome also showed higher Gal-3 levels at 1-year follow-up, and the ΔGal-3 was a prognostic marker at the prediction model, in line with the previous findings by Van Vark et al. (24).
When it comes to HF, heart and kidney functions are strictly intertwined. A renal dysfunction is very common in HFrEF, and it is acknowledged as a powerful predictor of survival (25). From a pathophysiological point of view, several mechanisms explain renal involvement in cardiac diseases, mainly attributable to renal congestion due to elevated venous pressure, decreased cardiac output, and activation of neurohormonal system (26). It has been described that the slight improvement in cardiac output after CRT may be associated with a concurrent improvement in renal function (27, 28). In our analysis, we confirm that the patients with adverse CV outcome show a significant decline of eGFR at follow-up compared with the patients without CV events. Moreover, eGFR is able to predict the absolute risk for adverse cardiac events. Maaten et al. demonstrated that the patients with chronic kidney disease undergoing CRT implantation, while experiencing a reverse remodeling in a lesser extent than those patients without renal dysfunction, also derive benefit on outcome at a lesser degree of remodeling. This could be related to the underlying pathogenesis of the renal dysfunction, such as nephrosclerosis, which is unlikely to respond to hemodynamic improvement (29).
4.2. Response to CRT and outcome
At follow-up, ΔLVESV and the reduction of more than 15% of LVESV did not show a significant relation with the outcome in our population. This finding seems to contrast with the large actual attention for the so-called CRT “response,” but lines up with a recent ESC position statement, which questions this arbitrary definition (30). Historically, the interest in literature for the research of variables to identify the patients who are less likely to benefit from CRT has always been alive. Also, a uniform way to define the desirable echocardiographic “response” to CRT is lacking, and echo improvement has been shown to be variable among different etiologies of HF. In fact, it has been argued that a binary definition of response underestimates the true benefits of CRT and that similar attention has not been posed to select patients for drug therapy. We challenge the idea that the selection of CRT candidates should be limited in base of the underlying etiology: while it is true that the patients with an ischemic etiology manifest less reverse remodeling, it should also be noticed that they have an equal relative risk reduction after CRT for HF admission and death as the non-ischemic group. Moreover, such a simple and cautious approach has resulted in a well-known undertreatment of dyssynchrony, preventing the patients to take advantage of a device that demonstrated to reduce morbidity and mortality (31). A lack of improvement in LVEF or in the symptoms of the patients (also considering the limitations of this evaluation) is not considered a good reason to withdraw one of the “drugs pillars” and accordingly should not be interpreted as a failure of CRT. The term “disease modification” (that may even imply a mere stabilization) should therefore replace the term “response” (32). Accordingly, our results corroborate the role of the laboratory values in CRT recipients, beyond the technical parameters used to define the efficacy of resynchronization. Importantly, the levels of these biomarkers, namely, sST2 and Gal-3, together with renal function, maintained their prognostic power also at 1-year follow-up. This should encourage clinicians to serially assess those values, especially when considering that many other parameters do not hold the same significance in advanced HF stages.
In our cohort, the risk stratification models incorporating one biomarker and E/e’ identified the patients at risk for CV outcome, confirming that the patients with higher left ventricular filling pressure (LVFP) at baseline before the device implantation show a worse prognosis. E/e’ is the most robust echocardiographic surrogate of an elevated LVFP, and several validation studies have confirmed the prediction of normal and abnormal LVFP when E/e’ ratio was <8 or >15 (33, 34). Elevated values of E/e’ ratio related to HF progression and worse prognosis as a consequence of an increased myocardial stiffness (35). Our data reproduce the findings of the REVERSE trial, where E/e’ ratio was associated with the endpoints of mortality and a new or recurrent HF in CRT recipients (36).
In summary, our results suggest that the laboratory parameters related to fibrosis production and extracellular matrix deposition, together with the concordant increase in echocardiographic surrogates of wall rigidity and chamber stiffness, are linked to an unfavorable outcome in CRT patients.
Our study comes with several limitations. All data were collected retrospectively from our single center, allowing the achievement of a small sample size with low cardiovascular events. However, we included an accurately screened population undergoing CRT implantation, following the indication of the latest guidelines. Gal-3 often increases in renal failure and chronic inflammatory diseases. Moreover, the so-called “response” to CRT depends on many different parameters: a role of underlying etiology, percentage of biventricular pacing, loss of LV capture, and compliance to medical therapy cannot be ruled out. In addition, notwithstanding that efforts should be made to optimize the efficacy of CRT capture after implantation, this does not affect the main finding of our study concerning the correlation between the biomarkers and the outcomes in these patients.
A total of four patients experienced the primary endpoint in the first year after the implantation, hence the correlation between echocardiographic and laboratory parameters at 1-year follow-up, and the outcome do not apply for them.
In conclusion, our study showed how, in a population of HFrEF patients implanted with CRT, a combined evaluation of biomarkers of cardiac inflammation, fibrosis, and renal function correlated with the combined outcome of CV death and HF hospitalization, as opposite to the echocardiographic definition of CRT response. The current findings cannot be extended to all HF patients with different etiologies and need to be confirmed in larger multi-center studies. However, despite the potential confounders, our results encourage clinicians to serially assess the levels of cardiac biomarkers to add significant prognostic implication.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
MB, APao, AG, MG, and MM performed the literature search and the data extraction. RB did the statistical analysis. MB, AG, and APal wrote the manuscript. MB and APal conceptualized and coordinated the study. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1180960/full#supplementary-material
References
1. Moulin T, Hamon D, Djouadi K, D’Humières T, Elbaz N, Boukantar M, et al. Impact of cardiac resynchronization therapy optimization inside a heart failure programme: a real-world experience. ESC Heart Fail. (2022) 9:3101–12. doi: 10.1002/ehf2.14043
2. Padeletti L, Paoletti Perini A, Gronda E. Cardiac resynchronization therapy: the issue of non-response. Heart Fail Rev. (2012) 17:97–105. doi: 10.1007/s10741-011-9250-6
3. O’Meara E, Zannad F. Fibrosis biomarkers predict cardiac reverse remodeling. JACC Heart Fail. (2023) 11:73–5. doi: 10.1016/j.jchf.2022.11.011
4. Aimo A, Januzzi JL, Vergaro G, Richards AM, Lam CS, Latini R, et al. Circulating levels and prognostic value of soluble ST2 in heart failure are less influenced by age than N-terminal pro-B-type natriuretic peptide and high-sensitivity troponin T. Eur J Heart Fail. (2020) 22:2078–88. doi: 10.1002/ejhf.1701
5. Beltrami M, Ruocco G, Ibrahim A, Lucani B, Franci B, Nuti R, et al. Different trajectories and significance of B-type natriuretic peptide, congestion and acute kidney injury in patients with heart failure. Intern Emerg Med. (2017) 12:593–603. doi: 10.1007/s11739-017-1620-1
6. Stolen C, Adourian A, Meyer T, Stein K, Solomon S. Plasma galectin-3 and heart failure outcomes in MADIT-CRT (multicenter automatic defibrillator implantation trial with cardiac resynchronization therapy). J Card Fail. (2014) 20:793–9. doi: 10.1016/j.cardfail.2014.07.018
7. Palazzuoli A, Beltrami M, Nodari S, McCullough PA, Ronco C. Clinical impact of renal dysfunction in heart failure. Rev Cardiovasc Med. (2011) 12:186–99. doi: 10.3909/ricm0581
8. McDonagh T, Metra M, Adamo M, Gardner R, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42:3599–726. doi: 10.1093/eurheartj/ehab368
9. Glikson M, Nielsen J, Kronborg M, Michowitz Y, Auricchio A, Barbash I, et al. 2021 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. (2021) 42:3427–520. doi: 10.1093/eurheartj/ehab364
10. Vardas P, Auricchio A, Blanc J, Daubert J, Drexler H, Ector H, et al. European Society of Cardiology; European Heart Rhythm Association. Guidelines for cardiac pacing and cardiac resynchronization therapy: the task force for cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology. Developed in collaboration with the European Heart Rhythm Association. Eur Heart J. (2007) 28:2256–95. doi: 10.1093/eurheartj/ehm305
11. Sassone B, Gambetti S, Bertini M, Beltrami M, Mascioli G, Bressan S, et al. Relation of QRS duration to response to cardiac resynchronization therapy. Am J Cardiol. (2015) 115:214–9. doi: 10.1016/j.amjcard.2014.10.024
12. Stiles M, Fauchier L, Morillo C, Wilkoff B, ESC Scientific Document Group. 2019 HRS/EHRA/APHRS/LAHRS focused update to 2015 expert consensus statement on optimal implantable cardioverter-defibrillator programming and testing. Eur Pacing Arrhythm Card Electrophysiol J Work Groups (2019) 21:1442–3. doi: 10.1093/europace/euz065
13. Lang R, Badano L, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. (2015) 16:233–70. doi: 10.1093/ehjci/jev014
14. Karayannis G, Triposkiadis F, Skoularigis J, Georgoulias P, Butler J, Giamouzis G. The emerging role of galectin-3 and ST2 in heart failure: practical considerations and pitfalls using novel biomarkers. Curr Heart Fail Rep. (2013) 10:441–9. doi: 10.1007/s11897-013-0169-1
15. Fung J, Szeto C, Chan J, Zhang Q, Chan H, Yip G, et al. Prognostic value of renal function in patients with cardiac resynchronization therapy. Int J Cardiol. (2007) 122:10–6. doi: 10.1016/j.ijcard.2006.11.015
16. Gehlken C, Suthahar N, Meijers WC, de Boer RA. Galectin-3 in heart failure: an update of the last 3 years. Heart Fail Clin. (2018) 14:75–92. doi: 10.1016/j.hfc.2017.08.009
17. Truong QA, Szymonifka J, Januzzi J, Contractor J, Deaño R, Chatterjee N, et al. Cardiorenal status using amino-terminal pro-brain natriuretic peptide and cystatin C on cardiac resynchronization therapy outcomes: from the BIOCRT study. Heart Rhythm. (2019) 16:928–35. doi: 10.1016/j.hrthm.2018.12.023
18. Spinale F, Meyer T, Stolen C, Van Eyk J, Gold M, Mittal S, et al. Development of a biomarker panel to predict cardiac resynchronization therapy response: results from the SMART-AV trial. Heart Rhythm. (2019) 16:743–53. doi: 10.1016/j.hrthm.2018.11.026
19. Pascual-Figal D, Ordoñez-Llanos J, Tornel P, Vázquez R, Puig T, Valdés M, et al. Soluble ST2 for predicting sudden cardiac death in patients with chronic heart failure and left ventricular systolic dysfunction. J Am Coll Cardiol. (2009) 54:2174–9. doi: 10.1016/j.jacc.2009.07.041
20. Skali H, Gerwien R, Meyer T, Snider J, Solomon S, Stolen C. Soluble ST2 and risk of arrhythmias, heart failure, or death in patients with mildly symptomatic heart failure: results from MADIT-CRT. J Cardiovasc Transl Res. (2016) 9:421–8. doi: 10.1007/s12265-016-9713-1
21. Beltrami M, Ruocco G, Dastidar A, Franci B, Lucani B, Aloia E, et al. Additional value of galectin-3 to BNP in acute heart failure patients with preserved ejection fraction. Clin Chim Acta Int J Clin Chem. (2016) 457:99–105. doi: 10.1016/j.cca.2016.04.007
22. Andre C, Piver E, Perault R, Bisson A, Pucheux J, Vermes E, et al. Galectin-3 predicts response and outcomes after cardiac resynchronization therapy. J Transl Med. (2018) 16:299. doi: 10.1186/s12967-018-1675-4
23. Lopez-Andrès N, Rossignol P, Iraqi W, Fay R, Nuée J, Ghio S, et al. Association of galectin-3 and fibrosis markers with long-term cardiovascular outcomes in patients with heart failure, left ventricular dysfunction, and dyssynchrony: insights from the CARE-HF (cardiac resynchronization in heart failure) trial. Eur J Heart Fail. (2012) 14:74–81. doi: 10.1093/eurjhf/hfr151
24. Van Vark L, Lesman-Leegte I, Baart S, Postmus D, Pinto Y, de Boer R, et al. Prognostic value of serial galectin-3 measurements in patients with acute heart failure. J Am Heart Assoc. (2017) 6:e003700. doi: 10.1161/JAHA.116.003700
25. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu Cy. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. (2004) 351:1296–305. doi: 10.1056/NEJMoa041031
26. Damman K, van Deursen V, Navis G, Voors A, van Veldhuisen D, Hillege H. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. (2009) 53:582–8. doi: 10.1016/j.jacc.2008.08.080
27. Boerrigter G, Costello-Boerrigter L, Abraham W, Sutton M, Heublein D, Kruger K, et al. Cardiac resynchronization therapy improves renal function in human heart failure with reduced glomerular filtration rate. J Card Fail. (2008) 14:539–46. doi: 10.1016/j.cardfail.2008.03.009
28. Adelstein E, Shalaby A, Saba S. Response to cardiac resynchronization therapy in patients with heart failure and renal insufficiency. Pacing Clin Electrophysiol PACE. (2010) 33:850–9. doi: 10.1111/j.1540-8159.2010.02705.x
29. Maaten JT, Martens P, L’hoyes W, Maass A, Damman K, Dupont M, et al. Response to cardiac resynchronization therapy across chronic kidney disease stages. J Card Fail. (2019) 25:803–11. doi: 10.1016/j.cardfail.2019.07.005
30. Mullens W, Auricchio A, Martens P, Witte K, Cowie M, Delgado V, et al. Optimized implementation of cardiac resynchronization therapy: a call for action for referral and optimization of care: a joint position statement from the Heart Failure association (HFA), European Heart Rhythm Association (EHRA), and European Association of Cardiovascular Imaging (EACVI) of the European Society of Cardiology. Eur J Heart Fail. (2020) 22:2349–69. doi: 10.1002/ejhf.2046
31. Sassone B, Bertini M, Beltrami M, Malagù M, Pasanisi G, Kuwornu H, et al. Relation of QRS duration to response to cardiac resynchronization therapy in patients with left bundle branch block. Am J Cardiol. (2017) 119:1803–8. doi: 10.1016/j.amjcard.2017.02.043
32. Cleland J, Freemantle N, Daubert JC, Toff W, Leisch F, Tavazzi L. Long-term effect of cardiac resynchronisation in patients reporting mild symptoms of heart failure: a report from the CARE-HF study. Heart Br Card Soc. (2008) 94:278–83. doi: 10.1136/hrt.2007.128991
33. Nagueh S, Smiseth O, Appleton C, Byrd B, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. (2016) 29:277–314. doi: 10.1016/j.echo.2016.01.011
34. Giudicatti L, Burrows S, Playford D, Strange G, Hillis G. Markers of elevated left ventricular filling pressure are associated with increased mortality in nonsevere aortic stenosis. J Am Soc Echocardiogr. (2021) 34:465–71. doi: 10.1016/j.echo.2020.12.017
35. Wang M, Yip G, Yu C, Zhang Q, Zhang Y, Tse D, et al. Independent and incremental prognostic value of early mitral annulus velocity in patients with impaired left ventricular systolic function. J Am Coll Cardiol. (2005) 45:272–7. doi: 10.1016/j.jacc.2004.09.059
36. St John Sutton M, Linde C, Gold M, Abraham W, Ghio S, Cerkvenik J, et al. Left ventricular architecture, long-term reverse remodeling, and clinical outcome in mild heart failure with cardiac resynchronization: results from the REVERSE trial. JACC Heart Fail. (2017) (3):169–78. doi: 10.1016/j.jchf.2016.11.012
Keywords: galectin-3, sST2, eGFR, biomarkers, heart failure, outcome, HF hospitalization, cardiovascular death
Citation: Beltrami M, Galluzzo A, Brocci RT, Paoletti Perini A, Pieragnoli P, Garofalo M, Halasz G, Milli M, Barilli M and Palazzuoli A (2023) The role of fibrosis, inflammation, and congestion biomarkers for outcome prediction in candidates to cardiac resynchronization therapy: is “response” the right answer?. Front. Cardiovasc. Med. 10:1180960. doi: 10.3389/fcvm.2023.1180960
Received: 6 March 2023; Accepted: 23 May 2023;
Published: 12 June 2023.
Edited by:
Alexander H. Maass, University Medical Center Groningen, NetherlandsReviewed by:
David Zizek, University Medical Centre Ljubljana, SloveniaErberto Carluccio, Heart Failure Unit, Italy
© 2023 Beltrami, Galluzzo, Brocci, Paoletti Perini, Pieragnoli, Garofalo, Halasz, Milli, Barilli and Palazzuoli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matteo Beltrami YmVsdHJhbWkubWF0dGVvMUBnbWFpbC5jb20=
 Matteo Beltrami
Matteo Beltrami Alessandro Galluzzo2
Alessandro Galluzzo2 Alessandro Paoletti Perini
Alessandro Paoletti Perini Geza Halasz
Geza Halasz Alberto Palazzuoli
Alberto Palazzuoli