
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 16 February 2023
Sec. Coronary Artery Disease
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1115463
This article is part of the Research TopicManagement of Ischemic CardiomyopathyView all 4 articles
 Long Yang1†
Long Yang1† Xia Dong2†
Xia Dong2† Baiheremujiang Abuduaini3
Baiheremujiang Abuduaini3 Nueraihemaiti Jiamali3
Nueraihemaiti Jiamali3 Zulihuma Seyiti1
Zulihuma Seyiti1 Xue-Feng Shan4*
Xue-Feng Shan4* Xiao-Ming Gao5,6,7*
Xiao-Ming Gao5,6,7*Background: Ischemic Heart Disease (IHD) is the leading cause of death from cardiovascular disease. Currently, most studies have focused on factors influencing IDH or mortality risk, while few predictive models have been used for mortality risk in IHD patients. In this study, we constructed an effective nomogram prediction model to predict the risk of death in IHD patients by machine learning.
Methods: We conducted a retrospective study of 1,663 patients with IHD. The data were divided into training and validation sets in a 3:1 ratio. The least absolute shrinkage and selection operator (LASSO) regression method was used to screen the variables to test the accuracy of the risk prediction model. Data from the training and validation sets were used to calculate receiver operating characteristic (ROC) curves, C-index, calibration plots, and dynamic component analysis (DCA), respectively.
Results: Using LASSO regression, we selected six representative features, age, uric acid, serum total bilirubin, albumin, alkaline phosphatase, and left ventricular ejection fraction, from 31 variables to predict the risk of death at 1, 3, and 5 years in patients with IHD, and constructed the nomogram model. In the reliability of the validated model, the C-index at 1, 3, and 5 years was 0.705 (0.658–0.751), 0.705 (0.671–0.739), and 0.694 (0.656–0.733) for the training set, respectively; the C-index at 1, 3, and 5 years based on the validation set was 0.720 (0.654–0.786), 0.708 (0.650–0.765), and 0.683 (0.613–0.754), respectively. Both the calibration plot and the DCA curve are well-behaved.
Conclusion: Age, uric acid, total serum bilirubin, serum albumin, alkaline phosphatase, and left ventricular ejection fraction were significantly associated with the risk of death in patients with IHD. We constructed a simple nomogram model to predict the risk of death at 1, 3, and 5 years for patients with IHD. Clinicians can use this simple model to assess the prognosis of patients at the time of admission to make better clinical decisions in tertiary prevention of the disease.
In many countries, cardiovascular disease is increasing annually and poses a serious threat to human life (1). Ischemic Heart Disease (IHD) is the leading cause of death from cardiovascular disease (2). IHD is caused by plaque accumulation along the inner wall of the coronary artery, resulting in partial or complete occlusion of the coronary artery, thereby reducing blood flow to the heart (3). Long-term IHD imbalances coronary blood flow regulatory mechanisms, including ion channels, leading to the development of hypoxia, fibrosis, and tissue death, which may determine the loss of myocardial function (4). These mechanisms will produce serious complications such as heart failure, ventricular arrhythmias, and myocardial infarction, ultimately leading to the death of patients (5). IHD mortality remains persistently elevated in most developing countries worldwide, placing a significant economic and resource burden on health and public health systems (6). Therefore, early identification of the risk of mortality in IHD patients becomes particularly important.
Numerous factors influence the risk of mortality in patients with IHD, including gender, age, blood pressure, smoking, alcohol consumption, and the presence of comorbidities, such as a history of diabetes and hypertension (7). Besides, the predictive role of leukocytes, neutrophils, lymphocytes, monocytes, eosinophils, basophils, erythrocytes, hemoglobin, and platelets for cardiovascular disease has also been reported (8). Furthermore, there are several recognized risk factors for cardiovascular disease, such as creatinine, uric acid, fasting blood glucose, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, total bilirubin, albumin, alanine aminotransferase, AST, lactate dehydrogenase, γ-glutamyl transferase, alkaline phosphatase, and creatine kinase, all of which play an important role in the progression of cardiovascular disease (9–12). In addition, the left ventricular ejection fraction is used to assess important echocardiographic parameters of cardiac function in IHD patients (13).
Most of the current research has focused on specific risk factors affecting death in IDH, and few predictive models have been used for the risk of death in patients with IHD. In this study, we constructed an effective nomogram prediction model to predict the risk of death in IHD patients by machine learning. The nomogram can be used to accurately predict the risk of disease or complications in many cases (14), and our study is based on data from the largest medical center in Xinjiang, China, to more comprehensively consider the impact of various factors on prognosis to develop and validate a simple, practical, and accurate risk prediction tool for IHD patients. The nomograms we developed can be used to assess the risk of death at 1, 3, and 5 years in patients with IHD, providing clinicians with a way to identify people at high risk of death from IDH at admission, and then appropriate interventions to prolong the survival time of patients.
We selected the data of patients who attended the First Affiliated Hospital of Xinjiang Medical University from 2010 to 2017, and a total of 2,043 patients with ischemic heart disease were included in this study. According to the Declaration of Helsinki, informed consent was obtained and the patients’ identities were completely concealed before including the data in the study. The inclusion and exclusion criteria were as follows.
Diagnosis on admission was ischemic heart disease: (1) angina pectoris or equivalent symptoms occurring during exercise, rest, or nitroglycerin relief. (2) Electrocardiogram showed significant myocardial ischemia type, and an exercise test revealed myocardial ischemia. (3) Coronary angiography showed that the degree of coronary artery stenosis was greater than 50%.
(1) Congenital heart disease. (2) Severe heart valve disease (mitral stenosis, mitral regurgitation, aortic stenosis, and aortic insufficiency); (3) Pericardial disease (acute pericarditis, constrictive pericarditis). (4) Heart failure caused by non-cardiac factors (severe infection, anemia); (5) Thyroid dysfunction. (6) Severe liver damage (alanine aminotransferase and aspartate aminotransferase three times higher than the upper limit of normal); (7) Severe renal impairment. (8) Malignant tumor.
We followed the patients for 5 years with the endpoint event of cardiovascular death. After excluding samples with missing follow-up and other causes of death, we finally included a sample of 1,663 cases (Figure 1).
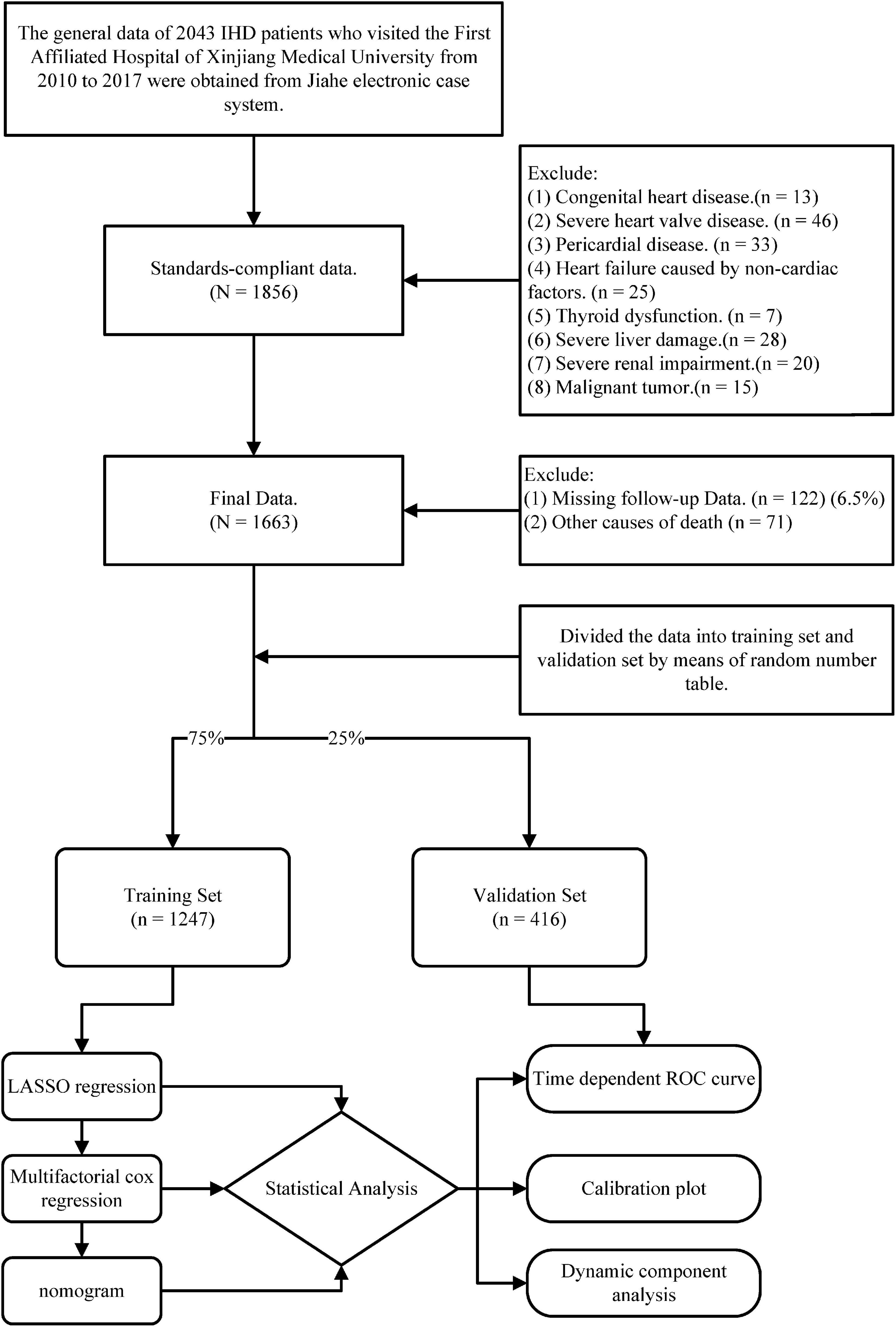
Figure 1. The flow chart presents the entire process of patient follow-up, data collection, and statistical analysis in this study.
Patient clinical data was derived from information stored in the Jiahe electronic case system at the time of initial presentation. Include gender, smoking history, alcohol history, diabetes history, hypertension history, age, systolic blood pressure, diastolic blood pressure, and other general information. All patients were asked to fast on the next day and 8 mL of venous blood was collected at admission, and routine blood data were measured using an automatic hematology analyzer XN-1000 (Sysmex, Japan). Biochemical data such as serum creatinine, uric acid, and fasting blood glucose were measured using an automatic biochemical analyzer DXC800 (Beckman Coulter, USA). Echocardiography was performed within 24 h after admission using a VIVID7 color Doppler ultrasound diagnostic apparatus (GE, USA) to measure left ventricular ejection fraction.
Statistical analysis of data from this study was performed using R software (The R Foundation).1 Two-sided P < 0.05 was considered statistically significant in all analyses. First, the patients were divided into training and validation sets for external validation with R software satisfying a 3:1 ratio. A normality test was performed on both data sets, and the study data were found to be non-normally distributed. Continuous variables are therefore presented as medians (quartiles) and categorical variables as frequencies (percentages). The Mann–Whitney U-test is used for all continuous variables and the Chi-squared test is used for all categorical variables. There were no statistically significant differences between the two groups in the above tests (P > 0.05). Data from the training set were then analyzed using least absolute shrinkage and selection operator (LASSO) regression. Variables with non-zero coefficients in the LASSO regression model were used to build nomogram prediction models. Multivariate Cox regression was used to verify whether the variables in the model were statistically significant. To test the accuracy of the risk prediction model, receiver operating characteristic (ROC) curves, C-Index, calibration plot, and dynamic component analysis (DCA) were calculated using data from the training and validation sets, respectively.
Ultimately, 1,663 participants with IHD were enrolled in the study. During a median follow-up of 42 months, 693 patients died. The median age of the patients was 47 years, with 1,264 (76%) males and 399 (24%) females. For external validation, the total number of cases was divided into a training set (1,247 patients) and a validation set (416 patients) in a ratio of approximately 3:1. The median age of the patients in the training set was 74 years, with 950 males and 297 females. During a median follow-up period of 42 months, 511 patients died. The median age of the patients in the validation set was 73 years, with 314 males and 102 females. During a median follow-up period of 42 months, 182 patients died. The two groups had no statistically significant difference in general information or laboratory test data (Table 1).
Least absolute shrinkage and selection operator regression analysis was performed on the training set data. By selecting non-zero features in the LASSO regression analysis results, the number of potential predictor variables was reduced from 18 to 6 (Figures 2A, B). Including age, uric acid, serum total bilirubin, albumin, alkaline phosphatase, and left ventricular ejection fraction. Multivariate Cox regression analysis was then used to verify whether each variable was statistically significant (Table 2). Finally, the above six risk profile factors were used to develop a predictive model to predict the risk of death at 1, 3, and 5 years in IHD patients and displayed in the form of nomograms (Figure 3).
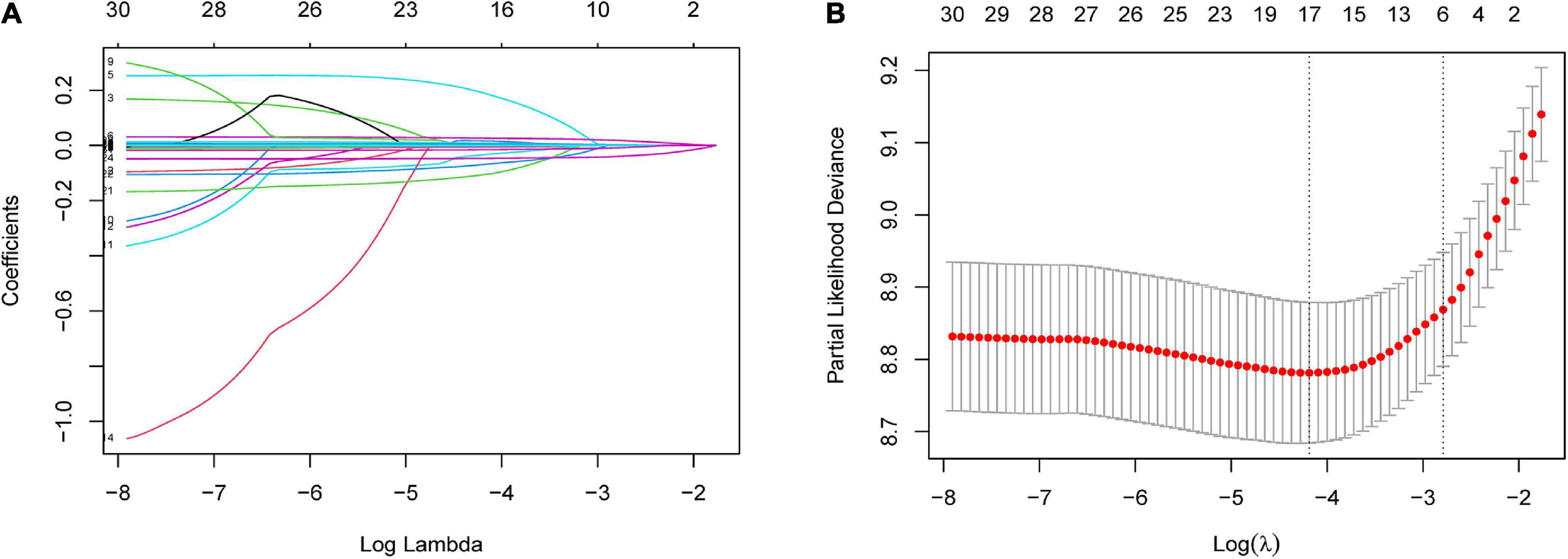
Figure 2. Risk factors contributing to death within 5 years in patients with IHD were selected using LASSO regression models. (A) LASSO coefficient profiles of the 31 features. A coefficient profile plot was generated against the log(lambda) sequence. (B) Optimal parameter (lambda) selection in the LASSO model used five-fold cross-validation based on minimum criteria. The partial likelihood deviance (binomial deviance) curve was plotted vs. log(lambda). Dotted vertical lines were drawn at the optimal values by using the minimum criteria and the 1 SE of the minimum criteria (the 1-SE criteria). LASSO coefficient profiles of the six features.
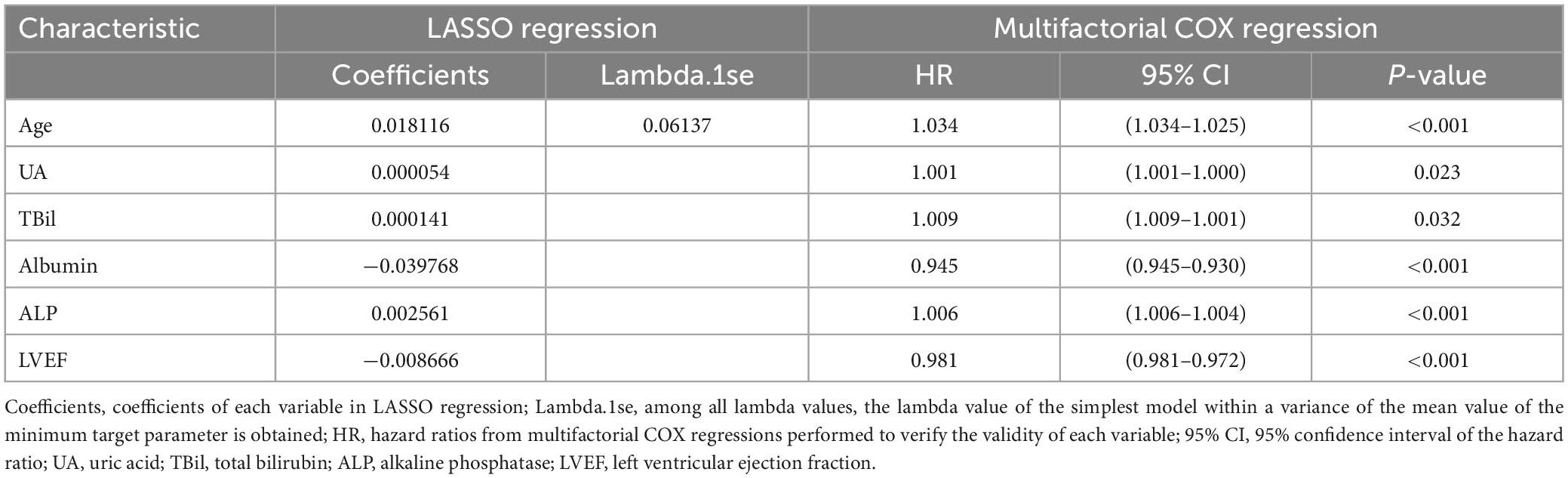
Table 2. Based on the coefficients and Lambda.1se values of the LASSO regression of the training set, multifactorial COX regression to validate the validity of each variable.
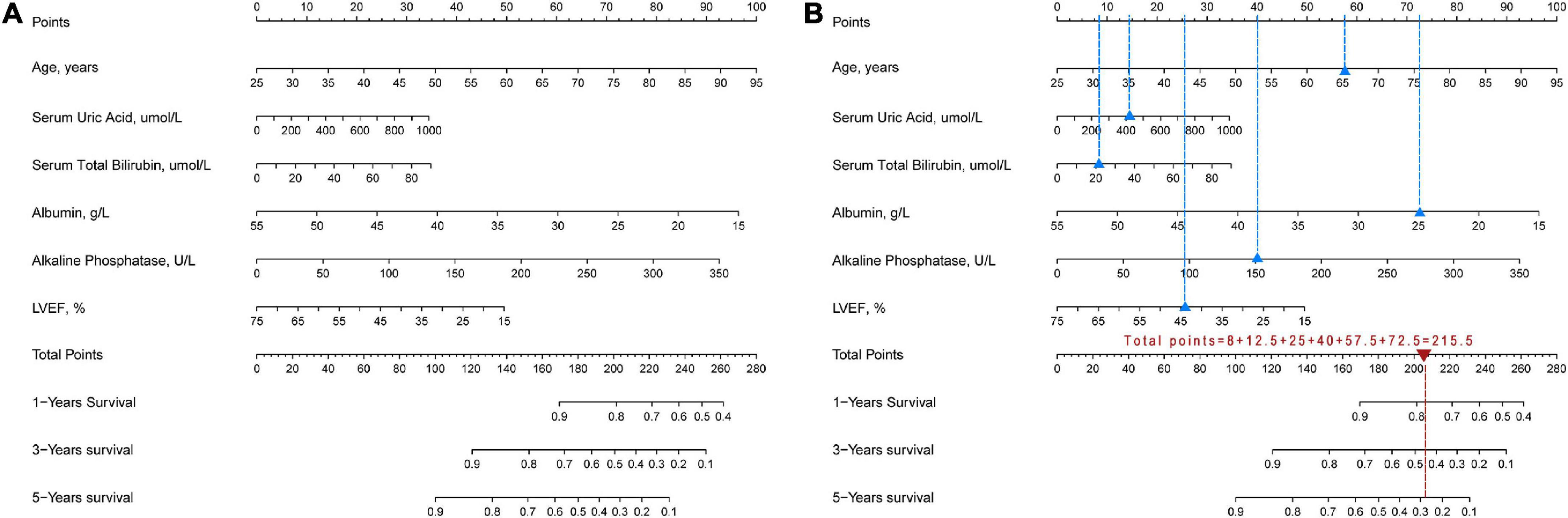
Figure 3. Nomogram for assessing the risk of death within 1, 3, 5 years in IHD patients. (A) Complete nomogram. (B) How to use the nomogram. Blue triangles show clinical indicators for a hypothetical IHD patient and blue dashed lines show the corresponding scores. Red triangles show the total scores and the red dashed lines show the corresponding survival rates.
To validate the reliability of the prediction model, the model is tested using data from the training and validation sets. As assessed by time-dependent receiver operating curve (ROC) analysis, the time-dependent accuracy of the nomogram model in predicting mortality risk at 1, 3, and 5 years is shown (Figure 4). C-index based on the training set 1, 3, and 5 years were 0.705 (0.658–0.751), 0.705 (0.671–0.739), and 0.694 (0.656–0.733), respectively; C-index based on the validation set 1, 3, and 5 years were 0.720 (0.654–0.786), 0.708 (0.650–0.765), and 0.683 (0.613–0.754), respectively (Table 3). In addition, to further validate the model, we analyzed the data from the training and validation sets using calibration plots (Figure 5) and decision curve analysis (Figure 6), and the results show that both calibration plots and DCA curves perform well.
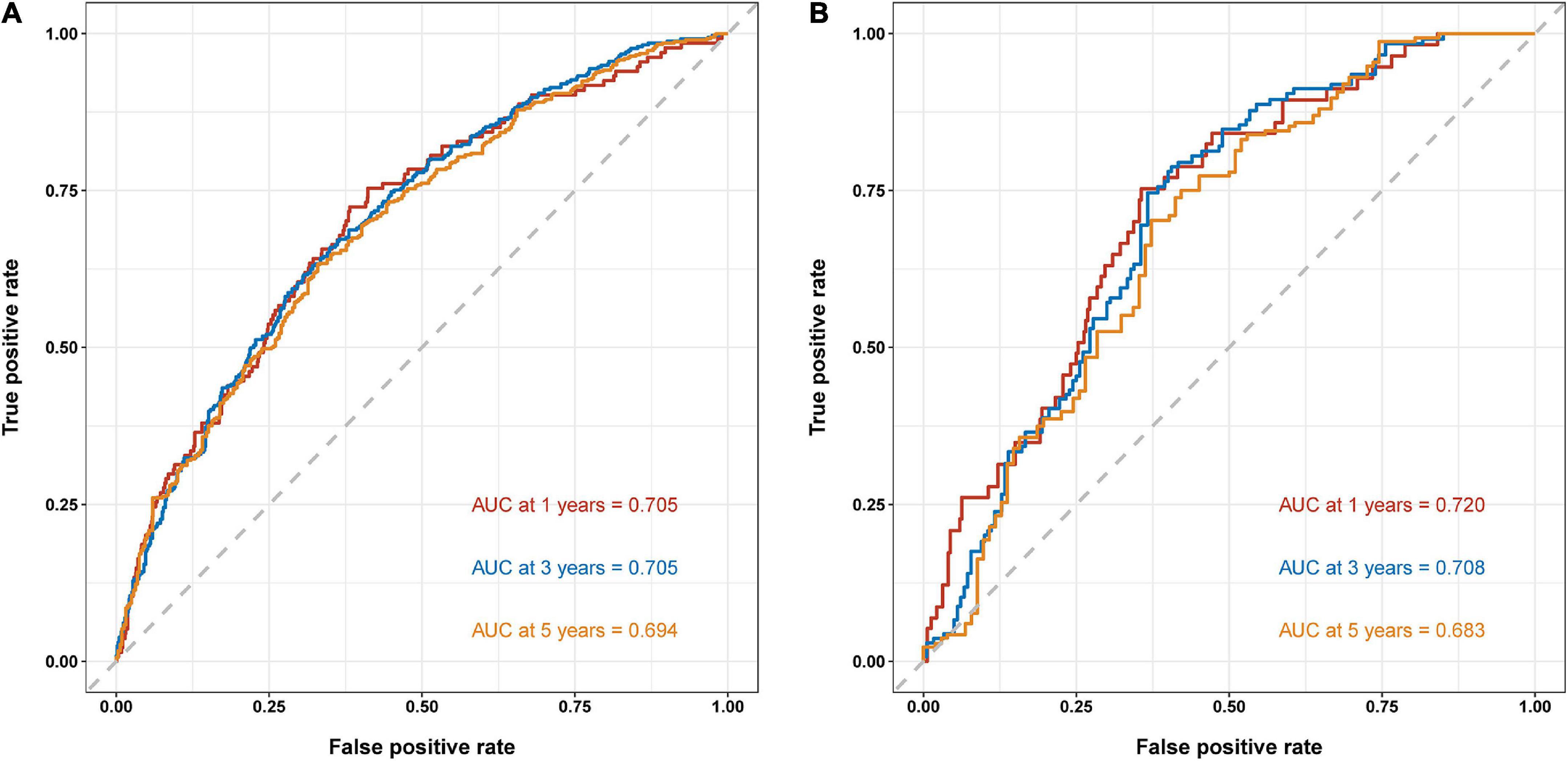
Figure 4. Time-dependent receiver operating curve (ROC) analysis of nomogram model. (A) Training set. (B) Validation set.
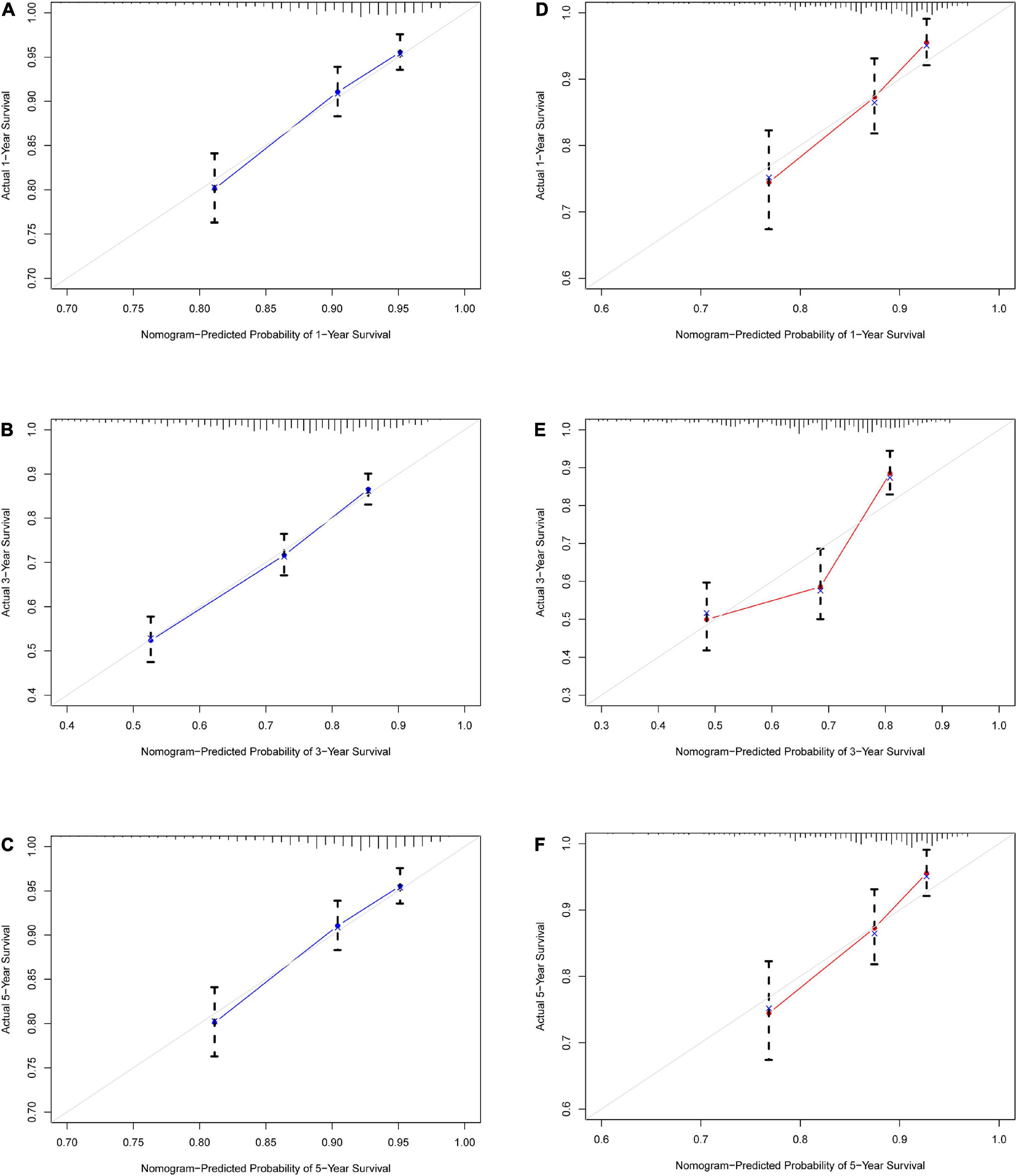
Figure 5. Calibration curve for IHD 1-, 3-, and 5-year mortality risk prediction in the array. (A) Training set 1 year. (B) Training set 3 years. (C) Training set 5 years. (D) Validation set 1 year. (E) Validation set 3 years. (F) Validation set 5 years. The x-axis represents the predicted incidence risk. The y-axis represents the actual diagnosed CHD. The diagonal dotted line represents a perfect prediction by an ideal model. The solid line represents the performance of the nomogram; a closer fit to the diagonal dotted line represents a better prediction.
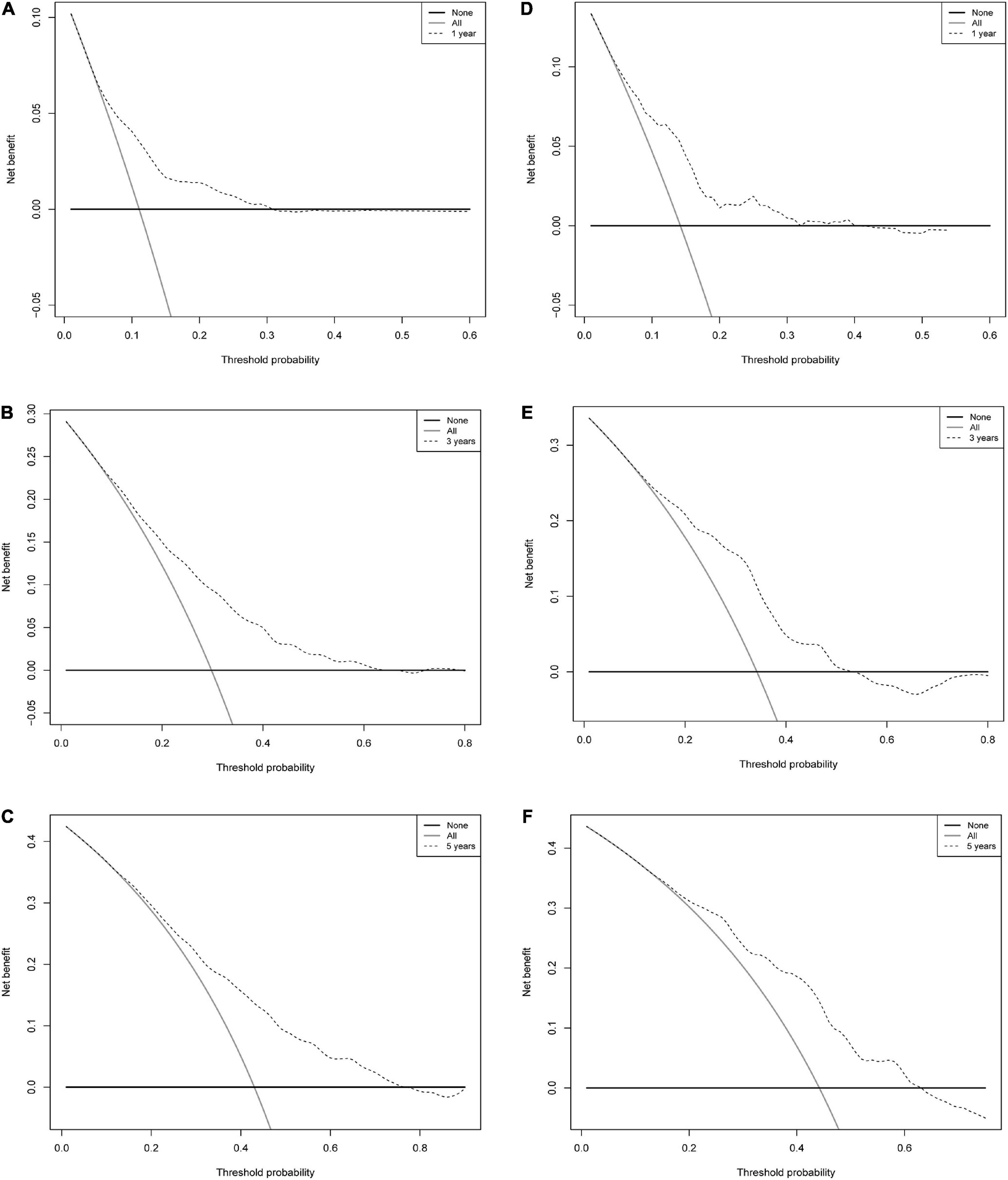
Figure 6. Decision curve analysis of a nomogram of mortality risk at 1, 3, and 5 years in patients with IHD. (A) Training set 1 year. (B) Training set 3 years. (C) Training set 5 years. (D) Validation set 1 year. (E) Validation set 3 years. (F) Validation set 5 years. The y-axis represents the net benefit. Dashed lines represent the IHD mortality risk nomogram. The thin solid line represents the hypothesis that all patients died. Thin solid lines represent the hypothesis of no patient death.
In this study, variables associated with mortality risk in IHD patients were screened by LASSO regression, a method that penalizes complex models with regularized parameters and identifies parameters that minimize mean square error, penalizing insignificant coefficients to zero (15). Finally, we screened six variables, age, uric acid, serum total bilirubin, albumin, alkaline phosphatase, and left ventricular ejection fraction, which had a significant impact on the risk of death in IHD patients, and then we constructed a nomogram prediction model, which is a two-dimensional graph, which uses approximate graph calculation of mathematical functions, is friendly in interface, more accurate, and has easily understood results and has been widely used in the medical field (16).
Our study showed that if an IHD patient was 65 years old, UA was 400 mmol/L, TBil was 20 mmol/L, albumin was 25 mmol/L, ALP was 150 U/L, and LVEF was 45%, the 1-, 3-, and 5-year survival rates of this patient were approximately 78, 45, and 29%, respectively (Figure 3B).
Through LASSO regression and Cox regression analysis, we concluded that the characteristics influencing the risk of death in IHD patients based on the training group in this study included age, uric acid, serum total bilirubin, albumin, alkaline phosphatase, and left ventricular ejection fraction, and the nomograms showed moderately good predictive ability by time-dependent ROC curve analysis, C-index, calibration plot, and DCA curve. Therefore, the impact of six factors cannot be ignored, and they are all strongly associated with the risk of mortality in IHD patients.
Our study suggests that age is one of the most important contributing factors to mortality risk in IHD patients. It has been shown that there is an exponential rise in mortality with increasing age in patients with IHD in both developed and developing countries (6). Increasing age is a major cause of vascular endothelial dysfunction, and aging endothelial cells accelerate the course of cardiovascular disease (17, 18). The mechanism may be that aging decreases circulating CD31 + T cell numbers and migration capacity, which leads to increased susceptibility to endothelial cell apoptosis, shortened telomere length, and decreased telomerase activity (19). In addition, aging leads to reduced cardiomyocyte turnover, increased levels of reactive oxygen species, DNA damage, organelle dysfunction, and accumulation of oxidized proteins and lipids, which leads to decreased cell quality control and accelerates the aging of the heart (20–22).
Uric acid is the end product of purine metabolism, and most scholars believe that the increase of uric acid will significantly increase the risk of cardiovascular disease, promote cardiovascular damage, and increase cardiovascular morbidity and mortality (23). In this study, uric acid was a risk factor for mortality in IHD patients. It has been shown that long-term hyperuricemia may lead to increased vascular sodium deposition and associated inflammatory burden (24). In addition, uric acid can lead to increased autophagy and cardiac hypertrophy in cardiomyocytes by increasing AMPK-ULK1 signaling pathway activity (25).
Previous studies have shown that mild increases in bilirubin protect against cardiovascular morbidity and mortality (26). Nevertheless, our study found that increased serum total bilirubin levels increased the risk of death in patients with IHD. However, several meta-analyzes have shown an L- or U-shaped association between serum bilirubin and the prognosis of coronary artery disease (27–29). In this study, the median bilirubin concentration in patients was 13.78 (9.90, 19.48), and one study demonstrated that the protective effect of bilirubin in patients with coronary heart disease increased with increasing bilirubin levels when bilirubin levels ranged from 3.42 to 13 mmol/L. When the bilirubin level exceeded 13 mmol/L, the protective effect of bilirubin was weakened, and the risk effect gradually emerged as the bilirubin level further increased (30). There is no specific mechanism to explain this phenomenon, but some studies suggest that stress-induced increases in heme oxygenase-1 activity may be exacerbating the process of coronary artery disease (29).
Serum albumin accounts for about 50% of the total plasma protein concentration and is one of the most abundant circulating proteins in the human body and an important indicator of nutritional status (31). Our study suggests an association between albumin and the risk of death in patients with IHD. Albumin has many physiological properties, including anti-inflammatory, antioxidant, and antiplatelet aggregation activities, and it also plays an important role in fluid exchange through capillary membranes (32, 33). Maintaining normal albumin levels can reduce the risk of death from ischemic heart disease (34). Low albumin levels decrease intravascular colloid osmotic pressure and increase inflammation and infectivity (35). This may accelerate the process of vascular atherosclerosis in patients with IHD, which in turn leads to serious complications such as myocardial infarction and heart failure (36, 37).
In this study, ALP was a risk factor for mortality risk in IHD patients. ALP is an enzyme responsible for hydrolyzing phosphates and releasing inorganic phosphates. Several studies have shown a significant linear association between ALP and the development of cardiovascular disease and death (38, 39). The mechanism may be that high serum phosphate levels lead to extracellular matrix degradation, osteochondrocyte changes, and increased production of reactive oxygen species, and stimulate osteoblast transcriptional programs in the vascular system in vascular smooth muscle (40). Calcification of vascular smooth muscle may therefore establish an association between ALP and the risk of death in IHD patients (41).
Left ventricular ejection fraction is the ratio of ventricular volume ejected during systole (stroke volume) to ventricular blood volume at the end of diastole (end-diastolic volume) and is widely used by clinicians as an important indicator for assessing cardiac function (42). Our study showed that LVEF was significantly associated with IHD mortality risk. LVEF is a powerful predictor of serious cardiovascular diseases such as heart failure, myocardial infarction, and arrhythmia (43, 44). In addition, it has been shown that ischemic heart disease patients with severe LVEF reduction will have a significantly increased risk of death (45).
Overall, we created a nomogram model to predict the 1-, 3-, and 5-year risk of death in patients with IHD, which helps clinicians understand the important risk factors affecting the development of death at the time of admission in patients with IHD, thereby taking reasonable tertiary preventive measures, which in turn reduces mortality and improves patient survival. In addition, the six indicators in the model are economical, non-invasive, simple, easily available, and the operation is not limited by hospital conditions.
The key strength of this study is that machine learning was used to screen out several important factors affecting the risk of death in a wide range of indicators, and these parameters were relatively easy to obtain. However, there are still some limitations in this study, first, this study is a retrospective study and there may be unknown confounding factors. Second, this study only collected the data of patients at admission and did not evaluate the way patients were treated for the disease such as through medication and surgery. Finally, this study is a single-center cohort evaluation, and multi-center, large-sample studies are still needed to optimize and validate the model in the future.
This study showed that age, uric acid, serum total bilirubin, albumin, alkaline phosphatase, and left ventricular ejection fraction were significantly associated with the risk of death in patients with IHD, and we constructed a simple nomogram model to predict the risk of death in patients with IHD at 1, 3, and 5 years, and clinicians could assess the prognosis of patients through this simple model at the time of patient admission to make better clinical decisions in the tertiary prevention of the disease.
The original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Medical Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University. The patients/participants provided their written informed consent to participate in this study.
LY and XD contributed to the conception and design, acquisition, drafting of the manuscript, and critical revision for important intellectual content. BA, NJ, and ZS contributed to the interpretation of the data and analysis. X-FS and X-MG contributed to the conception and design, reviewing of the manuscript, and critical revision for important intellectual content. All authors approved the final version and agreed to be accountable for all aspects of the work.
This work was supported by the National Natural Science Foundation of China (U1903212).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Tong C, Madhur M, Rzeszut A, Abdalla M, Abudayyeh I, Alexanderson E, et al. Status of early-career academic cardiology: A global perspective. J Am Coll Cardiol. (2017) 70:2290–303. doi: 10.1016/j.jacc.2017.09.030
2. Matsuda T, Zhai P, Sciarretta S, Zhang Y, Jeong J, Ikeda S, et al. NF2 activates hippo signaling and promotes ischemia/reperfusion injury in the heart. Circ Res. (2016) 119:596–606. doi: 10.1161/CIRCRESAHA.116.308586
3. Wang Y, Du Q, Ren F, Liang S, Lin D, Tian Q, et al. Spatio-temporal variation and prediction of ischemic heart disease hospitalizations in Shenzhen, China. Int J Environ Res Public Health. (2014) 11:4799–824. doi: 10.3390/ijerph110504799
4. Severino P, D’Amato A, Pucci M, Infusino F, Adamo F, Birtolo L, et al. Ischemic heart disease pathophysiology paradigms overview: From plaque activation to microvascular dysfunction. Int J Mol Sci. (2020) 21:8118. doi: 10.3390/ijms21218118
5. Jiang M, Xie X, Cao F, Wang Y. Mitochondrial metabolism in myocardial remodeling and mechanical unloading: Implications for ischemic heart disease. Front Cardiovasc Med. (2021) 8:789267. doi: 10.3389/fcvm.2021.789267
6. Finegold J, Asaria P, Francis D. Mortality from ischaemic heart disease by country, region, and age: Statistics from World Health Organisation and United Nations. Int J Cardiol. (2013) 168:934–45. doi: 10.1016/j.ijcard.2012.10.046
7. Nowbar A, Gitto M, Howard J, Francis D, Al-Lamee R. Mortality from ischemic heart disease. Circ Cardiovasc Qual Outcomes. (2019) 12:e005375. doi: 10.1161/CIRCOUTCOMES.118.005375
8. Lin G, Dai C, Xu K, Wu M. Predictive value of neutrophil to lymphocyte ratio and red cell distribution width on death for ST segment elevation myocardial infarction. Sci Rep. (2021) 11:11506. doi: 10.1038/s41598-021-91082-w
9. Ndrepepa G, Holdenrieder S, Cassese S, Xhepa E, Fusaro M, Laugwitz K, et al. Aspartate aminotransferase and mortality in patients with ischemic heart disease. Nutr Metab Cardiovasc Dis. (2020) 30:2335–42. doi: 10.1016/j.numecd.2020.07.033
10. Saag K, Choi H. Epidemiology, risk factors, and lifestyle modifications for gout. Arthritis Res Ther. (2006) 8(Suppl. 1):S2. doi: 10.1186/ar1907
11. Abi Khalil C, AlHabib K, Singh R, Asaad N, Alfaleh H, Alsheikh-Ali A, et al. beta-Blocker therapy prior to admission for acute coronary syndrome in patients without heart failure or left ventricular dysfunction improves in-hospital and 12-month outcome: Results from the GULF-RACE 2 (Gulf registry of acute coronary events-2). J Am Heart Assoc. (2017) 6:e007631. doi: 10.1161/JAHA.117.007631
12. Liu J, Au Yeung S, Lin S, Leung G, Schooling C. Liver enzymes and risk of ischemic heart disease and type 2 diabetes mellitus: A mendelian randomization study. Sci Rep. (2016) 6:38813. doi: 10.1038/srep38813
13. Suzuki S, Motoki H, Kanzaki Y, Maruyama T, Hashizume N, Kozuka A, et al. Prognostic impact of mineralocorticoid receptor antagonist in patients with heart failure with preserved ejection fraction. ESC Heart Fail. (2020) 7:2752–61. doi: 10.1002/ehf2.12867
14. Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. (2013) 31:1188–95. doi: 10.1200/JCO.2012.41.5984
15. Bertocci M, Bebko G, Versace A, Fournier J, Iyengar S, Olino T, et al. Predicting clinical outcome from reward circuitry function and white matter structure in behaviorally and emotionally dysregulated youth. Mol Psychiatry. (2016) 21:1194–201. doi: 10.1038/mp.2016.5
16. Shi R, Wu B, Niu Z, Sun H, Hu F. Nomogram based on risk factors for type 2 diabetes mellitus patients with coronary heart disease. Diabetes Metab Syndr Obes. (2020) 13:5025–36. doi: 10.2147/DMSO.S273880
17. Contreras A, Orozco A, Resende M, Schutt R, Traverse J, Henry T, et al. Identification of cardiovascular risk factors associated with bone marrow cell subsets in patients with STEMI: A biorepository evaluation from the CCTRN TIME and LateTIME clinical trials. Basic Res Cardiol. (2017) 112:3. doi: 10.1007/s00395-016-0592-z
18. Sun S, Cheng B, Sun P, Wu X, Wu Q, He P. RTEF-1 protects against oxidative damage induced by H2O2 in human umbilical vein endothelial cells through Klotho activation. Exp Biol Med. (2015) 240:1606–13. doi: 10.1177/1535370215587914
19. Kushner E, Weil B, MacEneaney O, Morgan R, Mestek M, Van Guilder G, et al. Human aging and CD31+ T-cell number, migration, apoptotic susceptibility, and telomere length. J Appl Physiol. (2010) 109:1756–61. doi: 10.1152/japplphysiol.00601.2010
20. Terman A, Brunk U. Autophagy in cardiac myocyte homeostasis, aging, and pathology. Cardiovasc Res. (2005) 68:355–65. doi: 10.1016/j.cardiores.2005.08.014
21. Leon L, Gustafsson A. Staying young at heart: Autophagy and adaptation to cardiac aging. J Mol Cell Cardiol. (2016) 95:78–85. doi: 10.1016/j.yjmcc.2015.11.006
22. Heallen T, Morikawa Y, Leach J, Tao G, Willerson J, Johnson R, et al. Hippo signaling impedes adult heart regeneration. Development. (2013) 140:4683–90. doi: 10.1242/dev.102798
23. van Dam E, van Leeuwen L, Dos Santos E, James J, Best L, Lennicke C, et al. Sugar-induced obesity and insulin resistance are uncoupled from shortened survival in Drosophila. Cell Metab. (2020) 31:710–725e717. doi: 10.1016/j.cmet.2020.02.016
24. Klauser A, Halpern E, Strobl S, Gruber J, Feuchtner G, Bellmann-Weiler R, et al. Dual-energy computed tomography detection of cardiovascular monosodium urate deposits in patients with gout. JAMA Cardiol. (2019) 4:1019–28. doi: 10.1001/jamacardio.2019.3201
25. Zhang X, Liu D, Sun Y, Li Y, Ma L, Kong X, et al. Potential risk of hyperuricemia: Leading cardiomyocyte hypertrophy by inducing autophagy. Am J Transl Res. (2020) 12:1894–903.
26. Yang T, Lin Y, Lin Y, Huang C, Chen H, Wu M. Total bilirubin in prognosis for mortality in end-stage renal disease patients on peritoneal dialysis therapy. J Am Heart Assoc. (2017) 6:e007507. doi: 10.1161/JAHA.117.007507
27. Horsfall L, Nazareth I, Petersen I. Cardiovascular events as a function of serum bilirubin levels in a large, statin-treated cohort. Circulation. (2012) 126:2556–64. doi: 10.1161/CIRCULATIONAHA.112.114066
28. Lai X, Fang Q, Yang L, Chen X, Wang H, Ma L, et al. Direct, indirect and total bilirubin and risk of incident coronary heart disease in the Dongfeng-Tongji cohort. Ann Med. (2018) 50:16–25. doi: 10.1080/07853890.2017.1377846
29. Jain V, Ghosh R, Bandyopadhyay D, Kondapaneni M, Mondal S, Hajra A, et al. Serum bilirubin and coronary artery disease: Intricate relationship, pathophysiology, and recent evidence. Curr Probl Cardiol. (2021) 46:100431. doi: 10.1016/j.cpcardiol.2019.06.003
30. Li C, Wu W, Song Y, Xu S, Wu X. The nonlinear relationship between total bilirubin and coronary heart disease: A dose-response meta-analysis. Front Cardiovasc Med. (2021) 8:761520. doi: 10.3389/fcvm.2021.761520
31. Polito C, Martin G. Albumin: Physiologic and clinical effects on lung function. Minerva Anestesiol. (2013) 79:1180–6.
32. Nicholson J, Wolmarans M, Park G. The role of albumin in critical illness. Br J Anaesth. (2000) 85:599–610. doi: 10.1093/bja/85.4.599
33. Nelson J, Liao D, Sharrett A, Folsom A, Chambless L, Shahar E, et al. Serum albumin level as a predictor of incident coronary heart disease: The atherosclerosis risk in communities (ARIC) study. Am J Epidemiol. (2000) 151:468–77. doi: 10.1093/oxfordjournals.aje.a010232
34. Yang Q, He Y, Cai D, Yang X, Xu H. Risk burdens of modifiable risk factors incorporating lipoprotein (a) and low serum albumin concentrations for first incident acute myocardial infarction. Sci Rep. (2016) 6:35463. doi: 10.1038/srep35463
35. Brioschi M, Gianazza E, Mallia A, Zoanni B, Altomare A, Martinez Fernandez A, et al. S-Thiolation targets albumin in heart failure. Antioxidants. (2020) 9:763. doi: 10.3390/antiox9080763
36. Sun T, Shen H, Guo Q, Yang J, Zhai G, Zhang J, et al. Association between neutrophil percentage-to-albumin ratio and all-cause mortality in critically ill patients with coronary artery disease. Biomed Res Int. (2020) 2020:8137576. doi: 10.1155/2020/8137576
37. Djoussé L, Rothman K, Cupples L, Levy D, Ellison R. Serum albumin and risk of myocardial infarction and all-cause mortality in the framingham offspring study. Circulation. (2002) 106:2919–24. doi: 10.1161/01.cir.0000042673.07632.76
38. Cheraghi M, Ahmadvand H, Maleki A, Babaeenezhad E, Shakiba S, Hassanzadeh F. Oxidative stress status and liver markers in coronary heart disease. Rep Biochem Mol Biol. (2019) 8:49–55.
39. Regidor D, Kovesdy C, Mehrotra R, Rambod M, Jing J, McAllister C, et al. Serum alkaline phosphatase predicts mortality among maintenance hemodialysis patients. J Am Soc Nephrol. (2008) 19:2193–203. doi: 10.1681/ASN.2008010014
40. Li J, Xu C, Fan Y, Wang Y, Xiao Y. Can serum levels of alkaline phosphatase and phosphate predict cardiovascular diseases and total mortality in individuals with preserved renal function? A systemic review and meta-analysis. PLoS One. (2014) 9:e102276. doi: 10.1371/journal.pone.0102276
41. Karwowski W, Naumnik B, Szczepański M, Myśliwiec M. The mechanism of vascular calcification - a systematic review. Med Sci Monit. (2012) 18:Ra1–11. doi: 10.12659/MSM.882181
42. de Graaf F, van Werkhoven J, van Velzen J, Antoni M, Boogers M, Kroft L, et al. Incremental prognostic value of left ventricular function analysis over non-invasive coronary angiography with multidetector computed tomography. J Nucl Cardiol. (2010) 17:1034–40. doi: 10.1007/s12350-010-9277-4
43. Mouhat B, Putot A, Hanon O, Eicher J, Chague F, Beer J, et al. Low systolic blood pressure and mortality in elderly patients after acute myocardial infarction. J Am Heart Assoc. (2020) 9:e013030. doi: 10.1161/JAHA.119.013030
44. Dong Y, Shi Y, Wang J, Dan Q, Gao L, Zhao C, et al. Development and validation of a risk prediction model for ventricular arrhythmia in elderly patients with coronary heart disease. Cardiol Res Pract. (2021) 2021:2283018. doi: 10.1155/2021/2283018
Keywords: nomogram, ischemic heart disease, LASSO, mortality, machine learning
Citation: Yang L, Dong X, Abuduaini B, Jiamali N, Seyiti Z, Shan X-F and Gao X-M (2023) Development and validation of a nomogram to predict mortality risk in patients with ischemic heart disease. Front. Cardiovasc. Med. 10:1115463. doi: 10.3389/fcvm.2023.1115463
Received: 04 December 2022; Accepted: 03 February 2023;
Published: 16 February 2023.
Edited by:
Faisal Rahman, Johns Hopkins University, United StatesCopyright © 2023 Yang, Dong, Abuduaini, Jiamali, Seyiti, Shan and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xue-Feng Shan,  eHVlZmVuZ3NoYW4xOTgyQDE2My5jb20=; Xiao-Ming Gao,
eHVlZmVuZ3NoYW4xOTgyQDE2My5jb20=; Xiao-Ming Gao,  eGlhb21pbmdhbzIwMTdAMTYzLmNvbQ==
eGlhb21pbmdhbzIwMTdAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.