
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 05 May 2023
Sec. Cardiovascular Imaging
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1137814
This article is part of the Research Topic Added Value of 3D Imaging in the Diagnosis and Prognostication of Patients with Right Ventricular Dysfunction View all 12 articles
 Alessandra M. Ferraro1,2,3
Alessandra M. Ferraro1,2,3 Kristin Bonello1,2
Kristin Bonello1,2 Lynn A. Sleeper1,2
Lynn A. Sleeper1,2 Minmin Lu1
Minmin Lu1 Melinda Shea1
Melinda Shea1 Gerald R. Marx1,2
Gerald R. Marx1,2 Andrew J. Powell1,2
Andrew J. Powell1,2 Tal Geva1,2
Tal Geva1,2 David M. Harrild1,2*
David M. Harrild1,2*
Background: Accurate measurement of ventricular volumes is an important clinical imaging goal. Three-dimensional echocardiography (3DEcho) is used increasingly as it is more available and less costly than cardiac magnetic resonance (CMR). For the right ventricle (RV), the current practice is to acquire 3DEcho volumes from the apical view. However, in some patients the RV may be better seen from the subcostal view. Therefore, this study compared RV volume measurements from the apical vs. the subcostal view, using CMR as a reference standard.
Methods: Patients <18 years old undergoing a clinical CMR examination were prospectively enrolled. 3DEcho was performed on the day of the CMR. 3DEcho images were acquired with Philips Epic 7 ultrasound system from apical and subcostal views. Offline analysis was performed with TomTec 4DRV Function for 3DEcho images and cvi42 for CMR ones. RV end-diastolic volume and end-systolic volume were collected. Agreement between 3DEcho and CMR was assessed with Bland-Altman analysis and the intraclass correlation coefficient (ICC). Percentage (%) error was calculated using CMR as the reference standard.
Results: Forty-seven patients were included in the analysis (age range 10 months to 16 years). The ICC was moderate to excellent for all volume comparisons to CMR (subcostal vs. CMR: end-diastolic volume 0.93, end-systolic volume 0.81; apical vs. CMR: end-diastolic volume 0.94, end-systolic volume 0.74).The 3DEcho mean % error vs. CMR for end-systolic volume was 25% for subcostal and 31% for apical; for end-diastolic volume it was 15% for subcostal and 16% for apical. The % error was not significantly different between apical vs. subcostal views for end-systolic and end-diastolic volume measurements.
Conclusions: For apical and subcostal views, 3DEcho-derived ventricular volumes agree well with CMR. Neither echo view has a consistently smaller error when compared to CMR volumes. Accordingly, the subcostal view can be used as an alternative to the apical view when acquiring 3DEcho volumes in pediatric patients, particularly when the image quality from this window is superior.
Measures of right ventricular (RV) volumes are critically important from a clinical perspective in the fields of pediatric and adult congenital heart disease (1–7), particularly in conditions providing a volume-loaded RV such as in the setting of an atrial septal defect or dysfunction of the pulmonary or tricuspid valves (7). Accurate measurement of these volumes is essential in the setting of tetralogy of Fallot (ToF), a common condition among pediatric patients (1, 3, 5, 6). In addition, RV volumes, together with RV function, are important parameters in patients status post Fontan palliation due to their correlation with mortality and heart transplant outcomes (8). Similarly, 3DEcho RV volume assessment was able to predict the severity of outcomes in patients with pulmonary hypertension (9). As well, 3DEcho RV volume assessment was the method used to assess differences in RV size and function after either Blalock-Taussig or Sano shunt in a multicenter study (9).
Cardiac magnetic resonance (CMR) is currently considered the reference standard for the measurement of ventricular volumes (10). However, this technology is relatively expensive and time-consuming. As an alternative, three-dimensional echocardiography (3DEcho) for the measurement of ventricular volumes is an emerging technique which has wider availability and lower expense relative to CMR, in addition to the fact that it may be used for patients with a contraindication to CMR. As well, 3DEcho images have a shorter acquisition time and time for analysis (with current semi-automated tools) as little as 3 min (11).
Traditionally, 3DEcho volumes have been acquired from an apical four-chamber view (12–18). However, there are limitations to this view including difficulty visualizing portions of the RV, particularly the outflow, and especially when ventricular dilation is present. These limitations result, in part, from the anterior position of the RV and its location just posterior to the sternum and rib cage (19, 20). Based on these challenges, recent data have called into question the practice of deriving 3DEcho RV volumes images based on apical view (20). An alternative to the apical view is the subcostal view. The potential advantage of this view is access to the entire RV, including the outflow, by avoiding acoustic shadowing from the sternum and rib cage (20, 21). On the other hand, it might happen that patients do not have adequate quality images from this view while having good apical ones. Therefore, in this study we sought to compare 3DEcho RV volume measurements from the apical vs. subcostal view, using contemporaneously acquired CMR measurements as a reference standard.
Patients who were referred for a clinical CMR were approached prospectively for acquisition of 3DEcho images immediately prior to or following the CMR examination. Inclusion criteria were age ≤18 years old, both a right and left ventricle were present, and adequate apical and subcostal imaging windows. The study protocol was approved by the local Institutional Review Board (IRB-P00033035). Informed consent was obtained from the patient's parent. The 3DEcho was acquired on the same day as the CMR in all cases.
Echocardiography images were obtained using the Philips EPIQ system (Philips Healthcare, Cambridge, MA, USA). Some patients were sedated for CMR for clinical indications and, in these cases, 3DEcho images were acquired while the patient was recovering from anesthesia; no additional sedation was administered for the 3DEcho images. The X5-1 or X-7-2 transducers were used based upon the patient's size. Images were acquired using standard techniques from subcostal and apical views by sonographers with expertise in 3DEcho (Figure 1). When possible, patients were instructed to hold their breath to minimize “stitch artifact” during imaging reconstruction. A 4 or 6 beat acquisition method was used in all cases.
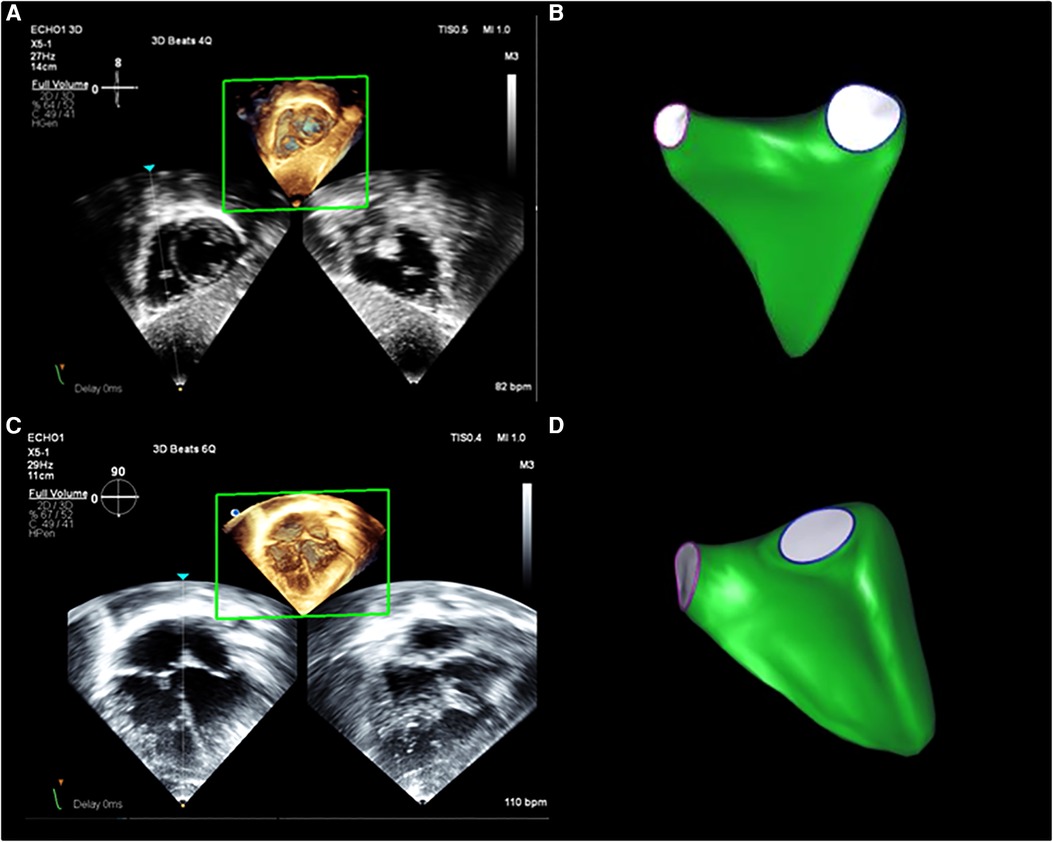
Figure 1. 3DEcho RV volume assessment. Example of RV volumes acquired from (A) subcostal and (C) apical views from a normal patient, as well as examples of the 3D surface resulting from analysis with the post-processing software from subcostal (B) and apical (D) views.
Deidentified images were stored in Digital Imaging and Communications in Medicine format. Offline volumetric analysis was carried out with 4D RV Function version 3 (TomTec, Unterschleißheim, Germany) according to the manufacturer's recommendations and prior descriptions (22). Manual adjustments of the endocardial borders as well as the tricuspid and pulmonary valves landmarks were made following the generation of the semi-automatic tracing (Figure 2). Custom bookmark tools were constructed to optimize the consistency of image alignment acquired from the subcostal view (Figure 3). End-diastolic volume (EDV) and end-systolic volume (ESV) were recorded. Intra- and inter-observer reproducibility was assessed for 10 randomly selected patients. For inter-observer measurements, the second analysis was performed at least 2 weeks after the first.
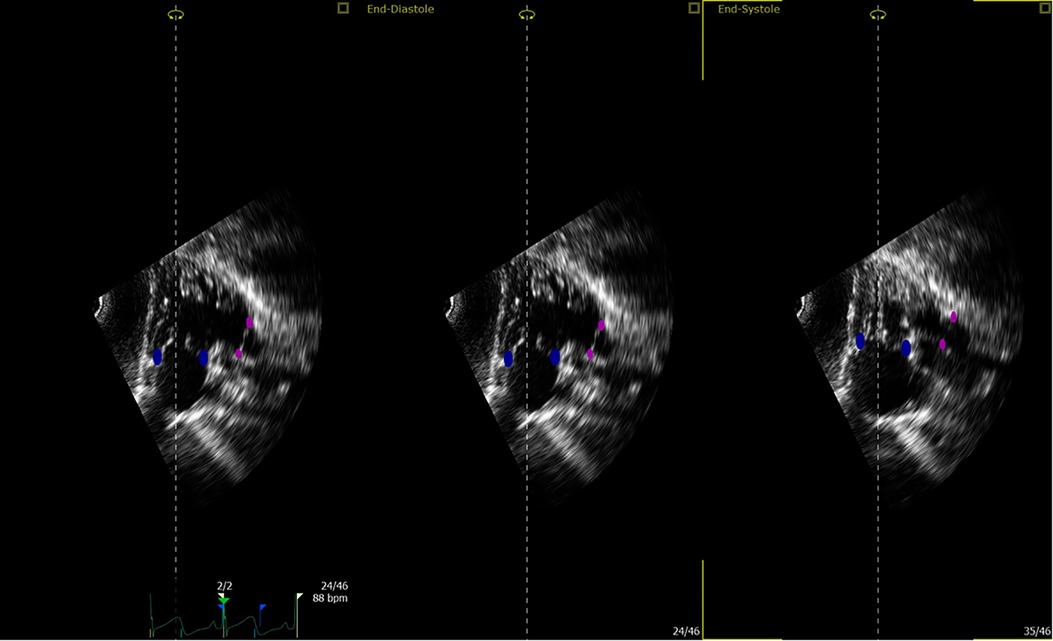
Figure 2. Tricuspid and pulmonary valve landmarks. Blue points define the tricuspid valve annulus; magenta points define the pulmonary valve annulus (this is true for both apical and subcostal views).
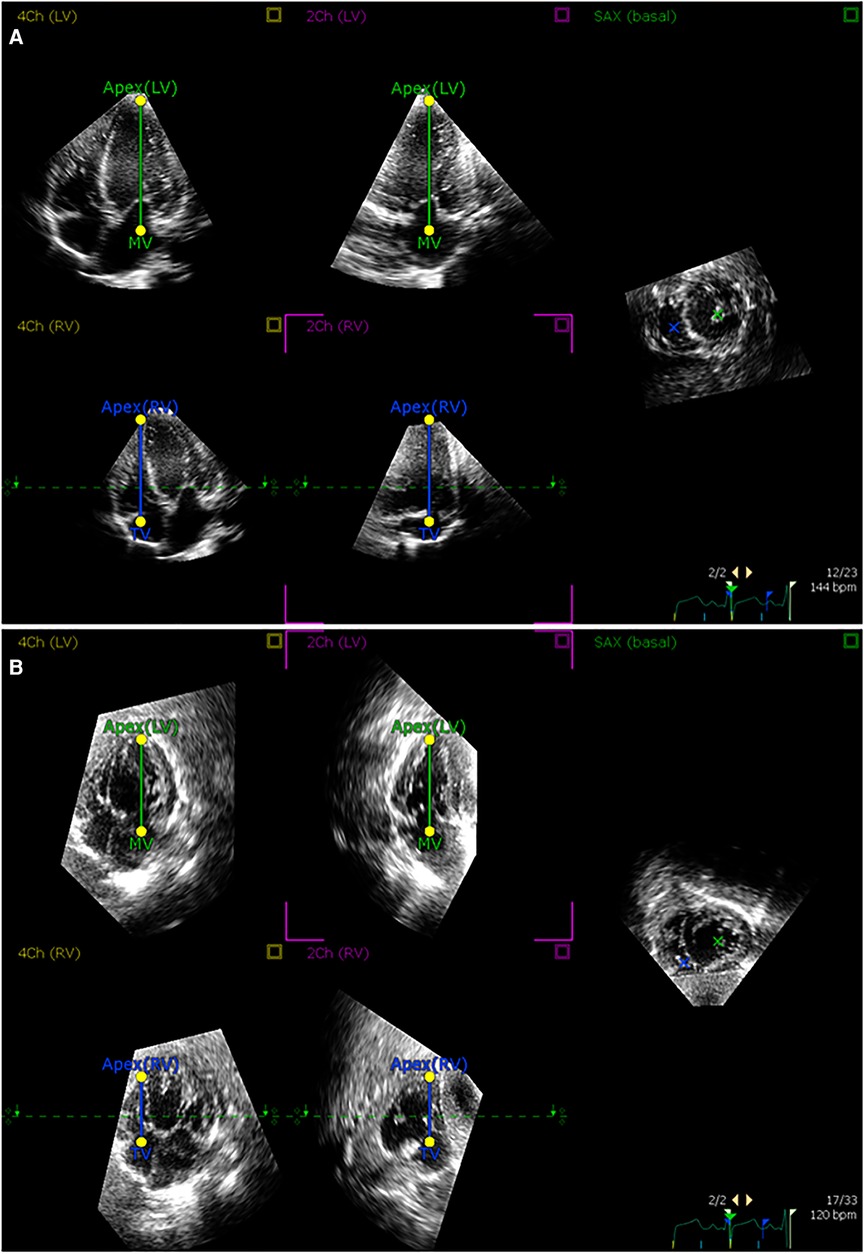
Figure 3. Alignment of 3D echocardiographic images from apical and subcostal views. An example of alignment of (A) apical and (B) subcostal views in the postprocessing software according to prespecified landmarks.
All CMR images were obtained from a 1.5 Tesla CMR scanner (Achieva, Phillips Healthcare, Best, the Netherlands). Imaging included a 12–14 slice stack (slice thickness 8–10 mm) of breath hold, ECG-gated, balanced steady-state free precession (bSSFP) cine acquisitions in the short-axis plane. Ventricular volumes were measured using commercially available software (cvi42, Circle Cardiovascular Imaging Inc., Calgary, Alberta, Canada; and QMass, Medis Medical Imaging Systems, Leiden, the Netherlands). Horizontal (four-chamber) and vertical (two-chamber) long-axis images were used as cross-references to aid with the identification of the ventricular myocardium to be included as chamber volume. The left ventricular papillary muscles and major trabeculations of the RV (e.g., septal band) were excluded from the blood pool and considered part of the myocardial mass as previously described (1, 2, 3).
Agreement between 3DEcho and CMR was assessed with Bland-Altman analysis and the intraclass correlation coefficient (ICC). To study if age may have an impact on agreement, we additionally divided our cohort in three age groups: <6 years old, 6–2 years old, and >12 years old. Percentage (%) error was calculated as [|(Echo − CMR)|/mean of Echo and CMR] × 100. Differences in raw values and % error for apical vs. subcostal views were compared with a paired t-test. In addition, differences in cardiac output using ventricular volumes calculated with apical vs. subcostal views were assessed using a Wilcoxon signed rank exact test.
Intra- and inter-observer reproducibility was assessed with a one-sample t-test and ICC. The Bland-Altman plots were used to display agreement between two readings (from the same observer) and between two readings (from different observers). Descriptive statistics include mean ± standard deviation and median with interquartile range. A p value 0.05 was considered to be statistically significant.
Fifty patients were consented for the study; in 3 of these, however, 3DEcho image quality from one of the views was judged to be inadequate for analysis upon subsequent review (apical view 1, subcostal view 2). Hence, the analytic cohort size for apical vs. subcostal comparisons was 47 patients. Demographic and clinical characteristics are presented in Table 1. Ages ranged from 10 months to 16 years. Eighteen patients underwent general anesthesia for CMR. Indications for CMR were for suspected or established congenital heart disease in nearly all patients (n = 43).
Bland-Altman plots for 3DEcho vs. CMR measurements of ventricular volumes for the two echo views are presented in Figure 4. In addition, differences in cardiac output using ventricular volumes calculated with apical vs. subcostal views were assessed using a Wilcoxon signed rank exact test. The biases were not significantly different for the comparison of end-diastolic measurements (panel A vs. panel C) (p value = 0.36), but they were different for end-systolic (panel B vs. panel D) (p-value <0.05). The biases were negative for all volume comparisons, reflecting an underestimation of 3DEcho ventricular volumes compared to CMR. Figure 5 presents box plots for the volume data analyzed in a grouped fashion. Volumes measured from the apical windows were significantly smaller than the CMR values (p values for EDV = 0.05 and ESV <0.001); the volumes measured by subcostal windows and the CMR values were not statistically different. When comparing mean volumes from subcostal to apical view: EDV volume from apical (98.5 ml) and subcostal (101.5 ml) views did not differ from each other, p = 0.36; ESV volume from apical (37.0 ml) and subcostal (43.9 ml) views differed one from the other (p < 0.05). Table 2 presents mean difference data between the two views compared to CMR, both as a raw value and as a percent error. The % error was not statistically different between apical vs. subcostal views for ESV and EDV measurements. Agreement as assessed by the ICC between 3DEcho and CMR for each view was high (ICC >0.7 for all), and higher for EDV compared to ESV (Table 2). When stratified by age, the patients in the middle age group (6 to 12 years) had a lower ICC than patients in both the youngest (<6 years) and oldest (>12 years) age groups. A factor contributing to these differences may be variations in patient diagnoses among the three patient groups; for example, the 6–12 year old cohort had a higher prevalence of patients with small left-sided structures than the other two.
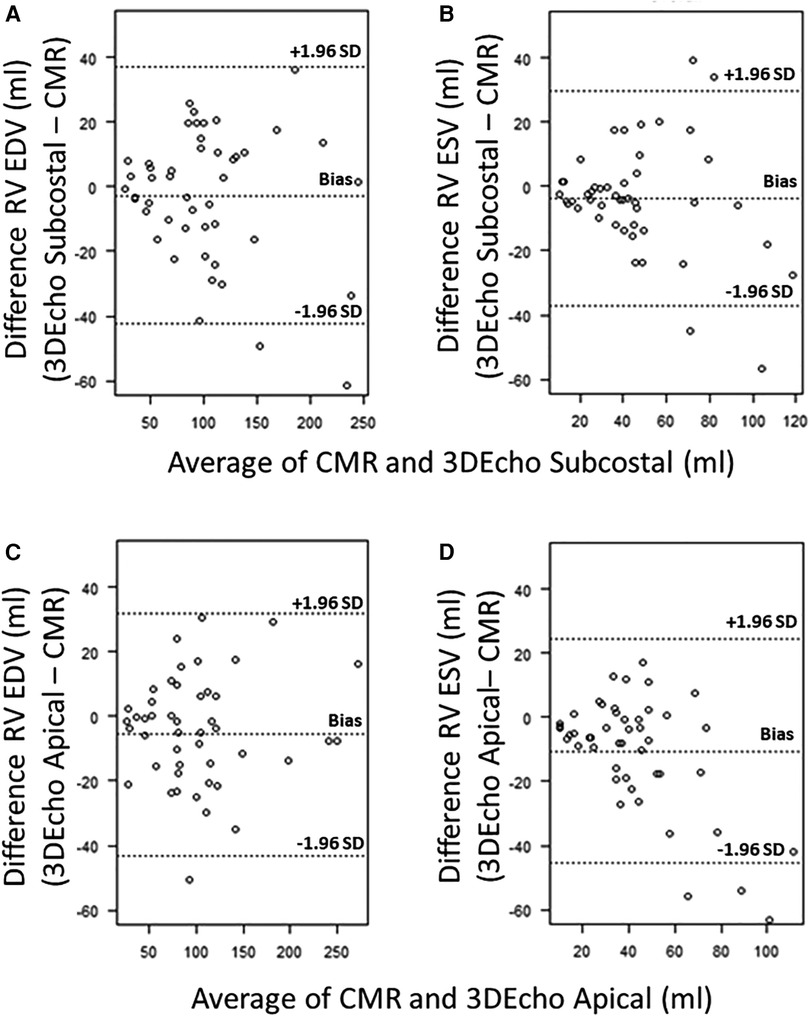
Figure 4. Bland–Altman plots for 3DEcho vs. CMR volumetric measurements (N = 47). Bland–Altman plots for 3DEcho vs. CMR for: end-diastolic measurements (panels A,C); end-systolic measurements (panels B,D). CMR, cardiac magnetic resonance; EDV, end diastolic volume; ESV, end systolic volume; ml, milliliter; RV, right ventricular.
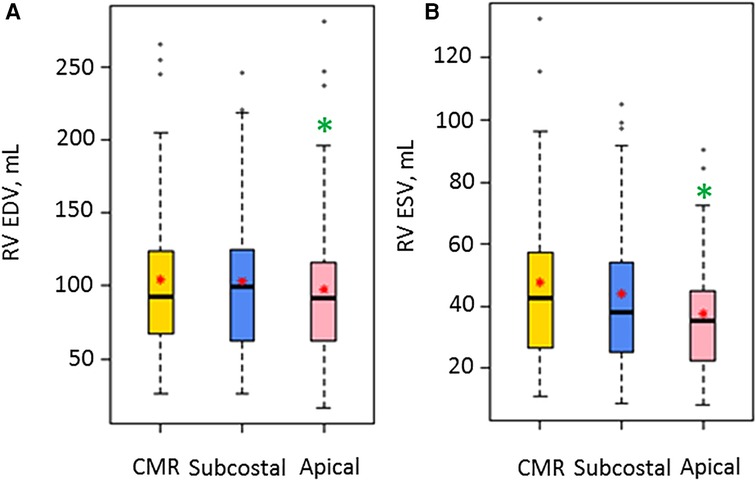
Figure 5. Box plots for volume measurements by CMR and the two 3D Echo views (N = 47). EDV, end diastolic volume; ESV, end systolic volume; ml, milliliter; RV, right ventricular. A red star indicates the mean value, and a solid box line indicates the median value. A green asterisk shows significant differences for the 3DEcho view relative to CMR.
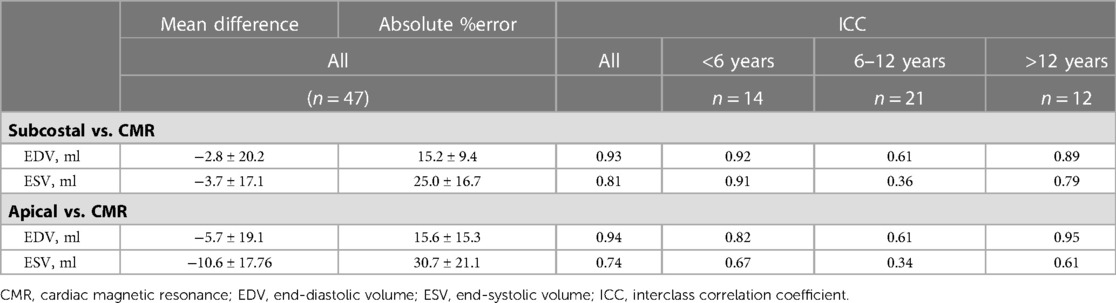
Table 2. Comparisons of RV volumes calculated by 3DEcho views vs. CMR; mean values, absolute percent error, and ICC.
The median and interquartile range for cardiac output were: subcostal view, 4,312 (2,893–5,711) ml/min; apical view, 4,296 (2,802–6,078) ml/min; these did not differ statistically (p = 0.42).
Inter- and intra-observer reproducibility data for RV EDV and RV ESV volume measurements using the two views are presented in Table 3. ICC values are in the good to excellent range (all >0.75) with slightly higher values for all EDV volumes measurements compared to ESV. The mean difference between the two measurements was statistically significant only for inter-observer subcostal RV ESV (p = 0.03).
In this study, we compared 3DEcho RV volume measurements from subcostal vs. apical views using CMR as the reference standard in a pediatric population. Overall, the volumes derived from echocardiography agreed well with those from CMR, for both systolic and diastolic measurements. In addition, we found no significant difference in the percent error of the measured volumes between the two views relative to CMR, thus, neither view clearly emerged as consistently inferior relative to the other.
The great majority of descriptions of 3DEcho RV volume acquisitions have used the apical view in a wide range of ages, in both normal and abnormal hearts (13–15, 20, 22, 24–26). In the pediatric population, the feasibility of quantifying 3D RV volumes from this view has varied widely with reported ranges between 20% (20) to 91% (14). 3DEcho RV volumes acquired from the apical view have been reported to correlate well with CMR (14, 22); however, there are limitations to acquisition from this view. For example, when the RV is dilated, portions of the ventricle may be incompletely captured from apical imaging, particularly the region of the heart adjacent to the transducer and the right ventricular outflow (14). In these cases, the 3D RV volumes may be significantly underestimated (13) (for example, inconsistent representation of the outflow tract). To overcome this issue, the subcostal view has been proposed as an alternative view for acquiring 3D RV volumes (20). In one early report describing its use in a pediatric cohort, the authors describe that analysis was feasible in 44% of the patients using this view, with only 20% feasibility in the same patients using the apical window. In our study, the feasibility of analysis was not examined; nearly all images could be analyzed as subjects had been preselected for having good-quality images from both views.
Comparisons of 3DEcho-based RV to CMR have been reported in multiple studies and nearly all have used the apical view for the echo-based quantification. For example, Dragulescu et al. compared 3DEcho RV volume images to CMR in 36 pediatric patients ages 7–18 years. They found that 3D RV EDV and ESV correlated very well with CMR (correlation coefficients were 0.99 for both EDV and ESV). In their report, Dragulescu et al. highlighted the importance of manually adjusting the endocardial borders and landmarks (such as tricuspid and pulmonary valves) to increase correlation of 3DEcho (14); this was similar to the techniques which we used in the current study. In a later report, Laser et al. (22) also found that 3DEcho RV volumes (EDV, ESV) were highly correlated to CMR (r values 0.98 for both volumes). Similarly, Muraru et al., in a cohort of congenital heart disease patients that included both pediatric and adult patients, showed that 3DEcho RV volumes correlated highly with CMR values (r = 0.92 and 0.93 for ESV and EDV, respectively) (11). Similar to these descriptions, our EDV measurements, from the apical view, agreed well with the CMR (ICC 0.94). ESV measurements, however, were less reliable (ICC 0.74). Some of the difference in our findings and those reported in the literature might be explained by differences in patient size and age, and the nature of the congenital heart disease in the included cohort, with variations in the degree of dilation of the portions of the RV that are particularly difficult to image by 3DEcho.
To the best of our knowledge, the only other study that has compared 3DEcho RV volumes acquired from the subcostal view to CMR measurements was also from our group (27). In this report, we studied a pediatric population ages 2 to 8 years with a variety of forms of single ventricle congenital heart disease. EDV and ESV agreed well with CMR values (ICCs 0.95 and 0.94, respectively) (27). In the current study, 3DEcho-based EDV from the subcostal view also agreed well with CMR (ICC = 0.93). ESV agreement was good (ICC 0.81) but somewhat less robust. In a subgroup analysis, agreement was best in the youngest cohort, perhaps reflecting enhanced image quality in this group of patients relative to the older cohort.
Prior reports of RV volume measurements (both EDV and ESV) have described good to excellent intra- and inter-observer reproducibility with less reproducibility for intra-observer readings (14, 17, 18, 20, 27, 28). Our results for intra- and intra-observer reproducibility follow a similar pattern with ICC values reflecting good agreement. Values were generally higher for intra-observer measurements, compared to inter-observer, as is typically the case, and were higher for end-diastolic volume compared to end-systolic volume. The latter finding is likely a reflection of the highly trabeculated nature of the RV leading to difficulty identifying the optimal location for placing the end-systolic contour.
While CMR was considered a reference standard, this technology itself has a certain amount of intrinsic variability in volume measurement. However, CMR is considered the most reliable imaging modality for the assessment of ventricular volumes. Our patients did not have the echo and CMR performed at precisely the same time. However, 3DEcho was performed soon before or after the CMR to mitigate change in patient conditions as much as possible. Finally, while differences are reported as “statistically significant”, these differences may not necessarily have clinical importance.
3DEcho measurements of RV volumes based on either subcostal or apical views agree well with corresponding values from CMR. There were no significant differences in the errors calculated by either view relative to CMR. These results support the use of the subcostal view as an alternative to the apical view when acquiring 3DEcho volumes in pediatric patients with good subcostal views, particularly if the RV outflow is not well seen in the apical view. These findings may encourage the use of 3DEcho measurement of RV volumes in the pediatric population, particularly when access to CMR is limited.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. The data and materials from this study will be available upon request and following approval from our Institutional Review Board.
The studies involving human participants were reviewed and approved by Institutional Review Board—Boston Children's Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
All authors provided substantial contribution to the conception of the study; AMF and DMH designed the study; AMF and KB collected data; AMF, ML, LAS, DMH analyzed and interpreted data; AMF, ML, and LAS prepared figures; AMF and DMH drafted the manuscript. All authors contributed to the article and approved the submitted version.
We would like to acknowledge funding from the Higgins Family Research Fund.
The authors would like to thank the sonographers who helped in acquiring the 3DEchos: Isabella Andrade, Efe Clode, Kathryn Cronin, Brenna Hardiman, Sara Keyser, Manouk Kirakosian, Linda Le, Rose Lipinski, Kaitlyn Lozier, Rebecca Odour, Stephen O'Neil, Stephanie Quealy, Nicole Rabideau, Marga Rivera, Ingrid Roth, Matthew Schildmeier, Eugenya Stantcheva, Stephanie Strout, Xiahong Su, Bridget Weygand, and Kara Zammito. In addition, we thank Dr. Sonal Jhaveri for her dedication in teaching scientific writing and for editing an early version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
bSSFP, balanced steady-state free precession; CMR, cardiac magnetic resonance; EDV, end-diastolic volume; ESV, end-systolic volume; ICC, interclass correlation coefficient; RV, right ventricular; ToF, Tetralogy of Fallot; 3DEcho, three-dimensional echocardiography.
1. Geva T. Repaired tetralogy of Fallot: the roles of cardiovascular magnetic resonance in evaluating pathophysiology and for pulmonary valve replacement decision support. J Cardiovasc Magn Reson. (2011) 13(1):9. doi: 10.1186/1532-429X-13-9
2. Williams RG, Pearson GD, Barst RJ, Child JS, del Nido P, Gersony WM, et al. Report of the national heart, lung, and blood institute working group on research in adult congenital heart disease. J Am Coll Cardiol. (2006) 47(4):701–7. doi: 10.1016/j.jacc.2005.08.074
3. Oosterhof T, van Straten A, Vliegen HW, Meijboom FJ, van Dijk AP, Spijkerboer AM, et al. Preoperative thresholds for pulmonary valve replacement in patients with corrected tetralogy of Fallot using cardiovascular magnetic resonance. Circulation. (2007) 116(5):545–51. doi: 10.1161/CIRCULATIONAHA.106.659664
4. Knauth AL, Gauvreau K, Powell AJ, Landzberg MJ, Walsh EP, Lock JE, et al. Ventricular size and function assessed by cardiac MRI predict major adverse clinical outcomes late after tetralogy of Fallot repair. Heart. (2008) 94(2):211–6. doi: 10.1136/hrt.2006.104745
5. Geva T, Sandweiss BM, Gauvreau K, Lock JE, Powell AJ. Factors associated with impaired clinical status in long-term survivors of tetralogy of Fallot repair evaluated by magnetic resonance imaging. J Am Coll Cardiol. (2004) 43(6):1068–74. doi: 10.1016/j.jacc.2003.10.045
6. Shimazaki Y, Kawashima Y, Mori T, Kitamura S, Matsuda H, Yokota K. Ventricular volume characteristics of single ventricle before corrective surgery. Am J Cardiol. (1980) 45(4):806–10. doi: 10.1016/0002-9149(80)90125-3
7. Davlouros PA, Niwa K, Webb G, Gatzoulis MA. The right ventricle in congenital heart disease. Heart. (2006) 92(Suppl 1):i27–38. doi: 10.1136/hrt.2005.077438
8. Piran S, Veldtman G, Siu S, Webb GD, Liu PP. Heart failure and ventricular dysfunction in patients with single or systemic right ventricles. Circulation. (2002) 105(10):1189–94. doi: 10.1161/hc1002.105182
9. Marx GR, Shirali G, Levine JC, Guey LT, Cnota JF, Baffa JM, et al. Multicenter study comparing shunt type in the Norwood procedure for single-ventricle lesions: three-dimensional echocardiographic analysis. Circ Cardiovasc Imaging. (2013) 6(6):934–42. doi: 10.1161/CIRCIMAGING.113.000304
10. Kilner PJ, Geva T, Kaemmerer H, Trindade PT, Schwitter J, Webb GD. Recommendations for cardiovascular magnetic resonance in adults with congenital heart disease from the respective working groups of the European society of cardiology. Eur Heart J. (2010) 31(7):794–805. doi: 10.1093/eurheartj/ehp586
11. Muraru D, Spadotto V, Cecchetto A, Romeo G, Aruta P, Ermacora D, et al. New speckle-tracking algorithm for right ventricular volume analysis from three-dimensional echocardiographic data sets: validation with cardiac magnetic resonance and comparison with the previous analysis tool. Eur Heart J Cardiovasc Imaging. (2016) 17(11):1279–89. doi: 10.1093/ehjci/jev309
12. Laser KT, Horst JP, Barth P, Kelter-Klöpping A, Haas NA, Burchert W, et al. Knowledge-based reconstruction of right ventricular volumes using real-time three-dimensional echocardiographic as well as cardiac magnetic resonance images: comparison with a cardiac magnetic resonance standard. J Am Soc Echocardiogr. (2014) 27(10):1087–97. doi: 10.1016/j.echo.2014.05.008
13. Khoo NS, Young A, Occleshaw C, Cowan B, Zeng IS, Gentles TL. Assessments of right ventricular volume and function using three-dimensional echocardiography in older children and adults with congenital heart disease: comparison with cardiac magnetic resonance imaging. J Am Soc Echocardiogr. (2009) 22(11):1279–88. doi: 10.1016/j.echo.2009.08.011
14. Dragulescu A, Grosse-Wortmann L, Fackoury C, Mertens L. Echocardiographic assessment of right ventricular volumes: a comparison of different techniques in children after surgical repair of tetralogy of Fallot. Eur Heart J Cardiovasc Imaging. (2012) 13(7):596–604. doi: 10.1093/ejechocard/jer278
15. Sato T, Calderon RJ, Klas B, Pedrizzetti G, Banerjee A. Simultaneous volumetric and functional assessment of the right ventricle in hypoplastic left heart syndrome after fontan palliation, utilizing 3-dimensional speckle-tracking echocardiography. Circ J. (2020) 84(2):235–44. doi: 10.1253/circj.CJ-19-0926
16. Kamińska H, Małek Ł A, Barczuk-Falęcka M, Werner B. Usefulness of three-dimensional echocardiography for the assessment of ventricular function in children: comparison with cardiac magnetic resonance, with a focus on patients with arrhythmia. Cardiol J. (2021) 28(4):549–57. doi: 10.5603/CJ.a2019.0026
17. Jone PN, Schäfer M, Pan Z, Bremen C, Ivy DD. 3D Echocardiographic evaluation of right ventricular function and strain: a prognostic study in paediatric pulmonary hypertension. Eur Heart J Cardiovasc Imaging. (2018) 19(9):1026–33. doi: 10.1093/ehjci/jex205
18. Bell A, Rawlins D, Bellsham-Revell H, Miller O, Razavi R, Simpson J. Assessment of right ventricular volumes in hypoplastic left heart syndrome by real-time three-dimensional echocardiography: comparison with cardiac magnetic resonance imaging. Eur Heart J Cardiovasc Imaging. (2014) 15(3):257–66. doi: 10.1093/ehjci/jet145
19. Wu VC, Takeuchi M. Echocardiographic assessment of right ventricular systolic function. Cardiovasc Diagn Ther. (2018) 8(1):70–9. doi: 10.21037/cdt.2017.06.05
20. Renella P, Marx GR, Zhou J, Gauvreau K, Geva T. Feasibility and reproducibility of three-dimensional echocardiographic assessment of right ventricular size and function in pediatric patients. J Am Soc Echocardiogr. (2014) 27(8):903–10. doi: 10.1016/j.echo.2014.04.008
21. Kurath-Koller S, Koestenberger M, Hansmann G, Cantinotti M, Tissot C, Sallmon H. Subcostal echocardiographic imaging in neonatal and pediatric intensive care. Front Pediatr. (2021) 9:471558. doi: 10.3389/fped.2021.471558
22. Laser KT, Karabiyik A, Körperich H, Horst JP, Barth P, Kececioglu D, et al. Validation and reference values for three-dimensional echocardiographic right ventricular volumetry in children: a multicenter study. J Am Soc Echocardiogr. (2018) 31(9):1050–63. doi: 10.1016/j.echo.2018.03.010
23. Alfakih K, Plein S, Thiele H, Jones T, Ridgway JP, Sivananthan MU. Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady-state free precession imaging sequences. J Magn Reson Imaging. (2003) 17(3):323–9. doi: 10.1002/jmri.10262
24. Nagata Y, Wu VC, Kado Y, Otani K, Lin FC, Otsuji Y, et al. Prognostic value of right ventricular ejection fraction assessed by transthoracic 3D echocardiography. Circ Cardiovasc Imaging. (2017) 10(2):e005384. doi: 10.1161/CIRCIMAGING.116.005384
25. Grewal J, Majdalany D, Syed I, Pellikka P, Warnes CA. Three-dimensional echocardiographic assessment of right ventricular volume and function in adult patients with congenital heart disease: comparison with magnetic resonance imaging. J Am Soc Echocardiogr. (2010) 23(2):127–33. doi: 10.1016/j.echo.2009.11.002
26. Medvedofsky D, Addetia K, Patel AR, Sedlmeier A, Baumann R, Mor-Avi V, et al. Novel approach to three-dimensional echocardiographic quantification of right ventricular volumes and function from focused views. J Am Soc Echocardiogr. (2015) 28(10):1222–31. doi: 10.1016/j.echo.2015.06.013
27. Soriano BD, Hoch M, Ithuralde A, Geva T, Powell AJ, Kussman BD, et al. Matrix-array 3-dimensional echocardiographic assessment of volumes, mass, and ejection fraction in young pediatric patients with a functional single ventricle: a comparison study with cardiac magnetic resonance. Circulation. (2008) 117(14):1842–8. doi: 10.1161/CIRCULATIONAHA.107.715854
Keywords: three-dimensional echocardiography, right ventricular volumes, congenital heart disease, pediatrics, apical view, subcostal view
Citation: Ferraro AM, Bonello K, Sleeper LA, Lu M, Shea M, Marx GR, Powell AJ, Geva T and Harrild DM (2023) A comparison between the apical and subcostal view for three-dimensional echocardiographic assessment of right ventricular volumes in pediatric patients. Front. Cardiovasc. Med. 10:1137814. doi: 10.3389/fcvm.2023.1137814
Received: 4 January 2023; Accepted: 19 April 2023;
Published: 5 May 2023.
Edited by:
Márton Tokodi, Semmelweis University, HungaryReviewed by:
Alisa Arunamata, Stanford University, United States© 2023 Ferraro, Bonello, Sleeper, Lu, Shea, Marx, Powell, Geva and Harrild. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David M. Harrild david.harrild@cardio.chboston.org
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.